Abstract
The primary hyperoxalurias are three rare inborn errors of the glyoxylate metabolism in the liver, which lead to massively increased endogenous oxalate production, thus elevating urinary oxalate excretion and, based on that, recurrent urolithiasis and/or progressive nephrocalcinosis. Frequently, especially in type 1 primary hyperoxaluria, early end-stage renal failure occurs. Treatment possibilities are scare, namely, hyperhydration and alkaline citrate medication. In type 1 primary hyperoxaluria, vitamin B6, though, is helpful in patients with specific missense or mistargeting mutations. In those vitamin B6 responsive, urinary oxalate excretion and concomitantly urinary glycolate is significantly decreased, or even normalized. In patients non-responsive to vitamin B6, RNA interference medication is now available. Lumasiran® is already available on prescription and targets the messenger RNA of glycolate oxidase, thus blocking the conversion of glycolate into glyoxylate, hence decreasing oxalate, but increasing glycolate production. Nedosiran blocks liver-specific lactate dehydrogenase A and thus the final step of oxalate production. Similar to vitamin B6 treatment, where both RNA interference urinary oxalate excretion can be (near) normalized and plasma oxalate decreases, however, urinary and plasma glycolate increases with lumasiran treatment. Future treatment possibilities are on the horizon, for example, substrate reduction therapy with small molecules or gene editing, induced pluripotent stem cell-derived autologous hepatocyte-like cell transplantation, or gene therapy with newly developed vector technologies. This review provides an overview of current and especially new and future treatment options.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-022-01735-x.
Key Points
| Primary hyperoxaluria is a rare metabolic disorder, with often a fatal outcome if not treated. |
| New medications based on RNA interference are available but need adequate adjustment into the current therapeutic approaches. |
| Future treatment options are on the horizon. |
Background
The primary hyperoxalurias (PHs) are a group of rare but underdiagnosed disorders of hepatic glyoxylate metabolism resulting in excessive endogenous oxalate production, which is their common biochemical hallmark [1, 2]. Three types (PH1–3) can be distinguished according to their specific enzymatic defect in glyoxylate metabolism [3–6]. It is important to understand that PHs per se are not renal diseases, but autosomal-recessive inborn errors of metabolism that usually manifest first as recurrent urolithiasis and/or nephrocalcinosis (Fig. 1).
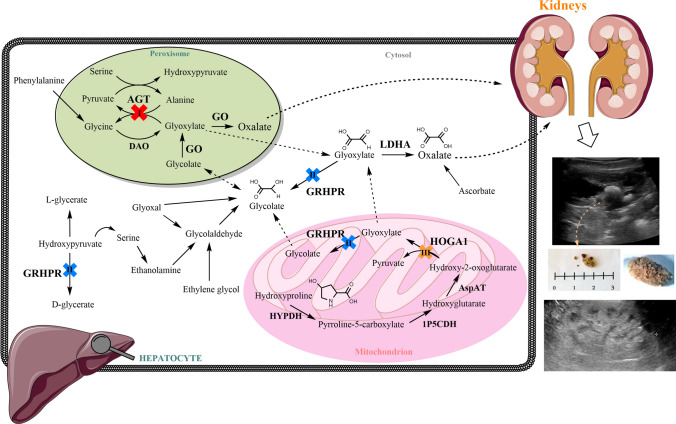
Fig. 1.
Glyoxylate metabolic pathway in the liver (modified from Martin-Higueras et al. [7]). The enzymatic deficits responsible for the three known types of primary hyperoxaluria are shown (I, II, and III). Oxalate is then secreted out of the liver, to be excreted by the kidneys. High levels of oxalate in the kidneys and urine lead to formation of calcium oxalate (CaOx) in the renal tissue and tubular system, causing urolithiasis (upper image) and/or nephrocalcinosis (lower image), which can be detected by ultrasound imaging procedures. 1P5CDH Δ1-pyrroline-5-carboxylate dehydrogenase, AGT alanine:glyoxylate aminotransferase, AspAT aspartate aminotransferase, DAO D-aminoacid oxidase, GO glycolate oxidase, GRHPR glyoxylate reductase/hydropyruvate reductase, HOGA 4-hydroxy-2- oxoglutarate aldolase 1, HYPDH hydroxyproline dehydrogenase, LDH L-lactate dehydrogenase
Mutations in the AGXT gene (encoding for liver-specific alanine: glyoxylate aminotransferase, AGT) cause PH1, mutations in the GRHPR gene (for ubiquitous glyoxylate reductase/hydroxypyruvate reductase) cause PH2, and mutations in the HOGA1 gene (for 4-hydroxy-2-oxoglutarate aldolase type 1, expressed in the liver and kidney) cause PH3. Variants in either gene increase glyoxylate, which is oxidized to oxalate by liver-specific peroxisomal glycolate oxidase (GO, upstream in PH1) and cytosolic lactate dehydrogenase A (LDHA) [downstream in all types, Fig. 1] [7].
Registry data show that PH1 is the most frequent (about 80% of cases) and most devastating type that regularly leads to end-stage kidney failure (ESKD) from infancy to late adulthood. Type 2 primary hyperoxaluria is much less frequent (<10% of cases) in Western registries, but it still bears a considerable risk of chronic kidney disease [CKD] (50%) and ESKD (25%) in adulthood [8]. Type 3 primary hyperoxaluria is regarded as the second most common type (>10% of cases), which also leads to CKD (20% of patients ≥CKD stage 2), while ESKD is only seldom reported so far [5, 6, 9–12].
Oxalate, the simplest dicarboxylic acid, is an end product of human metabolism. Hence, the elevated levels appearing, either endogenously (in PH) or exogenously from the gastrointestinal tract (in secondary hyperoxaluria), have to be eliminated predominantly via urine [1, 13].
Renal excretion allows the elimination of large quantities of oxalate; however, the window of calcium oxalate (CaOx) that can be excreted without causing harm to the kidney is rather narrow [1, 2]. In PH, increased oxalate levels (between 1.5-fold and > 10-fold the upper level of normal) lead to the precipitation of insoluble CaOx in the tubular lumen and interstitial tissue [14]. This deposition induces a strong inflammatory reaction that leads to a progressive decline in kidney function and finally ESKD [15, 16]. Plasma super saturation for calcium-oxalate (ßCaOx > 1 relative unit) is reached at plasma oxalate (Pox) levels > 30 µmol/L (depending on the analytical method used). A distinct correlation of the glomerular filtration rate and Pox was described, calculating the threshold for ßCaOx super saturation at a glomerular filtration rate of ≤ 30–40 mL/min*1.73 m2 body surface area [17]. Especially in the case of PH1, this constant elevation of Pox leads to massive deposition of CaOx in almost all tissues (bone, retina, myocardium, vessel walls, skin), which is defined as systemic oxalosis [1, 17, 18]. Systemic oxalosis also has been reported in a few individuals with PH2 [8, 19] and was also found in patients with PH3 [20].
Diagnosis
Diagnosis is established when a combination of clinical symptoms and laboratory data fit together. Clinically, patients experience recurrent kidney stone episodes and/or nephrocalcinosis, but also hematuria with or without the spontaneous passage of stone(s) (Fig. 1).
The level of urinary oxalate (Uox) excretion defining the primary range is commonly characterized as ≥ 0.8–1 mmol per 1.73 m2 body surface area per day (normal range is < 0.5 mmol/1.73 m2/day) (Tables 1 and 2). The excretion of related markers further clarifies a type-specific PH diagnosis: elevated glycolate suggests PH1, glycerate elevation suggests PH2, and excretion of either/or 4-hydroxy-2-oxoglutarate, 2,4-dihydroxy-glutarate, and 4-hydroxy-glutamate are pathognomonic of PH3 [21–24]. With that information, a confirmatory genetic analysis is performed by sequencing the suspected gene (Fig. 2).
Table 1.
Synopsis of clinical features and characteristics of PH types 1–3
| PH type | Clinical presentation | ESKD risk | Infantile oxalosis | Systemic oxalosis | Clinical remission |
|---|---|---|---|---|---|
| 1 |
UL, NC, UTI, hematuria, failure to thrive |
>50–100% | 10–20% of cases |
+++ In case of advanced CKD/ESKD |
none |
| 2 |
UL, UTI, hematuria NC↓ |
ca. 25% | Not reported | + Reported | None |
| 3 |
UL, UTI, hematuria NC↓↓ is reported |
CKD <20%, ESKD reported | Not reported | (+) Uncommon | Stones also in adulthood |
CCR complete clinical remission, ESKD end-stage renal disease, NC nephrocalcinosis, NC↓ indicates less frequent plus less severe NC in PH2, NC↓↓ indicates that (severe) nephrocalcinosis is a rare finding in PH3, PH primary hyperoxaluria, PH1 PH type 1, PH2 PH type 2, PH3 PH type 3, UL urolithiasis, UTI urinary tract infection
Table 2.
Synopsis of biochemical features in PH types 1–3
| PH type | Degree of hyperoxaluria | Concomitant/intermittent hypercalciuria | Glycolate excretion | L-glycerate excretion | HOG/DHG/4OHGlu excretion |
|---|---|---|---|---|---|
| 1 | +++ | −−− (rare) | +++ (high)a | −−−− (low) | −− (low) |
| 2 | ++ | −−− (rare) | −−− (low) | +++ (high)b | −−− (low) |
| 3 | ++ | rare | −−− (low) | −−− (low) | +++ (high)c |
4OHGlu 4-hydroxyglutamate, DHG 2,4-dihydroxyglutarate, HOG 4-hydroxy -2- oxoglutarate, PH primary hyperoxaluria, PH1 PH type 1, PH2 PH type 2, PH3 PH type 3
aNot all patients with PH1 have elevated glycolate, but all patients with elevated glycolate and oxalate are diagnosed as PH1
bNot all patients with PH2 have elevated glycerate, but all patients with elevated glycerate and oxalate are diagnosed as PH2
cIn patients with PH3, DHG and 4OHGlu appear always, but HOG does not appear in all of them
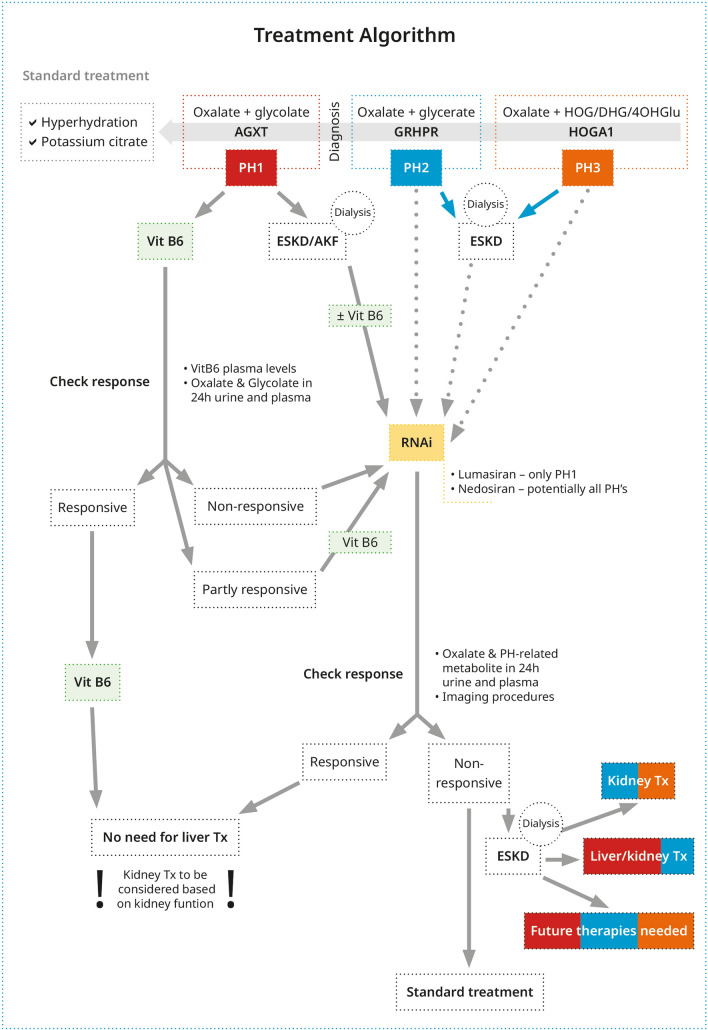
Fig. 2.
Schematic of treatment for patients with primary hyperoxaluria (PH). If diagnosis is suspected, all patients with PH must be given the standard treatment of care by means of hyperhydration and citrate medication. In addition, patients with PH type 1 (PH1) should receive vitamin B6 (Vit B6) until a genetic diagnosis is available. With a genetic diagnosis in hand, the therapeutical processes are depicted in the figure. AKF acute kidney failure, ESKD end-stage kidney failure, PH2 PH type 2, PH3 PH type 3, RNAi RNA interference, Tx transplantation. *Missense mutations that respond to VitB6: p.G170R, p.G41R, p.F152I
The median time from onset of the first symptom to an established diagnosis is 4 years [25], and diagnosis is often only made after a long odyssey. Additionally, not every hospital has the facilities necessary to measure the specific metabolites that assist the diagnostic algorithm. Thus, diagnosis is even more delayed or never established.
Current Treatment Options
The standard treatment of care has included, classically, hyperhydration, crystallization inhibitors, and vitamin B6 (VB6), the latter in PH1. If kidney function declines, dialysis and transplantation are performed. Here, we briefly address the classical treatment, to later focus with more detail on current and future pharmacological treatment options for patients with PH, and how they have changed the therapeutic algorithm in PH.
Standard Treatment
Hyperhydration
Hyperhydration remains the hallmark of conservative treatment for all stone diseases, here including all types of PH (Fig. 2). High fluid intake (> 3 L per 1.73 m2 body surface area, per day) is essential. If necessary, a gastrostomy tube can be installed in infants and toddlers to safeguard an adequate fluid administration day and night. Situations of fluid loss (fever, diarrhea/vomiting, urinary tract infections) or with compromised oral hydration (status post-surgery) require anticipated fluid administration intravenously [26, 27].
Crystallization Inhibitors
Of paramount importance for both the idiopathic kidney stone formers, and patients with PH is treatment with anti-lithogenic substances, for example, citrate and magnesium. Alkaline citrate increases the urinary pH and the citrate excretion (pH values > 7.5 should be avoided, as calcium-phosphate precipitation may happen). With that, CaOx precipitation is significantly reduced [28, 29]. Vitamin B6 is a well-established treatment option for patients with PH1 addressed below within the pharmacological options.
Dialysis
No renal replacement therapy, daily hemodialysis (HD), nor its combination with peritoneal dialysis is able to sufficiently remove the endogenously overproduced oxalate [30–33]. To keep the oxalate threshold as low as possible, frequent and shorter HD sessions, for example, five to six times a week for 3 hours are more efficient, than long, but less frequent dialysis regimens [31, 32]. High flux filters have a slight advantage in oxalate elimination [31]. Post-dialysis Pox of < 30 µmol/L should be achieved, as this is the cut-off value for plasma CaOx super-saturation (depending on the method used) [17]. Pox promptly rebounds after HD, thus nocturnal peritoneal dialysis is a valuable tool for further oxalate elimination [33]. Nevertheless, time on dialysis should be as short as possible to avoid post-transplantation problems by means of exaggerated systemic oxalate depositions.
Transplantation Strategies
Liver transplantation (LTx) cures the enzyme defect in PH1 and hence, sequential or combined liver/kidney transplantation (LKTx) and pre-emptive LTx are possible procedures. Combined LKTx is the method of choice, especially in ESKD and VB6 unresponsive patients without severe systemic oxalosis [34, 35]. Pre-emptive LTx may be an option in a patient with a more rapid decline in kidney function [36], but the timing of that procedure is difficult and sequential KTx may later be necessary [37, 38]. In patients with infantile oxalosis, sequential LKTx, based on anatomical reasons (e.g., small size, inadequate vessels for anastomosis), but also based on severe systemic oxalosis, should be considered to avoid prompt recurrence of oxalosis within the kidney graft [38].
Isolated KTx might be considered in elderly patients with late onset of ESKD and/or with a VB6-sensitive genotype [39, 40]. Isolated KTx was recently described to be equivalent in terms of long-term outcomes to the combined transplantation procedures [35]. Hence, personalized decisions on transplantation procedures are necessary, even more now, considering the new pharmacological options.
In PH2, isolated KTx is the transplant method of choice (8). Although the current follow-up of the tiny group of PH2 patients being transplanted is good, patients with oxalate- related graft dysfunction or problematic follow-up, which make a subsequent liver transplantation necessary, are described [40–42]. In patients with PH3 no data on transplantation procedures are currently available.
Pharmacological Options
Vitamin B6 in PH1: An Inexpensive Solution
One PH1-specific treatment option is the oral application of VB6. Pyridoxal 5’-phosphate (PLP) is one component of VB6, and is the cofactor of all body transaminases, therefore, also of the defective AGT in PH1. It was first described as a treatment option in two patients with PH1 in 1961, long before AGT deficiency was identified as the cause of the disease [44]. It has been observed for several decades that a variable number of patients with PH1 (up to 50%) with residual AGT activity show a reduction in Uox following administration of pharmacologic doses of PLP [45, 46]. Other authors, however, concluded pyridoxine treatment was not efficient [47, 48], opening a debate about the mode of action and response phenomena [49–51]. Data were mostly based on case series, single-case reports, and retrospective trials [52–57].
Side effects, for example, sensory polyneuropathy, are rarely seen even when higher dosages are used, as recorded in investigators brochures and retrospective case series [45]. In PH2 and PH3, PLP treatment is, obviously, inefficient (Fig. 2).
In more detail, VB6 entails six compounds (vitamers) with VB6 activity: pyridoxine, pyridoxal, pyridoxamine, and the activated phosphate esters pyridoxine 5’-phosphate, pyridoxal 5’-phosphate, and pyridoxamine 5’-phosphate [42]. Vitamin B6 is usually administered as pyridoxine hydrochloride and is passively absorbed in the jejunum. In the liver, the VB6 vitamers are enzymatically interconverted [58], but finally deliver pyridoxal 5’-phosphate, the activated form that interacts with AGT as its cofactor [59].
In PH1, missense variants such as p.G170R and p.F152I in the context of the minor haplotype (defined by the presence of the polymorphism p.P11L and p.I340M) generate misfolded AGT enzymes [60], resulting in mistargeted proteins in the mitochondria, with residual catalytic activity, but metabolically inefficient [61, 62]. Other AGT variants can cause protein aggregation (p.G41R, p.I244T), catalytic defects due to reduced PLP affinity (p.G82E, p.G41R), or synthesis defects due to reading frame shifts and an early stop codon (i.e., c.33dupC, splicing mutations, indels) [60].
There are different hypotheses as to why PLP might reduce endogenous oxalate production in PH1. Possible mechanisms are either an increase in AGT dimerization efficiency and intracellular stability, or reduced aggregation propensity, thus increasing AGT enzymatic activity [61, 62], or, most likely, a proper targeting of AGT into the peroxisomes, where it is metabolically efficient [63], or simply a combination of all these factors. Variant AGT proteins are not as stable as wild-type AGT and therefore, stabilizing compounds (i.e., pharmacological chaperones as PLP) might lead to re-stabilization of the AGT protein with an increase of enzymatic function (Fig. 3) [64].
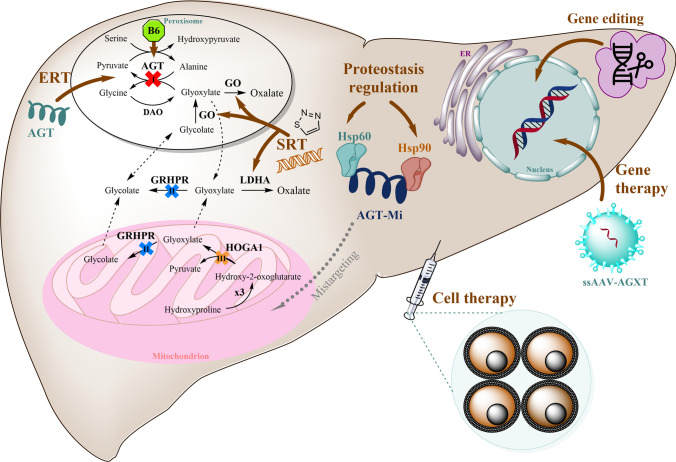
Fig. 3.
Strategies for molecular therapy in primary hyperoxaluria (updated from Martin-Higueras et al. [7]): gene therapy with single-stranded adeno-associated virus (ssAAV) carrying one copy of AGXT cDNA (here also applicable SV40 as a vehicle); cell therapy by hepatocyte transplantation, including the potential autologous transplantation of human induced pluripotent stem cell-derived hepatocytes; proteostasis regulation therapy targeting molecular chaperones (Hsp60 and Hsp90) such as dequalinium chloride, monesin, and emetine, or directly stabilizing the (AGT) enzyme with the cofactor pyridoxine (B6); enzyme replacement therapy (ERT) by delivery of polymer-conjugated AGT proteins into the peroxisomal compartment; and substrate reduction therapy (SRT) through inhibition of glycolate oxidase (GO) in the peroxisome and/or lactate dehydrogenase A (LDHA) in the cytosol either by RNA interference or by small molecules, or by editing the corresponding gene. AGT alanine:glyoxylate aminotransferase, responsible for PH1, AGT-Mi AGT in the minor haplotype, DAO D-amino acid oxidase, GRHPR glyoxylate reductase/hydroxypyruvate reductase, enzyme deficient in PH2, HOGA1 4-hydroxy-2-oxoglutarate aldolase 1, involved in PH3, LDH L-lactate dehydrogenase
In general, response to VB6 treatment seems to depend on the underlying variants [12]. Variants that lead to alterations at the active site directly interacting with PLP (e.g., p.W108R, p.S158L and p.D183N), and those resulting in mitochondrial mistargeting are likely to lead to responsiveness to PLP treatment [61, 65]. Thus, patients carrying one or two copies of p.G170R or p.F152I mutations are more likely to respond to pharmacological doses of pyridoxine, but other mutations are possibly similarly responsive [66, 67]. Classically, pyridoxine has been the VB6 vitamer administered in patients with PH1; however, pyridoxal and pyridoxamine have shown greater efficiency than pyridoxine in vitro and in vivo [61, 68, 69]. In humans, pyridoxamine hydrochloride has shown tolerability and efficacy in patients with diabetic nephropathy [70, 71], but unfortunately, no data are available about the effect of this drug in patients with PH and kidney stone formers from a clinical trial (NCT00490113).
Vitamin B6 should be administered primarily to any patient with suspected PH1 up until a genetic diagnosis is made. Thereafter, genotype-related administration is recommendable (Fig. 2) [34]. It is then first provided in increasing dosages of 5–20 mg/kg per day, aiming to decrease Uox by >30% [72, 73]. Responsiveness is defined by a decrease in Uox excretion (and urinary glycolate, Uglyc) after a test period of a minimum of 3 months at maximum dose [1, 72]. In some patients, normalization of Uox was reached during a long course of VB6 treatment [74, 75]. If responsive, patients remain on the VB6 medication, even if commencing dialysis [72, 76]. In cases of suspected infantile oxalosis, PLP is also given immediately as renal failure can sometimes be reversed in patients with a susceptible genotype (e.g., homozygous for p.G170R) [77]. Response to VB6 treatment is associated, not only with a specific genotype, but also with a better clinical course and manifestation at an older age of ESKD, and even death from ESKD, if ever, in VB6-sensitive patients [12, 67].
A prospective study reported that patients with missense mutations indeed showed a better VB6 sensitivity [72]. About 50% of patients showed a >30% reduction of Uox, but without reaching complete normalization, not even in patients homozygous for the p.G170R mutation. However, not all patients showed such a response, and serum VB6 levels did not correlate with Uox reduction [72]. In addition, intra-familial heterogeneity in response to VB6 was observed despite patients being identified with the same genotype [72]. AGXT/VB6 data need careful interpretation, as absorption and metabolism of VB6 have not been studied in vivo. Thus, the efficacy of VB6 may not totally depend on the AGXT genotype, but also on differences in absorption and metabolism between patients.
Substrate Reduction Therapies: The Expensive Approach
RNA interference (RNAi) therapeutics work at the level of messenger RNA (mRNA) translation. Synthetic small double-stranded RNA molecules (small interfering RNA) bind to a cytoplasmic protein complex (RNA-induced silencing complex), which specifically degrades the targeted mRNA and thus prevents translation into the corresponding protein [7, 78, 79]. This involves placing false information at the site that normally yields an enzymatic protein involved in oxalate metabolism (in the liver). If the protein is not formed, oxalate production can be significantly reduced or even completely blocked. A first RNAi drug, lumasiran (Oxlumo®; Alnylam Pharmaceuticals, Cambridge, MA, USA), was recently approved (end of 2020) by the US Food and Drug Administration and the European Medicines Agency for the treatment of PH1 and has been available via prescription since January 2021 in some countries (Germany, France, Luxembourg, Switzerland, Austria, Italy, Russia, Bulgaria, Poland, Israel, Qatar, and the USA) [80], or via an “early access program” directly from the company in those countries where it cannot be prescribed.
Oxlumo® targets the mRNA of GO, thus blocking the conversion of glycolate into glyoxylate, hence reducing oxalate but increasing glycolate production (Fig. 2). Subcutaneous administration in animals showed a reduction of Uox by 98% [79, 81]. In healthy volunteers, Oxlumo® blocked about 80% of the corresponding mRNA without relevant side effects, except for injection-site reactions (pain and erythema), headache, rhinitis, and upper respiratory tract infections as the most frequent compared to placebo [82, 83]. In patients > 20 kg body weight, Oxlumo® is injected subcutaneously in a dosage of 3 mg per kg body weight, monthly for the first dosages and quarterly after the fourth dose. In patients < 20 kg body weight, a dosage of 6 mg/kg body weight is given monthly for the first four dosages, followed by monthly 3 mg/kg body weight in children < 10 kg and of 6 mg/kg body weight quarterly in patients with a body weight from 10 to 20 kg. In patients with PH1, Uox was reduced by an average of 65.4% [80]. Based on the therapeutic mechanism, patients (and animals) showed an increase in Uglyc; however, it was, and still is, considered harmless [84].
So far, experiences with Oxlumo® treatment (outside long-term studies from the company itself) have been reported only in ten PH1 cases, both children (n = 7) [85–88] and adults (n = 3) [89–91] (Table 3) with the time under treatment reported as from 1 to 18 months. Good tolerance, reduced urinary oxalate/creatinine ratio, and stability (or even slight improvement) of renal function were reported only in the children treated. In contrast, the outcome was not successful in adult patients in whom systemic oxalosis already existed before Oxlumo® was started (Table 3) and, when related to the Pox follow-up. However, other clinical outcome parameters such as amelioration of oxalate osteopathy, or cardiac involvement and no worsening of retinal depositions may help in better interpretation of RNAi efficacy here. Glycolate values (in urine or plasma) were not provided in any case. In one case, however, metabolic acidosis was reported during a 5-month administration period (measured by low HCO3− values and negative base excess) and treatment with sodium bicarbonate was needed [88]. Here, we may speculate that acidosis might possibly be due to very high plasma glycolic acid levels (Table 3).
Table 3.
Patients with primary hyperoxaluria reported with RNAi medications outside clinical trials
| Patient no. and sex | Age of Dx Genetic Dx |
Clinical situation before RNAi | Age of starting RNAi medication and dose | Serum creatinine (Scr)/eGFR | Oxalate and Glycolate | Duration and dose of RNAi | Outcome/ Side effects | Other relevant information |
|---|---|---|---|---|---|---|---|---|
| 1 [88] |
7 years old (post KTx) c.33dupC Phe152Ile |
KTx + HD + B6 + hyperhydration + citrate Oxalosis in eyes |
7 years old (day 34 post-KTx) Lumasiran |
38 ml/min/1.73m2 at 4 months after Tx |
65.9% decrease Uox after 5 months. No raw value provided. Glycolate not measured |
>5 months Fourth dose injected 3 months after third dose + B6 + citrate + hyperhydration |
Slight elevation of GGT and alkaline phosphatase. Developed non-anion gap metabolic acidosis (HCO3- 14 mmol/L and anion gap 8mmol/L) after HD discontinuation due to elevated glycolate | Sodium bicarbonate resolved the metabolic acidosis |
|
2 [87] Male Twin A |
11 months old (p.Gly161Cys p.Gly170Arg) |
Passed stones at 6 weeks old. Kidney stone (100% CaOx) at 9 months old, bilateral UL and bilateral double-J stent. UTI. Bilateral ureteroscopic stone extraction with laser lithotripsy and a complicated bilateral ureteral stent removal + B6 + citrate |
12 months old Lumasiran |
Prior: Scr 0.47 mg/dl Follow up: Scr 0,18 mg/dl |
Prior: Uox/cr 0,19 mg/mg (normal <0.103) After: n/a Glycolate not measured |
8 months 6 mg/kg monthly for 3 months, 3 mg/kg monthly thereafter |
Improved psychologically, and emotionally expressive. Well tolerated |
Preeclampsia at week 31. B6 and citrate discontinued when lumasiran started |
|
3 [87] Male Twin B |
11 months old p.Gly161Cys p.Gly170Arg (assumed from dizygotic twin A) |
At 10 months old, bilateral renal (non-obstructive) and bladder calculi + B6 + citrate |
12 months old Lumasiran |
Prior: Scr 0.31 mg/dl Follow up: Scr 0,12 mg/dl |
Prior: Uox/cr 0,125 mg/mg After: n/a Glycolate not measured |
8 months 6 mg/kg monthly for 3 months, 3 mg/kg monthly thereafter |
Stopped crying episodes. No new stone formation. No metabolic acidosis. Well tolerated |
Preeclampsia at week 31. B6 and citrate discontinued when lumasiran started |
|
4 [86] Female |
9 years old Homozygous for duplication of entire exon 9 |
Recurrent kidney stones treated with ESWL and PNL since 3 years old. At 9 years old, bilateral coralliform stones. + hyperhydration + citrate + low-salt diet + B6 only for 3 months (Uox did not decrease) 5 urological interventions/year during 5 years (doubleJ in and out, ureteroscopy, laser therapy, and ESWL) |
13 years old 3 mg/kg monthly, then quarterly, starting one month after last loading dose Lumasiran |
Prior: Scr 0.75 mg/dl. eGFR 67-60 ml/min/1.73m2 After: eGFR 62 ml/min/1.73m2 at month 18 post 1st dose |
Prior: Uox/cr 0.21–0.28 mol/mol (normal <0.06 mol/mol) Pox: 20 µmol/L, and 32 µmol/L 4 years later (normal <27) After: Uox/cr 0.08 mol/mol (after first dose) 70% mean reduction of ox/cr ratio Pox: 13 µmol/L (after first dose). 16 µmol/L at month 18 60% mean reduction Glycolate not measured |
18 months |
No substantial changes in kidney US at month 18. No new stones. No increase of old stones. No requirement of urological interventions. Temporary and asymptomatic episodes of macroscopic hematuria after the first four injections |
No CaOx deposited in other tissue was observed prior to lumasiran No information provided about continuation of hyperhydration and/or citrate |
|
5 [85] Male |
Antenatal Dx (his sister was the index case) c.33dupC c.321_344del |
9 days old 6 mg/kg/month Lumasiran At month 2: + hyperhydration + citrate At month 4: 3 mg/kg + stiripentol At month 5: 6 mg/kg + stiripentol At month 6: stiripentol withdrawal At month 8: 3 mg/kg/month |
At birth: “normal” At month 10 of lumasiran: “normal” |
At birth: Uox/cr 1263 µmol/mmol Pox 36 µmol/L and marked elevated glycolate (no value) After first injection: Uox/cr 2853–1500 to 1060–1000 µmol/mmol at month 0.5, 1, 2, and 3. Pox 12 µmol/L at 3 months After seventh injection: 226 µmol/mmol No glycolate measured after lumasiran started |
10 months |
Grade III NC after 2 months At month 10, hyperechogenicity started to decrease Well tolerated |
Hyperhydration and citrate given from month 2 after starting lumasiran | |
|
6 [85] (no gender displayed) |
2.5 months old p.Ile244Thr p.Ile244Thr |
At age 2.5 months, grade III NC + hyperhydration + B6 + Mg citrate |
3 months old 6 mg/kg/month Lumasiran |
At Dx: Scr 243 µmol/L, eGFR 8 ml/min/1.73m2 After 9th: Scr 120 µmol/L, eGFR 20 ml/min/1.73m2 |
At Dx: Uox/cr 806 µmol/mmol and Pox 194 µmol/L Elevated glycolate (no value given) After 9th: Uox/cr 310 µmol/mmol (60% reduction) |
10 months | Grade III NC persisted | B6 withdrawn after 3 months under lumasiran |
| 7 [85] |
3.5 months (clinically) 5.5 months: p.Ile244Thr p.Ile244Thr |
At age 3.5 months, grade III NC + hyperhydration + B6 + citrate |
4 months old 6 mg/kg/month first 3 months 3 mg/kg/month other 3 months Lumasiran |
Prior: Scr 30 µmol/L (eGFR 77 ml/min/1.73m2) After: “remained stable” |
Prior: Uox/cr1651–2167 µmol/mmol Pox 36 µmol/L After: Uox/cr 1640–662 µmol/mmol after first and second dose. Elevated plasma glycolate (no value given) |
6 months | After fifth injection, nephrocalcinosis improved from grade III to grade II | Lumasiran started prior to genetic diagnosis, as allowed in France |
|
8 [91] Female |
Not mentioned |
At age 36 years: ESKD and systemic oxalosis. Conventional HD At age 39 years: HD daily |
39 years old (6 months after HD started) 3 mg/kg monthly loading dose and quarterly maintenance (started 1 month after last loading) Lumasiran |
Post KTx: Scr 140 µmol/L Methylprednisolone and antithymocyte globulin treatment resulted in Scr of 200 µmol/L (day 25 post KTx). 10 weeks post KTx, Scr 169 µmol/L |
Prior: Pox ~100 µmol/L (normal <33) After: Pox ~80 µmol/L after second dose, >100 µmol/L after third dose, and ~27 µmol/L after fifth dose (before KTx) After KTx (d25): Pox 24 µmol/L and Uox 1.15 mmol/day (normal < 0.45); 10 weeks after Pox 20.9 µmol/L Glycolate not measured |
6 months, and continued on the seventh week after KTx |
KTx after fifth dose. Inmunosuppression: basiliximab, steroids, tacrolimus, and everolimus + 1HD session + hyperhydration + citrate + B6 + indapamide + low-oxalate diet No adverse events or drug interactions |
Oxalate nephropathy and rejection day 25 post-KT due to release of systemic oxalate stores |
|
9 [90] Female |
40 years old c.680+1G>A c.680+1G>A (after kidney biopsy positive for CaOx crystals) |
NL at age 19 years and 33 years, requiring lithotripsy. At age 40 years: hypertension, acrocyanosis, ESKD, systemic oxalosis in skin, and subcutaneous vessels. HD + B6 |
41 years old (11 months after HD initiation) 1mg/kg/month for 3 months, then quarterly Lumasiran |
Prior: Scr 103 µmol/L (19 months before) and at presentation: Scr 1644 µmol/L (eGFR 2 ml/min/1.73m2) |
At presentation: Uox 319 µmol/day Pre-HD prior lumasiran: Pox 98.2 µmol/L Pre-HD post 3 months of lumasiran: 62.8 µmol/L Glycolate not measured |
3 months | No signs of renal recovery, increased of extra-renal involvement: bilateral swan-neck deformities, reduced cardiac systolic function, and pulmonary hypertension |
Stones of calcium, magnesium, oxalate, and phosphate Awaiting kidney-liver transplantation after lumasiran withdrawal |
|
10 [89] Male |
38 years old p.Arg122* p.Arg122* |
Long-standing HD for ESKD Severe calcified kidneys, osteosclerosis, and focal calcification of the iliac-femoral axis. Cardiac oxalate deposits |
38 years old (dose not reported) Lumasiran |
On non-HD days, Scr 6 mg/ml |
Prior: Pox 130 µmol/L No follow-up reported Glycolate not measured |
1 month | ||
|
11 [93] Female |
5 years old c.973delG c.973delG |
Symptomatic NL since 3 years old needing multiple ureteroscopy and ESWL. At age 20 years: ESKD and HD started |
20 years old (5 months after starting HD) 1.5 mg/kg Nedosiran |
Prior HD: Scr 6 mg/dL (eGFR 9 ml/min/1.73m2) |
Prior HD: Pox >60 µmol/L On HD: max 83 µmol/L Pre-nedosiran: 55.5 µmol/L Post-fifth dose: 13.9 µmol/L |
6 months |
Decreased weekly HD sessions over nedosiran treatment (from 6 per week to 3 per week) No new NL events Only temporary injection site discomfort as a side effect |
Patient elected to be removed from liver transplant waiting list during nedosiran treatment Awaiting renal transplantation |
B6 vitamin B6, CaOx calcium oxalate, Dx diagnosis, eGFR estimated glomerular filtration rate, ESKD end-stage kidney failure, ESWL extracorporeal shock wave lithotripsy, GGT gamma glutamyl transferase, HD hemodialysis, KT kidney transplantation, n/a not assessed, NC nephrocalcinosis, NL nephrolithiasis, Pox plasma oxalate, UL urolithiasis, Uox urinary oxalate, Uox/cr oxalate/creatinine ratio, US ultrasound, UTI urinary tract infection, PNL percutaneous lithotripsy, RNAi RNA interference
Another RNAi medication, nedosiran (DCR-PHXC; Dicerna/Novo Nordisk, Bagsværd, Denmark) interferes with the translation of liver-specific LDHA, preventing the conversion of glyoxylate to oxalate supposedly in all PH types [92]. Its efficacy in reducing Uox was also proven in animal models [81]. Nedosiran is also subcutaneously injected; however, in a fixed dose of 170 mg on a monthly basis in an adult patient weighing > 50 kg and of 136 mg in a patient weighing < 50 kg, but corrected to body weight when administered in children (3.5 mg/kg body weight in patients aged 11–18 years and 2 mg/kg in patients aged < 11 years). In a pilot study and a long-term follow-up of the same patients, nedosiran achieved a significant decrease in Uox, up to normalization [78], a result that was comparable to Oxlumo® [78, 92]. However, no conclusive data were seen in PH2, and data on PH3 are still unpublished from a recent phase I trial (NCT04555486). So far, only one case outside clinical trials has been published with experience under nedosiran treatment (compassionate use) [93]. The patient with PH1 was undergoing HD because of ESKD at age 20 years, when nedosiran was started, reporting some discomfort at the injection site. After five doses, Pox had decreased and HD sessions per week were consecutively reduced. The patient was withdrawn from the LTx list, and is awaiting isolated Tx [93].
The potential long-term benefits for patients of the substrate reduction therapy based on RNAi, namely, better preservation of renal function and, at best, prevention of progression to ESKD and systemic oxalosis, remain to be confirmed. Although the healthcare costs for patients with PH are significantly higher than those in matched cohorts without other diseases [94], the very high price of RNAi-based drugs (average expenses are, at least, $490,000 per patient per year) and the requirement for repeated dosing are major drawbacks. To reduce this socioeconomic burden, it may, at least for the body weight-related dosing of Oxlumo®, be recommendable, to provide medication via specialized/centralized pharmacies, which can adequately produce patient-related dosing, so that no medication needs to be thrown away. This procedure was already established by one insurance company in Germany. Especially problematic in children is their complaint about severe injection-site pain. The main differences between both RNAi medications are the dosage regimen during the maintenance phase (monthly vs quarterly), and the increased glycolate production when Oxlumo® is used. As long-term experience is still missing (longest treatment published is 18 months, Table 3), and nedosiran is still pending Food and Drug Administration and European Medicines Agency approval, it is currently impossible to make clear recommendations regarding the use of either medication for a patient with PH1, or even a combination of both, and the long-term effects of any of them.
VB6 versus RNAi: Which Treatment When?
So, what should be the current best treatment practice recommendations? Vitamin B6 is cheap and known to be helpful for patients with specific genotypes. RNA interference medications are expensive but appear to be highly effective in all patients with PH1. Thus, the genotype, which should be known, could be the decision marker. Therefore, VB6 should be administered as the first-line drug treatment in patients with sensitive genotypes. An effect should be visible relatively promptly, by analyzing Uox/Uglyc or Pox/Pglyc, respectively, according to kidney function (Fig. 2) [27]. Additional treatment is not necessary with normalization or near normalization of these parameters, especially in patients with good kidney function. Furthermore, in patients undergoing dialysis, an additive effect of RNAi treatment is not expected if a patient is truly sensitive to VB6 treatment. This is further supported by a recent paper from the OxalEurope registry showing the favorable results of isolated KTx in patients with VB6-sensitive mutations under treatment. Isolated KTx was equivalent to, or even better than combined LKTx in this group of patients [35].
From a mechanistic point of view, one would expect the effect of RNAi treatment (depletion of oxalate precursors/substrates) is independent of the effect of VB6 treatment (partial restoration and proper targeting of AGT to the peroxisome in susceptible genotypes) (Fig. 1). Thus, one could assume synergistic benefits from a combination of RNAi and VB6 in patients with susceptible missense genotypes. This may, at best, lead the way to a more profound normalization of Uox, but also to a reduction in the increase in Uglyc in those patients treated with Oxlumo®. In patients with suspected PH presenting with acute kidney insufficiency or with ESKD, they should receive VB6 until a diagnosis is definitely established. After a diagnosis of PH1, and no obvious VB6 responsiveness, or as an additional rescue medication, RNAi can be administered, together with renal replacement therapies, aiming to quickly reduce the bulk of oxalate from the body (Fig. 2). This is, of course, a very personalized treatment procedure, and also relates to the ability of performing diagnostic evaluations.
In all other patients, either those that cannot be treated with VB6 or in those for whom VB6 medication did not reach relative normalization, RNAi medication should be considered. Currently, there is only minor experience with Oxlumo® or compassionate use with nedosiran reported in the literature (Table 3). Reports show the decline in Uox and Pox, as per reported in the pivotal studies, but emerging evidence also shows that careful and personalized strategies have to be applied, to, for example, avoid recurrence of oxalosis in a kidney graft [85, 88, 91], or to adequately reduce Uox excretion by adaptation of medication [85].
Adequate dosing will be most problematic in patients with ESKD, in whom Pox is used currently as the primary outcome parameter. Even with our limited experience, it is recognized that Pox may initially decline, but increase very rapidly again when body oxalate stores are dissolving, thus making it problematic to only relate treatment success to a Pox follow-up. Here, further markers, such as repeated imaging procedures detecting changes in systemic oxalate deposition, are needed to declare the success or failure of treatment. In responsive patients, RNAi medication negates the need for liver transplantation. However, kidney transplantation may be still necessary depending on the clinical outcome (Fig. 2) [95].
If both RNAi medications fail singularly, combined administration of GO and LDHA blockers may be considered. Examples are the PH1 infant treated with lumasiran and stiripentol [85] (a small molecule that inhibits LDHA, see below) (Table 1), and new small molecules that inhibit both GO and LDHA enzymes, showing efficacy in primary hepatocytes from all PH animal models (1–3) [96, 97]. If these all fail, patients must remain on standard treatment of care and a close follow-up evaluation of the disease course. When kidney function declines, renal replacement therapy and transplantation may be necessary. This group of patients may benefit from future therapeutic strategies (see below), which might also prevent the need for a transplant (Fig. 2).
Other Approaches for Substrate Reduction
Glycolate oxidase inhibition can also be achieved with an oral small-molecule medication targeting the protein, after showing proof of principle in animal studies [98, 99]. The drug BBP-711 is currently being tested in a phase I trial by Cantero Therapeutics Inc. (Palo Alto, CA, USA) [NCT04876924]. Gene editing of the HAO1 gene, the gene coding for GO, has been developed in the animal model using Crispr-cas9 technology [100], and the applicability in humans is currently in development by Precision Biosciences (Durham, NC, USA).
Stiripentol (Diacomit), a LDHA-targeted oral commercial medication with the primary indication Dravet syndrome, was recently repurposed for the use in primary hyperoxaluria [101, 102]. Stiripentol significantly reduced Uox in one patient with PH1 with good kidney function after 10 weeks of treatment. However, in patients with PH1 with CKD or ESKD, this medication did not produce a significant decline in Uox or Pox [103, 104]. Currently, a pivotal study is ongoing for all types of PH, for which recruitment has been finished (NCT03819647).
Other LDHA-targeted small molecules are currently in the pipeline, such as CHK-336 from Chinook Therapeutics [105]. Silencing the LDHA gene using Crispr-Cas9 technology delivered in adeno-associated viral vectors has been recently proven to be safe and efficient in PH1 and PH3 mouse models, thus supporting that LDHA inhibition potentially treats all types of PH [106]. Regrettably, LDHA inhibition did not show efficacy in patients with PH2 when treated with nedosiran.
Oxalate-Degrading Formulations
It is well known that the intestinal tract plays an important role in oxalate homeostasis in states of health and disease. Here, Oxalobacter formigenes, an obligate anaerobe microbe, plays an important role, as oxalate is its sole source of energy [107]. Oxalobacter utilizes a symbiotic relationship concerning the adjustment of oxalic acid absorption in the gut [108, 109]. It was shown repeatedly in animal models of PH and secondary hyperoxaluria that oxalate can be eliminated via the intestinal tract [110–112]. Such treatment showed its efficacy in trials with orally administered O. formigenes, as a paste or capsule (Table 1 in the Electronic Supplementary Material [ESM]), in patients with PH with normal renal function or at ESKD [113, 114]. Urinary oxalate or Pox levels decreased significantly, and clinical symptoms were ameliorated in the ESKD population [110, 112, 113]. However, these results were not repeated in further phase III trials, thus the manufacturer stopped the development process [115].
Different approaches are currently in (pre-)clinical studies based on orally administered oxalate-degrading formulations to the gut (Table 1 of the ESM). All of them have the common aim of degrading oxalate in the intestinal tract and, hence, primarily are regarded as treatment options in patients with secondary hyperoxaluria (overview in Table 1 of the ESM about recent and current studies). However, these medications may have their place also in, at least, a supportive manner in patients with PH. The basis for this assumption is the study in patients with PH undergoing dialysis, who had received long-term Oxalobacter treatment and showed amelioration of clinical symptoms aside the reduction in plasma oxalate values [113]. We speculate though, that concomitant treatment, for example, alongside VB6 or RNAi and renal replacement therapy, might be helpful in patients with PH with severe systemic oxalosis.
Further Approaches
Induced pluripotent stem cell-derived hepatocytes have been explored to model human metabolic liver diseases [95, 116]. Recently, human induced pluripotent stem cells were generated from peripheral blood mononuclear cells and dermal fibroblasts of patients with PH1. The generated PH1-human induced pluripotent stem cell lines were reprogrammed, showing an ability to differentiate in vivo to the three germ layers [117, 118]. This may not only be useful to study the disease in detail but may, in the future, lead the way to an autologous hepatocyte-like cell transplantation (Fig. 3), when a proliferation advantage of the cells over the diseased hepatocytes is probably given [119]. This proliferation advantage was, however, not observed, when liver cell transplantation was performed in one patient with PH1 [120]. However, on the contrary, such a procedure has yielded quite successful results in an animal model of PH1, in which mice underwent procedures to destroy the native liver, but such measures are unsuitable for human application [121].
In the Agxt1-knockout mouse model, PH1 can be healed with gene therapy (Fig. 3) [122]. However, for use in humans, optimization of vector technologies is necessary, as a single dosage might not be sufficient because almost all hepatocytes must be transfected. Multiple dosage, however, could induce severe immune responses if the current vectors are used [27]. SVac, a vector derived from macaque polyomavirus SV40, was safely administered intravenously already in mice and non-human primates and resulted in adequate expression of the applied gene in their livers [123]. Because SVac is a non-integrating virus, which replication is defective and non-immunogenic, it is also safe for human use [124]. As the affected AGXT gene in PH1 is liver specific, patients might therefore benefit from such a gene therapy, currently in development by Amarna Therapeutics.
Pharmacological chaperone therapy aims to use small molecules to assist misfolded proteins in folding correctly and reaching the appropriate subcellular location, so that they function as proteostasis regulators [125] (Fig. 3), as it does with VB6 [61]. Dequalinium chloride and monensin both interfere with mitochondrial transport, preventing the mistargeting of AGT in cell models, and restoring AGT into the peroxisomes [126, 127], like VB6. Additionally, aminooxyacetic acid works as a pharmaco-chaperone for AGT in vitro, not only in relation to the misfolded-causing variant p.G170R, but also in aggregation-causing variants such as p.I244M and p.G41R [128]. Miniaturized and cost-effective in vitro tools for high-throughput screening may help in finding new or repurposing known molecules able to rescue the enzyme-trafficking defect in PH1 [129]. More recently, translation inhibition with nanomolar doses of the anti-parasitic drug emetine corrected the AGT mistargeting and mildly decreased oxalate excretion in expanded primary hepatocytes from healthy and PH1 donors (Upcyte®) exposed to glyoxylate [126]. However, no further in vivo progress was made, and it is unclear whether these compounds can be used for human treatment.
Outlook
The PHs are challenging diseases and diagnosis is often delayed. As with most inherited metabolic disorders, a timely and proper diagnosis is the basis of any therapeutic concept. Genetic testing should be offered to every patient after evaluation of metabolites in urine/plasma, as the genotype drives targeted treatment and transplantation strategies. Orthotopic liver transplantation is far from being an ideal curative therapy especially in disorders where global liver function remains intact. Newly available RNAi medications, but also adequately applied VB6 treatment may make liver transplantation in PH1 avoidable. Alternative therapeutic approaches are already in the pipeline, which will move a step forward in treating patients non-responsive to current medication. A rare disease with a severe clinical phenotype has now become treatable, but only with an early diagnosis and prompt treatment installment can chronic kidney failure be prevented.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Conflicts of Interest/Competing Interests
Bernd Hoppe is an employee of Dicerna/Novo Nordisk. Cristina Martin-Higueras is a consultant of Dicerna/Novo Nordisk. There are no additional conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
CM-H was invited to prepare the manuscript. Both authors contributed equally to the manuscript.
References
- 1.Cochat P, Rumsby G. Primary hyperoxaluria. N Engl J Med. 2013;369:649–658. doi: 10.1056/NEJMra1301564. [DOI] [PubMed] [Google Scholar]
- 2.Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012;8:467–475. doi: 10.1038/nrneph.2012.113. [DOI] [PubMed] [Google Scholar]
- 3.Danpure CJ. Peroxisomal alanine:glyoxylate aminotransferase and prenatal diagnosis of primary hyperoxaluria type 1. Lancet. 1986;2:1168. doi: 10.1016/S0140-6736(86)90584-2. [DOI] [PubMed] [Google Scholar]
- 4.Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP. The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum Mol Genet. 1999;8:2063–2069. doi: 10.1093/hmg/8.11.2063. [DOI] [PubMed] [Google Scholar]
- 5.Belostotsky R, et al. Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet. 2010;87:392–399. doi: 10.1016/j.ajhg.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monico CG, et al. Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol. 2011;6:2289–2295. doi: 10.2215/CJN.02760311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Higueras C, Torres A, Salido E. Molecular therapy of primary hyperoxaluria. J Inherit Metab Dis. 2017;40:481–489. doi: 10.1007/s10545-017-0045-3. [DOI] [PubMed] [Google Scholar]
- 8.Garrelfs SF, et al. Patients with primary hyperoxaluria type 2 have significant morbidity and require careful follow-up. Kidney Int. 2019;96:1389–1399. doi: 10.1016/j.kint.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Singh P, et al. Clinical characterization of primary hyperoxaluria type 3 in comparison with types 1 and 2. Nephrol Dial Transplant. 2022;37(5):869–875. doi: 10.1093/ndt/gfab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Higueras C, et al. A report from the European Hyperoxaluria Consortium (OxalEurope) Registry on a large cohort of patients with primary hyperoxaluria type 3. Kidney Int. 2021;100:621–635. doi: 10.1016/j.kint.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Singh P, et al. Primary hyperoxaluria type 3 can also result in kidney failure: a case report. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandrile G, et al. Data from a large European study indicate that the outcome of primary hyperoxaluria type 1 correlates with the AGXT mutation type. Kidney Int. 2014;86:1197–1204. doi: 10.1038/ki.2014.222. [DOI] [PubMed] [Google Scholar]
- 13.Siener R, Hoppe B, Löhr P, Müller SC, Latz S. Metabolic profile and impact of diet in patients with primary hyperoxaluria. Int Urol Nephrol. 2018;50:1583–1589. doi: 10.1007/s11255-018-1939-1. [DOI] [PubMed] [Google Scholar]
- 14.Vervaet BA, Verhulst A, de Broe ME, D’Haese PC. The tubular epithelium in the initiation and course of intratubular nephrocalcinosis. Urol Res. 2010;38:249–256. doi: 10.1007/s00240-010-0290-5. [DOI] [PubMed] [Google Scholar]
- 15.Mulay SR, et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest. 2013;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knauf F, et al. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013;84:895–901. doi: 10.1038/ki.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoppe B, et al. Plasma calcium oxalate super saturation in children with primary hyperoxaluria and end-stage renal failure. Kidney Int. 1999;56:268–274. doi: 10.1046/j.1523-1755.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 18.Marangella M, et al. Plasma profiles and dialysis kinetics of oxalate in patients receiving hemodialysis. Nephron. 1992;60:74–80. doi: 10.1159/000186708. [DOI] [PubMed] [Google Scholar]
- 19.Birtel J, et al. The ocular phenotype in primary hyperoxaluria type 1. Am J Ophthalmol. 2019;206:184–191. doi: 10.1016/j.ajo.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Hoppe B, Birtel J, Herberg U, Martin-Higueras C. Systemic oxalate deposition in patients with primary hyperoxaluria type 3 (PO1997). American Society of Nephrology Kidney Week; San Diego, Nov 2-7, 2021; p. 614.
- 21.Ventzke A, et al. Systematic assessment of urinary hydroxy-oxo-glutarate for diagnosis and follow-up of primary hyperoxaluria type III. Pediatr Nephrol. 2017;32:2263–2271. doi: 10.1007/s00467-017-3731-3. [DOI] [PubMed] [Google Scholar]
- 22.Pitt JJ, Willis F, Tzanakos N, Belostotsky R, Frishberg Y. 4-Hydroxyglutamate is a biomarker for primary hyperoxaluria type 3. JIMD Rep. 2015;15:1–6. doi: 10.21009/jimd.v15i1.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greed L, et al. Metabolite diagnosis of primary hyperoxaluria type 3. Pediatr Nephrol. 2018;33:1443–1446. doi: 10.1007/s00467-018-3967-6. [DOI] [PubMed] [Google Scholar]
- 24.Hulton S-A. The primary hyperoxalurias: a practical approach to diagnosis and treatment. Int J Surg. 2016;36:649–654. doi: 10.1016/j.ijsu.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Zhao F, et al. Predictors of incident ESRD among patients with primary hyperoxaluria presenting prior to kidney failure. Clin J Am Soc Nephrol. 2016;11:119–126. doi: 10.2215/CJN.02810315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weigert A, Hoppe B. Nephrolithiasis and nephrocalcinosis in childhood-risk factor-related current and future treatment options. Front Pediatr. 2018;6:98. doi: 10.3389/fped.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weigert A, Martin-Higueras C, Hoppe B. Novel therapeutic approaches in primary hyperoxaluria. Expert Opin Emerg Drugs. 2018;23:349–357. doi: 10.1080/14728214.2018.1552940. [DOI] [PubMed] [Google Scholar]
- 28.Leumann E, Hoppe B, Neuhaus T. Management of primary hyperoxaluria: efficacy of oral citrate administration. Pediatr Nephrol. 1883;7:207–211. doi: 10.1007/BF00864405. [DOI] [PubMed] [Google Scholar]
- 29.Hamm LL. Renal handling of citrate. Kidney Int. 1990;38:728–735. doi: 10.1038/ki.1990.265. [DOI] [PubMed] [Google Scholar]
- 30.Bergstralh EJ, et al. Transplantation outcomes in primary hyperoxaluria. Am J Transplant. 2010;10:2493–2501. doi: 10.1111/j.1600-6143.2010.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Illies F, Bonzel K-E, Wingen A-M, Latta K, Hoyer PF. Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int. 2006;70:1642–1648. doi: 10.1038/sj.ki.5001806. [DOI] [PubMed] [Google Scholar]
- 32.Hoppe B, et al. Oxalate elimination via hemodialysis or peritoneal dialysis in children with chronic renal failure. Pediatr Nephrol. 1996;10:488–492. doi: 10.1007/s004670050145. [DOI] [PubMed] [Google Scholar]
- 33.Bunchman TE, Swartz RD. Oxalate removal in type I hyperoxaluria or acquired oxalosis using HD and equilibration PD. Perit Dial Int. 1994;14:81–84. doi: 10.1177/089686089401400117. [DOI] [PubMed] [Google Scholar]
- 34.Cochat P, et al. Primary hyperoxaluria type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant. 2012;27:1729–1736. doi: 10.1093/ndt/gfs078. [DOI] [PubMed] [Google Scholar]
- 35.Metry EL, et al. Transplantation outcomes in patients with primary hyperoxaluria: a systematic review. Pediatr Nephrol. 2021;36:2217–2226. doi: 10.1007/s00467-021-05043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolkemper D, et al. Long-term results of pre-emptive liver transplantation in primary hyperoxaluria type 1. Pediatr Transplant. 2000;4:177–181. doi: 10.1034/j.1399-3046.2000.00107.x. [DOI] [PubMed] [Google Scholar]
- 37.Brinkert F, et al. Transplantation procedures in children with primary hyperoxaluria type 1: outcome and longitudinal growth. Transplantation. 2009;87:1415–1421. doi: 10.1097/TP.0b013e3181a27939. [DOI] [PubMed] [Google Scholar]
- 38.Jamieson NV, European PHI Transplantation Study Group. A 20-year experience of combined liver/kidney transplantation for primary hyperoxaluria (PH1): the European PH1 transplant registry experience 1984-2004. Am J Nephrol. 2005;25:282–9. [DOI] [PubMed]
- 39.Saborio P, Scheinman JI. Transplantation for primary hyperoxaluria in the United States. Kidney Int. 1999;56:1094–1100. doi: 10.1046/j.1523-1755.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 40.Monico CG, Milliner DS. Combined liver-kidney and kidney-alone transplantation in primary hyperoxaluria. Liver Transpl. 2001;7:954–963. doi: 10.1053/jlts.2001.28741. [DOI] [PubMed] [Google Scholar]
- 41.Hoppe B, Langman CB. A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18:986–991. doi: 10.1007/s00467-003-1234-x. [DOI] [PubMed] [Google Scholar]
- 42.Dhondup T, Lorenz EC, Milliner DS, Lieske JC. Combined liver-kidney transplantation for primary hyperoxaluria type 2: a case report. Am J Transplant. 2018;18:253–257. doi: 10.1111/ajt.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.del Bello A, Cointault O, Delas A, Kamar N. Primary hyperoxaluria type 2 successfully treated with combined liver-kidney transplantation after failure of isolated kidney transplantation. Am J Transplant. 2020;20:1752–1753. doi: 10.1111/ajt.15829. [DOI] [PubMed] [Google Scholar]
- 44.Mclaurin AW, Beisel WR, Mccormick GJ, Scalettar R, Herman RH. Primary hyperoxaluria. Ann Intern Med. 1961;55:70–80. doi: 10.7326/0003-4819-55-1-70. [DOI] [PubMed] [Google Scholar]
- 45.Milliner DS, Eickholt JT, Bergstralh EJ, Wilson DM, Smith LH. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N Engl J Med. 1994;331:1553–1558. doi: 10.1056/NEJM199412083312304. [DOI] [PubMed] [Google Scholar]
- 46.Toussaint C. Pyridoxine-responsive PH1: treatment. J Nephrol. 1998;11(Suppl. 1):49–50. [PubMed] [Google Scholar]
- 47.Gibbs DA, Watts RW. Biochemical studies on the treatment of primary hyperoxaluria. Arch Dis Child. 1967;42:505–508. doi: 10.1136/adc.42.225.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmgren G, Hörnström T, Johansson S, Samuelson G. Primary hyperoxaluria (glycolic acid variant): a clinical and genetical investigation of eight cases. Ups J Med Sci. 1978;83:65–70. doi: 10.3109/03009737809179114. [DOI] [PubMed] [Google Scholar]
- 49.Helin I. Primary hyperoxaluria: an analysis of 17 Scandinavian patients. Scand J Urol Nephrol. 1980;14:61–64. doi: 10.3109/00365598009181192. [DOI] [PubMed] [Google Scholar]
- 50.Harrison AR, Kasidas GP, Rose GA. Hyperoxaluria and recurrent stone formation apparently cured by short courses of pyridoxine. Br Med J (Clin Res Ed) 1981;282:2097–2098. doi: 10.1136/bmj.282.6282.2097-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alinei P, Guignard JP, Jaeger P. Pyridoxine treatment of type 1 hyperoxaluria. N Engl J Med. 1984;311:798–799. [PubMed] [Google Scholar]
- 52.Leumann E, Matasovic A, Niederwieser A. Pyridoxine in primary hyperoxaluria type I. Lancet. 1986;2:699. doi: 10.1016/S0140-6736(86)90221-7. [DOI] [PubMed] [Google Scholar]
- 53.Morgan SH, Maher ER, Purkiss P, Watts RW, Curtis JR. Oxalate metabolism in end-stage renal disease: the effect of ascorbic acid and pyridoxine. Nephrol Dial Transplant. 1988;3:28–32. [PubMed] [Google Scholar]
- 54.Edwards P, Nemat S, Rose GA. Effects of oral pyridoxine upon plasma and 24-hour urinary oxalate levels in normal subjects and stone formers with idiopathic hypercalciuria. Urol Res. 1990;18:393–396. doi: 10.1007/BF00297371. [DOI] [PubMed] [Google Scholar]
- 55.Shah GM, et al. Effects of ascorbic acid and pyridoxine supplementation on oxalate metabolism in peritoneal dialysis patients. Am J Kidney Dis. 1992;20:42–49. doi: 10.1016/S0272-6386(12)80315-5. [DOI] [PubMed] [Google Scholar]
- 56.Costello JF, Sadovnic MC, Smith M, Stolarski C. Effect of vitamin B6 supplementation on plasma oxalate and oxalate removal rate in hemodialysis patients. J Am Soc Nephrol. 1992;3:1018–1024. doi: 10.1681/ASN.V341018. [DOI] [PubMed] [Google Scholar]
- 57.Marangella M. Transplantation strategies in type 1 primary hyperoxaluria: the issue of pyridoxine responsiveness. Nephrol Dial Transplant. 1999;14:301–303. doi: 10.1093/ndt/14.2.301. [DOI] [PubMed] [Google Scholar]
- 58.Dakshinamurti K, Dakshinamurti S, Czubryt MP. Vitamin B6: effects of deficiency, and metabolic and therapeutic functions. In: Preedy V, Patel V (Eds), Handbook of famine, starvation, and nutrient deprivation. Springer International Publishing; Springer Cham, 2017: p. 1–23. 10.1007/978-3-319-40007-5_81-1.
- 59.Musayev FN, et al. Molecular basis of reduced pyridoxine 5’-phosphate oxidase catalytic activity in neonatal epileptic encephalopathy disorder. J Biol Chem. 2009;284:30949–30956. doi: 10.1074/jbc.M109.038372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salido E, Pey AL, Rodriguez R, Lorenzo V. Primary hyperoxalurias: disorders of glyoxylate detoxification. Biochim Biophys Acta. 2012;1822:1453–1464. doi: 10.1016/j.bbadis.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Oppici E, et al. Pyridoxamine and pyridoxal are more effective than pyridoxine in rescuing folding-defective variants of human alanine:glyoxylate aminotransferase causing primary hyperoxaluria type I. Hum Mol Genet. 2015;24:5500–5511. doi: 10.1093/hmg/ddv276. [DOI] [PubMed] [Google Scholar]
- 62.Fargue S, Lewin J, Rumsby G, Danpure CJ. Four of the most common mutations in primary hyperoxaluria type 1 unmask the cryptic mitochondrial targeting sequence of alanine:glyoxylate aminotransferase encoded by the polymorphic minor allele. J Biol Chem. 2013;288:2475–2484. doi: 10.1074/jbc.M112.432617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fodor K, Wolf J, Erdmann R, Schliebs W, Wilmanns M. Molecular requirements for peroxisomal targeting of alanine-glyoxylate aminotransferase as an essential determinant in primary hyperoxaluria type 1. PLoS Biol. 2012;10:e1001309. doi: 10.1371/journal.pbio.1001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hopper ED, Pittman AMC, Fitzgerald MC, Tucker CL. In vivo and in vitro examination of stability of primary hyperoxaluria-associated human alanine:glyoxylate aminotransferase. J Biol Chem. 2008;283:30493–30502. doi: 10.1074/jbc.M803525200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oppici E, et al. Biochemical analyses are instrumental in identifying the impact of mutations on holo and/or apo-forms and on the region(s) of alanine:glyoxylate aminotransferase variants associated with primary hyperoxaluria type I. Mol Genet Metab. 2012;105:132–140. doi: 10.1016/j.ymgme.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harambat J, Fargue S, Acquaviva C, et al. Genotype-phenotype correlation in primary hyperoxaluria type 1: the p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int. 2010;77:443–9. [DOI] [PubMed]
- 67.van Woerden CS, Groothoff JW, Wanders RJA, Davin J-C, Wijburg FA. Primary hyperoxaluria type 1 in The Netherlands: prevalence and outcome. Nephrol Dial Transplant. 2003;18:273–279. doi: 10.1093/ndt/18.2.273. [DOI] [PubMed] [Google Scholar]
- 68.Chetyrkin SV, et al. Pyridoxamine lowers kidney crystals in experimental hyperoxaluria: a potential therapy for primary hyperoxaluria. Kidney Int. 2005;7:53–60. doi: 10.1111/j.1523-1755.2005.00054.x. [DOI] [PubMed] [Google Scholar]
- 69.Scheinman JI, et al. Pyridoxamine lowers oxalate excretion and kidney crystals in experimental hyperoxaluria: a potential therapy for primary hyperoxaluria. Urol Res. 2005;33:368–371. doi: 10.1007/s00240-005-0493-3. [DOI] [PubMed] [Google Scholar]
- 70.Dwyer JP, et al. Pyridoxamine dihydrochloride in diabetic nephropathy (PIONEER-CSG-17): lessons learned from a pilot study. Nephron. 2015;129:22–28. doi: 10.1159/000369310. [DOI] [PubMed] [Google Scholar]
- 71.Williams ME, et al. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am J Nephrol. 2007;27:605–614. doi: 10.1159/000108104. [DOI] [PubMed] [Google Scholar]
- 72.Hoyer-Kuhn H, et al. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin J Am Soc Nephrol. 2014;9:468–477. doi: 10.2215/CJN.06820613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Latta K, Brodehl J. Primary hyperoxaluria type I. Eur J Pediatr. 1990;149:518–522. doi: 10.1007/BF01957682. [DOI] [PubMed] [Google Scholar]
- 74.Monico CG, Rossetti S, Olson JB, Milliner DS. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 2005;67:1704–1709. doi: 10.1111/j.1523-1755.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 75.Monico CG, Olson JB, Milliner DS. Implications of genotype and enzyme phenotype in pyridoxine response of patients with type I primary hyperoxaluria. Am J Nephrol. 2005;25:183–188. doi: 10.1159/000085411. [DOI] [PubMed] [Google Scholar]
- 76.Milliner DS. The primary hyperoxalurias: an algorithm for diagnosis. Am J Nephrol. 2005;25:154–160. doi: 10.1159/000085407. [DOI] [PubMed] [Google Scholar]
- 77.Leumann EP, Niederwieser A, Fanconi A. New aspects of infantile oxalosis. Pediatr Nephrol. 1987;1:531–535. doi: 10.1007/BF00849265. [DOI] [PubMed] [Google Scholar]
- 78.Hoppe B, et al. Safety, pharmacodynamics, and exposure-response modeling results from a first-in-human phase 1 study of nedosiran (PHYOX1) in primary hyperoxaluria. Kidney Int. 2022;101:626–634. doi: 10.1016/j.kint.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 79.Liebow A, et al. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J Am Soc Nephrol. 2017;28:494–503. doi: 10.1681/ASN.2016030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garrelfs SF, et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N Engl J Med. 2021;384:1216–1226. doi: 10.1056/NEJMoa2021712. [DOI] [PubMed] [Google Scholar]
- 81.Dutta C, et al. Inhibition of glycolate oxidase with dicer-substrate siRNA reduces calcium oxalate deposition in a mouse model of primary hyperoxaluria type 1. Mol Ther. 2016;24:770–778. doi: 10.1038/mt.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott LJ. Keam SJ. Lumasiran: first approval. Drugs. 2021;81:277–282. doi: 10.1007/s40265-020-01463-0. [DOI] [PubMed] [Google Scholar]
- 83.Frishberg Y, et al. Phase 1/2 study of lumasiran for treatment of primary hyperoxaluria type 1: a placebo-controlled randomized clinical trial. Clin J Am Soc Nephrol. 2021 doi: 10.2215/CJN.14730920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGregor TL, et al. Characterising a healthy adult with a rare HAO1 knockout to support a therapeutic strategy for primary hyperoxaluria. Elife. 2020;9:e54363. doi: 10.7554/eLife.54363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Méaux M-N, et al. The effect of lumasiran therapy for primary hyperoxaluria type 1 in small infants. Pediatr Nephrol. 2022;37:907–911. doi: 10.1007/s00467-021-05393-1. [DOI] [PubMed] [Google Scholar]
- 86.Chiodini B, et al. Case report: sustained efficacy of lumasiran at 18 months in primary hyperoxaluria type 1. Front Pediatr. 2021;9:791616. doi: 10.3389/fped.2021.791616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aldabek K, Grossman OK, Al-Omar O, Fox JA, Moritz ML. Infantile primary hyperoxaluria type 1 treated with lumasiran in twin males. Cureus. 2022;14:e21673. doi: 10.7759/cureus.21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stone HK, et al. Primary hyperoxaluria diagnosed after kidney transplant: a review of the literature and case report of aggressive renal replacement therapy and lumasiran to prevent allograft loss. Am J Transplant. 2021;21:4061–4067. doi: 10.1111/ajt.16762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.di Toro A, et al. Oxalic cardiomyopathy: could it influence treatment plans in patients with primary hyperoxaluria type 1? J Am Coll Cardiol. 2021;78:998–999. doi: 10.1016/j.jacc.2021.06.039. [DOI] [PubMed] [Google Scholar]
- 90.Poyah P, et al. Primary hyperoxaluria type 1 (PH1) presenting with end-stage kidney disease and cutaneous manifestations in adulthood: a case report. Can J Kidney Health Dis. 2021;8:20543581211058932. doi: 10.1177/20543581211058931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joher N, et al. Early post-transplant recurrence of oxalate nephropathy in a patient with primary hyperoxaluria type 1, despite pretransplant lumasiran therapy. Kidney Int. 2022;101:185–186. doi: 10.1016/j.kint.2021.10.022. [DOI] [PubMed] [Google Scholar]
- 92.Ariceta G, et al. Hepatic lactate dehydrogenase A: an RNA interference target for the treatment of all known types of primary hyperoxaluria. Kidney Int Rep. 2021;6:1088–1098. doi: 10.1016/j.ekir.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shee K, et al. Nedosiran dramatically reduces serum oxalate in dialysis-dependent primary hyperoxaluria 1: a compassionate use case report. Urology. 2021;156:e147–e149. doi: 10.1016/j.urology.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 94.Mucha L, et al. Clinical and economic impact of primary hyperoxaluria: a retrospective claims analysis. J Manag Care Spec Pharm. 2022;28:316–323. doi: 10.18553/jmcp.2022.28.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dejban P, Lieske JC. New therapeutics for primary hyperoxaluria type 1. Curr Opin Nephrol Hypertens. 2022 doi: 10.1097/MNH.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moya-Garzon MD, et al. New salicylic acid derivatives, double inhibitors of glycolate oxidase and lactate dehydrogenase, as effective agents decreasing oxalate production. Eur J Med Chem. 2022;237:114396. doi: 10.1016/j.ejmech.2022.114396. [DOI] [PubMed] [Google Scholar]
- 97.Ding J, et al. Dual glycolate oxidase/lactate dehydrogenase A inhibitors for primary hyperoxaluria. ACS Med Chem Lett. 2021;12:1116–1123. doi: 10.1021/acsmedchemlett.1c00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martin-Higueras C, Luis-Lima S, Salido E. Glycolate oxidase is a safe and efficient target for substrate reduction therapy in a mouse model of primary hyperoxaluria type I. Mol Ther. 2016;24:719–725. doi: 10.1038/mt.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moya-Garzón MD, et al. Salicylic acid derivatives inhibit oxalate production in mouse hepatocytes with primary hyperoxaluria type 1. J Med Chem. 2018;61:7144–7167. doi: 10.1021/acs.jmedchem.8b00399. [DOI] [PubMed] [Google Scholar]
- 100.Zabaleta N, et al. CRISPR/Cas9-mediated disruption of glycolate oxidase is an efficacious and safe treatment for primary hyperoxaluria type I. Mol Ther. 2018;26:384–385. [Google Scholar]
- 101.le Dudal M, et al. Stiripentol protects against calcium oxalate nephrolithiasis and ethylene glycol poisoning. J Clin Invest. 2019;129:2571–2577. doi: 10.1172/JCI99822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Letavernier E, Daudon M. Stiripentol identifies a therapeutic target to reduce oxaluria. Curr Opin Nephrol Hypertens. 2020;29:394–399. doi: 10.1097/MNH.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 103.Martin-Higueras C, Feldkötter M, Hoppe B. Is stiripentol truly effective for treating primary hyperoxaluria? Clin Kidney J. 2021;14:442–444. doi: 10.1093/ckj/sfaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kempf C, et al. Stiripentol fails to lower plasma oxalate in a dialysis-dependent PH1 patient. Pediatr Nephrol. 2020;35:1787–1789. doi: 10.1007/s00467-020-04585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shee K, Stoller ML. Perspectives in primary hyperoxaluria - historical, current and future clinical interventions. Nat Rev Urol. 2022;19:137–146. doi: 10.1038/s41585-021-00543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martinez-Turrillas R, et al. In vivo CRISPR-Cas9 inhibition of hepatic LDH as treatment of primary hyperoxaluria. Mol Ther Methods Clin Dev. 2022;25:137–146. doi: 10.1016/j.omtm.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allison MJ, Dawson KA, Mayberry WR, Foss JG. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol. 1985;141:1–7. [DOI] [PubMed]
- 108.Hatch M, Freel RW, Vaziri ND. Regulatory aspects of oxalate secretion in enteric oxalate elimination. J Am Soc Nephrol. 1999;10(Suppl. 1):S324–S328. [PubMed] [Google Scholar]
- 109.Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res. 2005;33:1–16. doi: 10.1007/s00240-004-0445-3. [DOI] [PubMed] [Google Scholar]
- 110.Hatch M, et al. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006;69:691–698. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 111.Hatch M, et al. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with oxalobacter. Am J Physiol Gastrointest Liver Physiol. 2011;86:G461–G469. doi: 10.1152/ajpgi.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grujic D, et al. Hyperoxaluria is reduced and nephrocalcinosis prevented with an oxalate-degrading enzyme in mice with hyperoxaluria. Am J Nephrol. 2009;29:86–93. doi: 10.1159/000151395. [DOI] [PubMed] [Google Scholar]
- 113.Hoppe B, et al. Effects of Oxalobacter formigenes in subjects with primary hyperoxaluria type 1 and end-stage renal disease: a phase II study. Nephrol Dial Transplant. 2020 doi: 10.1093/ndt/gfaa135. [DOI] [PubMed] [Google Scholar]
- 114.Hoppe B, et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 2006;70:1305–1311. doi: 10.1038/sj.ki.5001707. [DOI] [PubMed] [Google Scholar]
- 115.Hoppe B, et al. A randomised phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatr Nephrol. 2017;32:781–790. doi: 10.1007/s00467-016-3553-8. [DOI] [PubMed] [Google Scholar]
- 116.Rashid ST, et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127–3136. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martinez-Turrillas R, Rodriguez-Diaz S, Rodriguez-Marquez P,et al. Generation of an induced pluripotent stem cell line (CIMAi001-A) from a compound heterozygous primary hyperoxaluria type I (PH1) patient carrying p.G170R and p.R122* mutations in the AGXT gene. Stem Cell Res. 2019;41:101626. [DOI] [PubMed]
- 118.Zapata-Linares N, Rodriguez S, Salido E, et al. Generation and characterization of human iPSC lines derived from a primary hyperoxaluria type I patient with p.I244T mutation. Stem Cell Res. 2016;16:116–9. [DOI] [PubMed]
- 119.Estève J, et al. Generation of induced pluripotent stem cells-derived hepatocyte-like cells for ex vivo gene therapy of primary hyperoxaluria type 1. Stem Cell Res. 2019;38:101467. doi: 10.1016/j.scr.2019.101467. [DOI] [PubMed] [Google Scholar]
- 120.Beck BB, et al. Liver cell transplantation in severe infantile oxalosis: a potential bridging procedure to orthotopic liver transplantation? Nephrol Dial Transplant. 2012;27:2984–2989. doi: 10.1093/ndt/gfr776. [DOI] [PubMed] [Google Scholar]
- 121.Jiang J, et al. Correction of hyperoxaluria by liver repopulation with hepatocytes in a mouse model of primary hyperoxaluria type-1. Transplantation. 2008;85:1253–1260. doi: 10.1097/TP.0b013e31816de49e. [DOI] [PubMed] [Google Scholar]
- 122.Salido E, et al. Phenotypic correction of a mouse model for primary hyperoxaluria with adeno-associated virus gene transfer. Mol Ther. 2011;19:870–875. doi: 10.1038/mt.2010.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toscano MG, de Haan P. How simian virus 40 hijacks the intracellular protein trafficking pathway to its own benefit … and ours. Front Immunol. 2018;9:1160. doi: 10.3389/fimmu.2018.01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Toscano MG, et al. Generation of a Vero-based packaging cell line to produce SV40 gene delivery vectors for use in clinical gene therapy studies. Mol Ther Methods Clin Dev. 2017;6:124–134. doi: 10.1016/j.omtm.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fernández-Higuero JÁ, et al. Structural and functional insights on the roles of molecular chaperones in the mistargeting and aggregation phenotypes associated with primary hyperoxaluria type I. Adv Protein Chem Struct Biol. 2019;114:119–152. doi: 10.1016/bs.apcsb.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 126.Belostotsky R, et al. Translation inhibition corrects aberrant localization of mutant alanine-glyoxylate aminotransferase: possible therapeutic approach for hyperoxaluria. J Mol Med (Berl) 2018;96:621–630. doi: 10.1007/s00109-018-1651-8. [DOI] [PubMed] [Google Scholar]
- 127.Miyata N, et al. Pharmacologic rescue of an enzyme-trafficking defect in primary hyperoxaluria 1. Proc Natl Acad Sci. 2014;111:14406–14411. doi: 10.1073/pnas.1408401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moreno-Sánchez R, Marín-Hernández Á, del Mazo-Monsalvo I, Saavedra E, Rodríguez-Enríquez S. Assessment of the low inhibitory specificity of oxamate, aminooxyacetate and dichloroacetate on cancer energy metabolism. Biochim Biophys Acta Gen Subj. 2017;861:3221–3236. doi: 10.1016/j.bbagen.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 129.Wang M, et al. High throughput cell-based assay for identification of glycolate oxidase inhibitors as a potential treatment for primary hyperoxaluria type 1. Sci Rep. 2016;6:34060. doi: 10.1038/srep34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.