Abstract
We present a case series of three febrile episodes in neutropenic pediatric cancer patients who wore an FDA-approved high frequency temperature monitoring (HFTM) wearable device (WD) at home. The WD detected fever events when temperature monitoring by thermometer did not detect fever, or was not feasible to perform. Two of the episodes were associated with bloodstream infections and the WD detected fevers 5 and 12 hours prior to fevers detected by thermometer, triggering earlier medical evaluation and more prompt administration of antibiotics. These observations provide a basis for future investigation of home-based HFTM to improve infection-related outcomes in pediatric oncology.
Keywords: wearable device, febrile neutropenia, bacteremia, supportive care, digital biomarkers, home care
Introduction
Pediatric cancer patients are at risk of developing febrile neutropenia (FN), a known complication of myelosuppressive chemotherapy. Febrile neutropenia is historically associated with a high mortality rate due to a high incidence of bacteremia.1,2 Due to advancements in supportive care, the FN mortality rate has significantly improved despite the current rate of bloodstream infections (BSI) ranging from 11–24%.3–5 In pediatric sepsis, outcomes are improved with the prompt administration of antibiotics, leading to significant efforts to decrease the time-to-antibiotics.6 Specific to pediatric FN, fewer intensive care admissions occur when antibiotics are administered in <60 minutes of presentation to clinical facilities.7 Clinical guidelines currently recommend administration of antibiotics within 60 minutes of initial presentation for FN.8
Accurate vital sign monitoring for acutely ill patients is imperative for effective clinical assessment.9,10 More recently, wearable devices (WD) that enable high frequency temperature monitoring (HFTM) show promise as a feasible and easy-to-use monitoring technology for pediatric patients.11 A pilot study of pediatric cancer and hematopoietic cell transplant (HCT) patients showed the feasibility of using a Food and Drug Administration cleared class II approved, axillary “stick-on” patch WD known as TempTraqⓇ (BlueSpark Technologies, Inc.) for HFTM in the inpatient setting.12 In a subsequent inpatient study of adult patients receiving HCT or chimeric antigen receptor therapies, HFTM using the same WD detected fever several hours prior to detection by nursing staff using a thermometer, especially for fever episodes where a true infection was also found.13The early findings in the inpatient setting stimulate interest in the use of HFTM in the outpatient setting for pediatric oncology patients at high risk of infection. A recent study of a different type of wearable device that can also measure body temperature was conducted in both outpatient and inpatient pediatric oncology settings; however the focus was on feasibility and the authors did not present results on HFTM for fever detection14.
It is important to note that in the inpatient setting, it is convenient to obtain and transmit HFTM data to the cloud in a way that does not require involvement of the patient or caregiver, using electronic equipment known as a “gateway” that is placed in the patient’s room and transmits HFTM data through the hospital’s Wi-Fi network. As a result, patients also do not receive access to their own data. In the outpatient setting, in contrast, HFTM using the TempTraqⓇ WD is more practical to implement through use of a mobile app on the patient or caregivers’ personal smartphone. This requires active participation of the patient or caregiver to ensure the WD connection to the smartphone application, as well as to keep the smartphone app active on a daily basis to allow data transmission to a cloud-based server. Importantly, this implementation in the outpatient setting also provides the patient or caregiver access to the HFTM data in real-time through the smartphone app. The case series presented here describes the use of WD-based HFTM at home in pediatric oncology patients and examples of its impact on the early detection of fevers and administration of antibiotics in outpatient pediatric FN.
Methods
Data and setting
The cases are part of a larger observational study of HFTM using the TempTraq WD in pediatric oncology patients. Clinical information was manually abstracted from the electronic medical record (MiChart, EPIC) and included demographics, primary diagnosis and treatment, onset of fever, blood culture results, culture time-to-positivity, and length-of-fever.15
Definitions
Febrile neutropenia treatment at our institution follows society guidelines with administration of empiric anti-pseudomonal antibiotics at presentation.8,9 Patients and caregivers were recommended to obtain an oral or axillary temperature by thermometer based upon clinical symptoms or concern for infection per standard practice. Febrile neutropenia was defined as an absolute neutrophil count (ANC) less than 0.5 K/μL, or 1 K/μL with anticipation that it would decrease; and a fever of 38°C for greater than 1-hour or a single fever greater than 38.3°C.9 Culture time-to-positivity was manually calculated based on documented times of culture collection and abnormal report read-out in the medical record.
High frequency temperature monitoring wearable device use protocol and procedures
Patients, or caregivers as appropriate, were given instructions on correct application of the TempTraq® WD (BlueSpark Technologies, Inc.), which is a clinical-grade, non-invasive, disposable, bluetooth-enabled flexible patch that is applied to the skin using a silicone gel-based adhesive. Patients or caregivers were instructed to apply the patch after administration of granulocyte colony stimulating factor (G-CSF), or at any time the patient was known to be neutropenic or was ill-appearing. TempTraq® WD data is automatically uploaded via Bluetooth connection through a smartphone application and stored on a HIPAA secured web-based platform.
This study received Institutional Review Board (IRB) approval and was carried out under IRB supervision with informed consent or assent obtained as appropriate. All protected health information was stored on IRB-approved hardware and storage devices. Per protocol, this was an observational study that was not designed to use HFTM WD data for clinical decision-making. However, it ultimately influenced care indirectly, driven by the response of the family caregivers to the data.
Results
Case #1 (Figure 1, Panel A)
FIGURE 1. HFTM monitoring data for the three cases described in the main text.
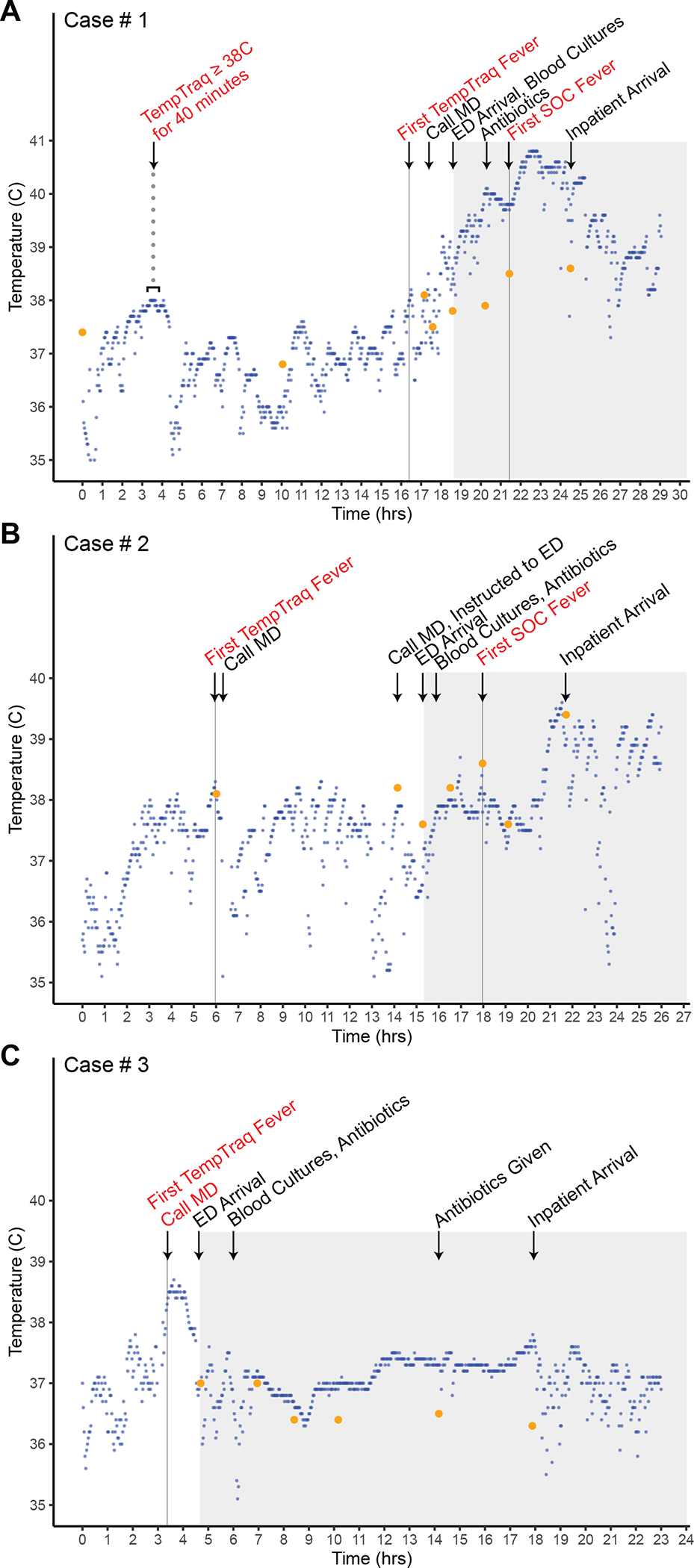
HFTM data points are plotted in blue, with temperature data points measured by family caregivers or nursing staff using a standard thermometer plotted in orange, for each of the cases described in the main text. The gray shaded area corresponds to time points when the patient was at the healthcare facility. MD = physician; ED = emergency department. SOC = standard of care, which refers to temperature measured using a standard thermometer by clinical staff.
A 17-year-old male with Burkitt Leukemia in remission presented 7 days after completing cycle 1 consolidation chemotherapy with rituximab, cytarabine, and etoposide with G-CSF support. Per electronic health records, his caregiver noted the night prior to admission he was feeling ill with persistent “low grade fevers” by thermometer which did not reach the fever definition (see Methods); during this period, the HFTM WD recorded persistent temperature readings ≥38°C for 40 minutes. The following morning, he presented to the outpatient infusion center to receive blood products, during which he was afebrile based on nursing assessments by thermometer. Upon returning home, the family caregiver contacted the physician because fever was detected by the HFTM WD, although oral temperature measurements by thermometer taken by the caregiver did not meet the fever definition. Due to the persistent caregiver concerns about the HFTM WD data, the family caregiver was instructed to bring the patient to the medical center for evaluation.
Upon arrival at the medical center, he received empiric anti-pseudomonal antibiotics and 2 liters of normal saline for clinical dehydration; the first fever documented by nursing staff using a thermometer was 1.5 hours after antibiotic administration. The first HFTM-detected fever (as per fever definition in Methods) was recorded 5 hours prior to the first thermometer-detected fever of 38.5°C by nursing staff. His central line blood cultures grew pan-sensitive Rothia mucilaginosa in 19 hours. He completed a treatment course of systemic antibiotics and was discharged home without complications.
Case #2 (Figure 1, Panel B)
The same adolescent male with Burkitt Leukemia presented with a second FN episode 8 days after completing cycle 2 of consolidation therapy with rituximab, cytarabine, and etoposide with G-CSF support. The caregiver noticed nasal congestion associated with intermittent fevers >38°C detected by the HFTM WD, which prompted a call to the physician. However, due to absence of fever based on family caregiver temperature measurement by thermometer, the family caregiver was instructed to continue outpatient observation. The next morning the caregiver called the physician again and due to persistent caregiver concerns about HFTM WD data and symptoms, was instructed to bring the patient to the medical center for evaluation. Of note, throughout this time, there were no fevers detected by thermometer taken by the caregiver at home.
At initial presentation to the medical center, he appeared mildly dehydrated with tachycardia receiving a normal saline bolus; empiric anti-pseudomonal antibiotics were given 2 hours prior to the first documented oral fever obtained by nursing staff. The first HFTM-detected fever was recorded 12 hours prior to the first thermometer-detected fever of 38.6°C obtained by nursing staff. His blood culture grew methicillin-sensitive Staphylococcus aureus in 20 hours, requiring removal of his central venous line and a therapeutic course of antibiotics. He was also diagnosed with recurrent Clostridium difficile colitis and treated with appropriate antibiotics without sequelae. He fully recovered from both infections and was discharged to home uneventfully.
Case #3 (Figure 1, Panel C)
A 1-year-old female with standard risk pre-B cell acute lymphoblastic leukemia in remission with pancytopenia was near the end of consolidation treatment when her family noted a new “headache” associated with fatigue. They were not able to obtain a temperature at home using a thermometer due to patient fussiness, but were concerned due to HFTM detection of fevers. On exam she was fussy, tachycardic, and mildly dehydrated without any focal findings, and received a normal saline bolus with empiric antibiotics. She had sustained febrile temperatures ≥38°C detected by the HFTM WD for over an hour, with a TMax of 38.6°C. She did not have a documented thermometer-detected fever at home or by nursing staff. A clinical or infectious cause for the fever was not identified.
Discussion
Pediatric cancer patients with FN remain at high risk for bacteremia and poor outcomes from serious infections. This case series highlights the potential impact early detection of fevers on patients at home could have on medical management and potentially on outcomes. In all 3 cases, the HFTM WD data raised caregiver concern and prompted them to seek medical attention earlier. This was the case even when oral temperature measured at home did not detect fever, or when oral temperature was not feasible to measure at home, as in the third case with an infant.
Per protocol, our study was observational and not designed to use HFTM WD data for clinical decision-making. However, this case series illustrates the potential impact of providing patients and caregivers access to continuous temperature data in real-time; the actions of the caregiver resulted in more rapid clinical evaluation and treatment for two FN episodes caused by bloodstream infections. The earlier administration of antibiotics is relevant given the association with improved clinical outcomes.6,7,16–18 Had caregivers been instructed to act on the HFTM WD data, the FN episodes secondary to BSI would have received empiric antibiotics even earlier, since HFTM-detected fevers occurred 5 and 12 hours prior to the first thermometer-detected fever in cases #1 and #2, respectively.
The highlighted cases in this report show proof-of-concept of the potential feasibility and value of extending HFTM to the outpatient setting, with patients and caregivers provided access to HFTM data in real-time, illustrated by the early detection and intervention in FN episodes. The importance of these cases is that they may stimulate subsequent larger studies, leading to a future randomized trial of hundreds of outpatients to evaluate the effect on clinical outcomes, of providing patients/caregivers access to real-time HFTM data, versus current standard-of-care monitoring. The clinical outcomes could include frequency of intensive care unit admission, length of hospital stay, frequency of secondary complications from bloodstream infections, among others. In addition, it would be important to capture measures related to patient and caregiver experience, including for example, levels of caregiver anxiety experienced with and without the use of HFTM. Evaluating the potential for healthcare cost-savings by the use of HFTM to reduce intensity of care needed for FN, will also be of value.
In conclusion, the cases described here indicate that HFTM may be a useful monitoring modality in the early detection of clinically significant fevers in outpatient cancer patients with febrile neutropenia. We hope that future, large-scale clinical studies motivated by this report will evaluate the clinical utility of HFTM data and lead to improves outcomes for pediatric oncology outpatients at risk for FN episodes.
Acknowledgements
C.N.N. acknowledges support from a NIH/NCI training grant (T32CA236621; PI: Christopher Friese). C.F. acknowledges funding from NIH Training Grant T32 HL007622. NIH/NHLBI (K24HL156896 – mentoring award), NIH/NHBLI (1R01HL146354), and NIH/NCI grants (R01CA249211) and the Edith S. Briskin and Shirley K. Schlafer Foundation supported the work of S.W.C.. The study was supported in part by funding from the A. Alfred Taubman Medical Research Institute through infrastructure for HFTM studies and data analysis, which was enabled by Taubman Institute funding of Grand Challenge and Innovation projects to S.W.C. and M.T..
Abbreviations Table
- FN
Febrile neutropenia
- ANC
Absolute neutrophil count
- HCT
Hematopoietic cell transplant
- BSI
Blood stream infection
- GCSF
Gram colony stimulating factor
- TMAX
Maximum temperature
- HFTM
high frequency temperature monitor
- WD
Wearable device
- IRB
Institutional review board
Footnotes
Disclosures of Conflicts of Interest
The authors have no relevant conflicts to disclose.
References
- 1.Bodey GP, Rodriguez V, Chang HY, Narboni. Fever and infection in leukemic patients: a study of 494 consecutive patients. Cancer. 1978;41(4):1610–1622. [DOI] [PubMed] [Google Scholar]
- 2.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64(2):328–340. [DOI] [PubMed] [Google Scholar]
- 3.Arif T, Phillips RS. Updated systematic review and meta-analysis of the predictive value of serum biomarkers in the assessment and management of fever during neutropenia in children with cancer. Pediatr Blood Cancer. 2019;66(10):e27887. [DOI] [PubMed] [Google Scholar]
- 4.Mueller EL, Croop J, Carroll AE. Fever and neutropenia hospital discharges in children with cancer: A 2012 update. Pediatric Hematology and Oncology. 2016;33(1):39–48. doi: 10.3109/08880018.2015.1102998 [DOI] [PubMed] [Google Scholar]
- 5.Choi SW, Chang L, Hanauer DA, et al. Rapid reduction of central line infections in hospitalized pediatric oncology patients through simple quality improvement methods. Pediatr Blood Cancer. 2013;60(2):262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salstrom JL, Coughlin RL, Pool K, et al. Pediatric patients who receive antibiotics for fever and neutropenia in less than 60 min have decreased intensive care needs. Pediatr Blood Cancer. 2015;62(5):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green AL, Yi J, Bezler N, et al. A Prospective Cohort Quality Improvement Study to Reduce the Time to Antibiotics for New Fever in Neutropenic Pediatric Oncology Inpatients. Pediatr Blood Cancer. 2016;63(1):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(7):882–913. [DOI] [PubMed] [Google Scholar]
- 10.Lehrnbecher T, Robinson P, Fisher B, et al. Guideline for the Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J Clin Oncol. 2017;35(18):2082–2094. [DOI] [PubMed] [Google Scholar]
- 11.Kakarmath SS, de Redon E, Centi AJ, et al. Assessing the Usability of an Automated Continuous Temperature Monitoring Device (iThermonitor) in Pediatric Patients: Non-Randomized Pilot Study. JMIR Pediatr Parent. 2018;1(2):e10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson M, Hickey V, Huber J, Alonso PB, Davies SM, Dandoy CE. Feasibility of continuous temperature monitoring in pediatric immunocompromised patients: A pilot study. Pediatr Blood Cancer. 2019;66(6):e27723. [DOI] [PubMed] [Google Scholar]
- 13.Flora C, Tyler J, Mayer C, et al. High-frequency temperature monitoring for early detection of febrile adverse events in patients with cancer. Cancer Cell. 2021;39(9):1167–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig C, Ammann RA, Kuehni CE, Roessler J, Brack E. Continuous recording of vital signs with a wearable device in pediatric patients undergoing chemotherapy for cancer—an operational feasibility study. Support Care Cancer, 2021. 29: 5283–5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patient Safety Component Manual: Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line Associated Bloodstream Infection). In: (NHSN) NHSN, ed. Atlanta, GA: Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 16.Mullen CA, Nair J, Sandesh S, Chan KW. Fever and neutropenia in pediatric hematopoietic stem cell transplant patients. Bone Marrow Transplant. 2000;25(1):59–65. [DOI] [PubMed] [Google Scholar]
- 17.Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Hematol Oncol Clin North Am. 2011;25(1):101–116. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. [DOI] [PubMed] [Google Scholar]


