Abstract
Necessary stages of cervical carcinogenesis include acquisition of a carcinogenic human papillomavirus (HPV) type, persistence associated with the development of precancerous lesions, and invasion. Using prospective data from immunocompetent women in the Guanacaste HPV Natural History Study (NHS), the ASCUS-LSIL Triage Study (ALTS), and the Costa Rica HPV Vaccine Trial (CVT), we compared the early natural history of HPV types to inform transition probabilities for health decision models. We excluded women with evidence of high-grade cervical abnormalities at any point during follow-up and restricted the analysis to incident infections in all women and prevalent infections in young women (aged <30 years). We used survival approaches accounting for interval-censoring to estimate the time to clearance distribution for 20,529 HPV infections (64% were incident and 51% were carcinogenic). Time to clearance was similar across HPV types and risk classes (HPV16, HPV18/45, HPV31/33/35/52/58, HPV 39/51/56/59, and non-carcinogenic HPV types); and by age group (18–29, 30–44, and 45–54 years), among carcinogenic and non-carcinogenic infections. Similar time to clearance across HPV types suggests that relative prevalence can predict relative incidence. We confirmed that there was a uniform linear association between incident and prevalent infections for all HPV types within each study cohort. In the absence of progression to precancer, we observed similar time to clearance for incident infections across HPV types and risk classes. A singular clearance function for incident HPV infections has important implications for the refinement of microsimulation models used to evaluate the cost-effectiveness of novel prevention technologies.
Keywords: Early natural history of HPV, HPV clearance, HPV prevalence, HPV incidence
Introduction
Human papillomavirus (HPV), the virus that causes cervical cancer, is one of the most common sexually transmitted infections worldwide, typically occurring soon after sexual initiation. The multistage carcinogenic pathway to cervical cancer includes the following necessary stages: 1) infection with a carcinogenic HPV type; 2) persistent, transforming HPV infection associated with high-grade lesions at a high likelihood of invasion if left untreated; and 3) invasive cervical cancer1. Corollary transitions between these respective stages are acquisition of a carcinogenic HPV type; viral clearance versus persistence; progression to precancer; and invasion2. Results from several longitudinal studies have shown that up to half of the HPV infections clear within 6 months and most infections clear within two years after acquisition3–6. Clearance is thought to be the consequence of cell-mediated immune suppression of a type-specific infection, which is either eliminated or kept in an undetectable latent state that may sporadically reappear later, due to a lapse in immune control or age-related “immune senescence”7. Recent findings suggest that systematic factors such as smoking8, long-term hormone exposure9 and local exposure to anti-inflammatory cytokines10, cervicovaginal microbiota11, 12 and HIV infection9, 13 may affect HPV clearance.
Estimating the transition rates between stages on the pathway to cervical cancer is essential to inform health decision models that simulate the lifetime natural history of HPV and cervical pathogenesis to project the long-term impact of cancer prevention strategies. Increasingly, policy makers rely on such models to identify the most effective and economical approaches to cervical cancer prevention. With novel screening technologies and algorithms on the horizon that may detect cervical precancer at earlier stages and with greater accuracy, there is an urgent need for the development of health decision models with greater fidelity to existing natural history data2 (Figure 1). While the risks of HPV acquisition may vary considerably between populations due to different sexual behavior patterns, the risks of HPV clearance versus progression to precancer are likely to be similar across immunocompetent populations2.
Figure 1.
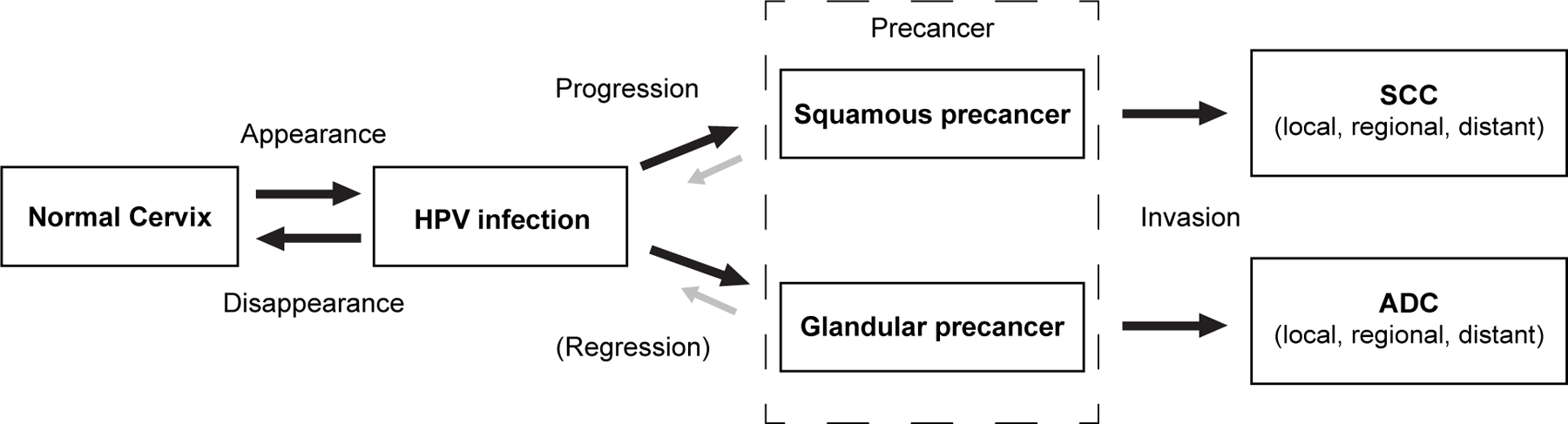
New health decision model schematic of HPV infection and cervical pathogenesis. Each box represents a necessary stage, or health state, on the path to cervical cancer, including acquisition of a carcinogenic HPV infection; progression of a persistent carcinogenic infection to precancer; and invasion to cervical cancer. Each arrow represents the risk of transitioning between stages. Appearance - the risk of transitioning from normal cervix to HPV infection— may include new acquisition of a particular genotype, re-infection with the same genotype, or reactivation of a latent infection. Disappearance— the risk of transitioning from HPV infection to normal cervix— refers to the shift from a would-be detectable infection to non-detectable by a clinical HPV DNA assay, whether attributable to complete viral clearance or viral latency. The model distinguishes progression to squamous precancer (which may transition to squamous cell carcinoma) from progression to glandular precancer (which may transition to adenocarcinoma). The probability of invasion of a precancer is a function of time and the accumulation of genetic changes needed to overcome the coded cellular safeguards against growth inward into the cervical stroma across the epithelial basement membrane. ADC: adenocarcinoma; HPV: human papillomavirus; SCC: squamous cell carcinoma.
Estimates of HPV type-specific time to clearance are influenced by study design and timing of measurement, including left censoring (i.e., for prevalent infections detected at baseline, the time of HPV acquisition is unknown) and interval censoring (i.e., the actual moment of clearance occurs in the interval between two observational visits). Studies that include prevalent infections in older women, which are often persistent infections at higher risk of precancer, may yield time-to-clearance estimates that are biased upward5. Similarly, if women with early precancerous lesions that are too small to be diagnosed are included in an analysis, the population’s average time to clearance may appear longer, particularly for HPV types that are more likely to progress to precancer. The length of the interval between measurements may also influence the apparent time to clearance, with longer intervals leading to seemingly greater clearance rates. Thus, to estimate and compare HPV type-specific clearance rates accurately, the criteria for defining an analytic cohort are critical.
A recent study among a population of well-screened women in the United States demonstrated that the type-specific clearance curves of carcinogenic and non-carcinogenic HPV types were indistinguishable over time, among infections that did not progress to precancer14. This finding challenges prior publications which may not have excluded progression events, concluding that incident infections with carcinogenic HPV types may clear more slowly than infections with non-carcinogenic types4. Specifically, that HPV16 and related alpha-9 types may clear more slowly than other types15.
To examine the early natural history of type-specific HPV infections in immunocompetent women, we used data from three prospective cohort studies: the Guanacaste HPV Natural History Study (NHS), the ASCUS-LSIL Triage Study (ALTS), and the Costa Rica HPV Vaccine Trial (CVT).
Methods
Study Population
Details of the study design, study procedures and characteristics of the study populations in the NHS16, ALTS17, and CVT18, 19 cohorts have been described in detail elsewhere. Brief descriptions are provided below. All the study participants were at least 18 years old, not pregnant and had no prior hysterectomy at the time of enrollment into each study. At study visits, clinical examinations were performed and specimens for HPV DNA testing were collected.
NHS was a population-based cohort set up in Guanacaste, Costa Rica, to study the natural history of HPV infection and cervical intraepithelial neoplasia (CIN). From 1993 to 1994, 10,049 women were recruited and assessed at enrollment and again at 5 to 7 years. A risk-stratified subset of 2,655 sexually active women were followed every 6 to 12 months (depending on clinical test results) to investigate the origins of incident CIN grade II or higher (CIN2+). In the present study, we considered only the women in this risk-stratified subset.
ALTS was established as a multicenter, randomized clinical trial, designed to evaluate the optimal strategies for managing low grade cervical abnormalities. In total, 5,060 women were enrolled from clinical centers in Alabama, Pennsylvania, Oklahoma, and Washington states between 1997 and 1998. Study participants were followed up every 6 months for 2 years, allowing for conduct of natural history studies.
CVT was a double-blind, randomized controlled trial to evaluate the efficacy of the HPV 16/18 AS04-adjuvanted vaccine (Cervarix®, GlaxoSmithKline Biologicals, Belgium) against cervical HPV16/18 infection and related precancerous lesions (NCT00128661). Women aged 18 to 25 years residing in Guanacaste Province, Costa Rica or selected areas of Puntarenas Province were enrolled in 2004 and 2005 and followed annually for 4 years. Concurrent with the year 4 visit, a screening-only unvaccinated control group (n=2,836) was enrolled from the same age groups and geographic areas to replace the original control group that received HPV vaccination at the end of the randomized phase of CVT. In this observational long-term follow-up phase, women in the HPV-vaccinated arm and in the new control group attended follow-up visits at years 7, 9, and 11 after the CVT baseline visit or more frequently according to the algorithms of their screening results. In the present study, we considered type-specific infections among women in the vaccine arm20, the control arm, and the unvaccinated control group over 11 years of follow-up, as a prior analysis demonstrated that the vaccine had no impact on prevalent infections.
HPV Detection and Genotyping
Several assays were used to test cervical cell samples collected from the study participants. For NHS, MY09/M11 L1 consensus primer polymerase chain reaction (PCR) 21 [10] with AmpliTaq Gold polymerase were used for HPV deoxyribonucleic acid (DNA) detection21, 22. Hybrid Capture®2 (HC2) HPV DNA assay (Digene-Qiagen) and PCR-based methods22, 23 were used in ALTS. In CVT, all samples were tested with SPF10 DNA enzyme immunoassay (DEIA) followed by LiPA2524, 25. A subset was tested by TypeSeq in parallel26. A validation study showed that major cervical HPV DNA screening and typing tests had similar analytic sensitivity for the HPV genotypes they distinguished.
Statistical Analyses
We defined prevalent infections as HPV-type positive at baseline and incident infections as HPV-type positive following a negative result at the previous visit. Clearance of HPV type-specific infections was defined as the first HPV-type negative result after a positive result.
Consistent with the multistage pathway of cervical pathogenesis, in which nearly all infections either clear or progress over time, we estimated time to clearance among infections destined to clear. This is the pure risk of clearance if the competing risk of progression could be hypothetically eliminated.27 We ruled out infections that may have already progressed to undiagnosed precancer by excluding study participants with any evidence of high-grade cervical abnormalities over the course of each study by histology (CIN2+), cytology (high-grade squamous intraepithelial lesions or worse [HSIL+]; atypical glandular cells [AGC]; atypical squamous cells, cannot rule out high-grade intraepithelial lesion [ASC-H]) or cervigram (P2 - high grade/P3 - cancer). HPV68 was excluded from the analysis because the SPF10-LiPA assay used in the CVT cannot distinguish between HPV68 and non-carcinogenic HPV73. The final analytic population included 7,146 women with HPV infections, all of whom had at least two consecutive non-missing HPV results. Of these women, 939 were from the NHS; 1,903 were from ALTS; and 4,304 were from the CVT (the HPV vaccinated arm and two control groups)20. The final analytic population contributed 20,529 HPV infections consisting of 10,020 non-carcinogenic and 10,509 carcinogenic infections.
To estimate time to clearance of incident infections for a health decision model (Figure 1), we focused the analytic cohort on apparently new infections. We considered all incident HPV infections in all women regardless of their age and prevalent HPV infections in women under 30 years old. We excluded all prevalent infections in women 30 years or older as these are more likely to already be persistent infections leading to longer left censoring5. Person-time accrual began at the time HPV was first detected. The time to clearance could not be precisely ascertained as it occurred within the interval between study visits (i.e., interval censoring). To avoid biased estimates assuming clearance occurred at the beginning, midpoint, or end of each interval, 28, 29 we used the Turnbull estimator, 30 a nonparametric procedure for estimating survival function for interval-censored survival data 31. We considered several parametric distributions to describe the clearance curve and found the Weibull distribution to be the best fit according to the Akaike Information Criterion (AIC).
Following direct estimation of HPV time to clearance, we evaluated whether the mathematical relationship between prevalence, incidence, and duration holds for the NHS, ALTS, and CVT study populations. Given a steady state, which is approximated by P=ID when prevalence is under 10%, where P refers to prevalence of a particular HPV type; I refers to the incidence rate of the HPV type; and D is the average duration of type-specific infection. For each of the study populations in this analysis, linear regression models were used to estimate the association between type-specific HPV prevalence at enrollment and type-specific HPV incidence over follow-up. We used the non-parametric Turnbull estimator to calculate type-specific HPV incidence on the interval between the last type-specific HPV-negative and first type-specific HPV positive study visit. As with the direct estimation of time to HPV clearance, we excluded women with evidence of high-grade cervical abnormalities during the study from the point prevalence calculation, as the duration estimate we derived only holds for infections that do not progress to precancer. Unlike the analytic population used for direct estimation of time to clearance, the CVT population used for estimation of incidence included only the control arm through year 4 (up until cross-over vaccination) and the unvaccinated control group for years 5 through 11, to reflect HPV incidence in the general population rather than in vaccinated women.
We grouped certain HPV types based on risk class to achieve greater statistical power. We defined HPV risk groups a priori as HPV16 (the predominant type in invasive cervical cancer); HPV18/45 (particularly present in adenocarcinomas); HPV31/33/35/52/58 (non-HPV16 alpha-9 types); HPV39/51/56/59 (found in only ~1% of invasive cervical cancers worldwide); and non-carcinogenic HPV (types rarely found alone in invasive cervical cancers). The assayed non-carcinogenic HPV types varied by study but overall included HPV6/11/26/32/34/40/42/43/44/53/54/55/57/61/62/64/66/67/69/70/72/73/74/74v/81/82/82v/83/84/85/89).
To assess the impact of age, carcinogenicity, and HPV risk group on time to clearance, we fit a Weibull model with potential predictors including age (18–29 years, 30–44 years, 45–54 years, and ≥ 55 years); carcinogenicity (12 carcinogenic types vs. non-carcinogenic types); and an interaction term for age and carcinogenicity.
Sensitivity Analyses
In the first sensitivity analysis, we defined clearance as the first HPV-type negative result after the last positive result, to determine the potential impact of apparently intermittent infections, on time to clearance. In the second sensitivity analysis, we relaxed restrictions to allow for evidence of high-grade abnormalities among incident HPV infections only, to consider the potential influence of incident progression events on clearance. In these analyses, evidence of high-grade abnormalities among prevalent infections in women under 30 years were excluded.
All p values are 2-sided, and the analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC) and R 4.1.0.
Results
The characteristics of the analytic cohort are shown in Table 1. At enrollment, the median age (interquartile range) of all study participants was 23 (20–26) years, with women from the NHS having a higher median age (29 years) and greater interval between age at first intercourse and study enrollment. Women in ALTS had a greater lifetime number of sexual partners (median = 5) compared to women in the CVT and NHS (median = 2). Characteristics of the HPV infections considered in the analysis are presented in Table 2.
Table 1.
Baseline Characteristics of the Analytic Cohort
| ALTS | CVT | NHS | All | |
|---|---|---|---|---|
| Characteristics | n = 1,903 | n = 4,304 | n = 939 | n = 7,146 |
| Study participants included in analytic cohort | Full study population | Vaccine and control arms; Unvaccinated control group | Active follow-up cohort | |
| Age (years) at enrollment, mean (SD); median (IQR) | 25.3 (7.3); 23 (20–27) |
22.3 (3.1); 22 (20–24) |
34.0 (13.7); 29 (23–41) | 24.6 (7.7); 23 (20–26) |
| Age at first sexual intercourse, mean (SD); median (IQR) | 16.3 (2.4); 16 (15–18) |
17.2 (2.8); 17 (15–19) |
18.0 (3.5); 17 (16–20) |
17.1 (2.8); 17 (15–18) |
| Lifetime number of sexual partners at enrollment, mean (SD); median (IQR) | 8.3 (8.6); 5 (3–10) |
2.6 (2.5); 2 (1–3) |
3.0 (2.9); 2 (1–4) |
4.2 (5.5); 2 (1–5) |
| Time (in years) between age at first intercourse and enrollment, mean (SD), median (IQR) | 9.0 (7.1); 7 (4–11) |
5.7 (3.7); 5 (3–8) |
17.4 (13.8); 14 (7–24) | 8.2 (7.9); 6 (4–10) |
| Incident HPV infections, n | 2,636 | 8,801 | 1,619 | 13,056 |
| Prevalent HPV infections among women <30 years, n |
3,434 | 3,426 | 613 | 7,473 |
| Carcinogenic HPV infections, n | 2,635 | 7,001 | 873 | 10,509 |
| Non-carcinogenic HPV infections, n | 3,435 | 5,226 | 1,359 | 10,020 |
IQR = Interquartile Range; SD = Standard Deviation
Table 2.
Baseline characteristics of the HPV infections at time of first detection
| HPV16 | HPV18/45 | HPV 31/33/35/52/58 | HPV 39/51/56/59 | Non-carcinogenic HPV | |
|---|---|---|---|---|---|
| Characteristics | n=1,126 | n = 1,209 | n = 4,027 | n = 4,147 | n=10,020 |
| Age (years) at first detection, mean (SD); median (IQR) | 24.4 (6.0); 23 (21–26) | 25.3 (6.5); 24 (21–27) | 25.2 (6.0); 24 (21–28) |
24.8 (5.5); 24 (21–27) |
26.3 (8.0); 25 (21–28) |
| Lifetime number of sexual partners, mean (SD); median (IQR) | 5.2 (6.7); 3 (2–6) |
5.5 (7.7); 3 (2–6) |
4.4 (4.8); 3 (2–5) |
4.6 (5.7); 3 (2–5) | 5.2 (6.3); 3 (2–6) |
| Time (in years) between age at first intercourse and enrollment, mean (SD), median (IQR) | 7.5 (6.0); 6 (4–10) |
8.4 (6.3); 7 (4–11) |
8.3 (6.2); 7 (4–11) |
7.8 (5.9); 7 (4–10) |
9.3 (8.1); 7 (4–11) |
| Incident HPV infections, n | 535 | 719 | 2,586 | 2,634 | 6,582 |
| Prevalent HPV infections among women <30 years, n | 591 | 490 | 1,441 | 1,513 | 3,438 |
Nonparametric time to clearance estimates by HPV risk group are presented in Figure 2. Overall, the time to clearance of HPV16, HPV18/45, HPV31/33/35/52/58, and HPV39/51/56/59 were similar. While non-carcinogenic types had a slightly higher probability of clearance within year 1, differences diminished for the remaining period of follow-up. Despite the similar time to clearance by carcinogenicity (Figure 3), the Weibull hazard ratio estimates yielded a slightly lower hazard of clearance among carcinogenic infections compared to non-carcinogenic types (HR: 0.88; 95% CI: 0.85–0.91), which appears to have been driven by HPV16, 18, 31, 33, 35, 45, 52 and 58.
Figure 2.
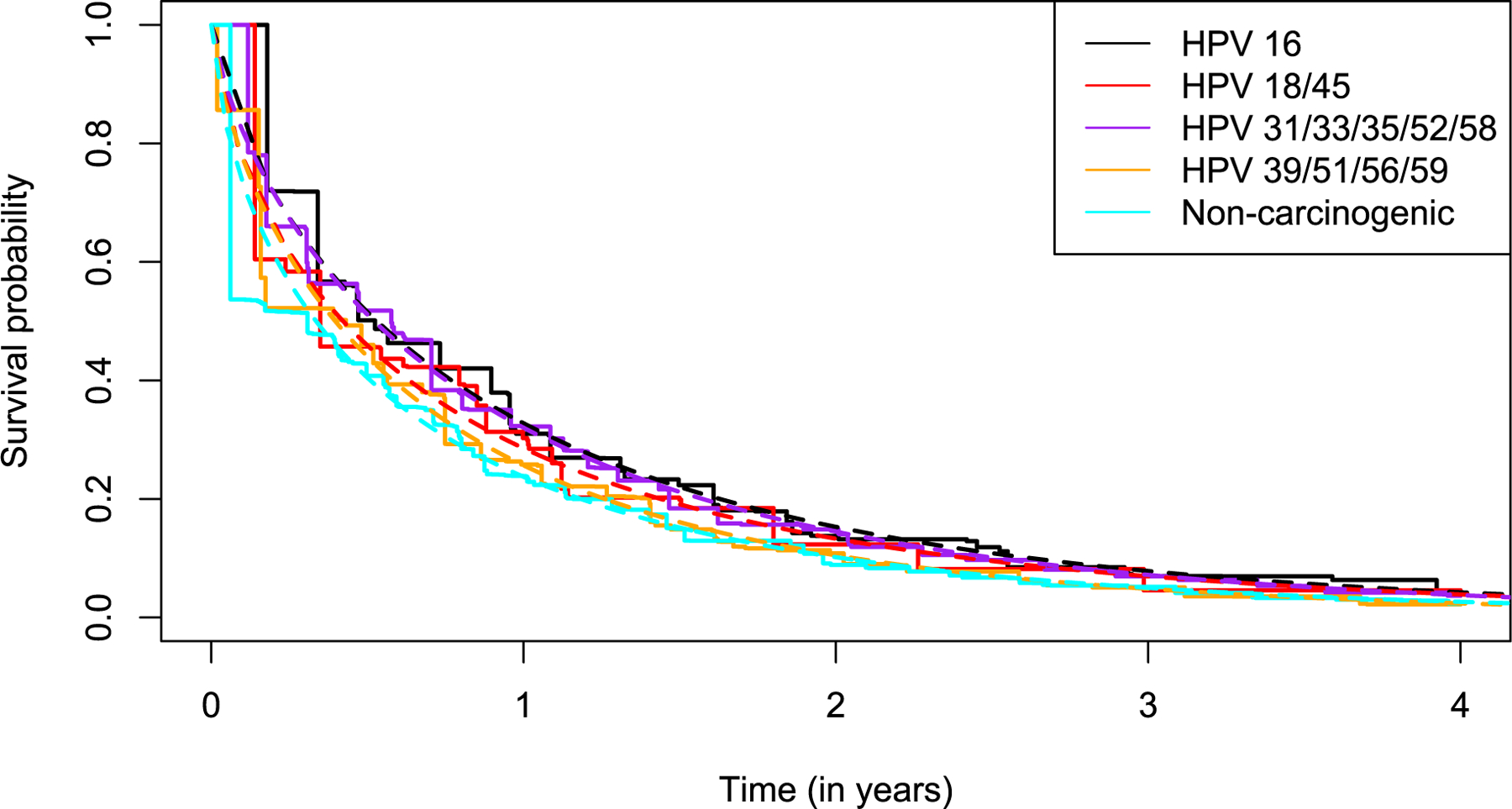
Time to clearance (non-parametric Turnbull estimator) of incident infections and prevalent infections in women under 30 years of age, by HPV risk class, in NHS, ALTS, and CVT. Follow-up was truncated at 4 years in the figure, by which point nearly all infections had cleared. HPV68 was excluded from the analysis, as the SPF10-LiPA assay used in the CVT cannot distinguish HPV68 and non-carcinogenic HPV73.
Figure 3.
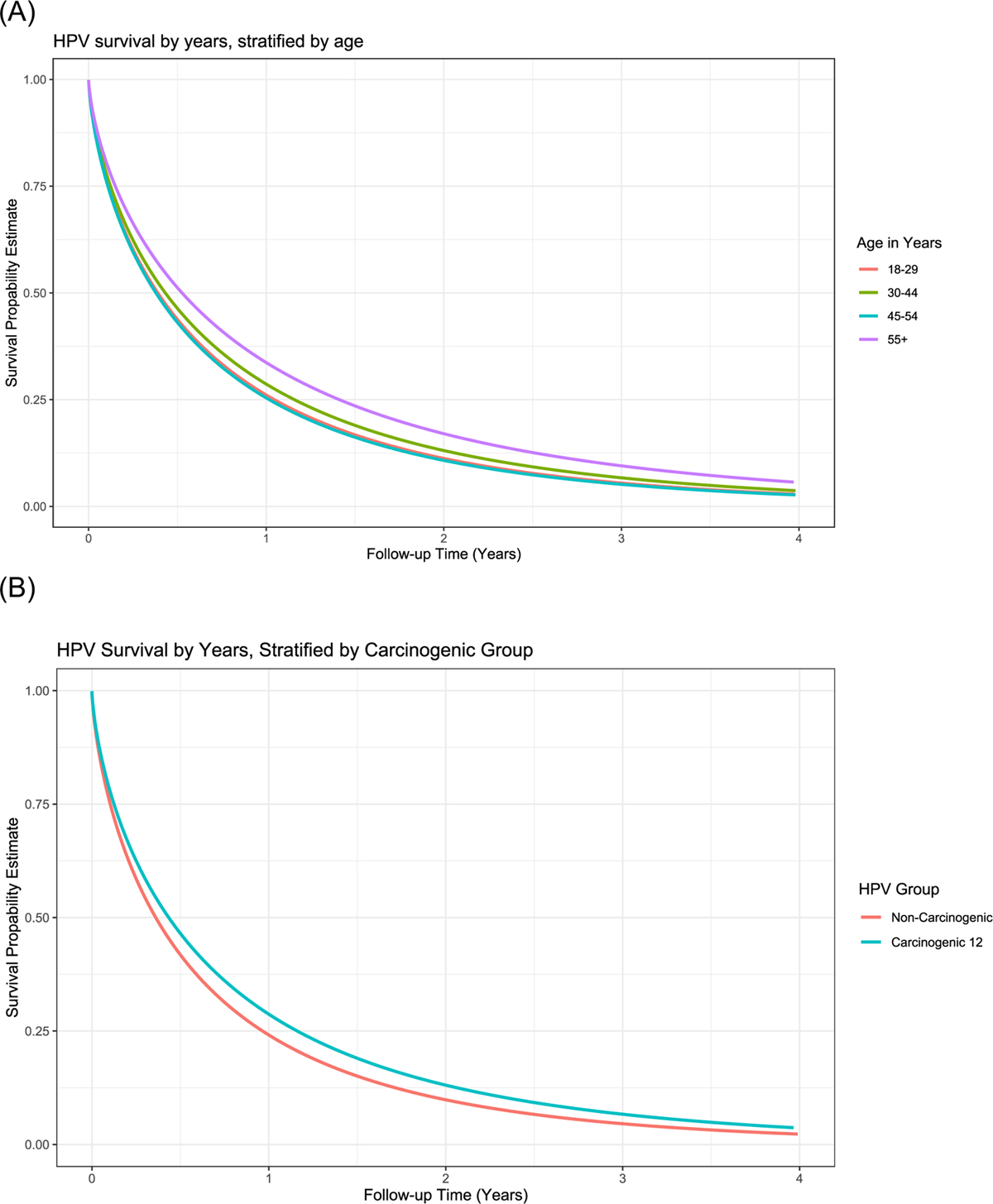
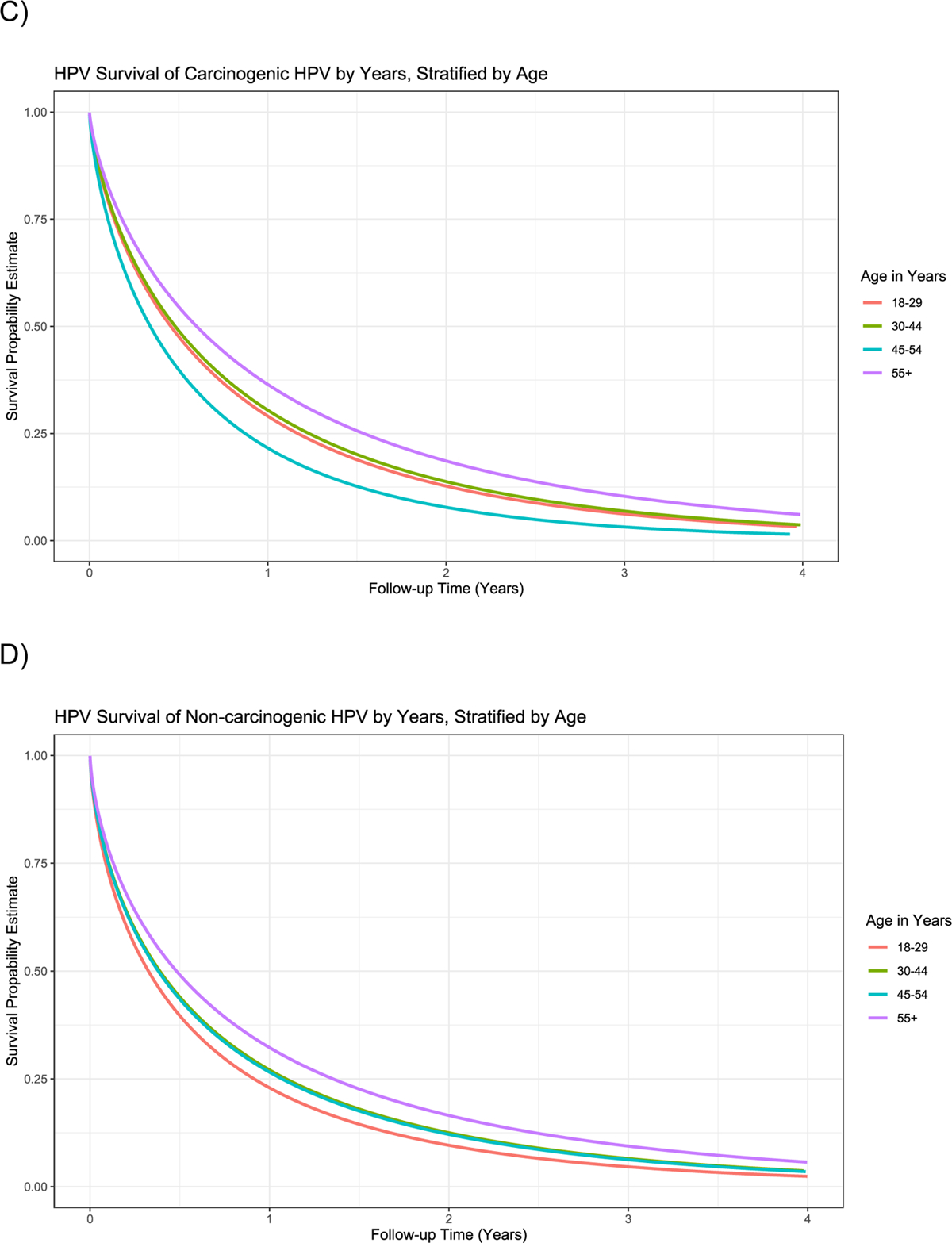
Time to clearance (Weibull model) of incident infections and prevalent infections in women under 30 years of age, by age group, in NHS, ALTS, and CVT. The Weibull distribution with shape parameters of 0.70–0.71 was the best parametric fit to the non-parametric clearance curve, indicating the hazards of clearance are decreasing with respect to time. Panel (A) all infections stratified by age group 18–29 years; 30–44 years; 45–54 years; and ≥55 years; (B) carcinogenic (HPV16/18/31/33/35/39/45/51/52/56/58/59) infections versus non-carcinogenic infections; (C) carcinogenic infections by age group; (D) non-carcinogenic infections by age group.
Time to clearance was similar by age group (18–29 years, 30–44 years, and 45–54 years) when we considered both carcinogenic and non-carcinogenic infections (Figure 3). Women aged 55 years and older were slightly less likely to clear their infections than women at younger ages (Supplemental Table 1) (HR: 0.81; 95% CI: 0.70–0.93).
For each study, Figure 4 plots type-specific HPV prevalence at study enrollment against the type-specific incidence rate per person-years of follow-up. Linear regression coefficients did not show a consistently different pattern when we stratified regression plots by age (<30 years versus ≥30 years at enrollment) or carcinogenicity (carcinogenic HPV versus non-carcinogenic HPV) (data not shown). However, study population did matter. In the CVT, we estimated that a 0.01 per person-year higher incidence rate was associated with a 1.02% (95% CI: 0.91–1.12) higher prevalence. In the ALTS population, we estimated that a 0.01 per person-year higher incidence rate was associated with a 1.63% (95% CI:1.48–1.79) higher prevalence. In the NHS, we estimated that a 0.01 per person-year higher incidence rate was associated with a 6.88% (95% CI:6.01–7.68) higher prevalence. Despite differences in the magnitude of correlation coefficients due to different baseline population risk and study visit intervals, there were clear and significant linear associations between HPV type-specific incidence and prevalence in each of these study populations, implying that prevalence ratios and incidence rate ratios between types are approximately equivalent for a given steady-state population.
Figure 4.
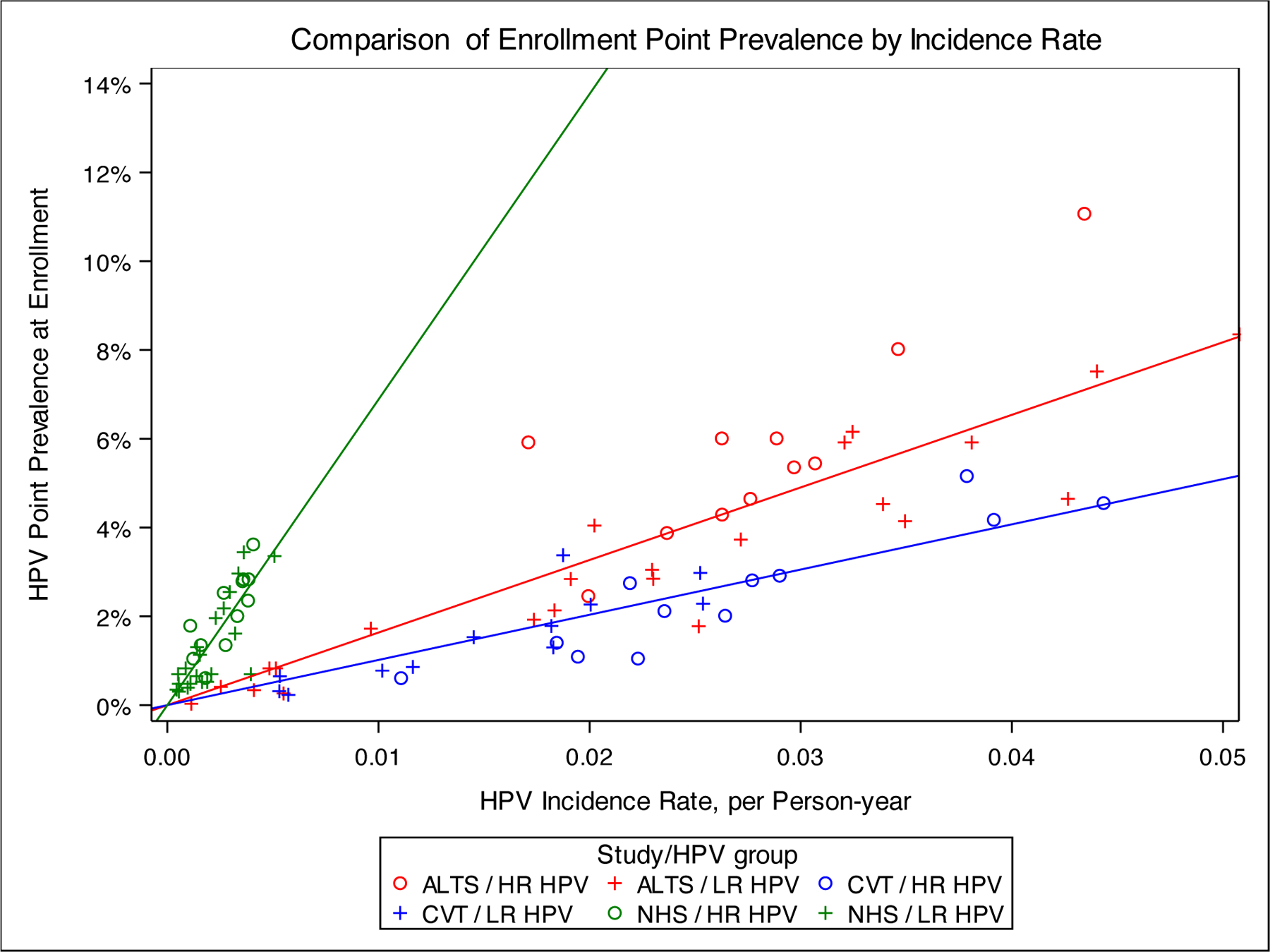
Linear regression plots, type-specific HPV prevalence at enrollment by type-specific incidence rate. Plots display the linear regression of type-specific HPV prevalence at enrollment by type-specific incidence rate per 100 person-years for A) NHS population (slope=6.88); B) ALTS population (slope=1.63); and C) CVT Population (slope=1.02). Carcinogenic types are displayed in blue and non-carcinogenic types are displayed in red. Women with evidence of high-grade disease were excluded, as described in the methods.
In the sensitivity analysis, when clearance was defined as the first HPV negative result after the last HPV positive result, the time to clearance remained similar across HPV risk groups (Supplemental Figure 1), compared to when clearance was defined as the first HPV negative result. Comparing the definition of clearance as the first HPV negative result vs the first HPV negative result after the last HPV positive result, the median times to clearance in months, were relatively similar for HPV 16 (6.4 vs 7.4), HPV18/45 (5.1 vs 5.7), HPV31/33/35/52/58 (6.3 vs 7.3) and HPV39/51/56/59 (4.8 vs 5.4). The Weibull hazard ratio estimate also showed a lower hazard of clearance among carcinogenic types compared to non-carcinogenic types (HR: 0.92; 95% CI: 0.89 – 0.94). When we relaxed the restrictions to allow for evidence of high-grade abnormalities among incident HPV infections only, the time to clearance remained similar across HPV risk groups (Supplemental Figure 2), compared to when the evidence of high-grade abnormalities was excluded. In addition, the median time to clearance in months was relatively similar for HPV 16 (6.4 vs 7.9), HPV18/45 (5.1 vs 5.7), HPV31/33/35/52/58 (6.3 vs 7.0) and HPV39/51/56/59 (4.8 vs 4.9). The Weibull hazard ratio estimate also showed a lower hazard of clearance among carcinogenic infections compared to non-carcinogenic types (HR: 0.88; 95% CI: 0.86 – 0.90).
Discussion
We analyzed time to clearance among new HPV type-specific infections in three independent prospective cohorts of immunocompetent women without evidence of high-grade cervical abnormalities at any time during follow up. We found that time to HPV clearance was similar regardless of HPV type or carcinogenicity, despite the slightly faster clearance rate among less carcinogenic HPV types that reached statistical significance due to the large number of infections. There was a modest effect of slower time to clearance among infections in women aged 55 years and older.
To estimate the risk of clearance for a health decision model, we defined the analytic population to estimate the pure clearance distribution27 that would describe time to clearance if no other risks were acting. Therefore, we restricted the analysis to infections in women without any evidence of precancer or high-grade cervical abnormalities over the course of each study because progression to precancer is associated with HPV type32, 33, and undetected precancer may increase time to clearance estimates among disease-free women for HPV types that are at greater risk of progression. Based on previous findings that prevalent infections first detected in older women are more likely to be persistent infections with a greater risk of progression5, 34, 35 or undetected precancer (and thus may yield estimates of clearance rates that are confounded by HPV type)3, we also restricted the present analysis to incident infections at all ages and prevalent infections in women under 30 years.
Our findings were consistent with a recent analysis using longitudinal data from Kaiser Permanente Northern California (KPNC), which found that clearance rates of most carcinogenic HPV types are indistinguishable, while the risk of progression to precancer is strongly associated with HPV type14. Of note, the analysis of HPV clearance in the KPNC study population could only assess HPV clearance, rather than type-specific clearance, as HPV genotyping was only conducted at one time point. However, some studies reported different findings. Results from the Population-Based Screening Amsterdam (POBASCAM) trial showed that in women with normal cytology, the lowest clearance rates were observed for HPV16, HPV18, HPV31, and HPV33, and the clearance curves for HPV16 and HPV31 were statistically distinct from the other carcinogenic types36, 37. Results from the PApilloma TRIal against Cancer In young Adults (PATRICIA) showed that the clearance curves for transient infections with HPV16 was slow, HPV18, HPV31, HPV33, and HPV52 were moderate, and the other carcinogenic HPV types were fast38, 39. The study design, population and methodology of these trials differed from the present study. A strength of the present analysis is the availability of HPV typing at multiple time points, which allows for type-specific clearance estimates and validation that clearance is similar across types. To produce comparable estimates between studies, it is critical that estimation of early natural history transition risks consider the potential for misclassification of transient versus persistent infections and undetected precancer. This distinction is particularly important for informing health decision models that will be used to evaluate novel screening and triage tests that identify transforming infections at increasingly early stages.
Following efforts to exclude precancers, the simplicity of a singular clearance function across HPV types and immunocompetent populations implies a linear relationship between HPV prevalence and incidence because the average duration of infections that are destined to clear is the same. In other words, prevalence and incidence are mathematically linked by a constant duration across HPV types when a population is in a steady state. We confirmed a linear relationship between HPV type-specific prevalence at enrollment and incidence over follow up within each study, even though different baseline risk and follow-up schedules between study populations likely led to between-study differences in measuring incidence. Between any two given types, prevalence ratios are approximately equivalent to incidence rate ratios. The early natural history of all genital HPV types appears to be similar, with type-specific incidence driving prevalence within a population due to the common mode of transmission; clearance usually occurs rapidly among infections that are destined to clear, regardless of type. Future work will leverage the mathematical relationship between HPV prevalence and a common clearance rate to estimate HPV incidence for health decision models when longitudinal data are not available.
There are several limitations to the current study. First, our definition of clearance may underestimate time to clearance if infections with fluctuating detectability are not separate re-infections with the same type. However, a sensitivity analysis defining clearance as the first HPV-type negative result following the last positive result showed relatively similar time to clearance. Second, in our rigorous attempts to exclude undetected precancers, we may have removed incident precancers and biased the analytic population toward infections most likely to clear. Third, we could not distinguish incident, first acquisition from potentially reactivated latent infections, and therefore could not evaluate whether time to clearance differs for first appearance versus reactivation. However, time to clearance of newly appearing infections was not different among women under the age of 55 years. Slower clearance among women aged 55 years and older may be due to immune senescence, or the possibility that these were not newly acquired infections but re-emergence of a prior infection that was below the limits of detection of the HPV genotyping assay(s). Lastly, for practical reasons we grouped women aged 55 years or older together, but it is unlikely that all women aged 55 years and older have the same time to clearance. The finding of slower clearance at older ages occurs at some point after age 55 years, but we are unable to distinguish a specific age range when it occurs due to insufficient power.
The present analysis focused on prospective cohorts of immunocompetent women in geographic regions characterized by HPV prevalence that declines with age. In some populations in Africa, Central and South America, meta-analysis show that HPV prevalence is relatively stable across ages, even when the HIV burden is low40–42. We have hypothesized that impairment of cell-mediated immunity, perhaps due to environmental conditions such as helminth infestation43, may lead to reduced HPV clearance in these populations, thus contributing to sustained HPV prevalence2. To determine whether there are, in fact, different rates of clearance by population, it will be necessary to analyze prospective data from settings that are underrepresented in the literature. We and others are analyzing data from the African Collaborative Center for Microbiome and Genomics Research (ACCME)41 and CONCEPT (Tanzania)44 cohorts. HPV clearance rates that differ by geographical region would have implications for microsimulation models of HPV natural history used for cost-effectiveness analysis, which to date rely on transition probabilities from populations with steadily declining age-specific HPV prevalence.
Our finding of a singular time-dependent clearance function in three independent prospective cohorts of immunocompetent women, as well as the implied linear relationship between HPV prevalence and incidence, supports the view that early HPV natural history is similar across HPV types. Type-specific HPV incidence is driven by prevalence, and clearance is similar across HPV types. As other studies have demonstrated, however, the risk of progression to precancer is strongly associated with HPV type. Present findings allow for greater parsimony in microsimulation models used for health decision and cost-effectiveness analysis of novel cervical cancer prevention technologies.
Supplementary Material
Novelty and Impact Statement.
Using data from three prospective cohorts, we compared the early natural history of HPV types to inform transition probabilities for health decision models. Our results show that the time to clearance was similar across HPV types and risk classes, and we confirmed that there was a uniform linear association between incident and prevalent infections for all HPV types.
Acknowledgements
We are grateful to the study participants are sponsors for their contribution to the study.
Funding
ALTS was supported by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (Rockville, MD) contracts CN-55153, CN-55154, CN55155, CN-55156, CN-55157, CN-55158, CN-55159, and CN55105. Some of the equipment and supplies used in this study were donated or provided at reduced cost by Digene Corporation, Gaithersburg, Md; Cytyc Corporation, Boxborough, Mass; National Testing Laboratories, Fenton, Mo; Denvu, Tucson, Ariz; and TriPath Imaging, Inc, Burlington, NC.
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals (GSK) provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the four-year, randomized blinded phase of our study.
NHS was supported by Public Health Service contracts N01CP21081 and N01CP31061 between the National Cancer Institute (NCI), National Institutes of Health, Department of Health and Human Services, and FUCODOCSA (Costa Rican Foundation for Training in Health Sciences), Caja Costarricense de Seguro Social, Costa Rica.
Sally Adebamowo was supported by the NIH/NHGRI grants U54HG006947 and U01HG011717; funding from the Division of Cancer Epidemiology and Genetics, NCI; The Maryland Department of Health’s Cigarette Restitution Fund; The University of Maryland Greenebaum Comprehensive Cancer Center Support Grant (P30CA134274); and The American Cancer Society Institutional Research Grant IRG-18–160-16.
The work of Brian Befano was supported by NCI/NIH under Grant T32CA09168.
Abbreviations
- AGC
atypical glandular cells
- ALTS
ASCUS-LSIL Triage Study ALTS
- ASC-H
high-grade intraepithelial lesion
- CIN
cervical intraepithelial neoplasia
- CIN
cervical intraepithelial neoplasia
- CVT
Costa Rica HPV Vaccine Trial
- DEIA
DNA enzyme immunoassay (DEIA)
- DNA
deoxyribonucleic acid
- HC2
Hybrid Capture®2
- HPV
Human papillomavirus
- HSIL
high-grade squamous intraepithelial lesions
- IRB
Institutional Review Board
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- NHS
Guanacaste HPV Natural History Study
- PCR
polymerase chain reaction
Footnotes
Conflict of Interest
Nicole Campos reports other salary support from the National Cancer Institute, and personal fees from Basic Health International, outside the submitted work. Philip Castle has received HPV tests and assays for research at a reduced or no cost from Roche, Becton Dickinson, Cepheid, and Arbor Vita Corporation.
Ethics Statement
All the study participants provided written informed consent before participating in each study. All the studies were approved by the Institutional Review Board (IRB) of the National Cancer Institute (NCI), National Institutes of Health (NIH). Additionally, ALTS was approved by local IRBs of the recruitment centers; CVT and NHS were approved by several IRBs in Costa Rica; CVT was registered with Clinicaltrials.gov NCT00128661.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev 2013;22:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campos NG, Demarco M, Bruni L, Desai KT, Gage JC, Adebamowo SN, de Sanjose S, Kim JJ, Schiffman M. A proposed new generation of evidence-based microsimulation models to inform global control of cervical cancer. Prev Med 2021;144:106438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM, Group A. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. The Journal of infectious diseases 2007;195:1582–9. [DOI] [PubMed] [Google Scholar]
- 4.Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, Ning L, Killeen J, Kamemoto L, Hernandez BY. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res 2008;68:8813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, Solomon D, Guillen D, Alfaro M, Morales J, Hutchinson M, Katki H, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010;102:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winer RL, Hughes JP, Feng Q, Xi LF, Cherne S, O’Reilly S, Kiviat NB, Koutsky LA. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev 2011;20:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammer A, de Koning MN, Blaakaer J, Steiniche T, Doorbar J, Griffin H, Mejlgaard E, Svanholm H, Quint WG, Gravitt PE. Whole tissue cervical mapping of HPV infection: Molecular evidence for focal latent HPV infection in humans. Papillomavirus Res 2019;7:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliano AR, Sedjo RL, Roe DJ, Harri R, Baldwi S, Papenfuss MR, Abrahamsen M, Inserra P. Clearance of oncogenic human papillomavirus (HPV) infection: effect of smoking (United States). Cancer Causes Control 2002;13:839–46. [DOI] [PubMed] [Google Scholar]
- 9.de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol 2018;47:2–13. [DOI] [PubMed] [Google Scholar]
- 10.Scott ME, Shvetsov YB, Thompson PJ, Hernandez BY, Zhu X, Wilkens LR, Killeen J, Vo DD, Moscicki AB, Goodman MT. Cervical cytokines and clearance of incident human papillomavirus infection: Hawaii HPV cohort study. International journal of cancer. Journal international du cancer 2013;133:1187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, Gradissimo A, Gonzalez P, Safaeian M, Schiffman M, Burk RD, Costa Rica HPVVTG. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog 2020;16:e1008376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dareng EO, Ma B, Adebamowo SN, Famooto A, Ravel J, Pharoah PP, Adebamowo CA. Vaginal microbiota diversity and paucity of Lactobacillus species are associated with persistent hrHPV infection in HIV negative but not in HIV positive women. Sci Rep 2020;10:19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adebamowo SN, Famooto A, Dareng EO, Olawande O, Olaniyan O, Offiong R, Adebamowo CA. Clearance of Type-Specific, Low-Risk, and High-Risk Cervical Human Papillomavirus Infections in HIV-Negative and HIV-Positive Women. J Glob Oncol 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demarco M, Hyun N, Carter-Pokras O, Raine-Bennett TR, Cheung L, Chen X, Hammer A, Campos N, Kinney W, Gage JC, Befano B, Perkins RB, et al. A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs. EClinicalMedicine 2020;22:100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz N, Mendez F, Posso H, Molano M, van den Brule AJ, Ronderos M, Meijer C, Munoz A, Instituto Nacional de Cancerologia HPVSG. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. The Journal of infectious diseases 2004;190:2077–87. [DOI] [PubMed] [Google Scholar]
- 16.Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, Balmaceda I, Greenberg MD, Alfaro M, Burk RD, Wacholder S, Plummer M, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000;92:464–74. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol 2000;44:726–42. [DOI] [PubMed] [Google Scholar]
- 18.Herrero R, Hildesheim A, Rodriguez AC, Wacholder S, Bratti C, Solomon D, Gonzalez P, Porras C, Jimenez S, Guillen D, Morales J, Alfaro M, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine 2008;26:4795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez P, Hildesheim A, Herrero R, Katki H, Wacholder S, Porras C, Safaeian M, Jimenez S, Darragh TM, Cortes B, Befano B, Schiffman M, et al. Rationale and design of a long term follow-up study of women who did and did not receive HPV 16/18 vaccination in Guanacaste, Costa Rica. Vaccine 2015;33:2141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hildesheim A, Gonzalez P, Kreimer AR, Wacholder S, Schussler J, Rodriguez AC, Porras C, Schiffman M, Sidawy M, Schiller JT, Lowy DR, Herrero R, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol 2016;215:212 e1–12 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castle PE, Schiffman M, Gravitt PE, Kendall H, Fishman S, Dong H, Hildesheim A, Herrero R, Bratti MC, Sherman ME, Lorincz A, Schussler JE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol 2002;68:417–23. [DOI] [PubMed] [Google Scholar]
- 22.Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, Klein RS, Burk RD. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol 1997;35:1304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol 1998;36:3020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Hamont D, van Ham MA, Bakkers JM, Massuger LF, Melchers WJ. Evaluation of the SPF10-INNO LiPA human papillomavirus (HPV) genotyping test and the roche linear array HPV genotyping test. Journal of clinical microbiology 2006;44:3122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang SH, Sampson JN, Schussler J, Porras C, Wagner S, Boland J, Cortes B, Lowy DR, Schiller JT, Schiffman M, Kemp TJ, Rodriguez AC, et al. Durability of Cross-Protection by Different Schedules of the Bivalent HPV Vaccine: The CVT Trial. J Natl Cancer Inst 2020;112:1030–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner S, Roberson D, Boland J, Kreimer AR, Yeager M, Cullen M, Mirabello L, Dunn ST, Walker J, Zuna R, Porras C, Cortes B, et al. Evaluation of TypeSeq, a Novel High-Throughput, Low-Cost, Next-Generation Sequencing-Based Assay for Detection of 51 Human Papillomavirus Genotypes. The Journal of infectious diseases 2019;220:1609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benichou J, Gail MH. Estimates of absolute cause-specific risk in cohort studies. Biometrics 1990;46:813–26. [PubMed] [Google Scholar]
- 28.Odell PM, Anderson KM, D’Agostino RB. Maximum likelihood estimation for interval-censored data using a Weibull-based accelerated failure time model. Biometrics 1992;48:951–9. [PubMed] [Google Scholar]
- 29.Dorey FJ, Little RJ, Schenker N. Multiple imputation for threshold-crossing data with interval censoring. Stat Med 1993;12:1589–603. [DOI] [PubMed] [Google Scholar]
- 30.Turnbull BW. The Empirical Distribution Function with Arbitrarily Grouped, Censored and Truncated Data. Journal of the Royal Statistical Society. Series B (Methodological) 1976;38:6. [Google Scholar]
- 31.Dehghan MH, Duchesne T. A generalization of Turnbull’s estimator for nonparametric estimation of the conditional survival function with interval-censored data. Lifetime Data Anal 2011;17:234–55. [DOI] [PubMed] [Google Scholar]
- 32.Wheeler CM, Hunt WC, Schiffman M, Castle PE, Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study G. Human papillomavirus genotypes and the cumulative 2-year risk of cervical precancer. The Journal of infectious diseases 2006;194:1291–9. [DOI] [PubMed] [Google Scholar]
- 33.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. International journal of cancer. Journal international du cancer 2007;121:621–32. [DOI] [PubMed] [Google Scholar]
- 34.Maucort-Boulch D, Plummer M, Castle PE, Demuth F, Safaeian M, Wheeler CM, Schiffman M. Predictors of human papillomavirus persistence among women with equivocal or mildly abnormal cytology. International journal of cancer. Journal international du cancer 2010;126:684–91. [DOI] [PubMed] [Google Scholar]
- 35.Trottier H, Mahmud S, Prado JC, Sobrinho JS, Costa MC, Rohan TE, Villa LL, Franco EL. Type-specific duration of human papillomavirus infection: implications for human papillomavirus screening and vaccination. The Journal of infectious diseases 2008;197:1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulkmans NW, Rozendaal L, Snijders PJ, Voorhorst FJ, Boeke AJ, Zandwijken GR, van Kemenade FJ, Verheijen RH, v Groningen K, Boon ME, Keuning HJ, van Ballegooijen M, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. International journal of cancer. Journal international du cancer 2004;110:94–101. [DOI] [PubMed] [Google Scholar]
- 37.Bulkmans NW, Berkhof J, Bulk S, Bleeker MC, van Kemenade FJ, Rozendaal L, Snijders PJ, Meijer CJ, Group PS. High-risk HPV type-specific clearance rates in cervical screening. British journal of cancer 2007;96:1419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, Skinner SR, Apter D, Naud P, Salmeron J, Chow SN, Kitchener H, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. The lancet oncology 2012;13:89–99. [DOI] [PubMed] [Google Scholar]
- 39.Vanska S, Auranen K, Leino T, Salo H, Nieminen P, Kilpi T, Tiihonen P, Apter D, Lehtinen M. Impact of vaccination on 14 high-risk HPV type infections: a mathematical modelling approach. PLoS One 2013;8:e72088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gage JC, Ajenifuja KO, Wentzensen N, Adepiti AC, Stoler M, Eder PS, Bell L, Shrestha N, Eklund C, Reilly M, Hutchinson M, Wacholder S, et al. Effectiveness of a simple rapid human papillomavirus DNA test in rural Nigeria. International journal of cancer. Journal international du cancer 2012;131:2903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adebamowo SN, Dareng EO, Famooto AO, Offiong R, Olaniyan O, Obende K, Adebayo A, Ologun S, Alabi B, Achara P, Bakare RA, Odutola M, et al. Cohort Profile: African Collaborative Center for Microbiome and Genomics Research’s (ACCME’s) Human Papillomavirus (HPV) and Cervical Cancer Study. International journal of epidemiology 2017;46:1745–45j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. The Journal of infectious diseases 2010;202:1789–99. [DOI] [PubMed] [Google Scholar]
- 43.Gravitt PE, Marks M, Kosek M, Huang C, Cabrera L, Olortegui MP, Medrano AM, Trigoso DR, Qureshi S, Bardales GS, Manrique-Hinojosa J, Cardenas AZ, et al. Soil-Transmitted Helminth Infections Are Associated With an Increase in Human Papillomavirus Prevalence and a T-Helper Type 2 Cytokine Signature in Cervical Fluids. The Journal of infectious diseases 2016;213:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McHome B, Swai P, Wu C, Katanga J, Kahesa C, Manongi R, Mwaiselage JD, Kjaer S, Rasch V, Linde DS. Comprehensive Cervical Cancer Prevention in Tanzania (CONCEPT) study: Cohort profile. BMJ Open 2020;10:e038531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


