Abstract
Many anxiety disorders can be characterized by abnormalities in detecting and learning about threats, and the inability to reduce fear responses in non-threatening environments. PTSD may be the most representative of context processing pathology, as intrusive memories are experienced in “safe” contexts. The ventral subiculum (vSUB), the main output of the ventral hippocampus, encodes environmental cues and is critical for context processing. The bed nucleus of the stria terminalis (BNST) contributes to anxiety-like behaviors as well as context fear conditioning. Given the important roles of the BNST and the vSUB in these anxiety and fear-related behaviors, and the anatomical connections between the two brain regions, the major aims of this study were to characterize the anatomy and function of the vSUB-BNST pathway. First, using the retrograde tracer cholera toxin, we mapped the topographical arrangement of the vSUB-BNST pathway. Dual retrograde tracing experiments revealed neurons projecting to the BNST and those projecting to the basolateral amygdala are distinct populations. Second, we assessed whether activity in this pathway, as indexed by FOS immunohistochemistry, was modulated by context fear conditioning. Our data reveal less activation of the vSUB-BNST pathway in both males and females in aversive contexts and the greatest activation when animals explored a neutral familiar context. In addition, the vSUB of females contained fewer GABAergic neurons compared to males. These findings suggest that the vSUB-BNST pathway is involved in eliciting appropriate responses to contexts.
Keywords: BNST, fear conditioning, amygdala, threat
Introduction
Many anxiety disorders can be characterized by an inability to reduce fear responses in non-threatening environments (Jovanovic and Ressler, 2010). In PTSD, for example, intrusive thoughts and memories are experienced in “safe” contexts, as if the person were re-experiencing the traumatic event. Deficits in context processing can lead to inflexible or inappropriate behavioral responses seen in multiple psychiatric disorders such as schizophrenia, depression and drug addiction (Maren et al., 2013). Thus, it is imperative to understand the neural circuits underlying normal adaptive behaviors and how these circuits become disordered in psychiatric illness.
The hippocampal formation is an anatomically complex brain region that consists of many different subregions, including the subiculum. The subiculum is composed of three principal neuronal layers: a molecular layer, a pyramidal cell layer containing the soma of principal neurons, and a polymorphic layer. The principal cell layer is populated with large pyramidal neurons among which are many small cells considered interneurons (Amaral & Witter, 1989, 1995; O’Mara, 2005). However, the anatomical and functional organization of the subiculum is still debated (Witter, 2006). The dorsal part contains navigation-specific cells (O’Mara et al., 2009) whereas the ventral part of the subiculum (vSUB) is involved in mediating stress and anxiety responses (Herman & Mueller, 2006; Kjelstrup et al., 2002). Indeed, lesions of the vSUB impair the expression of context fear conditioning (Biedenkapp & Rudy, 2009; Maren, 1999). However, some studies also show that the vSUB plays a role in spatial learning (Laxmi et al., 1999; Torromino et al., 2019). This anatomical dichotomy is also reflected by differences in gene expression and long-range connections (Cembrowski, Phillips, et al., 2018; Ding et al., 2020; Yao et al., 2021). The vSUB projects to neocortical areas such as the entorhinal cortex, as well as subcortical areas including the nucleus accumbens, amygdala, lateral hypothalamic area and the bed nucleus of the stria terminalis (BNST; Jennings et al., 2013; Kishi et al., 2006; Naber et al., 2000).
The vSUB sends a direct excitatory projection to regions of the BNST including the anteromedial and anteroventral areas (Canteras & Swanson, 1992; Cullinan et al., 1993; Radley & Sawchenko, 2011). The BNST contributes to an animal’s response to unpredictable stressful events and processes both adaptive and pathological anxiety (Dunn & Williams, 1995; Walker et al., 2003). The BNST also plays a crucial role in learned or conditioned fear responses. Lesions or reversible inactivation of the BNST impair the expression of context fear conditioning (Ledoux et al., 1988; Pelrine et al., 2016; Sullivan et al., 2004). Conversely, both FOS and ARC protein expression increase in the anterior BNST during the expression of context fear (Lemos et al., 2010; Urien et al., 2021).
One recent study revealed that high-frequency stimulation of vSUB inputs to anteromedial BNST neurons reduces anxiety in both basal and anxiogenic situations, using both the light-dark test and the elevated plus maze (Glangetas et al., 2017). As there is evidence that both the vSUB and BNST contribute to context conditioned fear, it is critical to determine whether the vSUB-BNST pathway contributes to the expression of learned, or conditioned fear.
Here, using infusions of the retrograde label cholera toxin subunit B (CT) infused into the BNST, we labeled neurons in the vSUB. We first characterized the projections from the vSUB to the BNST. These indeed followed a precise and specific topography. While it is thought that single subicular neurons project to only one or very few target areas in the brain (Wee & MacAskill, 2020), we confirmed that BNST-projecting neurons and amygdala-projecting neurons were indeed separate populations. After animals underwent context fear conditioning we analyzed the neuronal activation of the vSUB-BNST pathways using FOS immunohistochemistry. Our results reveal less activation of this pathway when animals are exposed to an aversive context vs. a non-aversive context, suggesting that the vSUB-BNST pathway is involved in encoding responses to anxiolytic contexts.
Experimental Procedures
Subjects:
Male (250–325g) and female (225–275g) CD Sprague Dawley rats were purchased from Charles River Laboratories. They were housed in pairs, with ad libitum access to food and water, and maintained on a 12 hour light/dark cycle. All behavioral experiments were performed between 8am and 11am during the light cycle of the animals. All procedures were approved by Columbia University’s Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgeries:
After being anesthetized with a mixture of isoflurane and oxygen, rats were mounted in a stereotaxic apparatus. Rats received the local anesthetic bupivacaine (5 mg/kg) injected under the scalp and an analgesic (carprofen, 5 mg/kg, i.p) before and 24 h after surgery. For cholera toxin CT tracing experiments, a 1 μL Hamilton syringe was lowered into the BNST (AP = −0.1; ML = ±3.5, DV = −6.2, 20 degree angle to avoid the ventricles) and/or BLA (AP = −2.8; ML = ±5.3, DV = −8) and left in place for 5 min. 0.2 μL of CT fused to Alexa 488 or Alexa 647 (Invitrogen) was injected at a rate of 0.5 μL/min. The needle stayed in place for an additional 5 minutes to avoid diffusion of CT along the needle track. Injections were made bilaterally. Animals with misplaced injections were not included in the analysis. Animals recovered for 14 days before behavioral testing.
Context fear conditioning:
On day 1, rats were habituated to the context for 10 min which consisted of a standard conditioning chamber with a metal grid floor (Coulbourn Instruments). On day 2, animals in the “shock” group were placed in the conditioning chamber for 8 min and received 3 footshocks (0.5mA, 1 sec). Animals in the “no-shock” group were placed in conditioning chambers for the same 8 minutes on day 2 but did not receive shocks. On day 3, shock and no-shock animals were returned to the conditioning chamber for 10 min. Total time spent freezing before the shock (baseline, 30 seconds), after the shock (30 seconds, post shock) during conditioning and to the context during testing (freezing time per minute over 10 minutes) was manually scored offline for each animal by an observer blind to group assignment. Preexposure to the context on Day 1 and a 5 min placement in the context before the first shock on Day 2 were used to reduce any potential behavioral differences between sexes (Wiltgen et al., 2001). A third group of animals received no behavioral manipulation and were naïve “home cage” controls (n=5 males, n=5 females).
Immunohistochemistry:
60 minutes after the completion of behavioral testing, animals were given an overdose of sodium pentobarbital (100mg/kg) and perfused transcardially with 0.1M PBS and 4% paraformaldehyde in 0.1M PB. After perfusions, brains were post-fixed for 4 hours and transferred to a 20% sucrose solution for at least 48 hours. Tissue was sectioned at 80μm using a Vibratome. 10–12 slices per animal were collected, covering the rostro-caudal extent of the BNST, and/or the BLA. For animals in which FOS activity and GAD labeling in the vSUB was analyzed, 18–24 slices were collected covering the rostro-caudal extent of the vSUB. Tissue was washed first in 0.1M PB 3 times, then in PB with 1% Triton (PBT) 3 times for 5 min each wash. Slices were blocked in 2% normal goat serum (Vector Laboratories) in PBT for 1 hr. Slices were then incubated for 48 hours at 4 degrees Celsius in primary polyclonal rabbit anti-cFOS antibody (1:1000, Abcam ab190289), mouse anti-GAD67 antibody (1:1000, Millipore MAB5406) and/or a mouse anti-NeuN antibody (1:2000, Millipore MAB377) in block solution. Slices were washed in PBT and incubated with corresponding secondary fluorescent goat anti-rabbit/mouse antibody (1:200; Invitrogen) for 1 hr at room temperature. Slices were washed in PB, mounted on slides and coverslipped.
Microscopy:
All imaging and counting procedures (for all regions) were performed with the experimenter(s) blind to the group assignments of the animals. BNST, BLA and vSUB images were generated (10X, 20X magnification) using a confocal A1000 Nikon microscope and NIS element software.
To verify CT injections in the BNST and BLA, fluorescent images of the BNST from +0.12 to −0.48 mm posterior to bregma of the skull, and BLA from −2.8 to −3.4, from both left and right hemispheres, were generated (10X magnification). For FOS-CT-GAD analysis in the ventral subiculum, images (10X- 20X magnification) were taken from AP= −5.5 to −6; ML = +/− 3 to 5.5 depending on the site of injection in the BNST and the subsequent infection of the vSUB. Three images of non-consecutive slices per successful injection were quantified for each animal. We categorized injections as either anterior or posterior. Injections in the dorsal anterior medial BNST (amBNST AP= 0.12 to −0.1) and resulting anterior vSUB projections (AP=−5.5 to −5.74) were considered anterior. Injections in the dorsal posterior BNST (AP= −0.12 to −0.36) and resulting posterior vSUB projections (AP=−5.76 to −6) were considered posterior (See Figure 1). Counts were analyzed using ImageJ software after delineating the boundaries of the vSUB according to the Paxinos and Watson Atlas 6th Edition and cytoarchitecture.
Figure 1:
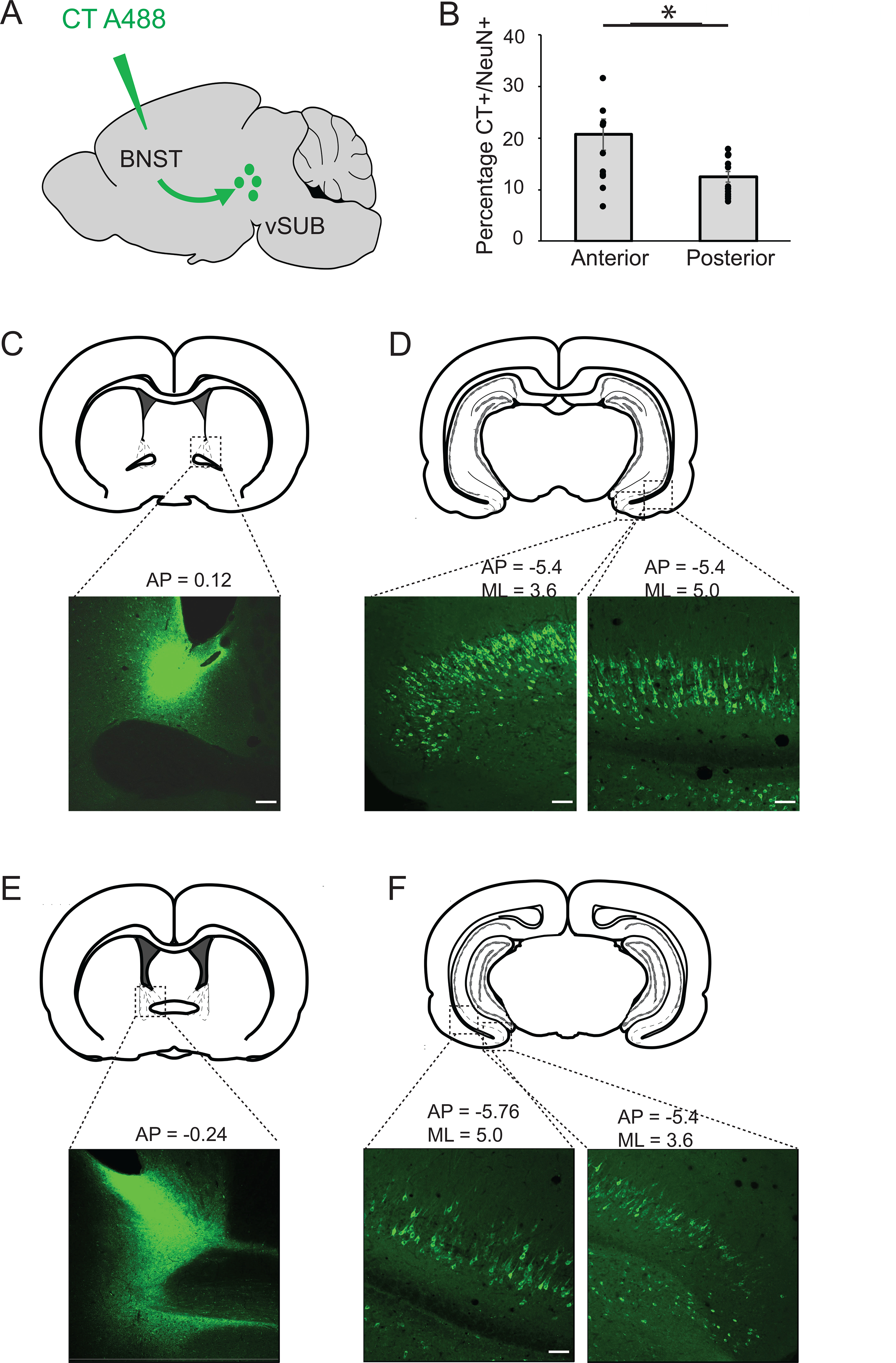
Topographic organization of projections from the vSUB to the BNST. A: Schematic representation of an injection of cholera toxin (CT) fused to Alexa 488, in the BNST. The toxin is transported retrogradely to label neurons in the vSUB. B: Percentage of anterior versus posterior vSUB neurons projecting to the BNST. Neurons located in the anterior, medial vSUB project to the dorsal anteromedial BNST; neurons located in the posterior, lateral vSUB project to the dorso-postero BNST *p=0.008. C: Example of a CT injection in the dorso antero-medial BNST. D: BNST-projecting neurons located in the anterior, medial vSUB. E: Example of a CT injection in the dorso-posterior BNST. F: BNST-projecting neurons located in the posterior vSUB.
Statistical analyses:
All data are presented as mean ± SEM. All statistical analyses were made using SPSS statistical software. Two-way or three-way ANOVAs assessed the effect of sex, behavior and vSUB region on FOS+ neurons. For behavioral analyses, we conducted two-way ANOVAs to compare groups during context fear training and expression. When appropriate, we conducted one-way ANOVAs and either Bonferroni or Tukey’s HSD post hoc tests. For all experiments, differences were considered significant when P < 0.05.
Results
Specificity of the projections from the vSUB to the BNST
To map the anatomical projections between the vSUB and the BNST, we infused 0.2μL of the retrograde tracer cholera toxin subunit B fused to the fluorophore Alexa 488 bilaterally into the BNST of adult male and female rats (n=6; Figure 1A). Our data show a general topographical organization of outputs from vSUB to BNST. Antero-medial, distal subicular neurons from all cellular layers project to the dorsal antero-medial BNST (BNST-AM) (Figure 1C–D), while more lateral and proximal (closer to vCA1) and posterior portions of the vSUB project to the posterior portions of the dorso medial BNST (Figure 1E–F). Moreover, we observe that different layers of the vSUB are infected depending on the projection to the BNST (Figure 1D, F). Quantification of these projections with the neuronal marker NeuN revealed that 16.14%±1.58% (n=6) of the vSUB neurons project to the BNST. Interestingly, we observed that a significantly higher percentage of neurons project from the antero-medial, distal vSUB to the BNST-AM compared to more posterior and lateral vSUB projections to the BNST-PM (20.7±2.86% versus 11.95±0.94%, p=0.008, Student’s unpaired t-test; Figure 1B).
As we investigate the contribution of the vSUB-BNST pathway to context fear expression, which involves the basolateral amygdala (BLA; Ciocchi et al., 2010; Fanselow & Ledoux, 1999) and knowing that the vSUB also sends projections to the BLA (Kishi et al., 2006), we asked whether individual vSUB neurons which project to the BNST also project to the BLA. Animals received infusions of the retrograde tracer CT-488 into the BNST and CT-647 into the BLA (n=8, Figure 2A). The brains were then stained for NeuN to quantify the proportion of vSUB neurons projecting to the BNST and/or BLA. Our data reveal that 21.58±2.98 % of the vSUB neurons project to the BNST and 20.6±2.76% of the vSUB neurons project to the BLA. Interestingly, only 2.4±0.33% of vSUB neurons send projections to both the BLA and the BNST (Figure 2B). Neurons projecting to the BLA appear to be more dorsal, and more abundant at the boundary between CA1 and the vSUB (Figure 2 D, E, G, H). As we observed a specific topographical organization of the vSUB-BNST projections, we also characterized the overlap between BNST and BLA projections along the anterior posterior axis. First, when injections were made in the anterior BNST, there were significantly more retrogradely labeled neurons in the vSUB than when injections were made in more posterior regions (t(14)=2.3; p=0.038; Student’s unpaired t-test, Figure 2C). There was no difference in the number of neurons projecting to the BLA along the anterior posterior axis (t(14)=1.94; p=0.073; Student’s unpaired t-test, Figure 2C). Finally, we found very little colocalization between vSUB-BNST and vSUB-BLA projections, although there were more neurons showing colocalization when injections were made in the anterior BNST and anterior BLA (t(14)=2.73; p=0.016; Student’s unpaired t-test; Figure 2C). These data suggest that vSUB neurons that project to the BNST are distinct from the vSUB-amygdala neural circuit and that vSUB neurons preferentially target anterior regions in the BNST and BLA.
Figure 2:
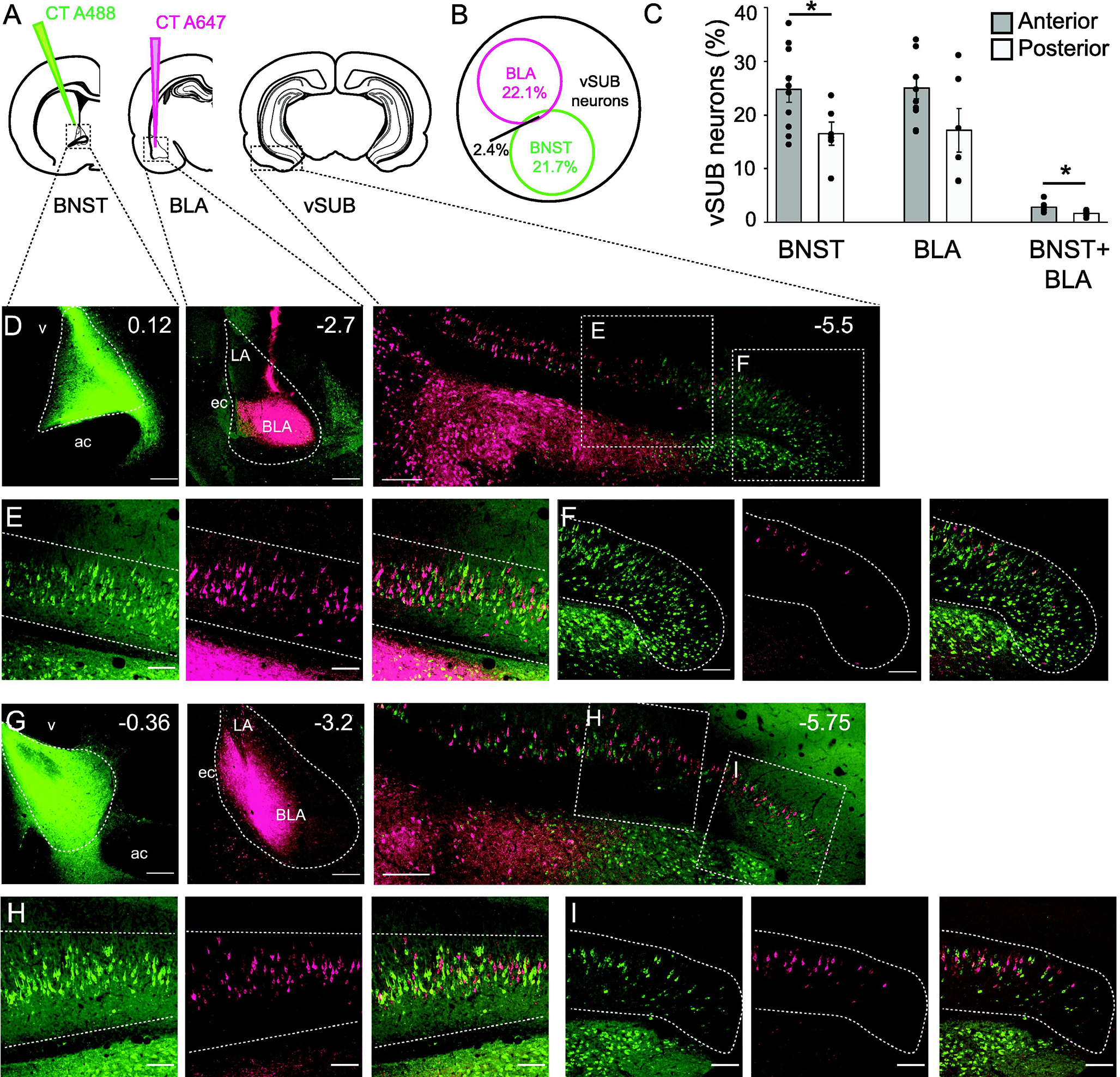
Specificity of vSUB-BNST projections. A: Schematic representation of an injection of cholera toxin fused to Alexa 488 in the BNST and an injection of cholera toxin fused to Alexa 647, in the BLA. B: Venn diagram representing the percentage of vSUB neurons projecting to the BNST, the percentage of neurons projecting to the BLA, and the percentage of neurons projecting to both structures. Only 2.4% of the vSUB neurons project to both structures, suggesting segregation of the neuronal projections. C: Histogram representing the percentage of vSUB neurons projecting to the BNST, BLA or both, according to the anterior or posterior injection in the BNST or BLA, *p<0.05. D: Confocal images of the injection site in the anterior BNST (left), BLA (middle) and resulting labeling in the vSUB (right). E: Magnification of the antero medial vSUB, neurons projecting to the BNST (green, left), to the BLA (pink, middle) and overlay (right). F: Magnification of the antero lateral vSUB, neurons projecting to the BNST (green, left), to the BLA (pink, middle) and overlay (right). G: Confocal images of the injection sites in the posterior BNST (left), BLA (middle) and resulting labeling in the vSUB (right). H: Magnification of the postero medial vSUB, neurons projecting to the BNST (green, left), to the BLA (pink, middle) and overlay (right). I: Magnification of the postero lateral vSUB, neurons projecting to the BNST (green, left), to the BLA (pink, middle) and overlay (right). Scale bars =100μm.
Expression of contextual fear decreases FOS expression in the vSUB-BNST pathway
As the vSUB and the BNST each contribute to context fear conditioning and anxiety-like behaviors we asked whether the vSUB-BNST pathway plays a role in encoding context fear. To answer this question, our first experiment was to measure the upregulation of the immediate early gene FOS in the neurons from the vSUB projecting to the BNST in both males and females after context fear expression (Figure 3). Animals were conditioned with 3 unsignaled footshocks (males n=7; females n=7) or no footshocks (males n=10, females n=8). Twenty-four hours later, animals were returned to the conditioning chambers for 10 minutes (Figure 3A). One hour after the completion of testing, animals were perfused and their brains collected.
Figure 3:
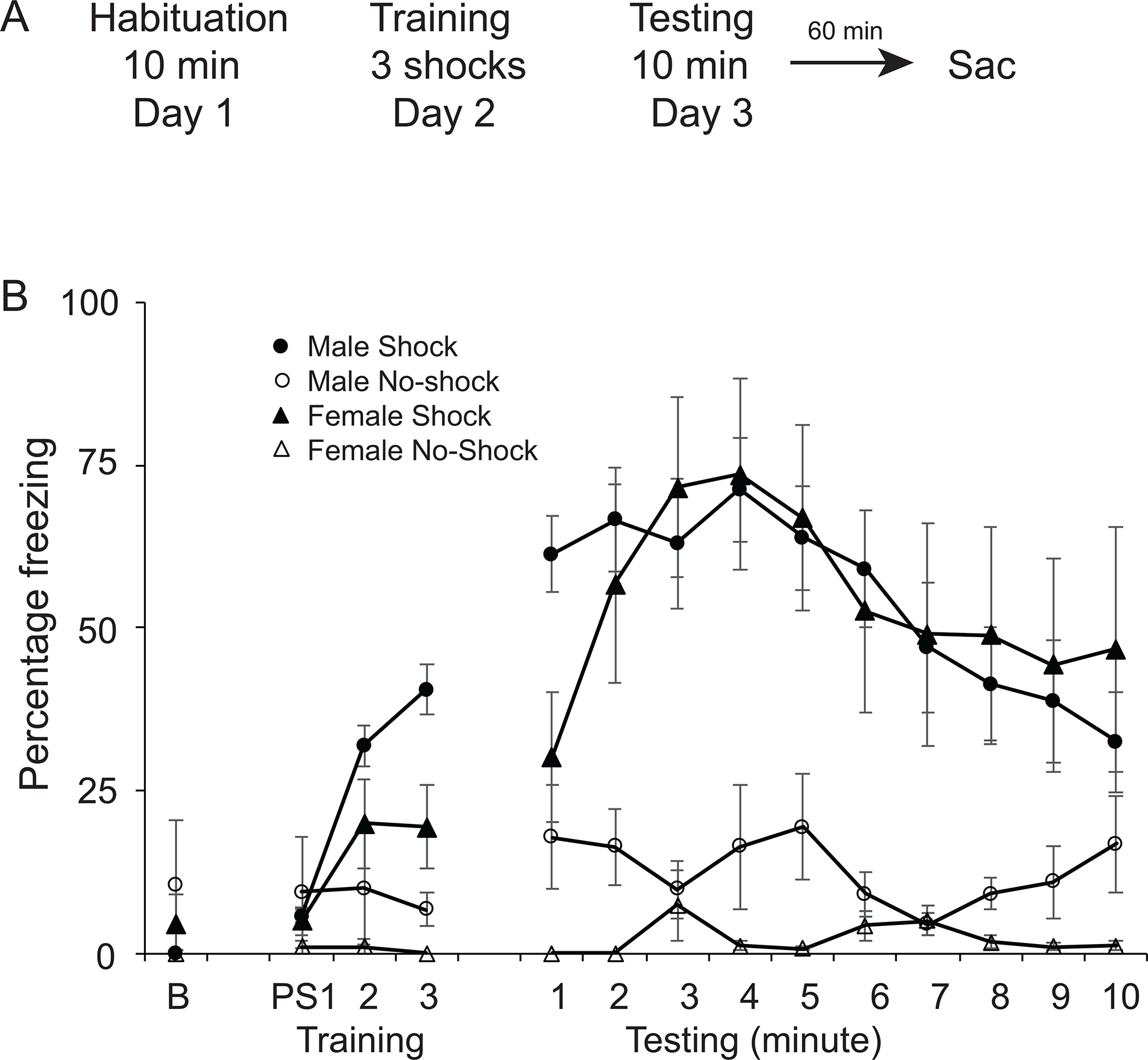
Context fear conditioning. A: Schematic representation of the behavioral protocol. On day 1, animals were habituated to the context for 10 minutes. Twenty-four hours later, on day 2, animals were placed in the same context for five minutes and received either 3 shocks (shock group) or no shocks (no shocks group). Twenty-four hours later, on day 3, animals were placed in the same context for 10 minutes. One hour after the end of the testing phase, the animals were perfused and their brains collected. B: Percent freezing (s.e.m.) during training at baseline (B) and after three footshocks (postshock PS1, PS2, PS3) or the equivalent time points in animals receiving no shocks. Percent freezing during the 10 minutes of testing in males and females in the shock and no shock behavioral groups.
To confirm that there were no a priori differences between the shock and no-shock groups, we analyzed freezing in the 30 seconds before the first shock on training day using a two-way ANOVA. We found no differences in freezing behavior, with no main effect of behavioral group (F(1,26)=0.101; p=0.753), sex (F(1,26)=0.169; p=0.69) or interaction (F(1,26)=1.08; p=0.308). To assess acquisition of context fear conditioning, we analyzed freezing during the 30 seconds after each shock (postshock freezing PS) and during the corresponding time points in the no shock animals. A two-way repeated measures ANOVA revealed a significant effect of time (F(2,52)=8.53; p<0.001), and behavioral group (F(1,26) = 6.41; p<0.05) with animals in the shock group freezing more than the animals in the no shock group over time (Figure 3B). To assess context fear expression, we analyzed freezing during the 10 min test in animals receiving shocks or no shocks using a two-way repeated measures ANOVA. We found a significant effect of behavioral group (F(1,26)=36.83; p<0.001) with shock animals freezing more than no shock animals. There was no significant main effect of sex (F(1,26)=0.53; p=0.47) or group X sex interaction (F(1,26)=0.45; p=0.51; Figure 3B).
The numbers of FOS positive, CT positive and FOS/CT positive neurons were quantified across the three behavioral groups in the vSUB: no shock, shock and naïve home cage controls. Three images of non-consecutive slices were quantified for each animal. We first ensured that the percentage of BNST-projecting neurons was equivalent in each behavioral group. A one-way ANOVA analysis of the percentage of CT+/NeuN neurons showed no effect of behavior (F(2,153)=1.16; p=0.32, data not shown), confirming that there were no a priori differences between the two behavioral groups. Across all behavioral groups, 11.9 ± 0.53% of the FOS+ neurons in the vSUB were labelled by the retrograde tracer CT. We then calculated the percentage of CT+FOS+ cells as a percentage of the total number of CT+ cells to determine how many neurons projecting to the BNST were activated during context fear expression. A two-way ANOVA revealed a significant effect of behavior (F(2,153)=17.49; p<0.0001), but no effect of sex (F(1,153)=0.86; p=0.36) or interaction between behavior and sex (F(2,153)=1.02; p=0.37; Fig 4C). Bonferroni post-hoc tests revealed a significant difference between the no shock and shock groups (p<0.001) and no shock and home cage groups (p<0.001). This result suggests that in both males and females the vSUB-BNST pathway is most active when an animal is a familiar neutral environment and less active in an aversive context. However, there was also less activity in the naïve home cage group. We then asked if this difference in neuronal activation was specific to the vSUB-BNST pathway or if the entire vSUB was less active. A two-way ANOVA of the percentage of FOS+/NeuN neurons revealed an effect of behavior (F(2,155)=11.61; p=<0.001), but no effect of sex (F(1,155)=2.06; p=0.15), and no sex X behavior interaction (F(2,155)=0.65; p=0.52; Fig 4D). Bonferroni post-hoc tests revealed significant differences between the no shock and shock groups (p=0.014), and between the home cage group and both the shock (p=0.023) and no shock (p<0.001) groups. Overall these results reveal the highest amount of vSUB activity in the no shock group in both males and females. They also reveal significantly less overall activity in the vSUB in naïve controls.
Figure 4:
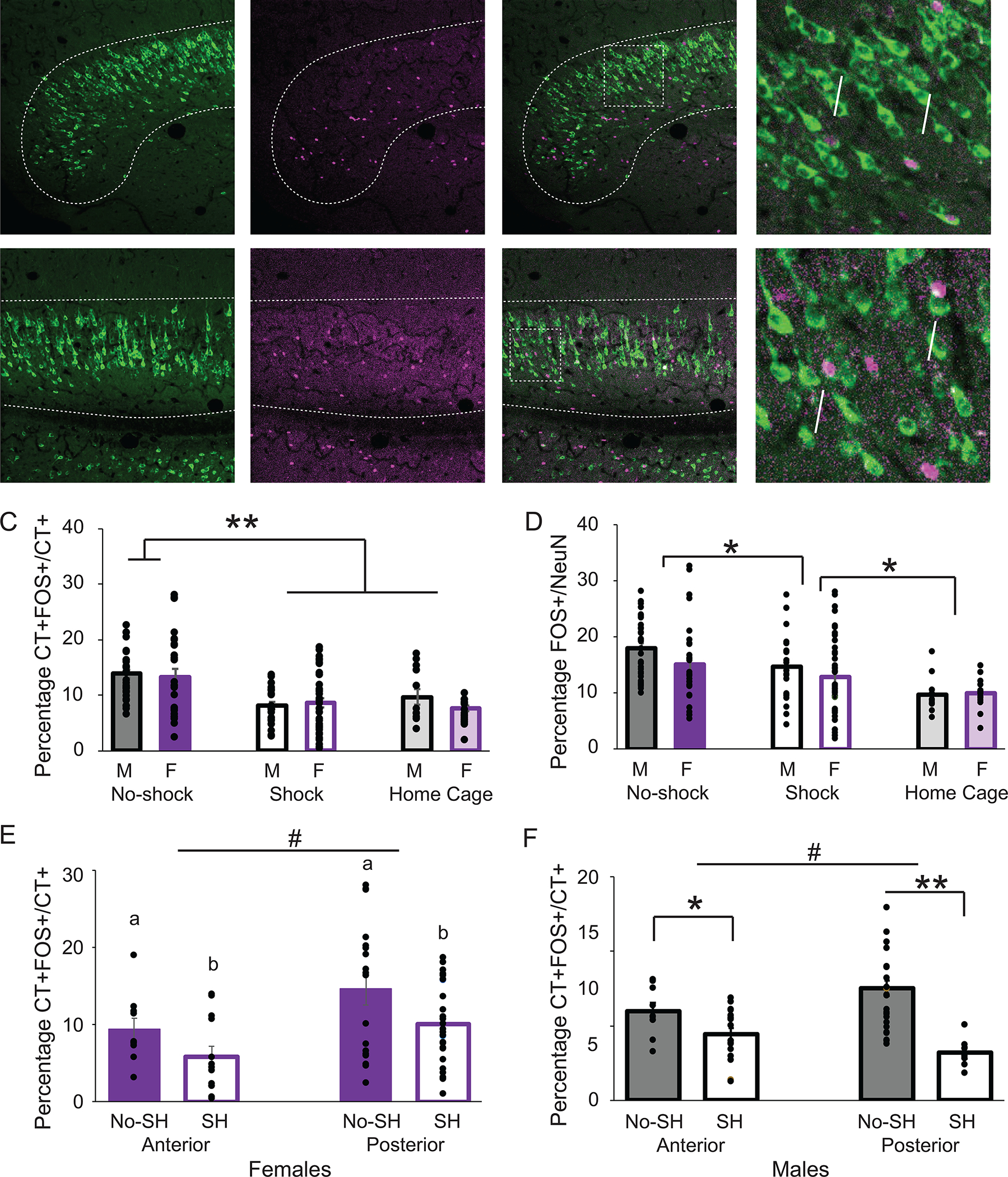
Differential effects of context fear expression on FOS immunoreactivity in the vSUB-BNST path in males and females. Confocal images of the anterior medial (A) and lateral (B) vSUB. CT A488 (left) FOS (middle) and overlay (right) Scale bars =100μm. Magnification of overlay (far right). Scale bars = 25μm. Arrows point to double-labeled cells. C: Double-labeled CT+/FOS+ cells as a percentage of CT+ cells in the vSUB in the no shock, shock and home cage groups in males (M) and females (F). Asterisks indicates a significant difference in the percentage of CT+FOS+/CT+ cells between the no shock group and the shock and home cage groups (p<0.0001). D: Percentage of FOS positive/NeuN (total number of neurons) in the vSUB in the no shock, shock and home cage groups in males (M) and females (F). Asterisk indicates a significant difference in the percentage of FOS+/NeuN cells between groups (p<0.05). E: Double-labeled CT+/FOS+ cells as a percentage of CT+ cells in the vSUB in the no shock (NS) and shock (S) groups in females in the anterior and posterior vSUB. Letters indicate significant differences (p<0.05). Hash indicates significantly more CT+FOS/CT+ in posterior regions than anterior (p<0.005). F: Double-labeled CT+/FOS+ cells as a percentage of CT+ cells in the vSUB in the no shock (NS), and shock (S) groups in males in the anterior and posterior vSUB. Asterisks indicate significantly more CT+FOS/CT+ in the no shock group than in the shock group in both the anterior (p<0.05) and posterior (p<0.001) vSUB. Hash indicates significantly more CT+FOS/CT+ in posterior regions than anterior (p<0.05).
Context fear processing along the anterior-posterior axis of the ventral subiculum
Because our previous anatomical analysis revealed different projections along the anterior posterior axis of the dorsal BNST and vSUB (Figure 1), we examined if upregulation of FOS in cells projecting to the BNST observed in the no shock group was specific to a subregion of the vSUB. We classified the slices we analyzed according to the anterior posterior axis. We considered as anterior, injections in the dorsal anterior medial BNST (amBNST AP= 0.12 to −0.1) and resulting anterior vSUB neurons (AP=−5.5 to −5.74) and we considered as posterior, injections in the dorsal posterior BNST (AP=−0.12 to −0.36,) and resulting posterior vSUB neurons (AP=−5.76 to −6).
A three way ANOVA again revealed a significant effect of behavior (F(2,129)=19.76; p<0.001), with a higher percentage of FOS in BNST-projecting neurons (FOS+CT+/CT+) in the no shock animals. However, there was also significant effect of region (F(2,129)=5.51;p=0.021), with a greater upregulation of FOS+CT+/CT+ in posterior sections, and a significant region X sex interaction (F(2,129)=4.45; p=0.037). We thus further analyzed males and females separately. For the females, we observed a significant effect of behavior (F(1,69)=5.389; p=0.023), with a higher percentage of FOS+CT+/CT+ in the no shock group, an effect of region F(1,69)=8.948, p=0.004), with a higher percentage of FOS+CT+/CT+ in the posterior vSUB, but no interaction between behavior X region (F(1,69)=0.019; p=0.89; Fig 4E). For the males, a two-way ANOVA revealed a significant interaction region X behavior (F(1,60)=4.323; p=0.042) (Figure 4F). We then looked at the anterior and posterior region separately for the males. One way ANOVAs showed a greater percentage of FOS+CT+/CT+ cells in the no shock group in both the anterior (F(1,27)=5.033; p=0.034) and posterior vSUB (F(1,33)=14.772; p<0.001). In addition, a one way ANOVA revealed a greater percentage of FOS+CT+/CT+ neurons in the posterior versus anterior vSUB of the males (F(1,60)=5.656; p=0.021). These results confirm that the no shock groups exhibit more FOS activity in vSUB-BNST neurons than the shock group in both males and females and that there was a greater percentage of FOS activity in the posterior vSUB in both sexes.
Sex differences in overall numbers of GABAergic inhibitory neurons
One possible mechanism for decreased activity in the shock animals is inhibition of the vSUB-BNST pathway by local GABAergic interneurons in the vSUB. We therefore asked whether the decrease in neuronal activation during context fear expression was due to a modulation of inhibition in the vSUB. The vast majority of vSUB neurons are glutamatergic but studies have reported the presence of inhibitory neurons expressing calbindin or parvalbumin (Fujise et al., 1995). We thus performed dual labeling of both FOS and GAD67 after the animals (n=15 males, n=15 females) completed behavioral testing and calculated the percentage of GAD+/NeuN+ neurons and GAD+FOS+/GAD+ neurons. First, as previously described (Fujise et al., 1995, O’Mara, 2005), only 3.55±0.13% of the vSUB neurons were GABAergic and 1.83±0.29% of the vSUB-BNST neurons were GABAergic (Figure 5A–B). Interestingly, we observed that males have more GAD+ neurons than females (males 3.93±0.02% vs females 3.08±0.04%, t(71)=3.4; p=0.001, Student’s unpaired t-test, Figure 5C). We then asked whether context fear expression affects activity of GABAergic neurons in the vSUB. A one-way ANOVA revealed a significant effect of sex (F(1,67)=10.02; p=0.002) with more GAD+FOS+/GAD+ neurons in males, but no significant effect of behavior (F(2,67)=1.31; p=0.28) and no interaction between behavior and sex (F(2,67)=1.04; p=0.36; Figure 5D). These results highlight a difference in the number of GABAergic inhibitory neurons and a difference of the percentage of active GABAergic neurons between males and females in the vSUB. However, context fear conditioning does not modulate GABAergic activity in the vSUB.
Figure 5:
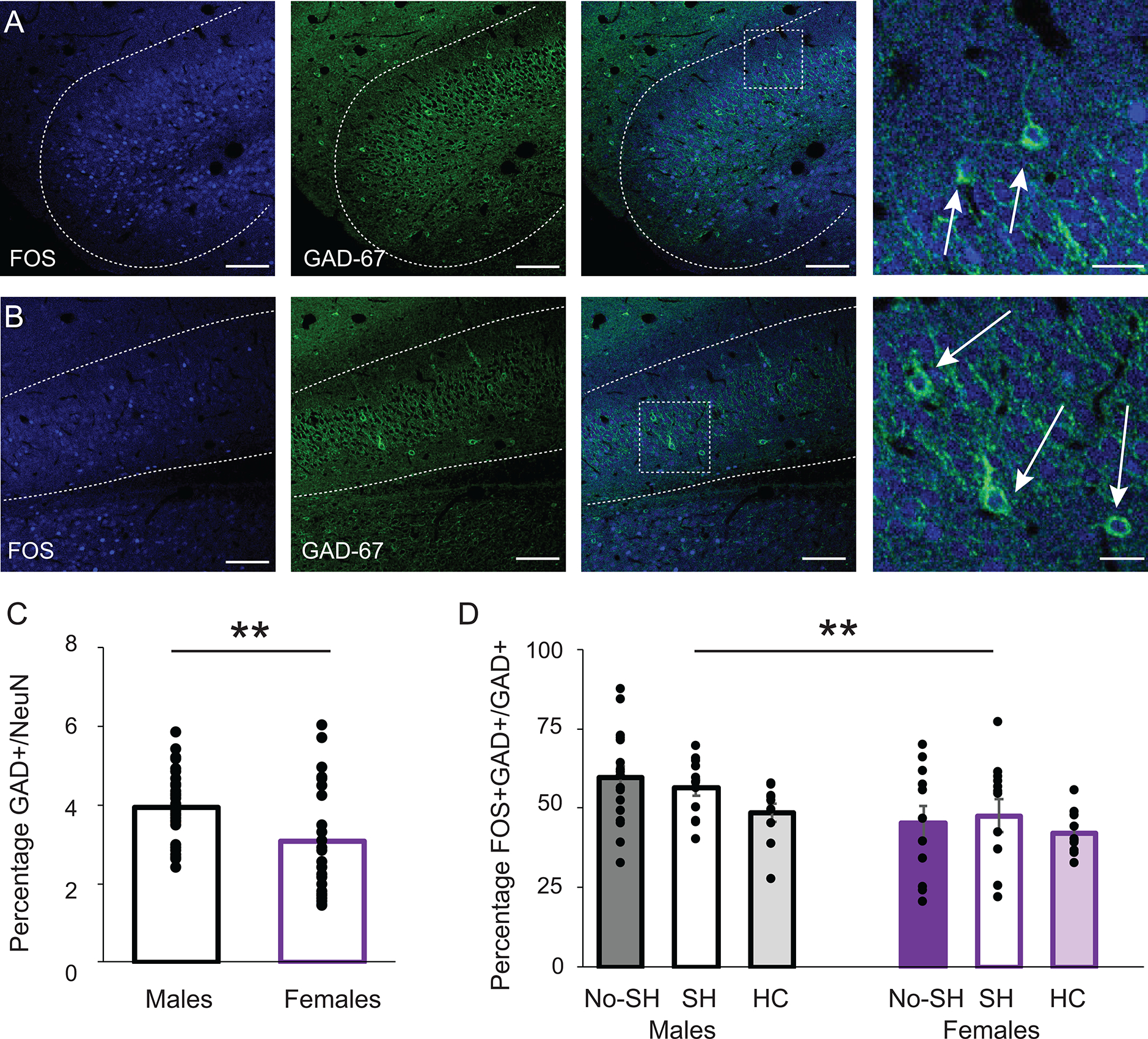
GABAergic neurons in the vSUB. Confocal images of the anterior medial (A) and lateral (B) vSUB. FOS (left), GAD67 (middle) overlay (right), scale bars =100μm, and magnification (far right) scale bars = 25μm. Arrows point to double-labeled cells. Anterior-posterior distance from Bregma (mm) = −5.5. C: Percentage of GAD+ neurons in the vSUB. Asterisk indicates a significant difference between males and females *p<0.001. B: Double-labeled GAD+/FOS+ cells as a percentage of GAD+ cells in the vSUB in the no shock (NS), shock (S) and home cage (HC) groups in males and females. Asterisk indicates a significant difference between males and females with no difference between behavioral groups *p<0.005.
Discussion
The aims of this study were to characterize the anatomy and behavioral function of the pathway between the vSUB and the BNST, two brain structures known to be involved in anxiety and fear-related behaviors. We labeled the projections from the vSUB to the BNST using the retrograde tracer cholera toxin fused to a fluorescent reporter in male and female rats. This anatomical pathway is topographically organized. We then asked whether the activity of this pathway was modulated by contextual fear expression. We found that neurons projecting from the vSUB to the BNST were less active in freezing animals and that males and females exhibited differences in GABAergic cell expression within the vSUB.
Specific topography of the vSUB projection to the BNST
The anatomical and functional organization of the subiculum is still debated (Witter, 2006). Indeed, some studies argue for a columnar organization in which subicular neurons are organized along different axes: longitudinal (dorso-ventral) and proximo-distal (mediolateral). A neuron’s position along those axes defines its efferent targets and its roles either in spatial encoding/learning or in emotional processing (Witter, 2006). On the other hand, models of a laminar organization (Bienkowski et al., 2018, 2021; Ding, 2013; Honda & Ishizuka, 2015; Ishizuka, 2001) describe deeply located neurons projecting to different brain areas than those located in more intermediate or superficial layers. Recent advances using transcriptomic tools describe specific gene expression in different cellular layers along the proximal-distal axis (Cembrowski, Wang, et al., 2018) synthesizing the two visions.
In our study, projections from the vSUB to the BNST indeed followed a precise topography. Projections from all the cellular layers of the anterior vSUB, with a decreasing mediolateral gradient, innervated the dorso anteromedial BNST (BNST-AM). More posterolateral projections, originating in some layers of the vSUB innervated the dorso-posterior BNST. Thus, our study supports both columnar and laminar organizational models of the vSUB.
It is generally thought that single subicular neurons project to only one or very few target areas in the brain (Wee & MacAskill, 2020). In agreement with this, we show that separate populations of vSUB neurons project to the BNST and the BLA and that they are localized in different layers and different parts of the mediolateral axis of the vSUB. This precise organization may have implications for the role of the vSUB in performing its unique behavioral function (Adhikari et al., 2010; Cembrowski, Phillips, et al., 2018; Ciocchi et al., 2015.; Jimenez et al., 2018). It is interesting to note that this organization of vSUB regions is different from other hippocampal areas where axon collaterals target multiple brain areas (Naber and Witter, 1998).
The ventral hippocampus is known to project to several downstream areas including the medial prefrontal cortex, nucleus accumbens and lateral hypothalamus involved in encoding anxiety, context fear expression and goal directed behaviors (Adhikari et al., 2010; Jimenez et al., 2018). Context fear conditioning selectively strengthens the inputs to the basal amygdala from the ventral hippocampus (Kim & Cho, 2020). Here we showed that the vSUB has distinct projections to the BNST and BLA, suggesting that these pathways may convey different information to contribute to an integrative behavioral response to contexts.
Modulation of the vSUB-BNST pathway by context fear expression
We next asked whether neuronal activity in the vSUB-BNST pathway, as indexed by FOS expression, is modulated by context fear expression. We found that this pathway was most active in animals in the no shock group. This suggests that this pathway is most active in animals exposed to a non-aversive environment which they are free to explore, and less active in aversive contexts.
The vSUB-BNST pathway was also less active in naïve controls not exposed to any behavioral manipulation. However, this group of animals exhibited much less FOS expression in the vSUB overall, which could explain the decrease in activity in the specific vSUB-BNST pathway. In addition, because the behavior of the naïve group was not manipulated, FOS expression in this group was not tied to a specific behavior. Indeed, animals were tested and perfused during the light cycle, when overall activity in the home cage is low. Animals in the no shock group were placed in the conditioning chambers for a total of 10 minutes on testing day, during which they actively explored the chamber. As the vSUB has been implicated in exploration and spatial learning (Trent and Menard, 2010; Laxmi et al., 1999; Torromino et al., 2019), one explanation of our results is that FOS activity in the vSUB as a whole and the vSUB-BNST pathway specifically is tied to exploration and activity in a familiar neutral environment. When animals are exposed to an aversive environment, FOS expression is not upregulated. Indeed the vSUB-BNST pathway was most active during free exploration and spatial learning of a neutral familiar environment, but was less active in threatening contexts. This suggests that the vSUB-BNST pathway may be inhibited in aversive contexts to allow freezing behavior to occur.
Although this is the first study to examine the contribution of the vSUB-BNST pathway to learned fear, there is evidence that stimulation of the vSUB-BNST alleviates anxiety under both basal and anxiogenic situations (Glangetas et al., 2017). Moreover, lesions of the ventral hippocampus/subiculum reduce anxiety (Kjelstrup et al., 2002) and impair the expression of context fear conditioning (Bannerman et al., 2003; Biedenkapp & Rudy, 2009; Maren, 1999), suggesting a role for this structure in conditioned fear responses.
Lesion and inactivation studies have established that the BNST is necessary for the expression of context fear conditioning (Duvarci et al., 2009; Sullivan et al., 2004; Walker et al., 2003). Moreover, both FOS and ARC are upregulated in the anterolateral BNST after context fear conditioning (Urien et al., 2021). However, there is also evidence that the BNST can inhibit fear expression (Campeau et al., 1997; Meloni et al., 2006). Within the anterolateral and anteromedial BNST, there are separate groups of neurons which increase or decrease their firing rates during both cued and context fear expression (Haufler et al., 2013). Together these data reveal that the BNST is functionally heterogeneous, and suggest that different populations of neurons in the BNST respond to aversive vs. non-aversive contexts. Our data here suggest that the vSUB input to the BNST contributes to an inhibition of fear output networks.
As the dorsal BNST has been implicated in the expression of context fear conditioning, we restricted our analysis to those animals who had injections of retrograde tracer infused into the dorsal BNST. Those who had injections solely in the ventral BNST were not included in this study. However, optogenetic activation of the anteroventral BNST produces anxiogenic or anxiolytic responses depending on whether glutamatergic or GABAergic neurons are activated, respectively (Jennings et al,. 2013). Thus, describing the vSUB-BNSTv projection is an important next step. Further, while the majority of BNST neurons are GABAergic (Cullinan et al., 1993; Poulin et al., 2009), the anteromedial BNST does contain a population of glutamatergic neurons (Poulin et al., 2009). Thus it is important to determine the postsynaptic targets of the vSUB projections to the BNST.
Interneurons play an important role in hippocampus physiology (Freundl & Buzsaki, 1996; Pelkey et al., 2017), however less is known about inhibitory interneurons in the subiculum. Fast-spiking interneurons, similar to those in the hippocampus, have been described (Kawaguchi et al., 1987). Both calbindin and parvalbumin-containing interneurons have been characterized (Fujise et al., 1995) but a comprehensive analysis for inhibitory interneurons is still lacking (Böhm et al., 2018). As we observed reduced activity in the vSUB-BNST pathway during context fear expression, we asked if this could be due to a difference in local subicular inhibition. However, we found no change in the percentage of activated GABAergic neurons across our three behavior groups suggesting that the modulation of the vSUB-BNST pathway may arise from afferents to the vSUB. These inputs could include the hippocampus (Fuentealba et al., 2008) thalamic structures (Prasad & Chudasama, 2013), or the amygdala (Pitkänen et al., 2000). Interestingly raphe inputs inhibit the bursting activity of pyramidal cells in the vSUB (Petersen et al., 2017) and another serotonergic system arising from the median raphé nucleus and projecting extensively and selectively to a ventral subiculum projection system has also been described (Lowry, 2002).
Both male and female animals in the shock groups exhibited decreased activity in the vSUB-BNST pathway, suggesting that this pathway contributes to behavior in similar ways in both sexes. While some find stronger context conditioning in males (Fanselow & Dong, 2010; Archer, 1975), others find stronger conditioning in females (Keiser et al., 2017). Fear conditioning in females can also produce differential fear responses such as darting, which correlates with low freezing levels (Gruene et al., 2015). Here, however, we did not observe any behavioral differences between males and females.
One sex difference observed was that females had fewer GABAergic vSUB neurons than males. Although the hippocampus of adolescent females contains fewer parvalbumin-containing GABAergic neurons (Wu et al., 2014), there are few studies of sex differences in the adult vSUB. The BNST of males and females, on the other hand, have both volumetric and neurochemical differences (Allen & Gorski, 1990; del Abril et al., 1987; Hinesa et al., 1992). Many of these differences are attributable to hormonal influences (Stefanova and Ovtscharoff, 2007).
This study is the first, to our knowledge, to examine the contribution of the vSUB-BNST pathway to conditioned fear, in both males and females. Our data suggest that this pathway is most active when animals are exposed to a non-aversive context and allowed to freely explore a neutral familiar environment. By understanding the neural circuits underlying normal adaptive behaviors, we can begin to explore how these circuits become disordered by psychiatric illness.
Projections from the ventral subiculum to the BNST are topographically organized
Separate neurons project from the ventral subiculum to the BNST and basolateral amygdala
Context fear expression reduces FOS expression in the ventral subiculum-BNST pathway.
The ventral subiculum of females contains fewer GABAergic neurons compared to males.
Funding:
This work was supported by NIH grant 1R15MH122969-01 to EPB and MRI#1828264 from the NSF Division of Biological Infrastructure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A, Topiwala MA, & Gordon JA (2010). Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety. Neuron, 65(2), 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LS, & Gorski RA (1990). Sex difference in the bed nucleus of the stria terminalis of the human brain. Journal of Comparative Neurology, 302(4), 697–706. [DOI] [PubMed] [Google Scholar]
- Amaral DG, & Witter MP (1989). The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience, 31(3):571–91. [DOI] [PubMed] [Google Scholar]
- Amaral D., & Witter MP (1995) Hippocampal formation. The Rat Nervous System, 2nd edn (ed. Paxinos G), pp. 247–291. New York: Academic Press. [Google Scholar]
- Archer J (1975), Rodent Sex Differences in Emotional and Related Behavior I. Behavioral Biology, 14(4):451–79. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, & Rawlins JNP (2003). Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural Brain Research, 139(1–2), 197–213. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, & Rudy JW (2009). Hippocampal and extrahippocampal systems compete for control of contextual fear: Role of ventral subiculum and amygdala. Learning and Memory, 16(1), 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Bowman I, Song MY, Gou L, Ard T, Cotter K, Zhu M, Benavidez NL, Yamashita S, Abu-Jaber J, Azam S, Lo D, Foster NN, Hintiryan H, & Dong HW (2018). Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nature Neuroscience, 21(11), 1628–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Sepehrband F, Kurniawan ND, Stanis J, Korobkova L, Khanjani N, Clark K, Hintiryan H, Miller CA, & Dong HW (2021). Homologous laminar organization of the mouse and human subiculum. Scientific Reports, 11(1): 3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorni M, Rovero NG, Yang ER, Holmes A, & Halladay LR (2020). Phasic signaling in the bed nucleus of the stria terminalis during fear learning predicts within- And across-session cued fear expression. Learning and Memory, 27(3), 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm C, Peng Y, Geiger JRP, & Schmitz D (2018). Routes to, from and within the - subiculum. In Cell and Tissue Research. Cell Tissue Research, 373(3):557–563 [DOI] [PubMed] [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, & Robbins TW (1996). Effects of Lesions to Amygdala, Ventral Subiculum, Medial Prefrontal Cortex, and Nucleus Accumbens on the Reaction to Novelty: Implication for Limbic-Striatal Interactions. Behavioral Neuroscience, 110(1):60–73. [DOI] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, & Watson SJ (1997). Elicitation and reduction of fear: Behavioral and neuroendocrine indices and brain induction of the immediate early gene c-fos. Neuroscience, 78(4):1087–104. [DOI] [PubMed] [Google Scholar]
- Canteras NS, & Swanson LW (1992). Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: A PHAL anterograde tract-tracing study in the rat. Journal of Comparative Neurology, 324(2), 180–194. [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Phillips MG, DiLisio SF, Shields BC, Winnubst J, Chandrashekar J, Bas E, & Spruston N (2018). Dissociable Structural and Functional Hippocampal Outputs via Distinct Subiculum Cell Classes. Cell, 173(5), 1280–1292.e18. [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Wang L, Lemire AL, Copeland M, Dilisio SF, Clements J, & Spruston N (2018). The subiculum is a patchwork of discrete subregions. Elife, 7:e37701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, & Lüthi A (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature, 468(7321), 277–282. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, & Klausberger T (2015). Selective information routing by ventral hippocampal CA1 projection neurons. Science, 348(6234):560–3. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, & Watson SJ (1993). Ventral Subicular Interaction With the Hypothalamic Paraventricular Nucleus: Evidence for a Relay in the Bed Nucleus of the Stria Terminalis. Journal of Comparative Neurology, 332(1):1–20. [DOI] [PubMed] [Google Scholar]
- del Abril A, Segovia S, & Guillamon A (1987). The bed nucleus of the stria terminalis in the rat” regional sex differences controlled by gonadal steroids early after birth. Brain Research, 429(2):295–300. [DOI] [PubMed] [Google Scholar]
- Ding SL (2013). Comparative anatomy of the prosubiculum, subiculum, presubiculum, postsubiculum, and parasubiculum in human, monkey, and rodent. In Journal of Comparative Neurology 521(18):- [DOI] [PubMed] [Google Scholar]
- Ding SL, Yao Z, Hirokawa KE, Nguyen TN, Graybuck LT, Fong O, Bohn P, Ngo K, Smith KA, Koch C, Phillips JW, Lein ES, Harris JA, Tasic B, & Zeng H (2020). Distinct Transcriptomic Cell Types and Neural Circuits of the Subiculum and Prosubiculum along the Dorsal-Ventral Axis. Cell Reports, 31(7): 107648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JD, & Williams TJ (1995). Cardiovascular Responses to Electrical Stimulation of the Bed Nucleus of the Stria Terminalis. Journal of Comparative Neurology, 352(2):227–34. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, & Paré D (2009). The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. Journal of Neuroscience, 29(33), 10357–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, & Dong HW (2010). Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron, 65(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, & Ledoux JE (1999). Why We Think Plasticity Underlying Viewpoint Pavlovian Fear Conditioning Occurs in the Basolateral, Neuron, 23(2):229–32. [DOI] [PubMed] [Google Scholar]
- Figel B, Brinkmann L, Buff C, Heitmann CY, Hofmann D, Bruchmann M, Becker MPI, Herrmann MJ, & Straube T (2019). Phasic amygdala and BNST activation during the anticipation of temporally unpredictable social observation in social anxiety disorder patients. NeuroImage: Clinical, 22:101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floriou-Servou A, von Ziegler L, Stalder L, Sturman O, Privitera M, Rassi A, Cremonesi A, Thöny B, & Bohacek J (2018). Distinct Proteomic, Transcriptomic, and Epigenetic Stress Responses in Dorsal and Ventral Hippocampus. Biological Psychiatry, 84(7), 531–541. [DOI] [PubMed] [Google Scholar]
- Freund TF, & Buzsaki G (1996). Interneurons of the Hippocampus. Hippocampus, 6(4):347–470. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Tomioka R, Dalezios Y, Márton LF, Studer M, Rockland K, Klausberger T, & Somogyi P (2008). Rhythmically active enkephalin-expressing GABAergic cells in the CA1 Area of the hippocampus project to the subiculum and preferentially innervate interneurons. Journal of Neuroscience, 28(40), 10017–10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujise N, Hunziker W, Heizmann CW, & Kosaka T (1995). Distribution of the calcium binding proteins, calbindin D-28K and parvalbumin, in the subicular complex of the adult mouse. Neuroscience Research 22(1):89–107 [DOI] [PubMed] [Google Scholar]
- Glangetas C, Massi L, Fois GR, Jalabert M, Girard D, Diana M, Yonehara K, Roska B, Xu C, Lüthi A, Caille S, & Georges F (2017). NMDA-receptor-dependent plasticity in the bed nucleus of the stria terminalis triggers long-term anxiolysis. Nature Communications, 8:14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Schafe GE, Orr PT, & Frick KM (2009). Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience, 159(2), 451–467. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD & Shansky RM (2015) Sexually divergent expression of active and passive fear responses in rats. Elife, 4:e11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, & Pare D (2013). Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learning and Memory, 20(11), 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, & Mueller NK (2006). Role of the ventral subiculum in stress integration. In Behavioural Brain Research 174(2):215–224. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, & Figueiredo H (2004). Role of GABA and glutamate circuitry in hypothalamo-pituitary- adrenocortical stress integration. Annals of the New York Academy of Sciences, 1018, 35–45. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA,. (1992). Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Research, 579(2):321–6. [DOI] [PubMed] [Google Scholar]
- Honda Y, & Ishizuka N (2015). Topographic distribution of cortical projection cells in the rat subiculum. Neuroscience Research, 92, 1–20. [DOI] [PubMed] [Google Scholar]
- Ishizuka N (2001). Laminar Organization of the Pyramidal Cell Layer of the Subiculum in the Rat. Journal of Comparative Neurology, 435(1):89–110. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, & Stuber GD (2013). Distinct extended amygdala circuits for divergent motivational states. Nature, 496(7444), 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, Paninski L, Hen R, & Kheirbek MA (2018). Anxiety Cells in a Hippocampal-Hypothalamic Circuit. Neuron, 97(3), 670–683.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic and Ressler, (2010). How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry, 167(6):648–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Katsumaru H, Kosaka T, Heizmann CW, & Hama K (1987). Fast spiking cells in rat hippocampus (CA 1 region) contain the calcium-binding protein parvalbumin. In Brain Research 416(2):369–74 [DOI] [PubMed] [Google Scholar]
- Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, & Tronson NC (2017) Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology, 42(2):397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WB, & Cho JH (2020). Encoding of contextual fear memory in hippocampal–amygdala circuit. Nature Communications, 11(1) :1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, & Yasui Y (2006). Topographical projection from the hippocampal formation to the amygdala: A combined anterograde and retrograde tracing study in the rat. Journal of Comparative Neurology, 496(3), 349–368. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach H-A, Murison R, Moser EI, & Moser M-B (2002). Reduced fear expression after lesions of the ventral hippocampus. PNAS, 99(16):10825–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmi TR, Bindu PN, Raju TR, & Meti BL (1999). Spatial memory impairment in ventral subicular lesioned rats. Brain Research, 816(1):245–8. [DOI] [PubMed] [Google Scholar]
- Ledoux JE, Iwata J, Cicchetti P, & Reis DJ (1988). Different Projections of the Central Amygdaloid Nucleus Mediate Autonomic and Behavioral Correlates of Conditioned Fear. The Journal of Neuroscience 8(7):2517–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JI, Resstel LB, & Guimarães FS (2010). Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behavioural Brain Research, 207(1), 105–111. [DOI] [PubMed] [Google Scholar]
- Lowry CA, (2002). Functional Subsets of Serotonergic Neurons: Implications for Control of the Hypothalamic-Pituitary-Adrenal Axis. Journal of Neuroendocrinology, 14(11):911–23 [DOI] [PubMed] [Google Scholar]
- Maren S (1999). Neurotoxic or Electrolytic Lesions of the Ventral Subiculum Produce Deficits in the Acquisition and Expression of Pavlovian Fear Conditioning in Rats. Behavioral Neuroscience 113(2):283–90. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, & Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci, 14(6):417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Jackson A, Gerety LP, Cohen BM, & Carlezon WA (2006). Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Annals of the New York Academy of Sciences, 1071, 538–541. [DOI] [PubMed] [Google Scholar]
- Monfort P, Gomez-Gimenez B, Llansola M, & Felipo V (2015). Gender Differences in Spatial Learning, Synaptic Activity, and Long-Term Potentiation in the Hippocampus in Rats: Molecular Mechanisms. ACS Chemical Neuroscience, 6(8), 1420–1427. [DOI] [PubMed] [Google Scholar]
- Mueller NK, Dolgas CM, & Herman JP (2006). Regulation of forebrain GABAergic stress circuits following lesion of the ventral subiculum. Brain Research, 1116(1), 132–142. [DOI] [PubMed] [Google Scholar]
- Naaz F, Knight LK, & Depue BE (2018). Explicit and ambiguous threat processing: Functionally dissociable roles of the amygdala and bed nucleus of the stria terminalis. Journal of Cognitive Neuroscience, 31(4), 543–559. [DOI] [PubMed] [Google Scholar]
- Naber PA, & Witter MP (1998). Subicular Efferents Are Organized Mostly as Parallel Projections: A Double-Labeling, Retrograde-Tracing Study in the Rat. J. Comp. Neurol 393(3):284–97. [PubMed] [Google Scholar]
- Naber PA, Witter MP, Lopes FH, & Silva FH (2000). Networks of the Hippocampal Memory System of the Rat The Pivotal Role of the Subiculum Ann N Y Acad Sci, 911:392–403. [DOI] [PubMed] [Google Scholar]
- O’Mara S (2005). The subiculum: What it does, what it might do, and what neuroanatomy has yet to tell us. Journal of Anatomy 207(3):271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara SM, Sanchez-Vives M. v., Brotons-Mas JR, & O’Hare E (2009). Roles for the subiculum in spatial information processing, memory, motivation and the temporal control of behaviour. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 33(5), 782–790. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, & Mcbain CJ (2017). Hippocampal GABAergic Inhibitory Interneurons. Physiol Rev, 97, 1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelrine E, Pasik SD, Bayat L, Goldschmiedt D, & Bauer EP (2016). 5-HT2C receptors in the BNST are necessary for the enhancement of fear learning by selective serotonin reuptake inhibitors. Neurobiology of Learning and Memory, 136, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AV, Jensen CS, Crépel V, Falkerslev M, & Perrier JF (2017). Serotonin regulates the firing of principal cells of the subiculum by inhibiting a T-type Ca2+ current. Frontiers in Cellular Neuroscience, 11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, & Ylinen A (2000). Reciprocal Connections between the Amygdala and the Hippocampal Formation, Perirhinal Cortex, and Postrhinal Cortex in Rat A Review. Ann N Y Acad Sci, 911:639–91. [DOI] [PubMed] [Google Scholar]
- Poulin J-F, Arbour D, Laforest S, & Drolet G, (2009) Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry, 33(8):1356–65. [DOI] [PubMed] [Google Scholar]
- Prasad JA, & Chudasama Y (2013). Viral tracing identifies parallel disynaptic pathways to the hippocampus. Journal of Neuroscience, 33(19), 8494–8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, & Sawchenko PE (2011). Behavioral/Systems/Cognitive A Common Substrate for Prefrontal and Hippocampal Inhibition of the Neuroendocrine Stress Response. Journal of Neuroscience, 31(26):9683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler RL, Goode TD, Evemy C, & Maren S (2020). NMDA receptors in the CeA and BNST differentially regulate fear conditioning to predictable and unpredictable threats. Neurobiology of Learning and Memory, 174:107281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, & Moser EI (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience 15(10):655–669. [DOI] [PubMed] [Google Scholar]
- Stefanova Nadya, and Ovtscharoff Wladimir. 2007. “Advances in Anatomy Embryology and Cell Biology.” Advances in Anatomy Embryology and Cell Biology 185: 1–73. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, & Ledoux JE (2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience, 128(1), 7–14. [DOI] [PubMed] [Google Scholar]
- Torromino G, Autore L, Khalil V, Mastrorilli V, Griguoli M, Pignataro A, Centofante E, Biasini GM, de Turris V, Ammassari-Teule M, Rinaldi A, & Mele A (2019). Offline ventral subiculum-ventral striatum serial communication is required for spatial memory consolidation. Nature Communications, 10(1) :5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent NL, & Menard JL (2010) The ventral hippocampus and the lateral septum work in tandem to regulate rats’ open-arm exploration in the elevated plus-maze. Physiol Behav, 101(1):141–52. [DOI] [PubMed] [Google Scholar]
- Tronson NC, & Keiser AA (2019). A Dynamic Memory Systems Framework for Sex Differences in Fear Memory. Trends in Neurosciences 42(10):680–692. [DOI] [PubMed] [Google Scholar]
- Urien L, Stein N, Ryckman A, Bell L, & Bauer EP (2021). Extended amygdala circuits are differentially activated by context fear conditioning in male and female rats. Neurobiology of Learning and Memory, 180:107401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urien L, and Bauer EP, (2021). Sex differences in BNST and amygdala activation by contextual, cued and unpredictable threats. eNeuro, 0233–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, & Davis M (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology, 463(1–3), 199–216. [DOI] [PubMed] [Google Scholar]
- Wee RWS, & MacAskill AF (2020). Biased Connectivity of Brain-wide Inputs to Ventral Subiculum Output Neurons. Cell Reports, 30(11), 3644–3654.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Behne NS, & Fanselow MS (2001). Sex Differences, Context Preexposure, and the Immediate Shock Deficit in Pavlovian Context Conditioning With Mice. Behavioral Neuroscience, 115(1), 26–32. [DOI] [PubMed] [Google Scholar]
- Witter MP (2006). Connections of the subiculum of the rat: Topography in relation to columnar and laminar organization. Behavioural Brain Research, 174(2), 251–264. [DOI] [PubMed] [Google Scholar]
- Wu YWC, Du X, van den Buuse M, & Hill RA (2014). Sex differences in the adolescent developmental trajectory of parvalbumin interneurons in the hippocampus: A role for estradiol. Psychoneuroendocrinology, 45, 167–178. [DOI] [PubMed] [Google Scholar]
- Yagi S, Chow C, Lieblich SE, & Galea LAM (2016). Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus, 26(1), 87–101 [DOI] [PubMed] [Google Scholar]
- Yao Z, van Velthoven CTJ, Nguyen TN, Goldy J, Sedeno-Cortes AE, Baftizadeh F, Bertagnolli D, Casper T, Chiang M, Crichton K, Ding SL, Fong O, Garren E, Glandon A, Gouwens NW, Gray J, Graybuck LT, Hawrylycz MJ, Hirschstein D, … Zeng H (2021). A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell, 184(12), 3222–3241.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]


