Abstract
Introduction:
Great variation exists in the progression and outcomes of cystic fibrosis (CF) lung disease, due to both genetic and environmental influences. Social determinants mediate environmental exposures and treatment success; people with CF from socioeconomically disadvantaged backgrounds have worse health and die younger than those in more advantaged positions.
Areas covered:
This paper reviews the literature on the mechanisms that are responsible for generating and sustaining disparities in CF health, and the ways by which social determinants translate into health advantages or disadvantages in people with CF. The authors make recommendations for addressing social risk factors in CF clinical practice.
Expert opinion:
Socioeconomic factors are not dichotomous and their impact is felt at every step of the social ladder. CF care programs need to adopt a systematic protocol to screen for health-related social risk factors, and then connect patients to available resources to meet individual needs. Considerations such as daycare, schooling options, living and working conditions, and opportunities for physical exercise and recreation as well as promotion of self-efficacy are often overlooked. In addition, advocacy for changes in public policies on health insurance, environmental regulations, social welfare, and education would all help address the root causes of CF health inequities.
Keywords: cystic fibrosis, respiratory outcomes, social determinants of health, social risk factors, socioeconomic position
1. INTRODUCTION
Cystic fibrosis (CF), the second most common autosomal recessive genetic disorder in the United States, is characterized by abnormal secretions in multiple organ systems and eventual respiratory failure [1]. Although the disease is caused by mutations in a single gene – the cystic fibrosis transmembrane conductance regulator (CFTR) – there is a substantial variation in disease progression and outcomes among individuals with identical CFTR genotypes [2–4].
While it is hypothesized that variants in several non-CFTR genes are influential, a number of non-genetic factors also have been implicated in this variability [2, 5–7]. These non-genetic factors are, for the most part, associated with social determinants of health, and contribute substantially to individual and group differences in health status [8–12]; it has been estimated that they account for approximately 50% of the clinical variation in CF [2].
The current paper summarizes the evidence of social determinants in relation to respiratory decline in CF, assesses implications for CF treatment strategies, and makes recommendations for clinical and population-based care and research to address the role of social determinants in CF lung health.
2. SOCIAL DETERMINANTS OF HEALTH
The World Health Organization (WHO) has defined social determinants of health as “the circumstances in which people are born, grow up, live, work, and age,” which are “shaped by the distribution of money, power, and resources locally, nationally, and globally [13].” In the WHO framework (Figure 1) [14], the high-level socioeconomic and political context (e.g., the labor market, the educational system, political institutions, and cultural and societal values) generate social stratification according to income, education, occupation, gender, race/ethnicity, and other factors. The socioeconomic and political context and the resultant social stratification are “structural determinants” that act as determinants of health inequity. One’s standing in the resulting social hierarchy shapes one’s material circumstances (living and working conditions, housing, food, transportation), environmental exposures, and associated behavioral and psychosocial factors, which may be conceptualized as proximal or intermediary determinants of an individual’s health. In this context, the health system may be seen as an intermediary determinant that mediates the differential consequences of illness, whereas social cohesion and social capital cut across the structural and intermediary dimensions.
Figure 1.
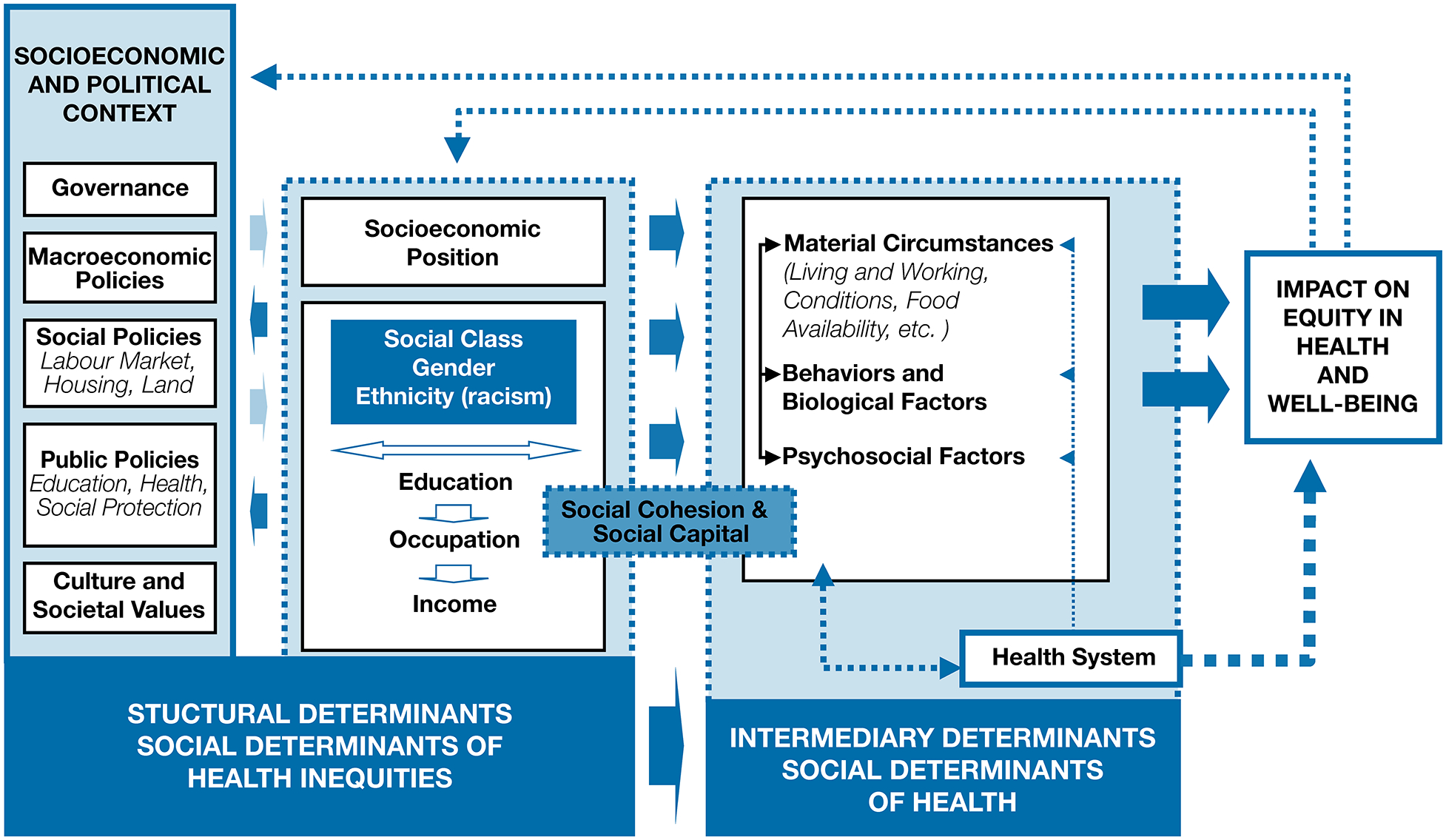
The World Health Organization Commission on Social Determinants of Health conceptual framework. Reproduced from A conceptual framework for action on the social determinants of health. Social Determinants of Health Discussion Paper 2 (Policy and Practice), Solar O, Irwin A, Copyright (2010). [14]
As seen in the WHO framework, the social determinants of health inequities, such as macroeconomic policy and policies on education, housing, and labor, impact the variation in the more proximal social determinants, such as food access and living conditions. Therefore, social determinants have both direct and indirect effects on health.
It is important to note that the term “social determinants of health” encompasses factors that, depending on their manifestation, can promote or undermine health. For example, as a structural determinant, income can affect health positively or negatively through either granting or limiting access to food, housing, educational opportunities, and health care. In other words, the social determinants of health affect everyone, not just the poor and vulnerable. In their adverse manifestation (e.g., poverty, housing instability, food insecurity), they may be classified as social risk factors, but the effect of social determinants is typically that of a non-binary gradient [15].
3. STRUCTURAL DETERMINANTS OF CF RESPIRATORY OUTCOMES
3.1. Social policies
The socioeconomic and political context, which encompasses the structural and functional aspects of the social system, is a powerful determinant of health, although its impact cannot be directly measured at the individual level. In general, there is a paucity of studies that quantify the effect of the economic, social, and political context on human health, with even fewer attempts in the field of respiratory health. Recently, an analysis of data from the U.S. CF Foundation Patient Registry (CFFPR) showed that both area-level socioeconomic characteristics and state-level child health play a role in the health of children with CF. Importantly, the residual association of state child health with CF outcomes after controlling for area-level socioeconomic deprivation reflects the ability of state policies and programs to mitigate the effect of poverty [16]. For example, policies regarding Supplemental Nutrition Assistant Program (SNAP) and Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) vary considerably by state in terms of eligibility, income limits, asset limits, benefit levels, and medical nutrition. Similarly, there is a significant state-level variation in health insurance policies, including expansion of Medicaid eligibility under the Affordable Care Act. Such differences in public policy have a major impact on health outcomes [17], and likely a significant impact on CF lung health. On a global level, analysis of data from 13 European countries in the European Cystic Fibrosis Society Patient Registry showed that, after adjusting for confounders, countries with higher health care spending had a 46% lower hazard of mortality than countries with lowest health care spending [18].
3.2. Socioeconomic position
As a powerful structural determinant of health, socioeconomic position, manifested though indicators such as income, education, and occupation, is linked in a stepwise manner to health outcomes across disease conditions at every point of the life course [19–25]. Below we present evidence for the role of social stratification in CF respiratory health.
3.2.1. Income.
CFFPR data from 1986 to 2011 showed that patients residing in zip codes with lower income had 3–10% lower ppFEV1 than those residing in higher-income zip codes [26]. Another study with zip code income data reported a 5.5% difference in first spirometry at 6 years of age between the lowest quintile (<$20,000/year) and the highest quintile (>$50,000/year) zip codes; the disparity persisted until 18 years of age without increasing significantly over time [27]. The authors also found a 44% increased risk of death for CF patients residing in the most deprived compared to the least deprived income quintiles [27]. Taylor-Robinson et al.[28] assessed the correlation between area-level social deprivation and CF outcomes in a longitudinal study of the UK CF population (1996–2009). The results showed that, compared with CF patients residing in the least deprived areas, those in the most deprived areas had 4% lower ppFEV1 and nearly twice the odds of having chronic P. aeruginosa infection. The lung function disparity was present at 5 years of age and remained constant over time. More recently, in longitudinal analysis of household income data from the CFFPR (2006–2016), Oates et al. reported a dose-response relationship between annual household income and lung function, where every additional $10,000 is associated with a 0.2% increase in ppFEV1 after controlling for demographic and clinical covariates [29]. Additionally, a retrospective analysis of single-center data from Mexico (2000–2020) revealed that, compared with high-income counterparts, low-income patients with CF are four times more likely to have shortened survival; median survival age for the low-income group was 15 years compared to 30 years in the high-income group [30].
3.2.2. Education.
Maternal education of high school or less was associated with 4.2% lower ppFEV1 at age 6–7 in a U.S. multicenter cohort of children with CF enrolled in the Early Pseudomonas Infection Control (EPIC) Observational Study [31]. A retrospective longitudinal analysis of CFFPR data (2006–2016) showed that lower paternal education is associated with a 4.9% lower ppFEV1 after adjusting for demographic and clinical covariates[29]; the disparity was present at age 6 and remained constant through age 18. A lung function deficit by paternal educational attainment has been observed as early as 12 months of age in infants diagnosed with CF through newborn screening [32]. In Denmark, a retrospective longitudinal study with data from the Danish CF patient registry (1969–2010) linked to the national administrative register showed that low parental education was associated with a 0.5% greater annual decline in ppFEV1 after adjusting for demographic, genetic, and clinical factors, resulting in approximately 4% gap between the most and least disadvantaged by 17 years of age [33]. In contrast to US data, however, the lung function deficit was not present at 6 years of age but developed over time. The authors opined that “the Danish welfare system, coupled with lower levels of child poverty, and universal access to high quality healthcare may reduce social differences in outcomes in early childhood [33].” They further acknowledged the potential contribution of monthly follow-up and aggressive treatment of infections in Denmark, which may protect the most disadvantaged in the early years.
3.2.3. Occupation.
One of the first CF studies that demonstrated the independent effect of socioeconomic position was conducted by Britton [34]. Using mortality data for England and Wales (1959–1986), he found that the odds of death from CF above the median age were 2.75 higher among individuals in non-manual occupations than among those in manual occupations. Updated with data through 2008, a subsequent analysis showed that individuals in the highest socioeconomic group had 2.5 higher odds of dying above the median age of death from CF than those in the lowest socioeconomic group [35].
3.2.4. Health insurance.
In the United States, Medicaid is a network of Statewide programs administered by State governments following broad national guidelines established by Federal statutes, regulations, and policies. It specifically provides health care coverage for low-income adults and children, using age-related eligibility criteria and benefits that vary from state to state. Schechter et al.[36, 37] used Medicaid coverage as an indicator for low socioeconomic position and found that the adjusted risk of death was 3.65 times higher for patients on Medicaid than for those not on Medicaid. In addition, the lung function (ppFEV1) of Medicaid patients was 9% lower than that of non-Medicaid patients. The lung function disparity was present at 5 years of age and widened only slightly up to 20 years of age [36]. More recently, Dickinson et al. reported that in the 2000–2011 CF birth cohort, children with intermittent private insurance and exclusively public insurance had, respectively, 3.3% and 6.6% lower ppFEV1 at age 6 compared to those with always private insurance [38].Transition from pediatric to adult CF care is a high-risk period for losing health insurance [39], and the group of 18–25 year olds have the highest uninsured rate of any CF patient age group [1]. During the transition to adulthood, public insurance coverage was associated with accelerated lung function decline among patients with CF: 3.1% and 2.4% per year among those with among patients with continuous and intermittent public insurance, respectively, compared to 2.1% per year among patients with continuous private coverage. These differences were not explained by differences in outpatient care [40]. Therefore, universal health coverage is critical for improving access to CF care.
3.3. Race and ethnicity
Members of racial or ethnic minorities make up a growing proportion of U.S. patients with CF. Between 2004 and 2019, the CFFPR reported an increase in minorities from 3.9 to 4.7% for African Americans, from 6.1 to 9.4% for Hispanics/Latinos, and from 1.9 to 3.8% for other designations [1]. When interpreting reports of racial/ethnic differences in CF respiratory outcomes, it is important to recognize that race and ethnicity are social constructs with little genetic basis [41–43]. As such, the association of race and ethnicity with CF outcomes is to a great extent attributable to unequal social conditions and long-standing structural inequalities. For example, both African Americans and Hispanics/Latinos with CF reside in neighborhoods with lower median household income[44] and have higher Medicaid coverage (52.2% and 41.8%, respectively) than non-Hispanic white counterparts [44]. The adverse effects of lower income, education, and public health insurance were described earlier.
CF patients from racial/ethnic minority backgrounds experience greater disease burden and worse outcomes that parallel the disparities by race/ethnicity in the general population. For example, African Americans with CF have lower lung function than Whites [45], and in the Southern U.S. they also have a higher risk of future hospitalization compared with Whites [46]. Similarly, after adjusting for demographic and clinical covariates, Hispanics/Latinos with CF have approximately 6% lower FEV1 than non-Hispanic/Latino counterparts [47], and in the Western U.S. they have lung function deficit as high as 9.0% [48]. Significant differences in CF mortality by race and ethnicity have also been reported [44,49].
It is notable that disparities in CF outcomes by Hispanic ethnicity occur in spite of higher BMI and a larger proportion of residual function pancreatic sufficient CFTR mutations in the Hispanic/Latino population [43, 50]. Understanding the racial/ethnic disparities in CF respiratory decline requires careful consideration of the effects of socioeconomic position as well as intermediary determinants, including environmental exposures, psychosocial factors, health literacy and acculturation, and the effects of stress and racism [51]. For example, African American and Hispanic patients report worse emotional and social functioning after controlling for disease severity and socioeconomic position [52].
4. INTERMEDIARY DETERMINANTS OF CF RESPIRATORY OUTCOMES
People’s position in the social hierarchy shapes their material circumstances (living and working conditions, housing, food, transportation), environmental exposures, and psychosocial context and greatly influences their health-related behaviors.
4.1. Material circumstances
4.1.1. Food access.
Measured on a household level, food insecurity is defined as limited or uncertain availability of nutritionally adequate foods, with either disrupted eating patterns or reduced food intake [53]. In 2016, 12.3% of all U.S. households experienced food insecurity, with higher prevalence in certain populations and geographic areas. Among people with CF, about 30% are food insecure [54]. Limited access to full-service supermarkets and farmers markets, as well as difficulty getting to grocery stores due to lack of transportation or unsafe neighborhoods are important environmental correlates of nutritional intake and food insecurity [55, 56]. There is ample evidence that the nutritional status of CF patients is closely associated with their socioeconomic status [28, 36, 57–59]. Because food insecurity has profound implication for the health of people with CF [60], the CF Foundation has recommended screening for food insecurity and linking individuals to programs and community resources for food assistance. Studies that evaluate the role of food access for CF respiratory outcomes are yet to be conducted.
4.1.2. Housing and living conditions.
Molds, particularly the filamentous fungus Aspergillus fumigatus, have been implicated in the pathogenesis of allergic bronchopulmonary aspergillosis (ABPA) and CF bronchiectasis [61, 62]. Housing instability has been associated with more severe chronic asthma and greater risk of emergency and hospital readmissions in pediatric patients [63]. In general, however, the role of housing and living conditions for CF respiratory outcomes remains underexplored.
4.1.3. Neighborhood characteristics.
Residential neighborhoods are often divided across socioeconomic and racial/ethnic lines [64]. Low-income and minority neighborhoods are typically characterized by limited access to healthy food, green spaces, and other health-promoting resources[65–68] and have higher rates of crime and violence [69–72]. There are also regional disparities: for example, states with the highest share of neighborhoods that are both low-income and have low access to food are mostly in the South [73].
The neighborhood social and physical environments, including residential segregation, social cohesion, blight, walkability, and food access, are a powerful determinant of health [74, 75]. Important health indicators have been shown to improve with moving people to areas of less concentrated poverty [76]. Specifically, neighborhood crime has been associated with worse respiratory outcomes [77–79], likely through stress [80–82], and with negative health behaviors such as smoking and poor adherence to medications [83]. Exposure to green space has shown protective effects against asthma hospitalizations[84] and bronchitis [85], after accounting for noise and air pollution. Residential segregation predicts asthma burden better than race/ethnicity[86] and has been adversely associated with dyspnea, lung function, emphysema, and air trapping in Black people with chronic obstructive pulmonary disease [87].
Currently, there is a paucity of research on the effect of neighborhood characteristics for CF respiratory outcomes. Area socioeconomic deprivation, calculated at the level of residential zip codes, has been associated with worse respiratory outcomes in pediatric patients with CF in the CFFPR. After adjusting for demographic and clinical covariates, children with CF residing in the worst tertile for area deprivation had 2.8% lower ppFEV1, 1.2 more intravenous treatment nights annually, and 20% higher odds of two or more pulmonary exacerbations [16]. The individual and cumulative impact of specific neighborhood characteristics is yet to be investigated.
4.1.4. Environmental exposures.
Exposures from the natural and built environment, such as outdoor and indoor air quality, allergens, and infectious agents, are important social determinants on CF lung health. A summary of current knowledge about the role of environmental exposures for CF outcomes was presented in a recent review by Szczesniak et al [88]. The mechanisms of this association include both direct damage to the lung tissue and indirect pathways via reactive oxygen species and systemic inflammation [89, 90]
Tobacco smoke exposure.
Aside from direct use of tobacco products, tobacco smoke exposure includes exposure to burning tobacco products or exhaled by a smoker (second-hand), as well as exposure to the residue from tobacco smoke that accumulates in dust, objects, and on surfaces and is reemitted into the air, ingested, or absorbed via skin contact (third-hand) [91]. Approximately one-third of U.S. children and adolescents with CF are regularly exposed [29, 92].
Compelling evidence from animal and human studies indicates that cigarette smoke reduces the expression of the CFTR gene and impairs anion transport and [93–102] and could result in acquired CFTR dysfunction among people without CF [97, 103]. Smoke exposure also increases airway inflammation and impairs pathogen clearance people with CF [104–106]. Because of associations in the prevalence of tobacco use with socioeconomic status, tobacco smoke exposure has been proposed as one of the mechanisms of the link between socioeconomic position and CF lung health [4, 107].
A dose-dependent association between tobacco smoke exposure and overall CF disease severity was first reported by Rubin [108]. These early findings have been corroborated in subsequent studies. A retrospective assessment of the U.S. Cystic Fibrosis Twin and Sibling Study reported a 6.1% decrease in mean ppFEV1 at age 20 attributable to smoke exposure [4]. More recently, analysis of data from the Early Pseudomonas Infection Control (EPIC) Observational Study found a 4-year decrease in mean ppFEV1 associated with smoke exposure (6.0% if mother smoked after birth, 4.6% if mother smoked during pregnancy, 3.2% if child ever around smokers, 2.6% if a household member smokes) [92]. A longitudinal study of CFFPR data (2006–2016) evaluated the contributions of tobacco smoke exposure and socioeconomic factors on initial spirometry at age 6 and change in ppFEV1 through age 18 years [29]. At age 6, ppFEV1 of smoke-exposed children was nearly 5% lower than among unexposed, and the deficit persisted through age 18. Smoke exposure and socioeconomic factors had independent, additive associations with lung function, with the effect of smoke exposure on ppFEV1 being larger in disadvantaged children compared to privileged counterparts (3.2% vs 1.2%) [29]. Routine assessments may present an opportunity to identify socio-environmental risk factors and prioritize children who are both low-income and smoke-exposed for targeted interventions [109].
Important recent evidence points to an interaction of smoke exposure with novel, highly effective modulator therapies for CF. A retrospective longitudinal analysis of encounter-based data from the CFFPR (2016–2018) showed that among individuals with CF aged 12–20 years old, tezacaftor/ivacaftor provided no benefit to smoke-exposed patients although it was associated with improved ppFEV1 among unexposed counterparts [110]. To maximize the therapeutic opportunity presented by highly effective CFTR modulators, every effort must be taken to eliminate environmental exposure to smoke for people with CF. The benefits of removing smoke exposure were demonstrated in a recent study of CFFPR data for children and adolescents with CF (2006–2018). The authors found that removing smoke exposure reduces the odds of having a pulmonary exacerbation by 17% in the first year and by another 6% in each additional year of non-exposure [111]. Stopping of exposure is also associated with respiratory and nutritional improvements: 0.7% ppFEV1 increase in the first year and 0.4% increase in each additional year of non-exposure; 1% increase in BMI percentile in the first year and 0.4% increase in each additional year. After three years of not being exposed to tobacco smoke, children and adolescents with CF have 8% lower predicted probability of an exacerbation and 2% higher ppFEV1 and BMI than counterparts who remain exposed [111]. These results provide further support for the need to prioritize smoking cessation and exposure prevention in CF care.
Ambient air pollution.
In general, residents of socioeconomically disadvantaged communities are exposed to greater short- and long-term air pollution [112–115], which compromises lung growth [116] and leads to increased mortality [115, 117]. These effects are ever stronger among populations with increased susceptibility, such as people with CF and other lung diseases [118]. For example, longitudinal CF studies outside of the US show that ozone is associated with an increased risk of pulmonary exacerbations [119] in people with CF, whereas higher concentrations of ozone, particulate matter, and nitrogen dioxide are associated with more prescriptions of IV antibiotics [120]. Analysis of CFFPR data shows similar results: higher exposure to ozone and particulate matter (PM2.5 and PM10) is linked to increased exacerbations and decreased lung function [121]. In addition, fine particulate matter (PM2.5) increases the risk of P. aeruginosa acquisition in young children with CF [122].
Infectious agents.
Socioeconomically disadvantaged communities are exposed to greater health risks through a disproportionate exposure to infectious agents. In the UK, people with CF residing in poor areas were nearly twice more likely to have chronic P. aeruginosa infection than counterparts in affluent areas [30]. In the US, the likelihood of P. aeruginosa acquisition among children with CF is also increased with low maternal education [123]. Similar disparities have been reported in Methicillin-resistant Staphylococcus aureus (MRSA) infection. In the US, private health insurance has been associated with 13% lower risk of having MRSA [124]. Neighborhood socioeconomic deprivation has been linked to more than 2-fold increase in the odds of having MRSA after adjusting for demographic and clinical covariates [125]. Multiple mechanisms may be contributing to this association. Collective and concentrated poverty may affect exposure to indoor and outdoor air quality and pathogens. Further study of potential mediators of the link between socioeconomic deprivation and infectious agents is warranted.
4.2. Behavioral factors
Due to high treatment burden in CF, maintenance of daily therapies is a challenge for all people with CF [126]; suboptimal adherence and associated adverse effects for disease outcomes have been reported across the entire CF population [127, 128]. Parental educational level is correlated with chronic disease self-management in the general population [129–132], and studies suggest that knowledge of the treatment regimen and an understanding of its rationale are a prerequisite for adherence in CF as well [133, 134]. Anthony et al.[135] reported that maternal nutritional knowledge specific to CF is a predictor of caloric intake and growth in children with CF, while Quittner et al.[136] found that nonadherence was explained by misunderstanding of the prescribed regimen. CF treatment adherence also correlates with optimism, family functioning [137, 138], and parental stress [139], discussed below. Thus, worse adherence is a likely contributor to poorer outcomes among disadvantaged children with CF [140, 141]
4.3. Psychosocial factors
4.3.1. Family structure.
Closely related to socioeconomic status, family structure is implicated in a range of health-related outcomes. For example, children with CF who are cared for by single mothers have worse respiratory and nutritional outcomes than children with dual caregivers [142, 143]. Relatedly, mothers of children with CF report higher levels of stress associated with decision-making and responsibility for parenting [144, 145].
4.3.2. Stress.
Socioeconomic position is associated with differential exposure to chronic stressors, such as financial strain, job insecurity, residential crowding, noise exposure, and social isolation [146–151]. Disproportionate exposure to continuous and repeated stressors, which results in physiologic ‘wear and tear’, is a known mechanism of health disparities [152–155]. Onerous and costly daily care, frequent interactions with the healthcare system, uncertainty about the future, and limited employment opportunities take a toll on the physical and mental health of people with CF and their caregivers [156–160]. A third of CF parents are clinically depressed [161], with low socioeconomic status being associated with a higher prevalence of depressive symptoms [162]. Depression is linked to worse health outcomes, including lower lung function [163, 164]. In a study by Quittner et al. [52], socioeconomic disadvantage was associated with worse quality of life for both CF children and their parents when adjusted for disease severity. A recent longitudinal study showed that 5-year mortality of people with CF screening positive for depression was twice that of those who did not, and nearly triple for those who screened in the severe range [165].
4.3.3. Social support.
The harmful effects of stress on health can be buffered by sense of control and other stress-mitigating resources, including social support. Although social support has been linked to a variety of health outcomes in people with and without chronic illness, relatively few studies focus on the importance of social support in CF [166, 167]. A study of 250 adults with CF showed that greater social support was associated with fewer self-reported mental and physical health symptoms, lower treatment burden, better body image, and higher emotional, social, and role functioning [168]. Social support is protective against lung function decline[169] and hospitalizations[170] after transfer from pediatric to adult care.
4.4. Health system factors
Access to health care is an important social determinant of health. To determine whether socioeconomic disparities in CF outcomes in the US can be explained by differences in medical treatment, Schechter et al.[171] performed a cross-sectional analysis of data on pediatric patients in the Epidemiologic Study of Cystic Fibrosis. Disease severity showed a similar inverse correlation with all measures of socioeconomic status, but the number of clinic visits was unrelated to socioeconomic status, and disadvantaged patients were prescribed more – rather than less – chronic therapies. These results demonstrate that the socioeconomic disparities in CF health are not due to differential therapy prescription or use of health services. Another similar study found that pediatric patients of lower socioeconomic status are prescribed more – rather than less – antibiotic treatments for pulmonary exacerbations [172]. Among adults with CF, public insurance also was associated with equal or greater use of CF care compared to private insurance [173].
Although generally there are no socioeconomic differences in prescribed therapies or treatment of pulmonary exacerbations in CF [171, 172, 174], significant disparities have been observed in lung transplantation. Low socioeconomic status as measured by zip-code income, education level, and Medicaid insurance has been independently associated with not being referred to evaluation for lung transplantation [175] or accepted for lung transplantation [176] despite meeting all the criteria. A case-control study of in the linked CFFPR/Scientific Registry of Transplant Recipients found that accrual of socioeconomic barriers (race, marital status, education, health insurance, zip-code income, and distance to transplant program) limits access to lung transplant irrespective of disease severity [177]. Individuals with greater socioeconomic barriers accessed transplant about half as often as those with less barriers at the same level of medical severity [177]. Consequently, CF patients with Medicaid insurance have higher risk of death while awaiting lung transplantation compared to those with Medicare or private insurance [178]. They also have 22% worse survival after lung transplantation than those with private insurance [179].
The advent of cystic fibrosis transmembrane conductance regulator (CFTR) modulators, genomic-specific medications that target the malfunctioning protein made by the CFTR gene, is revolutionizing the treatment of CF due to their effectiveness in mitigating the downstream adverse effects of CFTR dysfunction in patients with responsive mutations. While the overall positive impact of this new treatment modality is clearly welcome, it may also serve to increase disparities. First, these drugs are not effective for “nonsense mutations” that are associated with premature stop codons, and this mutation class is more common is people with CF who are of non-European ancestry. Second, as noted earlier, there is some suggestion that tobacco smoke exposure, which is more common in lower socioeconomic groups, may have an inhibitory effect on CFTR modulators. Finally, an increased inventory of CFTR modulators and mutation-eligible patients may trigger cost concerns among public and private payors alike[180]. This could result in restricted coverage or increased cost-sharing and out-of-pocket expenses [181], both of which will limit patient access to highly effective modulator therapies. It is imperative that the pharmaceutical industry, insurers, health-care providers, and CF stakeholders engage in a deliberate process to make sure that CF precision medicine is available to all.
A discussion of the therapeutic pipeline that led to the development of CFTR modulators must also touch on disparities in participation in clinical trials. In 2006, Goss et al. reported that, compared to the overall CF population in the US, clinical trial participants were more likely to have private insurance and to be White [182]. They further pointed out that clinical trial participants tended to have a lower average rate of decline in lung function than non-participants, an effect that seemed to be linked to closer clinical follow-up. In 2016, McGarry et al. further documented the absolute underrepresentation of minorities in CF clinical trials [183]. Inadequate inclusion of all population subgroups in clinical research may bias trial results and inhibit our understanding of factors that influence drug response.
In summary, multiple studies report an association between socioeconomic disadvantage and worse CF outcomes, which begins in early childhood and persists throughout the life course. The mechanisms of this association are varied and complex. Improved understanding of all various pathways will require in-depth studies that integrate clinical, socioeconomic, and environmental data. Such studies can provide critical evidence for policy, social, and healthcare initiatives to reduce disparities as well as for clinical interventions to optimize treatments.
5. IMPLICATIONS FOR TREATMENT
Abundant evidence supports the relationship between structural and intermediate social determinants and CF respiratory outcomes, with incremental improvements of CF health at every step of the socioeconomic ladder. The importance of economic and government-level policies for improving the social and environmental context is indisputable. The conceptual framework of the WHO Commission on the Social Determinants of Health illustrates that interventions must not be limited to addressing the intermediary determinants, but must include policies that tackle the social mechanisms that systematically generate an inequitable distribution of health-related resources among population groups. To tackle structural, as well as intermediary, determinants of health requires inter-sectoral and multilevel approaches [14].
5.1. Social policies
Population-level policy, system, and environmental interventions may be more difficult for clinicians to take on, but are more impactful than individual, patient-level interventions to improve CF health [184, 185]. Multi-level strategies that address both structural and intermediary determinants are particularly needed. Table 1 provides examples of such strategies for the general population and specifically for people with CF, organized according to the framework for action of the WHO Commission on the Social Determinants of Health.
Table 1.
Examples of interventions on the social determinants of health
| Level | Strategies | |
|---|---|---|
| General Population | People with CF | |
|
Social stratification:
Policies to reduce social inequalities and mitigate the effects of stratification |
|
|
|
Environmental exposures: Policies to reduce exposures of disadvantaged people to health-damaging factors |
|
|
|
Vulnerability:
Policies to reduce vulnerability |
|
|
5.2. Community-level interventions
Community interventions on social determinants that can affect the respiratory health of people with CF include urban planning policies that promote physical separation from pollution sources, complete streets policies that promote active transportation, and urban planning efforts that increase walkability and access to parks and recreational facilities. Several interventions developed specifically for the CF community have been particularly successful, as described further.
5.2.1. The CF Foundation patient assistance program (CF Compass).
Compass is a CF Foundation-sponsored personalized service to help with insurance, financial, legal, and other issues faced by people with CF, their families, or their care teams [186]. Compass’s team of case managers provide free expert advice and connect individuals to resources offered by community organizations, local and state governments, foundations, or other groups.
5.2.2. Patient and Family Advisory Councils.
These groups advise CF care centers on the needs of people with CF and on implementing the types of support most needed by CF families.
5.2.3. CF Chapter and care center partnerships.
CF Foundation chapters are uniquely positioned to create close relationships with local community organizations and CF care centers. In recent years, the role of chapters has expanded to include outreach and support programs for people with CF and their families.
5.3. Clinical interventions
5.3.1. Screening for unmet social needs.
Under the umbrella of the social determinants of health, unmet social needs are defined as the social risks factors that an individual both recognizes and prioritizes [15]. For instance, a screening tool may uncover multiple social risks, such as inadequate food, housing, utilities, and transportation, but the individual may consider that her most pressing need is to find a safe place away from an abusive partner. Identifying unmet social needs can inform providers that a patient needs more support to manage their condition or could benefit from referrals to social services [187]. Screening for social needs can also make patients feel supported and understood even if their needs cannot be directly addressed by the clinic [188]. Although the feasibility of screening for unmet social needs in CF care has not been formally established, CF clinics may offer a natural setting for social needs evaluation because of routine visits and established relationships between patients and providers. However, there are both feasibility and ethical issues associated with social needs screening in clinical settings. Perceived lack of time is among the most significant barriers [189, 190]. Furthermore, while clinicians are aware of the importance of social determinants of health, most of them have inadequate training in extracting information related to sensitive social needs, such as housing and food insecurity, in a respectful and culturally appropriate way. Finally, screening for social needs can detect adverse social circumstances that require resources beyond the scope of clinical care. Referring families to nonmedical organizations to resolve social needs requires specialized training and dedicated staff that few clinicians have at their disposal [189, 191, 192]. Garg et al. warned about the unintended consequences of screening for SDOH in clinical care, especially when referral resources are unavailable for addressing identified needs [193].
5.3.2. Referrals to community organizations.
Screening in clinical settings has limited impact unless it is followed by an action to help alleviate the identified social needs. One common intervention is the referral of patients to community organizations that routinely provide such assistance. However, the availability of such community resources is often limited. A 2020 study analyzed the capacity of social service agencies to meet the needs of those who called 211, a toll-free number that connects callers with community services [194]. There was both a high prevalence of and high capacity to meet food needs. Needs with high prevalence but a low capacity of resources included public transportation and housing assistance. Similarly, in 2019 there were nearly 600 requests to CFF Compass program for assistance with food, housing, transportation, or utilities. In 19% of these cases, there was not an existing community resource to meet the caller’s needs.
5.3.3. Screening for tobacco smoke exposure and delivery of tobacco treatment services.
Cessation of tobacco smoke exposure improves pediatric CF outcomes, so this is low-hanging fruit [109]. CF care teams can screen for exposure and deploy evidence-based smoking cessation services, such as behavioral counseling, nicotine replacement therapy, and pharmacotherapy [91, 195, 196]. Determining what cessation strategies are most effective for CF families will be critical. Identifying and intervening with smoke-exposed CF patients may also require changes to current CF clinical guidelines regarding screening and interventions. Barriers to such efforts include lack of trained staff to deliver smoking cessation interventions, inadequate reimbursement for cessation services, and a perception of low levels of success [197]. Still, smoke exposure is one of few modifiable risk factors in CF that can be targeted to optimize therapies and maximize the health potential of people with CF. Programs that can be initiated in the setting of pediatric practices have been shown to reduce second-hand smoke exposure, and surveys show that advice on smoking cessation from their child’s physician would be welcomed by most parents [198].
5.3.4. Ensuring equitable access to treatment, including transplant.
Although previous studies have indicated that access to care and differential treatment of patients from lower socioeconomic status do not appear to be the problem typically seen in other disease populations, there may still be nuance and important unmeasured differences. For example, uptake of new medications seems slower in disadvantaged CF patients. In the 2009 study by Schechter et al., the only medication prescribed less often to patients with public insurance was azithromycin, which at the time was a relatively new therapy [199]. More recently, patients with public insurance received prescriptions for lumacaftor-ivacaftor more slowly after its approval than those with private insurances [200]; race but not insurance status was associated with slower uptake of ivacaftor following its approval [201]. Given the lack of differential prescribing of other medications, it is unlikely that slower prescribing of new medications is intentional. Rather, delays may be due to insurance barriers to approval of the new medications, but it is also possible that wealthier and/or better-educated patients are more aware of new breakthroughs and more skilled at self-advocating, thus ensuring that their physicians promptly prescribe these newer medications. The relationship between health literacy and self-advocacy in families of lower socioeconomic status and its effect on treatment have been noted [202]. Therefore, quality improvement efforts by CF care teams to ensure consistently optimal treatment to all patients may have a differential impact on disadvantaged patients. The literature on this topic in the general population is mixed, but preliminary findings regarding a reduction in lung function disparities following successful institution of a quality improvement program that focused on increasing the consistency of antibiotic treatment for pulmonary exacerbations support the concept [203, 204].
It was noted previously that there is a differential referral for lung transplant, and acceptance into the program, by socioeconomic status [175–179], largely due to a perception that the resources and social support needed to successfully undergo organ transplantation are beyond the reach of people with limited finances and education. Although such concerns may be legitimate, the resulting disparities in transplantation access and outcomes are unacceptable. Transplant programs must focus additional resources to make the process more equitable.
5.3.5. Screening for and treatment of depression.
International CF care guidelines recommend universal screening and treatment for anxiety and depression in people with CF [205], and those guidelines have generally been adopted in the US [206]. However, screening, as well as the diagnosis and treatment of mental health disorders, continues to vary across care centers [1]. Improvements in the rate of screening through QI efforts and ensuring access to mental health services to those in need would likely have a positive effect on people with CF from lower socioeconomic status.
5.3.6. Improving enrollment in clinical trials.
The downside to excluding socioeconomically disadvantaged and minority patients from clinical trials is the introduction of bias into study results and extrapolating findings to populations that may not necessarily respond to the treatments in the same way as those enrolled in the trials. This should be a concern to study sponsors and pharmaceutical companies. Furthermore, inclusion in clinical trials will benefit these populations, especially given the possibility that some drugs may behave differently in certain ethnic and racial subgroups with non-European ancestry (or, as noted for the CFTR modulators, in disadvantaged populations with higher tobacco smoke exposure). The sociodemographic characteristics of all study participants should be reported, with necessary steps taken to ensure adequate representation of vulnerable populations in clinical trials. This may require extra efforts aimed at building trust, particularly for individuals belonging to racial and ethnic communities that have experienced a history of insidious and pervasive mistreatment in research settings.
6. EXPERT OPINION
With advancements in early diagnosis and medical treatment, survival in CF has improved rapidly, yet variations in disease progression persist. People with CF from socioeconomically disadvantaged backgrounds have worse health and die younger than those in more advantaged positions. Multiple mechanisms are responsible for producing disparities in CF health, and we outlined some of the ways by which social determinants translate into health advantages or disadvantages in people with CF.
As discussed, the health effect of socioeconomic and environmental factors is not dichotomous but exist at every step of the social ladder. Therefore, we need interventions that not only reduce disparities but optimize outcomes for people with CF across the entire socioeconomic spectrum. CF care programs in the U.S. need to adopt a systematic, protocolized screening for health-related social risk factors such as food insecurity, housing instability, lack of transportation needs, utilities insecurity, and harmful environmental exposures, and then connect patients to available resources to meet identified needs. Federal and state government benefits, such as food assistance programs, supplemental security insurance, and social security disability, as well as local resources and patient assistance programs can help ensure a level of material well-being that is a prerequisite for CF health. Screening for social risk factors in people with CF and their families should also include often-overlooked aspects of daily life with CF such as daycare needs, schooling options, living and working conditions, and opportunities for physical exercise and recreation. For example, subsidized day care and home-based nursing assistance programs can relieve major daily stressors for CF families. Health insurance plans or patient assistance programs that cover nutritional supplements, exercise equipment, gym memberships, medical equipment, and all medically indicated therapies, can minimize inequities in CF health. Finally, CF care programs and patient advocacy organizations such as the CF Foundation need to support changes in public policies on economic affairs and taxation, health insurance, environmental regulations, social welfare, and education – that can address the root causes of CF health inequities.
6.1. Five-year view:
In the era of highly effective CFTR modulator therapies, as the mean age of the CF patient population increases, socioeconomic disparities in CF will become more prominent. Consequently, we expect that care teams and patient advocacy organizations will show growing interest in identifying and intervening on the social determinants of CF outcomes, from unmet basic needs such as food, housing, and utilities, to preventable environmental exposures, to self-management factors. We anticipate increased focus on developing interventions that target modifiable aspects of the social environment and expanded use of social and behavioral science to support daily CF care. Finally, we foresee interest in research on race, ethnicity, socioeconomic status, sexual orientation, and other social vulnerabilities in CF, and efforts to ensure equitable treatment and care for all CF patient population subgroups.
Article highlights.
Factors termed social determinants of health have both direct and indirect implications for CF health and contribute to the observed variability in CF respiratory outcomes.
Structural social determinants that indirectly impact CF respiratory outcomes include social policies, socioeconomic position (income, education, occupation, health insurance), and race/ethnicity.
Intermediate social determinants that directly affect CF respiratory outcomes include food, housing and living conditions, environmental exposures (tobacco smoke, outdoor and indoor air quality, infectious agents), psychosocial factors (family structure, stress, social support), and health system factors.
Multi-level strategies that address both structural and intermediary social determinants through policy, system, and environment changes are particularly needed.
Interventions that can be adopted in clinical settings include screening for unmet social needs and referral to available resources, screening for smoke exposure and provision of tobacco treatment services, screening for and treatment of depression, and ensuring equitable access to care and representation in clinical trials.
Declaration of funding
This paper was supported by grants from the National Institutes of Health (P30DK072482) and the CF Foundation (OATES20A0-I).
Declaration of financial/other relationships
Gabriela R. Oates and Michael S. Schechter receive grant support from the Cystic Fibrosis Foundation and NIH
Michael S. Schechter receives consultancy fees from Vertex, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Cystic Fibrosis Foundation Patient Registry 2019 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation, 2020. [Google Scholar]
- 2.*.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the relative contribution of environmental and genetic factors to variation in cystic fibrosis lung function. J Pediatr. 2010;157(5):802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first paper to quantify the contribution of genetic and non-genetic factors to disease heterogeneity in cystic fibrosis
- 3.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361(9358):681–9. [DOI] [PubMed] [Google Scholar]
- 4.Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, et al. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299(4):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfenden LL, Schechter MS. Genetic and non-genetic determinants of outcomes in cystic fibrosis. Paediatr Respir Rev. 2009;10(1):32–6. [DOI] [PubMed] [Google Scholar]
- 6.Oates GR, Schechter MS. Socioeconomic status and health outcomes: cystic fibrosis as a model. Expert Rev Respir Med. 2016;10(9):967–77. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor GT, Quinton HB, Kahn R, Robichaud P, Maddock J, Lever T, et al. Case-mix adjustment for evaluation of mortality in cystic fibrosis. Pediatr Pulmonol. 2002;33(2):99–105. [DOI] [PubMed] [Google Scholar]
- 8.Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public health reports. 2014;129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marmot M, Allen JJ. Social determinants of health equity. Am J Public Health. 2014;104(S4):S517–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–98. [DOI] [PubMed] [Google Scholar]
- 11.Braveman PA, Egerter SA, Mockenhaupt RE. Broadening the focus: the need to address the social determinants of health. Am J Prev Med. 2011;40(1):S4–S18. [DOI] [PubMed] [Google Scholar]
- 12.Brennan Ramirez L, Baker EA, Metzler M. Promoting Health Equity: A Resource to Help Communities Address Social Determinants of Health. 2008.
- 13.Closing the gap in a generation: health equity through action on the social determinants of health. Final Report of the Commission on Social Determinants of Health. Geneva, Switzerland: World Health Organization, 2008. [DOI] [PubMed] [Google Scholar]
- 14.*.Solar O, Irwin A. A conceptual framework for action on the social determinants of health. Social Determinants of Health Discussion Paper 2 (Policy and Practice): World Health Organization, Geneva, Switzerland, 2010. [Google Scholar]; A definitive WHO summary of the conceptual framework for understanding social determinants of health
- 15.Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. The Milbank Quarterly. 2019;97(2):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oates GR, Rutland S, Juarez L, Friedman A, Schechter MS. The association of area deprivation and state child health with respiratory outcomes of pediatric patients with cystic fibrosis in the United States. Pediatr Pulmonol. 2021;56(5):883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guth M, Garfield R, Rudowitz R. The Effects of Medicaid Expansion under the ACA: Studies from January 2014 to January 2020. 2020. Mar 17, 2020. [Google Scholar]
- 18.McKone EF, Ariti C, Jackson A, Zolin A, Carr SB, Orenti A, et al. Survival estimates in European cystic fibrosis patients and the impact of socioeconomic factors: a retrospective registry Cohort study. Eur Respir J. 2021; 58: 2002288. [DOI] [PubMed] [Google Scholar]
- 19.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. [DOI] [PubMed] [Google Scholar]
- 20.Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56(4):769–84. [DOI] [PubMed] [Google Scholar]
- 21.Spilerman S. Wealth and stratification processes. Annu Rev Sociology. 2000;26:497–524. [Google Scholar]
- 22.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100 Suppl 1:S186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitagawa EM, Hauser PM. Differential mortality in the United States: a study in socioeconomic epidemiology. Cambridge, Mass.: Harvard University Press, 1973. [Google Scholar]
- 24.Pappas G, Queen S, Hadden W, Fisher G. The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. N Engl J Med. 1993;329(2):103–9. [DOI] [PubMed] [Google Scholar]
- 25.Adler NE, Ostrove JM. Socioeconomic status and health: What we know and what we don’t. Socioeconomic Status and Health in Industrial Nations. 1999;896:3–15. [DOI] [PubMed] [Google Scholar]
- 26.Johnson B, Ngueyep R, Schechter MS, Serban N, Swann J. Does distance to a cystic fibrosis center impact health outcomes? Pediatr Pulmonol. 2018;53(3):284–92. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor GT, Quinton HB, Kneeland T, Kahn R, Lever T, Maddock J, et al. Median household income and mortality rate in cystic fibrosis. Pediatrics. 2003;111(4):e333–9. [DOI] [PubMed] [Google Scholar]
- 28.*.Taylor-Robinson DC, Smyth RL, Diggle PJ, Whitehead M. The effect of social deprivation on clinical outcomes and the use of treatments in the UK cystic fibrosis population: a longitudinal study. Lancet Respir Med. 2013;1(2):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reflecting social disparities in the UK
- 29.**.Oates GR, Baker E, Rowe SM, Gutierrez HH, Schechter MS, Morgan W, et al. Tobacco smoke exposure and socioeconomic factors are independent predictors of pulmonary decline in pediatric cystic fibrosis. J Cyst Fibros. 2020. Feb 17;S1569–1993(20)30049–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Showing the role played by tobacco smoke exposure in CF social disparities
- 30.Bustamante AE, Fernandez LT, Rivas LC, Mercado-Longoria R. Disparities in cystic fibrosis survival in Mexico: Impact of socioeconomic status. Pediatr Pulmonol. 2021;56(6):1566–72. [DOI] [PubMed] [Google Scholar]
- 31.Sanders DB, Emerson J, Ren CL, Schechter MS, Gibson RL, Morgan W, et al. Early Childhood Risk Factors for Decreased FEV1 at Age Six to Seven Years in Young Children with Cystic Fibrosis. Ann Am Thorac Soc. 2015;12(8):1170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Britton LJ, Oates GR, Oster RA, Self ST, Troxler RB, Hoover WC, et al. Risk stratification model to detect early pulmonary disease in infants with cystic fibrosis diagnosed by newborn screening. Pediatr Pulmonol. 2016;51(11):1168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor-Robinson DC, Thielen K, Pressler T, Olesen HV, Diderichsen F, Diggle PJ, et al. Low socioeconomic status is associated with worse lung function in the Danish cystic fibrosis population. Eur Respir J. 2014;44(5):1363–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Britton JR. Effects of social class, sex, and region of residence on age at death from cystic fibrosis. BMJ. 1989;298(6672):483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barr HL, Britton J, Smyth AR, Fogarty AW. Association between socioeconomic status, sex, and age at death from cystic fibrosis in England and Wales (1959 to 2008): cross sectional study. Br Med J. 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.*.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–7. [DOI] [PubMed] [Google Scholar]; The initial paper reporting on social disparities in CF
- 37.Schechter MS, Margolis PA. Relationship between socioeconomic status and disease severity in cystic fibrosis. J Pediatr. 1998;132(2):260–4. [DOI] [PubMed] [Google Scholar]
- 38.*.Dickinson KM, Psoter KJ, Riekert KA, Collaco JM. Association between insurance variability and early lung function in children with cystic fibrosis. J Cyst Fibros. 2021. Jun 23:S1569–1993(21)01290-X. doi: 10.1016/j.jcf.2021.06.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; Emphasizing that socioeconomic disparities align along a continuum rather than just a dichotomy
- 39.Tuchman LK, Schwartz LA, Sawicki GS, Britto MT. Cystic fibrosis and transition to adult medical care. Pediatrics. 2010;125(3):566–73. [DOI] [PubMed] [Google Scholar]
- 40.Tumin D, Crowley EM, Li SS, Wooten W, Ren CL, Hayes D Jr. Patterns of Health Insurance Coverage and Lung Disease Progression in Adolescents and Young Adults with Cystic Fibrosis. Ann Am Thorac Soc. 2021;18(2):290–9. [DOI] [PubMed] [Google Scholar]
- 41.Caldwell SH, Popenoe R. Perceptions and misperceptions of skin color. Ann Intern Med. 1995;122(8):614–7. [DOI] [PubMed] [Google Scholar]
- 42.Kahn J How a drug becomes “ethnic”: law, commerce, and the production of racial categories in medicine. Yale J Health Policy Law Ethics. 2004;4(1):1–46. [PubMed] [Google Scholar]
- 43.Hoover EL. There is no scientific rationale for race-based research. J Natl Med Assoc. 2007;99(6):690–2. [PMC free article] [PubMed] [Google Scholar]
- 44.Buu MC, Sanders LM, Mayo JA, Milla CE, Wise PH. Assessing Differences in Mortality Rates and Risk Factors Between Hispanic and Non-Hispanic Patients With Cystic Fibrosis in California. Chest. 2016;149(2):380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamosh A, FitzSimmons SC, Macek M Jr., Knowles MR, Rosenstein BJ, Cutting GR. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J Pediatr. 1998;132(2):255–9. [DOI] [PubMed] [Google Scholar]
- 46.Kopp BT, Nicholson L, Paul G, Tobias J, Ramanathan C, Hayes D Jr. The Geographic Impact on Hospitalization in Patients with Cystic Fibrosis. J Pediatr. 2016;170:246–52. [DOI] [PubMed] [Google Scholar]
- 47.*.McGarry ME, Neuhaus JM, Nielson DW, Burchard E, Ly NP. Pulmonary function disparities exist and persist in Hispanic patients with cystic fibrosis: A longitudinal analysis. Pediatr Pulmonol. 2017;52(12):1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pointing out that ethnic disparities are superimposed upon socioeconomic disparities
- 48.McGarry ME, Neuhaus JM, Nielson DW, Ly NP. Regional variations in longitudinal pulmonary function: A comparison of Hispanic and non-Hispanic subjects with cystic fibrosis in the United States. Pediatr Pulmonol. 2019;54(9):1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rho J, Ahn C, Gao A, Sawicki GS, Keller A, Jain R. Disparities in Mortality of Hispanic Patients with Cystic Fibrosis in the United States. A National and Regional Cohort Study. Am J Respir Crit Care Med. 2018;198(8):1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watts KD, Seshadri R, Sullivan C, McColley SA. Increased prevalence of risk factors for morbidity and mortality in the US Hispanic CF population. Pediatr Pulmonol. 2009;44(6):594–601. [DOI] [PubMed] [Google Scholar]
- 51.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Annals of the New York Academy of Sciences. 1999;896:173–88. [DOI] [PubMed] [Google Scholar]
- 52.Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. Impact of socioeconomic status, race, and ethnicity on quality of life in patients with cystic fibrosis in the United States. Chest. 2010;137(3):642–50. [DOI] [PubMed] [Google Scholar]
- 53.Definitions of Food Insecurity. United States Department of Agriculture Economic Research Service. Available at: https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security.aspx.
- 54.McDonald CM, Christensen NK, Lingard C, Peet KA, Walker S. Nutrition Knowledge and Confidence Levels of Parents of Children With Cystic Fibrosis. ICAN: Infant, Child, & Adolescent Nutrition. 2009;1(6):325–31. [Google Scholar]
- 55.Dubowitz T, Zenk SN, Ghosh-Dastidar B, Cohen DA, Beckman R, Hunter G, et al. Healthy food access for urban food desert residents: examination of the food environment, food purchasing practices, diet and BMI. Public Health Nutr. 2015;18(12):2220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: A review of food deserts literature. Health Place. 2010;16(5):876–84. [DOI] [PubMed] [Google Scholar]
- 57.Taylor-Robinson D, Whitehead M, Diggle P, Smyth R. The Effect of Social Deprivation on Weight in the UK Cystic Fibrosis Population. J Epidemiol Community Health. 2011;65:A389–A. [Google Scholar]
- 58.Pinto IC, Silva CP, Britto MC. Nutritional, clinical and socioeconomic profile of patients with cystic fibrosis treated at a referral center in northeastern Brazil. J Bras Pneumol. 2009;35(2):137–43. [DOI] [PubMed] [Google Scholar]
- 59.Balmer DF, Schall JI, Stallings VA. Social disadvantage predicts growth outcomes in preadolescent children with cystic fibrosis. J Cyst Fibros. 2008;7(6):543–50. [DOI] [PubMed] [Google Scholar]
- 60.Brown PS, Durham D, Tivis RD, Stamper S, Waldren C, Toevs SE, et al. Evaluation of Food Insecurity in Adults and Children With Cystic Fibrosis: Community Case Study. Front Public Health. 2018;6:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moss RB. Fungi in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med. 2015;36(2):207–16. [DOI] [PubMed] [Google Scholar]
- 62.Rocchi S, Richaud-Thiriez B, Barrera C, Grenouillet F, Dalphin JC, Millon L, et al. Evaluation of mold exposure in cystic fibrosis patients’ dwellings and allergic bronchopulmonary risk. J Cyst Fibros. 2015;14(2):242–7. [DOI] [PubMed] [Google Scholar]
- 63.Molina AL, Molina Y, Walley SC, Wu CL, Zhu A, Oates GR. Residential instability, neighborhood deprivation, and pediatric asthma outcomes. Pediatr Pulmonol. 2020;55(6):1340–8. [DOI] [PubMed] [Google Scholar]
- 64.Anderson KM, Institute of Medicine Roundtable on the Promotion of Health Equity and the Elimination of Health Disparities. How far have we come in reducing health disparities? Progress since 2000: Workshop summary. Washington, D.C.: National Academies Press; 2012. [PubMed] [Google Scholar]
- 65.Franco M, Diez Roux AV, Glass TA, Caballero B, Brancati FL. Neighborhood characteristics and availability of healthy foods in Baltimore. Am J Prev Med. 2008;35(6):561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore LV, Diez Roux AV. Associations of neighborhood characteristics with the location and type of food stores. Am J Public Health. 2006;96(2):325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller M, Middendorf G, Wood SD. Food availability in the heartland: Exploring the effects of neighborhood racial and income composition. Rural Sociology. 2015;80(3):340–61. [Google Scholar]
- 68.Jennings V, Baptiste AK, Osborne Jelks N, Skeete R. Urban Green Space and the Pursuit of Health Equity in Parts of the United States. Int J Environ Res Public Health. 2017;14(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sampson RJ, Morenoff JD, Raudenbush S. Social anatomy of racial and ethnic disparities in violence. Am J Public Health. 2005;95(2):224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salas-Wright CP, Nelson EJ, Vaughn MG, Reingle Gonzalez JM, Córdova D. Trends in Fighting and Violence Among Adolescents in the United States, 2002–2014. Am J Public Health. 2017;107(6):977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bogar S, Beyer KM. Green Space, Violence, and Crime: A Systematic Review. Trauma Violence Abuse. 2016;17(2):160–71. [DOI] [PubMed] [Google Scholar]
- 72.Han B, Cohen DA, Derose KP, Li J, Williamson S. Violent Crime and Park Use in Low-Income Urban Neighborhoods. Am J Prev Med. 2018;54(3):352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rhone A, Ver Ploeg M, Williams R, Breneman V. Understanding Low-Income and Low-Access Census Tracts Across the Nation Subnational and Subpopulation Estimates of Access to Healthy Food. United States Department of Agriculture, Economic Research Service, 2019. [Google Scholar]
- 74.Kolak M, Bhatt J, Park YH, Padron NA, Molefe A. Quantification of Neighborhood-Level Social Determinants of Health in the Continental United States. JAMA Netw Open. 2020;3(1):e1919928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gustafsson PE, San Sebastian M, Janlert U, Theorell T, Westerlund H, Hammarstrom A. Life-course accumulation of neighborhood disadvantage and allostatic load: empirical integration of three social determinants of health frameworks. Am J Public Health. 2014;104(5):904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ludwig J, Duncan GJ, Gennetian LA, Katz LF, Kessler RC, Kling JR, et al. Neighborhood effects on the long-term well-being of low-income adults. Science. 2012;337(6101):1505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shmool JL, Kubzansky LD, Newman OD, Spengler J, Shepard P, Clougherty JE. Social stressors and air pollution across New York City communities: a spatial approach for assessing correlations among multiple exposures. Environ Health. 2014;13:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beck AF, Huang B, Ryan PH, Sandel MT, Chen C, Kahn RS. Areas with High Rates of Police-Reported Violent Crime Have Higher Rates of Childhood Asthma Morbidity. J Pediatr. 2016;173:175–82 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koinis-Mitchell D, Kopel SJ, Salcedo L, McCue C, McQuaid EL. Asthma indicators and neighborhood and family stressors related to urban living in children. Am J Health Behav. 2014;38(1):22–30. [DOI] [PubMed] [Google Scholar]
- 80.Vangeepuram N, Galvez MP, Teitelbaum SL, Brenner B, Wolff MS. The association between parental perception of neighborhood safety and asthma diagnosis in ethnic minority urban children. J Urban Health. 2012;89(5):758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramratnam SK, Han YY, Rosas-Salazar C, Forno E, Brehm JM, Rosser F, et al. Exposure to gun violence and asthma among children in Puerto Rico. Respir Med. 2015;109(8):975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosas-Salazar C, Han YY, Brehm JM, Forno E, Acosta-Perez E, Cloutier MM, et al. Gun Violence, African Ancestry, and Asthma: A Case-Control Study in Puerto Rican Children. Chest. 2016;149(6):1436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen E, Chim LS, Strunk RC, Miller GE. The role of the social environment in children and adolescents with asthma. Am J Respir Crit Care Med. 2007;176(7):644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alcock I, White M, Cherrie M, Wheeler B, Taylor J, McInnes R, et al. Land cover and air pollution are associated with asthma hospitalisations: A cross-sectional study. Environ Int. 2017;109:29–41. [DOI] [PubMed] [Google Scholar]
- 85.Tischer C, Gascon M, Fernandez-Somoano A, Tardon A, Lertxundi Materola A, Ibarluzea J, et al. Urban green and grey space in relation to respiratory health in children. Eur Respir J. 2017;49(6). [DOI] [PubMed] [Google Scholar]
- 86.Alexander D, Currie J. Is it who you are or where you live? Residential segregation and racial gaps in childhood asthma. J Health Econ. 2017;55:186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woo H, Brigham EP, Allbright K, Ejike C, Galiatsatos P, Jones MR, et al. Racial Segregation and Respiratory Outcomes among Urban Black Residents with and at Risk of COPD. Am J Respir Crit Care Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.*.Szczesniak R, Rice JL, Brokamp C, Ryan P, Pestian T, Ni Y, et al. Influences of environmental exposures on individuals living with cystic fibrosis. Expert Rev Respir Med. 2020;14(7):737–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. Chest. 2019;155(2):409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. Chest. 2019;155(2):417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smoking Cessation: A Report of the Surgeon General. Publications and Reports of the Surgeon General. Washington, DC. 2020. [Google Scholar]
- 92.Ong T, Schechter M, Yang J, Peng L, Emerson J, Gibson RL, et al. Socioeconomic Status, Smoke Exposure, and Health Outcomes in Young Children With Cystic Fibrosis. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raju SV, Rasmussen L, Sloane PA, Tang LP, Libby EF, Rowe SM. Roflumilast reverses CFTR-mediated ion transport dysfunction in cigarette smoke-exposed mice. Respir Res. 2017;18(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rab A, Rowe SM, Raju SV, Bebok Z, Matalon S, Collawn JF. Cigarette smoke and CFTR: implications in the pathogenesis of COPD. American journal of physiology Lung cellular and molecular physiology. 2013;305(8):L530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rasmussen JE, Sheridan JT, Polk W, Davies CM, Tarran R. Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. The Journal of biological chemistry. 2014;289(11):7671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188(11):1321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One. 2012;7(6):e39809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173(10):1139–44. [DOI] [PubMed] [Google Scholar]
- 99.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L894–902. [DOI] [PubMed] [Google Scholar]
- 100.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(2):533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Savitski AN, Mesaros C, Blair IA, Cohen NA, Kreindler JL. Secondhand smoke inhibits both Cl- and K+ conductances in normal human bronchial epithelial cells. Respir Res. 2009;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin VY, Fain MD, Jackson PL, Berryhill TF, Wilson LS, Mazur M, et al. Vaporized E-Cigarette Liquids Induce Ion Transport Dysfunction in Airway Epithelia. Am J Respir Cell Mol Biol. 2019;61(2):162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, et al. Acquired CFTR Dysfunction in the Lower Airways in COPD. Chest. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ni I, Ji C, Vij N. Second-hand cigarette smoke impairs bacterial phagocytosis in macrophages by modulating CFTR dependent lipid-rafts. PLoS One. 2015;10(3):e0121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kopp BT, Thompson R, Kim J, Konstan R, Diaz A, Smith B, et al. Secondhand smoke alters arachidonic acid metabolism and inflammation in infants and children with cystic fibrosis. Thorax. 2019;74(3):237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kopp BT, Sarzynski L, Khalfoun S, Hayes D Jr., Thompson R, Nicholson L, et al. Detrimental effects of secondhand smoke exposure on infants with cystic fibrosis. Pediatr Pulmonol. 2015;50(1):25–34. [DOI] [PubMed] [Google Scholar]
- 107.Schechter MS. Nongenetic influences on cystic fibrosis outcomes. Curr Opin Pulm Med. 2011;17(6):448–54. [DOI] [PubMed] [Google Scholar]
- 108.Rubin BK. Exposure of children with cystic fibrosis to environmental tobacco smoke. N Engl J Med. 1990;323(12):782–8. [DOI] [PubMed] [Google Scholar]
- 109.Schechter MS. Speaking of pandemics. J Cyst Fibros. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.**.Baker E, Harris WT, Rowe SM, Rutland SB, Oates GR. Tobacco smoke exposure limits the therapeutic benefit of tezacaftor/ivacaftor in pediatric patients with cystic fibrosis. J Cyst Fibros. 2021. Jul;20(4):612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]; **On the potential detrimental effect of tobacco exposure on response to CFTR modulators
- 111.**.Oates GR, Baker E, Collaco JM, Rowe SM, Rutland SB, Fowler CM, et al. Cessation of smoke exposure improves pediatric CF outcomes: Longitudinal analysis of CF Foundation Patient Registry data. J Cyst Fibros. 2021. Jul;20(4):618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]; **Showing that eliminating second hand tobacco exposure improves outcomes in effected children
- 112.Wheeler BW, Ben-Shlomo Y. Environmental equity, air quality, socioeconomic status, and respiratory health: a linkage analysis of routine data from the Health Survey for England. J Epidemiol Community Health. 2005;59(11):948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hajat A, Hsia C, O’Neill MS. Socioeconomic Disparities and Air Pollution Exposure: a Global Review. Curr Environ Health Rep. 2015;2(4):440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ard K Trends in exposure to industrial air toxins for different racial and socioeconomic groups: A spatial and temporal examination of environmental inequality in the U.S. from 1995 to 2004. Soc Sci Res. 2015;53:375–90. [DOI] [PubMed] [Google Scholar]
- 115.Deguen S, Petit C, Delbarre A, Kihal W, Padilla C, Benmarhnia T, et al. Neighbourhood Characteristics and Long-Term Air Pollution Levels Modify the Association between the Short-Term Nitrogen Dioxide Concentrations and All-Cause Mortality in Paris. PLoS One. 2015;10(7):e0131463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–67. [DOI] [PubMed] [Google Scholar]
- 117.Pope CA, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, et al. Particulate Air-Pollution as a Predictor of Mortality in a Prospective-Study of Us Adults. Am J Respir Crit Care Med. 1995;151(3):669–74. [DOI] [PubMed] [Google Scholar]
- 118.Hooper LG, Kaufman JD. Ambient Air Pollution and Clinical Implications for Susceptible Populations. Ann Am Thorac Soc. 2018;15(Suppl 2):S64–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Farhat SCL, Almeida MB, Silva-Filho LVRF, Farhat J, Rodrigues JC, Braga ALF. Ozone is associated with an increased risk of respiratory exacerbations in patients with cystic fibrosis. Chest. 2013. Oct;144(4):1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.*.Goeminne PC, Kicinski M, Vermeulen F, Fierens F, De Boeck K, Nemery B, et al. Impact of air pollution on cystic fibrosis pulmonary exacerbations: a case-crossover analysis. Chest. 2013;143(4):946–54. [DOI] [PubMed] [Google Scholar]; *Exposures to ozone and particulates leads to increased CF pulmonary exacerbations.
- 121.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–21. [DOI] [PubMed] [Google Scholar]
- 122.Psoter KJ, De Roos AJ, Mayer JD, Kaufman JD, Wakefield J, Rosenfeld M. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann Am Thorac Soc. 2015;12(3):385–91. [DOI] [PubMed] [Google Scholar]
- 123.Kosorok MR, Jalaluddin M, Farrell PM, Shen G, Colby CE, Laxova A, et al. Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr Pulmonol. 1998;26(2):81–8. [DOI] [PubMed] [Google Scholar]
- 124.Jennings MT, Dasenbrook EC, Lechtzin N, Boyle MP, Merlo CA. Risk factors for persistent methicillin-resistant Staphylococcus aureus infection in cystic fibrosis. J Cyst Fibros. 2017;16(6):681–6. [DOI] [PubMed] [Google Scholar]
- 125.Oates GR, Harris WT, Rowe SM, Solomon GM, Dey S, Zhu A, et al. Area Deprivation as a Risk Factor for Methicillin-resistant Staphylococcus aureus Infection in Pediatric Cystic Fibrosis. Pediatr Infect Dis J. 2019;38(11):e285–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros. 2009;8(2):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eakin MN, Bilderback A, Boyle MP, Mogayzel PJ, Riekert KA. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cystic Fibrosis. 2011;10(4):258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Briesacher BA, Quittner AL, Saiman L, Sacco P, Fouayzi H, Quittell LM. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med. 2011;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1810–7. [DOI] [PubMed] [Google Scholar]
- 130.Rapoff MA, Belmont JM, Lindsley CB, Olson NY. Electronically monitored adherence to medications by newly diagnosed patients with juvenile rheumatoid arthritis. Arthritis & Rheumatism-Arthritis Care & Research. 2005;53(6):905–10. [DOI] [PubMed] [Google Scholar]
- 131.Oates GR, Juarez LD, Hansen B, Kiefe CI, Shikany JM. Social Risk Factors for Medication Nonadherence: Findings from the CARDIA Study. Am J Health Behav. 2020;44(2):232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goldman DP, Smith JP. Can patient self-management help explain the SES health gradient? Proc Natl Acad Sci U S A. 2002;99(16):10929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ievers CE, Brown RT, Drotar D, Caplan D, Pishevar BS, Lambert RG. Knowledge of physician prescriptions and adherence to treatment among children with cystic fibrosis and their mothers. J Dev Behav Pediatr. 1999;20(5):335–43. [DOI] [PubMed] [Google Scholar]
- 134.Lask B Non-adherence to treatment in cystic fibrosis. J R Soc Med. 1994;87:25–7. [PMC free article] [PubMed] [Google Scholar]
- 135.Anthony H, Paxton S, Bines J, Phelan P. Psychosocial predictors of adherence to nutritional recommendations and growth outcomes in children with cystic fibrosis. J Psychosom Res. 1999;47(6):623–34. [DOI] [PubMed] [Google Scholar]
- 136.Quittner AL, Drotar D, Ievers-Landis C. Adherence to medical treatments in adolescents with cystic fibrosis: the development and evaluation of family based interventions. In: Drotar D, ed. Promoting adherence to medical treatment in childhood chronic illness: concepts, methods, and interventions. Hillsdale, New Jersey: Erlbaum Associates, Inc; 2000. [Google Scholar]
- 137.Patterson JM, Budd J, Goetz D, Warwick WJ. Family correlates of a 10-year pulmonary health trend in cystic fibrosis. Pediatrics. 1993;91(2):383–9. [PubMed] [Google Scholar]
- 138.Patterson JM, McCubbin HI, Warwick WJ. The impact of family functioning on health changes in children with cystic fibrosis. Soc Sci Med. 1990;31(2):159–64. [DOI] [PubMed] [Google Scholar]
- 139.Eddy ME, Carter BD, Kronenberger WG, Conradsen S, Eid NS, Bourland SL, et al. Parent relationships and compliance in cystic fibrosis. J Pediatr Health Care. 1998;12(4):196–202. [PubMed] [Google Scholar]
- 140.Pendleton DA, David TJ. The compliance conundrum in cystic fibrosis. J R Soc Med. 2000;93 Suppl 38:9–13. [PMC free article] [PubMed] [Google Scholar]
- 141.Schechter MS. Non-genetic influences on cystic fibrosis lung disease: the role of sociodemographic characteristics, environmental exposures, and healthcare interventions. Semin Respir Crit Care Med. 2003;24(6):639–52. [DOI] [PubMed] [Google Scholar]
- 142.Macpherson C, Redmond AO, Leavy A, McMullan M. A review of cystic fibrosis children born to single mothers. Acta Paediatr. 1998;87(4):397–400. [DOI] [PubMed] [Google Scholar]
- 143.Oates GR, Stepanikova I, Gamble S, Gutierrez HH, Harris WT. Adherence to airway clearance therapy in pediatric cystic fibrosis: Socioeconomic factors and respiratory outcomes. Pediatr Pulmonol. 2015;50(12):1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hodgkinson R, Lester H. Stresses and coping strategies of mothers living with a child with cystic fibrosis: implications for nursing professionals. J Adv Nurs. 2002;39(4):377–83. [DOI] [PubMed] [Google Scholar]
- 145.Quittner AL, Opipari LC, Regoli MJ, Jacobsen J, Eigen H. The impact of caregiving and role strain on family life: comparisons between mothers of children with cystic fibrosis and matched controls. Rehabil Psychol. 1992;37:275–90. [Google Scholar]
- 146.Pearlin L The Sociological Study of Stress. Journal of Health and Social Behavior 1989;30(3):241–56. [PubMed] [Google Scholar]
- 147.Turner RJ, Wheaton B, Lloyd DA. The Epidemiology of Social Stress. Am Sociol Rev. 1995;60(1):104–25. [Google Scholar]
- 148.Kubzansky LD, Kawachi I, Sparrow D. Socioeconomic status, hostility, and risk factor clustering in the Normative Aging Study: any help from the concept of allostatic load? Ann Behav Med. 1999;21(4):330–8. [DOI] [PubMed] [Google Scholar]
- 149.Pearlin L, Schieman S, Fazio EM, Meersman SC. Stress, Health, and the Life Course: Some Conceptual Perspectives. J Health Soc Behav. 2005;46(2):205–19 [DOI] [PubMed] [Google Scholar]
- 150.Berkman LF, Kawachi I. Social epidemiology. New York: Oxford University Press; 2000. [Google Scholar]
- 151.Thoits PA. Stress and health: major findings and policy implications. J Health Soc Behav. 2010;51 Suppl:S41–53. [DOI] [PubMed] [Google Scholar]
- 152.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. [DOI] [PubMed] [Google Scholar]
- 153.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98(8):4770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann NY Acad Sci. 2010;1186:174–89. [DOI] [PubMed] [Google Scholar]
- 155.Harper S, Lynch J, Hsu WL, Everson SA, Hillemeier MM, Raghunathan TE, et al. Life course socioeconomic conditions and adult psychosocial functioning. Int J Epidemiol. 2002;31(2):395–403. [PubMed] [Google Scholar]
- 156.Everhart RS, Fiese BH, Smyth JM, Borschuk A, Anbar RD. Family Functioning and Treatment Adherence in Children and Adolescents with Cystic Fibrosis. Pediatr Allergy Immunol Pulmonol. 2014;27(2):82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cruz I, Marciel KK, Quittner AL, Schechter MS. Anxiety and depression in cystic fibrosis. Semin Respir Crit Care Med. 2009;30(5):569–78. [DOI] [PubMed] [Google Scholar]
- 158.Bittman M, Hill T, Thomson C. The impact of caring on informal carers’ employment, income and earnings: a longitudinal approach. Australian Journal of Social Issues. 2007;42(2):255–72. [Google Scholar]
- 159.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129 946–72. [DOI] [PubMed] [Google Scholar]
- 160.Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18:250–67. [DOI] [PubMed] [Google Scholar]
- 161.Neri L, Lucidi V, Catastini P, Colombo C, Group LS. Caregiver burden and vocational participation among parents of adolescents with CF. Pediatr Pulmonol. 2016;51(3):243–52. [DOI] [PubMed] [Google Scholar]
- 162.Schechter MS, Cruz I, Blackwell LS, Quittner AL. Risk Factors for Anxiety and Depression in Cystic Fibrosis. Pediatr Pulmonol. 2010:109–10. [Google Scholar]
- 163.Besier T, Goldbeck L. Anxiety and depression in adolescents with CF and their caregivers. Journal of Cystic Fibrosis. 2011;10(6):435–42. [DOI] [PubMed] [Google Scholar]
- 164.Yohannes AM, Willgoss TG, Fatoye FA, Dip MD, Webb K. Relationship between anxiety, depression, and quality of life in adult patients with cystic fibrosis. Respir Care. 2012;57(4):550–6. [DOI] [PubMed] [Google Scholar]
- 165.Schechter MS, Ostrenga JS, Fink AK, Barker DH, Sawicki GS, Quittner AL. Decreased survival in cystic fibrosis patients with a positive screen for depression. J Cyst Fibros. 2021;20(1):120–6. [DOI] [PubMed] [Google Scholar]
- 166.Foster C, Eiser C, Oades P, Sheldon C, Tripp J, Goldman P, et al. Treatment demands and differential treatment of patients with cystic fibrosis and their siblings: patient, parent and sibling accounts. Child Care Health Dev. 2001;27(4):349–64. [DOI] [PubMed] [Google Scholar]
- 167.Barker DH, Driscoll KA, Modi AC, Light MJ, Quittner AL. Supporting cystic fibrosis disease management during adolescence: the role of family and friends. Child Care Health Dev. 2012;38(4):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Flewelling KD, Sellers DE, Sawicki GS, Robinson WM, Dill EJ. Social support is associated with fewer reported symptoms and decreased treatment burden in adults with cystic fibrosis. J Cyst Fibros. 2019;18(4):572–6. [DOI] [PubMed] [Google Scholar]
- 169.Crowley EM, Bosslet GT, Khan B, Ciccarelli M, Brown CD. Social complexity negatively influences lung function in cystic fibrosis after transfer to adult care. Pediatr Pulmonol. 2020;55(1):24–6. [DOI] [PubMed] [Google Scholar]
- 170.Crowley EM, Bosslet GT, Khan B, Ciccarelli M, Brown CD. Impact of social complexity on outcomes in cystic fibrosis after transfer to adult care. Pediatr Pulmonol. 2018;53(6):735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.*.Schechter MS, McColley SA, Silva S, Haselkorn T, Konstan MW, Wagener JS, et al. Association of socioeconomic status with the use of chronic therapies and healthcare utilization in children with cystic fibrosis. J Pediatr. 2009;155(5):634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Socioeconomic disparities in CF do not appear to be associated with differences in access or prescribing patterns
- 172.*.Schechter MS, McColley SA, Regelmann W, Millar SJ, Pasta DJ, Wagener JS, et al. Socioeconomic status and the likelihood of antibiotic treatment for signs and symptoms of pulmonary exacerbation in children with cystic fibrosis. J Pediatr. 2011;159(5):819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li SS, Hayes D Jr., Tobias JD, Morgan WJ, Tumin D. Health insurance and use of recommended routine care in adults with cystic fibrosis. Clin Respir J. 2018;12(5):1981–8. [DOI] [PubMed] [Google Scholar]
- 174.Stephenson A, Hux J, Tullis E, Austin PC, Corey M, Ray J. Socioeconomic status and risk of hospitalization among individuals with cystic fibrosis in Ontario, Canada. Pediatr Pulmonol. 2011;46(4):376–84. [DOI] [PubMed] [Google Scholar]
- 175.Ramos KJ, Quon BS, Psoter KJ, Lease ED, Mayer-Hamblett N, Aitken ML, et al. Predictors of non-referral of patients with cystic fibrosis for lung transplant evaluation in the United States. J Cyst Fibros. 2016;15(2):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Quon BS, Psoter K, Mayer-Hamblett N, Aitken ML, Li CI, Goss CH. Disparities in access to lung transplantation for patients with cystic fibrosis by socioeconomic status. Am J Respir Crit Care Med. 2012;186(10):1008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.**.Lehr CJ, Fink AK, Skeans M, Faro A, Fernandez G, Dasenbrook E, et al. Impact of Socioeconomic Position on Access to the U.S. Lung Transplant Waiting List in a Matched Cystic Fibrosis Cohort. Ann Am Thorac Soc. 2020;17(11):1384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailing socioeconomic barriers to lung transplantion in CF
- 178.Krivchenia K, Tumin D, Tobias JD, Hayes D Jr. Increased Mortality in Adult Cystic Fibrosis Patients with Medicaid Insurance Awaiting Lung Transplantation. Lung. 2016;194(5):799–806. [DOI] [PubMed] [Google Scholar]
- 179.Merlo CA, Clark SC, Arnaoutakis GJ, Yonan N, Thomas D, Simon A, et al. National Healthcare Delivery Systems Influence Lung Transplant Outcomes for Cystic Fibrosis. Am J Transplant. 2015;15(7):1948–57. [DOI] [PubMed] [Google Scholar]
- 180.Hyde R, Dobrovolny D. Orphan drug pricing and payer management in the United States: are we approaching the tipping point? Am Health Drug Benefits. 2010;3(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 181.Kanavos P, Nicod E. What is wrong with orphan drug policies? Suggestions for ways forward. Value Health. 2012;15(8):1182–4. [DOI] [PubMed] [Google Scholar]
- 182.Goss CH, Rubenfeld GD, Ramsey BW, Aitken ML. Clinical trial participants compared with nonparticipants in cystic fibrosis. Am J Respir Crit Care Med. 2006;173(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McGarry ME, McColley SA. Minorities Are Underrepresented in Clinical Trials of Pharmaceutical Agents for Cystic Fibrosis. Ann Am Thorac Soc. 2016;13(10):1721–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Final Report of the Commission on Social Determinants of Health. Geneva, Switzerland: World Health Organization, 2008. [Google Scholar]
- 185.Taylor-Robinson D, Schechter MS. Health inequalities and cystic fibrosis. BMJ. 2011;343:d4818. [DOI] [PubMed] [Google Scholar]
- 186.What Is Compass? Cystic Fibrosis Foundation. Available from: https://www.cff.org/Assistance-Services/About-Compass/What-Is-Compass.
- 187.Baer TE, Gottlieb L, Sandel M. Addressing social determinants of health in the adolescent medical home. Curr Opin Pediatr. 2013;25(4):447–53. [DOI] [PubMed] [Google Scholar]
- 188.Gottlieb L, Sandel M, Adler NE. Collecting and applying data on social determinants of health in health care settings. JAMA Intern Med. 2013;173(11):1017–20. [DOI] [PubMed] [Google Scholar]
- 189.Schickedanz A, Hamity C, Rogers A, Sharp AL, Jackson A. Clinician Experiences and Attitudes Regarding Screening for Social Determinants of Health in a Large Integrated Health System. Med Care. 2019;57 Suppl 6 Suppl 2:S197–S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Byhoff E, Garg A, Pellicer M, Diaz Y, Yoon GH, Charns MP, et al. Provider and Staff Feedback on Screening for Social and Behavioral Determinants of Health for Pediatric Patients. J Am Board Fam Med. 2019;32(3):297–306. [DOI] [PubMed] [Google Scholar]
- 191.Colvin JD, Bettenhausen JL, Anderson-Carpenter KD, Collie-Akers V, Plencner L, Krager M, et al. Multiple Behavior Change Intervention to Improve Detection of Unmet Social Needs and Resulting Resource Referrals. Acad Pediatr. 2016;16(2):168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Herrera CN, Brochier A, Pellicer M, Garg A, Drainoni ML. Implementing Social Determinants of Health Screening at Community Health Centers: Clinician and Staff Perspectives. J Prim Care Community Health. 2019;10:2150132719887260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Garg A, Boynton-Jarrett R, Dworkin PH. Avoiding the Unintended Consequences of Screening for Social Determinants of Health. JAMA. 2016;316(8):813–4. [DOI] [PubMed] [Google Scholar]
- 194.Kreuter M, Garg R, Thompson T, McQueen A, Javed I, Golla B, et al. Assessing The Capacity Of Local Social Services Agencies To Respond To Referrals From Health Care Providers. Health Aff (Millwood). 2020;39(4):679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fiore M, United States. Tobacco Use and Dependence Guideline Panel. Treating tobacco use and dependence: 2008 update. Rockville, Md.: U.S. Dept. of Health and Human Services, 2008. [Google Scholar]
- 196.Prochaska JJ, Benowitz NL. The Past, Present, and Future of Nicotine Addiction Therapy. Annu Rev Med. 2016;67:467–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Oates GR, Harris WT, Gutierrez HH, Mims C, Rutland SB, Ott C, et al. Tobacco smoke exposure in pediatric cystic fibrosis: A qualitative study of clinician and caregiver perspectives on smoking cessation. Pediatr Pulmonol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Frankowski BL, Weaver SO, Secker-Walker RH. Advising parents to stop smoking: pediatricians’ and parents’ attitudes. Pediatrics. 1993;91(2):296–300. [PubMed] [Google Scholar]
- 199.Schechter MS, McColley SA, Silva S, Haselkorn T, Konstan MW, Wagener JS, et al. Association of Socioeconomic Status with the Use of Chronic Therapies and Healthcare Utilization in Children with Cystic Fibrosis. J Pediatr. 2009;155(5):634–U67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.*.Sawicki GS, Fink AK, Schechter MS, Loeffler DR, Mayer-Hamblett N. Rate and predictors of prescription of lumacaftor-ivacaftor in the 18 months following approval in the United States. J Cyst Fibros. 2018;17(6):742–6. [DOI] [PubMed] [Google Scholar]; Showing that uptake of the newly approved CFTR modifiers is slower in patients on Medicaid
- 201.Sawicki GS, Dasenbrook E, Fink AK, Schechter MS. Rate of Uptake of Ivacaftor Use after U.S. Food and Drug Administration Approval among Patients Enrolled in the U.S. Cystic Fibrosis Foundation Patient Registry. Ann Am Thorac Soc. 2015;12(8):1146–52. [DOI] [PubMed] [Google Scholar]
- 202.Yin HS, Dreyer BP, Vivar KL, MacFarland S, van Schaick L, Mendelsohn AL. Perceived barriers to care and attitudes towards shared decision-making among low socioeconomic status parents: role of health literacy. Acad Pediatr. 2012;12(2):117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schechter M, Hendrickson K. Impact of Improved Reliability and Outcomes of CF Care on Socioeconomic Disparities. Pediatr Pulmonol 2019;54:835. [Google Scholar]
- 204.DiGirolamo M, Kang L, Schechter M. QI-Induced Improvements May Lead to a Reduction of Socioeconomic Disparities in CF Health Outcomes. Pediatr Pulmonol. 2020;55:331. [Google Scholar]
- 205.Quittner AL, Abbott J, Georgiopoulos AM, Goldbeck L, Smith B, Hempstead SE, et al. International Committee on Mental Health in Cystic Fibrosis: Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus statements for screening and treating depression and anxiety. Thorax. 2016;71(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Quittner AL, Abbott J, Hussain S, Ong T, Uluer A, Hempstead S, et al. Integration of mental health screening and treatment into cystic fibrosis clinics: Evaluation of initial implementation in 84 programs across the United States. Pediatr Pulmonol. 2020;55(11):2995–3004. [DOI] [PubMed] [Google Scholar]


