Abstract
This study reports on the identification and characterization of a novel abortive infection system, AbiU, from Lactococcus lactis. AbiU confers resistance to phages from the three main industrially relevant lactococcal phage species: c2, 936, and P335. The presence of AbiU reduced the efficiency of plaquing against specific phage from each species as follows: 3.7 × 10−1, 1.0 × 10−2, and 1.0 × 10−1, respectively. abiU involves two open reading frames, abiU1 (1,772 bp) and abiU2 (1,019 bp). Evidence indicates that AbiU1 is responsible for phage resistance and that AbiU2 may downregulate phage resistance against 936 and P335 type phages but not c2 type phage. AbiU appeared to delay transcription of both phage 712 and c2, with the effect being more marked on phage c2.
Lactococcus lactis is the main bacterial species used as starter cultures in dairy fermentations. However, phage infection of this species is a problem that causes significant economic loss. Research on natural phage resistance systems encoded by Lactococcus led to the identification of four different mechanisms: adsorption inhibition, DNA injection blocking, restriction-modification (R/M), and abortive infection (abi) (for a recent review, see reference 12). The first three systems operate at steps early in the phage infection process, whereas abortive infection can target various different phases of phage development, including DNA replication, RNA transcription, protein synthesis, packaging, and morphogenesis.
To date, 18 abortive infection systems have been sequenced and are designated AbiA through AbiR (7, 12). DNA and protein sequence analysis indicates that none of the abi systems has any significant homology with other genes and proteins in the databanks. In addition, few motifs have been identified, making it difficult to hypothesize the function(s) of each protein (12). While comparison of the different abi systems revealed that AbiD, AbiD1, and AbiK proteins are related, they have been reported to have very different modes of action (1, 11, 22). Fourteen of the eighteen abi systems sequenced are encoded by single genes. Four abi systems—AbiE, AbiG, AbiL, and AbiR—involve two genes (6, 7, 13, 26). DNA sequence analysis, Northern hybridization, and reverse transcription-PCR demonstrated that the two genes of AbiE, AbiG, and AbiL are cotranscribed (6, 13, 26). AbiR is encoded by two separate genetic loci (7).
The mode of action of various different abi systems have been studied. AbiA, AbiF, and AbiK were found to interfere with phage DNA replication (11, 13, 19). AbiQ was found to cause accumulation of the replicative form of phage DNA so that it could not be cleaved into the mature form. AbiB and AbiG were demonstrated to interfere with phage RNA transcription (26, 27). AbiD1 has been shown to interact with a phage operon consisting of four genes (1). AbiD1 appears to act in cooperation with Orf1 to decrease the level of Orf3 to below that required for proper phage development (1). In the presence of AbiA, the appearance of major capsid protein (MCP) of phage ul36 was delayed compared to that produced in the sensitive host (24). AbiC reduces the amount of MCP production (9). Like AbiA, AbiK also inhibited phage ul36 DNA replication. As a consequence, MCP of phage ul36 could not be detected in AbiK+ cells. It is believed that AbiK acts prior to phage DNA replication (11). MCP was produced normally in AbiQ+ cells, but the lytic cycle of the phage was blocked after MCP synthesis (12). The mechanistic studies clearly indicate that the different abi systems act on different phage targets at various stages of development.
The various abi systems affect the three main species of lactococcal phages (c2, 936, and P335) differently. Some Abis, such as AbiB, AbiE, AbiH, and AbiJ, are effective against only one of the three main species of lactococcal phages. Others, such as AbiC, AbiD, AbiD1, AbiF, AbiG, AbiI, and AbiL, inhibit propagation of two species of phage. For AbiA and AbiK, representatives of all three phage species are inhibited.
This study describes the isolation and molecular characterization of a novel abortive infection phage resistance system, AbiU, from a native plasmid that was isolated from a phage-resistant industrial strain, L. lactis LL51-1.
MATERIALS AND METHODS
Bacterial strains, plasmids, phages, and media.
The strains and plasmids used in this study are listed in Table 1. Escherichia coli was grown at 37°C in Luria-Bertani medium (30). L. lactis was grown at 30°C in M17 medium (31) supplemented with 0.5% glucose (M17G). For propagation of phages, calcium chloride (10 mM) was added to M17G medium. For selection and plasmid maintenance, antibiotics were added to the medium as follows: for E. coli, 100 μg of spectinomycin per ml; for L. lactis, 500 μg of spectinomycin, 300 IU of nisin, and 500 μg of streptomycin per ml.
TABLE 1.
Bacterial strains, plasmids, and phages
| Strain, plasmid, or phage | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strain | ||
| L. lactis subsp. lactis | ||
| LL51-1 | Industrial strain, lac+prt+ Nisr | DSM Food Specialties |
| LM0230 | lac prt plasmid-free derivative of c2 | 10 |
| LM0230Smr | Smr derivative of LM0230 | 8 |
| L. lactis subsp. cremoris UL8 | 25 | |
| E. coli NM522 | Transformation host | 17 |
| Plasmids | ||
| pDL278 | SpecrE. coli-L. lactis shuttle vector (6.6 kb) | 21 |
| pND001 | Native plasmid of LL51-1, 75 kb | This study |
| pND002 | 5.2-kb HindIII fragment from pND001 cloned into pDL278 | This study |
| pND003 | BamHI deletion of pND002 (abiU1 deletion) | This study |
| pND006 | abiU2 deletion of pND002 | This study |
| Phage | ||
| φ712 | Small isometric-headed phage, 936 species propagated on LM0230 | 14 |
| φc2 | Prolate-headed phage, c2 species propagated on LM0230 | 12 |
| φul36 | Small isometric-headed phage, P335 species propagated on UL8 | 25 |
Specr, Nisr, and Smr, spectinomycin, nisin, and streptomycin resistance, respectively.
Conjugation and transformation.
Conjugation was carried out by filter mating (15). L. lactis was transformed by electroporation as described by Powell et al. (28). For E. coli, the CaCl2 transformation method was used as described by Sambrook et al. (30).
Phage techniques.
Cross-streaking was employed for the initial screening of phage-resistant and phage-sensitive isolates. Phage preparations were titered by a standard plaque assay (20). The efficiency of plaquing (EOP) was calculated by dividing the phage titer on test strains by that on the sensitive host LM0230. The efficiency of center of infection (ECOI) assays were conducted as described by Deng et al. (6). By convention, it is assumed that 100% of the infected sensitive cells result in progeny phage (ECOI = 1). Cell survival was determined as described by Behnke and Malke (3). The cell survival rate was calculated as the colony count of infected culture divided by colony count of uninfected control. One-step growth curves were carried out by the method of Klaenhammer and Sanozky (20).
Plasmid DNA techniques.
Lactococcal plasmid DNA was isolated by the method of Anderson and McKay (2). Plasmid was isolated from E. coli by alkaline lysis and purified by cesium chloride-ethidium bromide density gradient centrifugation (30). Molecular cloning was essentially carried out as described by Sambrook at al. (30). Restriction endonucleases and T4 DNA ligase were purchased from Roche Molecular Biochemicals (Roche Diagnostics Australia Pty., Ltd.) or New England BioLabs (Genesearch, Pty., Ltd.) and used as recommended by the manufacturers.
DNA cloning.
Restriction endonuclease, calf intestinal alkaline phosphatase, and T4 DNA ligase were purchased from Roche and used in cloning work according to the manufacturer's instructions. DNA cloning procedures were as described by Sambrook et al. (30). Plasmid pND002 was obtained by shotgun cloning a HindIII digest of pND001 into the HindIII site of pDL278.
Inactivating abiU1 was achieved by digesting pND002 with BamHI, gel purifying the largest fragment, and religation to produce pND003 (see Fig. 3). Plasmid pND002 was double digested with HindIII and ScaI, and the 2.9-kb fragment was gel purified and cloned into the HindIII/ScaI sites of pDL278 to create pND006 (see Fig. 3). The constructs were confirmed by restriction enzyme mapping.
FIG. 3.
Gene organization of the 5.2-kb insert in pND002 and the subcloned DNA fragments.
Nucleotide sequencing and analysis.
Both DNA strands were sequenced by using an Applied Biosystems 377 DNA sequencer according to the manufacturer's protocol. Sequencing of the phage resistance determinant was initiated by using the M13mp19 primers (New England Biolabs). Based on the sequences obtained, 17- to 21-mer oligonucleotide primers were synthesized and used to “walk” along the DNA template. Recording and analysis of the nucleotide sequence were carried out by using AutoAssembler DNA sequence assembly software (Applied Biosystems) and the Australian National Genomic Information Service (ANGIS) Software System operated by ANGIS at the University of Sydney.
Northern blot hybridization.
Total RNA was isolated from L. lactis at various times during the phage infection cycle by using the RNeasy Mini Kit (Qiagen, Pty., Ltd.) according to the manufacturer's instructions. RNA preparations were digested with RQ1 DNase (Promega Corp., Sydney, Australia) prior to use. RNA concentrations were determined spectrophotometrically at 260 nm. Northern hybridization was carried out by using the ECL Direct Nucleic Acid Labeling and Detection System (Amersham Pharmacia Biotech) according to the manufacturer's instructions. Total RNA (12 μg) from each sample was loaded onto a 1.2% denaturing gel. Phage DNA probes were isolated by the method of Grosserberger (18) and purified by using the QIAEX II gel extraction kit (Qiagen).
Nucleotide sequence accession number.
The GenBank accession number for the DNA sequence of the AbiU abortive infection system from L. Lactis LL51-1 is AF188839.
RESULTS
Isolation and cloning of the phage resistance determinant.
L. lactis LL51-1 is an industrial cheese starter strain that shows good phage resistance (φr). Characterization of this strain also revealed that it contains nine plasmids (of ca. 75, 60, 50, 45, 27, 10, 9, 8, and 3 kb) and is sensitive to streptomysin (Sms) and resistant to nisin (Nisr).
In an attempt to identify the plasmid encoding phage resistance, conjugation experiments were conducted between LL51-1 (Nisr φr Sms) and LM0230Smr (Niss φs Smr), and transconjugants were obtained at 10−7 per donor on M17G plates containing streptomycin and nisin. Nine transconjugants were resistant to phages φc2 and φ712, which are representatives of the c2 and 936 lactococcal phage species, respectively. Two of the nine transconjugants contained a single plasmid of ca. 75 kb, and HindIII digests of plasmid DNA from both transconjugants produced identical restriction patterns. This 75-kb plasmid was designated pND001. When LM0230(pND001) was infected separately with φc2 and φ712, reductions in EOP were observed (3 × 10−1 and 2 × 10−4, respectively), and plaque sizes were reduced relative to LM0230 (Table 2).
TABLE 2.
Phage reaction on L. lactis LM0230(pDL278), LM0230(pND002), UL8(pDL278), UL8(pND002), and derivative plasmids at 30°C
| Strain | Phage | EOP | Plaque size (mm), morphology | Adsorption (%) | Cell survival (%) |
|---|---|---|---|---|---|
| LM0230 | φ712 | 1 | 1.5, clear | 99.3 | |
| φc2 | 1 | 2.5, clear | |||
| LM0230(pDL278) | φ712 | 1 | 1.5, clear | 99.4 | 0.3 |
| φc2 | 1 | 2.5, clear | 17 | ||
| LM0230(pND001) | φ712 | 2.0 × 10−4 | 0.2, clear | 99.4 | |
| φc2 | 3.0 × 10−1 | 1, clear | |||
| LM0230(pND002) | φ712 | 1.0 × 10−2 | 0.3, hazy | 99.5 | 1.5 |
| φc2 | 3.7 × 10−1 | 1, hazy | 14 | ||
| LM0230(pND003) | φ712 | 1 | 1.5, clear | ||
| φc2 | 1 | 2.5, clear | |||
| LM0230(pND006) | φ712 | 1.0 × 10−4 | 0.3, hazy | ||
| φc2 | 3.0 × 10−1 | 1, hazy | |||
| UL8(pDL278) | φul36 | 1 | 1.5, clear | 5.1 | |
| UL8(pND002) | φul36 | 1.0 × 10−1 | 1.5, clear | 5.3 | |
| UL8(pND006) | φul36 | 4.0 × 10−2 | 1.2, clear | 5.7 |
To isolate the region of pND001 responsible for phage resistance, HindIII restriction fragments of pND001 were shotgun cloned into pDL278 and introduced into LM0230. Recombinant clones were tested for resistance to both phages, and the plasmid content of resistant clones was analyzed. The smallest fragment from pND001 that conferred resistance to both phages was 5.2 kb. This construct was designated pND002.
Characterization of the phage resistance mechanism in pND002.
Various assays were conducted to characterize the phage resistance mechanism encoded by pND002. To investigate whether the phage resistance phenotype encoded by pND002 was due to the inability of phage to adsorb to the host cells, adsorption inhibition assays were carried out by using φ712. The results are listed in Table 2. Phage φ712 adsorbed efficiently to the sensitive control strain LM0230(pDL278) and to the resistant strains LM0230(pND001) and LM0230(pND002), indicating that the phage resistance encoded by pND001 and pND002 did not affect φ712 adsorption.
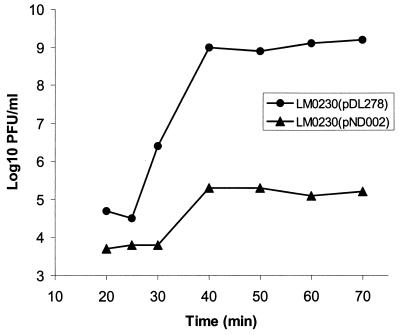
To investigate the effect of pND002 on phage infection, one-step phage growth curves of φ712 in LM0230(pDL278) and LM0230(pND002) were conducted at 30°C (Fig. 1). Compared to the sensitive control strain, LM0230(pDL278), the latent period for LM0230(pND002) was extended by 5 min (30 versus 25 min). More importantly, the burst size obtained with LM0230(pND002) was significantly reduced relative to the control. Furthermore, the size of the plaques obtained on LM0230(pND002) were much smaller than those obtained on LM0230(pDL278) (0.3 versus 1.5 mm; Table 2). These results indicate that pND002 may encode an abortive infection system.
FIG. 1.
One-step growth curve of φ712 in LM0230(pDL278) and LM0230(pND002).
Another characteristic of abortive infection is death of the host cells after phage infection. The number of cells that died as a result of φ712 infection was examined (Table 2). Compared to the sensitive host, the presence of pND002 in LM0230 did not improve the cell survival rate after φ712 infection. The LM0230(pND002) culture, however, did not lyse, whereas the LM0230(pDL278) culture did. Similar results were observed after φc2 infection (Table 2).
ECOI assays with φ712 were performed on both sensitive LM0230(pDL278) and resistant LM0230(pND002). It was shown that 3.2 × 108 infectious centers were formed on LM0230(pDL278), while only 5.0 × 106 infectious centers were formed on LM0230(pND002) under the same conditions. Therefore, the ECOI of LM0230(pND002) was 1.6%. This extremely low efficiency of infectious center formation is another typical characteristic of abortive infection systems.
Sequence analysis of the 5.2-kb insert in pND002.
Sequence data showed that the exact length of the insert in pND002 was 5,213 bp. Use of the GCG programs Frame and Mapping, available through ANGIS, predicted the presence of three complete open reading frames (ORFs): orf1, orf2 (designated abiU1 and abiU2 after characterization), and orf3 and one truncated ORF, orf4 (Fig. 3). The sequence of all ORFs, together with the flanking control regions, is given in Fig. 2. All three complete ORFs are read in the same direction, while orf4 is in the opposite direction. abiU1 is 1,766 bp long, starting at bp 1078 with AUG as the start codon and ending at bp 2844, followed by two stop codons, UAG and UAA. It has a G+C content of 26% and encodes a predicted 589-amino-acid protein of 67.9 kDa. abiU1 is preceded by a putative promoter (−35 box [TTGATT], 16-bp spacer, −10 box [ATAAAC]) and a putative ribosome-binding site (GGA; calculated free energy [ΔG] = −9.4 kcal) located 5 bp ahead of the start codon. In addition to the putative transcriptional signals, two other promoter-like structures (−35 and −10 box) were identified upstream of abiU1. abiU2 is 1,023 bp in length, starting at bp 3105 with AUG as the start codon and ending at bp 4127, followed by one stop codon (UAA). It has a G+C content of 25% and encodes a predicted protein of 40.6 kDa. Upstream of abiU2 are a consensus −10 box (TATAAT) and a partial consensus −35 box (TTGAAA) separated by 20 bp. A putative ribosome-binding site (AGGAG) with a ΔG of −12.8 kcal is located 6 bp upstream of the abiU2 start codon. orf3 starts at bp 4275 and ends at bp 5012. It is 737 bp long and has a G+C content of 37%. A consensus −10 region (TATAAT) was identified 35 bp upstream of this ORF. At 17 bp upstream of this −10 region there is a typical −35 region (TTGAAT). A typical ribosome-binding site was also identified 4 bp ahead of the ATG start codon (AGGAGG). No ρ-dependent or ρ-independent terminator was identified between abiU1 and abiU2 or downstream of abiU2. No significant homology with abiU1 was found at the protein level when searched in NR proteins by BLASTP and FASTA. No transmembrane regions were detected by using the DAS program (5), thus suggesting that AbiU1 is a cytoplasmic protein.
FIG. 2.
Nucleotide sequence of the insert in pND002 and the deduced amino acid sequence. The deduced amino acid sequences are shown below the nucleotide sequence. Indicated are the putative ribosome-binding sites (RBS) (double underline), −35 and −10 promoter sequences (single underline), stop codons (asterisks), and possible transcriptional terminators (converging arrows), start and stop codons, and restriction enzyme sites (shading).
The nucleotide and protein sequences resulting from the 5.2-kb fragment were compared to the nonredundant nucleotide and protein databases by using the BLAST and FASTA programs. The amino acid sequence from positions 25 to 201 of the AbiU2 protein has 26% identity and 43% similarity with the predicted protein of AbiGii (26). The DNA sequence from bp 1 to 849, which includes the truncated orf4 (orf4 starts at bp 453 and is read into the opposite direction), was found to have 98% identity with the DNA sequence of the L. lactis ATP binding protein (GenBank accession numbers M90969, M77093, and M98400). The derived amino acid sequence of orf4 has 97% identity with the same protein. The protein encoded by orf3 was found to have 72% identity with the derived product of orfU, the function of which is unknown. The orfU gene is adjacent to abiF in plasmid pNP40 (13).
Identification of the ORF(s) that encodes phage resistance in pND002.
To determine what is responsible for the phage-resistant phenotype, two deletion plasmids were constructed. abiU1 and orf4 were deleted, resulting in the pND003 (Fig. 3). To study the phenotype of abiU1, abiU2 and orf3 were removed by constructing pND006 (Fig. 3). Cross-streaking with φ712 and φc2 showed that LM0230(pND003) was sensitive to both phages, but LM0230(pND006) was resistant to both (Table 2). These results suggest that abiU1 is the primary phage resistance determinant in pND002.
Possible downregulation function of AbiU2 on AbiU1.
The degree of phage resistance obtained with pND002 and pND006 was compared (Table 2). With φ712, the EOP obtained on LM0230(pND006) was much lower than that obtained with LM0230(pND002) (10−4 versus 10−2). When the same experiment was repeated with φc2, however, there was very little difference observed between the EOPs obtained with LM0230(pND006) and LM0230(pND002) (Table 2).
To determine whether the difference observed between LM0230(pND006) and LM0230(pND002) was evident against the P335 phage species, the same experiment was performed with φul36. Plasmids pND002 and pND006, as well as the vector pDL278, were electroporated into L. lactis UL8, which is the host for φul36. A similar phenomenon to that reported with φ712 was observed: the EOP obtained with UL8(pND006) was 10−1 lower than that obtained with UL8(pND002) (Table 2). It should also be noted that the sizes of the φul36 plaques on UL8(pND006) were smaller than those obtained on UL8(pND002). The cell survival rate after φul36 infection of the two hosts UL8(pND002) and UL8(pND006) was similar to the sensitive host UL8(pDL278), further indicating that abiU is an abortive infection system.
The data suggest that this abortive infection system acts differently on phage from different species. The presence of abiU1 in pND006 confers a low EOP against all phage species. In comparison to pND006, the presence of the complete 5.2-kb fragment in pND002 actually reduces phage resistance against 936 and P335 species but does not affect the phage resistance observed with φc2 (Table 2). The presence of the sequence downstream of abiU1 appears to downregulate phage resistance in pND002. Given the homology of the AbiU2 protein to AbiGii, it is tempting to speculate that this protein may be responsible for this effect.
Effect of pND002 on phage DNA transcription.
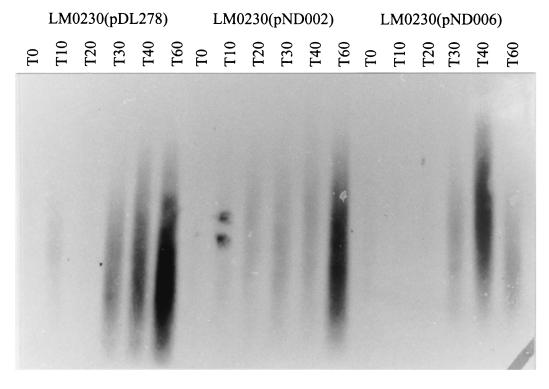
To study the effect on phage φ712 transcription, total RNA was isolated from LM0230(pDL278), LM0230(pND002) and LM0230(pND006) at 10-min intervals after φ712 infection and Northern blotted. The blot was probed with labeled genomic DNA from φ712 (Fig. 4). Transcripts of φ712 first appeared 10 min after infection of strains LM0230(pDL278) and LM0230(pND002). Transcripts were first detected in LM0230(pND006) 30 min after infection, and the degree of transcription increased by 40 min. Plasmids pND002 and pND006 appear to delay phage transcription relative to the control strain.
FIG. 4.
Hybridization of total RNA isolated from φ712-infected L. lactis hosts by using φ712 genomic DNA as the probe.
The effect of pND006 on φc2 RNA transcription was also investigated. Samples were withdrawn 0, 45, and 75 min after φc2 infection and analyzed by Northern hybridization by using labeled genomic DNA of φc2 (Fig. 5). Phage c2 transcripts were detected 45 and 75 min after infection in the sensitive host LM0230(pDL278) but not in the resistant host LM0230(pND006). Apparently, the presence of pND006 significantly reduced or delayed φc2 RNA synthesis.
FIG. 5.
Hybridization of total RNA isolated from phage c2-infected hosts by using φc2 genomic DNA as the probe.
DISCUSSION
In this study, aspects of the molecular characterization of a lactococcal phage abortive infection system (AbiU) have been described. AbiU was isolated from the plasmid pND001 found in the industrial strain L. lactis LL51-1. pND001 conferred resistance to small isometric-headed φ712 (936 species) and prolate-headed φc2 (c2 species). EOP tests of LM0230(pND001) and LM0230(pND002) on φc2 showed a similar level of resistance from both plasmids. However, EOP values of LM0230(pND001) to φ712 were 2 orders of magnitude less than that of LM0230(pND002) (Table 2). This suggests that another mechanism of phage resistance against φ712 exists or that the existing mechanism is enhanced on pND001 but not on pND002.
The phage resistance phenotype conferred by pND002 was characterized. Plasmid pND002 conferred reduced plaque size (Table 2), EOP (Table 2), burst size (Fig. 1), and ECOI against φ712. Plasmid pND002 conferred reduced plaque size and EOP against φc2 and φul36; this plasmid prevented lysis of the host but conferred a very low survival rate against all three phage tested. These observations are typical of those observed with abortive infection systems.
Analysis of the 5.2-kb fragment in pND002 revealed the presence of three complete ORFs and one incomplete ORF. Cloning experiment were used to determine that abiU1 is directly involved in phage resistance. Comparison of the EOPs obtained with pND002 and pND006 against phages φ712 and φul36 suggest that sequences downstream of abiU1 interfere with or downregulate phage resistance. Like other abi genes (12), both abiU1 and abiU2 have low G+C contents of 26 and 25%, respectively. In contrast, the G+C content of the truncated orf4 is 32%, and that of orf3 is 37%, values which are close to the average G+C content of lactococcal chromosome. The homology of the AbiU2 and AbiGii proteins adds support to the hypothesis that abiU2 is involved in phage resistance. Stronger resistance to φ712 and φul36 was conferred by pND006 relative to pND002, suggesting that abiU2 or its protein might be involved in abiU1 or AbiU1 downregulation (Table 2). This downregulation function of abiU2 on abiU1 seems to affect resistance against only isometric-headed phage from the 936 and P335 species and has no effect on prolate-headed phage from the c2 species.
The possible downregulation function of AbiU2 on AbiU1 resembles the negative control of PifC on pif gene expression in F exclusion of phage T7 in E. coli cells (23). The Pif system, located on the F plasmid in E. coli, is one of the abi systems identified in E. coli and confers host resistance to phage T7 infection. The pif region contains at least three genes—pifC, pifA, and pifB (29)—in which pifA encodes phage resistance. It is known that pifA and pifC lie within a polycistronic operon (4) and that the promoter is upstream of pifC. Construction of fusion proteins of PifA-LacZ, PifB-LacZ, and PifC-LacZ showed that pifC expression is autoregulated. PifC, in trans, significantly decreases the level of β-galactosidase activity produced by PifA-LacZ, PifB-LacZ, and PifC-LacZ. In addition, inactivating pifC in cis dramatically increased the resistance to T7 conferred by PifA, just as deleting abiU2 increases the phage resistance conferred by AbiU1. Interestingly, PifC is a 40-kDa protein (23), which is similar in size to the predicted 40.6 kDa of AbiU2, whereas PifA is a 70-kDa protein (29), a size similar to the predicted 67.9 kDa of AbiU1. The DNA of the pif region has not been sequenced to allow a more detailed comparison.
One important step in understanding the mechanism of an abortive infection system is to locate the stage at which it inhibits the phage life cycle. Detecting phage mRNA transcription by Northern hybridization demonstrated that AbiU appeared to delay transcription of both φc2 and φ712. The effect was more extreme for φc2 (Fig. 4 and 5). However, the Northern hybridization signals that were observed were not discrete. Likewise, O'Connor et al. also observed similar signals when studying AbiG and φsk1 (26). These authors tentatively explained it as a result of nonspecific processing of transcripts, transcript degradation, the presence of fragments of increasing size with a common 5′ end, or some combination of these factors.
Among the three lactococcal mechanisms reported to inhibit or delay phage transcription (16, 26, 27), AbiU resembles most closely pBu1-8 (16). However, since pBu1-8 is a native plasmid and the phage resistance determinant was not subcloned, it is not known whether other phage resistance systems exist on pBu1-8 that may provide additional phage resistance. On the basis of sequence homology, AbiU appears related to AbiG, which is part of another mechanism that interferes with phage transcription.
The phage resistance spectrum of an abi system is varied. Most abi systems confer resistance to one or two phage species, whereas only AbiA and AbiK have been reported to confer resistance to three species of phage: c2, 936, and p335 (11, 19). AbiU is the third abi system that encodes resistance against representative phages from all three of these phage species.
ACKNOWLEDGMENTS
This work was supported by the Australian Cooperative Research Center for Food Industry Innovation and by DSM Food Specialties, Australia.
REFERENCES
- 1.Anba J, Bidnenko E, Hillier A, Ehrlich S D, Chopin M-C. Characterization of the lactococcal abiD1 gene coding for phage abortive infection. J Bacteriol. 1995;177:3818–3823. doi: 10.1128/jb.177.13.3818-3823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D G, McKay L L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983;46:549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behnke D, Malke H. Bacteriophage interference in Streptococcus pyogenes. I. Characterization of prophage-host systems interfering with the virulent phage A25. Virology. 1978;85:118–128. doi: 10.1016/0042-6822(78)90416-6. [DOI] [PubMed] [Google Scholar]
- 4.Cram D, Ray A, Skurray R. Molecular analysis of F plasmid pif region specifying abortive infection of T7 phage. Mol Gen Genet. 1984;197:137–142. doi: 10.1007/BF00327934. [DOI] [PubMed] [Google Scholar]
- 5.Cserzo M, Wallin E, Simon I, Heijne von G, Elofsson A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997;10:673–676. doi: 10.1093/protein/10.6.673. [DOI] [PubMed] [Google Scholar]
- 6.Deng Y-M, Liu C-Q, Dunn N W. Genetic organisation and functional analysis of a novel phage abortive infection system, AbiL, from Lactococcus lactis. J Biotech. 1999;67:135–149. doi: 10.1016/s0168-1656(98)00175-8. [DOI] [PubMed] [Google Scholar]
- 7.Denis P D, Twomey P, Urraza P J, McKay L L, O'Sullivan D J. Characterization of AbiR, a novel multicomponent abortive infection mechanism encoded by plasmid pKR223 of Lactococcus lactis subsp. lactis KR2. Appl Environ Microbiol. 2000;66:2647–2651. doi: 10.1128/aem.66.6.2647-2651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan K, Harvey M L, Liu C-Q, Dunn N W. Identification and characterization of a mobilizing plasmid pND300, in Lactococcus lactis M189 and its encoded nisin resistance determinant. J Appl Bacteriol. 1996;81:493–500. doi: 10.1111/j.1365-2672.1996.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 9.Durmaz E, Higgins D L, Klaenhammer T R. Molecular characterization of a second abortive phage resistance gene present in Lactococcus lactis subsp. lactis ME2. J Bacteriol. 1992;174:7463–7469. doi: 10.1128/jb.174.22.7463-7469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efstathiou J D, McKay L L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977;130:257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emond E, Holler B J, Boucher I, Vandenbergh P A, Vedamuthu E R, Kondo J K, Moineau S. Phenotypic and genetic characterization of the bacteriophage abortive infection mechanism AbiK from Lactococcus lactis. Appl Environ Microbiol. 1997;63:1274–1283. doi: 10.1128/aem.63.4.1274-1283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford A, Fitzgerald G F. Bacteriophage defense systems in lactic acid bacteria. Antonie Leeuwenhoek. 1999;76:89–113. [PubMed] [Google Scholar]
- 13.Garvey P, Fitzgerald G F, Hill C. Cloning and DNA sequence analysis of two abortive infection phage resistance determinants from the lactococcal plasmid pNP40. Appl Environ Microbiol. 1995;61:4321–4328. doi: 10.1128/aem.61.12.4321-4328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasson M J, Davis F L. High-frequency conjugation associated with Streptococcus lactis donor cell aggregation. J Bacteriol. 1980;143:1260–1264. doi: 10.1128/jb.143.3.1260-1264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geis A, Janzen T, Teuber M, Wirsching F. Mechanism of plasmid-mediated bacteriophage resistance in lactococci. FEMS Microbiol Lett. 1992;94:7–14. [Google Scholar]
- 17.Gough J, Murray N. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983;166:1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- 18.Grossberger D. Minipreps of DNA from bacteriophage λ. Nucleic Acids Res. 1987;15:6737. doi: 10.1093/nar/15.16.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill C, Miller L A, Klaenhammer T R. Nucleotide sequence and distribution of the pTR2030 resistance determinant (hsp) which aborts bacteriophage infection in lactococci. Appl Environ Microbiol. 1990;56:2255–2258. doi: 10.1128/aem.56.7.2255-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaenhammer T R, Sanozky R B. Conjugal transfer from Streptococcus lactis ME2 of plasmids encoding phage resistance, nisin resistance and lactose-fermenting ability: evidence for a high-frequency conjugative plasmid responsible for abortive infection of virulent bacteriophage. J Gen Microbiol. 1985;131:1531–1541. doi: 10.1099/00221287-131-6-1531. [DOI] [PubMed] [Google Scholar]
- 21.LeBlanc D, Lee L N, Abu-Al-Jaibat A. Molecular, genetic, and functional analysis of the basis replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid. 1992;28:130–145. doi: 10.1016/0147-619x(92)90044-b. [DOI] [PubMed] [Google Scholar]
- 22.McLandsborough L A, Kolaetis K M, Requena T, McKay L L. Cloning and characterization of the abortive infection genetic determinant abiD isolated from pBF61 of Lactococcus lactis subsp. lactis KR5. Appl Environ Microbiol. 1995;61:2023–2026. doi: 10.1128/aem.61.5.2023-2026.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J F, Malamy M H. Identification of the pifC gene and its role in negative control of F factor pif gene expression. J Bacteriol. 1983;156:338–347. doi: 10.1128/jb.156.1.338-347.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moineau S, Durmaz E, Pandian E S, Klaenhammer T R. Differentiation of two abortive mechanisms by using monoclonal antibodies directed toward lactococcal bacteriophage capsid proteins. Appl Environ Microbiol. 1993;59:208–212. doi: 10.1128/aem.59.1.208-212.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moineau S, Fortier J, Ackermann H W, Pandian S. Characterization of lactococcal bacteriophages from Quebec cheese plants. Can J Microbiol. 1992;38:875–882. [Google Scholar]
- 26.O'Connor L, Tangney M, Fitzgerald G F. Expression, regulation, and mode of action of the AbiG. Appl Environ Microbiol. 1999;65:330–335. doi: 10.1128/aem.65.1.330-335.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parreira R, Ehrlich S D, Chopin M-C. Dramatic decay of phage transcripts in lactococcal cells carrying the abortive infection determinant AbiB. Mol Microbiol. 1996;19:221–230. doi: 10.1046/j.1365-2958.1996.371896.x. [DOI] [PubMed] [Google Scholar]
- 28.Powell B I, Achen G M, Hiller J A, Davidson E B. A simple and rapid method for genetic transformation of lactic streptococci by electroporation. Appl Environ Microbiol. 1988;54:655–660. doi: 10.1128/aem.54.3.655-660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotman G S, Cooney R, Malamy M H. Cloning of the pif region of the F sex factor and identification of a pif protein product. J Bacteriol. 1983;155:254–264. doi: 10.1128/jb.155.1.254-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Environ Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]