Abstract
Pseudoxanthoma elasticum (PXE) is a heritable ectopic calcification disorder with multi-organ clinical manifestations. The gene at default, ABCC6, encodes an efflux transporter, ABCC6, which is a new player regulating the homeostasis of inorganic pyrophosphate (PPi), a potent endogenous anti-calcification factor. Previous studies suggested that systemic PPi deficiency is the major, but not the exclusive, cause of ectopic calcification in PXE. In this study, we demonstrate that the DNA damage response (DDR) and poly(ADP-ribose) (PAR) pathways are involved locally in PXE at sites of ectopic calcification. Genetic inhibition of PARP1, the predominant PAR-producing enzyme, showed a 54% reduction of calcification in the muzzle skin in Abcc6−/−Parp1−/− mice, as compared to age-matched Abcc6−/−Parp1+/+ littermates. Subsequently, oral administration of minocycline, an inhibitor of DDR/PAR signaling, resulted in an 86% reduction of calcification in the muzzle skin of Abcc6−/− mice. Minocycline treatment also attenuated the DDR/PAR signaling and reduced calcification of dermal fibroblasts derived from PXE patients. The anti-calcification effect of DDR/PAR inhibition was not accompanied by alterations in plasma PPi concentrations. These results suggest that local DDR/PAR signaling in calcification-prone tissues contributes to PXE pathogenesis, and its inhibition might provide a promising treatment strategy for ectopic calcification in PXE, a currently intractable disease.
Keywords: Pseudoxanthoma elasticum, ectopic calcification, DNA damage response, mouse model, minocycline
INTRODUCTION
Pseudoxanthoma elasticum (PXE; OMIM 264800) is an autosomal recessive metabolic disorder characterized by fragmentation and progressive calcification of elastic fibers in connective tissue of the skin, Bruch’s membrane of the retina, and the cardiovascular system (Luo et al., 2020, Neldner, 1988). PXE is caused by pathogenic variants in the ABCC6 gene, encoding a transmembrane transporter ABCC6 primarily expressed in the liver (Belinsky and Kruh, 1999, Scheffer et al., 2002). Although ABCC6 was identified as the causative gene for PXE two decades ago (Bergen et al., 2000, Le Saux et al., 2000, Ringpfeil et al., 2000), the metabolic nature of PXE, i.e., how the lack of ABCC6 transporter activity in the liver drives aberrant calcium hydroxyapatite deposition in peripheral connective tissues, was only recently unveiled. Specifically, it was demonstrated that ABCC6 mediates extracellular release of adenosine triphosphate (ATP) from hepatocytes, providing a crucial source of systemic inorganic pyrophosphate (PPi), a powerful physicochemical inhibitor of hydroxyapatite crystal formation (Jansen et al., 2014, Jansen et al., 2013). Loss of hepatic ABCC6 transporter activity in PXE results in a decreased extracellular ATP pool and subsequently reduced circulating concentrations of PPi, allowing ectopic calcification to ensue in the skin, retina, and arterial blood vessels. Thus, plasma PPi deficiency is considered a critical pathogenic feature of PXE, and efforts have been made to raise its levels to counteract ectopic calcification (Li et al., 2019b, Luo et al., 2020). However, restoration of plasma PPi to the control levels does not completely prevent calcification, suggesting that PPi deficiency in the circulation does not fully explain the pathology in the distant tissues, and other yet unidentified factor(s) may be at play in the pathogenesis of PXE (Zhao et al., 2017). In this context, while the altered behavior of PXE fibroblasts in culture manifests features of an in vivo imbalance of a circulating factor, there is evidence that inherent, cell-autonomous defects, are operative locally in calcified areas of PXE (Boraldi et al., 2014a, Boraldi et al., 2014b, Le Saux et al., 2006, Ziegler et al., 2017). A better understanding of the pathophysiology is warranted as there are currently no effective or specific treatments capable of altering the systemic disease course of PXE.
Recently, activation of oxidative stress and/or DNA damage response (DDR) pathways, in particular poly(ADP-ribose) polymerase 1 (PARP1) signaling, was found to play an important role in the pathophysiology of vascular calcification, including atherosclerosis, diabetes, and chronic kidney disease (Bartoli-Leonard et al., 2021, Li et al., 2020, Muller et al., 2019, Wang et al., 2019). Poly(ADP-ribosylation), discovered in 1963, is a post-translational modification of proteins that occurs in most eukaryotic organisms in response to DNA damage and oxidative stress (Chambon et al., 1963). The reaction is carried out by enzymes of the family of poly(ADP-ribose) polymerases (PARPs) by using NAD+ as a substrate to synthesize the linear or branched biopolymer poly(ADP-ribose) (PAR), which consists of up to 200 ADP-ribose subunits. PARP1, the most abundant isoform and founding member of the PARP family, accounts for approximately 90% of the total cellular PARP activity (Schreiber et al., 2002). PARP1, the most extensively and intensively studied member of the PARP family (Gibson et al., 2020, Ryu et al., 2015, Xu et al., 2014), is activated by both single-strand and double-strand DNA breaks and initiates events that attract DNA damage repair machinery to the sites of damage. PARP1 exhibits key roles in a variety of cellular processes including, most prominently, deposition of PAR at sites of DNA damage, forming a nidus of ectopic calcification. Recent studies have demonstrated that DDR/PAR was involved in pathological calcification in both experimental animal models and in calcified human arteries, and its inhibition by molecular or pharmacological means attenuated ectopic calcification (Bartoli-Leonard et al., 2021, Li et al., 2020, Muller et al., 2019, Wang et al., 2019).
In light of the emerging role of the DDR/PAR pathway in ectopic calcification, early studies reported the presence of chronic oxidative stress in an Abcc6−/− mouse model of PXE and in PXE patients (Pasquali-Ronchetti et al., 2006), as well as increased apoptotic rate in cultured PXE dermal fibroblasts (Boraldi et al., 2009, Hosen et al., 2014, Li et al., 2008). These observations could be linked to excessive DDR/PAR signaling at sites of ectopic calcification. In this study, we hypothesize that activation of the DDR/PAR signaling pathway contributes to the pathogenesis of PXE, and its genetic and pharmacologic inhibition has the potential to attenuate ectopic calcification in an Abcc6−/− mouse model of PXE and dermal fibroblasts cultured from patients with PXE.
RESULTS
The DDR/PAR pathway is activated locally in the calcified areas in Abcc6−/− mice
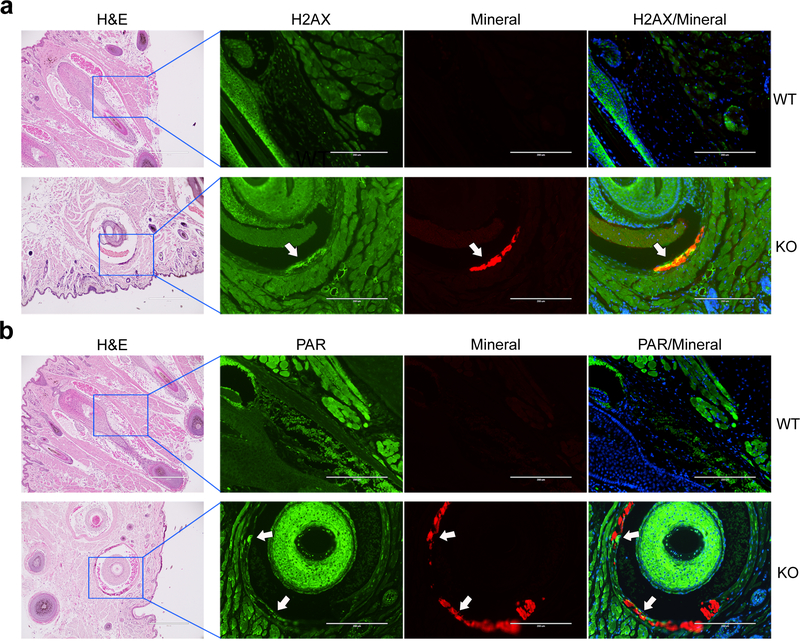
To examine whether the DDR/PAR signaling pathway is involved at sites of ectopic calcification, we first performed immunofluorescent staining to examine the juxtaposition of DNA damage, PAR production, and mineral deposition in calcified tissues of Abcc6−/− mouse model of PXE. As the unique and characteristic early biomarker of the ectopic calcification process in these mice is calcification of the dermal connective tissue sheath of vibrissae in muzzle skin, immunofluorescence was performed on muzzle skin sections from Abcc6−/− mice with evident ectopic calcification and from wild-type mice negative for ectopic calcification. Sections were also stained with alizarin complexone, a calcium-binding dye that labels calcification in red fluorescence (Siu et al., 2016). The results demonstrated that cells lining the mineral deposits in Abcc6−/− mice were stained positive for histone H2AX phosphorylation, ɤH2AX, a marker of DNA damage (Figure 1a), consistent with PAR being synthesized as part of the DDR (Figure 1b). PAR and H2AX were barely detectable in the vibrissae dermal sheath in the wild-type mice and in areas without calcification in the Abcc6−/− mice (Figure 1).
Figure 1. Immunofluorescent staining of H2AX and PAR shows the presence of DNA damage response and PAR deposition in the muzzle skin.
Paraffin sections of muzzle skin from 5 wild-type and 5 Abcc6−/− mice were analyzed. Bar = 200 μm. Arrows indicate the positivity of the green fluorescent signals of H2AX (a) and PAR (b) in association with mineral deposits (red fluorescence), as shown as yellow in the overlay. Cell nuclei were stained with DAPI. Adjacent slides were stained with hematoxylin and eosin for mineral deposits. WT, wild-type; KO, Abcc6−/−.
Genetic ablation of Parp1 attenuates connective tissue calcification in Abcc6−/− mice
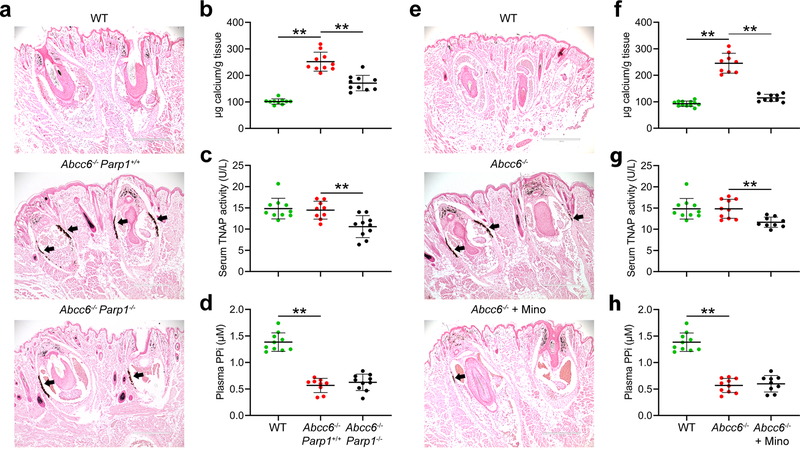
In mammalian cells, PARP1, the first described and most extensively characterized member of the PARP family, accounts for more than 90% of the overall cellular PARP activity in response to DNA damage (Los et al., 2002, Schreiber et al., 2002). Therefore, to explore the potential efficacy of inhibition of PARP activity for counteracting ectopic calcification in PXE, Abcc6−/− mice were crossed with Parp1−/− mice to generate Abcc6−/−Parp1+/+ and Abcc6−/−Parp1−/− double mutant mice. Wild-type mice served as negative controls of ectopic connective tissue calcification. At 12 weeks of age, calcification in the muzzle skin containing vibrissae, an early and reliable biomarker of the overall calcification process in the Abcc6−/− mice (Klement et al., 2005), was examined by two independent assays. One piece of muzzle skin was processed for semi-quantitative histopathologic examinations. A mineralization-specific stain, von Kossa, revealed robust calcification in the dermal sheath of vibrissae in the Abcc6−/− Parp1+/+ mice (Figure 2a). By contrast, the Abcc6−/−Parp1−/− mice showed significantly reduced calcification (Figure 2a). Another piece of muzzle skin was decalcified and the amount of solubilized calcium was quantitated using a chemical assay. The calcium content in the muzzle skin corroborated with the degree of ectopic calcification examined histologically, with a significant reduction of 54% in the amount of calcium in the muzzle skin in the Abcc6−/−Parp1−/− mice, as compared to age-matched Abcc6−/−Parp1+/+ mice (Figure 2b).
Figure 2. Genetic and pharmacologic studies show that abolished PARP1 activity in the Parp1−/− mice inhibits ectopic connective tissue calcification in the Abcc6−/− mice. All mice were analyzed at 12 weeks of age. (a-d), genetic proof-of-concept study. (e-h), minocycline treatment study.
(a) Muzzle skin biopsy samples were collected and processed for histopathology, followed by von Kossa stains. The WT mice were negative for ectopic calcification (top panel). The Abcc6−/−Parp1−/− mice (bottom panel) developed less ectopic calcification of the dermal connective tissue sheath of vibrissae in the muzzle skin compared with Abcc6−/−Parp1+/+ mice (middle panel). (b) The calcium content in the muzzle skin of Abcc6−/−Parp1−/− mice was significantly decreased compared with Abcc6−/−Parp1+/+ mice. (c) Serum TNAP activity was significantly reduced in Abcc6−/−Parp1−/− mice. (d) Plasma PPi levels in the Abcc6−/−Parp1+/+ mice were 43% of those in wild-type mice; however, plasma PPi levels in the Abcc6−/−Parp1−/− mice were not statistically different from those in Abcc6−/−Parp1+/+ mice. (e) The Abcc6−/− mice treated with 100 mg/kg/day minocycline had marked decrease of calcification (bottom panel), as compared to untreated Abcc6−/−mice (middle panel). (f) The chemical assay of calcium showed significant reduction in the amount of calcium in the muzzle skin in the Abcc6−/− mice treated with 100 mg/kg/day minocycline. (g) Serum TNAP activity was significantly decreased in Abcc6−/− mice as a result of minocycline treatment. (h) Plasma PPi levels in the Abcc6−/− mice treated with minocycline were not statistically different from those in Abcc6−/− control mice. n = 9–12 mice per group. Bar = 400 μm. Mino, minocycline; PPi, inorganic pyrophosphate; TNAP, tissue non-specific alkaline phosphatase; WT, wild-type. **P < 0.01.
To explore additional mechanisms and downstream targets of PARP1 ablation on ectopic calcification, we quantified serum activities of tissue non-specific alkaline phosphatase (TNAP), a driver of ectopic calcification. Genetic ablation of Parp1 in the Abcc6−/−Parp1−/− mice showed significantly reduced, approximately 27%, serum TNAP activity, as compared to age-matched Abcc6−/−Parp1+/+ mice (Figure 2c). To examine whether PPi homeostasis was affected, plasma concentrations of PPi in the Abcc6−/−Parp1+/+, Abcc6−/−Parp1−/−, and wild-type mice were determined. The results demonstrated that the Abcc6−/−Parp1−/− mice had plasma PPi concentrations indistinguishable from the Abcc6−/−Parp1+/+ mice, being approximately 43% of that in the wild-type mice (Figure 2d).
Minocycline attenuates connective tissue calcification in Abcc6−/− mice
Based on the demonstration in the genetic proof-of-concept study which showed that reduced PARP1 activity attenuated ectopic calcification in Abcc6−/− mice, a treatment trial with minocycline, a prototypic orally bioavailable PARP inhibitor, was initiated. To investigate the effects of minocycline on PXE, the Abcc6−/− mice were treated with minocycline at a dose of 100 mg/kg/day supplemented in drinking water, starting at 4 weeks of age, a time point just before ectopic calcification of the dermal connective tissue sheath of vibrissae starts to take place, and continued for an additional 8 weeks. Histologic examination of untreated Abcc6−/− control mice at 12 weeks of age revealed robust calcification in the muzzle skin (Figure 2e). By contrast, the Abcc6−/− mice treated with minocycline had only residual calcification (Figure 2e). The therapeutic effect of minocycline was substantiated by measuring the calcium content in muzzle skin biopsies using a chemical assay. The results revealed a significant, 86% reduction in the amount of calcium in the Abcc6−/− mice treated with minocycline, as compared to the Abcc6−/− control mice (Figure 2f).
As a result of minocycline treatment in Abcc6−/− mice, serum TNAP activity was reduced by 21% as compared to untreated Abcc6−/− control mice (Figure 2g). Plasma PPi concentrations, however, were not significantly altered, remaining as low as at approximately 43% of that in the wild-type mice (Figure 2h).
The DDR/PAR pathway is activated in patients with PXE
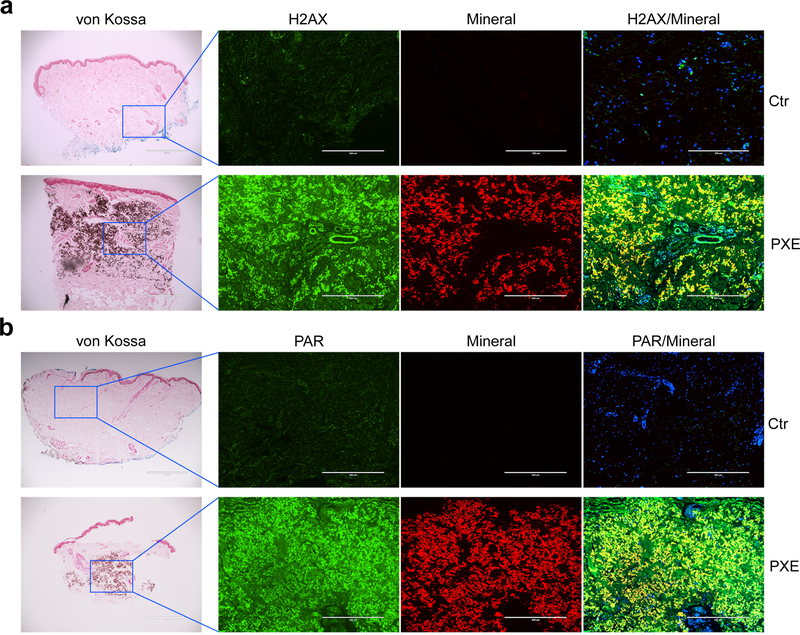
To examine whether the DDR/PAR signaling pathway is involved in patients with PXE, we performed immunofluorescent staining of PAR and H2AX in lesional skin biopsies from patients with PXE. Staining of the PXE patients’ skin sections with alizarin complexone revealed robust mineral deposition in the mid-dermis of specimen from PXE patients (Figure 3). Both PAR and H2AX co-localized with the mineral deposits and they were barely detected in areas without calcification and in skin sections from healthy individuals (Figure 3).
Figure 3. Immunofluorescent staining of H2AX and PAR shows co-localization of DNA damage response, PAR production, and mineral deposits in the lesional skin of patients with PXE.
Paraffin sections of skin biopsies from three healthy controls and three PXE patients were analyzed. Bar = 400 μm. Please note the positivity of the green fluorescent signals of H2AX (a) and PAR (b) in association with mineral deposits (red fluorescence), as shown as yellow in the overlay. Cell nuclei were stained with DAPI.
Minocycline attenuates the DDR/PAR signaling in cultured PXE dermal fibroblasts
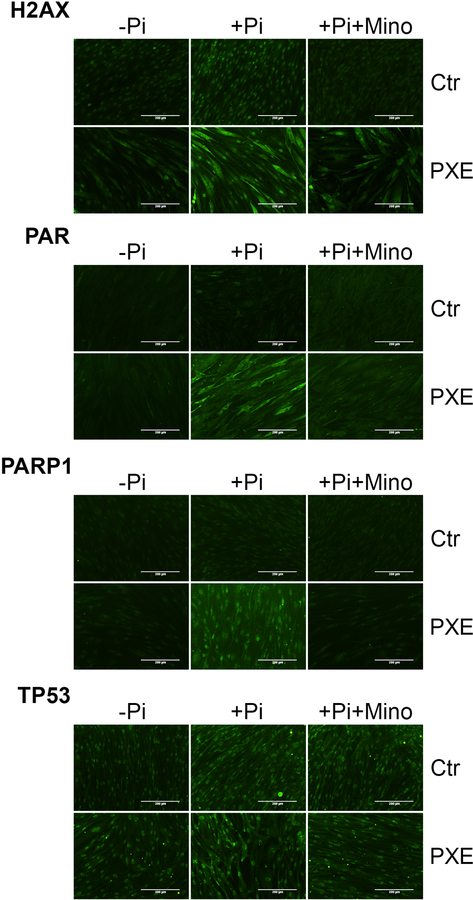
Human dermal fibroblasts were cultured from healthy controls and patients with PXE to examine whether the DDR/PAR signaling is activated in PXE and whether minocycline treatment would attenuate it. Immunofluorescent staining of H2AX and PAR, as well as PARP1 and TP53, key mediators of DDR/PAR signaling, was performed on dermal fibroblasts incubated for 4 days in standard culture medium or in calcification-inducing medium containing 4 mM inorganic phosphate (Pi) with or without minocycline at final concentration of 3 μM. The results demonstrated increased expression of H2AX, PAR, PARP1, and TP53 in cells cultured in the calcifying medium as compared to control medium (Figure 4). Minocycline treatment attenuated their expression (Figure 4). While most of the signals were confined to the cell nuclei, there was increased fluorescence in the cytoplasm of cells when incubated in the calcifying medium (Supplementary Figure S1).
Figure 4. Immunofluorescent staining of H2AX, PAR, PARP1, and TP53 in cultured dermal fibroblasts.
Dermal fibroblasts from three healthy controls and three patients with PXE were cultured in DMEM medium containing 10% fetal bovine serum. Immunofluorescent staining (green fluorescence) was performed in cells cultured in normal medium and medium supplemented with 4 mM Pi with or without 3 μM minocycline for 4 days. The cellular localization of H2AX, PAR, PARP1, and TP53 in cells cultured in medium containing 4 mM Pi was provided in Supplementary Figure S1. Ctr, control; Mino, minocycline; Pi, inorganic phosphate; Bar = 200 μm.
Minocycline attenuates calcium deposition in cultured PXE dermal fibroblasts
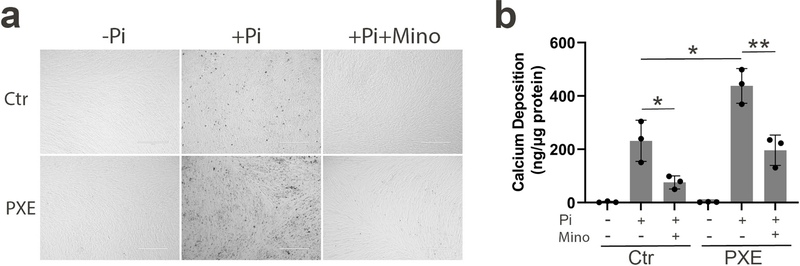
An in vitro calcification assay was utilized to examine whether minocycline treatment is beneficial to calcification of PXE dermal fibroblasts. Specifically, dermal fibroblasts from healthy human volunteers and patients with PXE were incubated in calcification-inducing medium containing 4 mM inorganic phosphate (Pi). During the subsequent 10 days of incubation, significant cell layer-associated calcification was observed, as determined by phase contrast microscopy of the cells (Figure 5a). Consistent with prior findings (Boraldi et al., 2014a, Dabisch-Ruthe et al., 2014), the PXE dermal fibroblasts had increased calcification potential in comparison to control fibroblasts (Figure 5a). The addition of minocycline at a final concentration of 3 μM dramatically decreased Pi-induced calcification for both control and PXE fibroblasts (Figure 5a). When the calcium content was quantified, a marked, 67% and 55% decrease was found if Pi and minocycline were added to the control and PXE cultures, respectively, as compared to their respective cultures incubated with Pi alone (Figure 5b).
Figure 5. Minocycline prevents Pi-induced calcification of dermal fibroblasts cultured from PXE patients.
Dermal fibroblasts from three healthy controls and three patients with PXE were cultured on 24-well plates in DMEM medium containing 10% fetal bovine serum. At day 0, some cultures were supplemented with 4 mM Pi with or without 3 μM minocycline. At day 10, the degree of calcification was assessed either (a) by phase contrast light microscopy or (b) by chemical assay of calcium deposition in the cell layer normalized by the protein content. Data were represented as mean ± SD; *P < 0.05, **P < 0.01. n = 3 replicate cultures for each fibroblast. Ctr, control; Mino, minocycline; Pi, inorganic phosphate; Bar = 400 μm.
DISCUSSION
Although plasma PPi deficiency is considered as a major determinant of ectopic calcification, providing a critical molecular mechanism for PXE, therapies aimed at targeted increase of PPi concentrations could only partially prevent the formation of these lesions in murine models of PXE. Specifically, daily intraperitoneal injections of PPi or administration of PPi in the drinking water to Abcc6−/− mice inhibited ectopic calcification by approximately 50% and 90%, respectively (Dedinszki et al., 2017, Pomozi et al., 2017). Although an inadvertent discovery, increasing the amount of PPi 7.1-fold over the standard chow, attenuated ectopic calcification by 57% in Abcc6−/− mice (Pomozi et al., 2019). In addition, when administered at high dose to Abcc6−/− mice, etidronate, a stable, non-hydrolyzable analog of PPi, prevented ectopic calcification by 73% (Li et al., 2018, Li et al., 2015). Furthermore, restoration of plasma PPi levels via genetic overexpression of human ENPP1 reduced ectopic calcification by 90% in the Abcc6−/− mice (Zhao et al., 2017). These results suggest that systemic PPi deficiency is not the sole mechanism of the pathogenesis in PXE (Zhao et al., 2017). Consistent with these findings, previous studies showed that deletion of Abcc6 in mice in both the liver and local cells in the dermal sheath surrounding the bulb of vibrissae was required to phenocopy the early-onset calcification seen upon constitutive gene ablation in Abcc6−/− mice (Ziegler et al., 2017). These observations suggest that in addition to deficiency of systemic PPi, other factors, possibly imposed by local cells at sites of ectopic calcification in peripheral connective tissues, might contribute to the pathogenesis of PXE.
Poly(ADP-ribosylation) is an immediate and transient cellular repair response to DNA damage (Liu et al., 2017). As the role of the DDR/PAR signaling pathway has been recently implicated in the ectopic calcification process, in this study we tested the hypothesis that the DDR/PAR pathway contributed to ectopic calcification in PXE, and that its inhibition would prevent the formation of these lesions. We report here the potential importance of local events in PXE pathogenesis ˗ H2AX and PAR were detected in association with mineral deposits in both the mouse model and human PXE. A genetic model, Abcc6−/−Parp1−/− double mutant mice, showed significantly reduced ectopic calcification. Minocycline, a semi-synthetic tetracycline that has been in therapeutic use for over 30 years because of its antibiotic properties, recently emerged as a potent inhibitor of PARP. Treatment of Abcc6−/− mice with minocycline significantly reduced ectopic calcification. The inhibitory effects of minocycline were confirmed in a cell-based calcification assay in cultured dermal fibroblasts from PXE patients. While these findings are consistent with those reported in a recent study in which the efficacy of minocycline treatment was performed in abcc6a knockout zebrafish developing skeletal hypermineralization (Nollet et al., 2021), our studies used Abcc6−/− mice which fully faithfully recapitulate the features of human PXE (Klement et al., 2005). In contrast to PAR being exclusively localized in nuclei of PXE dermal fibroblasts under normal culture conditions, increased cytoplasmic deposition of PAR was found in these cells under calcifying conditions. These results mirror those reported in calcified human carotid arteries with increased cytoplasmic PAR deposition, thereby facilitating ectopic calcification by acting as a nidus for crystal formation in the extracellular matrix (Muller et al., 2019). In the current study, the more pronounced inhibition of ectopic calcification by minocycline over Parp1 genetic inhibition could be contributed to the multiple biological actions of minocycline beyond its PARP-inhibition activity (Garrido-Mesa et al., 2013). In addition, although PARP1 is the predominant PAR-producing enzyme, the contribution of other PARP proteins, such as PARP2, in supplying a minor portion of the total PARP activity in response to DNA damage, cannot be excluded.
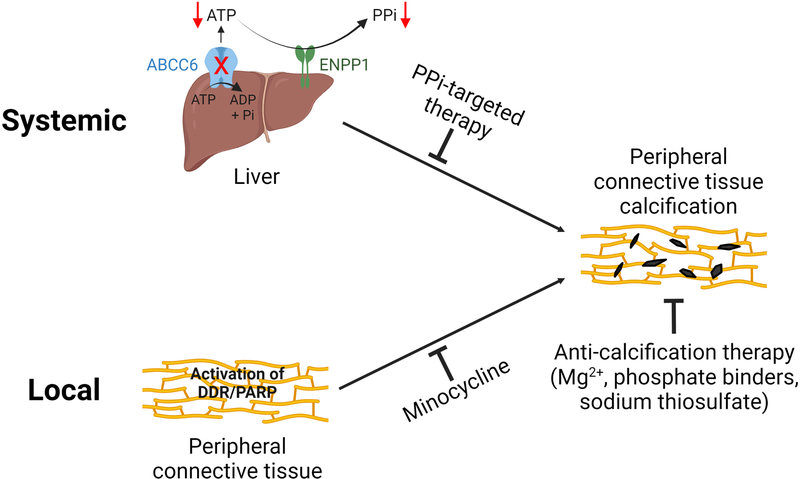
The anti-calcification effects of the inhibition of the DDR/PAR pathway were accompanied by reduced serum activity of TNAP – increased activities of this enzyme are known to promote ectopic calcification. The mouse models that overexpress human TNAP in vascular smooth muscle cells and endothelial cells demonstrated aortic calcification and cardiac hypertrophy (Sheen et al., 2015; Romanelli et al., 2017). On the other hand, genetic and pharmacologic inhibition of TNAP attenuated ectopic calcification in mouse models of ectopic calcification, including PXE mice (Li et al., 2019a, Opdebeeck et al., 2020, Tani et al., 2020, Ziegler et al., 2017). Inhibition of the DDR/PAR pathway, either via genetic or pharmacological means, did not alter plasma PPi concentrations. These results suggest that in addition to systemic PPi deficiency that acts at distantly affected sites, activation of the DDR/PAR signaling in the calcification-prone connective tissues is another driving force of ectopic calcification in PXE (Figure 6). Although the two pathways do not currently intersect, their potential interactions cannot be excluded.
Figure 6. Proposed pathomechanisms of PXE – both systemic and local factors contribute to ectopic calcification in PXE.
The metabolic nature of PXE is supported by the reduced circulating concentrations of PPi, a potent endogenous inhibitor of calcification. Systemic PPi deficiency in PXE is caused by the reduced ABCC6 transport activity in hepatocytes, reducing the extracellular ATP pool which serves as substrate for ENPP1, an ectonucleotidase/pyrophophotase 1, to generate PPi. Targeted therapies to raise plasma PPi levels have been shown to be effective to counteract ectopic calcification in murine models of PXE. On the other hand, local activation of DNA damage response and PAR deposition triggers ectopic calcification in peripheral connective tissues. Minocycline, a potent inhibitor of DDR/PAR, prevents ectopic calcification in PXE. Collectively, both systemic and local factors contribute to the pathogenesis of ectopic calcification in PXE. The potential interaction between these two pathways cannot be excluded. Furthermore, other anti-calcification therapies such as magnesium, phosphate binders, and sodium thiosulfate have therapeutic effects on ectopic calcification independent of the underlying pathomechanisms of PXE.
Taken together, ectopic calcification in PXE can be attenuated by inhibition of the DDR/PAR pathway genetically or pharmacologically without altering systemic PPi concentrations. The results derived from the preclinical mouse studies and cultured human PXE dermal fibroblasts suggest that inhibition of the DDR/PAR pathway could reduce or slow down progression of the devastating consequences of ectopic calcification in patients with PXE. While the anti-calcification effect of minocycline was independent of plasma PPi concentrations, we can envision that a combined therapy of minocycline and PPi is superior to each treatment alone, with the potential to achieve a complete arrest of ectopic calcification in PXE. In addition to minocycline as a potent PARP inhibitor, additional PARP inhibitors have been developed and approved by U.S. Food and Drug Administration as anti-cancer drugs ˗ olaparib, niraparib, veliparib and rucaparib (Walsh, 2018, Walsh and Hodeib, 2016). In this context, these drugs with PARP-inhibiting capabilities have the potential to reduce ectopic calcification in patients with PXE as well as other clinical conditions accompanied with ectopic calcification, both genetic and acquired. However, controlled clinical trials are needed to test this hypothesis with careful evaluation of long-term systemic effects.
MATERIALS AND METHODS
Immunostaining of paraffin sections
Immunofluorescent staining of muzzle skin from wild-type mice (n=5) and Abcc6−/− mice (n=5), as well as skin biopsies from PXE patients (n=3; lesional skin) and healthy controls (n=3) was performed on tissue specimen embedded in paraffin. Sections were incubated with primary antibodies against PAR (Abcam ab14459, Waltham, MA) and H2AX (Abcam ab11174). The secondary antibodies conjugated with Alexa Fluor 488 (Invitrogen, Carlsbad, CA) were applied to generate green fluorescence for PAR and H2AX. Sections were also stained with alizarin complexone, a calcium-binding dye that labels mineral deposits with red fluorescence (Siu et al., 2016). Images were acquired with EVOS FL Auto Imaging Microscopy (Thermo Scientific, Waltham, MA).
Mice and treatments
The Abcc6−/− mouse was generated and described previously (Klement et al., 2005). The Parp1−/− mice, described previously (Waymire et al., 1995), were obtained from The Jackson Laboratory (Bar Harbor, ME). The Abcc6 and Parp1 double mutant mice were generated by intercrossing Abcc6−/− mice with Parp1−/− mice. In the proof-of-concept genetic study, the wild-type, Abcc6−/−Parp1+/+, and Abcc6−/−Parp1−/− mice were fed a standard rodent diet (Lab Diet 5010; PMI Nutrition, Brentwood, MO) throughout the experiments. In the minocycline treatment experiments, Abcc6−/− mice were treated with 0.5 mg/mL minocycline administered in the drinking water. This dose corresponds to 100 mg/kg body weight/day, assuming that a 20-g mouse consumes 4 mL of water per day. The treatment was initiated in mice at 4 weeks of age and continued for an additional 8 weeks. All mice were killed at 12 weeks of age for analysis. The protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Histopathological analysis
Left muzzle skin biopsies from euthanized mice were collected and processed for histopathology. Paraffin sections were examined for calcification by von Kossa staining (Mastertech Scientific KTVKO, Lodi, CA).
Chemical quantitation of calcium
Right muzzle skin biopsies were decalcified in 1.0 mol/L HCl for 48 hours at room temperature. Solubilized calcium was determined using a colorimetric assay kit (Stanbio Laboratory 0150–250, Boerne, TX). The values were normalized to tissue weight.
Serum TNAP activity and plasma PPi concentrations
Whole blood was collected by cardiac puncture. The alkaline phosphatase activity was determined in serum using a colorimetric kit (Abcam ab83369). PPi concentration was quantified in plasma as previously described (Huang et al., 2019).
Cell culture, immunostaining, induction of calcification, and minocycline treatment
Fibroblast cultures were obtained through biopsies from macroscopic skin lesions in three PXE patients and from three age- and sex-matched healthy controls. Fibroblasts were cultured in a DMEM growth medium in the presence of 10% fetal bovine serum. At approximately 80% confluence, some cells were switched to calcification-inducing medium (DMEM supplemented with 4 mM Pi). Minocycline was added to some calcifying cultures at a final concentration of 3 μM. The medium was changed every other day. Immunofluorescent staining was performed on cells after 4 days in the calcification-inducing medium with or without minocycline. Cells were incubated with primary antibodies against PAR (Abcam ab14459), H2AX (Abcam ab11174), PARP1 (ProteinTech 13371–1-AP, Rosemont, IL), and TP53 (Abcam ab131442). The secondary antibodies conjugated with Alexa Fluor 488 (Invitrogen, Carlsbad, CA) were applied to generate green fluorescence. Calcification was examined in additional cultures after 10 days in the calcification-inducing medium and minocycline. At day 10, the media were removed, and the amount of calcium in the cell layer was determined (Stanbio Laboratory) and normalized to the protein content (Thermo Scientific 23225). All experiments were performed in triplicate.
Statistical analysis
The data were analyzed using one-way ANOVA with multiple group comparisons with Tukey’s corrections. Statistical significance was reached with P < 0.05. All statistical analyses were completed using Prism 8 (GraphPad, San Diego, CA).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Ida Joely Jacobs, Jason Lee, Mark Pawlowski, Amy Terry, and Viral Patel for technical assistance.
FUNDING SOURCES
This study was supported by PXE International, NIH/NIAMS grants R01AR072695 (JU and QL) and R21AR077332 (QL).
Abbreviations:
- DDR
DNA damage response
- PXE
pseudoxanthoma elasticum
- PPi
inorganic pyrophosphate
- WT
wild-type
- KO
knockout
- PAR
poly(ADP-ribose)
- PARP
poly(AD-Pribose) polymerase
- Pi
inorganic phosphate
- TNAP
tissue non-specific alkaline phosphatase
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT
All data associated with this study are presented in the paper.
REFERENCES
- Bartoli-Leonard F, Wilkinson FL, Schiro A, Serracino Inglott F, Alexander MY, Weston R. Loss of SIRT1 in diabetes accelerates DNA damage-induced vascular calcification. Cardiovasc Res 2021;117(3):836–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky MG, Kruh GD. MOAT-E (ARA) is a full-length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer 1999;80(9):1342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet 2000;25(2):228–31. [DOI] [PubMed] [Google Scholar]
- Boraldi F, Annovi G, Bartolomeo A, Quaglino D. Fibroblasts from patients affected by Pseudoxanthoma elasticum exhibit an altered PPi metabolism and are more responsive to pro-calcifying stimuli. J Dermatol Sci 2014a;74(1):72–80. [DOI] [PubMed] [Google Scholar]
- Boraldi F, Annovi G, Guerra D, Paolinelli Devincenzi C, Garcia-Fernandez MI, Panico F, et al. Fibroblast protein profile analysis highlights the role of oxidative stress and vitamin K recycling in the pathogenesis of pseudoxanthoma elasticum. Proteomics Clin Appl 2009;3(9):1084–98. [DOI] [PubMed] [Google Scholar]
- Boraldi F, Bartolomeo A, Li Q, Uitto J, Quaglino D. Changes in dermal fibroblasts from Abcc6(−/−) mice are present before and after the onset of ectopic tissue mineralization. J Invest Dermatol 2014b;134(7):1855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P, Weill JD, Mandel P. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun 1963;11:39–43. [DOI] [PubMed] [Google Scholar]
- Dabisch-Ruthe M, Kuzaj P, Gotting C, Knabbe C, Hendig D. Pyrophosphates as a major inhibitor of matrix calcification in Pseudoxanthoma elasticum. J Dermatol Sci 2014;75(2):109–20. [DOI] [PubMed] [Google Scholar]
- Dedinszki D, Szeri F, Kozak E, Pomozi V, Tokesi N, Mezei TR, et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol Med 2017;9(11):1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol 2013;169(2):337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BHY, Duvernay MT, Moore-Lotridge SN, Flick MJ, Schoenecker JG. Plasminogen activation in the musculoskeletal acute phase response: Injury, repair, and disease. Res Pract Thromb Haemost 2020;4(4):469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosen MJ, Coucke PJ, Le Saux O, De Paepe A, Vanakker OM. Perturbation of specific pro-mineralizing signalling pathways in human and murine pseudoxanthoma elasticum. Orphanet J Rare Dis 2014;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Snook AE, Uitto J, Li Q. Adenovirus-Mediated ABCC6 Gene Therapy for Heritable Ectopic Mineralization Disorders. J Invest Dermatol 2019;139(6):1254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Varadi A, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol 2014;34(9):1985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Kucukosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, et al. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci U S A 2013;110(50):20206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, et al. Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol 2005;25(18):8299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Bunda S, VanWart CM, Douet V, Got L, Martin L, et al. Serum factors from pseudoxanthoma elasticum patients alter elastic fiber formation in vitro. J Invest Dermatol 2006;126(7):1497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet 2000;25(2):223–7. [DOI] [PubMed] [Google Scholar]
- Li P, Wang Y, Liu X, Liu B, Wang ZY, Xie F, et al. Loss of PARP-1 attenuates diabetic arteriosclerotic calcification via Stat1/Runx2 axis. Cell Death Dis 2020;11(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Huang J, Pinkerton AB, Millan JL, van Zelst BD, Levine MA, et al. Inhibition of Tissue-Nonspecific Alkaline Phosphatase Attenuates Ectopic Mineralization in the Abcc6(−/−) Mouse Model of PXE but Not in the Enpp1 Mutant Mouse Models of GACI. J Invest Dermatol 2019a;139(2):360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Uitto J. Pseudoxanthoma elasticum: oxidative stress and antioxidant diet in a mouse model (Abcc6−/−). J Invest Dermatol 2008;128(5):1160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kingman J, Sundberg JP, Levine MA, Uitto J. Etidronate prevents, but does not reverse, ectopic mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6(−/−) ). Oncotarget 2018;9(56):30721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sundberg JP, Levine MA, Terry SF, Uitto J. The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6 gene. Cell Cycle 2015;14(7):1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, van de Wetering K, Uitto J. Pseudoxanthoma Elasticum as a Paradigm of Heritable Ectopic Mineralization Disorders: Pathomechanisms and Treatment Development. Am J Pathol 2019b;189(2):216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Vyas A, Kassab MA, Singh AK, Yu X. The role of poly ADP-ribosylation in the first wave of DNA damage response. Nucleic Acids Res 2017;45(14):8129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, Renz A, et al. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell 2002;13(3):978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Li Q, Cao Y, Uitto J. Therapeutics Development for Pseudoxanthoma Elasticum and Related Ectopic Mineralization Disorders: Update 2020. J Clin Med 2020;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KH, Hayward R, Rajan R, Whitehead M, Cobb AM, Ahmad S, et al. Poly(ADP-Ribose) Links the DNA Damage Response and Biomineralization. Cell Rep 2019;27(11):3124–38 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol 1988;6(1):1–159. [DOI] [PubMed] [Google Scholar]
- Nollet L, Van Gils M, Willaert A, Coucke PJ, Vanakker OM. Minocycline attenuates excessive DNA damage response and reduces ectopic calcification in pseudoxanthoma elasticum. J Invest Dermatol 2021. [DOI] [PubMed] [Google Scholar]
- Opdebeeck B, Neven E, Millan JL, Pinkerton AB, D’Haese PC, Verhulst A. Pharmacological TNAP inhibition efficiently inhibits arterial media calcification in a warfarin rat model but deserves careful consideration of potential physiological bone formation/mineralization impairment. Bone 2020;137:115392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali-Ronchetti I, Garcia-Fernandez MI, Boraldi F, Quaglino D, Gheduzzi D, De Vincenzi Paolinelli C, et al. Oxidative stress in fibroblasts from patients with pseudoxanthoma elasticum: possible role in the pathogenesis of clinical manifestations. J Pathol 2006;208(1):54–61. [DOI] [PubMed] [Google Scholar]
- Pomozi V, Brampton C, van de Wetering K, Zoll J, Calio B, Pham K, et al. Pyrophosphate Supplementation Prevents Chronic and Acute Calcification in ABCC6-Deficient Mice. Am J Pathol 2017;187(6):1258–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomozi V, Julian CB, Zoll J, Pham K, Kuo S, Tokesi N, et al. Dietary Pyrophosphate Modulates Calcification in a Mouse Model of Pseudoxanthoma Elasticum: Implication for Treatment of Patients. J Invest Dermatol 2019;139(5):1082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A 2000;97(11):6001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu KW, Kim DS, Kraus WL. New facets in the regulation of gene expression by ADP-ribosylation and poly(ADP-ribose) polymerases. Chem Rev 2015;115(6):2453–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest 2002;82(4):515–8. [DOI] [PubMed] [Google Scholar]
- Schreiber V, Ame JC, Dolle P, Schultz I, Rinaldi B, Fraulob V, et al. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem 2002;277(25):23028–36. [DOI] [PubMed] [Google Scholar]
- Siu SY, Dyment NA, Rowe DW, Sundberg JP, Uitto J, Li Q. Variable patterns of ectopic mineralization in Enpp1asj-2J mice, a model for generalized arterial calcification of infancy. Oncotarget 2016;7(51):83837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani T, Fujiwara M, Orimo H, Shimizu A, Narisawa S, Pinkerton AB, et al. Inhibition of tissue-nonspecific alkaline phosphatase protects against medial arterial calcification and improves survival probability in the CKD-MBD mouse model. J Pathol 2020;250(1):30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C Targeted therapy for ovarian cancer: the rapidly evolving landscape of PARP inhibitor use. Minerva Ginecol 2018;70(2):150–70. [DOI] [PubMed] [Google Scholar]
- Walsh CS, Hodeib M. Leveraging DNA repair deficiency in gynecologic oncology. Curr Opin Obstet Gynecol 2016;28(1):24–31. [DOI] [PubMed] [Google Scholar]
- Wang C, Xu W, An J, Liang M, Li Y, Zhang F, et al. Poly(ADP-ribose) polymerase 1 accelerates vascular calcification by upregulating Runx2. Nat Commun 2019;10(1):1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waymire KG, Mahuren JD, Jaje JM, Guilarte TR, Coburn SP, MacGregor GR. Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet 1995;11(1):45–51. [DOI] [PubMed] [Google Scholar]
- Xu S, Bai P, Little PJ, Liu P. Poly(ADP-ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med Res Rev 2014;34(3):644–75. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kingman J, Sundberg JP, Uitto J, Li Q. Plasma PPi Deficiency Is the Major, but Not the Exclusive, Cause of Ectopic Mineralization in an Abcc6(−/−) Mouse Model of PXE. J Invest Dermatol 2017;137(11):2336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SG, Ferreira CR, MacFarlane EG, Riddle RC, Tomlinson RE, Chew EY, et al. Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci Transl Med 2017;9(393). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are presented in the paper.