Abstract
Objective:
Despite limited evidence supporting atherectomy alone over stenting/angioplasty as the index peripheral vascular intervention (PVI), the use of atherectomy has rapidly increased in recent years. We previously identified a wide distribution of atherectomy practice patterns among US physicians. The aim of this study was to investigate the association of index atherectomy with reintervention.
Methods:
100% Medicare fee-for-service claims were used to identify all beneficiaries who underwent elective first-time femoropopliteal peripheral vascular intervention (PVI) for claudication between 1/1/2019–12/31/2019. Subsequent PVI reinterventions were examined through 6/30/2021. Kaplan-Meier curves were used to compare the rate of PVI reinterventions for patients who received index atherectomy vs. non-atherectomy procedures. Reintervention rates were also described for physicians by their overall atherectomy use (by quartile). A hierarchical Cox proportional hazard model was used to evaluate patient and physician-level characteristics associated with reinterventions.
Results:
A total of 15,246 patients underwent index PVI for claudication in 2019, of which 59.7% were atherectomy. After a median of 603 days (IQR 77, 784) of follow-up, 41.2% of patients underwent a PVI reintervention, including 48.9% of patients who underwent index atherectomy vs. 29.8% of patients who underwent index non-atherectomy (P<0.001). Patients treated by high physician users of atherectomy (quartile 4) received more reinterventions than patients treated by standard physician users (quartiles 1–3) (56.8% vs. 39.6%, P<0.001). After adjustment, patient factors association with PVI reintervention included receipt of index atherectomy (aHR 1.33, 95% CI 1.21–1.46), Black race (vs. White, aHR 1.18, 95% CI 1.03–1.34), diabetes (aHR 1.13, 95% CI 1.07–1.21), and urban residence (aHR 1.11, 95% CI 1.01–1.22). Physician factors associated with reintervention included male sex (aHR 1.52, 95% CI 1.12–2.04), high-volume PVI practices (aHR 1.23, 95% CI 1.10–1.37), and physicians with high use of index atherectomy (aHR 1.49, 95% CI 1.27–1.74). Vascular surgeons had a lower risk of PVI reintervention than Cardiologists (vs. Vascular, aHR 1.22, 95% CI 1.09–1.38), Radiologists (aHR 1.55, 95% CI 1.31–1.83), and other specialties (aHR 1.59, 95% CI 1.20–2.11). Location of services delivered was not associated with reintervention (P>0.05).
Conclusions:
The use of atherectomy as an index PVI for claudication is associated with higher PVI reintervention rates compared to non-atherectomy procedures. Similarly, high physician users of atherectomy perform more PVI reinterventions than their peers. The appropriateness of using atherectomy for initial treatment of claudication needs critical reevaluation.
Keywords: atherectomy, endovascular, femoropopliteal disease, claudication, peripheral vascular interventions
Table of Contents Summary:
Atherectomy was associated with more reinterventions in this retrospective analysis of 15,246 patients who underwent index femoropopliteal peripheral vascular interventions for treatment of claudication. These findings suggest the critical need for re-evaluation of atherectomy use for claudication.
Introduction
Current professional guidelines recommend that intermittent claudication be managed initially with antiplatelet therapy, statin therapy, smoking cessation, optimization of comorbid medical conditions, and supervised exercise therapy1. With optimal medical therapy, less than 5% of patients with claudication eventually progress to chronic limb threatening ischemia1. However, a subset of patients with claudication experience lifestyle-limiting symptoms that can severely impede their quality of life. When intervention is needed, endovascular therapy is widely accepted as the initial intervention of choice, and may include balloon angioplasty, stenting or atherectomy1.
Although some studies have shown that atherectomy decreases rates of bail-out stenting after balloon angioplasty2–5, there have been no data showing a significant advantage in patency or limb salvage outcome for atherectomy compared to other peripheral vascular interventions (PVI) alone5–10. Furthermore, use of atherectomy is associated with distal embolization with varying reported rates and clinical significance8,11. Current guidelines regard atherectomy as non-superior to other PVI technologies for definitive therapy of most femoropopliteal lesions (Grade C level of evidence) with the exception of in-stent restenosis treatment (Grade B level of evidence)12,13.
Atherectomy has contributed to the rising rate of PVIs in the US, particularly in outpatient settings14. We have previously described a wide distribution of physician practice patterns for the use of atherectomy during index femoropopliteal PVI among Medicare beneficiaries in 2019, ranging from 0% to 100%15. $240.6 million (90%) of 2019 Medicare spending for PVI was for atherectomy, which comprised only 54% of PVI cases15. Given these data, there is a critical need for comparative effective analyses evaluating the efficacy of atherectomy vs. non-atherectomy PVI therapies for the treatment in PAD in specific subsets of patients16.
The aim of this study was to investigate the association of index femoropopliteal atherectomy performed for claudication with subsequent PVI reintervention among Medicare beneficiaries. We used the same Medicare patient cohort from our initial atherectomy analysis and analyzed data for up to 30 months post-intervention15.
Methods
Study Cohort
We used 100% Medicare fee-for-service claims to identify Medicare beneficiaries who underwent index PVI (balloon angioplasty, stenting or atherectomy) for claudication between January 1, 2019, to December 31, 2019, using Current Procedural Terminology codes (Supplementary Table 1). Patients who had prior femoropopliteal PVI, prior diagnosis of acute limb ischemia within one year prior to intervention, less than 12 months enrollment, missing demographic information were excluded15. We also excluded patients with chronic limb-threatening ischemia at the time of index reintervention to reduce the heterogeneity of our longitudinal study cohort (Supplementary Table 2).
The Johns Hopkins University School of Medicine Institutional Review Board approved the study. Informed consent was waived as this was a retrospective analysis of claims data.
Outcomes
All patients’ data were captured from the time of their index PVI until the first repeat PVI date, patient death date, or end of study follow-up period (06/30/2021), whichever came first. The primary outcome of the study was any repeat PVI performed after the index PVI (termed reintervention; Supplementary Table 3). Secondary outcomes included subsequent open surgical bypass and subsequent major amputation (Supplementary Table 4).
Main Exposure of Interest
Patients were stratified based on their index femoropopliteal intervention procedure into index atherectomy and index non-atherectomy groups. Patients who underwent any atherectomy during their procedure (alone or with concomitant balloon angioplasty or stent) were defined as receiving an atherectomy PVI. Otherwise, the procedure was classified as a non-atherectomy PVI. This definition was applied for both index procedures (Supplementary Table 1) as well as reintervention procedures, although reintervention CPT codes were expanded to include both femoropopliteal and tibial PVI (Supplementary Table 3).
As a secondary analysis, patients were also stratified based on whether or not their index PVI was performed by a high atherectomy physician user vs. standard atherectomy physician user. We previously reported the national distribution of the physician-level atherectomy metric in a histogram and divided physicians into quartiles on the basis of their proportion of atherectomy use15. Physicians who fell in quartile 4 (performed atherectomy in ≥87.5% of cases) were classified as high atherectomy physician users. Physicians who fell in quartiles 1–3 (performed atherectomy in 0–87.4% of cases) were classified as standard atherectomy physician users.
Other Patient and Physician Covariates
We have previously described our classification of patient demographics and comorbidities and physician practice patterns in detail15. All patient and physician characteristics in the current analysis were derived from 2019 claims data to be consistent with our initial analysis.
In brief, patient demographic data including age, sex, race and ZIP code were collected from 2019 Medicare Master Beneficiary Summary File17. State, census region of residence, and population density of residence were determined using Federal Information Processing Standard (FIPS) code and core-based statistical area (CBSA) codes with the aid of the CBSA to FIPS County Crosswalk18,19. Patient comorbidities were identified using patients’ claims within one year prior to their index PVI.
Physicians were identified using the National Provider Identification (NPI) number. Only physicians who performed greater than 10 index PVI procedures during the study period were included in the analysis according to our data use agreement with the Centers for Medicare and Medicaid. Physician characteristics including sex, years since graduation from medical school, primary specialty, region of practice, population density of practice, number of patients treated with index PVI, and settings of services delivered were obtained from Medicare Data on Provider Practice and Specialty (MD-PPAS) and the Medicare Physician National Downloadable File17,20. Medicare Data on Provider Practice and Specialty data reports summary statistics on each physician’s number of service line items rendered for Medicare beneficiaries by setting each year, which we used for estimating a physician’s percentage of services delivered in a freestanding ambulatory surgical center (ASC) or office-based laboratory (OBL).
Statistical Analysis
We performed two levels of analysis for this study. The first was a patient-level analysis, which included all patients who underwent index femoropopliteal PVI for claudication in 2019. The second was a physician-level analysis, which analyzed reintervention rates for patients treated by high atherectomy physician users (quartile 4) vs. standard atherectomy physician users (quartiles 1–3). The physician-level analysis was limited by our data user agreement; physicians who performed ≤10 femoropopliteal PVI during the study period were excluded from the analysis.
For both analyses, descriptive statistics were used to describe patient demographic, comorbidity, residency characteristics, and physician characteristics. We used Kaplan-Meier curves to estimate the cumulative incidence of reinterventions for patients who received index atherectomy PVI vs. index non-atherectomy PVI (patient-level analysis); and for patients treated by high atherectomy physician users vs. standard atherectomy physician users (physician-level analysis). We compared the incidence of reintervention for each group using log-rank tests. We compared the overall proportion of patients who underwent subsequent open interventions and major amputation between groups using Chi-squared tests.
We then used hierarchical cox proportional hazard models to assess patient- and physician-level characteristics associated with reintervention. Patient characteristics were included as the first level in the model and included receipt of index atherectomy PVI (vs. index non-atherectomy PVI), age, sex, race, comorbidities, population density of residence, and census region of residence. Physician level characteristics were included as the second level in the model and included sex, years since medical school graduation, census region of practice location, population density of practice location, primary specialty, number of patients treated with index femoropopliteal PVI, overall percentage of services delivered in ASC or OBL, and physician quartile for index atherectomy use. We used robust standard error estimates to account for patient clustering within a given physician.
Notably, intervention laterality is coded in the Medicare database, but there is a large amount of missing data around this. We therefore performed a sensitivity analysis limited to patients who had intervention laterality coded at the time of their index intervention and follow-up reinterventions. Cumulative ipsilateral reintervention rates were compared for patients who underwent index atherectomy vs. non-atherectomy PVI, and for high atherectomy vs. standard atherectomy physician users, using Kaplan-Meier curves and log-rank tests.
All statistical analysis was performed using SAS Enterprise version 7.1 (SAS Institute, Cary, North Carolina). All analyses were two-sided, and P<0.05 was defined as statistically significant.
Results
Patient-Level Analysis
There were 15,246 patients who underwent index PVI for claudication included in the analysis, 9,100 (59.7%) of whom received index atherectomy. After a median of 603 days (IQR 77, 784) of follow-up, 41.2% of patients underwent a PVI reintervention, including 48.9% of patients who underwent index atherectomy vs. 29.8% of patients who underwent index non-atherectomy (P<0.001). The estimated 2.5-year incidence of reintervention was 51.4% (95% CI 49.7%, 53.1%) in the index atherectomy group vs. 32.7% (95% CI 30.9%–34.5%) in the index non-atherectomy PVI group (P<0.001; Figure 1) Baseline demographic, comorbidity, and residency characteristics of patients who underwent a reintervention vs. those who did not are shown in Table 1.
Figure 1.
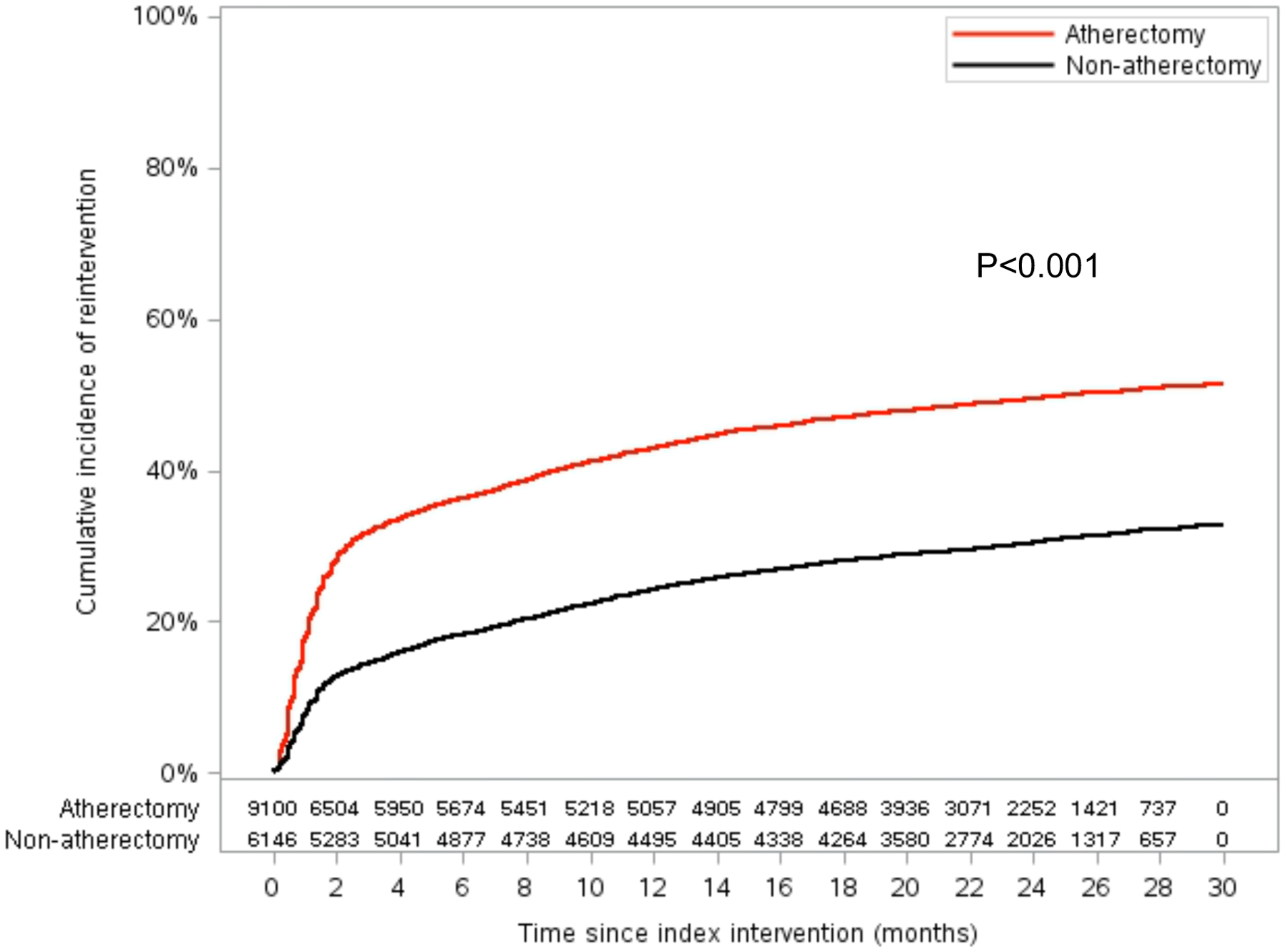
Kaplan-Meier Curve Showing Cumulative PVI Reinterventions for Patients Who Underwent Index Femoropopliteal Atherectomy vs. Non-Atherectomy Peripheral Vascular Intervention
Table 1.
Characteristics of Medicare Patients who Underwent Index Femoropopliteal Intervention for Claudication and Subsequent Reintervention vs. No Reintervention Overall and Stratified by Receipt of Index Atherectomy
| Patient Characteristics | All Patients (N=15,246) | Index Atherectomy PVI (N=9,100) | Index Non-Atherectomy PVI (N=6,146) | |||
|---|---|---|---|---|---|---|
| Reintervention (N=6,279) | No Reintervention (N=8,967) | Reintervention (N=4,448) | No Reintervention (N=4,652) | Reintervention (N=1,831) | No Reintervention (N=4,315) | |
| Age (years) | ||||||
| median, IQR | 74.1 (69.0, 79.6) | 74.3 (69.6, 79.9) | 74.3 (69.2, 79.8) | 74.3 (69.7, 80.0) | 73.6 (68.6, 78.6) | 74.2 (69.5, 79.9) |
| ≤64 | 610 (9.71) | 777 (8.67) | 427 (9.60) | 385 (8.28) | 183 (9.99) | 392 (9.08) |
| 65–74 | 2,817 (44.86) | 4,039 (45.04) | 1,948 (43.79) | 2,098 (45.10) | 869 (47.46) | 1,941 (44.98) |
| 75–84 | 2,292 (36.50) | 3,235 (36.08) | 1,656 (37.23) | 1,686 (36.24) | 636 (34.74) | 1,549 (35.90) |
| ≥85 | 560 (8.92) | 916 (10.22) | 417 (9.38) | 483 (10.38) | 143 (7.81) | 433 (10.03) |
| Sex | ||||||
| Female | 2,521 (40.15) | 3,631 (40.49) | 1,793 (40.31) | 1,849 (39.75) | 728 (39.76) | 1,782 (41.30) |
| Male | 3,758 (59.85) | 5,336 (59.51) | 2,655 (59.69) | 2,803 (60.25) | 1,103 (60.24) | 2,533 (58.70) |
| Race | ||||||
| White | 4,886 (77.81) | 7,455 (83.14) | 3,351 (75.34) | 3,757 (80.76) | 1,535 (83.83) | 3,698 (85.70) |
| Black | 922 (14.68) | 1,011 (11.27) | 728 (16.37) | 618 (13.28) | 194 (10.60) | 393 (9.11) |
| Asian | 65 (1.04) | 86 (0.96) | 47 (1.06) | 50 (1.07) | 18 (0.98) | 36 (0.83) |
| Hispanic | 207 (3.30) | 184 (2.05) | 182 (4.09) | 111 (2.39) | 25 (1.37) | 73 (1.69) |
| Other | 199 (3.17) | 231 (2.58) | 140 (3.15) | 116 (2.39) | 59 (3.22) | 115 (2.67) |
| Comorbidities | ||||||
| ESRD | 1,627 (25.91) | 2,251 (25.10) | 1,199 (26.96) | 1,114 (23.95) | 428 (23.38) | 1,137 (26.35) |
| Diabetes | 3,161 (50.34) | 3,999 (44.60) | 2,283 (51.33) | 2,119 (45.55) | 878 (47.95) | 1,880 (43.57) |
| Hypertension | 5,624 (89.57) | 8,001 (89.23) | 3,970 (89.25) | 4,141 (89.02) | 1,654 (90.33) | 3,860 (89.46) |
| Smoking | 1,961 (31.23) | 2,944 (32.83) | 1,350 (30.35) | 1,501 (32.27) | 611 (33.37) | 1,443 (33.44) |
| Population density of residence | ||||||
| Urban | 5,008 (79.76) | 6,957 (77.58) | 3,592 (80.76) | 3,618 (77.77) | 1,416 (77.33) | 3,339 (77.38) |
| Rural | 1,271 (20.23) | 2,010 (22.42) | 856 (19.24) | 1,034 (22.23) | 415 (22.67) | 976 (22.62) |
| Census region of residence | ||||||
| Midwest | 1,196 (19.05) | 1,934 (21.57) | 756 (17.00) | 882 (18.96) | 440 (24.03) | 1,052 (24.38) |
| Northeast | 716 (11.40) | 1,269 (14.15) | 428 (9.62) | 500 (10.75) | 288 (15.73) | 769 (17.82) |
| South | 3,196 (50.90) | 4,373 (48.77) | 2,379 (53.48) | 2,536 (54.51) | 817 (44.62) | 1,837 (42.57) |
| West | 1,163 (18.52) | 1,382 (15.41) | 879 (19.76) | 727 (15.63) | 284 (15.51) | 655 (15.18) |
| Other | 8 (0.13) | 9 (0.10) | 6 (0.13) | 7 (0.15) | 2 (0.11) | 2 (0.05) |
PVI, peripheral vascular intervention; ESRD, end-stage renal disease
Of the patients who underwent reintervention, 69.7% received atherectomy for their reintervention. 85.7% of patients who received index atherectomy also received a reintervention atherectomy, whereas 30.9% of patients who received index non-atherectomy PVI received subsequent reintervention atherectomy (Figure 2A; P<0.001). The median time to first reintervention overall was 56 days (IQR 24, 240 days), including 48 days (IQR 21, 201 days) in the index atherectomy group vs. 105 days (IQR 29, 316) in the index non-atherectomy PVI group (P<0.001; Table 2). The mean number of reinterventions performed during the study period was higher for patients who received index atherectomy vs. those who did not (2.54±2.42 vs. 1.95±1.67; P<0.001).
Figure 2.
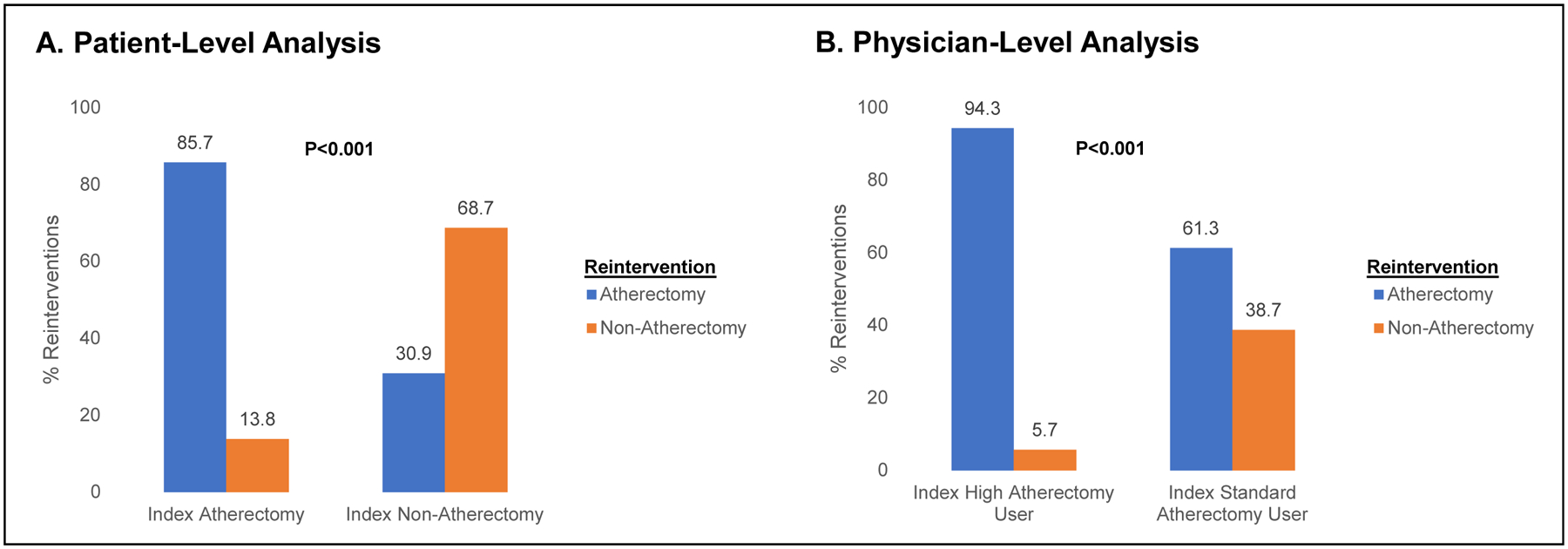
Proportion of Patients Undergoing Reintervention after Index PVI who Received an Atherectomy vs. Non-Atherectomy Reintervention Stratified By Index Intervention Type (Panel A) and Index Physician Atherectomy Use (Panel B)
Table 2.
Description of Reinterventions Performed Overall and Stratified By Receipt of Index Atherectomy
| All Patients | Index Atherectomy PVI | Index Non-Atherectomy PVI | P-value | |
|---|---|---|---|---|
| First PVI Reintervention | N=6,279 (95.60) | N=4,448 (70.84) | N=1,831 (29.16) | |
| Atherectomy PVI (N, %) | 4,377 (69.71) | 3,811 (85.68) | 566 (30.91) | <0.001 |
| Non-atherectomy PVI (N, %) | 1,902 (30.29) | 637 (14.32) | 1,265 (69.09) | |
| Time to first PVI reintervention (days), median (IQR) | 56 (24, 240) | 48 (21, 201) | 105 (29, 316) | <0.001 |
| Median number of PVI reinterventions (IQR) | 2 (1,3) | 2 (1, 3) | 1 (1. 2) | <0.001 |
| Mean number of PVI reinterventions (STD) | 2.37 (2.24) | 2.54 (2.42) | 1.95 (1.67) | <0.001 |
The frequency of open surgical interventions after index PVI for claudication was low overall (N=289, 1.9%), and did not significantly differ between the index atherectomy vs. Non-atherectomy PVI groups (P=0.86). Major amputation occurred in 0.22% of index atherectomy patients and 0.37% of index non-atherectomy patients (P=0.65).
Physician-Level Analysis
There were 9,414 patients treated by physicians who performed >10 PVI procedures during the study years. 62.4% patients were treated with index PVI for claudication by standard atherectomy use physicians and 37.7% were treated by high atherectomy use physicians. The estimated 2.5-year incidence of reintervention was 60.0% (95% CI 56.7%–63.6%) in the high atherectomy use physician group vs. 42.4% (95% CI 40.5%–44.3%) in the standard atherectomy physician use group (P<0.001; Figure 3). Of those patients who underwent reintervention, reintervention with atherectomy was more common for patients who were treated by a high atherectomy physician user compared to those treated by a standard atherectomy physician user (94.3% vs. 61.3%; P<0.001; Figure 2B).
Figure 3.
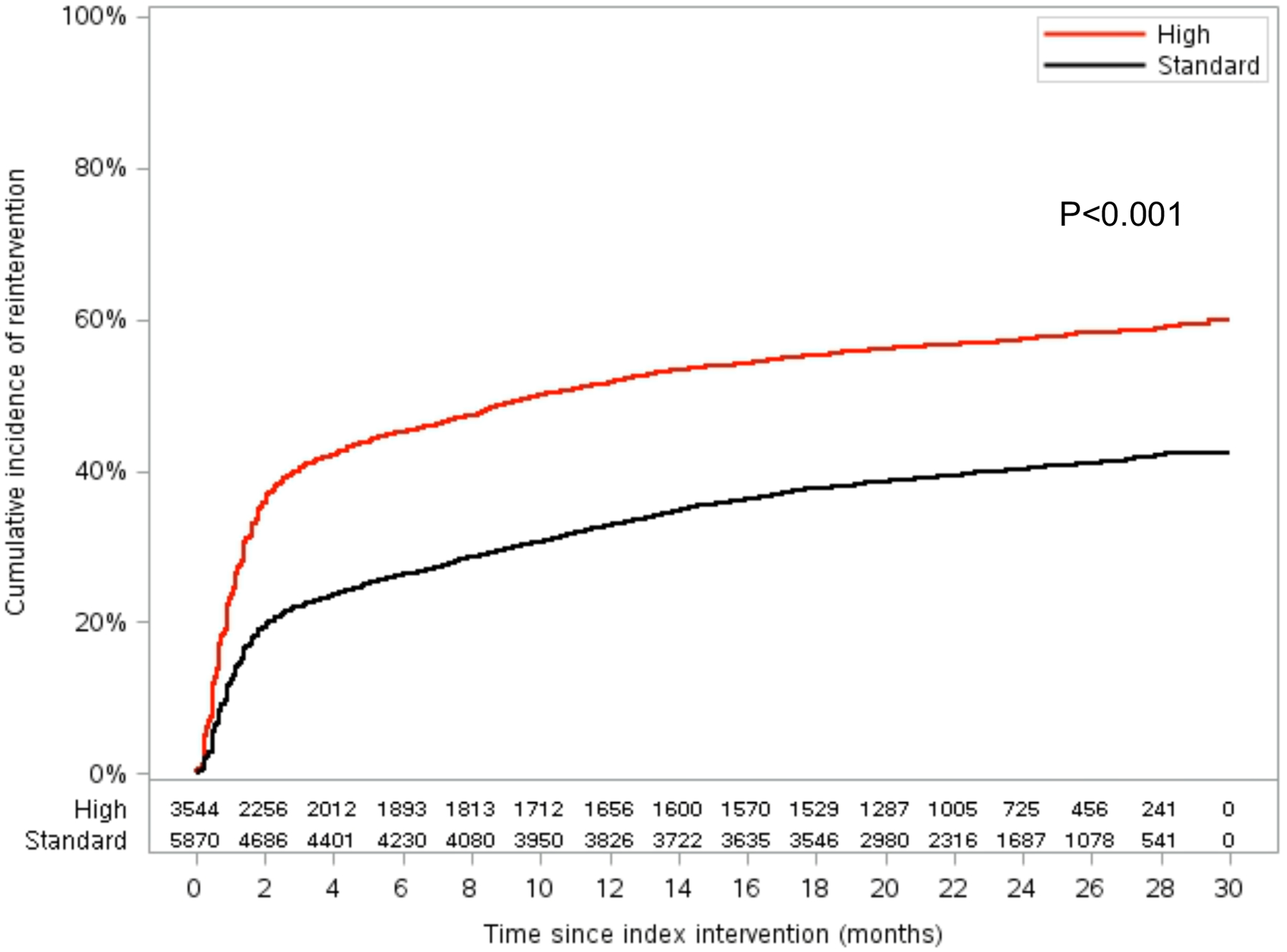
Kaplan-Meier Curve Showing Cumulative Reinterventions for Patients Who Underwent Index Femoropopliteal Peripheral Vascular Intervention by High Atherectomy Physician Users (High) vs. Standard Atherectomy Physician Users (Standard)
Hierarchical Regression Analysis
The crude (unadjusted) hazard ratio (HR) for reintervention was higher for patients who received index atherectomy vs. those who received index non-atherectomy PVI (HR 1.85, 95% CI 1.72, 1.99). The crude HR for reintervention for highest quartile of atherectomy use physicians vs. lowest quartile of atherectomy use physicians was 2.37 (95% CI 2.15, 2.61). After adjusting for patient- and physician-level characteristics (Table 3), the HR for reintervention was persistently higher for patients who received index atherectomy vs. those who received index non-atherectomy PVI (adjusted HR 1.33, 95% CI 1.21, 1.46) and for highest quartile of atherectomy physician user vs. lowest quartile of atherectomy physician user (aHR 1.49, 95% CI 1.27, 1.74). Other patient factors associated with reintervention included Black race (vs. White, aHR 1.18, 95% CI 1.03, 1.34), urban residence (aHR 1.11, 95% CI 1.01, 1.22), and diabetes (aHR 1.13, 95% CI 1.07, 1.21). Other physician factors associated with reintervention included male sex (aHR 1.52, 95% CI 1.12, 2.04) and high-volume PVI practices (aHR 1.23, 95% CI 1.10–1.37). Vascular surgeons had a lower risk of PVI reintervention than Cardiologists (vs. Vascular, aHR 1.22, 95% CI 1.09–1.38), Radiologists (aHR 1.55, 95% CI 1.31–1.83), Cardiothoracic Surgery (aHR 1.51, 95% CI 1.08, 2.13), and other specialties (aHR 1.59, 95% CI 1.20–2.11).
Table 3.
Hierarchical Cox Proportional Hazards Model Assessing Patient- and Physician-Level Characteristics Associated with PVI Reinterventions after Femoropopliteal PVI in the Medicare Population, 2019–2021
| Covariate | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) |
|---|---|---|
| Patient-Level Characteristics | ||
| Index PVI | ||
| Atherectomy | 1.85 (1.72, 1.99) | 1.33 (1.21, 1.46) |
| Non-Atherectomy | Ref | Ref |
| Age (years) | ||
| ≤64 | 1.05 (0.94, 1.18) | 1.01 (0.90, 1.14) |
| 65–74 | Ref | Ref |
| 75–84 | 1.08 (1.01, 1.16) | 1.06 (0.99, 1.14) |
| ≥85 | 0.99 (0.87, 1.10) | 0.92 (0.82, 1.04) |
| Sex | ||
| Female | 1.00 (0.94, 1.06) | 0.96 (0.91, 1.03) |
| Male | Ref | Ref |
| Race | ||
| White | Ref | Ref |
| Black | 1.33 (1.22, 1.45) | 1.18 (1.03, 1.34) |
| Asian | 1.20 (0.89, 1.62) | 0.91 (0.67, 1.23) |
| Hispanic | 1.65 (1.42, 1.93) | 1.04 (0.87, 1.23) |
| Other | 1.31 (1.10, 1.56) | 1.20 (1.00, 1.44) |
| Comorbidities | ||
| ESRD | 1.13 (1.05, 1.21) | 1.03 (0.95, 1.12) |
| Diabetes | 1.21 (1.14, 1.28) | 1.13 (1.07, 1.21) |
| Hypertension | 1.04 (0.94, 1.14) | 1.01 (0.92, 1.11) |
| Smoking | 0.92 (0.86, 0.98) | 0.96 (0.90, 1.03) |
| Population density of residence | ||
| Urban | 1.16 (1.09, 1.27) | 1.11 (1.01, 1.22) |
| Rural | Ref | Ref |
| Census region of residence | ||
| Midwest | 0.89 (0.82, 0.97) | 0.92 (0.69, 1.23) |
| Northeast | 0.84 (0.76, 0.93) | 0.87 (0.59, 1.28) |
| South | Ref | Ref |
| West | 1.31 (1.21, 1.42) | 1.44 (1.03, 2.01) |
| Other | 1.72 (0.55, 5.34) | 9.50 (2.44, 36.99) |
| Physician-Level Characteristics | ||
| Sex | ||
| Male | 1.79 (1.33, 2.38) | 1.52 (1.12–2.04) |
| Female | Ref | Ref |
| Years since medical school graduation | ||
| ≤10 | 0.92 (0.76, 1.12) | 1.04 (0.83, 1.30) |
| 11–20 | 1.16 (1.07, 1.25) | 1.05 (0.93, 1.20) |
| 21–30 | 1.08 (0.99, 1.17) | 1.02 (0.91, 1.14) |
| ≥31 | Ref | Ref |
| Census region of practice location | ||
| Midwest | 0.90 (0.83, 0.98) | 1.11 (0.83, 1.50) |
| Northeast | 0.85 (0.76, 0.94) | 1.06 (0.73, 1.55) |
| South | Ref | Ref |
| West | 1.28 (1.19, 1.39) | 0.82 (0.58, 1.17) |
| Other | 0.63 (0.09, 4.43) | 0.08 (0.02, 0.32) |
| Population density of practice location | ||
| Urban | Ref | Ref |
| Rural | 0.94 (0.83, 1.06) | 0.97 (0.82, 1.15) |
| Primary specialty | ||
| Vascular surgery | Ref | Ref |
| Cardiology | 1.26 (1.18, 1.35) | 1.22 (1.09, 1.38) |
| General surgery | 0.90 (0.76, 1.06) | 0.99 (0.80, 1.23) |
| Radiology | 1.82 (1.64, 2.02) | 1.55 (1.31, 1.83) |
| Cardiothoracic surgery | 1.44 (1.16, 1.79) | 1.51 (1.08, 2.13) |
| Other | 1.81 (1.51, 2.17) | 1.59 (1.20, 2.11) |
| Number of patients treated with index femoropopliteal PVI | ||
| 11–14 | Ref | Ref |
| 15–22 | 1.17 (1.06, 1.28) | 1.11 (0.99, 1.24) |
| 23–189 | 1.49 (1.37, 1.62) | 1.23 (1.10, 1.37) |
| Overall percentage of services delivered in ASC or OBL | ||
| 0–39% | Ref | Ref |
| 40–70% | 1.12 (1.03, 1.21) | 1.07 (0.96, 1.20) |
| 71–100% | 1.43 (1.32, 1.54) | 1.10 (0.96, 1.24) |
| Physician quartile for index atherectomy use | ||
| Q1 | Ref | Ref |
| Q2 | 1.31 (1.17, 1.47) | 1.06 (0.92, 1.22) |
| Q3 | 1.72 (1.54, 1.91) | 1.22 (1.05, 1.42) |
| Q4 | 2.37 (2.15, 2.61) | 1.49 (1.27, 1.74) |
PVI, peripheral vascular intervention; ESRD, end-stage renal disease; ASC, ambulatory surgical center; OBL, office-based laboratory
Cost Analysis
Overall, $129,209,234 USD was reimbursed by Medicare for initial claudication PVI and subsequent reinterventions during the study period. Of this, $118,745,943 USD was reimbursed for index femoropopliteal atherectomy and $10,463,291 USD was reimbursed for index non-atherectomy PVI. Medicare reimbursement was significantly higher for reinterventions after index atherectomy vs. non-atherectomy PVI, both overall ($41,932,458 vs. $2,840,478) and on a per-patient basis ($9,427 per patient vs. $1,551 per patient) (both, P<0.001; Table 4)
Table 4.
Medicare Reimbursement (USD) For Patients who Underwent Index Femoropopliteal Intervention for Claudication Stratified by Receipt of Index Atherectomy (2019–2021)
| Index Atherectomy PVI (N=9,100) | Index Non-Atherectomy PVI (N=6146) | |
|---|---|---|
| Overall, total costs | $118,745,943 | $10,463,291 |
| Index procedure, total costs | $76,813,486 | $7,622,813 |
| Reinterventions, total costs | $41,932,458 | $2,840,478 |
| Reinterventions, costs per patient | $9,427 | $1,551 |
Sensitivity Analysis
A sensitivity analysis limited to patients who had intervention laterality coded at the time of their index PVI was similar to the overall analysis. Patients who underwent index atherectomy had a higher estimated 2.5-year incidence of ipsilateral reintervention compared to the index non-atherectomy PVI group (24.7% vs. 16.6%, P<0.001; Supplementary Figure 1A). The estimated 2.5-year incidence of ipsilateral reintervention was also persistently higher in the high atherectomy use physician group vs. the standard atherectomy physician use group (30.4% vs, 20.5%; P<0.001; Supplementary Figure 1B).
Discussion
Despite limited evidence supporting atherectomy over balloon angioplasty/stenting alone during index PVI2–4,6–10, the use of atherectomy has rapidly increased in recent years21–24. We previously identified a wide distribution of atherectomy practice patterns among US physicians15. The aim of this study was to investigate the association of index femoropopliteal atherectomy with reintervention among patients with claudication. We found that, compared to claudication patients who received an index non-atherectomy PVI, claudication patients who received an index atherectomy are more likely to undergo another PVI and their first reintervention is more likely to be a repeat atherectomy. Furthermore, claudication patients treated by high atherectomy physician users were more likely to require a reintervention, and that reintervention was also more likely to be atherectomy. Overall, our data suggest that the efficacy and appropriateness of using atherectomy for index femoropopliteal interventions for the treatment of claudication needs critical reevaluation.
Our finding that index atherectomy was associated with more frequent reinterventions than other index PVI therapies is concerning, but consistent with recent literature showing a non-superior effect of atherectomy on patency compared to balloon angioplasty with or without stenting5,10,25. In a 2020 Cochrane review evaluating the effectiveness of atherectomy compared to other PVI therapies, Wardle et al. found no significant difference in six-month and twelve-month primary patency or mortality rates between atherectomy and balloon angioplasty or stenting, with or without drug-eluting therapy10. Atherectomy was associated with a slightly lower risk of dissection and need for bailout stenting, although this was based on very low-certainty evidence10. These findings are supported by a 2021 meta-analysis by Wu et al., which evaluated the efficacy and safety of atherectomy for patients with de novo femoropopliteal lesions. This more recent meta-analysis found that atherectomy did not improve primary patency, target lesion revascularization, mortality, or ankle brachial index compared to plain balloon angioplasty alone5.
In our study, we found no significant differences in open surgery or major amputation risk for index atherectomy vs. non-atherectomy PVI, but higher rates of reintervention with atherectomy. Our data suggests that not only is atherectomy not superior to alternative PVI therapies, but when applied to femoropopliteal lesions for claudication it is associated with a 33% higher risk of reintervention. Bath et al. showed similar results in an analysis of Vascular Quality Initiative Data; among patients who underwent index PVI for claudication between 2004 to 2017, use of atherectomy was associated with shorter time to claudication recurrence and more repeat procedures compared to non-atherectomy therapies26. Mukherjee et al. have also documented that 42.7% of office-based and 36.9% of hospital outpatient-based patients who underwent femoropopliteal atherectomy for claudication had a repeat PVI within 18 months27, which is aligned with the 51.4% reintervention rate at 2.5 years that we report. Overall, there is limited evidence endorsing the benefits of atherectomy over other endovascular therapies for femoropopliteal disease, and the high reintervention rates relative the historically low risk of disease progression associated with claudication raises questions about its application in non-limb threatening disease1.
Although there is lack of evidence indicating superiority of atherectomy compared to other endovascular treatments for peripheral artery disease, current practice patterns continue to show an increase in atherectomy procedures, especially in the outpatient setting15. Several studies have also reported on the trend of early PVI in claudicants15,21,28. In a study by Siracuse et al. using commercial insurance from 2007 to 2016, atherectomy use in the ambulatory setting increased from 0.7% in 2007 to 29% in 2016, with a reported 57.5% atherectomy use in ASC/OBLs in 201621. In the current study, we found that 59.6% of patients received atherectomy as their index intervention for claudication, which is similar to the prevalence reported by Siracuse et al21. While physicians working primarily at ASCs or OBLs were associated with a higher risk of reintervention after PVI on unadjusted analysis, this association was attenuated after risk adjustment. Outpatient settings have previously come under scrutiny for overuse of high-paying technologies including atherectomy15,23,24. The current data suggest that post-atherectomy outcomes for claudication are not significantly related to site of service.
Our study identified an increasing risk for reintervention among physicians with high index atherectomy use. Risk of reintervention in patients who were treated by the highest atherectomy users was 49% higher than patients treated by first quartile atherectomy physician users. Furthermore, high atherectomy physician users were more likely to employ atherectomy during reinterventions than standard users. There are a number of prior reports documenting outlier behavior in PVI. Sheaffer et al. have shown that one percent of vascular surgeons received an inordinate amount of total Medicare payments to the specialty in 2016, largely due to disproportionate use of atherectomy29. We have previously shown that a small subgroup of physicians received disproportionately higher payments from Medicare for PVI in 2019 than their peers, also largely as a result of seemingly high atherectomy use practices15. Whether these observations reflect a higher or more complex burden of disease, a difference in specialty representation, or non-efficacious or inappropriate use of atherectomy by a small subset of physicians is unclear.
In addition to index treatment modality and physician atherectomy use patterns, we also found a significant association of physician specialty with reinterventions for claudication. It is well documented that a wide variety of physician specialists perform PVIs for claudication21,30. Our analysis found that vascular surgeons had a lower risk of PVI reintervention compared to cardiologists, radiologists, and other specialties. The reasons behind this are likely multifactorial. We previously found that radiologists and cardiologists are more likely to be high atherectomy users than vascular surgeons, indicating a specialty preference associated with atherectomy15. Cardiologists are trained in the use of atherectomy for percutaneous coronary interventions, as vessel preparation with atherectomy is known to be associated with better outcomes in the setting of severely calcified coronary lesions31–33. As a result, cardiologists may have more of a training bias toward the use of atherectomy than vascular surgeons16. It is also possible that other specialties are not as aware of the treatment algorithm for claudication as vascular surgeons; while the use of medical management as the first-line treatment for claudication is a core component of vascular curriculum34, it may not be as prominent in the fields of radiology or cardiology. The higher rates of reintervention PVIs for claudication among non-vascular specialties is concerning, and deserves additional, dedicated investigation.
Limitations to our study include the inherent limitations of Medicare fee-for-service claim data, which does not include data on symptom severity, indication for intervention, or vascular anatomy (lesions location, length, severity of calcification). Due to lack of anatomic data, our study cannot address the possibility that more severe patient disease, rather than atherectomy use, drove the differences in PVI technology selection and need for reintervention. The database also does not have information on indication for reintervention; we captured endovascular and open reinterventions using claims codes, but specific patient data such as symptom recurrence, restenosis, or other indications for reintervention are not captured. It is possible that not all reinterventions occurred on the ipsilateral side from the index PVI. We performed a sensitivity analysis that showed consistent results in a subgroup of patients with intervention laterality, but there is a large amount of laterality data missing in the claims database. We used CPT does to identify procedures; we do not have further information regarding specific technologies used (plain balloon angioplasty versus drug coated balloon, bare metal stent versus drug eluting stent). We are also unable to comment on medication management prior to or after initial intervention. Aspirin is frequently not prescribed and therefore not reliably available in claims database, and if we were to limit our analysis to patients with Medicare Part D (medication claims), our sample size would drop by more than half35. Finally, the data we report is specific to patients who underwent index femoropopliteal interventions for claudication; we focused our analysis on this population to decrease study heterogeneity. We did not evaluate reinterventions following index tibial interventions for claudication or among patients receiving index PVI for chronic limb-threatening ischemia. The strengths of our study include our use of a national database with a large sample size, our use of very contemporary data (2019 through June 2021), and our ability to track patients longitudinally from their index atherectomy through follow-up.
Conclusions
The use of atherectomy during index PVI performed for claudication is associated with more reintervention rates compared to other non-atherectomy technologies. High physician users of atherectomy and non-vascular surgeons also perform more PVI reinterventions than their peers. The appropriateness of using atherectomy for initial treatment of claudication needs critical reevaluation.
Supplementary Material
Article Highlights:
Type of Research:
Retrospective analysis of Medicare fee-for-service claims data.
Key Findings:
9100 patients treated with atherectomy during their index femoropopliteal peripheral vascular intervention for claudication were at 33% higher risk for reintervention compared to 6146 patients who received non-atherectomy index femoropopliteal peripheral vascular interventions.
Take home Message:
Atherectomy use in index femoropopliteal peripheral vascular interventions for the treatment of claudication needs critical re-evaluation.
Funding:
Dr. Hicks receives funding from the NIH.NIDDK (K23DK124515), American College of Surgeons, and Society for Vascular Surgery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented at the 46th annual Southern Association for Vascular Surgery, Manalapan, Florida, January 19–22, 2022.
Disclosures: The authors have no related conflicts of interest to disclose.
References
- 1.Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication. J Vasc Surg. 2015. Mar 1;61(3):2S–41S.e1. [DOI] [PubMed] [Google Scholar]
- 2.Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360° Trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol. 2014. Aug;26(8):355–60. [PubMed] [Google Scholar]
- 3.Shammas NW, Coiner D, Shammas GA, Dippel EJ, Christensen L, Jerin M. Percutaneous lower-extremity arterial interventions with primary balloon angioplasty versus Silverhawk atherectomy and adjunctive balloon angioplasty: randomized trial. J Vasc Interv Radiol JVIR. 2011. Sep;22(9):1223–8. [DOI] [PubMed] [Google Scholar]
- 4.Shammas NW, Lam R, Mustapha J, Ellichman J, Aggarwala G, Rivera E, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther Off J Int Soc Endovasc Spec. 2012. Aug;19(4):480–8. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Huang Q, Pu H, Qin J, Wang X, Ye K, et al. Atherectomy Combined with Balloon Angioplasty versus Balloon Angioplasty Alone for de Novo Femoropopliteal Arterial Diseases: A Systematic Review and Meta-analysis of Randomised Controlled Trials. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2021. Jul;62(1):65–73. [DOI] [PubMed] [Google Scholar]
- 6.Zeller T, Langhoff R, Rocha-Singh KJ, Jaff MR, Blessing E, Amann-Vesti B, et al. Directional Atherectomy Followed by a Paclitaxel-Coated Balloon to Inhibit Restenosis and Maintain Vessel Patency: Twelve-Month Results of the DEFINITIVE AR Study. Circ Cardiovasc Interv. 2017. Sep;10(9):e004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott I, Cassese S, Groha P, Steppich B, Hadamitzky M, Ibrahim T, et al. Randomized Comparison of Paclitaxel-Eluting Balloon and Stenting Versus Plain Balloon Plus Stenting Versus Directional Atherectomy for Femoral Artery Disease (ISAR-STATH). Circulation. 2017. Jun 6;135(23):2218–26. [DOI] [PubMed] [Google Scholar]
- 8.Diamantopoulos A, Katsanos K. Atherectomy of the femoropopliteal artery: a systematic review and meta-analysis of randomized controlled trials. J Cardiovasc Surg (Torino). 2014. Oct;55(5):655–65. [PubMed] [Google Scholar]
- 9.Mukherjee D, Liu C, Jadali A, Lewis E, Neville R. Effects of Peripheral Arterial Disease Interventions on Survival: A Propensity-Score Matched Analysis Using VQI Data. Ann Vasc Surg. 2021. Oct 10;S0890–5096(21)00654–3. [DOI] [PubMed] [Google Scholar]
- 10.Wardle BG, Ambler GK, Radwan RW, Hinchliffe RJ, Twine CP. Atherectomy for peripheral arterial disease. Cochrane Database Syst Rev. 2020. Sep 29;9:CD006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhen Y, Chang Z, Wang C, Liu Z, Zheng J. Directional Atherectomy with Antirestenotic Therapy for Femoropopliteal Artery Disease: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol JVIR. 2019. Oct;30(10):1586–92. [DOI] [PubMed] [Google Scholar]
- 12.Bailey SR, Beckman JA, Dao TD, Misra S, Sobieszczyk PS, White CJ, et al. ACC/AHA/SCAI/SIR/SVM 2018 Appropriate Use Criteria for Peripheral Artery Intervention: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine. J Am Coll Cardiol. 2019. Jan 22;73(2):214–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman DN, Armstrong EJ, Aronow HD, Gigliotti OS, Jaff MR, Klein AJ, et al. SCAI consensus guidelines for device selection in femoral-popliteal arterial interventions. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2018. Jul;92(1):124–40. [DOI] [PubMed] [Google Scholar]
- 14.Jones WS, Mi X, Qualls LG, Vemulapalli S, Peterson ED, Patel MR, et al. Trends in settings for peripheral vascular intervention and the effect of changes in the outpatient prospective payment system. J Am Coll Cardiol. 2015. Mar 10;65(9):920–7. [DOI] [PubMed] [Google Scholar]
- 15.Hicks CW, Holscher CM, Wang P, Dun C, Abularrage CJ, Black JH, et al. Use of Atherectomy During Index Peripheral Vascular Interventions. JACC Cardiovasc Interv. 2021. Mar 22;14(6):678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman DN, Klein AJP. Atherectomy in Peripheral Vascular Interventions. JACC Cardiovasc Interv. 2021. Mar 22;14(6):689–91. [DOI] [PubMed] [Google Scholar]
- 17.Research data assistance center. Master beneficiary summary file base. [Internet] [cited 2020 Jun 12]. Available from: https://resdac.org/cms-data/files/mbsf-base
- 18.Health Resources and Survey Administration. Defining rural population [Internet]. Official web site of the U.S. Health Resources & Services Administration. 2017. [cited 2020 Jun 12]. Available from: https://www.hrsa.gov/rural-health/about-us/definition/index.html [Google Scholar]
- 19.National Bureau of Economic Research. CBSA to FIPS County Crosswalk. [Internet] NBER. [cited 2020 Jun 12]. Available from: https://www.nber.org/research/data/census-core-based-statistical-area-cbsa-federal-information-processing-series-fips-county-crosswalk [Google Scholar]
- 20.Centers for Medicare & Medicaid Services. Physician Compare Datasets [Internet]. [cited 2020 Jun 12]. Available from: https://data.medicare.gov/data/physician-compare
- 21.Siracuse JJ, Woodson J, Ellis RP, Farber A, Roddy SP, Kalesan B, et al. Intermittent claudication treatment patterns in the commercially insured non-Medicare population. J Vasc Surg. 2021. Aug;74(2):499–504. [DOI] [PubMed] [Google Scholar]
- 22.Bai H, Fereydooni A, Zhang Y, Tonnessen BH, Guzman RJ, Chaar CIO. Trends in Utilization and Outcomes of Orbital, Laser, and Excisional Atherectomy for Lower Extremity Revascularization. J Endovasc Ther Off J Int Soc Endovasc Spec. 2021. Oct 13;15266028211050328. [DOI] [PubMed] [Google Scholar]
- 23.Smith ME, Sutzko DC, Beck AW, Osborne NH. Provider Trends in Atherectomy Volume between Office-Based Laboratories and Traditional Facilities. Ann Vasc Surg. 2019. Jul;58:83–90. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee D, Hashemi H, Contos B. The disproportionate growth of office-based atherectomy. J Vasc Surg. 2017. Feb;65(2):495–500. [DOI] [PubMed] [Google Scholar]
- 25.Gupta R, Siada S, Lai S, Al-Musawi M, Malgor EA, Jacobs DL, et al. Critical appraisal of the contemporary use of atherectomy to treat femoropopliteal atherosclerotic disease. J Vasc Surg. 2021. Jul 22;S0741–5214(21)01668–2. [DOI] [PubMed] [Google Scholar]
- 26.Bath J, Lawrence PF, Neal D, Zhao Y, Smith JB, Beck AW, et al. Endovascular interventions for claudication do not meet minimum standards for the Society for Vascular Surgery efficacy guidelines. J Vasc Surg. 2021. May 1;73(5):1693–1700.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee D, Contos B, Emery E, Collins DT, Black JH. High Reintervention and Amputation Rates After Outpatient Atherectomy for Claudication. Vasc Endovascular Surg. 2018. Aug;52(6):427–33. [DOI] [PubMed] [Google Scholar]
- 28.Hicks CW, Holscher CM, Wang P, Black JH, Abularrage CJ, Makary MA. Overuse of early peripheral vascular interventions for claudication. J Vasc Surg. 2020. Jan 1;71(1):121–130.e1. [DOI] [PubMed] [Google Scholar]
- 29.Sheaffer WW, Davila VJ, Money SR, Soh IY, Breite MD, Stone WM, et al. Practice Patterns of Vascular Surgery’s “1%.” Ann Vasc Surg. 2021. Jan;70:20–6. [DOI] [PubMed] [Google Scholar]
- 30.Ahn SS, Tahara RW, Jones LE, Carr JG, Blebea J. Preliminary Results of the Outpatient Endovascular and Interventional Society National Registry. J Endovasc Ther Off J Int Soc Endovasc Spec. 2020. Dec;27(6):956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdel-Wahab M, Toelg R, Byrne RA, Geist V, El-Mawardy M, Allali A, et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ Cardiovasc Interv. 2018. Oct;11(10):e007415. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Wahab M, Richardt G, Joachim Büttner H, Toelg R, Geist V, Meinertz T, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013. Jan;6(1):10–9. [DOI] [PubMed] [Google Scholar]
- 33.Chambers JW, Feldman RL, Himmelstein SI, Bhatheja R, Villa AE, Strickman NE, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014. May;7(5):510–8. [DOI] [PubMed] [Google Scholar]
- 34.SCORE | Curriculum Outline [Internet]. [cited 2021 Nov 13]. Available from: https://www.surgicalcore.org/public/curriculum
- 35.Medicare Part D Charts - Chronic Conditions Data Warehouse [Internet]. [cited 2021 Nov 21]. Available from: https://www2.ccwdata.org/web/guest/medicare-charts/medicare-part-d-charts
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


