Abstract
OBJECTIVE:
Fine needle aspiration (FNA) is an early step in the work-up of lymphadenopathy in people living with HIV (PLWH). We set out to characterize the FNA cytology in PLWH and report on the time to lymphoma diagnosis through the FNA clinics in the public healthcare system in Johannesburg, South Africa.
DESIGN:
Retrospective review of laboratory database
METHODS:
A retrospective chart review of patients undergoing FNA through the department of cytopathology at the National Health Laboratory Service (NHLS) was undertaken. Results of FNAs performed between March to May 2018 were reviewed. Medical record chart abstraction included general demographics, HIV status, site and results of FNA, prior history of malignancy and other laboratory data.
RESULTS:
Five hundred and thirty-nine lymph node FNAs were performed on PLWH. Pathological findings included tuberculosis 47% (252), inadequate sampling 14% (75), reactive adenopathy 13% (71), benign pathology 12% (63), suspicious for lymphoproliferative neoplasm 8% (45), other malignancy 4% (21) and inflammation 2% (n= 12). Only 53% (24) of lymphomas were confirmed by biopsy. Those not confirmed had a high mortality (57%) and loss to follow-up rate (29%) over the following year. The median diagnostic interval exceeded 8 weeks from time of FNA to lymphoma diagnosis.
CONCLUSIONS:
FNA is an important screening modality in this high HIV and TB burden region. Patients with cytology suggestive for lymphoma, but without biopsy confirmation, have a high mortality rate suggesting undiagnosed lymphoma. A better understanding of the barriers to appropriate diagnostic triage for lymphoma is needed.
Keywords: Lymphadenopathy, lymphoma diagnosis, fine-needle aspiration, tuberculosis, PLWH
INTRODUCTION:
In low-resource settings, fine needle aspiration (FNA) is an important tool to determine the cause of suspicious lymphadenopathy.1–3 Previous reports highlight the utility of this procedure in high HIV and tuberculosis (TB) burden regions.4–6 In this setting, TB is the primary diagnosis in people living with HIV (PLWH)2,4–6 and the diagnostic yield of the FNA procedure is enhanced by the incorporation of GeneXpert molecular testing for TB.7,8 Apart from diagnosing TB and reactive adenopathy, FNA is an important first step in the work-up of lymphoma in low resource settings.
Over the past two decades, South Africa has seen an increasing incidence of HIV-associated lymphomas despite the introduction of ART in the early 2000s.9–12 Several factors contribute to this including, PLWH living longer on ART and a high proportion of patients either not receiving ART or not virally suppressed.13 At the time of diagnosis, nearly 75% of non-Hodgkin lymphomas (NHLs) are advanced stage in sub-Saharan Africa (SSA), with two-thirds of patients having a poor performance status (≥ 2) and 80% presenting with B-symptoms.14 Reports from South Africa suggest that advanced stage, poor performance status and B-symptoms are more common in PLWH.10,15 These adverse prognostic factors16 limit treatment options for this patient population resulting in an inferior prognosis.
Given the association of HIV with the development of aggressive B-cell lymphomas,17–20 a better understanding of the diagnostic pathway and barriers to diagnosis in low resource settings is needed. Two reasons for delayed diagnosis that have been proposed include the empiric treatment of TB for overlapping symptoms21–24 and a prolonged diagnostic interval,21 defined as the time from when a patient presents to the healthcare system with signs and symptoms of lymphoma until a diagnosis is confirmed. When this interval exceeds 6 weeks, patients are more likely to be diagnosed with late-stage disease.21
For many, the first diagnostic test for lymphoma is a FNA. However, FNA is often insufficient to accurately subclassify and grade lymphoma. In South Africa, all lymphoma diagnoses on FNA require confirmation on a lymph node biopsy. This two-step process serves to decrease the total number of lymph node biopsies performed in a low-resource setting, many of which may not be necessary due to the preponderance of TB. However, the linkage to care between the FNA and biopsy procedures can be fraught with delays. A clearer understanding of the steps that occur after the initial FNA result is needed. In the current study, we describe the lymph node pathology in PLWH presenting to a FNA clinic associated with the National Health Laboratory Service (NHLS) in Johannesburg, South Africa. Moreover, in those patients with cytology suggestive of lymphoma, the diagnostic work-up and duration of the diagnostic interval are reported.
METHODS:
Study design and ethics
We conducted a retrospective review of all lymph node FNAs submitted and reviewed by the National Health Laboratory Service (NHLS) from March to May of 2018 in Johannesburg, South Africa. FNAs were identified by performing a Systemized Nomenclature of Medicine (SNOMED) search of the laboratory information system (LIS) (TrakCare, Intersystems, Cambridge, MA). The NHLS provides laboratory services to the public sector hospitals throughout the country and these results are searchable in the LIS database. This study was approved by the Johns Hopkins School of Medicine Institutional Review Board (IRB - #00214981) and the University of the Witwatersrand Human Research Ethics Committee (HREC - #190206).
Patient population and data abstraction
All patients undergoing a FNA during the 3-month period were eligible for the retrospective review. Those undergoing a FNA on a non-lymphoid site and pediatric patients (age <18 years) were excluded. As our outcome of interest was the use of FNA for lymphoma diagnosis, we excluded patients with a prior history of malignancy.
Data abstraction was limited to the LIS database and included age, gender, HIV status, anatomical site of FNA procedure, pathologic diagnosis including microbiologic evaluation for TB. Through the NHLS all lymph node FNAs undergo evaluation for TB using the GeneXpert platform (Cepheid, Sunnyvale, CA) with either Xpert MTB/RIF or Xpert MTB/RIF Ultra cartridges, mycobacterial culture and Ziehl Neelsen smear microscopy. From the provided clinical vignette, prior history of malignancy and existing diagnoses were recorded. HIV status was confirmed by review of HIV testing result, review of HIV viral load testing or through documented clinical history provided by the pathologist. Review of the LIS also included prior procedures within the preceding year and further work-up after the FNA procedure was performed. In this setting, all cases with a cytology diagnosis of lymphoma/suspect for lymphoma routinely have a tissue biopsy for confirmation (lymph node, bone marrow or extranodal site). Morphology is routinely accompanied by immune-peroxidase staining for confirmation and sub-typing. Polymerase chain reaction (PCR) is employed to confirm B and T cell clonality in difficult cases. When indicated, fluorescent in-situ hybridization (FISH) for Myc, BCL2 and BCL6 is performed to identify double and triple hit lymphomas. Flow cytometry is often used to aid diagnosis of bone marrow disease. For the purpose of this review, all procedures leading to a lymphoma diagnosis were reviewed including dates. Date of lymphoma diagnosis was defined as the date the pathologic result from either a biopsy or flow cytometry was available and revealed a lymphoma diagnosis. For patients in which a diagnosis of lymphoma was suggested based on cytology but not confirmed, further laboratory data was evaluated to help predict clinical course as follows: alive based on further laboratory results, loss to follow-up with no further laboratory studies, or death. For the purpose of this study, death was defined as laboratory evidence of significant clinical deterioration followed by no further laboratory results.
Data and statistical analyses
Descriptive statistics were summarized using frequencies and percentages and categorical variables were compared using the Fishers exact test. Time to lymphoma diagnosis was calculated as the time from FNA collection until date of diagnostic biopsy result. This time interval was summarized in terms of medians and interquartile range (IQR). All statistical analyses were performed using STATA version 17.0 (College Station, TX, USA).
RESULTS:
Between March and May of 2018 a total of 2360 FNAs were performed by the NHLS in Johannesburg, South Africa (Figure 1). Nine-hundred and forty-one lymph node FNAs were performed in adults without a prior history of malignancy. HIV status of the patient was known for 688 FNAs, including 539 in PLWH and 149 in HIV negative individuals. Among PLWH, the median CD4 count was 203 (IQR 81 – 355; n=223). HIV viral load was known for 205 PLWH, 77 of whom had an undetectable viral load. Median HIV viral load for those with detectable viremia was 20442 (IQR 232 – 383250; n=128). The median age for PLWH undergoing FNA was 39 years (IQR 32–46; n=512), with 52% females (n=280) and 46% male (n=249). The distribution of diagnoses for the 539 FNAs performed in PLWH included 47% TB (n = 252), 14% inadequate sampling (n = 75),13% reactive adenopathy (n = 71), 12% benign pathology (n = 63), 8% lymphoproliferative disorder (n = 45), 4% other malignancy (n = 21) and 2% inflammation (n = 12). Nine PLWH were ultimately diagnosed with a lymphoproliferative neoplasm despite an initial FNA not suggestive of lymphoma (Figure 2A). FNA results in these 9 patients included inadequate sampling (n = 6), reactive adenopathy (n = 2), benign (n = 1), inflammation (n = 1) and other malignancy (n = 1). The FNA result was suggestive of a lymphoproliferative disorder in 9% (13/149) of HIV negative patients and 2% (4/253) among those with an unknown HIV status.
Figure 1. Chart review profile.
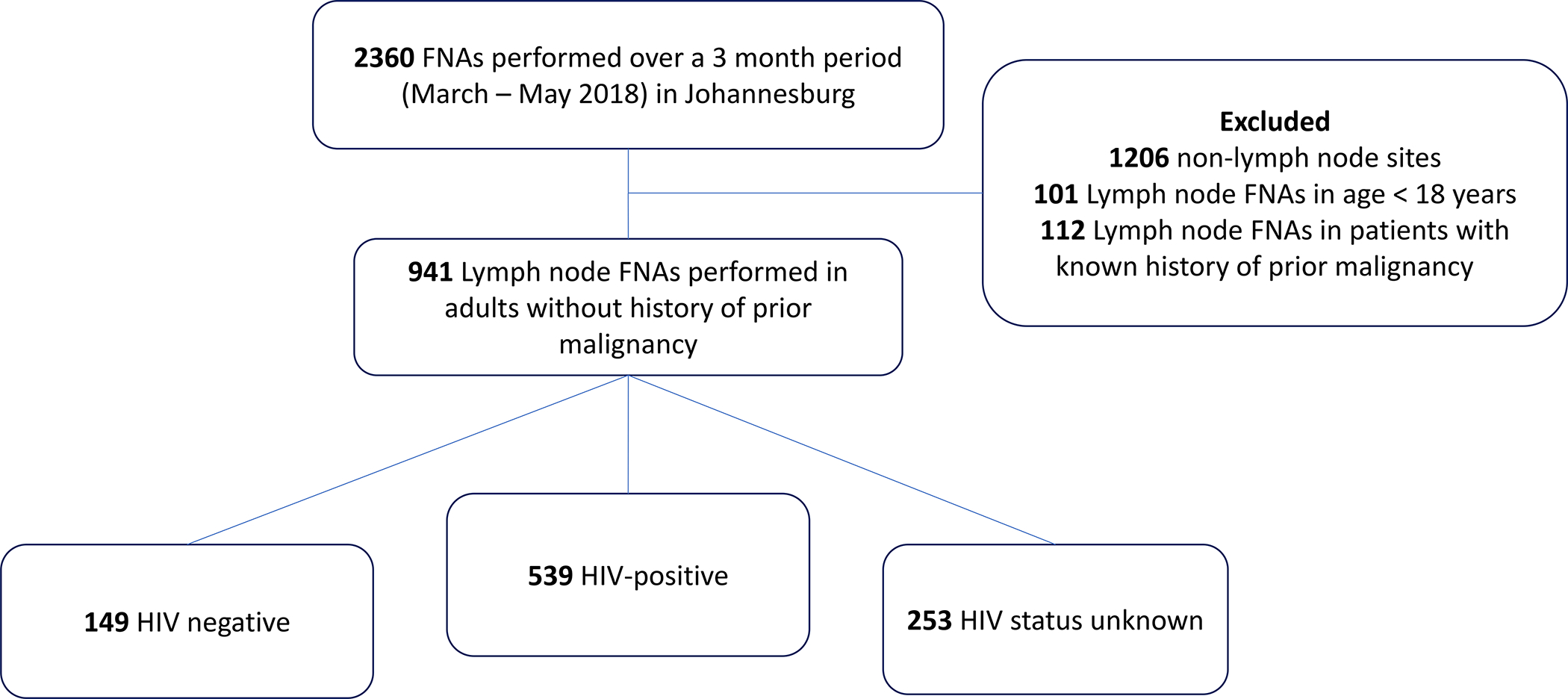
Abbreviations: FNA – fine needle aspiration
Figure 2. Diagnostic interval between FNA and biopsy for patients with lymphoma.
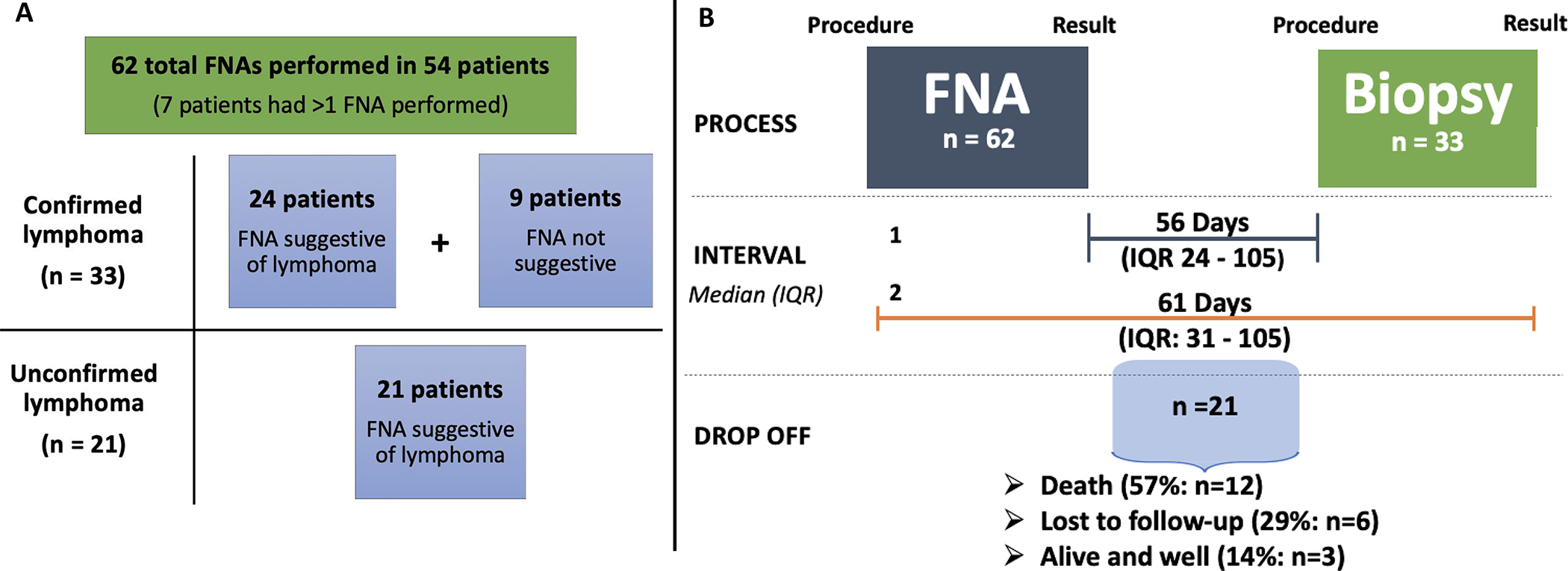
A. Total number of FNAs performed that were suggestive of lymphoma or performed in patients diagnosed with a lymphoproliferative disorder. Confirmed lymphoma is defined as lymphoma that was confirmed on a biopsy sample. Unconfirmed lymphoma is defined as cytology suggestive of lymphoma without pathologic confirmation on biopsy. B. Diagnostic interval for patients diagnosed with lymphoma is shown. Interval labeled 1 represents the time from FNA result until diagnostic biopsy (procedure) performed (n = 23). Interval labeled 2 represents the time from FNA procedure until a lymphoma diagnosis was confirmed (biopsy result; n = 33). Ten patients had the biopsy procedure performed prior to the FNA result was finalized. Median pathology turnaround time was 12 days for the FNA procedure and 13 days for the biopsy (not shown on figure). Total number of FNAs exceeds number of patients as some patients had more than 1 FNA performed during their work-up. Twenty-one patients that did not proceed to biopsy are represented under drop off.
Abbreviations: FNA – fine needle aspiration; IQR – interquartile range.
Among the 45 PLWH with cytology concerning for lymphoma, 24 (53%) patients had a biopsy confirming the diagnosis. There was no statistically significant difference between the rate of lymphoma confirmation by HIV status. The diagnoses for the 33 lymphoproliferative disorders among PLWH were as follows: 13 diffuse large B-cell lymphoma, 10 Hodgkin lymphoma, 4 high-grade non-Hodgkin lymphoma, 2 plasmablastic lymphoma, 1 HHV8 – related lymphoma, 1 Burkitt lymphoma, 1 Castleman’s disease and 1 chronic lymphocytic leukemia. The remaining 21 patients did not proceed to biopsy. Review of the laboratory database suggests that among these 21 patients, 12 (57%) patients died within one year, 6 (29%) were lost to follow-up and only 3 (14%) were alive at 1 year (Figure 2B). As a comparison, the 1 year survival rate among the 71 patients with reactive adenopathy was 58% (41), with 13% (9) mortality and 29% (21) loss to follow-up. Among the 54 PLWH with either a suspected or confirmed lymphoproliferative disorder the median CD4 count was 227 (IQR 117 – 451; n=35) and median HIV viral load for those with detectable viremia was 48000 (IQR 162 – 379500; n=21), with an additional 18 patients with an undetectable viral load.
The median pathology turn-around-time for the FNA procedure in lymphoma patients was 12 days (IQR: 7–17 days). Among the 33 patients with a confirmed lymphoma diagnosis, the diagnostic interval was 61 days (IQR: 31–105 days; Figure 2B). The median time between FNA result and biopsy procedure was 56 days (IQR 24 – 105; n=23). The median pathology turn-around-time for the biopsy was 13 days (IQR: 7–17 days). Clinical outcomes for the 33 patients with a confirmed lymphoma diagnosis include 17 alive at 1 year (52%), 12 deaths (36%) and 4 lost to follow-up (12%). Lymphoma diagnosis was associated with a statistically significant improvement in 1-year survival as compared to those with an unconfirmed diagnosis (59% vs 20%; p = 0.02)
DISCUSSION:
FNA represents an early step in the work-up of lymphadenopathy in Johannesburg, South Africa. It is readily available and serves as an important screening tool for PLWH. When combined with upfront GeneXpert and mycobacterial culture testing, a diagnosis of TB was confirmed in half of PLWH undergoing a lymph node FNA and is similar to prior reports.2,4–6
For patients with a FNA suspicious for lymphoma who never made it to biopsy, the mortality was 57%–86% (with the uncertainty reflecting a 29% lost to follow-up rate). These grim outcomes are consistent with aggressive lymphoma being associated with a high fatality when left untreated. A limitation of our current study is an inability to understand why patients never made it to a diagnostic biopsy given the retrospective study design. These barriers, whether patient, provider or health-system related should be evaluated and could inform the development of new diagnostic approaches to lymphoma in this setting. Additionally, given the large number of FNAs performed in this region, we limited our initial review to 3 months of data. A larger study to capture an entire year of data would be useful to account for any seasonal variation in lymphoma diagnosis.25,26
For patients who had a lymphoma diagnosis biopsy confirmed, the diagnostic interval exceeded the 6-week interval shown to predict advanced stage of disease. This finding is consistent with other retrospective reviews of lymphoma patients referred to a subspecialist for treatment in South Africa that suggest the median diagnostic interval in PLWH is 8–11 weeks.21,27 From our experience in Johannesburg, FNA result dissemination to both the patient and healthcare provider and poor provider continuity appear to be major barriers to further work-up being performed in the outpatient setting and may contribute to the prolonged interval between FNA and diagnostic biopsy. It should be noted that our interval was defined as time from FNA procedure until result of the diagnostic biopsy and likely underestimates the true diagnostic interval.
Aggressive lymphomas in general are treatable and often curable even in this low-resource setting. We report a 52%, 1-year survival for those patients with an established lymphoma diagnosis which is consistent with prior reports from this region.24,28 Among the 36% of patients who died within 1-year, it is unknown whether they were “well enough” at the time of diagnosis to receive curative intent chemotherapy. A high mortality rate within the first 3 months of diagnosis due to non-treatment has been previously reported for HIV-associated lymphomas in South Africa.24
For patients with FNA results suspicious for lymphoma, improved prioritization for biopsy represents an opportunity to save lives. The challenge is how to ensure that a follow-up biopsy happens expeditiously or to improve the information gathered from the FNA such that it is adequate for treatment. To address this problem, two interventions could be considered: 1) Upfront core-needle29 or prioritized excisional biopsy based on initial FNA findings. This type of intervention would result in a significant increase in pathologic specimens requiring triage and could overwhelm the pathology infrastructure. 2) Enhancement of the FNA process with molecular studies to facilitate rapid diagnosis and perhaps obviate the need for biopsy in many cases. We note that with the incorporation of GeneXpert technology, TB diagnosis has been greatly facilitiated.8 Molecular characterization of FNA specimens might similarly assist with lymphoma diagnosis.30,31 Exactly how and when this testing should be incorporated into the diagnostic paradigm will require further evaluation, but the present study highlights the extent of the opportunity in low-resource settings where pathology infrastructure is limiting. New molecular techniques such as the analysis of clonal immunoglobulin DNA, while requiring sophisticated equipment, are scalable in a way that histopathologic interpretation of specimens are not.
In low resource settings with a high burden of HIV and TB, FNA alone is a useful tool for the diagnosis of TB and reactive adenopathy. However, for lymphoma diagnosis it is inadequate to inform treatment decisions and the linkage to diagnostic biopsy and further care is poor.
Acknowledgments
Research Support: This work was supported in part by the following grants: Fogarty International Center (R25TW009340), National Cancer Institute (NCI) for the Training in the Molecular Targets for Cancer Detection and Treatment (5T32 CA09071), NCI (R01CA250069; R21CA232891; P30CA006973, P30AI094189), and the AIDS Malignancy Consortium (UM1CA121947).
Footnotes
Conflicts of Interest Disclosure: The authors have no relevant disclosures.
References
- 1.Carrilho C, Ismail M, Lorenzoni C, et al. : Fine needle aspiration cytology in Mozambique: Report of a 15-year experience. Diagn Cytopathol 47:166–171, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muyanja D, Kalyesubula R, Namukwaya E, et al. : Diagnostic accuracy of fine needle aspiration cytology in providing a diagnosis of cervical lymphadenopathy among HIV-infected patients. Afr Health Sci 15:107–16, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan S, Liomba G, Rosenberg NE, et al. : Utilization of fine needle aspiration cytology at Kamuzu central hospital. PLoS One 13:e0196561, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suresh PK, Poojary S, Basavaiah SH, et al. : Utility of fine-needle aspiration cytology in the diagnosis of HIV lymphadenopathy. Diagn Cytopathol 47:1011–1017, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Mandal R, Mondal K, Datta S, et al. : A clinicopathological study of peripheral lymph nodes in HIV-infected patients with special reference to CD4+ T-cell counts: Experience from a tertiary care institution in Darjeeling (India). Diagn Cytopathol 43:971–7, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Kamana NK, Wanchu A, Sachdeva RK, et al. : Tuberculosis is the leading cause of lymphadenopathy in HIV-infected persons in India: results of a fine-needle aspiration analysis. Scand J Infect Dis 42:827–30, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Antel K, Oosthuizen J, Malherbe F, et al. : Diagnostic accuracy of the Xpert MTB/Rif Ultra for tuberculosis adenitis. BMC Infect Dis 20:33, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denkinger CM, Schumacher SG, Boehme CC, et al. : Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 44:435–46, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Abayomi EA, Somers A, Grewal R, et al. : Impact of the HIV epidemic and Anti-Retroviral Treatment policy on lymphoma incidence and subtypes seen in the Western Cape of South Africa, 2002–2009: preliminary findings of the Tygerberg Lymphoma Study Group. Transfus Apher Sci 44:161–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel M, Philip V, Omar T, et al. : The Impact of Human Immunodeficiency Virus Infection (HIV) on Lymphoma in South Africa. Journal of Cancer Therapy 06:527–535, 2015 [Google Scholar]
- 11.Vaughan J, Perner Y, McAlpine E, et al. : Brief Report: HIV-Associated Hodgkin Lymphoma Involving the Bone Marrow Identifies a Very High-Risk Subpopulation in the Era of Widescale Antiretroviral Therapy Use in Johannesburg, South Africa. J Acquir Immune Defic Syndr 83:345–349, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Wiggill T, Mayne E, Perner Y, et al. : Changing Patterns of Lymphoma in the Antiretroviral Therapy Era in Johannesburg, South Africa. J Acquir Immune Defic Syndr 88:252–260, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Johnson LF, Dorrington RE. Modelling the impact of HIV in South Africa’s provinces: 2021 update. Centre for infectious Disease Epidemiology and Research working paper, March 2021. Assessed online at https://www.thembisa.org/downloads on Dec 1st, 2019. [Google Scholar]
- 14.Mezger NCS, Feuchtner J, Griesel M, et al. : Clinical presentation and diagnosis of adult patients with non-Hodgkin lymphoma in Sub-Saharan Africa. Br J Haematol 190:209–221, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Patel M, Philip V, Fazel F: Human Immunodeficiency Virus Infection and Hodgkin’s Lymphoma in South Africa: An Emerging Problem. Adv Hematol 2011:578163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barta SK, Xue X, Wang D, et al. : A new prognostic score for AIDS-related lymphomas in the rituximab-era. Haematologica 99:1731–7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engels EA, Biggar RJ, Hall HI, et al. : Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 123:187–94, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Grulich AE, van Leeuwen MT, Falster MO, et al. : Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 370:59–67, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Patel P, Hanson DL, Sullivan PS, et al. : Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 148:728–36, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Silverberg MJ, Lau B, Achenbach CJ, et al. : Cumulative Incidence of Cancer Among Persons With HIV in North America: A Cohort Study. Ann Intern Med 163:507–18, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antel K, Levetan C, Mohamed Z, et al. : The determinants and impact of diagnostic delay in lymphoma in a TB and HIV endemic setting. BMC Cancer 19:384, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buyego P, Nakiyingi L, Ddungu H, et al. : Possible misdiagnosis of HIV associated lymphoma as tuberculosis among patients attending Uganda Cancer Institute. AIDS Res Ther 14:13, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puvaneswaran B, Shoba B: Misdiagnosis of tuberculosis in patients with lymphoma. S Afr Med J 103:32–3, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Swart L, Novitzky N, Mohamed Z, et al. : Hodgkin lymphoma at Groote Schuur Hospital, South Africa: the effect of HIV and bone marrow infiltration. Ann Hematol 98:381–389, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Borchmann S, Muller H, Engert A: Hodgkin Lymphoma has a seasonal pattern of incidence and mortality that depends on latitude. Sci Rep 7:14903, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koutros S, Holford TR, Hahn T, et al. : Excess diagnosis of non-Hodgkin’s lymphoma during spring in the USA. Leuk Lymphoma 48:357–66, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Vogt S, Ashmore P, Patel M, Martinson N, Ambinder F. Delay in South African AIDS lymphoma diagnosis. Presented at the 16th International Conference on Malignancies in HIV/AIDS, Washington, DC, USA, 2017 [Google Scholar]
- 28.Milligan MG, Bigger E, Abramson JS, et al. : Impact of HIV Infection on the Clinical Presentation and Survival of Non-Hodgkin Lymphoma: A Prospective Observational Study From Botswana. J Glob Oncol 4:1–11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antel K, Louw VJ, Maartens G, et al. : Diagnosing lymphoma in the shadow of an epidemic: lessons learned from the diagnostic challenges posed by the dual tuberculosis and HIV epidemics. Leuk Lymphoma 61:3417–3421, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogt SL, Patel M, Lakha A, et al. : Feasibility of Cell-Free DNA Collection and Clonal Immunoglobulin Sequencing in South African Patients With HIV-Associated Lymphoma. JCO Glob Oncol 7:611–621, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogt SL, Patel M; Omar T, et al. : Molecular Diagnostics for AIDS Lymphoma Diagnosis in South Africa and the Potential for Other Low- and Middle- Income Countries. Journal of Global Oncology 4:1–6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]


