Abstract
Mesophotic coral ecosystems (MCEs, reefs between 30 and 150 m depth) have been hypothesized to contribute to shallow reef recovery through the recruitment of larvae. However, few studies have directly examined this. Here we used mesophotic colonies of Seriatopora hystrix, a depth generalist coral, to investigate the effect of light intensity on larval behavior and settlement through ex situ experiments. We also investigated juvenile survival, growth, and physiological acclimation in situ. Bleached larvae and a significant reduction in settlement rates were found when the mesophotic larvae were exposed to light conditions corresponding to shallow depths (5 and 10 m) ex situ. The in situ experiments showed that mesophotic juveniles survived well at 20 and 40 m, with juveniles in shaded areas surviving longer than three months at 3–5 m during a year of mass bleaching in 2016. Juvenile transplants at 20 m showed a sign of physiological acclimation, which was reflected by a significant decline in maximum quantum yield. These results suggest that light is a significant factor for successful recolonization of depth-generalist corals to shallow reefs. Further, recolonization of shallow reefs may only occur in shaded habitats or potentially through multigenerational recruitments with intermediate depths acting as stepping stones.
Subject terms: Ecology, Ecology
Introduction
Severe coral bleaching events caused by high surface seawater temperature have repeatedly occurred over the last few decades, leading to degradation and loss of diversity in coral reefs1. However, some reef habitats may act as a shelter from stressors, for instance the deeper parts of the reef, known as mesophotic coral ecosystems (MCEs), since these habitats have lower exposure to ultraviolet radiation and fluctuations in temperature compared to shallower reefs2,3. MCEs, characterized by limited light intensity (≤ 10% of surface irradiance), occur at depths below 30–40 m and extend to over 150 m, depending on the region4. The deep reef refugia hypothesis (DRRH) asserts that MCEs are protected from disturbances that affect shallow-water reefs and could act as a larval source for shallow reefs2,5. However, MCEs may not be entirely protected from natural and human threats6,7 and contrasting arguments on the role of MCEs as a larval source for shallow reef recovery have been discussed8–10.
Larval dispersal and recruitment of corals are essential for coral reefs to maintain and renew their populations11,12. Some studies support the DRRH, suggesting larval dispersal may occur from mesophotic to shallow reefs. For instance, population genetic studies suggest that larval migration between mesophotic and shallow reefs varies among reef sites and species13,14. Studies on mesophotic coral reproductive biology9 and larval dispersal modeling8 further support the potential for mesophotic corals to act as larval sources to recolonize shallow reefs. However, these studies are based on indirect evidence of larval migration, and currently there is no information on the tolerance of larvae and juveniles from mesophotic corals to the environmental conditions of shallow reefs.
After dispersal, coral larvae need to acclimatize to their settlement environments. Since mesophotic larvae dispersing to shallow reefs will be exposed to intense light, photo-acclimation responses are essential for their survival. In this context, the tolerance of mesophotic corals to shallow water conditions has been shown for adult colonies in several studies15–17. For instance, altered maximum quantum yield (Fv/Fm), algal density, and chlorophyll pigments were observed in the surviving adult corals Stylophora pistillata (Esper, 1792)15 and Fimbriaphyllia (formerly Euphyllia) paradivisa (Veron, 1990)16,17 from mesophotic depth when transplanted to shallow reefs. These adjustments are vital for corals to prevent the loss of photosynthetic activity of the algal symbionts after exposure to an excessive amount of absorbed light energy18,19. However, larvae and juveniles may be more flexible to environmental changes. For instance, coral larvae20 and juveniles21 have the flexibility to associate with multiple algal symbiont types that shape their survival and growth. Hence, their physiological responses to depth transplantation should be tested.
Seriatopora hystrix Dana 1846, is a widespread scleractinian coral in the Indo-Pacific region. This coral is an ideal species model since it is abundant at an MCE in Okinawa22,23 while it disappeared locally in a nearby shallow reef following bleaching events in 199824 and 2001 with no recovery in the shallow reef observed until 201025 and later26. Here we examined the effect of light on mesophotic coral larval behavior and settlement in a laboratory experiment as well as on the survival, growth, and physiological acclimation of juveniles in a field experiment. These experiments clarify whether coral larvae from mesophotic depth (from ca. 40 m depth) can settle in shallower reefs and whether the settled juveniles can survive and grow in this environment.
Results
Light and seawater temperature at different depths
In 2015 and 2016, mean maximum daily light intensities (± SE) in exposed orientation at 3–5, 20, and 40 m depths were 401.8 ± 22.2, 71.4 ± 2.7, and 34.5 ± 1.3 µmol photon m−2 s−1, respectively (Table 1; Fig. 1a). In shaded environments, the light intensities (± SE) were 4.8 ± 0.3, 1.9 ± 0.1, and 0.6 ± 0.04 µmol photon m−2 s−1 at the depths of 3–5, 20, and 40 m, respectively (Table 1; Fig. 1a).
Table 1.
Mean daily maximum irradiance (PAR, Photosynthetically Active Radiation) between June 2015 and February 2017 and mean seawater temperature during the crucial acclimation period (i.e., the first month) of coral juvenile transplant experiments in August–September 2015 and 2016 at the depths of 3–5, 20, and 40 m in exposed and shaded orientations.
| Depth | Orientation | Mean maximum daily irradiance (µmol quanta m−2 s−1) | Mean seawater temperature (°C) in August—September 2015 | Mean seawater temperature (°C) in August—September 2016 |
|---|---|---|---|---|
| 3–5 m | Exposed | 401.8 | 28.4 | 29.4 |
| Shaded | 4.8 | |||
| 20 m | Exposed | 71.4 | 28.5 | 28.9 |
| Shaded | 1.9 | |||
| 40 m | Exposed | 34.5 | 27.7 | 28.0 |
| Shaded | 0.6 |
Figure 1.
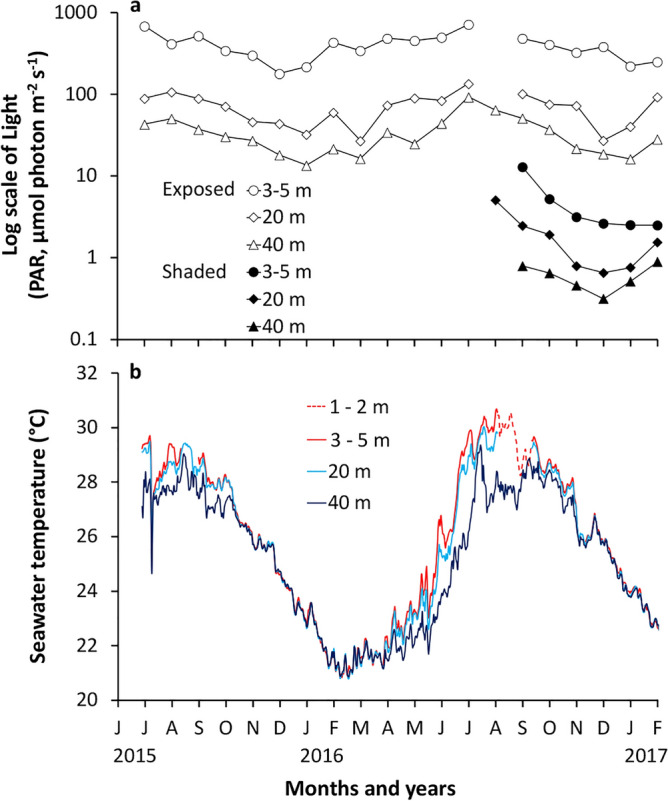
(a) Mean of daily maximum irradiance (PAR, Photosynthetically Active Radiation) per month at exposed and shaded orientation, and (b) Mean daily seawater temperature (°C) in different reef habitats (1–2, 3–5, 20, and 40 m depths) between June 2015 and February 2017 (mean ± SE). Light loggers at shaded orientation were installed between August 2016 to February 2017. A temperature logger at 1–2 m depth was installed from August to September 2016.
During the first month of juvenile transplant experiments (from August to September), the mean seawater temperatures (± SE) were 28.4 ± 0.02, 28.5 ± 0.02, and 27.7 ± 0.02 °C at 3, 20, and 40 m, respectively in 2015 and they were 29.4 ± 0.02, 28.9 ± 0.03, and 28.0 ± 0.02 °C at 1–5, 20, and 40 m, respectively in 2016 (Table 1; Fig. 1b). In 2016, high (> 3 °C) temperature differences (hourly average) between shallow and mesophotic depths were observed day and night. The temperature differences lasted for most of planula release periods in July (between 18 and 28 July 2016) and August (between 15 and 20 August 2016)9. Seawater temperature was relatively similar at all depths from November to February in both years of juvenile transplant experiments.
Larval behavior, settlement, and survival (laboratory experiment)
Regardless of the light conditions, planulae were mostly found at the bottom layer of the 80-cm tall acrylic columns, averaging between 68% (40 m) and 79% (5 m) (Supplementary Fig. 1). The descending speeds (downward swimming) of the planulae, between 1.6 mm s−1 (20 m) and 2.1 mm s−1 (40 m), were faster than the ascending speeds (upward swimming), between 0.5 mm s−1 (40 m) and 0.7 mm s−1 (20 m). There was no significant effect of light conditions on larval swimming speed at each direction (One-Way ANOVA, downward, F = 0.495, df = 3, P = 0.688; upward, F = 0.445, df = 3, P = 0.728).
The planulae were actively crawling and settled rapidly within hours after release under control (i.e., 40 m depth) light conditions (Fig. 2). Percent of settled larvae differed significantly between light conditions both in 2015 (Mann–Whitney U, P = 0.004, pairwise comparison: 40 m > 10 m) and 2016 (One-Way ANOVA, F = 9.008, df = 3, P = 0.001; Fig. 2). The percentage of settled larvae reduced significantly in 5 and 10 m light conditions compared to control, while the percentage of settled larvae in 20 m light condition was similar (40 m = 20 m ≥ 10 m = 5 m lights; Bonferroni, P = 0.001). All larvae survived in 40 and 20 m conditions, while in 10 and 5 m conditions, survival rates of both crawling/swimming and settled larvae reduced by 1.1 and 7.8%, respectively (Fig. 2). In addition, 16.9 and 100% of the surviving pre- and post-settled larvae became pale or bleached when exposed to 10 and 5 m light conditions, respectively (Supplementary Fig. 2).
Figure 2.
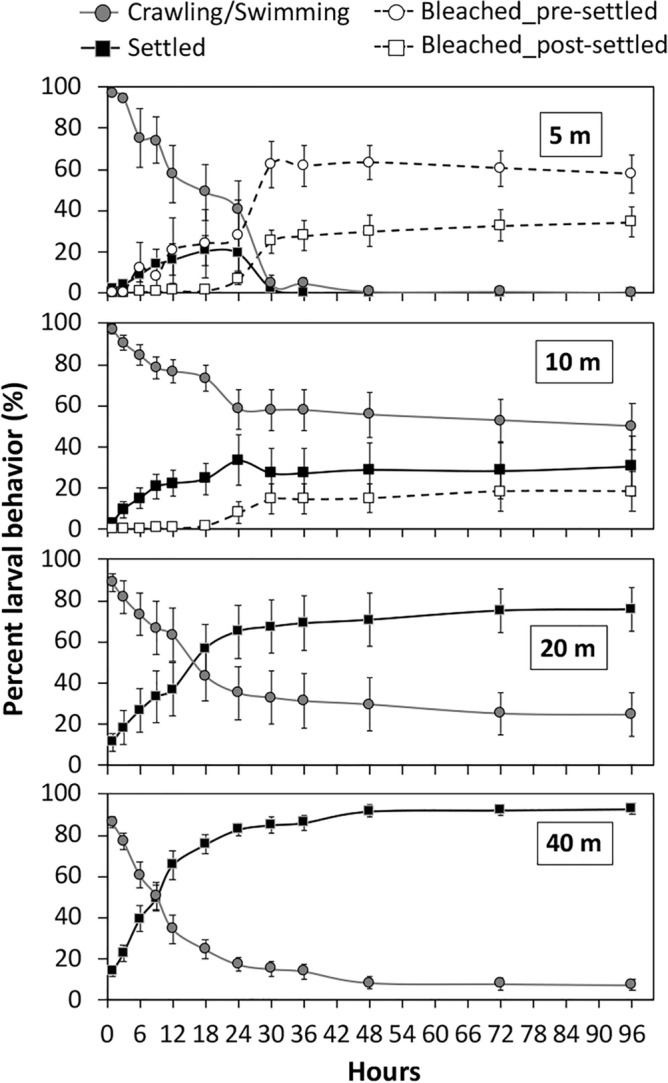
Mean number of larval behavior (± SE; n = 6) of S. hystrix larvae from mesophotic colonies. Larvae identified as settled, crawling/swimming, bleached (pre- and post-settled), and survived (pre- and post-settled) under light conditions representing the depth of 5, 10, 20 and 40 m in 2016.
Survival and growth rate of juvenile (field experiment)
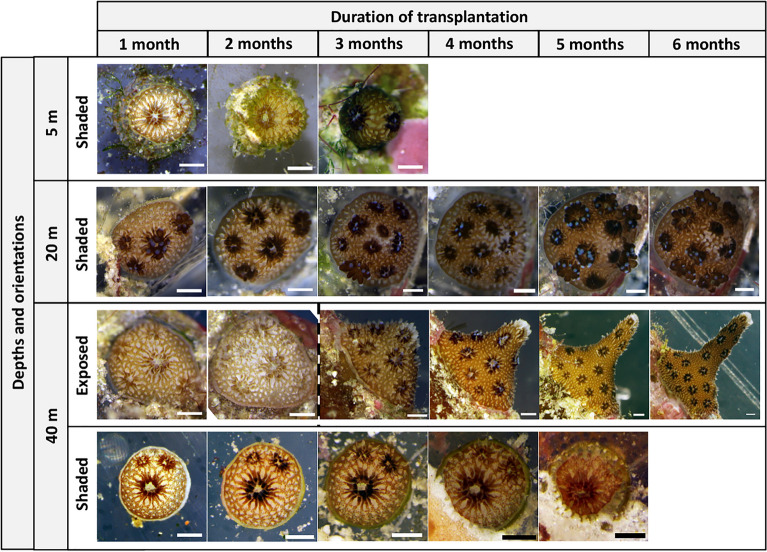
Most juveniles in exposed orientation at 3–5 m (in 2015, 2016) and 20 m (in 2016) did not survive the first month (Fig. 3). In 2015, the survival of juveniles at 3–5 m depth (0%) was significantly lower than other depths (i.e., 20 and 40 m) (Mantel-cox log-rank test, P < 0.001; Fig. 3; Supplementary Table 1). Depth also influenced juvenile survival at the deeper sites in an exposed orientation in 2015, where the 20 m juveniles had significantly lower survival (9.5%) than the 40 m control juveniles (11.1%) after six months (Mantel-cox log-rank test, P = 0.005; Fig. 3; Supplementary Table 1). In 2016, juveniles at 20 m in a shaded orientation (12.1%) and the control juveniles (40 m depth, exposed orientation; 6.3%) survived after six months, while the juveniles at 3–5 m shaded orientation only survived three months (3.6%) (Fig. 3). Importantly, orientation influenced juvenile survival, where the survival at 3–5 m and 20 m in a shaded orientation was similar to survival at 40 m in an exposed orientation (Mantel-cox log-rank test; P > 0.05; Supplementary Table 1).
Figure 3.
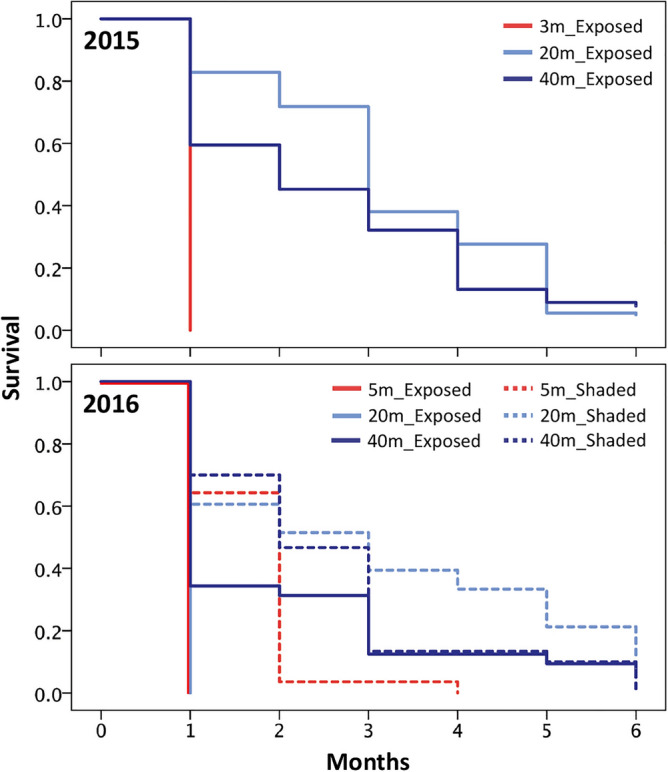
Survival of S. hystrix juveniles over six months in 2015 experiment from August 2015 to February 2016 (3, 20, and 40 m depth; only in exposed orientation) and in 2016 experiment from August 2016 to February 2017 (5, 20, and 40 m depth; exposed and shaded orientation).
In 2015, the number of polyps in juveniles in an exposed orientation at 20 and 40 m increased (Fig. 4), and the mean geometric diameter also increased (Supplementary Fig. 3), although differences were not significant. An average (± SE) of 5.7 ± 0.33 and 5.5 ± 0.78 polyps per juvenile was observed after two months at 20 m and 40 m, respectively (Student’s t-test, t = -0.209, df = 53, P = 0.835). Mean geometric diameter (± SE) of juveniles at 20 and 40 m frames was 1.52 ± 0.04 and 1.44 ± 0.06 mm, respectively, with no significant difference observed (Student’s t-test, t = -0.911, df = 53, P = 0.366, Supplementary Fig. 3).
Figure 4.
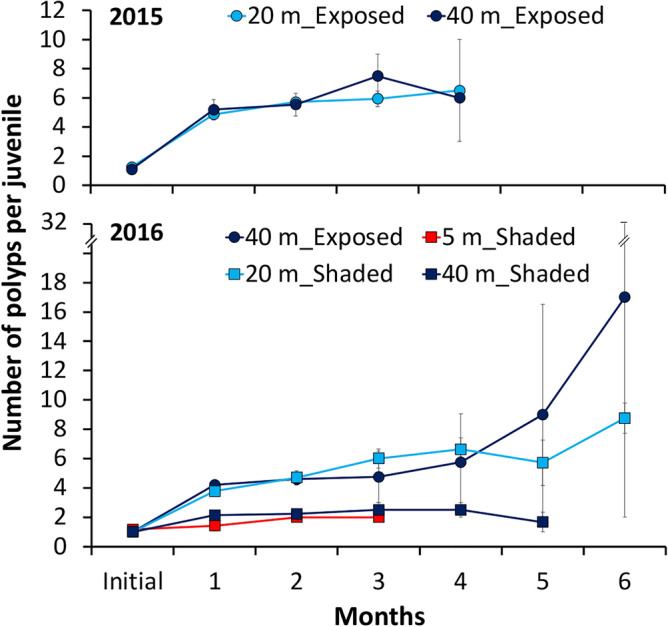
Mean number of S. hystrix polyps per juvenile (± SE) in 2015 experiment from August to December 2015 at 20 and 40 m depth and in 2016 experiment from August 2016 to February 2017 at 5, 20, and 40 m depth in exposed orientation (circles) and shaded orientation (squares).
Similarly, in 2016, the number of polyps in juveniles increased in both exposed and shaded orientations at all depths (Figs. 4, 5). After two months, the shaded juveniles at 20 m (mean ± SE of 4.7 ± 0.46 polyps per juvenile) and the exposed juveniles at 40 m (mean ± SE of 4.6 ± 0.40 polyps per juvenile) had a significantly greater number of polyps per juvenile than the juveniles at 40 m in a shaded orientation (mean ± SE of 2.2 ± 0.20 polyps per juvenile) (Kruskal–Wallis followed by Dunn tests, Chi-squared = 17.92, df = 2, P < 0.001). The shaded juveniles at 20 m had a significantly larger mean geometric diameter (1.40 mm) compared to the 40 m juveniles in exposed (1.24 mm) and shaded orientations (1.23 mm) (Kruskal–Wallis followed by Dunn tests, Chi-squared = 7.43, df = 2, P = 0.020).
Figure 5.
S. hystrix juveniles from mesophotic colonies transplanted at 5 m shaded orientation, 20 m shaded orientation, and 40 m exposed and shaded orientation over six months transplantation from August 2016 to February 2017. For the 40 m exposed orientation, the juvenile at the 1st and 2nd month is different individual with the juvenile from the 3rd to 6th month of transplantation. Scale bars = 400 µm.
Maximum quantum yield, algal density, and chlorophyll pigments of juveniles (field experiment)
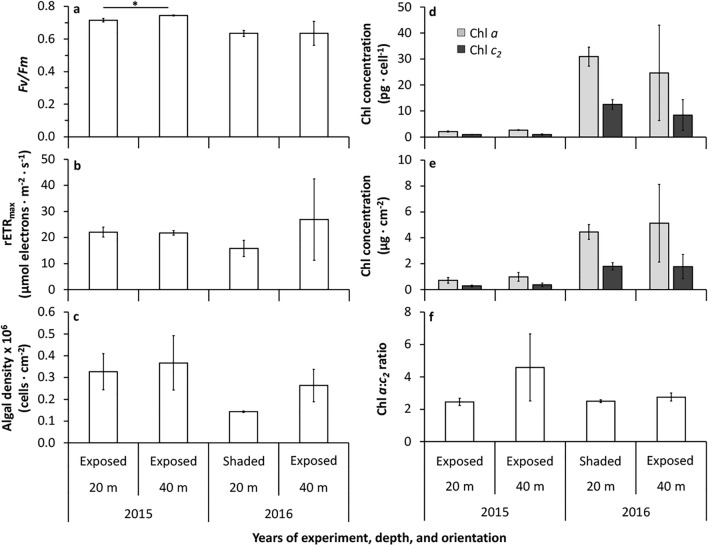
Among the surviving juveniles at the end of 2015 experiment, those at 20 m had significantly lower Fv/Fm (0.715 ± 0.01; mean ± SE; n = 5) compared to juveniles at 40 m (0.744 ± 0.003; n = 5), while similar maximum relative electron transport rates (rETRmax) were observed (Welch’s t-test, P = 0.049 for Fv/Fm; P = 0.898 for rETRmax; Fig. 6a, b; Supplementary Table 2). In 2016, shaded juveniles at 20 m had a Fv/Fm of 0.635 ± 0.02 (mean ± SE; n = 4) and rETRmax of 15.8 ± 3.1 µmol electrons m−2 s−1 (mean ± SE; n = 4), while exposed juveniles at 40 m had a Fv/Fm of 0.635 ± 0.07 (n = 2) and rETRmax of 26.9 ± 15.6 (n = 2), respectively (Fig. 6a, b). No statistical comparison was conducted in the 2016 experiment, due to insufficient number of surviving replicates.
Figure 6.
Maximum quantum yield of Photosystem II (Fv/Fm) (a), rETRmax (b), algal density per surface area (c), chlorophyll (a and c2) per algal cell (d) and per surface area (e), and the ratio of chlorophyll a and c2 (f) of the surviving S. hystrix juveniles at the end of the experiment (after 6 months) in 2015 (20 and 40 m depth both in exposed orientation) and in 2016 (20 m shaded orientation and 40 m exposed orientation) (mean ± SE). The asterisk indicates a significant difference (p = 0.028).
Algal density, chlorophyll a and c2 per cell and surface area, and chlorophyll ratio (a:c2) were similar for juveniles at 20 and 40 m in 2015 (Fig. 6c–f, Supplementary Table 2). In 2016, symbiont density in the shaded juveniles at 20 m and exposed juveniles at 40 m was 1.4 and 2.6 × 105 cells cm−2, respectively. Concentrations of chlorophyll a and c2 per cell were 31.0 and 12.5 pg cell−1, respectively, for those shaded at 20 m, while those exposed at 40 m had 24.7 and 8.4 pg cell−1, respectively (Fig. 6d). An insufficient number of surviving replicates in 2016 prevented statistical comparisons.
Discussion
Our results provide several insights into the potential recovery of S. hystrix in shallow reefs by means of larvae released from colonies living at mesophotic depths. In our laboratory-based experiments, we observed most larvae rapidly crawling at the bottom of the acrylic columns used, settling within 24 h; suggesting most S. hystrix larvae from mesophotic colonies settle close to their parent colonies at mesophotic depths. This behavior is consistent with previous observations on this species9,27. However, our results also suggest some larvae could disperse far from their natal reefs since up to 16% of the larvae remained in the water column or at the surface (Supplementary Fig. 1), and up to 9.7% of the larvae kept swimming four days after release (Fig. 2). This suggests that a non-negligible portion of S. hystrix larvae from mesophotic depth may swim vertically or are passively transported by currents in the water column to find a suitable place to settle further away.
The upward swimming behavior of mesophotic coral larvae and their position in the water column could partly contribute to vertical larval dispersal to shallow reefs. In the present study, since the upward swimming speed of S. hystrix larvae from mesophotic depth was 0.6 mm s−1, in theory, larvae could reach the surface from 40 m depth after 18 h. This lies within the timeframe of larval settlement competency periods. However, the swimming speed of coral larvae is much slower than horizontal currents in the reefs28. Larval dispersal distance is primarily determined by the larval competency period, their position in the water column and the coincident water currents29–31. The longer larvae stay in the water column, the more likely they will disperse. In the case of mesophotic larvae, water movement will also affect their vertical migration. For example, off North Carolina’s coast, upwelling influenced the shoreward migration of larval invertebrates and fish32. No information is available on upwelling around Okinawa during the coral reproductive season. However, at the time of larval release, typhoons could create enough vertical water movement to potentially transport S. hystrix larvae from mesophotic depths to shallow reefs, although the frequency and paths of strong storms vary across years9,33. This stochastic connectivity may explain the absence of genetic partitioning within the water column for S. hystrix in Okinawa26. Future studies on current patterns during spawning/larval release are necessary to understand larval dispersal processes from mesophotic reefs.
Mesophotic larvae that are dispersed to shallower areas of the reef face several challenges to their survival. The laboratory experiments showed that settlement rates of S. hystrix larvae from mesophotic colonies were reduced when exposed to light conditions corresponding to depths shallower than 20 m (i.e., 10 and 5 m) (Fig. 2). This supports previous findings where the larvae of shallow corals preferred to settle in similar light conditions as parental colonies34,35. In addition, most of the pre- and post-settled larvae partially or completely bleached under light conditions representing depths shallower than 20 m (Supplementary Fig. 2). The vertical transmission of algal symbionts into larvae from mesophotic corals like S. hystrix might be unfavorable for larvae dispersed to shallower water since the higher light conditions increased DNA damage in the symbiont algae36. Likewise, excess algal symbionts increases the susceptibility of adult corals to bleaching37. Therefore, in shallow reefs, settlement of larvae from mesophotic corals may be limited to shaded microhabitats such as steep spurs or overhangs. However, it is essential to note that light conditions cannot be solely interpreted as light intensity alone due to differences in the light spectrum used in our experiments; hence, further investigation into the independent effect of light quantity and quality is required.
In addition to high light stress, S. hystrix larvae from mesophotic depth will likely be exposed to thermal stress when dispersing to shallow reefs. In 2016, many corals bleached between 0 to 20 m depth in Okinawa38,39 (pers. Obs.), where high (> 3 °C) temperature differences between shallow and mesophotic depths occurred for the majority of the planula release period. Elevated temperatures (> 3 °C above average seasonal temperature) can lower larval survival and reduce dispersal40. In the case of S. hystrix from mesophotic depths in Okinawa, high temperature discrepancy between shallow and mesophotic depths during thermal stress events will likely reduce settlement success in shallow reefs.
While laboratory experiments on larvae focused on light stress, field experiments on juveniles combined light and thermal stresses. The overall survival of juveniles significantly increased with depth, and those in a shaded orientation showed higher survival than juveniles in exposed orientations (Supplementary Table 1). As observed for the larvae in laboratory experiments, the high light intensity may cause stress on juveniles at 3–5 and 20 m depths in exposed orientation (light intensity was ~ 12 and ~ 2 fold higher than 40 m depth, respectively). Similarly, for adult corals, most of the low light-adapted corals transplanted to high light environments showed bleaching41 and high mortality42,43 (but see cases for the S. pistillata15 and F. paradivisa16 for contrasting responses).
The survival of juveniles from mesophotic depth in shallower reefs was also affected by substrate orientation. In the 2016 field experiments, in shallower reefs (i.e., 3–5 and 20 m depths), most juveniles survived when in a shaded orientation (Fig. 3), i.e. in low light environments created by crevices and overhangs. These observations support previous studies showing the frequent occurrence of juveniles on shaded or vertical surfaces in shallow reefs44,45. Such surfaces also provide protection from sediment accumulation46 and fish grazing47. Conversely, almost all juveniles at 3–5 m and those at 20 m in an exposed orientation died within the first month of transplantation (Fig. 3). This mortality corresponds with exceptional thermal stress. Indeed, around Sesoko Island, sea surface temperature in 2016 was the second-highest in the last three decades after the 1998 bleaching event39. Since mortality in adult corals has been attributed to thermal and high light stresses48, this combination most likely affected the survival of S. hystrix from mesophotic depth in shallow and intermediate depths in 2016.
Surprisingly, the coral juveniles that survived at 20 m depth showed significantly increased growth rate at 20 m, or remained relatively similar, to those at 40 m, both at the exposed and shaded orientation (Fig. 4; Supplementary Fig. 3). A similar result has been reported previously, where juveniles of Stylophora kuehlmanni Sheer and Pillai 1983 were almost twice as large at 5 m as those at their natural depth of 45 m49. Likewise, adult colonies of S. hystrix (Pers. Obs.), S. pistillata15, and Orbicella franksi (Gregory 1895)50 grew significantly faster at shallow reefs (< 20 m) than those at MCEs. In these cases, higher light intensity in the shallower reefs may have enhanced calcification in corals by light activation51. However, this is unlikely to have occurred in our study since a significant increase in growth rate was only found at the 20 m shaded orientation with extremely low light intensity (~ 18 fold lower than 40 m exposed orientation). A possible explanation is that warmer seawater temperature (~ 0.5 °C warmer at 20 m than at 40 m) during the first two months of juvenile transplant experiments enhanced juvenile growth52,53. The juvenile growth rates observed in our study indicate that the intermediate depth of 20 m is adequate for the successful recruitment of S. hystrix from mesophotic depth.
Photoacclimation responses are also likely to play an essential role in juvenile survival in shallow reef environments. Here, the response of the algal symbiont to the higher light conditions can be seen through the decrease in maximum quantum yield, Fv/Fm (Fig. 6a). It should be noted that measurements of Fv/Fm were performed in the laboratory and, as such, changes in seawater temperature during the transfer from the field sites could have affected the results. To minimize this, samples were carefully transferred under dark conditions and immediately measured upon arrival to the laboratory. Minor variations were observed between samples (Fig. 6a), likely due to slight differences in light intensity between 20 and 40 m depth at the time of measurement (the light intensity differences between 20 and 40 m was ~ 37 µmol photon m−2 s−1), however the seawater temperature was similar at both depths. The differences in light intensity between 20 and 40 m depths may also be responsible for similar variations in the other indicators measured (algal density and chlorophyll concentrations). Other studies have also reported a decrease in Fv/Fm as one of the photoacclimatory processes of MCEs adult colonies of S. pistillata (from ~ 0.67 at 30 m to ~ 0.64 at 3 m) and F. paradivisa (from 0.66 at 50 m to 0.49 at 5 m) when transplanted to shallower depths15,17. A decrease in maximum quantum yield under high light conditions reflects potential damage to photosystem II or adaptation to reduce photo-oxidative damage to the photosynthetic apparatus in the symbiotic algae within corals54. Thus, adjustment of maximum quantum yield, Fv/Fm, in the symbiont can be considered a strategy for coral juveniles from mesophotic depth to acclimate to different light environments.
Green fluorescent proteins (GFPs) in corals have been suggested to play a role in photoprotective mechanism55, avoidance from predators56, and prey attraction57. While no study on corals from mesophotic depths has investigated fluorescent proteins in juveniles, a few studies focused on adults58–61. However, no specific conclusion could be drawn regarding the potential role of fluorescent proteins in mesophotic corals even though ~ 70% of corals fluoresced over the entire part of the corals observed60. In the Red Sea, mesophotic Galaxea fascicularis (Linnaeus 1767) from mesophotic depth increased its GFPs when exposed to light representing 3 and 20 m depth, suggesting a photoprotective function of GFPs59. In the present study, the juveniles at both 40 and 20 m (exposed and shaded orientation) had GFP with similar fluorescent characteristics (see ESM). Therefore, GFP for juveniles in a low light environment is likely to have other roles than photoprotection. Further studies are needed to quantify the intensity of coral fluorescence at mesophotic depth, investigating the role of fluorescent proteins as the coral’s defense mechanism at high light intensity during acclimation to shallow reefs.
In terms of recolonization of shallow reefs, our results showed that a small portion (up to 16%) of S. hystrix larvae from mesophotic colonies might disperse to shallower reef habitats. A shorter reproductive season and smaller planula size compared to their shallow counterparts9 also supports limited dispersal. Moreover, as light conditions at shallower depths impede direct recolonization of coral larvae from mesophotic depth, rugosity or tridimensional complexity of the shallow reef will play an essential role for mesophotic larvae by providing suitable shaded habitats. Around our study site at intermediate depth (~ 20 m), in 2015 and 2016 a few small colonies of S. hystrix were observed, and more recently, the occurrence of Seriatopora colonies at 20 m and 10 m appears to be increasing (pers. obs.). In 2021, a single, well-developed colony at 4.8 m was observed within the field experiment area (pers. obs.). No evidence yet whether those new colonies at a shallower depth originated from settlement of larvae from mesophotic colonies. Our results, and the absence of genetic structure related to depth between shallow and mesophotic Seriatopora in the region26, suggest that 20 m depth may act as a stepping stone to connect mesophotic corals to shallow reefs. We suggest that recolonization from mesophotic depths to shallow reefs occurs through multigenerational recruitment over a long-term period. However, anthropogenic stressors exacerbating coral reefs’ degradation62 will limit the potential of mesophotic corals to reseed shallow reefs. Indeed, S. hystrix juveniles from mesophotic depth transplanted to shallower depths during the thermal stress event of 2016 suffered high mortality, even at 20 m. If such events occur more frequently in the future63, they may reduce the stepping stone capacity of 20 m reef habitats. Given the ecological importance of mesophotic corals, the origin of shallow water colonies and the process for recolonization of these habitats deserves further investigation. To fully understand the contribution of MCEs to shallow reef recovery, future studies should include a range of corals with differing spawning mechanisms and agal transmission modes.
Methods
Study sites, coral collection, and environmental measurements
Six to ten mature colonies (> 15 cm diameter) of S. hystrix were collected several times between June and August 2015 and 2016 from an MCE site (40 m depth) north of Sesoko Island, Okinawa, Japan (Supplementary Fig. 4, Supplementary Table 3). Each colony was maintained in indoor seawater systems at the Sesoko Station of the University of the Ryukyus (described in9). Seriatopora hystrix is a brooder whose larvae contain symbiotic algae when released (vertical transmission)27 and, in Okinawan mesophotic reefs, S. hystrix release larvae monthly from May to August9. The larvae released during the peak planula release period9 were used for larval behavior and settlement experiments ex situ and juvenile acclimation experiments in situ. They were kept in filtered seawater (0.2 µm) until the experiments to avoid the presence of larvae and symbiotic algae from other species coming through the seawater supply and keep better water quality. In situ juvenile acclimation experiments were conducted at three sites: at the MCE site (40 m; where the original adult parent colonies originated) and at two shallower sites (3–5 m and 20 m, within 750 m from the MCE site; Supplementary Fig. 4). Seawater temperatures and light intensity were measured hourly at each site using loggers (HOBO Pendant/Light Data Logger, Onset Computer Corporation, USA).
Effect of different light conditions on larval behavior and settlement (laboratory experiment)
Fifty to a hundred larvae colony−1 day−1 were released on 18–20 July 2016 (5 colonies). They were all pooled in a 10 L tank filled with filtered seawater daily. Larvae were used in the following two experiments within 6 h of release. To examine the larval position in the water column, ten larvae were exposed to light conditions representing environments at 40 m, 20 m, 10 m, and 5 m depths (i.e., 50–60, 250, 450 and 600 µmol quanta m−2 s−1, respectively) in 80-cm-tall acrylic columns using LED lights (Hydra 52, C2 Development Inc., USA) (Supplementary Table 4). After 10 min, the planulae were counted at the surface (0–2 cm deep), the upper layer (2–40 cm deep), the lower layer (40–78 cm deep), and the bottom layer (0–2 cm above the bottom). The experiment was replicated eight (for 5 and 40 m conditions) or ten times (for 10 and 20 m conditions) using new individuals. In addition, the swimming speeds of larvae were measured by tracking their movement for up to 2 min in each light condition (n = 3, 7, 7, and 8 larvae in total in 5, 10, 20, and 40 m light conditions, respectively). The tracks were traced to transparent plastic sheets on the side of the acrylic column using a marker.
To examine larval settlement and survival, 30 newly released planulae were transferred to 700 ml plastic containers. Plastic settlement plates (circular shape, diameter: 6 cm, circle polyethylene V-type container V-1, AS ONE, Japan) were placed in each container since S. hystrix larvae prefer to settle on plastic9. The containers were exposed to 13 h:11 h (light:dark) photoperiods under the light environment at 40 m, 20 m, 10 m, and 5 m depths, see above). Six replicates were performed in each light condition. The larvae were counted over four days and classified as (i) settled, (ii) crawling/swimming, or (iii) dead. Pre- and post-settled larval conditions were monitored as healthy or bleached (Supplementary Fig. 2). Seawater temperature was kept at 27 ± 1 °C (i.e., the temperature at 40 m) and was replaced twice a day (i.e., 8:00–10:00 and 18:00–20:00) with 50–60% FSW.
Juvenile acclimation to different depths (field experiment)
Hundreds of larvae per colony released on 27 July–6 August 2015 (8 colonies) and 18–28 July 2016 (9 colonies) were pooled and kept in a 10 L filtered seawater tank. The plastic settlement plates (same as above) were then placed in the 10 L tank to facilitate settlement. A total of 477 and 181 planulae settled on 29 and 46 settlement plates in 2015 and 2016, respectively (ranging between 1 and 50 planulae per plate). The location of juveniles on the plates was mapped under a dissecting microscope. In both years, plates with 1-week-old juveniles were fixed horizontally to the top of rectangular PVC frames and deployed in August at three depths (3–5, 20, and 40 m sites). In 2015, n = 146, 163, and 168 juveniles on the plates were distributed at 3–5, 20, and 40 m sites, respectively, and all plates were placed upward on the frames exposed to direct sunlight (hereafter, this position is referred to as exposed orientation). In 2016, the plates were placed upward and downward (hereafter referred to as shaded orientation) on the frames (Supplementary Fig. 5). The plates hosted between 28 and 32 juveniles at each depth and orientation.
Survival and growth of juveniles
Monthly observations were performed to examine juvenile survival and growth in 2015 and 2016. The juveniles were carefully transported to Sesoko Station (in the dark, covered by shade cloth) and maintained under their respective depths’ light conditions. Juvenile survival was examined as either alive or dead based on their color and the presence of coral tissue under a dissecting microscope. The number of polyps and geometric diameter were measured under a dissecting microscope to examine juvenile growth. In 2015, 291 out of 477 juveniles were selected for growth measurements. In 2016, all the juveniles were measured. After the observations (within two days), the juveniles were returned to the originally transplanted depths.
Maximum quantum yield, algal density, chlorophyll pigments of juveniles
At the end of the experiment in both years (February 2015 and 2016), the juveniles were transferred under dark conditions to the laboratory to measure the maximum quantum yield of the symbiotic algae using a pulse-amplitude modulated chlorophyll fluorometer (Diving-PAM, Walz, Germany). A total of ten juveniles (5 each for 20 m and 40 m exposed orientation; 2015) and six juveniles (4 for 20 m shaded orientation; 2 for 40 m exposed orientation; 2016) were dark-adapted for 30 min before maximum quantum yield (Fv/Fm) and maximum relative electron transport rate (rETRmax) measurements (Supplementary Materials and Methods). Soft tissues of each juvenile were then removed using an airbrush. The tissues were then extracted for algal density, chlorophyll pigments (described in58) and fluorescent protein analyses (Supplementary Materials and Methods).
Statistical analyses
Before analyses, the statistical assumptions were verified with Shapiro–Wilk (normality) and Levene’s (homogeneity) tests and transformed if necessary (log, inverse sine, and square root transformation). All statistical analyses were conducted using SPSS (version 11.5).
For laboratory experiments, one-way ANOVA tests were performed to examine differences in larval swimming speeds across light conditions (40, 20, 10, and 5 m) for each downward (n = 9, 9, 12, and 4 measurements, respectively) and upward direction (n = 3, 3, 3, and 2 measurements, respectively). A one-way ANOVA, followed by Bonferroni post hoc tests, was performed to test for significant differences in larval settlement rate after 96 h under different light conditions (40, 20, 10, and 5 m light conditions); the container was the experimental unit (n = 6), the light condition was considered as a fixed factor.
For the field experiments, survival rates of juveniles were estimated using Kaplan–Meier (K–M) survival analysis. Each juvenile was assumed to be independent of the other. Mantel-cox log-rank tests were compared the estimated K-M survival curves among depths in 2015 and depths and orientations in 2016. Pairwise comparisons of Mantel-cox log-rank tests were used to quantify differences in survival curves for a given year. Student’s t-tests were used to detect the significance of the number of polyps and geometric diameter between depths (20 m, n = 44; 40 m, n = 11) at two months in 2015. In 2016, a Kruskal–Wallis test, followed by Dunn post hoc tests, was used to detect significant differences between depths and orientation (20 m shaded orientation, n = 17; 40 m exposed, n = 10; and 40 m shaded, n = 14). Statistical analyses for growth in both years were only performed during the first two months of transplantation due to an insufficient number of surviving juveniles beyond these periods. Welch’s t-tests were used to examine significance between depths (20 and 40 m; both n = 5) in 2015 on maximum quantum yield (Fv/Fm and rETRmax) and chlorophyll (a and c2) per surface area and per cell. A Mann–Whitney U test examined the significance of chlorophyll (a:c2) ratio between depths (20 and 40 m; both n = 5).
Ethics approval
Coral colonies were sampled under permits issued by Okinawa prefectural government, Japan (No. 27-28 and 28-21).
Supplementary Information
Acknowledgements
We thank Drs. M. Yorifuji, H. Rouzé, M. Morita and Mr. S. Kadena, Mr. M. Jinza, Mr. Y. Nakano from the University of the Ryukyus, and Mr. Shigeki Nakamura for field assistance. We also thank the Center for Research Advancement and Collaboration, University of the Ryukyus, for their assistance with fluorescent protein analyses and two anonymous reviewers for their constructive comments on this manuscript. Thanks to Emu Editing for editing English. This research was funded by Grant-in-Aid for Scientific Research of the Japan Society for the Promotion of Science, KAKENHI to SH (No. 16H02490, No. 16KK0164 and No. 21H04943), to FS (No. 20K06210) and Sasakawa Scientific Research Grants from the Japan Science Society to RP (No. 27–749).
Author contributions
R.P., F.S., S.H. conceptualized, performed sample collection and experiments; R.P. performed analyses and wrote the first draft; R.P., F.S., T.N., S.H. reviewed and edited the manuscript. All authors contributed to the manuscript and gave final approval for publication.
Data availability
All data needed to evaluate the conclusions are present in the paper and/or the Supplementary Materials. The raw data analyzed in this study are available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in the Results section. Full information regarding the correction made can be found in the correction notice for this Article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/12/2022
A Correction to this paper has been published: 10.1038/s41598-022-20029-6
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-16024-6.
References
- 1.Hughes TP, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 2.Glynn PW. Coral reef bleaching: facts, hypotheses and implications. Glob. Chang. Biol. 1996;2:495–509. [Google Scholar]
- 3.Riegl B, Piller WE. Possible refugia for reefs in times of environmental stress. Int. J. Earth Sci. 2003;92:520–531. [Google Scholar]
- 4.Hinderstein LM, et al. Theme section on ‘Mesophotic Coral Ecosystems: Characterization, Ecology, and Management’. Coral Reefs. 2010;29:247–251. [Google Scholar]
- 5.Bongaerts P, Ridgway T, Sampayo EM, Hoegh-Guldberg O. Assessing the ‘deep reef refugia’ hypothesis: Focus on Caribbean reefs. Coral Reefs. 2010;29:309–327. [Google Scholar]
- 6.Smith TB, et al. Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Global Change Biol. 2016;22:2756–2765. doi: 10.1111/gcb.13175. [DOI] [PubMed] [Google Scholar]
- 7.Frade PR, et al. Deep reefs of the Great Barrier Reef offer limited thermal refuge during mass coral bleaching. Nat. Commun. 2018;9:3447. doi: 10.1038/s41467-018-05741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holstein DM, Paris CB, Vaz AC, Smith TB. Modeling vertical coral connectivity and mesophotic refugia. Coral Reefs. 2016;35:23–37. [Google Scholar]
- 9.Prasetia R, Sinniger F, Hashizume K, Harii S. Reproductive biology of the deep brooding coral Seriatopora hystrix: Implications for shallow reef recovery. PLoS ONE. 2017;12:e0177034. doi: 10.1371/journal.pone.0177034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shlesinger T, Grinblat M, Rapuano H, Amit T, Loya Y. Can mesophotic reefs replenish shallow reefs? Reduced coral reproductive performance casts a doubt. Ecology. 2018;99:421–437. doi: 10.1002/ecy.2098. [DOI] [PubMed] [Google Scholar]
- 11.Gleason DF, Hofmann DK. Coral larvae: From gametes to recruits. J. Exp. Mar. Bio. Ecol. 2011;408:42–57. [Google Scholar]
- 12.Hughes TP, Tanner JE. Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology. 2000;81:2250–2263. [Google Scholar]
- 13.Bongaerts P, et al. Deep reefs are not universal refuges: Reseeding potential varies among coral species. Sci. Adv. 2017;3:e1602373. doi: 10.1126/sciadv.1602373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Oppen MJH, Bongaerts P, Underwood JN, Peplow LM, Cooper TF. The role of deep reefs in shallow reef recovery: An assessment of vertical connectivity in a brooding coral from west and east Australia. Mol. Ecol. 2011;20:1647–1660. doi: 10.1111/j.1365-294X.2011.05050.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen I, Dubinsky Z. Long term photoacclimation responses of the coral Stylophora pistillata to reciprocal deep to shallow transplantation: Photosynthesis and calcification. Front. Mar. Sci. 2015;2:45. [Google Scholar]
- 16.Eyal G, et al. Euphyllia paradivisa, a successful mesophotic coral in the northern Gulf of Eilat/Aqaba, Red Sea. Coral Reefs. 2016;35:91–102. [Google Scholar]
- 17.Ben-Zvi O, et al. Photophysiology of a mesophotic coral 3 years after transplantation to a shallow environment. Coral Reefs. 2020;39:903–913. [Google Scholar]
- 18.Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerget. 2007;1767:414–421. doi: 10.1016/j.bbabio.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi S, Murata N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008;13:178–182. doi: 10.1016/j.tplants.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Cumbo VR, Baird AH, van Oppen MJH. The promiscuous larvae: Flexibility in the establishment of symbiosis in corals. Coral Reefs. 2013;32:111–120. [Google Scholar]
- 21.Little AF, Van Oppen MJH, Willis BL. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;304:1492–1494. doi: 10.1126/science.1095733. [DOI] [PubMed] [Google Scholar]
- 22.Sinniger F, Morita R, Harii S. ‘Locally extinct’ coral species Seriatopora hystrix found at upper mesophotic depths in Okinawa. Coral Reefs. 2013;32:153. [Google Scholar]
- 23.Sinniger F, et al. Overview of the mesophotic coral ecosystems around Sesoko Island, Okinawa, Japan. Galaxea J. Coral Reef Stud. 2022;24:69–76. [Google Scholar]
- 24.Loya Y, et al. Coral bleaching: the winners and the losers. Ecol. Lett. 2001;4:122–131. [Google Scholar]
- 25.van Woesik R, Sakai K, Ganase A, Loya Y. Revisiting the winners and the losers a decade after coral bleaching. Mar. Ecol. Prog. Ser. 2011;434:67–76. [Google Scholar]
- 26.Sinniger F, Prasetia R, Yorifuji M, Bongaerts P, Harii S. Seriatopora diversity preserved in upper mesophotic coral ecosystems in Southern Japan. Front. Mar. Sci. 2017;4:155. [Google Scholar]
- 27.Atoda K. The larva and postlarval development of some reef-building corals. V. Seriatopora hystrix. Sci. Rep. Tohoku Univ. 1951;19:33–39. [Google Scholar]
- 28.Hata T, et al. Coral larvae are poor swimmers and require fine-scale reef structure to settle. Sci. Rep. 2017;7:2249. doi: 10.1038/s41598-017-02402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harii S, Kayanne H. Larval dispersal, recruitment, and adult distribution of the brooding stony octocoral Heliopora coerulea on Ishigaki Island, southwest Japan. Coral Reefs. 2003;22:188–196. [Google Scholar]
- 30.Mulla AJ, Lin CH, Takahashi S, Nozawa Y. Photo-movement of coral larvae influences vertical positioning in the ocean. Coral Reefs. 2021;40:1297–1306. [Google Scholar]
- 31.Figueiredo J, Baird AH, Harii S, Connolly SR. Increased local retention of reef coral larvae as a result of ocean warming. Nat. Clim. Chang. 2014;4:498–502. [Google Scholar]
- 32.Shanks AL, Largier J, Brink L, Brubaker J, Hooff R. Demonstration of the onshore transport of larval invertebrates by the shoreward movement of an upwelling front. Limnol. Oceanogr. 2000;45:230–236. [Google Scholar]
- 33.Singh T, et al. Long-term trends and seasonal variations in environmental conditions in Sesoko Island, Okinawa, Japan. Galaxea J. Coral Reef Stud. 2022;24:121–133. [Google Scholar]
- 34.Roth MS, Fan T-Y, Deheyn DD. Life history changes in coral fluorescence and the effects of light intensity on larval physiology and settlement in Seriatopora hystrix. PLoS ONE. 2013;8:e59476. doi: 10.1371/journal.pone.0059476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mundy CN, Babcock RC. Role of light intensity and spectral quality in coral settlement: Implications for depth-dependent settlement? J. Exp. Mar. Bio. Ecol. 1998;223:235–255. [Google Scholar]
- 36.Nesa B, Baird AH, Harii S, Yakovleva I, Hidaka M. Algal symbionts increase DNA damage in coral planulae exposed to sunlight. Zool. Stud. 2012;51:12–17. [Google Scholar]
- 37.Cunning R, Baker AC. Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat. Clim. Change. 2013;3:259–262. [Google Scholar]
- 38.Nakamura T. Mass coral bleaching event in Sekisei lagoon observed in the summer of 2016. J. Jpn. Coral Reef Soc. 2017;19:29–40. [Google Scholar]
- 39.Sakai K, Singh T, Iguchi A. Bleaching and post-bleaching mortality of Acropora corals on a heat-susceptible reef in 2016. PeerJ. 2019;7:e8138. doi: 10.7717/peerj.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edmunds PJ, Gates RD, Gleason DF. The biology of larvae from the reef coral Porites astreoides, and their response to temperature disturbances. Mar. Biol. 2001;139:981–989. [Google Scholar]
- 41.Baker AC. Reef corals bleach to survive change. Nature. 2001;411:765–766. doi: 10.1038/35081151. [DOI] [PubMed] [Google Scholar]
- 42.Bongaerts P, et al. Adaptive divergence in a scleractinian coral: Physiological adaptation of Seriatopora hystrix to shallow and deep reef habitats. BMC Evol. Biol. 2011;11:303. doi: 10.1186/1471-2148-11-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Einbinder S, et al. Novel adaptive photosynthetic characteristics of mesophotic symbiotic microalgae within the reef-building coral, Stylophora pistillata. Front. Mar. Sci. 2016;3:195. [Google Scholar]
- 44.Rogers CS, Fitz HC, Gilnack M, Beets J, Hardin J. Scleractinian coral recruitment patterns at Salt River submarine canyon, St. Croix, U.S. Virgin Islands. Coral Reefs. 1984;3:69–76. [Google Scholar]
- 45.Maida M, Collb JC, Sammarco PW. Shedding new light on scleractinian coral recruitment. J. Exp. Mar. Biol. Ecol. 1994;180:189–202. [Google Scholar]
- 46.Sato M. Mortality and growth of juvenile coral Pocillopora damicornis (Linnaeus) Coral Reefs. 1985;4:27–33. [Google Scholar]
- 47.Nozawa Y. Micro-crevice structure enhances coral spat survivorship. J. Exp. Mar. Biol. Ecol. 2008;367:127–130. [Google Scholar]
- 48.Gleason DF, Wellington GM. Ultraviolet radiation and coral bleaching. Nature. 1993;365:836–838. [Google Scholar]
- 49.Shlesinger T, Loya Y. Depth-dependent parental effects create invisible barriers to coral dispersal. Commun. Biol. 2021;4:1–10. doi: 10.1038/s42003-021-01727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groves SH, et al. Growth rates of Porites astreoides and Orbicella franksi in mesophotic habitats surrounding St. Thomas, US Virgin Islands. Coral Reefs. 2018;37:345–354. [Google Scholar]
- 51.Al-Horani FA, Al-Moghrabi SM, De Beer D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2003;142:419–426. [Google Scholar]
- 52.Jiang L, et al. Increased temperature mitigates the effects of ocean acidification on the calcification of juvenile Pocillopora damicornis, but at a cost. Coral Reefs. 2018;37:71–79. [Google Scholar]
- 53.Jurriaans S, Hoogenboom MO. Thermal performance of scleractinian corals along a latitudinal gradient on the Great Barrier Reef. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20180546. doi: 10.1098/rstb.2018.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown BE, et al. Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs. 1999;18:99–105. [Google Scholar]
- 55.Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O. Fluorescent pigments in corals are photoprotective. Nature. 2000;408:850–853. doi: 10.1038/35048564. [DOI] [PubMed] [Google Scholar]
- 56.Matz MV, Marshall NJ, Vorobyev M. Are corals colorful? Photochem. Photobiol. 2006;82:345–350. doi: 10.1562/2005-08-18-RA-653. [DOI] [PubMed] [Google Scholar]
- 57.Haddock SHD, Dunn CW. Fluorescent proteins function as a prey attractant: Experimental evidence from the hydromedusa Olindias formosus and other marine organisms. Biol. Open. 2015;4:1094–1104. doi: 10.1242/bio.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eyal G, et al. Spectral diversity and regulation of coral fluorescence in a mesophotic reef habitat in the Red Sea. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0128697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ben-Zvi O, Eyal G, Loya Y. Light-dependent fluorescence in the coral Galaxea fascicularis. Hydrobiologia. 2015;759:15–26. [Google Scholar]
- 60.Roth M, et al. Fluorescent proteins in dominant mesophotic reef-building corals. Mar. Ecol. Prog. Ser. 2015;521:63–79. [Google Scholar]
- 61.Ben-Zvi O, Eyal G, Loya Y. Response of fluorescence morphs of the mesophotic coral Euphyllia paradivisa to ultra-violet radiation. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-41710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes TP, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 63.Oliver ECJ, et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018;9:1324. doi: 10.1038/s41467-018-03732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakamura T, van Woesik R, Yamasaki H. Photoinhibition of photosynthesis is reduced by water flow in the reef-building coral Acropora digitifera. Mar. Ecol. Prog. Ser. 2005;301:109–118. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions are present in the paper and/or the Supplementary Materials. The raw data analyzed in this study are available from the corresponding author on request.