Abstract
Background:
Improved predictive models are needed in lung transplantation in the setting of a proposed allocation system that incorporates longer-term post-transplant survival in the United States. Allocation systems require accurate mortality predictions to justly allocate organs.
Methods:
Utilizing the United Network for Organ Sharing database (2005–2017), we fit models to predict 1-year mortality based on the Lung Allocation Score (LAS), the Chan, et al., 2019 model, a novel “clinician” model (a priori clinician selection of pre-transplant covariates), and two machine learning models (LASSO and Random Forests) for predicting 1-year and 3-year post-transplant mortality. We compared predictive accuracy among models. We evaluated the calibration of models by comparing average predicted probability versus observed outcome per decile. We repeated analyses fit for 3-year mortality, disease category, including donor covariates, and LAS era.
Results:
The area under the cure (AUC) for all models was low, ranging from 0.55 to 0.62. All exhibited reasonable negative predictive values (0.87–0.90), but the positive predictive value for was poor (all PPV <0.25). Evaluating LAS calibration found 1-year post-transplant estimates consistently overestimated risk of mortality, with greater differences in higher deciles. LASSO, Random Forests, and clinician models showed no improvement when evaluated by disease category or with the addition of donor covariates and performed worse for 3-year outcomes.
Conclusions:
The LAS overestimated patients’ risk of post-transplant death, thus underestimating transplant benefit in the sickest candidates. Novel models based on pre-transplant recipient covariates failed to improve prediction. There should be wariness in post-transplant survival predictions from available models.
Introduction
Lung transplant (LTx) can improve quality of life and increase survival for patients with advanced lung disease. However, many potential candidates for LTx die before organs become available, with waiting list mortality (14.6%) the highest of any solid organ1 and donor supply being the rate limiting resource in providing this effective therapy.2
Greater than 60% of LTx worldwide3 utilize the Lung Allocation Score (LAS).4 In designing the LAS, quantitative variables from the Organ Procurement and Transplantation Network (OPTN) Scientific Registry of Transplant Recipients (SRTR) database were selected to create disease-specific models to predict 1-year mortality without transplant, i.e. waitlist mortality, and 1-year post-transplant survival.5 The LAS is the difference between predicted waitlist survival (weighted double) and post-transplant survival, net transplant benefit,6 normalized to a range from 0–100. Lungs are prioritized to those with higher scores. Its 2005 implementation in the United States resulted in decreased waiting list mortality, increased LTx rates, and an initial small improvement in 1-year post-transplant survival.7
Despite the improvements seen in the LAS era, the score’s ability to allocate lungs justly remains highly dependent on its performance in predicting pre- and post-transplant outcomes. This, and an interest in broader organ sharing, have led to re-examination of the current allocation system. Recent literature suggests a need for improved predictive models. A 2021 United Network for Organ Sharing (UNOS) proposal suggested transition towards utilizing longer-term survival in order to better reflect meaningful transplant benefit.8,9 In this study, our primary aim was to assess the accuracy of the LAS and other models for predicting 1-year post-transplant mortality. In addition to refitting the Houston Methodist model by Chan, et al.,10 we also developed and validated novel models: a “clinician” model based on a priori selection of covariates by transplant pulmonology experts and two statistical machine learning models based on Least Absolute Shrinkage and Selection Operator (LASSO) and Random Forests, respectively. Finally, at a time when the United States allocation system transitions towards estimating longer-term survival,9,11 our secondary aim was to evaluate the predictive performance of all models by considering 3-year post-transplant outcomes, fit by disease category, with inclusion of donor covariates, or by LAS era.
Materials and Methods
Study Design and Participants
This retrospective cohort study included adult patients (≥18-years-old) from the UNOS dataset provided by the OPTN who had their first LTx between May 1, 2005, and May 1, 2017. UNOS follow-up data was available through mid-June, 2020. Patients lost to follow-up were designated as such in the UNOS dataset (n=309, “UNOS PX_STAT”) and were removed from our analyses. The University of Washington IRB approved this study (#9226). This study complied with the ISHLT ethics statement.
Variables of Interest
Our initial models considered all pre-transplant recipient covariates from the UNOS dataset. A complete list of UNOS variables is included in the online supplement (Table S-1). For the clinician model, 27 predictors of interest were selected a priori by transplant pulmonology experts (co-authors KJR, SGK, and CAM). These covariates included transplant year, forced expiratory volume in one second (FEV1) percent predicted, forced vital capacity (FVC) percent predicted, six minute walk (SMW) distance, oxygen requirement, diagnosis, mean pulmonary arterial pressure, arterial partial pressure of carbon dioxide (PCO2), body mass index (BMI), albumin, total bilirubin, creatinine, dialysis, mechanical ventilation at time of transplant, hospitalization status at time of transplant, sex category, education, diabetes, waitlist time, cigarette use, functional status, insurance, cytomegalovirus (CMV) status, glomerular filtration rate (GFR), steroid usage, bilateral versus single LTx, and multi-organ transplant (Table 2). Age was removed from the clinician model due to collinearity with diagnosis. We then utilized the statistical machine learning approaches, LASSO12 and Random Forests,13 to identify covariates of significance from the same UNOS dataset (Table 3 and Figure 3).Statistical significance for a priori selected covariates in the clinician model was adjusted for multiple comparisons using Bonferroni correction of the p-values. Analyses were performed in R version 3.6.3.
Table 2:
Coefficients from the Clinician Model
| Unit | Odds Ratio (95% CI) | Raw P-value | Bonferroni P-value | |
|---|---|---|---|---|
| Female | 0.77 (0.70, 0.85) | <0.01 | <0.01 | |
| Education | High School (Baseline) | |||
| None | 1.25 (0.42, 3.76) | 0.73 | 1.00 | |
| Grade School | 0.93 (0.70, 1.25) | 0.64 | 1.00 | |
| Some College | 0.90 (0.80, 1.01) | 0.08 | 1.00 | |
| College Graduate | 0.91 (0.80, 1.03) | 0.14 | 1.00 | |
| Post Graduate | 1.02 (0.87, 1.20) | 0.81 | 1.00 | |
| Unknown | 1.02 (0.86, 1.21) | 0.82 | 1.00 | |
| Insurance | Private (Baseline) | |||
| None | 0.70 (0.36, 1.37) | 0.30 | 1.00 | |
| Medicare | 1.11 (1.01, 1.23) | 0.03 | 1.00 | |
| Medicaid | 0.88 (0.72, 1.07) | 0.19 | 1.00 | |
| Other | 0.79 (0.60, 1.05) | 0.10 | 1.00 | |
| BMI | Normal (18.5–25) (Baseline) | |||
| Low (<18.5) | 1.45 (1.22, 1.73) | <0.01 | <0.01 | |
| High (>25 – 30) | 1.09 (0.98, 1.22) | 0.11 | 1.00 | |
| Obese (>30) | 1.02 (0.89, 1.17) | 0.74 | 1.00 | |
| Diagnosis | COPD (Baseline) | |||
| CF | 0.70 (0.56, 0.87) | <0.01 | 0.08 | |
| IPF | 1.03 (0.87, 1.22) | 0.73 | 1.00 | |
| Other | 1.08 (0.91, 1.27) | 0.37 | 1.00 | |
| Functional Status | No Assistance (Baseline) | |||
| Some Assistance | 1.18 (0.93, 1.51) | 0.18 | 1.00 | |
| Total Assistance | 0.99 (0.72, 1.35) | 0.95 | 1.00 | |
| Unknown | 1.58 (1.22, 2.05 | <0.01 | 0.02 | |
| Medical Condition | Not Hospitalized (Baseline) | |||
| Hospitalized | 1.32 (1.13, 1.54) | <0.01 | 0.03 | |
| ICU | 2.00 (1.67, 2.38) | <0.01 | <0.01 | |
| Dialysis Prior to LTx | No (Baseline) | |||
| Yes | 2.12 (1.29, 3.48) | <0.01 | 0.15 | |
| Unknown | 0.85 (0.76, 0.94) | <0.01 | 0.13 | |
| Steroids | No (Baseline) | |||
| Yes | 1.06 (0.97, 1.16) | 0.22 | 1.00 | |
| Unknown | 1.03 (0.77, 1.37) | 0.86 | 1.00 | |
| Cigarette Use | No (Baseline) | |||
| Yes | 0.91 (0.82, 1.01) | 0.09 | 1.00 | |
| Unknown | 1.05 (0.75, 1.45) | 0.79 | 1.00 | |
| CMV | Negative (Baseline) | |||
| Positive | 1.00 (0.91, 1.09) | 0.94 | 1.00 | |
| Unknown | 1.07 (0.85, 1.33) | 0.58 | 1.00 | |
| Ventilator Use | 1.38 (1.14, 1.68) | <0.01 | 0.05 | |
| Wait List Time | 100 days | 1.02 (1.00, 1.03) | 0.02 | 0.12 |
| Multi Organ Transplant | 1.49 (0.72, 3.08) | 0.29 | 1.00 | |
| O2 Used | 1L | 1.00 (0.99, 1.01) | 0.73 | 1.00 |
| FEV1 Percent Predicted | 5% | 1.00(0.98, 1.02) | 0.76 | 1.00 |
| FVC Percent Predicted | 5% | 0.99 (0.97, 1.00) | 0.13 | 1.00 |
| Six Minute Walk | 100ft | 0.98 (0.97, 1.00) | 0.01 | 0.41 |
| GFR | 5 mL/min | 0.97 (0.96, 0.98) | <0.01 | 0.01 |
| LAS | 1 unit | 1.00 (0.99, 1.00) | 0.33 | 1.00 |
| Mean Pulmonary Arterial Pressure | 1 mmHg | 1.01 (1.00, 1.01) | 0.02 | 1.00 |
| Serum Albumin | 1 g/dL | 0.81 (0.75, 0.88) | <0.01 | <0.01 |
| Total Bilirubin | 1 g/dL | 1.08 (1.04, 1.11) | <0.01 | <0.01 |
| Serum Creatinine | 1 mg/dL | 1.02 (0.85, 1.21) | 0.86 | 1.00 |
| PCO2 | 1 mmHg | 1.00 (0.99, 1.00) | 0.01 | 0.64 |
The risk score from the clinician model can be calculated by taking the natural log of the odds ratios and summing over each row to get a composite score for each patient.
Table 3:
Coefficients from the LASSO Model
| Unit | Odds Ratio | |
|---|---|---|
| Female | 0.93 | |
| Ventilator | 1.02 | |
| Age | 1 year | 1.01 |
| Six Minute Walk | 100 ft | 0.99 |
| Medical Condition | Not Hospitalized (Baseline) | |
| Hospitalized | 1.28 | |
| ICU | 1.58 | |
| GFR | 5 | 0.96 |
| Serum Albumin | 1 g/dL | 0.77 |
| Total Bilirubin | 1 g/dL | 1.10 |
| Serum Creatinine | 1 mg/dL | 1.20 |
| PCO2 | Normal (Baseline) | |
| Low | 1.00 | |
| High | 0.93 | |
| Waitlist Time | 100 days | 1.35 |
| Dialysis Prior to Transplant | No (Baseline) | |
| Yes | 1.00 | |
| Unknown | 0.92 | |
| EBV | Negative (Baseline) | |
| Positive | 0.98 | |
| Unknown | 1.00 | |
| BMI | Normal (Baseline) | |
| Low | 1.04 | |
| High | 1.04 | |
| Obese | 1.04 | |
| Steroid | No (Baseline) | |
| Yes | 1.02 | |
| Unknown | 1.02 | |
| Functional Status | No Assistance (Baseline) | |
| Some Assistance | 1.00 | |
| Total Assistance | 1.00 | |
| Unknown | 1.25 |
The risk score from the LASSO model can be calculated by taking the natural log of the odds ratios and summing over each row to get a composite score for each patient.
Figure 3:
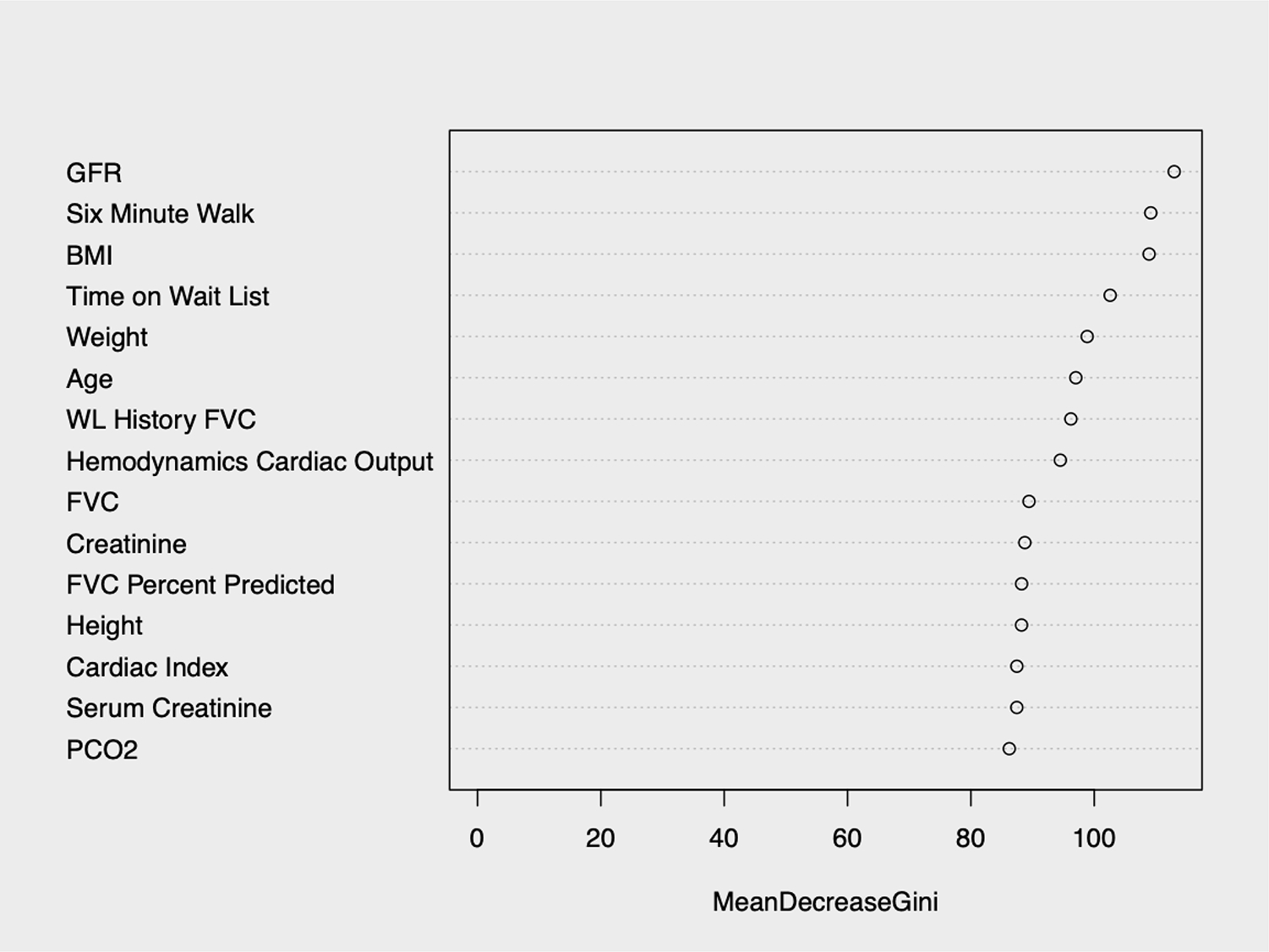
The 15 most important variables (by mean decrease in Gini index) from the Random Forests model
Outcomes of Interest
In designing our new models, we used death or re-transplant within the indicated timeframe as the outcome of interest.
Model Development
A random 85% of patients were selected to form a development set to fit the clinician, LASSO, and Random Forests models. The remaining 15% formed the validation set. We felt this split balanced the tradeoff between model development and evaluation precision. We fit logistic regression models on the development set with covariates chosen using the methods denoted above. Additionally, we evaluated the Chan, et al.,10 model, which used patients age, diagnosis, BMI, diabetes, total bilirubin, GFR, cardiac index, and SMW to develop a risk score.
For binary and categorical variables, patients with missing measurements were grouped into their own category. Missing measurements for continuous variables were estimated using mean imputation.
Primary Analysis
On the validation set, we compared the clinician, LASSO, and Random Forests models to the LAS at time of transplant and the Chan, et al,10 model. Prediction performance of the five different models was assessed by the area under the receiver-operator characteristic (ROC) curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) using the cutoff value that maximized combined sensitivity and specificity. Calibration of each model was evaluated by calibration slope14 using logistic regression with the predicted probability as the independent variable and the observed outcome as the dependent variable.
Secondary Analyses
We next explored various methods to improve the predictive performance of the prognostic models by repeating the primary analysis: 1. fitting separate models on each disease [cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and other] individually; 2. including donor covariates; and 3. evaluating 3-year instead of 1-year post-transplant survival.
To account for potential changes over time, prediction performance of the five models was also analyzed by LAS era, 2005–2010, 2011–2014, and 2015–2017. Calibration of the LASSO, Random Forests, and clinician models was repeated for each LAS era as well. Notably, the patient cohort to determine the LAS was updated in 201215 and the LAS equation was updated in 2015.16
Results
Cohort Characteristics
The UNOS dataset included 19,900 adults who received their first LTx between May 1, 2005, and May 1, 2017. Of the total cohort, 2,765 (14%) died and 171 (1%) were re-transplanted within one year. Compared to those who survived at least one year, those who died or were re-transplanted were more likely to have been male, have only a single lung transplanted, use pre-transplant mechanical ventilation, have had dialysis prior to LTx, and used pre-transplant steroids (Table 1). They were also more likely to be transplanted for IPF, hospitalized or in the intensive care unit, have high or low BMI, or required total assistance. Patients who died were older and had higher LAS, oxygen requirements, mean pulmonary arterial pressure, total bilirubin, and serum creatinine but lower SMW distance, serum albumin, and GFR.
Table 1:
Descriptive Statistics
| Variable | Unit | Alive without re-transplant after 1 years (n=16,964) | Death or re-transplant within 1 years (n=2,936) | p-value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | Raw | Bonferroni | ||
| Gender | Female | 7,019 | 41% | 1,090 | 37% | <0.001 | <0.001 |
| Mechanical Ventilation | Yes | 930 | 5% | 314 | 11% | <0.001 | <0.001 |
| Number of Lungs | Both Lungs | 11,604 | 68% | 1,884 | 64% | <0.001 | 1.9e-<0.001 |
| Multi-Organ Transplant | More than one | 50 | <1% | 15 | <1% | 0.085 | >0.99 |
| Medical Condition | ICU | 1,171 | 9% | 831 | 13% | <0.001 | <0.001 |
| Hospitalized | 1,148 | 8% | 595 | 9% | |||
| Not Hospitalized | 11,293 | 83% | 4,862 | 77% | |||
| Diabetes | None | 11,027 | 81% | 4,972 | 79% | 0.3 | >0.99 |
| Type I | 431 | 3% | 240 | 4% | |||
| Type II | 1,551 | 11% | 789 | 13% | |||
| Other | 531 | 4% | 242 | 4% | |||
| Unknown | 72 | 1% | 45 | 1% | |||
| Dialysis prior to LTx | Yes | 52 | <1% | 46 | 1% | <0.001 | <0.001 |
| No | 9,399 | 69% | 4,621 | 73% | |||
| Unknown | 4,161 | 31% | 1,621 | 26% | |||
| Cigarette Use | Yes | 8,053 | 59% | 3,722 | 59% | 0.86 | >0.99 |
| No | 5,252 | 39% | 2,400 | 38% | |||
| Unknown | 307 | 2% | 166 | 3% | |||
| Steroid | Yes | 6,117 | 45% | 3,051 | 49% | <0.001 | <0.001 |
| No | 7,212 | 53% | 3,104 | 49% | |||
| Unknown | 283 | 2% | 133 | 2% | |||
| CMV Status | Positive | 7,415 | 54% | 3,437 | 55% | 0.23 | >0.99 |
| Negative | 5,673 | 42% | 2,603 | 41% | |||
| Unknown | 524 | 4% | 248 | 4% | |||
| Functional Status | No assistance | 632 | 5% | 244 | 4% | <0.001 | <0.001 |
| Some assistance | 9,282 | 68% | 3,936 | 63% | |||
| Total Assistance | 744 | 5% | 443 | 7% | |||
| Unknown | 2,954 | 22% | 1,665 | 26% | |||
| Race | White | 11,316 | 83% | 5,239 | 83% | 0.86 | >0.99 |
| Black | 1,184 | 9% | 552 | 9% | |||
| Asian | 212 | 2% | 90 | 1% | |||
| Pacific Islander | 10 | <1% | 7 | 1% | |||
| American Indian | 46 | <1% | 16 | <1% | |||
| Hispanic | 804 | 6% | 370 | 6% | |||
| Multi-Racial | 46 | <1% | 17 | <1% | |||
| Insurance | Private | 7,250 | 53% | 3,198 | 51% | 0.12 | >0.99 |
| Medicaid | 873 | 6% | 449 | 7% | |||
| Medicare | 4,970 | 37% | 2,448 | 39% | |||
| Other | 447 | 3% | 165 | 2% | |||
| None | 72 | <1% | 28 | <1% | |||
| Education | No High School | 337 | 2% | 161 | 3% | 0.2 | >0.99 |
| High School or GED | 4,936 | 36% | 2,349 | 37% | |||
| Attended College | 3,446 | 25% | 1,575 | 25% | |||
| Associate/Bachelor | 2,742 | 20% | 1,111 | 18% | |||
| Post-College Graduate | 1,217 | 9% | 589 | 9% | |||
| Unknown | 918 | 7% | 492 | 8% | |||
| Disease | CF | 1,697 | 12% | 702 | 11% | <0.001 | <0.001 |
| COPD | 4,028 | 30% | 1,723 | 27% | |||
| IPF | 5,201 | 38% | 2,588 | 41% | |||
| Other | 2,686 | 20% | 1,275 | 20% | |||
| BMI | Very Low (<17) | 317 | 2% | 206 | 3% | <0.001 | 0.016 |
| Low (17–18.5) | 733 | 5% | 378 | 6% | |||
| Normal (18.5–25) | 5,359 | 39% | 2,252 | 36% | |||
| Overweight (25–30) | 5,055 | 37% | 2,366 | 38% | |||
| Obese (>30) | 2,128 | 16% | 1,076 | 17% | |||
| Unknown | 20 | <1% | 10 | <1% | |||
| N | Mean (SD) | N | Mean (SD) | ||||
| Age | Years | 16,964 | 55.1 (13.1) | 2,936 | 56.5 (13.1) | <0.001 | <0.001 |
| Lung Allocation Score | 16,102 | 41.5 (14.6) | 2,727 | 43.7 (16.5) | <0.001 | <0.001 | |
| FEV1 percent predicted | 16,687 | 38.4 (20.9) | 2,853 | 39.7 (20.2) | 0.002 | 0.052 | |
| FVC percent predicted | 16,748 | 48.6 (17.5) | 2,865 | 47.96 (18.0) | 0.093 | >0.99 | |
| Six Minute Walk | 16,238 | 812.5 (407.0) | 2,764 | 764.1 (425.0) | <0.001 | <0.001 | |
| Pulmonary Arterial Pressure | 15,577 | 27.6 (10.2) | 2,652 | 28.5 (11.2) | <0.001 | 0.004 | |
| PCO2 | 15,064 | 47.9 (13.6) | 2,534 | 47.2 (14.1) | 0.017 | 0.49 | |
| Serum Alb | 11.542 | 3.9 (0.6) | 2,118 | 3.8 (0.7) | <0.001 | <0.001 | |
| Total Bilirubin | 16,764 | 0.61 (0.91) | 2,892 | 0.78 (1.70) | <0.001 | <0.001 | |
| Serum Creatinine | 16,934 | 0.85 (0.43) | 2,928 | 0.90 (0.43) | <0.001 | <0.001 | |
| Time on Waiting List | Days | 16,964 | 196.1 (368.0) | 2,936 | 205.3 (409.0) | 0.25 | >0.99 |
| Oxygen Requirement | 16,521 | 5.1 (4.8) | 2,841 | 5.8 (5.5) | <0.001 | <0.001 | |
| GFR | 16,964 | 93.4 (23.2) | 2,936 | 89.6 (26.0) | <0.001 | <0.001 | |
Differences between binary variables was tested using a test of proportions. For categorical variables, the difference in distributions was tested using a chi-square test. For continuous variables, the difference in means was tested by a t-test. We report both the p-value for each test individually (Raw), and the Bonferroni adjusted p-value (Bonferroni).
Assessment of Diagnostic Accuracy in the Validation Cohort
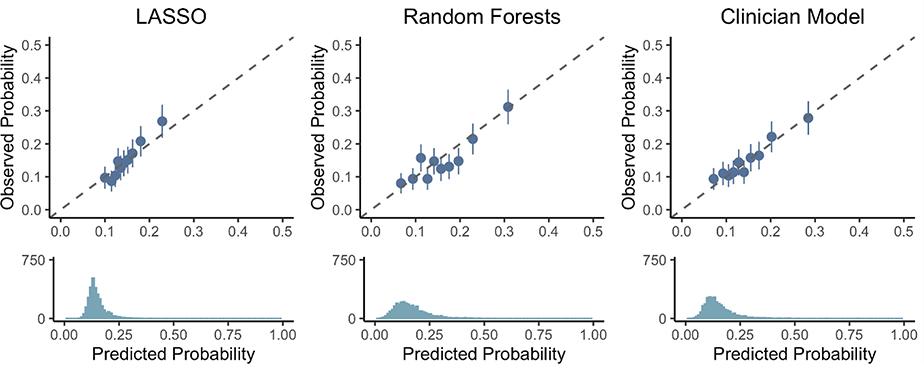
While the clinician and Random Forests models showed significant improvements in AUC over the LAS (Figure 1), the AUC for all models were low, ranging from 0.55 to 0.62. All exhibited reasonable NPV (0.87–0.90), but the PPV for was poor (all PPV <0.25). Calibration plots (Figure 2) for the clinician and Random Forests models suggested that they can identify those at the highest and lowest risks of 1-year death or re-transplant with reasonable calibration, but do not discriminate patients well in the middle quantiles. The calibration slope for the LASSO model (1.45, 95% CI [1.10, 1.80]) indicated poor calibration, where risk is underestimated for high-risk patients and overestimated for low-risk patients. No significant evidence for poor calibration was seen among the clinician (0.85, 95% CI [0.67, 1.10]) and the Random Forests models (0.96, 95% CI [0.76, 1.16]).
Figure 1:
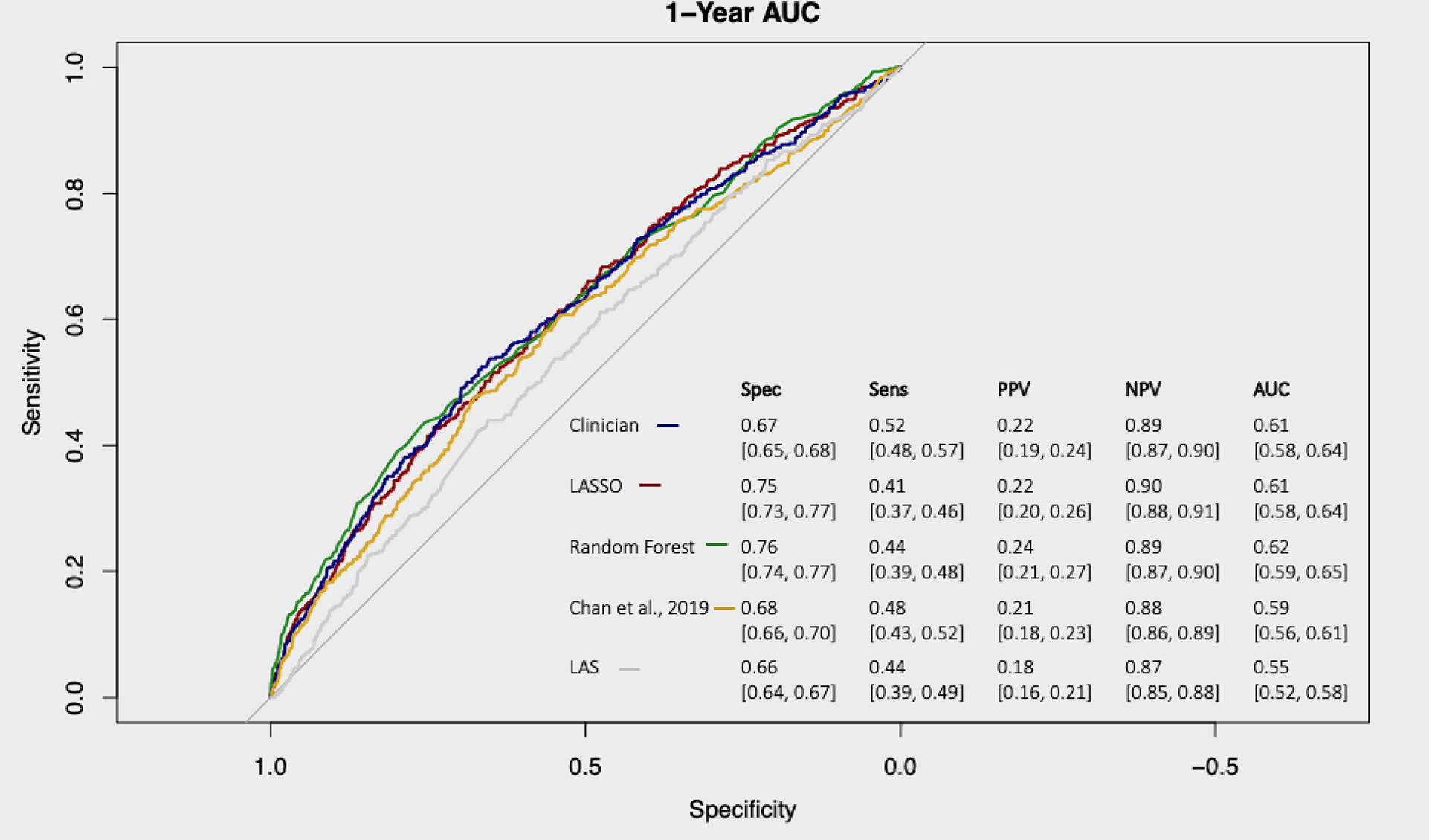
1-year survival receiver-operator curves for the LASSO (red), Random Forests (green), clinician (blue), Chan, et al.,10 (orange), and LAS (grey). Specificity (Spec), sensitivity (Sens), positive predictive value (PPV), and negative predictive value (NPV) for each model at cut-points selected to maximize the total sensitivity and specificity for each model are displayed in the table.
Figure 2:
Calibration of the LASSO, Random Forests, and clinician models at predicting 1-year survival. Patients are sorted into deciles, and each dot represents one decile. The x-axis represents the average predicted probability of death or re-transplant over each decile, while the y-axis shows the observed proportions (with standard errors) of death or re-transplant in each decile. The dotted line represents ‘perfect calibration’, where the predicted probabilities of death match the observed percentages. The density plot below each scatter plot shows the distribution of predicted probabilities of death or re-transplant for each model.
Among the 27 variables considered in the clinician model, only gender, medical condition (ICU, hospitalized, or not hospitalized), BMI, GFR, serum albumin, and total bilirubin were found to be significantly associated with death or re-transplant in the multivariable model (Table 2). The LASSO model, which considered all variables available in UNOS, selected the same six variables along with age, mechanical ventilation, SMW distance, PCO2, waitlist time, dialysis prior to LTx, EBV, steroid usage, functional status, and serum creatinine (Table 3). Random Forests, which allows for non-linearity and interactions between covariates, identified GFR, SMW distance, and BMI to be most important in predicting death or re-transplant along with smaller importance for time on waitlist, weight, age, FVC, hemodynamics cardiac (Figure 3).
The LAS Post-Transplant Survival Measurement
LAS post-transplant survival measures were available for patients who underwent LTx after February 2015, of which there were 3,964 in the training set and 706 in the validation set. While there was no statistically significant evidence for poor calibration slopes among LASSO (1.27, 95% CI [0.29, 2.26]), clinician (0.94, 95% CI [0.35,1.53]), and Random Forests (0.90, 95% CI [0.38, 1.43]) models, the confidence intervals were wide indicating large uncertainty on their accuracy (Figure 4). The calibration slope for the LAS post-transplant survival measure (0.38, 95% CI [0.03, 0.73]) indicated poor calibration, with the LAS overestimating the risk of post-transplant mortality (Figure 4). Evaluating the LAS post-transplant survival at 3-, 6-, 9-, and 12-months demonstrated that the risk of death was overestimated at each interval (Figure 5).
Figure 4:
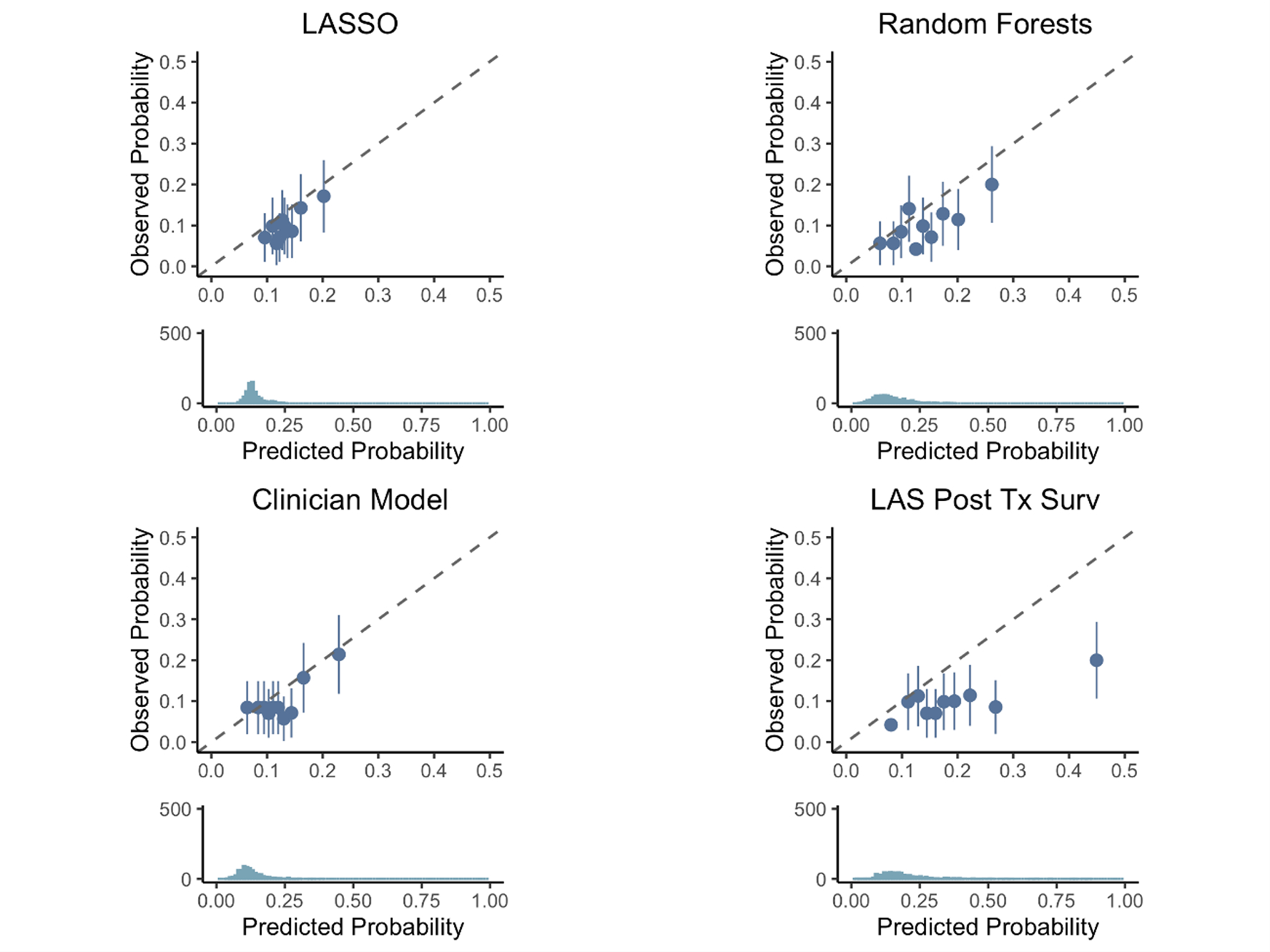
Calibration of the LASSO model (top left), Random Forests (top right), clinician model (bottom left), and LAS Post Transplant Survival Probability (bottom right) at predicting 1-year survival among patients who were transplanted from 2015–2017 with an available LAS post-transplant survival measure. Patients are sorted into deciles, and each dot represents one decile. The x-axis represents the average predicted probability of death or re-transplant over each decile, while the y-axis shows the observed proportions (with standard errors) of death or re-transplant in each decile. The dotted line represents ‘perfect calibration’, where the predicted probabilities of death match the observed percentages. The density plot below each scatter plot shows the distribution of predicted probabilities of death or re-transplant for each model.
Figure 5:
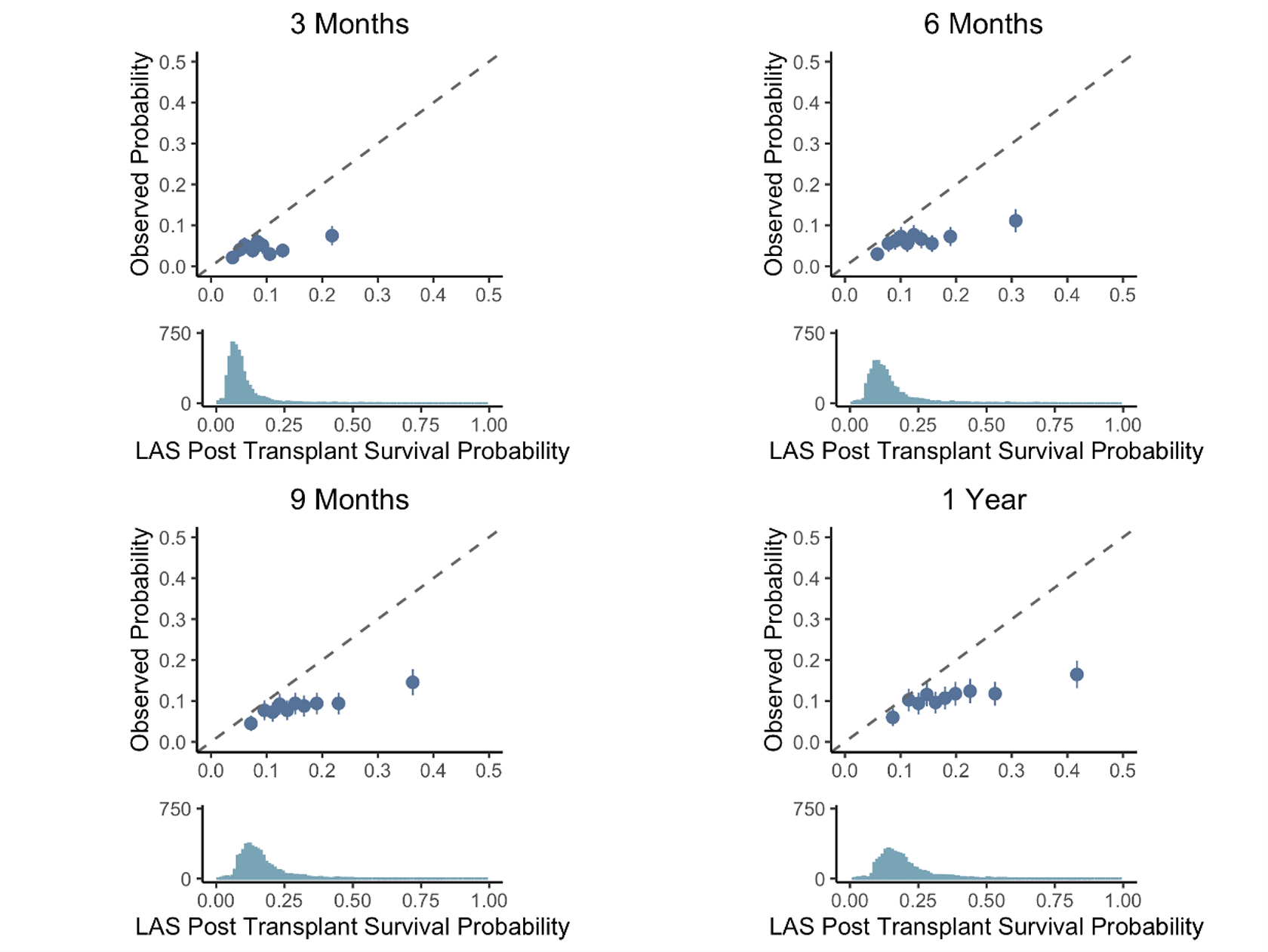
Calibration of the LAS Post Transplant Survival Probability at predicting 3-month (top left), 6-month (top right), 9-month (bottom left), and 1-year (bottom right) survival. Patients are sorted into deciles, and each dot represents one decile. The x-axis represents the average predicted probability of death or re-transplant over each decile, while the y-axis shows the observed proportions (with standard errors) of death or re-transplant in each decile. The dotted line represents ‘perfect calibration’, where the predicted probabilities of death match the observed percentages. The density plot below each scatter plot shows the distribution of predicted probabilities of death or re-transplant for each model.
Secondary Analysis – Disease Specific Models, Donor Covariates, Long Term (3-year) Survival, and LAS Era
The LASSO and Random Forests models re-fit on disease-specific subgroups (CF, IPF, COPD, and other) separately did not show any improvement in prediction accuracy (Figure S-1). No significant improvement in AUC was observed using disease specific models rather than fitting a single model on all patients. Adding donor covariates to the LASSO and Random Forests models did not improve AUC over using recipient covariates alone (Figures S-2 and S-3). LASSO, Random Forests, and clinician models re-fit using 3-year survival instead of 1-year survival demonstrated that longer term survival is more difficult to predict than shorter term survival, and every model considered had lower AUC on 3-year predictions than 1-year predictions (Figure S-4).
There were no differences in predictive accuracy at 1-year by LAS era: 2005–2010, 2011–2014, and 2015–2017 (Figure S-5). Calibration plots for the LASSO, Random Forests, and clinician models for each LAS era (Figure S-6) discriminated only those at the highest and lowest risks of 1-year death or re-transplant with reasonable calibration in the 2005–2010 era, but not in the 2011–2014 or 2015–2017 era.
Discussion
In this large retrospective analysis, we demonstrated that the LAS and several other models lacked a meaningful ability to accurately predict post-transplant mortality using pre-transplant covariates. The LAS overestimated patients’ risk of death (particularly for those in the highest deciles of LAS) and thus may disadvantage some waitlisted patients. Machine learning models, disease-specific models, and models with donor characteristics did not improve predictive accuracy. Because of the importance of longer-term survival after LTx, in keeping with proposed UNOS policy changes, we assessed 3-year survival models, but found the predictive accuracy was even worse than that seen in the 1-year models. Several variables individually were associated with re-transplant or death within 1-year. Unfortunately, aggregate models designed by expert selection or machine learning, even when incorporating variables with significance when isolated, were unable to meaningfully predict post-transplant mortality. The available covariates do not capture the data needed to do so. These findings are disappointing, but very relevant as the US attempts to revise its lung allocation system with a focus on prioritizing post-transplant survival.
Our findings confirm an earlier study by Gries, et al.,17 who used an ISHLT dataset and demonstrated that a model based on pre-transplant covariates poorly predicted 1-year or 5-year survival (AUC 0.553 and 0.591, respectively), the LAS had poor predictive ability at 1-year and 5-years (AUC 0.58 and 0.566, respectively), and there was no improvement when fitting these models for individual disease groups.17
In the 2019 study by Chan, et al.,10 the Houston Methodist model was predictive of 1-year mortality and was able to designate patients into risk categories, but we were unable to replicate these findings in our larger, more contemporary dataset. The Houston Methodist model was designed by Chan, et al.,10 using the UNOS dataset, containing 10533 patients who underwent LTx between 1994–2014, to identify and randomly cohort the 633 patients who underwent LTx at Houston Methodist Hospital into equal development and validation cohorts with which to form a predictive model. The Chan, et al.,10 model had an AUC for 1-year mortality of 0.74 and 0.67 for the development cohort and validation cohort, respectively. On our attempt to recreate the Chan, et al.,10 model within our development cohort, we found an AUC for 1-year mortality of only 0.59. The difference in populations and size of our cohorts may account for the difference in AUC of the original model to our recreation. Chan, et al.,10 also found that the LAS had a lower AUC for 1-year mortality, at 0.58 and 0.55 for their development and validation cohorts respectively, similar to the AUC for the LAS for our cohort (0.55).
In a recent study by Parker, et al.,8 the predictive accuracy of the LAS was analyzed using a Cox proportional hazard model and post-transplant survival Cox proportional hazard model which comprise the LAS. They, too, found poor calibration between predicted and observed waitlist survival, post-transplant survival, and LAS, respectively. The authors suggested that prediction could be better with updated models, specifically mentioning machine learning, and noting that the lack of donor variables may have contributed to the LAS not being effective for predicting post-transplant mortality.8 Unfortunately, the novel models assessed in our study did not show improved predictive accuracy despite utilizing machine learning techniques, including alternate pre-transplant recipient covariates, including available donor covariates, or stratifying by diagnosis.
We were unable to identify a model which provided better 1-year or 3-year accuracy in predicting survival. The OPTN Board of Directors established continuous organ distribution as the preferred framework to distribute all organs18 to improve transparency and equity in organ allocation. Lung was selected as the first organ to make this change, leading to the proposal of the composite allocation score (CAS) by the OPTN Lung Transplantation Committee.9 The Lung CAS will utilize 5-year predicted post-transplant outcomes model, rather than the 1-year predicted post-transplant outcomes model currently utilized.11 The 5-year model, much like the 3-year model considered in this study, did not have better predictive accuracy than the 1-year model, but was demonstrated to have similar level of confidence as the 1-year models in the report by the Scientific Registry of Transplant Recipients.11 The 5-year model allows consideration of a longer outcome period and, in the context of continuous allocation, showed greater variability across age groups than 1-year models, which may allow for stratification by age.11
Numerous studies have endeavored to validate the effectiveness of the LAS as a system to allocate organs to those most in need of a donor lung and its success in predicting post-transplant mortality. Earlier studies suggested that those with higher LAS experienced worse absolute survival after transplant than patients with lower LAS.19–24 Since its introduction, the mean LAS in the upper quartile of patients who receive transplants has steadily risen,25 suggesting sicker patients being listed for transplant. Earlier studies looked at absolute survival and worse outcomes may have reflected a sicker population being listed. Later studies show that recipients with higher LAS experienced greater survival benefit than those with lower LAS and that nuances exist between various diagnostic groups.25–27
Though several studies have shown modifiable pre-transplant variables, such as weight and albumin levels, that correlate with post-LTx mortality, our study did not find significant correlation when considering these as covariates aggregated within models.28–33 Several scoring systems, such as the Oto-Score34,35 and the Louisville-UNOS scale in conjunction with the LAS,36 predict post-transplant outcomes including 1-year mortality of the organ recipient based on donor and organ variables. The inclusion of donor covariates in a unifying model to predict post-LTx outcomes did not improve predictive accuracy in our study.
Our study involved a thorough approach to evaluating the available predictive models for post-LTx survival in a contemporary national sample of LTx recipients. There are, however, limitations to this study. First, we are limited to variables available in the OPTN registry. The data available may be insufficient to design a model with highly predictive accuracy. There may be factors we do not capture, whether in the OPTN registry or otherwise, which impact long-term mortality (see Table S-6). Second, while our data set was up to date, we have fewer outcomes, particularly 3-year survival from the most recent years, which could have impacted our assessment of 3-year survival prediction.
Our findings serve several cautionary tales. The LAS can overestimate an individual patient’s risk of death (particularly those with the highest LAS) and potentially limit access to transplant for certain patients. There should be wariness in the clinical utility of short-term survival predictions from the LAS and other models based on pre-transplant recipient covariates, and there is no identifiable model to reliably predict medium- and long-term survival after transplant. This may be due, in part, to the limitations of which variables are available in the OPTN registry. Additionally, we must consider whether the diversity of pulmonary diagnoses leading to end-stage lung disease lend to a single, unifying model for predicting post-transplant outcomes – though models stratified by diagnosis do not perform more accurately.
Stewardship of donated organs while caring for vulnerable patients is a solemn responsibility. Maintaining equity in allocating these organs while assuring that recipients and donated organs have the best chance for long-term survival are inexorable tenets. Though implementation of the LAS improved waitlist mortality and resulted in increased transplant rates, there continues to be room for improvement. Developing accurate models to predict long-term post-transplant survival is vitally important for ethical organ allocation and scarce resource-utilization.
Supplementary Material
Acknowledgements
Grant/Research Support; Current/Ongoing - CHEST Foundation Grant in CF in partnership with Vertex Pharmaceuticals, Cystic Fibrosis Foundation (CFF), and the National Institutes of Health (NIH) (K23HL138154).
A portion of the findings were presented during the 2022 ISHLT 42nd Annual Meeting and Scientific Sessions as a Mini Oral presentation by JMB.
List of Non-Standard Abbreviations
- AUC
Area Under the Curve
- BMI
Body Mass Index
- CAS
Composite Allocation Score
- CF
Cystic Fibrosis
- CMV
Cytomegalovirus
- COPD
Chronic Obstructive Pulmonary Disease
- FEV1
Forced Expiratory Volume in One Second
- FVC
Forced Vital Capacity
- GFR
Glomerular Filtration Rate
- IPF
Idiopathic Pulmonary Fibrosis
- LAS
Lung Allocation Score
- LASSO
Least Absolute Shrinkage and Selection Operator
- LTx
Lung Transplant
- NPV
Negative Predictive Value
- OPTN
Organ Procurement and Transportation Network
- PCO2
Arterial Partial Pressure of Carbon Dioxide
- PPV
Positive Predictive Value
- SMW
Six Minute Walk
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
Footnotes
Financial Conflict of Interest Statement
CHG, EDL, CAM, and KJR report additional grant support from the United States CFF. CAM reports grant support from the NIH Lung Transplant Outcomes Group. CHG reports grant support from the European Commission and NIH National Heart, Lung, and Blood Institute; National Institute of Diabetes and Digestive and Kidney Diseases; and National Center for Research Resources. CHG reports personal or other fees from Gilead Sciences, Novartis, Boehringer Ingelheim, and Vertex Pharmaceuticals. KJR reports grant support from the NIH. None of these financial relationships influenced the interpretation or reporting of the current study.
Contributor Information
Jay M. Brahmbhatt, Division of General Internal Medicine, Department of Medicine, University of Washington, Seattle, WA, USA.
Travis Hee Wai, University of Washington.
Christopher H. Goss, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, WA, USA.
Erika D. Lease, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, WA, USA.
Christian A. Merlo, Johns Hopkins University School of Medicine, Division of Pulmonary and Critical Care, Baltimore, MD, USA.
Siddhartha G. Kapnadak, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, University of Washington, Seattle, WA, USA.
Kathleen J. Ramos, University of Washington.
References
- 1.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2019 Annual Data Report: Lung. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2021;21 Suppl 2:441–520. doi: 10.1111/ajt.16495 [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez P, Veenstra D, Heagerty P, Goss CH, Ramos KJ, Bansal A. A framerwork for using real world data and health outcomes modeling to evaluate machine-learning based risk prediction models. [In Press]. Value Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Mark SC, Hoek RAS, Hellemons ME. Developments in lung transplantation over the past decade. Eur Respir Rev Off J Eur Respir Soc. 2020;29(157):190132. doi: 10.1183/16000617.0132-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x [DOI] [PubMed] [Google Scholar]
- 5.Patricia George M, Pipeling MR. Prognostic Markers and the LAS for Lung Transplantation: Impact of New Revisions for Successful Outcome. In: Raghu G, Carbone RG, eds. Lung Transplantation. Springer International Publishing; 2018:93–109. doi: 10.1007/978-3-319-91184-7_7 [DOI] [Google Scholar]
- 6.Davis SQ, Garrity ER. Organ allocation in lung transplant. Chest. 2007;132(5):1646–1651. doi: 10.1378/chest.07-0011 [DOI] [PubMed] [Google Scholar]
- 7.Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2016;35(4):433–439. doi: 10.1016/j.healun.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 8.Parker WF, Dussault NE, Jablonski R, Garrity ER, Churpek MM. Assessing the accuracy of the lung allocation score. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. Published online October 28, 2021:S1053-2498(21)02570-5. doi: 10.1016/j.healun.2021.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller E Establish Continuous Distribution of Lungs; Public Comment Proposal. Organ Procurement and Transplantation Network; :139. Accessed November 20, 2021. https://optn.transplant.hrsa.gov/policies-bylaws/public-comment/establish-continuous-distribution-of-lungs/ [Google Scholar]
- 10.Chan EY, Nguyen DT, Kaleekal TS, et al. The Houston Methodist Lung Transplant Risk Model: A Validated Tool for Pretransplant Risk Assessment. Ann Thorac Surg. 2019;108(4):1094–1100. doi: 10.1016/j.athoracsur.2019.03.108 [DOI] [PubMed] [Google Scholar]
- 11.Wey A, Skeans M, Valapour M. The Impact of Extending Follow-up for the PTAUC Model from 1 Year to 5 Years after Transplant. Scientific Registry of Transplant Recipients; 2021:14. [Google Scholar]
- 12.Tibshirani R Regression Shrinkage and Selection Via the Lasso. J R Stat Soc Ser B Methodol. 1996;58(1):267–288. doi: 10.1111/j.2517-6161.1996.tb02080.x [DOI] [Google Scholar]
- 13.Breiman L Random Forests. Mach Learn. 2001;45(1):5–32. doi: 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 14.Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016;25(4):1692–1706. doi: 10.1177/0962280213497434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller E Updated Cohort for Calculation of the Lung Allocation Score (LAS). Organ Procurement and Transplantation Network; :33. Accessed March 13, 2022. https://optn.transplant.hrsa.gov/media/4206/bp_202012_updated-cohort-for-calculation-of-the-lung-allocation-score.pdf [Google Scholar]
- 16.Preparing Your patients for changes to the lung allocation system - OPTN. Changes to the lung allocation system. Published February 17, 2015. Accessed March 13, 2022. https://optn.transplant.hrsa.gov/news/changes-to-the-lung-allocation-system/
- 17.Gries CJ, Rue TC, Heagerty PJ, Edelman JD, Mulligan MS, Goss CH. Development of a predictive model for long-term survival after lung transplantation and implications for the lung allocation score. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2010;29(7):731–738. doi: 10.1016/j.healun.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ad Hoc Geography Committee. OPTN Policy Notice Frameworks for Organ Distribution. Organ Procurement and Transplantation Network; 2018:2. Accessed November 19, 2021. https://optn.transplant.hrsa.gov/media/2789/geography_policynotice_201901.pdf [Google Scholar]
- 19.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Lung allocation score predicts survival in lung transplantation patients with pulmonary fibrosis. Ann Thorac Surg. 2009;88(6):1757–1764. doi: 10.1016/j.athoracsur.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 20.Merlo CA, Weiss ES, Orens JB, et al. Impact of U.S. Lung Allocation Score on Survival After Lung Transplantation. J Heart Lung Transplant. 2009;28(8):7. [DOI] [PubMed] [Google Scholar]
- 21.Liu V, Zamora MR, Dhillon GS, Weill D. Increasing lung allocation scores predict worsened survival among lung transplant recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2010;10(4):915–920. doi: 10.1111/j.1600-6143.2009.03003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo MJ, Iribarne A, Hong KN, et al. High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest. 2010;137(3):651–657. doi: 10.1378/chest.09-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo MJ, Worku B, Iribarne A, et al. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg. 2011;141(5):1270–1277. doi: 10.1016/j.jtcvs.2010.12.028 [DOI] [PubMed] [Google Scholar]
- 24.Maxwell BG, Levitt JE, Goldstein BA, et al. Impact of the lung allocation score on survival beyond 1 year. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2014;14(10):2288–2294. doi: 10.1111/ajt.12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawford TC, Grimm JC, Magruder JT, et al. Lung Transplant Mortality Is Improving in Recipients With a Lung Allocation Score in the Upper Quartile. Ann Thorac Surg. 2017;103(5):1607–1613. doi: 10.1016/j.athoracsur.2016.11.057 [DOI] [PubMed] [Google Scholar]
- 26.Vock DM, Durheim MT, Tsuang WM, et al. Survival Benefit of Lung Transplantation in the Modern Era of Lung Allocation. Ann Am Thorac Soc. 2017;14(2):172–181. doi: 10.1513/AnnalsATS.201606-507OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li SS, Miller R, Tumin D, Stewart WCL, Tobias JD, Hayes D. Lung Allocation Score Thresholds Prioritize Survival After Lung Transplantation. Chest. 2019;156(1):64–70. doi: 10.1016/j.chest.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 28.Singer JP, Peterson ER, Snyder ME, et al. Body composition and mortality after adult lung transplantation in the United States. Am J Respir Crit Care Med. 2014;190(9):1012–1021. doi: 10.1164/rccm.201405-0973OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos KJ, Kapnadak SG, Bradford MC, et al. Underweight Patients With Cystic Fibrosis Have Acceptable Survival Following Lung Transplantation: A United Network for Organ Sharing Registry Study. Chest. 2020;157(4):898–906. doi: 10.1016/j.chest.2019.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lederer DJ, Kawut SM, Wickersham N, et al. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med. 2011;184(9):1055–1061. doi: 10.1164/rccm.201104-0728OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandrashekaran S, Keller CA, Kremers WK, Peters SG, Hathcock MA, Kennedy CC. Weight loss prior to lung transplantation is associated with improved survival. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2015;34(5):651–657. doi: 10.1016/j.healun.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldwin MR, Arcasoy SM, Shah A, et al. Hypoalbuminemia and early mortality after lung transplantation: a cohort study. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2012;12(5):1256–1267. doi: 10.1111/j.1600-6143.2011.03965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto H, Sugimoto S, Soh J, et al. The prognostic nutritional index is correlated negatively with the lung allocation score and predicts survival after both cadaveric and living-donor lobar lung transplantation. Surg Today. 2021;51(10):1610–1618. doi: 10.1007/s00595-021-02244-2 [DOI] [PubMed] [Google Scholar]
- 34.Oto T, Levvey BJ, Whitford H, et al. Feasibility and utility of a lung donor score: correlation with early post-transplant outcomes. Ann Thorac Surg. 2007;83(1):257–263. doi: 10.1016/j.athoracsur.2006.07.040 [DOI] [PubMed] [Google Scholar]
- 35.Smits JM, van der Bij W, Van Raemdonck D, et al. Defining an extended criteria donor lung: an empirical approach based on the Eurotransplant experience. Transpl Int Off J Eur Soc Organ Transplant. 2011;24(4):393–400. doi: 10.1111/j.1432-2277.2010.01207.x [DOI] [PubMed] [Google Scholar]
- 36.Whited WM, Trivedi JR, van Berkel VH, Fox MP. Objective Donor Scoring System for Lung Transplantation. Ann Thorac Surg. 2019;107(2):425–429. doi: 10.1016/j.athoracsur.2018.08.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.