Abstract
Purpose
The relationship between peritumoral neuronal activity, early onset clinical seizures, and glioma survival outcomes remains poorly understood. Hyperexcitability on continuous EEG in the peri-operative period was studied as a prognostic biomarker in patients with newly diagnosed IDH-wildtype diffuse glioma.
Methods
A retrospective observational cohort study was performed including adults with newly diagnosed diffuse glioma, absence of IDH1/2 mutations, and continuous EEG monitoring prior to chemoradiation and within one month of initial resection. EEG hyperexcitability was defined by the presence of lateralized periodic discharges and/or electrographic seizures. The primary outcome of overall survival was estimated using the Kaplan-Meier method and compared between groups using multivariate Cox proportional hazards model.
Results
There were 424 patients without continuous EEG and 32 with continuous EEG, of whom lateralized periodic discharges and/or electrographic seizures were seen in 17 (53%). Peri-operative EEG hyperexcitability was associated with decreased overall survival in multivariate analysis (median 12.5 [95% CI 6.2–25.6] months with hyperexcitability versus median 19.9 [95% CI 8.9–53.5] months without hyperexcitability, p=0.043). Compared to patients without continuous EEG, overall survival was decreased in patients with hyperexcitability (p<0.0001) and similar in patients without hyperexcitability (p=0.193). Patients with and without hyperexcitability had similar rates of exposure to anti-seizure medication at baseline, and in long-term follow-up had no difference in number of medications required for seizure control.
Conclusions
These findings indicate the potential prognostic value of a clinical EEG biomarker of glioma aggressiveness prior to the initiation of chemoradiation.
Keywords: Glioblastoma, Electroencephalography, Hyperexcitability, Prognosis, Outcome
Introduction
Seizures are a frequent initial presentation of diffuse gliomas. In low grade gliomas, seizure frequency is associated with disease progression and anti-tumor treatment, and therefore seizure activity has been proposed as a potential biomarker of disease control [1]. Seizures at presentation have been reported as a favorable prognostic factor in glioblastoma [2–5], although the mechanisms are uncertain. The presence of the IDH mutation and other molecular features carrying prognostic value may partially contribute to this effect [3, 6]. However, animal models of high-grade glioma demonstrate that excessive peritumoral neuronal activity promotes activation of pathways, including PI3K-mTOR upregulation, which contribute to tumor progression and decreased survival [7–9]. Thus, the relationship between neuronal activity in the tumor microenvironment, early onset clinical seizures, and survival outcomes remains poorly understood, in part due to a lack of clinically validated biomarkers of peritumoral hyperexcitability and limited epilepsy phenotyping in large-scale neuro-oncology data sets.
Clinically available EEG-based biomarkers may be useful in reconciling the effects of peritumoral hyperexcitability, clinical seizures, and anti-seizure medication exposure on oncologic outcomes. Here we evaluate the prognostic value of epileptiform activity on continuous EEG recording in the peri-operative period prior to initiation of chemoradiation therapy in patients with newly diagnosed IDH-wildtype diffuse glioma.
Methods
Study Design
This was a retrospective observational cohort study of patients treated for newly diagnosed glioma at Dana-Farber Cancer Institute (DFCI) between 2013–2018. All protocols for Human Subjects Research were approved by the Institutional Review Board at DFCI with waiver of informed consent (Protocol #21-425).
Patient Selection
Clinical data from the previously validated DFCI Neuro-Oncology Cohort (dataset publicly available from DFCI-AACR GENIE cBioPortal or by contacting KLL) were used for the study [10]. Inclusion criteria were adult patients (at least 18 years old) with a pathology integrated diagnosis of newly diagnosed, diffuse glioma, and histological grade 2–4 (e.g. glioblastoma, diffuse glioma NEC) by the 2021 WHO Classification of Tumors of the Central Nervous System criteria. Exclusion criteria were the presence of IDH1/2 mutations by immunohistochemistry and/or sequencing (e.g. no oligodendroglioma or astrocytoma by the 2021 WHO Classification) and pediatric-type diffuse high-grade gliomas. Patients who had continuous EEG monitoring prior to chemoradiation and within one month from the time of initial resection were included in the EEG cohort for the primary analysis. An independent internal reference cohort consisted of adults with an integrated diagnosis of diffuse glioma and absence of IDH1/2 mutations by sequencing without continuous EEG data.
Clinical Variables
Baseline clinical and demographic data were obtained for the EEG cohort by review of neuro-oncology notes at the time of glioma diagnosis. Tumor classifications and grading were re-coded for consistency based on the 2021 WHO criteria [11]. Performance status was determined at the time of the initial post-operative pre-treatment oncology evaluation.
EEG data were obtained by cross-referencing with a previously validated large-scale critical care EEG database containing inpatient continuous EEG (cEEG) monitoring records [12]. All EEGs for a given patient performed within the timeframe of interest were reviewed, however, if both pre-operative and post-operative recordings were available, only post-operative studies were included in the analysis. EEG hyperexcitability was defined by the presence of lateralized periodic discharges and/or electrographic seizures (including either clinical or subclinical) according to American Clinical Neurophysiology Society Standardized Critical Care EEG Terminology [13].
Tumor Sequencing
Tumor sequencing was performed in all patients using the OncoPanel genomic assay, and single-nucleotide mutation analysis reviewed for variants in IDH1, IDH2, and PI3K-mTOR pathway genes MTOR, PIK3CA, PIK3C2B, and PIK3R1. As previously described, DNA was extracted and fragmented for library preparation and PCR enrichment in formalin-fixed paraffin-embedded tissue specimens, followed by hybrid capture with the OncoPanel DNA bait set and Illumina sequencing [14, 15].
Statistical Analysis
Univariate comparisons between categorical and continuous variables were performed using the Fisher Exact Test and Mann-Whitney U-test, respectively. Overall survival (OS) probabilities were estimated with the Kaplan-Meier method. Patients were censored at the time of last encounter. Survival times were compared between groups using the log-rank test and multivariate regression performed with Cox proportional hazards model. Proportional hazards assumptions were tested by calculating Schoenfeld residuals. Performance status was stratified as a binary variable to correct for violation of proportional hazards assumption. All tests were two-sided with a significance threshold of 0.05. Analyses were performed using R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
After excluding cases with IDH1/2 mutations and cEEG recording greater than one month from the time of initial glioma resection, there were 32 patients in the cEEG cohort and 424 patients without cEEG (Figure 1). Among the cEEG cohort, lateralized periodic discharges and/or electrographic seizures (LPD/ES) were seen in 17 (53%). In 9/17 (53%) patients with LPD/ES and in 11/15 (73%) patients without LPD/ES, cEEG recording was performed post-operatively (p=0.291). Overall cEEG was performed for evaluation of altered mental status in 18 patients (10/15 without LPD/ES vs 8/17 with LPD/ES), clinical status epilepticus in 10 patients (2/15 without LPD/ES vs 8/17 with LPD/ES), and characterization of spells and/or background activity in 4 patients (3/15 without LPD/ES vs 1/17 with LPD/ES). Demographic and baseline prognostic characteristics were similar between patients with and without LPD/ES on cEEG (Table 1, Figure 2).
Fig. 1.
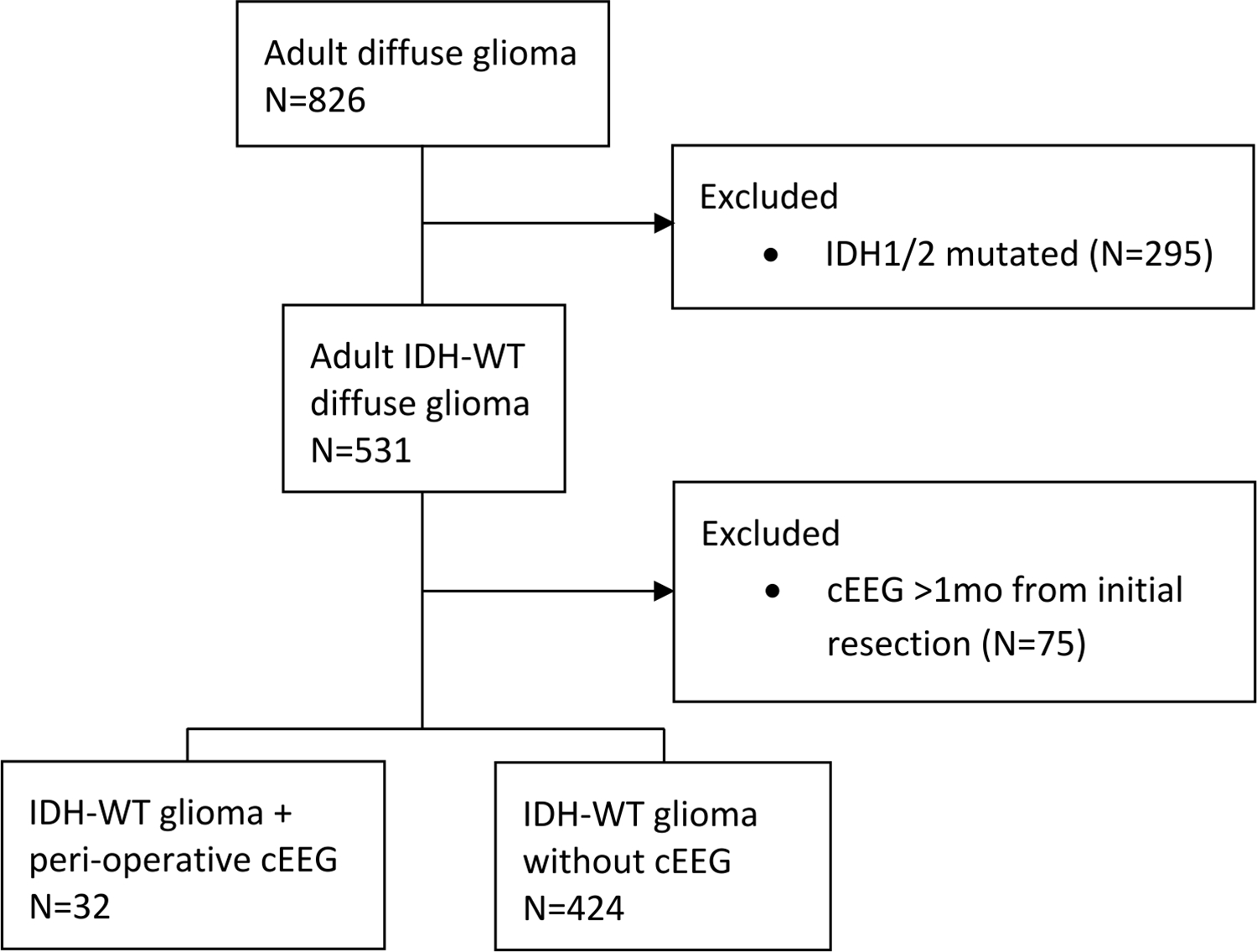
Flow diagram of patient inclusion. Adult patients with newly diagnosed diffuse gliomas were analyzed after exclusion of those with IDH1/2 mutations or cEEG >1 month from initial surgery. cEEG, continuous EEG; IDH, isocitrate dehydrogenase; WT, wildtype
Table 1.
Demographic and baseline characteristics
| EEG +LPD/ES (N=17) | EEG −LPD/ES (N=15) | p-value | |
|---|---|---|---|
| Age at diagnosis, mean (range) | 62 (41–82) | 67 (50–86) | 0.32 |
| Female sex, N(%) | 7 (41%) | 3 (20%) | 0.27 |
| KPS, median (range)a | 80 (20–100) | 80 (20–90) | 0.76 |
| Pathology integrated diagnosis, N(%)b | |||
| Glioblastoma | 13 (76%) | 15 (100%) | 0.10 |
| Diffuse glioma NEC | 4 (24%) | 0 (0%) | |
| WHO grade, N(%)b | |||
| Grade 4 | 13 (76%) | 15 (100%) | 0.10 |
| Grade 3 | 3 (18%) | 0 (0%) | |
| Grade 2 | 1 (6%) | 0 (0%) | |
| MGMT promoter methylated, N(%)c | 10 (77%) | 5 (38%) | 0.14 |
| Gross total resection, N(%) | 5 (29%) | 7 (47%) | 0.47 |
| Completed chemoradiation, N(%) | 13 (76%) | 12 (80%) | 1.00 |
| Tumor localization, N(%)d | |||
| Temporal/Insular | 9 (53%) | 10 (67%) | 0.49 |
| Frontal | 6 (35%) | 3 (20%) | |
| Parietal | 5 (29%) | 2 (13%) | |
| Occipital | 1 (6%) | 1 (7%) | |
| Thalamic | 1 (6%) | 0 (0%) | |
| Clinical seizures at presentation, N(%) | 14 (82%) | 10 (67%) | 0.42 |
| Clinical seizures at anytime, N(%) | 16 (94%) | 11 (73%) | 0.16 |
| ASMs received peri-operatively, N(%) | 17 (100%) | 15 (100%) | 1.00 |
| ASMs at start of continuous EEG, N(%) | 16 (94%) | 14 (93%) | 1.00 |
ASM, anti-seizure medication; KPS, Karnofsky Performance Status; LPD/ES, lateralized periodic discharge and/or electrographic seizure; MGMT, O[6]-methylguanine-DNA methyltransferase; WHO, World Health Organization
KPS determined at initial post-operative pre-treatment evaluation
Re-coded to align with 2021 WHO Classification criteria
Percent of cases with available data; −LPD/SZ (N=13), +LPD/SZ (N=13)
Multi-lobar cases counted in each applicable category
Fig. 2.
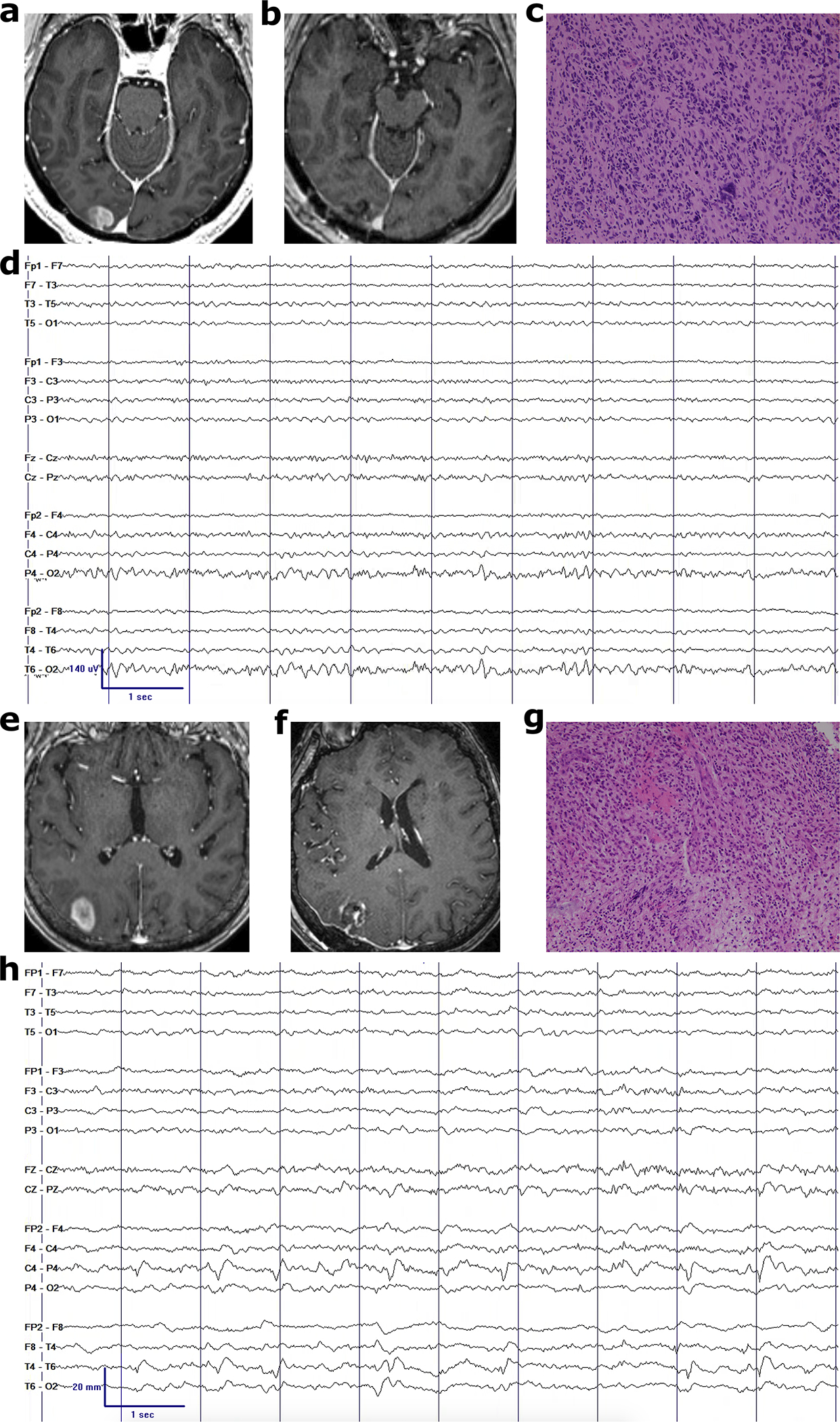
Examples of IDH-WT gliomas with and without continuous EEG hyperexcitability. Pre-operative MRI (a,e), post-operative MRI (b,f), representative glioblastoma histology at 20x magnification (c,g), and post-operative EEG (d,h) without and with lateralized periodic discharges in the right posterior peritumoral region, respectively
All patients received at least one anti-seizure medication peri-operatively, indicated for clinical seizures in 24 (75%) and for prophylaxis in 8 (25%) patients. At the initiation of cEEG recording, 16 (94%) patients with LPD/ES and 14 (93%) patients without LPD/ES were receiving treatment with anti-seizure medications, including levetiracetam as monotherapy (53% with LPD/ES vs 67% without LPD/ES) and in polytherapy (35% with LPD/ES vs 20% without LPD/ES). As a proxy metric for seizure control, patients in each group were being treated with a similar number of anti-seizure medications (median 1 [range 0–4] with LPD/ES vs median 1 [range 0–4] without LPD/ES, p=0.630) at the time of last available follow-up.
Prognostic Value of EEG Hyperexcitability
In long-term follow-up, the clinical endpoint of death was met in 17/17 (100%) patients with LPD/ES and 14/15 (93%) patients without LPD/ES. Patients with LPD/ES had a median OS of 12.5 [95% CI 6.2–25.6] months while patients without LPD/ES had a median OS of 19.9 (95% CI 8.9–53.5] months (X2=3.0, p=0.084, Table 2, Figure 3). In a multivariate Cox proportional hazards model stratified by performance status, LPD/ES on cEEG was significantly associated with OS (p=0.043), adjusting for the presence of WHO grade 4, MGMT promoter methylation, and gross total resection (Table 2).
Table 2.
Univariate and Multivariate Analysis of Overall Survival Outcomes
| Parameter | Median OS (months) | Unadjusted Model | Adjusted Model | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| LPD/ES | Yes No (ref) |
12.5 19.9 |
1.96 | 0.90–4.26 | 0.08 | 3.06 | 1.03–9.03 | 0.04 |
| KPSa | ≥90 <90 (ref) |
25.8 8.7 |
0.43 | 0.20–0.92 | 0.03 | NA | NA | NA |
| WHO grade | 4 2–3 (ref) |
11.8 18.4 |
1.05 | 0.36–3.05 | 0.93 | 0.51 | 0.08–3.05 | 0.46 |
| MGMT promoter | Methylated Unmethylated (ref) |
12.5 19.1 |
0.62 | 0.26–1.47 | 0.28 | 0.50 | 0.17–1.42 | 0.19 |
| GTR | Yes No (ref) |
14.1 15.1 |
1.05 | 0.50–2.21 | 0.90 | 1.87 | 0.74–4.70 | 0.18 |
GTR, gross total resection; HR, hazard ratio; KPS, Karnofsky Performance Status; LPD/ES, lateralized periodic discharges and/or electrographic seizures; MGMT, O[6]-methylguanine-DNA methyltransferase; OS, overall survival; WHO, World Health Organization
Stratified in multivariate analysis for violation of proportional hazards assumption
Fig. 3.
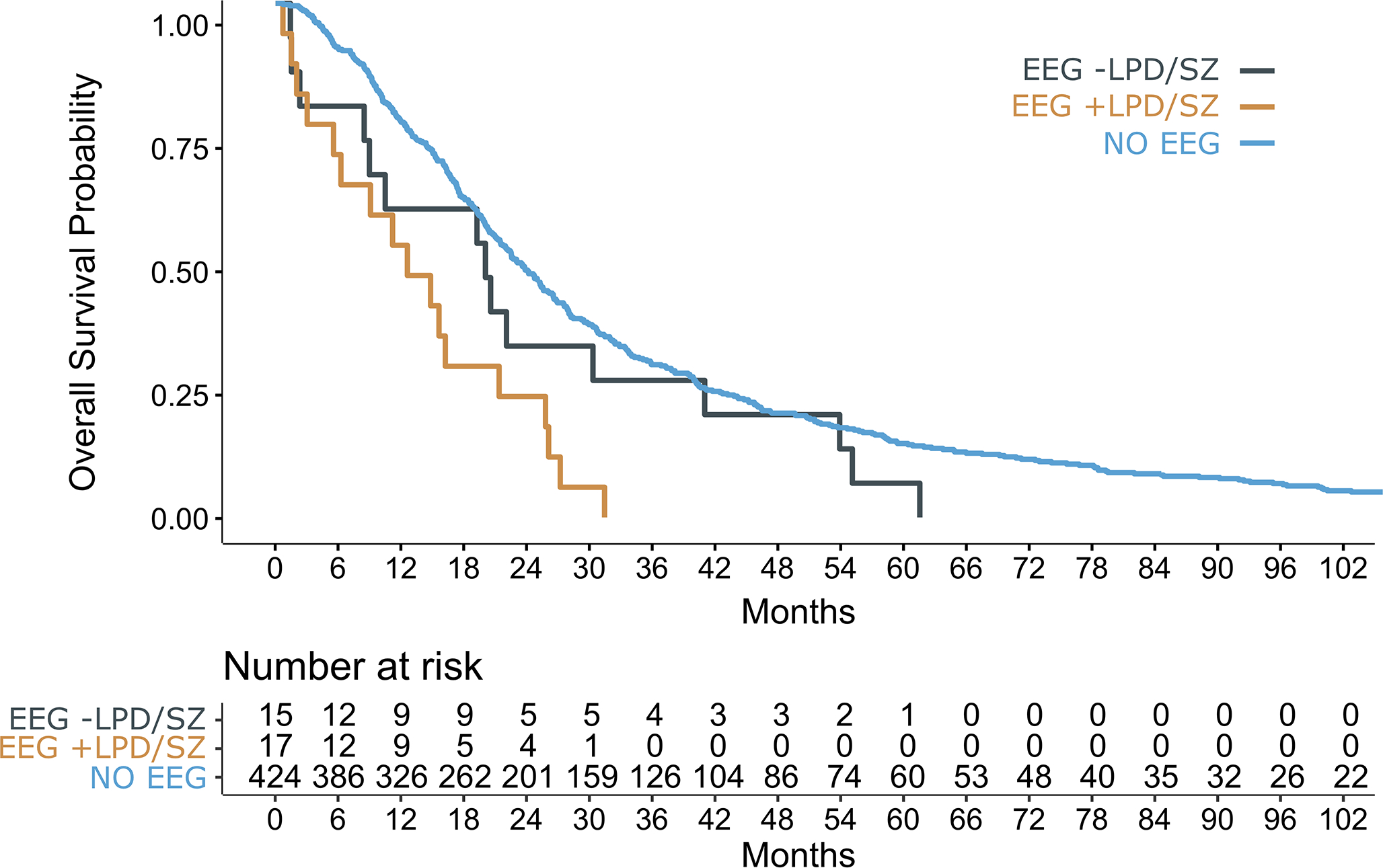
Survival curves for patients with IDH-WT glioma and peri-operative continuous EEG with LPD/SZ (orange), without LPD/SZ (gray), as well as an independent reference cohort without continuous EEG (blue). LPD/SZ, lateralized periodic discharges and/or electrographic seizures
To evaluate whether patients requiring cEEG had a worse prognosis due to the underlying clinical indication for cEEG monitoring, OS of patients with and without LPD/ES were compared to an internal reference cohort without cEEG. OS was decreased in patients with LPD/ES (X2=18.1, p<0.0001) compared to the reference cohort (median OS 22.8 [95% CI 20.8–25.2] months), whereas OS was similar in patients without LPD/ES (X2=1.7, p=0.193, Figure 3).
EEG Hyperexcitability Associated with PI3K-mTOR Pathway Hetereogeneity
Tumor somatic mutation variants in the PI3K-mTOR pathway were evaluated in relation to peri-operative cEEG hyperexcitability. Among patients with LPD/ES, 6/17 (35%) had at least one variant in MTOR, PIK3CA, PIK3C2B, and/or PIK3R1, compared to 1/15 (7%) without LPD/ES (p=0.088) and compared to 97/424 (23%) in the reference cohort without cEEG (p=0.246). The complete list of PI3K-mTOR variants is included in Supplementary Table 1.
Discussion
In this series of patients with newly diagnosed IDH-wildtype glioma, early EEG hyperexcitability was associated with decreased OS. These findings indicate the potential prognostic value of a clinical EEG biomarker of glioma aggressiveness prior to the initiation of chemoradiation.
EEG is not routinely required for the clinical management of seizures, and in many hospitals prolonged EEG monitoring may not be available. Therefore, as expected, cEEG data was available in only a minority of this cohort. Post-operative brain oscillatory activity measured by magnetoencephalography (MEG) has been associated with glioma survival outcomes [16, 17], although conflicting reports exist [18], which may be attributable to different glioma populations or methodologies studied. In contrast to using a quantitative oscillatory power metric of functional connectivity, hyperexcitability in this study reflected standardized EEG patterns on the ictal-interictal continuum and presumably corresponded to neuronal hyperactivity at the extreme of the spectrum [19].
While patients for whom cEEG is clinically indicated may hypothetically have more severe medical conditions, we found no difference in OS between patients without hyperexcitability on peri-operative cEEG and patients who did not undergo cEEG at any time. Furthermore, patients with and without EEG hyperexcitability had similar rates of clinical seizures at presentation, tumor characteristics, exposure to anti-seizure medications, and post-operative performance statuses. All patients received anti-seizure medications as clinically indicated based on clinical and electrographic seizure activity, and in long-term follow-up had the same medication burden regardless of the presence or absence of EEG hyperexcitability. Although pre-operative glioblastoma tumor size has a variable association with prognosis based on the specific imaging metrics evaluated [20–25], a prior quantitative analysis of morphological characteristics found that smaller tumor size was associated with seizures at presentation in high-grade gliomas [26]. All patients in the EEG cohort had supratentorial tumors with an overall similar distribution in location between cases with and without EEG hyperexcitability, with the majority localized to the temporal lobe. Focal epileptiform discharges on cEEG are commonly associated with brain tumors, with higher rates reported in temporal than frontal lobe tumors [27, 28]. Clinical seizures in high-grade gliomas have been previously associated with cortical involvement in the frontal, temporal, and parietal lobes [29–31]. Temporal lobe involvement is an independent risk factor for clinical seizures in low-grade gliomas [32], however to a much lesser extent in high-grade gliomas [26]. Overall, these factors argue against a significant confounding bias to explain the association between EEG hyperexcitability and OS.
In the absence of IDH mutations and controlling for other prognostic genetic features incorporated into the latest WHO tumor grading classification [11], it is likely that other genetic and molecular features contribute to the development of peritumoral hyperexcitability and glioma aggressiveness. A relative increase in prevalence of PI3K-mTOR variants were seen in patients with EEG hyperexcitability in this study. Activating mutations in the PI3K-mTOR pathway have been previously associated with peritumoral hyperexcitability, clinical seizure activity, and glioblastoma recurrence [33–36]. In animal models of glioblastoma, peritumoral neuronal activity promotes the release of soluble neuroligin-3, induction of PI3K-mTOR activity, and glioma cellular proliferation [9]. Peritumoral hyperexcitability by MEG has also been correlated with neuroligin-3 expression and associated with decreased progression-free survival [16]. These studies suggest the potential for multiple glioma variants to selectively influence neuronal activity and tumor growth in a bidirectional feedforward manner.
Limitations of this study included the small sample size of patients in the EEG cohort as well as the lack of epilepsy phenotyping in the larger reference cohort. As such, this study was not designed or powered to detect any potential impact of EEG hyperexcitability on survival independent from glioma progression. Furthermore, epileptiform activity on post-operative EEG may be attributable to cortical injury due to surgery or post-surgical hemorrhage and edema, although this risk factor was shared by all patients. Continuous EEG was performed as clinically indicated and only captured a select period in time. Given the small sample size, both pre-operative and post-operative cEEGs were evaluated together, however whether glioma-induced hyperexcitability is an enduring feature before and after tumor resection and later tumor treatments remains uncertain. Additional larger controlled studies are necessary to determine the mechanisms by which EEG hyperexcitability is associated with both epilepsy and oncologic outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Dana-Farber Cancer Institute Oncology Data Retrieval System (OncDRS) for the aggregation, management, and delivery of the clinical and operational research data used in this project. The content is solely the responsibility of the authors.
Funding:
This work was supported by the National Institutes of Health (NIH/NCI 2P50CA165962-06A1 Sub-Project 5140).
Footnotes
Competing Interests: The authors have no financial or proprietary interests in any material discussed in this article.
References
- 1.Avila EK, Chamberlain M, Schiff D, et al. (2017) Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro-Oncol 19:12–21. 10.1093/neuonc/now190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toledo M, Sarria-Estrada S, Quintana M, et al. (2015) Prognostic implications of epilepsy in glioblastomas. Clin Neurol Neurosurg 139:166–171. 10.1016/j.clineuro.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 3.Toledo M, Sarria-Estrada S, Quintana M, et al. (2017) Epileptic features and survival in glioblastomas presenting with seizures. Epilepsy Res 130:1–6. 10.1016/j.eplepsyres.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 4.Lote K, Stenwig AE, Skullerud K, Hirschberg H (1998) Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer Oxf Engl 1990 34:98–102. 10.1016/s0959-8049(97)00374-2 [DOI] [PubMed] [Google Scholar]
- 5.Berendsen S, Varkila M, Kroonen J, et al. (2016) Prognostic relevance of epilepsy at presentation in glioblastoma patients. Neuro-Oncol 18:700–706. 10.1093/neuonc/nov238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Judkins J, Thomas C, et al. (2017) Mutant IDH1 and seizures in patients with glioma. Neurology 88:1805–1813. 10.1212/WNL.0000000000003911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkataramani V, Tanev DI, Strahle C, et al. (2019) Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573:532–538. 10.1038/s41586-019-1564-x [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh HS, Morishita W, Geraghty AC, et al. (2019) Electrical and synaptic integration of glioma into neural circuits. Nature 573:539–545. 10.1038/s41586-019-1563-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatesh HS, Johung TB, Caretti V, et al. (2015) Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 161:803–816. 10.1016/j.cell.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touat M, Li YY, Boynton AN, et al. (2020) Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 580:517–523. 10.1038/s41586-020-2209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis DN, Perry A, Wesseling P, et al. (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncol 23:1231–1251. 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JW, LaRoche S, Choi H, et al. (2016) Development and Feasibility Testing of a Critical Care EEG Monitoring Database for Standardized Clinical Reporting and Multicenter Collaborative Research. J Clin Neurophysiol 33:133–140. 10.1097/WNP.0000000000000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch LJ, Fong MWK, Leitinger M, et al. (2021) American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol 38:1–29. 10.1097/WNP.0000000000000806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagle N, Berger MF, Davis MJ, et al. (2012) High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov 2:82–93. 10.1158/2159-8290.CD-11-0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia EP, Minkovsky A, Jia Y, et al. (2017) Validation of OncoPanel: A Targeted Next-Generation Sequencing Assay for the Detection of Somatic Variants in Cancer. Arch Pathol Lab Med 141:751–758. 10.5858/arpa.2016-0527-OA [DOI] [PubMed] [Google Scholar]
- 16.Derks J, Wesseling P, Carbo EWS, et al. (2018) Oscillatory brain activity associates with neuroligin-3 expression and predicts progression free survival in patients with diffuse glioma. J Neurooncol 140:403–412. 10.1007/s11060-018-2967-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belgers V, Numan T, Kulik SD, et al. (2020) Postoperative oscillatory brain activity as an add-on prognostic marker in diffuse glioma. J Neurooncol 147:49–58. 10.1007/s11060-019-03386-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Numan T, Kulik SD, Moraal B, et al. (2021) Non-invasively measured brain activity and radiological progression in diffuse glioma. Sci Rep 11:18990. 10.1038/s41598-021-97818-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schevon CA, Tobochnik S, Eissa T, et al. (2019) Multiscale recordings reveal the dynamic spatial structure of human seizures. Neurobiol Dis 127:303–311. 10.1016/j.nbd.2019.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliadis G, Kotoula V, Chatzisotiriou A, et al. (2012) Volumetric and MGMT parameters in glioblastoma patients: survival analysis. BMC Cancer 12:3. 10.1186/1471-2407-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henker C, Kriesen T, Glass Ä, et al. (2017) Volumetric quantification of glioblastoma: experiences with different measurement techniques and impact on survival. J Neurooncol 135:391–402. 10.1007/s11060-017-2587-5 [DOI] [PubMed] [Google Scholar]
- 22.Hammoud MA, Sawaya R, Shi W, et al. (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol 27:65–73. 10.1007/BF00146086 [DOI] [PubMed] [Google Scholar]
- 23.Pope WB, Sayre J, Perlina A, et al. (2005) MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol 26:2466–2474 [PMC free article] [PubMed] [Google Scholar]
- 24.Li W-B, Tang K, Chen Q, et al. (2012) MRI manifestions correlate with survival of glioblastoma multiforme patients. Cancer Biol Med 9:120–123. 10.3969/j.issn.2095-3941.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palpan Flores A, Vivancos Sanchez C, Roda JM, et al. (2020) Assessment of Pre-operative Measurements of Tumor Size by MRI Methods as Survival Predictors in Wild Type IDH Glioblastoma. Front Oncol 10:1662. 10.3389/fonc.2020.01662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JW, Wen PY, Hurwitz S, et al. (2010) Morphological characteristics of brain tumors causing seizures. Arch Neurol 67:336–342. 10.1001/archneurol.2010.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaatreh MM, Firlik KS, Spencer DD, Spencer SS (2003) Temporal lobe tumoral epilepsy: characteristics and predictors of surgical outcome. Neurology 61:636–641. 10.1212/01.wnl.0000079374.78589.1b [DOI] [PubMed] [Google Scholar]
- 28.Zaatreh MM, Spencer DD, Thompson JL, et al. (2002) Frontal lobe tumoral epilepsy: clinical, neurophysiologic features and predictors of surgical outcome. Epilepsia 43:727–733. 10.1046/j.1528-1157.2002.39501.x [DOI] [PubMed] [Google Scholar]
- 29.Chaichana KL, Parker SL, Olivi A, Quiñones-Hinojosa A (2009) Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg 111:282–292. 10.3171/2009.2.JNS081132 [DOI] [PubMed] [Google Scholar]
- 30.van Breemen MSM, Wilms EB, Vecht CJ (2007) Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 6:421–430. 10.1016/S1474-4422(07)70103-5 [DOI] [PubMed] [Google Scholar]
- 31.Skardelly M, Brendle E, Noell S, et al. (2015) Predictors of preoperative and early postoperative seizures in patients with intra-axial primary and metastatic brain tumors: A retrospective observational single center study. Ann Neurol 78:917–928. 10.1002/ana.24522 [DOI] [PubMed] [Google Scholar]
- 32.Chang EF, Potts MB, Keles GE, et al. (2008) Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg 108:227–235. 10.3171/JNS/2008/108/2/0227 [DOI] [PubMed] [Google Scholar]
- 33.Yu K, Lin C-CJ, Hatcher A, et al. (2020) PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 578:166–171. 10.1038/s41586-020-1952-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka S, Batchelor TT, Iafrate AJ, et al. (2019) PIK3CA activating mutations are associated with more disseminated disease at presentation and earlier recurrence in glioblastoma. Acta Neuropathol Commun 7:66. 10.1186/s40478-019-0720-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobochnik S, Pisano W, Lapinskas E, et al. (2021) Effect of PIK3CA variants on glioma-related epilepsy and response to treatment. Epilepsy Res 175:106681. 10.1016/j.eplepsyres.2021.106681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Y, Xiang W, Yanhui L, et al. (2017) Activation of the mTOR signaling pathway in peritumoral tissues can cause glioma-associated seizures. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 38:61–66. 10.1007/s10072-016-2706-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


