Abstract
To elucidate the mechanisms underlying the divergent clinicopathologic spectrum of EWSR1/FUS-CREB translocation-associated tumors, we performed a comprehensive genomic analysis of fusion transcript variants, recurrent genetic alterations (mutations, copy number alterations), gene expression, and methylation profiles across a large cohort of tumor types. The distribution of the EWSR1/FUS fusion partners – ATF1, CREB1, and CREM – and exon involvement was significantly different across different tumor types. Our targeted sequencing showed that secondary genetic events are associated with tumor type rather than fusion type. Of the 39 cases that underwent targeted NGS testing, 18 (46%) had secondary OncoKB mutations or copy number alterations (29 secondary genetic events in total), of which 15 (52%) were recurrent. Secondary recurrent, but mutually exclusive, TERT promoter and CDKN2A mutations were identified only in clear cell sarcoma (CCS) and associated with worse overall survival. CDKN2A/B homozygous deletions were recurrent in angiomatoid fibrous histiocytoma (AFH) and restricted to metastatic cases. mRNA upregulation of MITF, CDH19, PARVB, and PFKP was found in CCS, compared to AFH, and correlated with a hypomethylated profile. In contrast, S100A4 and XAF1 were differentially upregulated and hypomethylated in AFH but not CCS. A sarcoma methylation classifier was able to accurately match 100% of CCS cases to the correct methylation class; however, it was suboptimal when applied to other histologies. In conclusion, our comprehensive genomic profiling of EWSR1/FUS-CREB translocation-associated tumors uncovered mostly histotype, rather than fusion-type associated correlations in transcript variants, prognostically significant secondary genetic alterations, and gene expression and methylation patterns.
Keywords: CREB, ATF1, secondary recurrent genetic alterations, gene expression, methylation, fusion transcripts
Introduction
Recurrent gene fusions involving EWSR1/FUS with members of the cAMP response element binding protein (CREB) family (ATF1, CREB1 and CREM) are shared amongst multiple tumor-types spanning a wide clinicopathologic spectrum. Despite sharing related gene fusions, members of the EWSR1-CREB family of translocation-associated tumors exhibit significantly different clinicopathologic characteristics. The prototypic example is angiomatoid fibrous histiocytoma (AFH) vs clear cell sarcoma (CCS)—two morphologically distinct tumors, the former mostly associated with a benign behavior, while the latter being an aggressive sarcoma with a high metastatic potential and poor outcome, as illustrated by the survival analysis of our cohort. Clear cell sarcoma-like tumor of gastrointestinal tract (GICCS, also known as gastrointestinal neuroectodermal tumor) and hyalinizing clear cell carcinoma of salivary gland (HCCC) had intermediate overall survival relative to AFH and CCS.
To elucidate the molecular mechanisms underlying their differences, we performed a comprehensive genomic analysis of fusion transcript variants, secondary recurrent genetic alterations (mutations, copy number alterations), gene expression and methylation profiles across a large cohort of tumour-types defined by EWSR1/FUS-CREB gene fusions. Specifically, the tumors included in this study encompassed AFH, CCS, GICCS, HCCC, clear cell odontogenic carcinoma (CCOC), malignant epithelioid neoplasm with predilection for mesothelial-lined cavities (ME), mesothelioma (Meso), myxoid mesenchymal tumor (MMT), and primary pulmonary myxoid sarcoma (PPMS).
Materials and Methods
Case selection and study cohort
After approval from the Institutional Review Board, cases were identified from the Memorial Sloan Kettering Cancer Center (MSKCC) surgical pathology archives, or from collaborating institutions, based on tumor types and/or presence of EWSR1/FUS-ATF1/CREB1/CREM fusions. The diagnosis of all 137 cases included molecular confirmation of both fusion partners: 37 cases by fluorescence in situ hybridization, 24 cases by reverse transcription PCR (RT-PCR), 29 cases by Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) only, 10 cases by MSK-Fusion only, 10 cases by both MSK-IMPACT and MSK-Fusion, 21 cases by TruSight RNA Fusion Panel (Illumina, San Diego, CA), with the remaining cases based on NGS testing performed by referring institutions. The meta-analysis of the published literature was based on an exhaustive literature search on any fusions or gene rearrangements reported in all of the listed entities in Supplementary Table 1 that we could identify on PubMed.
DNA Seq and RNA Seq
Detailed descriptions of MSK-IMPACT workflow and data analysis, a hybridization capture-based targeted DNA NGS assay for solid tumor, and MSK-Fusion, an amplicon-based targeted RNA NGS assay using the Archer™ FusionPlex™ standard protocol, were described previously1,2.
850k methylation array
Details of the methylation array protocol were described previously3. Briefly, for each sample, 250 ng of input DNA was used for bisulfite conversion (EZ DNA Methylation Kit; Zymo Research; catalog number D5002), followed by an FFPE restoration step using the Infinium HD FFPE DNA Restore Kit (Illumina; catalog number WG-321–1002). All samples were processed on the Infinium methylationEPIC 850k BeadChip array and scanned using the Illumina iScan. Each CpG site interrogated by the Infinium array was identified by a unique cg identifier in the format of cg#, where # is a number. The methylation level for each CpG site was quantified using β values (continuous values between zero and one), calculated as the ratio of methylated signal/total signal plus an offset. 850k methylation array profiling was performed in a total of 80 samples, including: 7 AFH, 4 CCS, 8 GICCS, and compared to 51 soft tissue tumors of various histotypes (4 angiosarcomas, 27 gastrointestinal stromal tumors, 1 HCCC, 1 ME, 3 Meso, 11 paragangliomas, 4 small blue round cell tumors) and 10 normal tissues (8 human peripheral white blood cell samples, 2 normal adrenal medulla). A minimum cutoff of log2FC (fold change) > 1.0 and FDR < 0.01 was used for statistical analysis of differential methylation analysis using t-test, focusing on comparison of the 7 AFH against all other samples and 4 CCS against all other samples. Unsupervised hierarchical clustering was performed using the pheatmap R package version 1.0.12 with Ward’s linkage and Euclidean distance for clustering.
Sarcoma classification by DNA methylation profiling
Details of the DNA methylation-based machine learning sarcoma classification algorithm were described in Koelsche et al4. This random forest-based machine learning algorithm was developed at the German Cancer Research Center (DKFZ) in Heidelberg, Germany. Briefly, the method defines 62 methylation classes based on a reference cohort of 1077 samples encompassing a broad range of sarcomas. The classifier quantifies the confidence of the sample’s assigned methylation class using a calibrated score between 0 and 1. The sum of all calibrated scores across all methylation classes is 1.0. A confident match is generally considered > 0.9 and a poor match < 0.54. The 22 cases that underwent analysis with the DNA methylation classification algorithm corresponded to 6 AFH, 4 CCS, 8 GICCS, 1 ME, 3 Meso among the 80 samples that underwent methylation profiling.
Affymetrix microarray gene expression analysis
Details of the microarray protocol were described previously5,6. RNA was isolated using RNAwiz RNA isolation reagent (Ambion) and run through a column with RNase-free DNase (Qiagen). Ten micrograms of labeled and fragmented cRNA were then hybridized onto a Human Genome U133A expression array (Affymetrix, Santa Clara, CA). Post-hybridization staining, washing, and scanning were done according to instructions from the manufacturer (Affymetrix). The raw expression data were derived using the Affymetrix Microarray Analysis 5.0 (MAS 5.0) software. The data were normalized using a scaling target intensity of 500 to account for differences in the global chip intensity. The expression values were transformed using the logarithm base two. Affymetrix U133A gene expression array analysis was performed in a total of 58 samples, including 3 AFH, 4 CCS, 1 GICCS, and compared to 44 soft tissue tumors of various histotypes (3 adult fibrosarcomas, 5 angiosarcomas, 3 leiomyosarcomas, 10 gastrointestinal stromal tumors, 3 myxoid liposarcomas, 6 paragangliomas, 4 small blue round cell tumors, 4 solitary fibrous tumors, 3 synovial sarcoma, 3 undifferentiated pleomorphic sarcomas) and 6 normal tissues (adrenal gland, brain, kidney, small intestine, stomach, testis). For differential gene expression analysis, a minimum cutoff of log2FC (fold change) > 1.0 and FDR adjusted p-value < 0.01 were used for t-test. We compared one histotype against all other tumors for each respective analysis. For example: CCS (4 cases) vs all others (54 cases) in one analysis, and AFH (3 cases) vs all others (55 cases) in a different analysis. Unsupervised hierarchical clustering was performed using the pheatmap R package version 1.0.12 with Ward’s linkage and Euclidean distance for clustering.
Integration of gene expression and methylation analysis
First, we performed differential gene expression and differential methylation analysis by setting a false discovery rate (FDR) adjusted p-value of 0.01 and a minimum log2FC (fold change) of 1.0 for t-test, comparing one histotype against all other tumors each time (e.g., CCS vs all others, AFH vs all others). Thereafter, for integration of transcriptomic and methylation data, we matched all the genes that were both differentially expressed based on log2FC > 1.0 and FDR < 0.01 and differentially methylated based on log2FC > 2.0 and FDR < 0.01 for the CCS vs all others and AFH vs all others comparisons. Out of the 3 AFH, 4 CCS and 1 GICCS on the Affymetrix U133 microarray, 1 AFH and 2 CCS did not overlap with the samples used for the methylation array.
Results
Clinicopathologic summary
A total of 137 cases were identified [76 females, 61 males, mean age 37 (range 2–86)], including: 40 CCS (29%), 36 AFH (26%), 20 GICCS (15%), 14 ME (10%), 10 HCCC (7%), 8 Meso (6%), 5 MMT (4%), 3 PPMS (2%), and 1 clear cell odontogenic carcinoma (CCOC) (1%) (Figure 1A). The mean ages in HCCC, PPMS and CCOC were higher than those in AFH, CCS, GICCS, MMT, and ME (Table 1). As expected, the primary sites were predominantly soft tissue for AFH and CCS, gastrointestinal tract/pelvis for GICCS, brain for MMT, thoracic or abdominopelvic cavities for Meso and ME, lung for PPMS, and major and minor salivary glands for HCCC.
Figure 1.
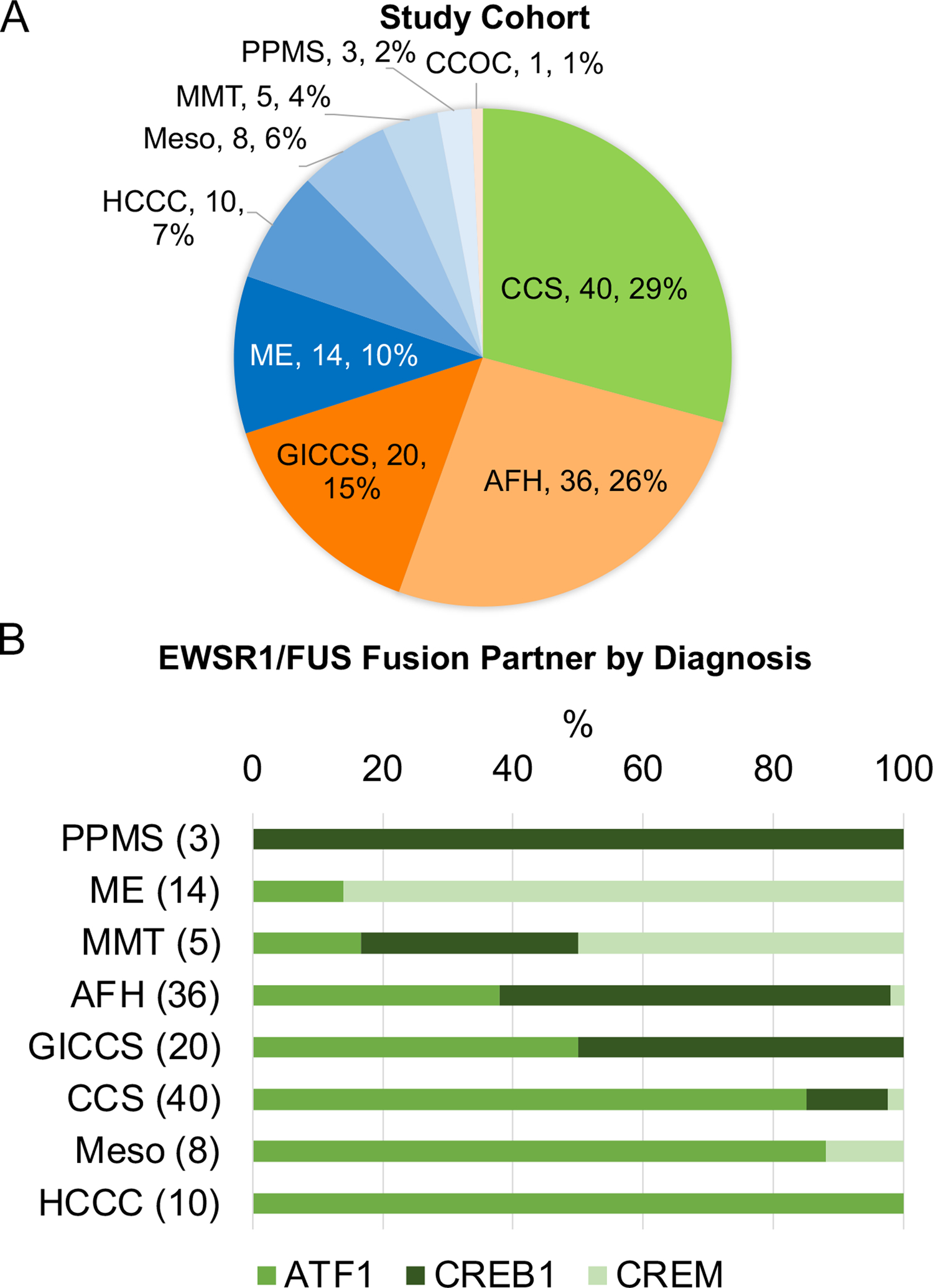
A, Study cohort showing number of cases and percentages by tumor type. B, Distribution of EWSR1/FUS fusion partners (ATF1, CREB1, CREM) by diagnosis (number of cases for each histotype indicated between parenthesis). Abbreviations—AFH: angiomatoid fibrous histiocytoma; CCS: clear cell sarcoma; CCOS: clear cell odontogenic carcinoma; GICCS: clear cell sarcoma-like tumor of gastrointestinal tract; HCCC: hyalinizing clear cell carcinoma of salivary gland; ME: malignant epithelioid neoplasm with predilection for mesothelial-lined cavities; Meso: mesothelioma; PPMS: primary pulmonary myxoid sarcoma.
Table 1.
Clinicopathologic summary of the study cohort
| Diagnosis | Female, n (%) | Male, n (%) | Mean age in years (range) | Median tumor size in cm (range) | Top primary site | Metastasis, n (%) | Top metastatic site | Total |
|---|---|---|---|---|---|---|---|---|
| Angiomatoid fibrous histiocytoma | 19 (53) | 17 (47) | 28.3 (2–79) | 3.4 (1.0–10.5) | Lower extremities, upper extremities, head and neck, trunk | 4 (11) | Lung, bone, adrenal | 36 |
| Clear cell odontogenic carcinoma | 1 (100) | 0 | 57.0 | 2.7 | Head and neck | 0 | N/A | 1 |
| Clear cell sarcoma | 16 (40) | 24 (60) | 32.9 (7–71) | 4.7 (2.5–12.0) | Lower extremities, upper extremities | 18 (45) | Lymph node, lung | 40 |
| Clear cell sarcoma-like tumor of the gastrointestinal tract | 14 (70) | 6 (30) | 39.0 (18–76) | 4.5 (2.5–6.0) | Gastrointestinal tract | 8 (40) | Liver, abdominopelvic | 20 |
| Hyalinizing clear cell carcinoma of salivary gland | 8 (80) | 2 (20) | 67.6 (55–86) | 2.1 (1.3–3.3) | Salivary gland | 3 (30) | Lymph node, trunk, abdominopelvic | 10 |
| Malignant epithelioid neoplasm with predilection for mesothelial-lined cavities | 7 (50) | 7 (50) | 37.2 (9–63) | 9.0 (4.5–15.0) | Abdominopelvic | 3 (21) | Lymph node, liver abdominopelvic | 14 |
| Mesothelioma | 5 (62) | 3 (38) | 39.0 (15–78) | 3.8 (2.6–8.5) | Abdominopelvic, pleura | 2 (25) | Lymph node, abdominopelvic | 8 |
| Myxoid mesenchymal tumor | 3 (60) | 2 (40) | 27.0 (12–48) | 2.9 (2.8–3.0) | Brain | 0 | N/A | 5 |
| Primary pulmonary myxoid sarcoma | 3 (100) | 0 | 59.0 (43–82) | 1.4 | Lung | 1 (33) | Lymph node | 3 |
| Total | 76 | 61 | 36.4 (5–86) | 4.0 | 39 (28) | 137 |
Fusion types and transcript variants by diagnosis
The distribution of the EWSR1/FUS fusion partners, ATF1, CREB1, and CREM, was significantly different across different tumor types (chi-square P < 0.0001) (Figure 1B). Specifically, EWSR1-ATF1 fusions were the only fusion type in HCCC (100%) and the predominant fusion type in CCS (85%) and Meso (88%); EWSR1-CREB1 fusions were the only fusion type in PPMS (100%) and the predominant fusion type in AFH (60%); EWSR1/FUS-CREM fusions were the predominant fusion type in ME (86%) and MMT (50%). ATF1 and CREB1 fusions were equally distributed in GICCS. The single case of CCOC had a EWSR1-ATF1 fusion. Of the 137 cases, only 5 (4%) harbored FUS fusions: four were FUS-CREM fusions in ME, one was a FUS-ATF1 fusion in a Meso.
The exon usage for the fusion transcript variants for each tumor type was derived from either MSK-Fusion and/or MSK-IMPACT testing and available in 48 cases (8 AFH, 18 CCS, 9 GICCS, 7 HCCC, 3 Meso, 1 PPMS, 2 ME) (Table 2). The predominant fusion transcript variants were EWSR1ex8-ATF1ex4 in CCS and GICCS; EWSR1ex7-ATF1ex5 and EWSR1ex7-CREB1ex7 in AFH; EWSR1ex11-ATF1ex3 in HCCC; FUSex8-CREMex5/7 for ME (Figure 2). Supplementary Table 1 summarizes the CREB family fusion variants of various tumor types derived from our meta-analysis of published studies in comparison to those detected in the current cohort.
Table 2.
Distribution of the most prevalent fusion transcript variants by exon usage in the current study
| Histotype | Fusion transcript variant | Number | Percentage |
|---|---|---|---|
| Angiomatoid fibrous histiocytoma (AFH) | EWSR1-ATF1 ex7-ex5 | 3 | 37.5 |
| EWSR1-ATF1 ex7-ex7 | 1 | 12.5 | |
| EWSR1-CREB1 ex7-ex7 | 3 | 37.5 | |
| Clear cell sarcoma (CCS) | EWSR1-ATF1 ex7-ex4 | 1 | 5.6 |
| EWSR1-ATF1 ex7-ex5 | 3 | 16.7 | |
| EWSR1-ATF1 ex8-ex4 | 10 | 55.6 | |
| EWSR1-ATF1 ex9-ex4 | 2 | 11.1 | |
| EWSR1-CREB1 ex7-ex7 | 1 | 5.6 | |
| EWSR1-CREM ex8-ex7 | 1 | 5.6 | |
| Clear cell sarcoma-like tumor of the gastrointestinal tract (GICCS) | EWSR1-ATF1 ex7-ex5 | 1 | 11.1 |
| EWSR1-ATF1 ex8-ex4 | 5 | 55.6 | |
| EWSR1-CREB1 ex6-ex6 | 1 | 11.1 | |
| EWSR1-CREB1 ex7-ex6 | 1 | 11.1 | |
| EWSR1-CREB1 ex7-ex7 | 1 | 11.1 | |
| Hyalinizing clear cell carcinoma (HCCC) | EWSR1-ATF1 ex7-ex4 | 1 | 14.3 |
| EWSR1-ATF1 ex8-ex4 | 1 | 14.3 | |
| EWSR1-ATF1 ex9-ex2 | 1 | 14.3 | |
| EWSR1-ATF1 ex10-ex3 | 1 | 14.3 | |
| EWSR1-ATF1 ex11-ex3 | 3 | 42.9 | |
| Malignant epithelioid neoplasm with predilection for mesothelial-lined cavities (ME) | FUS-CREM ex8-ex5 | 1 | 50.0 |
| FUS-CREM ex8-ex7 | 1 | 50.0 | |
| Mesothelioma (Meso) | EWSR1-ATF1 ex7-ex5 | 1 | 33.3 |
| EWSR1-ATF1 ex13-ex5 | 1 | 33.3 | |
| EWSR1-CREM ex10-ex5 | 1 | 33.3 |
Figure 2.
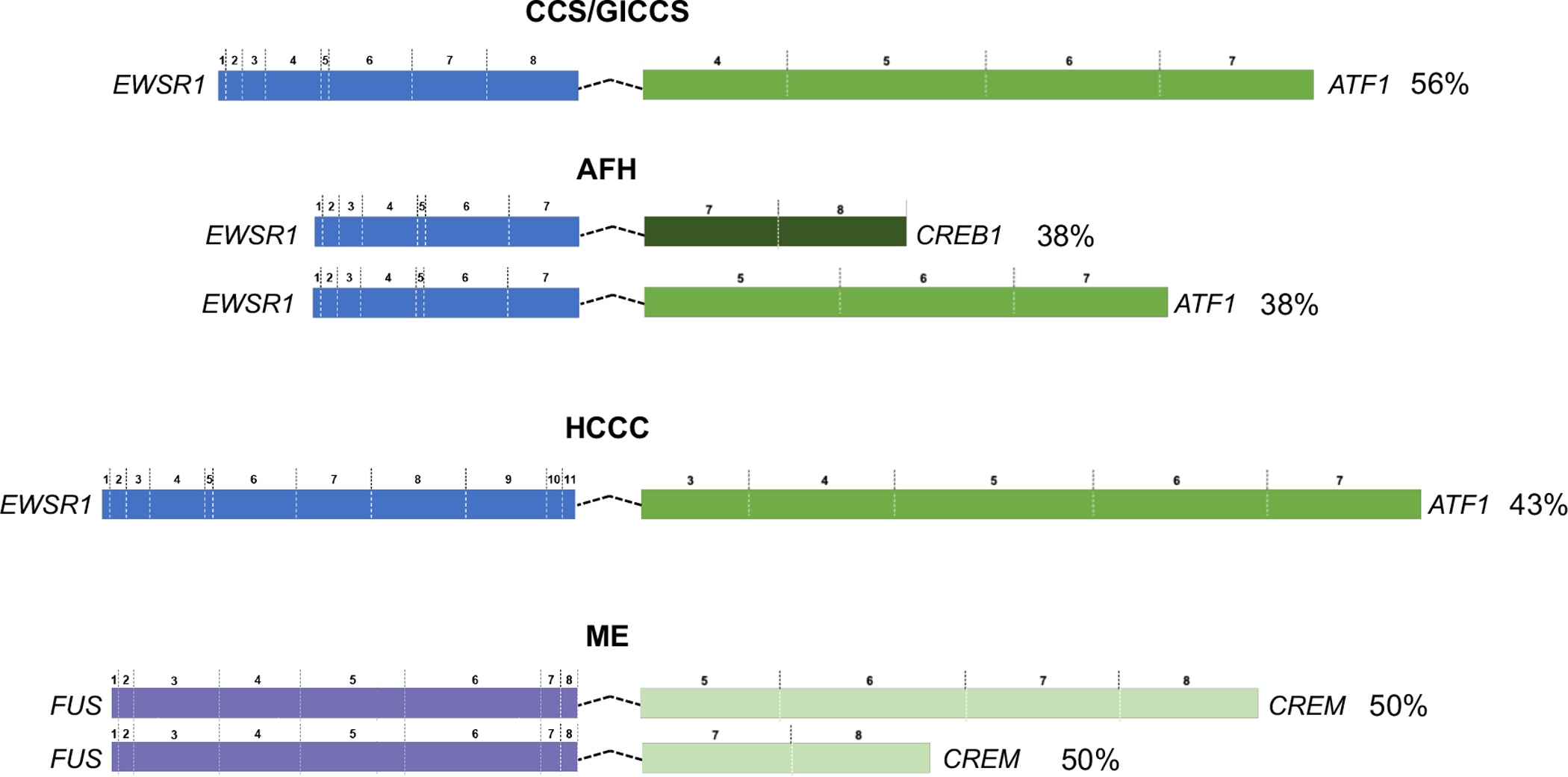
Schematics of predominant fusion transcript variants, for AFH, CCS, GICCS, HCCC, and ME. Exon numbers were based on canonical transcripts for each gene. Percentage indicates frequency of the fusion transcript variant within the corresponding histotype subgroup. RefSeq accession number: ATF1 (NM_005171); CREB1 (NM_134442); CREM (NM_181571); EWSR1 (NM_005432); FUS (NM_004960). Abbreviations—AFH: angiomatoid fibrous histiocytoma; CCS: clear cell sarcoma; GICCS: clear cell sarcoma-like tumor of gastrointestinal tract; HCCC: hyalinizing clear cell carcinoma of salivary gland; ME: malignant epithelioid neoplasm with predilection for mesothelial-lined cavities; Meso: mesothelioma.
Clinically significant recurrent genetic alterations
39 cases [6 AFH, 14 CCS, 9 GICCS, 5 HCCC, 3 Meso, 1 PMMS, 1 clear cell odontogenic carcinoma (CCOS)] were analyzed by MSK-IMPACT. Only clinically significant variants with OncoKB annotations (Chakravarty 2017) (or known recurrent hotspots) and secondary recurrent genetic alterations (events that occur > 1 in our cohort) were included. Variants of unknown significance were excluded. Of the 39 cases that underwent targeted NGS testing, 18 (46%) had OncoKB mutations or copy number alterations (29 secondary genetic events in total), of which 15 (52%) were recurrent. Specifically, TERT promoter hotspot mutations (n=5) and CDKN2A X51_splice and P81Lfs*30 mutations (n=2) were mutually exclusive and identified in CCS only. Other secondary recurrent genetic alterations identified were: TP53 R248Q and T155Pfs*15 mutations (n=2, 1 CCS, 1 GICCS), 9p21.3 (CDKN2A/CDKN2B) copy number loss (homozygous deletion) (n=4, 2 AFH, 1 CCS, 1 HCCC), and DIS3 D479G and D488N mutations (n=2, both GICCS) (Figure 3A). No secondary recurrent genetic alterations were identified in any of the 3 Meso, 1 PMMS, or 1 CCOC. The type of secondary recurrent genetic alterations did not correlate with the EWSR1/FUS fusion partner type (Supplementary Figure 1).
Figure 3.
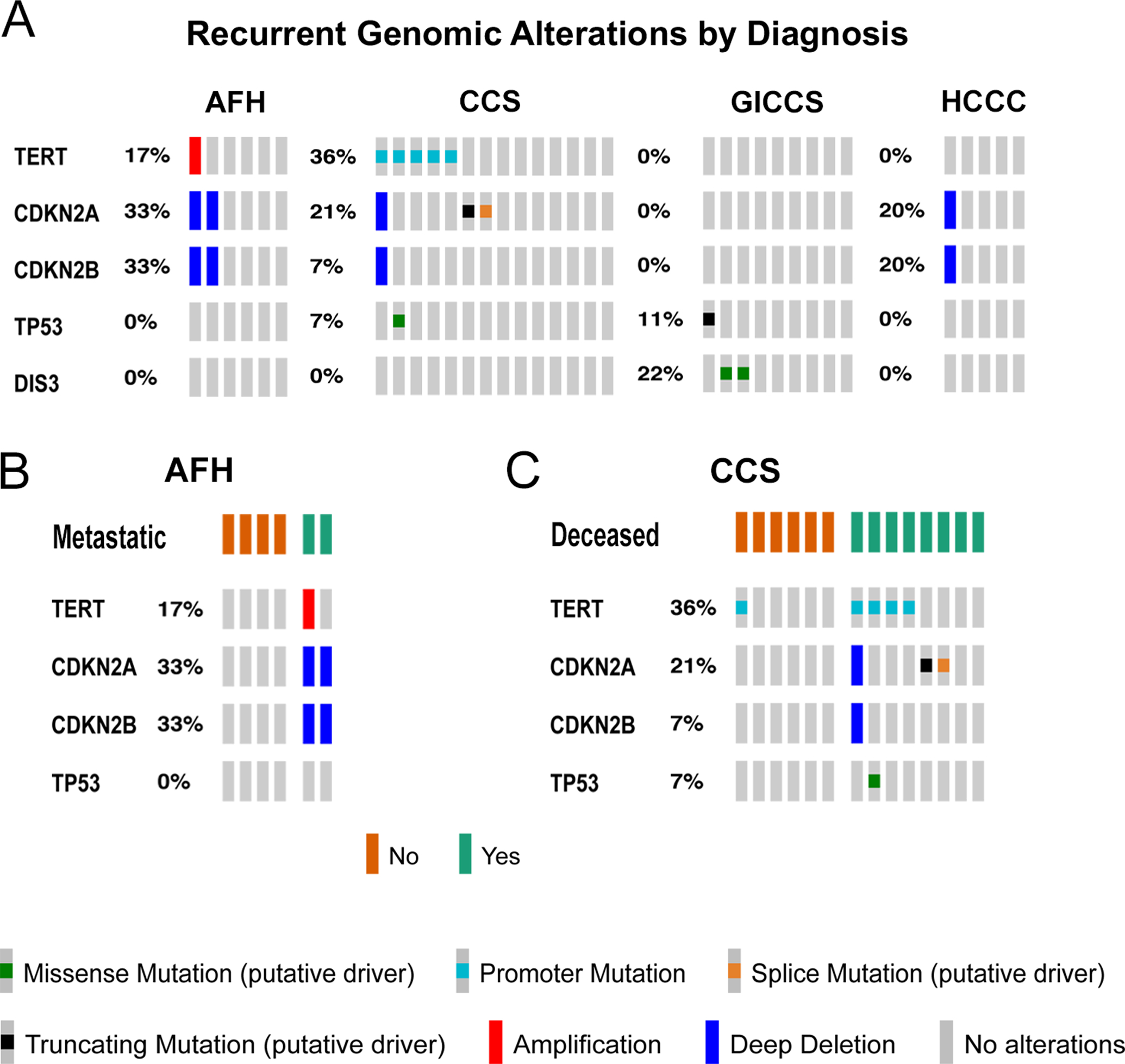
A, Recurrent genomic alterations identified by MSK-IMPACT, including OncoKB mutations and copy number alterations84, in 6 AFH, 14 CCS, 9 GICCS, and 5 HCCC. Only genomic alteration events occurring > 1 were included. B, Presence of TERT, CDKN2A, and CDKN2B alterations in AFH with or without metastatic disease. C, Presence of TERT, CDKN2A, CDKN2B and TP53 alterations in living vs deceased CCS patients. Data generated from cBioPortal and visualized using OncoPrint85. Abbreviations—AFH: angiomatoid fibrous histiocytoma; CCS: clear cell sarcoma; GICCS: clear cell sarcoma-like tumor of gastrointestinal tract; HCCC: hyalinizing clear cell carcinoma of salivary gland.
Interestingly, AFH cases with CDKN2A/CDKN2B homozygous deletion (n=2, 33%) were exclusively found in metastatic cases, whereas the remaining CDKN2A/CDKN2B non-altered AFH cases were non-metastatic (Figure 3B). On the other hand, CCS cases with TERT promoter mutations and CDKN2A loss-of-function mutations (frameshift and splice site mutations) (n=7, 50%) were significantly correlated with decreased overall survival (Mantel Haenszel chi-square P = 0.0196) (Figure 3C), with a median survival of 5.13 vs 22.85 months in non-altered CCS cases (n=7, 50%). The presence of DIS3 mutations were not correlated with metastatic nor survival status in GICCS.
Methylation and gene expression correlation
Gene expression profiling were performed on the Affymetrix U133A expression array comparing 3 AFH, 4 CCS cases, and 1 GICCS to a group of 44 soft tissue tumors of various histotypes and 6 normal tissue samples (Supplementary Figure 2 and Supplementary Table 2). Methylation profile testing was performed comparing 7 AFH, 4 CCS, 4 GICCS to a group of 51 soft tissue tumors of various histotypes and 10 normal tissue samples on the 850k methylation array (Supplementary Figure 3 and Supplementary Table 3). The goal is to identify correlates between differential gene expression (1.5 log2 FC, FDR 0.01) and differential methylation (4 log2 FC and FDR 0.01) for EWSR1-ATF1-rearranged CCS and EWSR1-CREB1-rearranged AFH, respectively, in relation to other tumor types. Gene expression profiling revealed upregulation of PMP22, MITF, SLC7A5, CDH19, WIPI1, FYN, PARVB, and PFKP in CCS but not AFH, and upregulation of SGK1, S100A4, XAF1 and LY96 expression in AFH but not CCS. However, despite differential gene expression, CREB family translocation tumors mostly cluster together in terms of methylation profile in relation to other tumor types (Supplementary Figure 3 and Supplementary Table 3).
Thereafter, differentially expressed genes were matched to CpG sites based on chromosomal locations. We matched all the genes that were both differentially expressed based on log2FC > 1.0 and FDR < 0.01 and differentially methylated based on log2FC > 2.0 and FDR < 0.01. We focused on upregulated genes with corresponding hypomethylation. Our analyses revealed genes (MITF, CDH19, PARVB, and PFKP) with increased expression and hypomethylation in CCS but not AFH (Figure 4A), and genes (S100A4, XAF1) with increased expression and hypomethylation in AFH but not CCS (Figure 4B). MITF is involved in melanogenesis and overexpressed in CCS as part of its core gene signature5,7. CDH19 and PARVB are involved in cell adhesion and were highly expressed in primary melanoma8. S100A4 has been implicated in cell migration and cancer metastases9. XAF1 is a proapoptotic tumor suppressor gene10.
Figure 4.
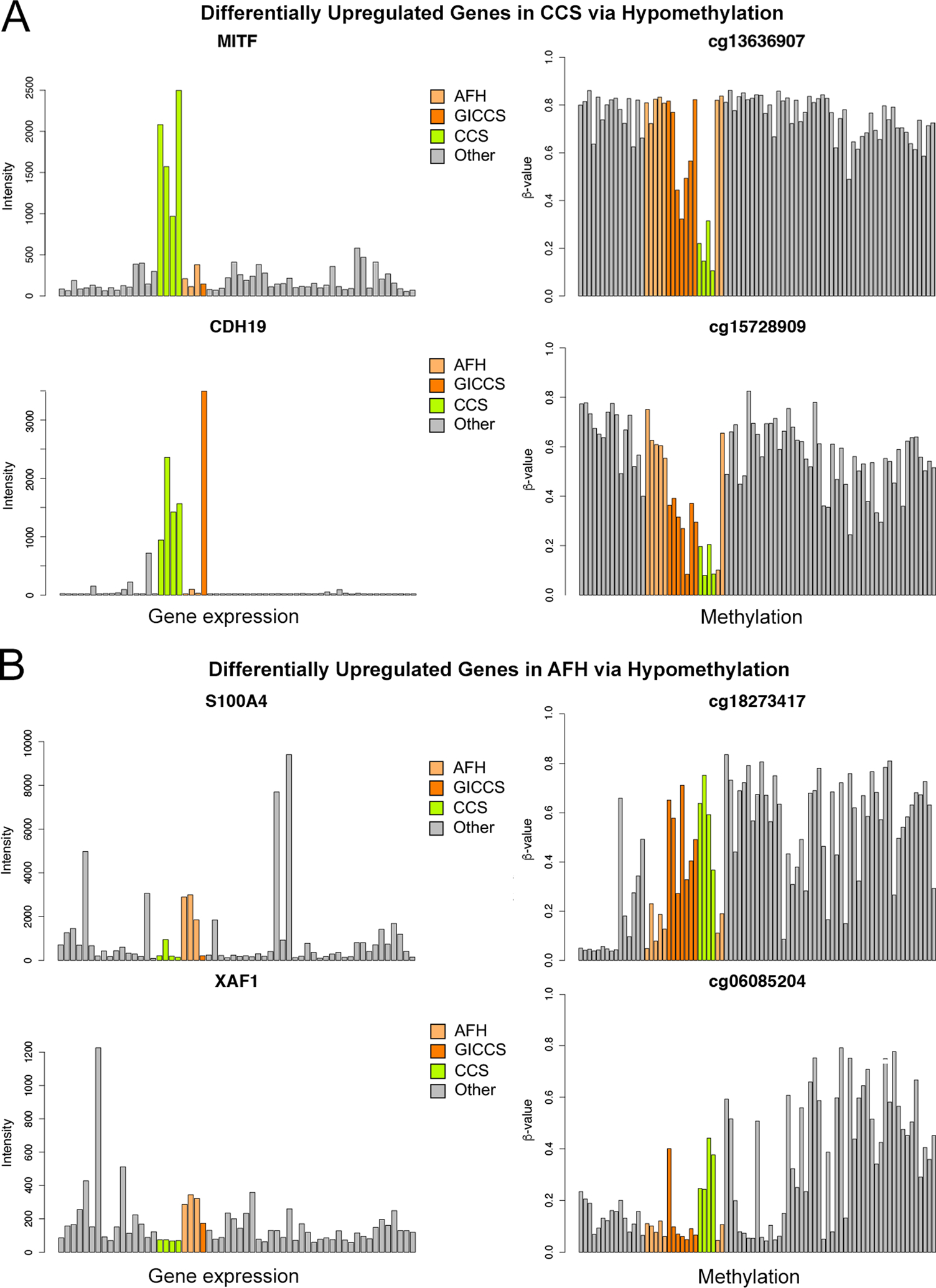
Differential gene upregulation corresponding to hypomethylation on matched CpG sites in CCS (A) and AFH (B). Affymetrix U133A was performed comparing 3 AFH, 4 CCS cases, and 1 GICCS to a group of 44 tumors of various histotypes and 6 normal tissues, using log2-fold change threshold of 1 and P < 0.01. Infinium 850k methylation array was performed comparing 7 AFH, 4 CCS, 4 GICCS to a group of 29 tumors of various histotypes and 8 normal tissues, using a log2-fold change threshold of 2 and P < 0.01. Differentially expressed genes were matched to CpG site identified by a unique cg identifier in the format of cg#. The numbers of CpG sites assigned to each of these 4 genes on the 850k array were as follows: 8 for S100A4, 14 for XAF1, 27 for MITF, and 3 for CDH19. Out of these, the numbers of CpG sites that showed negative correlation with gene expression are as follows: 3 for S100A4, 6 for XAF1, 3 for MITF, and 3 for CDH19. The most representative CpG site from each gene is displayed in this figure. Abbreviations—AFH: angiomatoid fibrous histiocytoma; CCS: clear cell sarcoma; GICCS: clear cell sarcoma-like tumor of gastrointestinal tract.
Tumor type prediction by the Sarcoma Methylation Classifier
The DNA-methylation based sarcoma classification algorithm described in Koelsche et al4 was applied to 22 cases (6 AFH, 4 CCS, 8 GICCS, 1 ME, and 3 Meso) (Table 3). This algorithm was able to accurately match 100% of four CCS cases to the correct methylation class (calibrated score = 0.99 in all cases), but only 33% (2 of 6) of AFH cases (calibrated score = 0.75 and 0.33, respectively). GICCS was not a methylation class in the original classifier. Interestingly, the algorithm matched 1 GICCS to AFH (calibrated score 0.56) and 2 GICCS to CCS (calibrated score = 0.65 and 0.96, respectively).
Table 3.
Tumor type prediction by sarcoma methylation classifier*
| Diagnosis | Age/Sex | Site | Fusion | Calibrated score | Matching methylation class |
|---|---|---|---|---|---|
| AFH | 21/M | groin | EWSR1-CREB1 | 0.33 | angiomatoid fibrous histiocytoma |
| AFH | 16/M | scalp | EWSR1-CREB1 | 0.75 | angiomatoid fibrous histiocytoma |
| AFH | 79/F | knee | EWSR1-ATF1 | < 0.3 | no matching methylation class |
| AFH | 22/F | inguinal | EWSR1-CREB1 | < 0.3 | no matching methylation class |
| AFH | 34/M | foot | EWSR1-ATF1 | < 0.3 | no matching methylation class |
| AFH | 17/M | axilla | EWSR1-CREB1 | 0.74 | squamous cell carcinoma (cutaneous) |
| CCS | 20/F | heel | EWSR1-ATF1 | 0.99 | clear cell sarcoma of soft parts |
| CCS | 30/M | knee | EWSR1-ATF1 | 0.99 | clear cell sarcoma of soft parts |
| CCS | 24/M | arm | EWSR1-ATF1 | 0.99 | clear cell sarcoma of soft parts |
| CCS | 46/F | hip | EWSR1-ATF1 | 0.99 | clear cell sarcoma of soft parts |
| GI CCS | 42/F | small bowel | EWSR1-CREB1 | 0.56 | angiomatoid fibrous histiocytoma |
| GI CCS | 47/F | small bowel | EWSR1-CREB1 | 0.65 | clear cell sarcoma of soft parts |
| GI CCS | 57/M | stomach | EWSR1-CREB1 | 0.96 | clear cell sarcoma of soft parts |
| GI CCS | 42/F | small bowel | EWSR1-CREB1 | 0.51 | neurofibroma (plexiform) |
| GI CCS | 19/F | mesentery | EWSR1-ATF1 | < 0.3 | no matching methylation class |
| GI CCS | 76/F | colon | EWSR1-CREB1 | < 0.3 | no matching methylation class |
| GI CCS | 18/F | small bowel | EWSR1-ATF1 | < 0.3 | no matching methylation class |
| GI CCS | 25/M | stomach | EWSR1-CREM | 0.68 | sclerosing epithelioid fibrosarcoma |
| ME | 20/F | peri-rectal | EWSR1-CREM | < 0.3 | no matching methylation class |
| Meso | 26/F | peritoneal | FUS-ATF1 | < 0.3 | no matching methylation class |
| Meso | 34/F | pleura | EWSR1-ATF1 | < 0.3 | no matching methylation class |
| Meso | 79/F | mediastinum | EWSR1-ATF1 | < 0.3 | no matching methylation class |
Percentage matched: AFH 33.3%, CCS 100%, GICCS/ME/meso (methylation class doesn’t exist in the classifier)
Abbreviations—AFH: angiomatoid fibrous histiocytoma; CCS: clear cell sarcoma; GICCS: clear cell sarcoma-like tumor of gastrointestinal tract; ME: malignant epithelioid neoplasm with predilection for mesothelial-lined cavities; Meso: mesothelioma.
Survival analysis
The overall survival across AFH, CCS, GICCS, HCCC was significantly different (log rank P = 0.023), with CCS associated with the worse survival (median survival 15 months), followed by HCCC (median survival 36 months) and then GICCS (median survival 43 months). All AFH patients remained alive across the follow-up period of 42 months (Figure 5).
Figure 5.
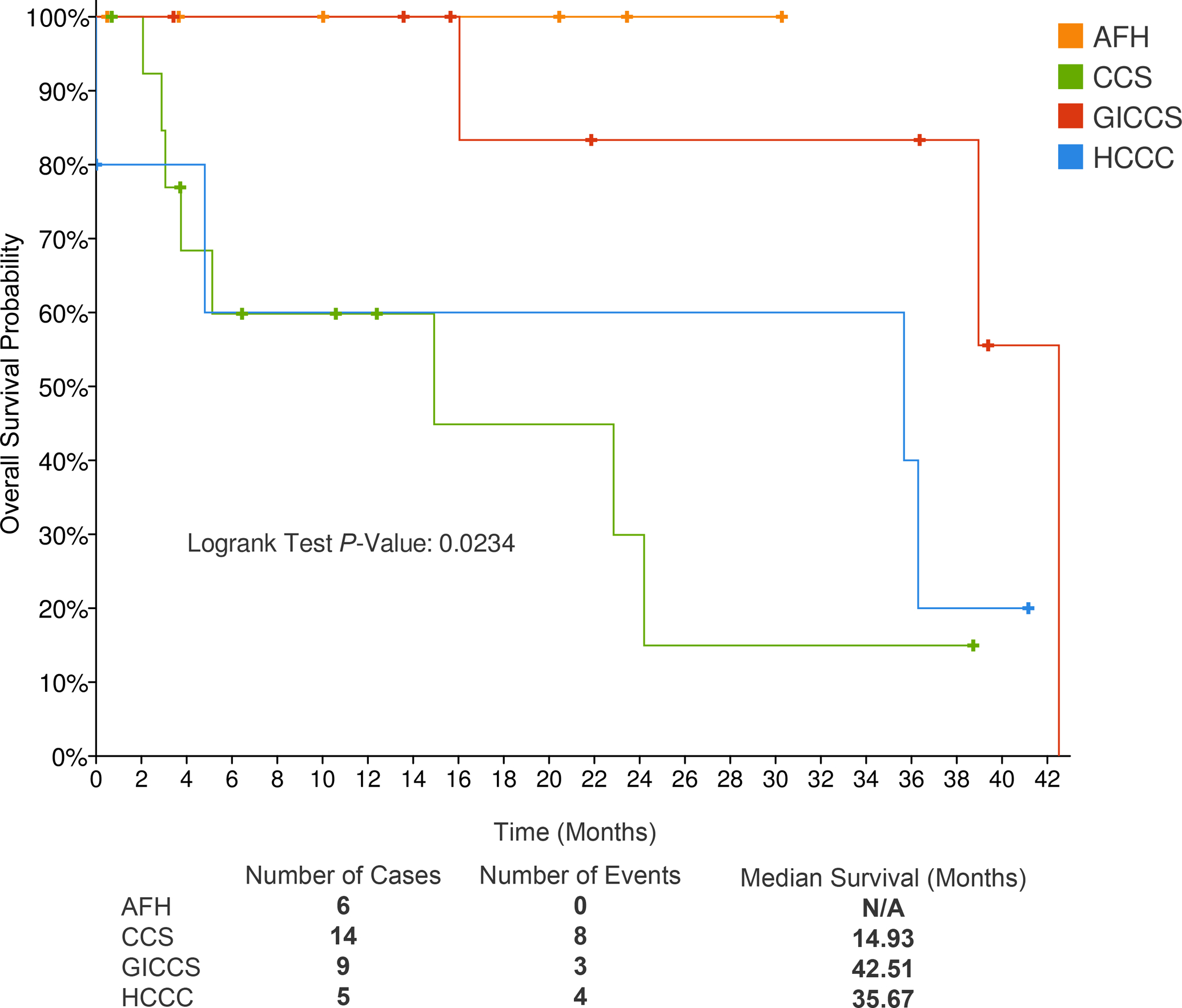
Comparison of overall survival in 6 AFH, 14 CCS, 9 GICCS, and 5 HCCC. Median survival time (months) for each tumor type listed beneath Kaplan-Meier curves. Hazard ratios compared using log-rank analysis. Abbreviations—AFH: angiomatoid fibrous histiocytoma; CCS: clear cell sarcoma; GICCS: clear cell sarcoma-like tumor of gastrointestinal tract; HCCC: hyalinizing clear cell carcinoma of salivary gland.
Discussion
The EWSR1-CREB family of translocation-associated tumors encompasses a wide and heterogenous clinicopathologic spectrum. To understand the pathogenesis that sets them apart, we performed comprehensive genomic analysis of fusion variants, secondary recurrent genetic alterations (mutations, copy number alterations), gene expression and methylation profiles across a large cohort of EWSR1-CREB family of translocation-associated tumors, with emphasis on AFH, CCS, GICCS, and HCCC.
Although the analysis of fusion transcript variants in our cohort largely paralleled the published literature, some new interesting findings emerged. For AFH, the most common reported fusion transcript variant is EWSR1-CREB1 (ex7-ex7) (58%)7,11–15. However, we identified a significant proportion (39%) of AFH cases with EWSR1-ATF1 (largely ex7-ex5). A minority (3%) of AFH harbored EWSR1-CREM fusions. Interestingly, MMT16–21, which remains disputed by some authors to be related to a myxoid, intracranial variant of AFH22–27, harbor roughly equal proportions of EWSR1-ATF1 and EWSR1-CREB1 fusions, with a significant minority harboring EWSR1-CREM. For CCS, the predominant fusion transcript is EWSR1ex8-ATF1ex415,28–34. This pattern is mirrored by a subtype of Meso, initially described by our group and occurs in younger patients without asbestos exposure history, which are driven predominantly by EWSR1-ATF1ex535–37. Of interest, in contrast to prior data, GICCS showed similar proportions of EWSR1-ATF1 (mostly ex8-ex4) and EWSR1-CREB1 fusions5,34,38. On the other hand, the recently described distinct tumor type, so-called ‘malignant epithelioid neoplasm with predilection for mesothelial-lined cavities’39 and subsequently validated by Shibayama et al40, most commonly harbor either fusions between EWSR1 or FUS and exon 7 of CREM. In contrast, PPMS is almost exclusively driven by EWSR1-CREB1 (mostly ex7-ex7)41–48 except for a rare case with EWSR1-ATF149. Some authors proposed that PPMS and AFH exist on a morphologic and molecular spectrum43,49. Finally, both HCCC50–55 and CCOC56–60 harbor only EWSR1-ATF1, supporting the notion that HCCC and CCOC are likely related tumors. Nevertheless, it is evident from our meta-analysis of the published literature and from the current study that there is significant intertumoral overlap as well as intratumoral heterogeneity of fusion transcript variants and exon usage across the different CREB family translocation tumors. Here, intratumoral heterogeneity refers to variation of fusion transcript variants, e.g., EWSR1-ATF1 and EWSR1-CREB1 in GICCS, and exon usage within the same histotype, e.g., FUS-CREM exon 5 and FUS-CREM exon 7 in ME.
This is the first study to report secondary recurrent genomic alterations in CCS, AFH and GICCS. In CCS, we identified the presence of recurrent TERT promoter and CDKN2A hotspot mutations, which were mutually exclusive but in combination strongly associated with worse overall survival. TERT promoter somatic mutations and amplifications are frequently found across multiple tumor types61,62. In soft tissue tumors, TERT promoter mutations have been identified in myxoid liposarcomas63, atypical fibroxanthoma/pleomorphic dermal sarcomas64, chondrosarcomas65, and solitary fibrous tumors66; this is reported to be associated with a worse prognosis in the latter. Our findings suggest that TERT promoter and CDKN2A mutations may serve as prognostic biomarkers for worse survival in CCS.
In AFH, we identified CDKN2A/CDKN2B homozygous deletions exclusively amongst cases with metastasis. Genomic profiling of multiple sarcoma types has revealed secondary recurrent CDKN2A alterations67,68, with a role suggested as a biomarker for poor prognosis67. Compared to other CREB family translocations tumors, AFH is a soft tissue tumor of borderline malignant potential and a relatively good prognosis; metastasis is usually < 2%. In fact, all the patients whose AFH were sequenced in our cohort remain alive at the time of reporting. Our finding of CDKN2A/CDKN2B deletions in the two AFH cases with biopsy-proven metastasis, but not in the non-metastatic AFH cases, raises the possibility of CDKN2A/CDKN2B deletion testing as a biomarker for metastatic potential. Although not a recurrent abnormality in this cohort, one of the metastatic AFH case showed a co-existing BRAF V600E mutation which was confirmed by immunohistochemistry to have diffuse and strong VE1 expression. This was the only case in our cohort to have a BRAF mutation detected.
Gene expression profiling revealed differential gene expression in AFH vs CCS, which clustered in distinct genomic groups by unsupervised analysis. A number of genes involved in melanocyte regulation and cellular membrane/migration were upregulated in CCS compared to AFH, including PMP22, MITF, SLC7A5, CDH19, WIPI1, FYN, PARVB, and PFKP, while upregulation of SGK1, S100A4, XAF1 and LY96 mRNA expression was detected in AFH but not CCS. An expression profiling analysis of CCS cell lines revealed upregulation of S100A11 (encoding for S100 protein), MITF (microphthalmia-associated transcription factor), and Pmel17 (SILV) (silver mouse homologue-like melanosomal protein detected by the IHC marker HMB45)69. Moreover, in an in vitro CCS induced pluripotent stem cell model, Komura et al reported expression of several Schwann cell marker genes, such as P75NTR, S100b, Mbp, Plp1, and Pmp2270. They proposed that S100-expressing peripheral nerve cells could be a cell of origin for EWS/ATF1-induced CCS. In a recent study using human embryonic stem (hES) cell models, hES cells driven by EWSR1-CREB1 and EWSR1-ATF1 fusions recapitulate the core gene signatures, respectively, of AFH (SGK1 and MXRA5 upregulation) and GICCS (SGK1, MXRA5, SOX10, and DUSP4 upregulation)71. Our gene expression profiling of patient samples validates a subset of the findings of these preclinical studies.
Our methylation analysis did not reveal significant differential methylation profiles among different tumor types within the CREB family translocation-associated tumors. Rather, these tumors clustered together and displayed methylation profiles distinct from other soft tissue tumor types and normal tissue. However, when matching the differentially expressed genes to the corresponding methylation probes/CpG sites, we found significant correlations between upregulated genes that were hypomethylated in CCS but not AFH. These genes included MITF, CDH19, PARVB, and PFKP in CCS. MITF is involved in melanogenesis and found to be overexpressed in CCS as part of its core gene signature, but not in AFH or GICCS5,7. More recently, a Cre-loxP-induced Ewsr1-Atf1-driven CCS model demonstrated that Mitf and Myc can contribute to sarcomagenesis72. Both CDH19 and PARVB are involved in cell adhesion and were highly expressed in primary melanoma, associated with worse survival8. It is interesting how they were found to show increased expression and hypomethylation in CCS in our study. On the other hand, we identified upregulation and hypomethylation of S100A4 and XAF1 in AFH but not CCS. S100A4 protein is a member of the S100 calcium binding protein family, also known as metastasin, and has been implicated in cell migration and cancer metastases9. XAF1 is a proapoptotic, interferon-stimulated tumor suppressor gene that suppresses tumorigenesis10. While XAF1 is usually hypermethylated and downregulated in most cancers, it was found to be paradoxically hypomethylated in glioblastoma with adaptive temozolomide resistance73. These findings serve as a proof-of-concept example of how integrative gene expression and methylation profiling may provide interesting biological insights into the different pathogenesis underlying tumors sharing the same driver gene fusions. Integrated DNA methylation and gene expression studies have been performed in Ewing sarcoma74, pediatric rhabdomyosarcomas75, myxoid, dedifferentiated, and pleomorphic liposarcomas76, which identified sets of genes with inverse methylation and gene expression relationship. In a comprehensive molecular and genomic study of undifferentiated sarcomas (USARC), DNA methylation profiling failed to identify distinct USARC subgroups and did not correlate with gene expression, but showed MSH2 and TERT promoter hypermethylation77. On the other hand, DNA methylation profiling also revealed epigenetic heterogeneity within the same tumor type, e.g., Ewing sarcoma78. Unfortunately, the sample size of individual tumor types used for methylation profiling in the current study is insufficient to perform differential methylation analysis within the same tumor type, which could be explored in future studies.
Genome-wide DNA methylation profiling has largely been performed for tumor classification purposes in a wide range of mesenchymal tumors, with varying degree of success. These include: benign and malignant nerve sheath tumors79, osteosarcomas80, undifferentiated small round blue cell tumors81, CIC-rearranged undifferentiated sarcomas82. Most recently, a Random Forest machine learning sarcoma classifier from the German Cancer Research Center (DKFZ) in Heidelberg were developed to classify a wide spectrum of 66 soft tissue and bone tumors using a large reference and validation cohort4. The limitations of using methylation profiling alone to differentiate soft tissue tumors were illustrated by the inability of the Heidelberg methylation classifier to accurately classify tumor entities in our cohort, with the exception of CCS. There are several major shortcomings to the applicability of this methylation classifier for soft tissue tumors with EWSR1/FUS fusions with CREB family transcription factors. First, several tumor types, including GICCS and Meso, were not included in the reference cohort that was used to develop the classifier. Second, although the reference cohort included 8 cases of AFH, only 1 case was used in the validation cohort, which was misclassified as desmoplastic small round cell tumor4. In our study, the classifier was able to correctly classify one-third of the AFH cases. On the other hand, the methylation classifier performed very well, both in the Koelsche et al study and in our experience, in the classification of CCS: classifying 100% of the cases correctly. It is also interesting that when we applied the classifier to GICCS, 2 cases were classified as CCS and 1 case as AFH, illustrating their overlapping methylation profiling as described above. All 3 of these GICCS cases were located in the gastrointestinal tract (1 stomach, 2 small bowel), and were diffusely and strongly positive for S100 and negative for HMB45. The combined clinical and immunohistochemical profile essentially excludes CCS and AFH. These findings highlight the existing limitation of methylation profiling in soft tissue tumor classification, which may require further algorithm refinement as well as larger reference and validation cohorts83.
In addition to these molecular mechanisms, the nature of the initial stem cell host in which the fusion, and its degree of commitment / plasticity, arose may also play a significant role in ultimate tumor type (i.e., depending on location / extent of totipotency). These are interesting questions that are beyond the scope of the current study. Recent studies using Cre inducible mouse and human embryonic stem cell models have begun to address these questions71,72.
The lack of consistency in the sample sizes of the cases with each technique is a major drawback of our paper. Further studies focusing on specific molecular profiling techniques with deeper genomic characterization utilizing a larger sample size of some of the rarer histotypes would be beneficial to validate or expand on our findings.
In conclusion, our comprehensive genomic profiling of EWSR1/FUS-CREB translocation-associated tumors uncover fusion transcript variant heterogeneity, prognostically significant secondary recurrent genetic alterations, and differentially hypomethylated and upregulated genes. These findings underscore the utility of integrative genomic approaches in the study of translocation-associated tumors with diverse clinicopathologic features, and whether some of the entities in this family could be unified under the same morphologic/molecular spectrum (e.g., CCS and GICCS, AFH and PPMS).
Supplementary Material
Supplementary Table 1. Summary of the most prevalent fusion transcript variants in the literature
Supplementary Figure 1. Recurrent genetic alterations by EWSR1/FUS fusion partner type (ATF1, CREB1, CREM).
Supplementary Figure 2. Heatmap showing differential gene expression of EWSR1-ATF1-rearranged clear cell sarcoma compared to other tumor types compared to other tumor types identified on the Affymetrix platform (1.5 log2 fold change and P-value 0.01).
Supplementary Figure 3. Heatmap showing differential methylation of EWSR1-ATF1 rearranged clear cell sarcoma compared to other tumor types identified on the 850k platform (4 log2 fold change and P-value 0.01).
Supplementary Table 2. Spreadsheet showing differential gene expression of EWSR1-ATF1-rearranged clear cell sarcoma compared to other tumor types compared to other tumor types identified on the Affymetrix platform (1.5 log2 fold change and P-value 0.01).
Supplementary Table 3. Spreadsheet showing differential methylation of EWSR1-ATF1 rearranged clear cell sarcoma compared to other tumor types identified on the 850k platform (4 log2 fold change and P-value 0.01).
Acknowledgement
We would like to acknowledge the Center Core grant (P30 CA008748), the Molecular Diagnostics Service in the Department of Pathology, and the Marie-Josee and Henry R. Kravis Center for Molecular Oncology for use of MSK-IMPACT data.
Funding Statement
This work was supported by P50 CA 140146-01 (SS, WT, CRA), P50 CA217694 (SS, WT, CRA), P30 CA008748 (SS, WT, CRA), Cycle for Survival (CRA, FV), Kristin Ann Carr Foundation (CRA). All other authors report no funding sources related to this study.
Footnotes
Ethics Approval / Consent to Participate
This study was approved by the Memorial Sloan Kettering Cancer Institute Institutional Review Board.
Conflict of Interest Statement
All authors report no conflict of interests related to this study.
Data Availability Statement
The datasets generated or analyzed during this study are included in this published article [and its supplementary information files]. The raw Affymetrix and Illumina 850k methylation array data generated are not publicly available due to lack of access to indefinite hosting capabilities, but are available from the corresponding author on reasonable request.
References
- 1.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23, 703–13 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benayed R, Offin M, Mullaney K, Sukhadia P, Rios K, Desmeules P, et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin Cancer Res 25, 4712–22 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benhamida JK, Hechtman JF, Nafa K, Villafania L, Sadowska J, Wang J, et al. Reliable Clinical MLH1 Promoter Hypermethylation Assessment Using a High-Throughput Genome-Wide Methylation Array Platform. J Mol Diagn 22, 368–375 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koelsche C, Schrimpf D, Stichel D, Sill M, Sahm F, Reuss DE. Sarcoma classification by DNA methylation profiling. Nat Commun 12, 498 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonescu CR, Nafa K, Segal NH, Dal Cin P, Ladanyi M. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma–association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res 12, 5356–62 (2006) [DOI] [PubMed] [Google Scholar]
- 6.Huang SC, Alaggio R, Sung YS, Chen CL, Zhang L, Kao YC, et al. Frequent HRAS Mutations in Malignant Ectomesenchymoma: Overlapping Genetic Abnormalities With Embryonal Rhabdomyosarcoma. Am J Surg Pathol 40, 876–85 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonescu CR, Dal Cin P, Nafa K, Teot LA, Surti U, Fletcher CD, et al. EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 46, 1051–60 (2007) [DOI] [PubMed] [Google Scholar]
- 8.Eriksson J, Le Joncour V, Nummela P, Jahkola T, Virolainen S, Laakkonen P, et al. Gene expression analyses of primary melanomas reveal CTHRC1 as an important player in melanoma progression. Oncotarget 7, 15065–92 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helfman DM, Kim EJ, Lukanidin E, Grigorian M. The metastasis associated protein S100A4: role in tumour progression and metastasis. Br J Cancer 92, 1955–8 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liston P, Fong WG, Kelly NL, Toji S, Miyazaki T, Conte D, et al. Identification of XAF1 as an antagonist of XIAP anti-Caspase activity. Nat Cell Biol 3, 128–33 (2001) [DOI] [PubMed] [Google Scholar]
- 11.Waters BL, Panagopoulos I, Allen EF. Genetic characterization of angiomatoid fibrous histiocytoma identifies fusion of the FUS and ATF-1 genes induced by a chromosomal translocation involving bands 12q13 and 16p11. Cancer Genet Cytogenet 121, 109–16 (2000) [DOI] [PubMed] [Google Scholar]
- 12.Raddaoui E, Donner LR, Panagopoulos I. Fusion of the FUS and ATF1 genes in a large, deep-seated angiomatoid fibrous histiocytoma. Diagn Mol Pathol 11, 157–62 (2002) [DOI] [PubMed] [Google Scholar]
- 13.Hallor KH, Mertens F, Jin Y, Meis-Kindblom JM, Kindblom LG, Behrendtz M, et al. Fusion of the EWSR1 and ATF1 genes without expression of the MITF-M transcript in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 44, 97–102 (2005) [DOI] [PubMed] [Google Scholar]
- 14.Rossi S, Szuhai K, Ijszenga M, Tanke HJ, Zanatta L, Sciot R, et al. EWSR1-CREB1 and EWSR1-ATF1 fusion genes in angiomatoid fibrous histiocytoma. Clin Cancer Res 13, 7322–8 (2007) [DOI] [PubMed] [Google Scholar]
- 15.Yoshida A, Wakai S, Ryo E, Miyata K, Miyazawa M, Yoshida KI. Expanding the Phenotypic Spectrum of Mesenchymal Tumors Harboring the EWSR1-CREM Fusion. Am J Surg Pathol 43, 1622–1630 (2019) [DOI] [PubMed] [Google Scholar]
- 16.Kao YC, Sung YS, Zhang L, Chen CL, Vaiyapuri S, Rosenblum MK, et al. EWSR1 Fusions with CREB Family Transcription Factors Define a Novel Myxoid Mesenchymal Tumor with Predilection for Intracranial Location. Am J Surg Pathol 41, 482–490 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sciot R, Jacobs S, Calenbergh FV, Demaerel P, Wozniak A, Debiec-Rychter M. Primary myxoid mesenchymal tumour with intracranial location: report of a case with a EWSR1-ATF1 fusion. Histopathology 72, 880–883 (2018) [DOI] [PubMed] [Google Scholar]
- 18.Komatsu M, Yoshida A, Tanaka K, Matsuo K, Sasayama T, Kojita Y. Intracranial myxoid mesenchymal tumor with EWSR1-CREB1 gene fusion: a case report and literature review. Brain Tumor Pathol 37, 76–80 (2020) [DOI] [PubMed] [Google Scholar]
- 19.Ballester LY, Meis JM, Lazar AJ, Prabhu SS, Hoang KB, Leeds NE, et al. Intracranial Myxoid Mesenchymal Tumor with EWSR1-ATF1 Fusion. J Neuropathol Exp Neurol 79, 347–351 (2020) [DOI] [PubMed] [Google Scholar]
- 20.Valente Aguiar P, Pinheiro J, Lima J, Vaz R, Linhares P. Myxoid mesenchymal intraventricular brain tumour with EWSR1-CREB1 gene fusion in an adult woman. Virchows Arch 478, 1019–1024 (2021) [DOI] [PubMed] [Google Scholar]
- 21.Ward B, Wang CP, Macaulay RJB, Liu JKC. Adult Intracranial Myxoid Mesenchymal Tumor with EWSR1-ATF1 Gene Fusion. World Neurosurg 143, 91–96 (2020) [DOI] [PubMed] [Google Scholar]
- 22.Bale TA, Oviedo A, Kozakewich H, Giannini C, Davineni PK, Ligon K, et al. Intracranial myxoid mesenchymal tumors with EWSR1-CREB family gene fusions: myxoid variant of angiomatoid fibrous histiocytoma or novel entity? Brain Pathol 28, 183–191 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sloan EA, Chiang J, Villanueva-Meyer JE, Alexandrescu S, Eschbacher JM, Wang W. Intracranial mesenchymal tumor with FET-CREB fusion-A unifying diagnosis for the spectrum of intracranial myxoid mesenchymal tumors and angiomatoid fibrous histiocytoma-like neoplasms. Brain Pathol 31, 12918 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan NJH, Pratiseyo PD, Wahjoepramono EJ, Kuick CH, Goh JY, Chang KTE, et al. Intracranial myxoid angiomatoid fibrous histiocytoma with ‘classic’ histology and EWSR1-CREM fusion providing insight for reconciliation with intracranial myxoid mesenchymal tumors. Neuropathology (2021) [DOI] [PubMed] [Google Scholar]
- 25.Dunham C, Hussong J, Seiff M, Pfeifer J, Perry A. Primary intracerebral angiomatoid fibrous histiocytoma: report of a case with a t (12; 22) (q13; q12) causing type 1 fusion of the EWS and ATF-1 genes. Am J Surg Pathol 32, 478–84 (2008) [DOI] [PubMed] [Google Scholar]
- 26.Gareton A, Pierron G, Mokhtari K, Tran S, Tauziède-Espariat A, Pallud J. ESWR1-CREM Fusion in an Intracranial Myxoid Angiomatoid Fibrous Histiocytoma-Like Tumor: A. Case Report and Literature Review. J Neuropathol Exp Neurol 77, 537–541 (2018) [DOI] [PubMed] [Google Scholar]
- 27.Konstantinidis A, Cheesman E, O’Sullivan J, Pavaine J, Avula S, Pizer B, et al. Intracranial Angiomatoid Fibrous Histiocytoma with EWSR1-CREB Family Fusions: A Report of 2 Pediatric Cases. World Neurosurg 126, 113–119 (2019) [DOI] [PubMed] [Google Scholar]
- 28.Antonescu CR, Tschernyavsky SJ, Woodruff JM, Jungbluth AA, Brennan MF, Ladanyi M. Molecular diagnosis of clear cell sarcoma: detection of EWS-ATF1 and MITF-M transcripts and histopathological and ultrastructural analysis of 12 cases. J Mol Diagn 4, 44–52 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panagopoulos I, Mertens F, Dêbiec-Rychter M, Isaksson M, Limon J, Kardas I, et al. Molecular genetic characterization of the EWS/ATF1 fusion gene in clear cell sarcoma of tendons and aponeuroses. Int J Cancer 99, 560–7 (2002) [DOI] [PubMed] [Google Scholar]
- 30.Coindre JM, Hostein I, Terrier P, Bouvier-Labit C, Collin F, Michels JJ. Diagnosis of clear cell sarcoma by real-time reverse transcriptase-polymerase chain reaction analysis of paraffin embedded tissues: clinicopathologic and molecular analysis of 44 patients from the French sarcoma group. Cancer 107, 1055–64 (2006) [DOI] [PubMed] [Google Scholar]
- 31.Hisaoka M, Ishida T, Kuo TT, Matsuyama A, Imamura T, Nishida K. Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol 32, 452–60 (2008) [DOI] [PubMed] [Google Scholar]
- 32.Wang WL, Mayordomo E, Zhang W, Hernandez VS, Tuvin D, Garcia L. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts. Mod Pathol 22, 1201–9 (2009) [DOI] [PubMed] [Google Scholar]
- 33.Jakubauskas A, Valceckiene V, Andrekute K, Seinin D, Kanopka A, Griskevicius L. Discovery of two novel EWSR1/ATF1 transcripts in four chimerical transcripts-expressing clear cell sarcoma and their quantitative evaluation. Exp Mol Pathol 90, 194–200 (2011) [DOI] [PubMed] [Google Scholar]
- 34.Segawa K, Sugita S, Aoyama T, Kubo T, Asanuma H, Sugawara T. Detection of specific gene rearrangements by fluorescence in situ hybridization in 16 cases of clear cell sarcoma of soft tissue and 6 cases of clear cell sarcoma-like gastrointestinal tumor. Diagn Pathol 13, 73 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desmeules P, Joubert P, Zhang L, Al-Ahmadie HA, Fletcher CD, Vakiani E, et al. A Subset of Malignant Mesotheliomas in Young Adults Are Associated with Recurrent EWSR1/FUS-ATF1 Fusions. Am J Surg Pathol 41, 980–988 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ajelero O, Zhang PL, Collingwood R, Fortuna D. A rare case of malignant peritoneal mesothelioma with EWSR-ATF1 fusion transcription and unusual immunophenotype. Hum Pathol Case Rep 25, 200542 (2021) [Google Scholar]
- 37.Ren H, Rassekh LSR, Lacson A, Lee CH, Dickson BC, Chung CT, Lee AF. Malignant Mesothelioma With EWSR1-ATF1 Fusion in Two Adolescent Male Patients. Pediatr Dev Pathol 12, 10935266211021222 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stockman DL, Miettinen M, Suster S, Spagnolo D, Dominguez-Malagon H, Hornick JL. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol 36, 857–68 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Argani P, Harvey I, Nielsen GP, Takano A, Suurmeijer AJH, Voltaggio L, et al. EWSR1/FUS-CREB fusions define a distinctive malignant epithelioid neoplasm with predilection for mesothelial-lined cavities. Mod Pathol 33, 2233–2243 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibayama T, Shimoi T, Mori T, Noguchi E, Honma Y, Hijioka S. Cytokeratin-positive Malignant Tumor in the Abdomen With EWSR1/FUS-CREB Fusion: A Clinicopathologic Study of 8 Cases. Am J Surg Pathol (2021) [DOI] [PubMed] [Google Scholar]
- 41.Thway K, Nicholson AG, Lawson K, Gonzalez D, Rice A, Balzer B, et al. Primary pulmonary myxoid sarcoma with EWSR1-CREB1 fusion: a new tumor entity. Am J Surg Pathol 35, 1722–32 (2011) [DOI] [PubMed] [Google Scholar]
- 42.Matsukuma S, Hisaoka M, Obara K, Kono T, Takeo H, Sato K, et al. Primary pulmonary myxoid sarcoma with EWSR1-CREB1 fusion, resembling extraskeletal myxoid chondrosarcoma: Case report with a review of Literature. Pathol Int 62, 817–22 (2012) [DOI] [PubMed] [Google Scholar]
- 43.Smith SC, Palanisamy N, Betz BL, Tomlins SA, Mehra R, Schmidt LA, et al. At the intersection of primary pulmonary myxoid sarcoma and pulmonary angiomatoid fibrous histiocytoma: observations from three new cases. Histopathology 65, 144–6 (2014) [DOI] [PubMed] [Google Scholar]
- 44.Jeon YK, Moon KC, Park SH, Chung DH. Primary pulmonary myxoid sarcomas with EWSR1-CREB1 translocation might originate from primitive peribronchial mesenchymal cells undergoing (myo)fibroblastic differentiation. Virchows Arch 465, 453–61 (2014) [DOI] [PubMed] [Google Scholar]
- 45.Yanagida R, Balzer BL, Mckenna RJ, Fuller CB. Primary pulmonary myxoid sarcoma, a potential mimic of metastatic extraskeletal myxoid chondrosarcoma. Pathology 49, 792–794 (2017) [DOI] [PubMed] [Google Scholar]
- 46.Prieto-Granada CN, Ganim RB, Zhang L, Antonescu C, Mueller J. Primary Pulmonary Myxoid Sarcoma: A Newly Described Entity-Report of a Case and Review of the Literature. Int J Surg Pathol 25, 518–525 (2017) [DOI] [PubMed] [Google Scholar]
- 47.Chen Z, Yang Y, Chen R, Ng CS, Shi H. Primary pulmonary myxoid sarcoma with EWSR1-CREB1 fusion: a case report and review of the literature. Diagn Pathol 15, 15 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koelsche C, Tavernar L, Neumann O, Heußel CP, Eberhardt R, Winter H, et al. Primary pulmonary myxoid sarcoma with an unusual gene fusion between exon 7 of EWSR1 and exon 5 of CREB1. Virchows Arch 476, 787–791 (2020) [DOI] [PubMed] [Google Scholar]
- 49.Gui H, Sussman RT, Jian B, Brooks JS, Zhang PJL. Primary Pulmonary Myxoid Sarcoma and Myxoid Angiomatoid Fibrous Histiocytoma: A Unifying Continuum With Shared and Distinct Features. Am J Surg Pathol 44, 1535–1540 (2020) [DOI] [PubMed] [Google Scholar]
- 50.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer 50, 559–70 (2011) [DOI] [PubMed] [Google Scholar]
- 51.Nakano T, Yamamoto H, Nishijima T, Tamiya S, Shiratsuchi H, Nakashima T, et al. Hyalinizing clear cell carcinoma with EWSR1-ATF1 fusion gene: report of three cases with molecular analyses. Virchows Arch 466, 37–43 (2015) [DOI] [PubMed] [Google Scholar]
- 52.Chapman E, Skalova A, Ptakova N, Martinek P, Goytain A, Tucker T. Molecular Profiling of Hyalinizing Clear Cell Carcinomas Revealed a Subset of Tumors Harboring a Novel EWSR1-CREM Fusion: Report of 3 Cases. Am J Surg Pathol 42, 1182–1189 (2018) [DOI] [PubMed] [Google Scholar]
- 53.Hirose K, Usami Y, Kohara M, Sato S, Iwamoto Y, Murakami S. Clear Cell Carcinoma of Palatal Minor Salivary Gland Harboring a Novel EWSR1-ATF1 Fusion Gene: Report of a Case and Review of the Literature. Head Neck Pathol 15, 676–681 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nojima S, Kohara M, Harada H, Kajikawa H, Hirose K, Nakatsuka SI. Clear Cell Carcinoma in the Oral Cavity with Three Novel Types of EWSR1-ATF1 Translocation: A Case Report. Head Neck Pathol (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heft Neal ME, Gensterblum-Miller E, Bhangale AD, Kulkarni A, Zhai J, Smith J, et al. Integrative sequencing discovers an ATF1-motif enriched molecular signature that differentiates hyalinizing clear cell carcinoma from mucoepidemoid carcinoma. Oral Oncol 117, 105270 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S, et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol 37, 1001–1005 (2013) [DOI] [PubMed] [Google Scholar]
- 57.Yancoskie AE, Sreekantaiah C, Jacob J, Rosenberg A, Edelman M, Antonescu CR, et al. EWSR1 and ATF1 rearrangements in clear cell odontogenic carcinoma: presentation of a case. Oral Surg Oral Med Oral Pathol Oral Radiol 118, 115–8 (2014) [DOI] [PubMed] [Google Scholar]
- 58.Vogels R, Baumhoer D, Gorp J, Eijkelenboom A, Verdijk M, Cleef P, et al. Clear Cell Odontogenic Carcinoma: Occurrence of EWSR1-CREB1 as Alternative Fusion Gene to EWSR1-ATF1. Head Neck Pathol 13, 225–230 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santana T, Andrade FL, Sousa Melo MC, Rocha GBL, Trierveiler M. Clear Cell Odontogenic Carcinoma Harboring the EWSR1-ATF1 Fusion Gene: Report of a Rare Case. Head Neck Pathol 14, 847–851 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breik O, Higginson J, Al-Ajami AK, Mohamed A, Martin T, Amel-Kashipaz R. Clear Cell Odontogenic Carcinoma: First Report of Novel EWSR1-CREM Fusion Gene in Case of Long-Term Misdiagnosis. Head Neck Pathol (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bell RJ, Rube HT, Xavier-Magalhães A, Costa BM, Mancini A, Song JS, et al. Understanding TERT Promoter Mutations: A Common Path to Immortality. Mol Cancer Res 14, 315–23 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta S, Vanderbilt CM, Lin YT, Benhamida JK, Jungbluth AA, Rana S. A Pan-Cancer Study of Somatic TERT Promoter Mutations and Amplification in 30,773 Tumors Profiled by Clinical Genomic Sequencing. J Mol Diagn 23, 253–263 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koelsche C, Renner M, Hartmann W, Brandt R, Lehner B, Waldburger N. TERT promoter hotspot mutations are recurrent in myxoid liposarcomas but rare in other soft tissue sarcoma entities. J Exp Clin Cancer Res 33, 33 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griewank KG, Schilling B, Murali R, Bielefeld N, Schwamborn M, Sucker A. TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas. Mod Pathol 27, 502–8 (2014) [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Chen Y, Yang C, Seger N, Hesla AC, Tsagkozis P, et al. TERT promoter mutation is an objective clinical marker for disease progression in chondrosarcoma. Mod Pathol (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bahrami A, Lee S, Schaefer IM, Boland JM, Patton KT, Pounds S, et al. TERT promoter mutations and prognosis in solitary fibrous tumor. Mod Pathol 29, 1511–1522 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bui NQ, Przybyl J, Trabucco SE, Frampton G, Hastie T, Rijn M, et al. A clinico-genomic analysis of soft tissue sarcoma patients reveals CDKN2A deletion as a biomarker for poor prognosis. Clin Sarcoma Res 9, 12 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groisberg R, Hong DS, Holla V, Janku F, Piha-Paul S, Ravi V. Clinical genomic profiling to identify actionable alterations for investigational therapies in patients with diverse sarcomas. Oncotarget 8, 39254–39267 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaefer KL, Brachwitz K, Wai DH, Braun Y, Diallo R, Korsching E. Expression profiling of t (12; 22) positive clear cell sarcoma of soft tissue cell lines reveals characteristic up-regulation of potential new marker genes including ERBB3. Cancer Res 64, 3395–405 (2004) [DOI] [PubMed] [Google Scholar]
- 70.Komura S, Ito K, Ohta S, Ukai T, Kabata M, Itakura F. Cell-type dependent enhancer binding of the EWS/ATF1 fusion gene in clear cell sarcomas. Nat Commun 10, 3999 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanoli F, Meskauskaite B, Herviou L, Mallen W, Sung YS, Fujisawa Y. Generation of human embryonic stem cell models to exploit the EWSR1-CREB fusion promiscuity as a common pathway of transformation in human tumors. Oncogene (2021) doi: 10.1038/s41388-021-01843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panza E, Ozenberger BB, Straessler KM, Barrott JJ, Li L, Wang Y. The clear cell sarcoma functional genomic landscape. J Clin Invest 131, 146301 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Q, Berglund AE, Wang D, MacAulay RJ, Mulé JJ, Etame AB. Paradoxical epigenetic regulation of XAF1 mediates plasticity towards adaptive resistance evolution in MGMT-methylated glioblastoma. Sci Rep 9, 14072 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel N, Black J, Chen X, Marcondes AM, Grady WM, Lawlor ER, et al. DNA methylation and gene expression profiling of Ewing sarcoma primary tumors reveal genes that are potential targets of epigenetic inactivation. Sarcoma 2012, 498472 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahoney SE, Yao Z, Keyes CC, Tapscott SJ, Diede SJ. Genome-wide DNA methylation studies suggest distinct DNA methylation patterns in pediatric embryonal and alveolar rhabdomyosarcomas. Epigenetics 7, 400–8 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Renner M, Wolf T, Meyer H, Hartmann W, Penzel R, Ulrich A. Integrative DNA methylation and gene expression analysis in high-grade soft tissue sarcomas. Genome Biol 14, 137 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steele CD, Tarabichi M, Oukrif D, Webster AP, Ye H, Fittall M. Undifferentiated Sarcomas Develop through Distinct Evolutionary Pathways. Cancer Cell 35, 441–456 8 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheffield NC, Pierron G, Klughammer J, Datlinger P, Schönegger A, Schuster M. DNA methylation heterogeneity defines a disease spectrum in Ewing sarcoma. Nat Med 23, 386–395 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Röhrich M, Koelsche C, Schrimpf D, Capper D, Sahm F, Kratz A. Methylation-based classification of benign and malignant peripheral nerve sheath tumors. Acta Neuropathol 131, 877–87 (2016) [DOI] [PubMed] [Google Scholar]
- 80.Wu SP, Cooper BT, Bu F, Bowman CJ, Killian JK, Serrano J. DNA Methylation-Based Classifier for Accurate Molecular Diagnosis of Bone Sarcomas. JCO Precis Oncol 17 00031 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koelsche C, Kriegsmann M, Kommoss FKF, Stichel D, Kriegsmann K, Vokuhl C. DNA methylation profiling distinguishes Ewing-like sarcoma with EWSR1-NFATc2 fusion from Ewing sarcoma. J Cancer Res Clin Oncol 145, 1273–1281 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miele E, De Vito R, Ciolfi A, Pedace L, Russo I, De Pasquale MD. DNA Methylation Profiling for Diagnosing Undifferentiated Sarcoma with Capicua Transcriptional Receptor (CIC) Alterations. Int J Mol Sci 21, 1818 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lyskjaer I, De Noon S, Tirabosco R, Rocha AM, Lindsay D, Amary F. DNA methylation-based profiling of bone and soft tissue tumours: a validation study of the ‘DKFZ Sarcoma Classifier’. J Pathol Clin Res 7, 350–360 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol 17 00011 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6, 1 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Summary of the most prevalent fusion transcript variants in the literature
Supplementary Figure 1. Recurrent genetic alterations by EWSR1/FUS fusion partner type (ATF1, CREB1, CREM).
Supplementary Figure 2. Heatmap showing differential gene expression of EWSR1-ATF1-rearranged clear cell sarcoma compared to other tumor types compared to other tumor types identified on the Affymetrix platform (1.5 log2 fold change and P-value 0.01).
Supplementary Figure 3. Heatmap showing differential methylation of EWSR1-ATF1 rearranged clear cell sarcoma compared to other tumor types identified on the 850k platform (4 log2 fold change and P-value 0.01).
Supplementary Table 2. Spreadsheet showing differential gene expression of EWSR1-ATF1-rearranged clear cell sarcoma compared to other tumor types compared to other tumor types identified on the Affymetrix platform (1.5 log2 fold change and P-value 0.01).
Supplementary Table 3. Spreadsheet showing differential methylation of EWSR1-ATF1 rearranged clear cell sarcoma compared to other tumor types identified on the 850k platform (4 log2 fold change and P-value 0.01).
Data Availability Statement
The datasets generated or analyzed during this study are included in this published article [and its supplementary information files]. The raw Affymetrix and Illumina 850k methylation array data generated are not publicly available due to lack of access to indefinite hosting capabilities, but are available from the corresponding author on reasonable request.


