Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive cyst formation that ultimately leads to kidney failure in most patients. Approximately 10% of patients who receive kidney replacement therapy suffer from ADPKD. To date, a vasopressin V2 receptor antagonist (V2RA) is the only drug that has been proven to attenuate disease progression. However, aquaresis-related adverse events limit its widespread use. Data on the renoprotective effects of somatostatin analogues differ largely between studies and medications. This review discusses new drugs that are investigated in clinical trials to treat ADPKD, such as cystic fibrosis transmembrane conductance regulator (CFTR) modulators and micro RNA inhibitors, and drugs already marketed for other indications that are being investigated for off-label use in ADPKD, such as metformin. In addition, potential methods to improve the tolerability of V2RAs are discussed, as well as methods to select patients with (likely) rapid disease progression and issues regarding the translation of preclinical data into clinical practice. Since ADPKD is a complex disease with a high degree of interindividual heterogeneity, and the mechanisms involved in cyst growth also have important functions in various physiological processes, it may prove difficult to develop drugs that target cyst growth without causing major adverse events. This is especially important since long-standing treatment is necessary in this chronic disease. This review therefore also discusses approaches to targeted therapy to minimize systemic side effects. Hopefully, these developments will advance the treatment of ADPKD.
Key points
| The pathophysiology of ADPKD is complex and various potential therapeutic targets are available for which novel treatments are being developed or repurposed, but as yet V2RAs are currently the only widely accepted renoprotective treatment for this disease. |
| Because ADPKD is a chronic disease that requires lifelong treatment, and targets involved in cyst growth also have important functions in various physiological processes, there is a need to develop (targeted) therapies to minimize systemic side effects. |
| Since various treatment options have shown discrepant results between preclinical and clinical studies, we suggest the use of at least 2 different rodent polycystic kidney disease models before proceeding to clinical trials or before aborting drugs for clinical use. |
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) typically leads to progressive cyst formation in the kidneys, which causes kidney enlargement (shown in Fig. 1), pain, hematuria, and progressive loss of kidney function that ultimately leads to kidney failure [1]. In addition to local manifestations in the kidney, the disease is associated with systemic manifestations such as hypertension; cyst formation in the liver, pancreas and spleen; valvular heart disease; and intracerebral aneurysms. Although ADPKD has an estimated prevalence of around 4:10,000 [2] and is therefore not very common, the disease is seen quite frequently in clinical routine because of its progressive character. It is estimated that around 10% of patients who are on kidney replacement therapy suffer from ADPKD [3].
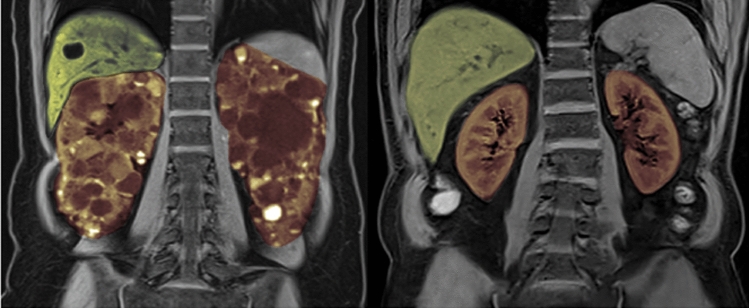
Fig. 1.
MRI scan of a 33-year-old female with autosomal dominant polycystic kidney disease (ADPKD). Innumerable cysts are present throughout both kidneys (depicted in orange), and a few cysts are present in the liver (depicted in green). The total kidney volume (TKV) of this patient is 1800 mL; roughly eight times larger than normal. Two normal sized kidneys are shown on the right for reference (normal MRI-measured TKV in females: approximately 260–300 mL [4])
In the majority of ADPKD patients, the disease is caused by a mutation in the PKD1 or PKD2 gene. To date, approximately 1500 different pathogenic mutations in the PKD1 and PKD2 genes have been recorded. The PKD1 gene, located on chromosome 16, encodes the polycystin 1 protein, and mutations in this gene account for around 78% of ADPKD cases [5]. The PKD2 gene is located on chromosome 4 and encodes the protein polycystin 2 [6]. Polycystin 1 and 2 form a complex that is located on the primary cilia of renal tubular cells. This complex is thought to be involved in transmission of information from the external environment to the cell. The idea is that polycystin 1 and 2 inhibit cystogenesis in a dose-dependent way, and that cystogenesis occurs when the concentration of polycystin 1 or 2 falls below a critical threshold [7, 8]. More recently identified mutations, such as mutations in the GANAB gene [9] and the DNAJB11 gene [10], as well as mutations in genes associated with polycystic liver disease (PRKCSH, SEC63, LRP5, ALG8, and SEC61B), lead to maturation defects of polycystin 1 and 2 [11]. These maturation defects interfere with the attainment of a fully functional and appropriately localized polycystin complex [12]. When concentrations of polycystin 1 or 2 fall below the aforementioned threshold, the intracellular calcium concentration decreases, leading to, among others, increased cyclic AMP (cAMP) concentrations in renal tubular cells and ultimately to cystogenesis through activation of proliferative signaling pathways.
In this review, we discuss new treatment options that are under investigation in clinical trials as well as some issues that merit attention when developing drugs to treat ADPKD, namely which patients are at risk of rapid disease progression and should be treated, study sample size considerations, potential solutions to reduce side effects of current and upcoming treatment options, and the problems that are faced when extrapolating data from animal models to the human situation. Relevant articles and trials were selected from three online databases (PubMed, Clinicaltrials.gov and the Cochrane Central Register of Controlled Trials) using as search terms (“ADPKD” OR “PKD”) AND (“treatment” OR “therapy”), with a focus on recent works from 2010 up until April 2022. For an overview of symptomatic treatment of hypertension and other cardiovascular complications of ADPKD, as well as of non-pharmacological options and treatment of polycystic liver disease, we refer to other recent review articles [13–15].
Selection of Patients for Treatment (in Trials) and Sample Size Considerations for Interventional Autosomal Dominant Polycystic Kidney Disease (ADPKD) Trials
The disease course of ADPKD is highly variable. Some patients develop rapid kidney enlargement and subsequent progression to end stage kidney disease (ESKD) at a young age, while others will never develop significant renal impairment. The inclusion of patients with rapidly progressive disease in clinical trials ensures that beneficial effects on endpoints like total kidney volume (TKV) growth or renal function decline can be detected. Identifying patients at risk for rapidly progressive disease is also necessary in clinical practice to select patients who will benefit the most from treatment, and to protect patients who do not require treatment against treatment-related costs and side effects.
There is no international consensus on the definition of rapidly progressive ADPKD [16]. The European Renal Association (ERA) has recently published an updated position statement regarding in which ADPKD patients to prescribe tolvaptan [17]. This paper defines rapidly progressive disease as an annual estimated glomerular filtration rate (eGFR) decline of ≥ 3.0 mL/min/1.73 m2. That cut-off value was chosen based on the observed rates of eGFR decline in unselected cohorts of ADPKD patients [18]. An algorithm is presented to identify patients who in the future will likely show an eGFR loss that is more rapid than this cut-off, i.e., have rapidly progressive disease. This algorithm relies on several indicators of disease severity like kidney function indexed for age, historical kidney function decline, Mayo Clinic classification, and the PROPKD score. These disease severity indicators are also used to select patients with rapidly progressive disease for inclusion in clinical ADPKD trials that investigate the renoprotective efficacy of other drugs. Individually, the predictive value of these disease severity indicators is relatively limited [19], but integrating multiple disease indicators will likely improve the predictive capacity and should be considered when developing similar algorithms. Several plasma or urinary biomarkers have also been investigated for this purpose, for example plasma copeptin [19] and urinary monocyte chemoattractant protein-1 excretion [20], but reliable algorithms that incorporate these biomarkers are not yet available. The development of such an algorithm would be a substantial advancement, especially to enable selection of rapidly progressive patients early in the disease course (when kidney function is still intact) and to further refine risk assessment for patients in whom volumetric indices are ambiguous (e.g., patients with Mayo class 1C, of whom at least half will have an annual rate of eGFR loss of less than 3.0 mL/min/1.73 m2) [21].
The required sample size for clinical trials depends on a number of factors, including two fixed assumptions, namely the chance of making a type I error (i.e., the chance of reaching a false positive conclusion, that is typically set at 5% or an α of 0.05) and the chance of a type II error (i.e., the chance of reaching a false negative conclusion, that is usually set at 20%, or a β of 0.20). In addition to these assumptions, the required number of patients also depends on the expected treatment effect on the decline of eGFR, which in the field of nephrology, including PKD, is often set at a reduction of 30% [22–24]. Although TKV can be used as a surrogate outcome that may even be used to obtain conditional drug approval, a trial with eGFR decline as the primary endpoint should always be performed, since renoprotection is the ultimate goal of ADPKD treatment. The study sample size also depends on the incidence of the outcome and its variance. This re-emphasizes that inclusion of rapidly progressive ADPKD patients is important to be able to detect a treatment effect. As an example, it can be calculated that for a phase 3 clinical ADPKD trial with an α of 0.05 and β of 0.20 (a power of 80%), assuming a mean eGFR decline of − 3.5 mL/min/1.73 m2 per year in the placebo group [22, 25, 26], with a standard deviation of ± 3.0 mL/min/1.73 m2 per year and an estimated treatment effect of 30%, a total sample size of 256 patients would be appropriate. When, for instance for registration purposes, a more stringent β is used (0.13) and adjustments are made to account for drop-out rate, multiplicity of tests and an interim analysis, in total 560 patients would be needed. This number was calculated for the STAGED-PKD trial [23], of which the design was US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved.
In hindsight, it therefore seems that some ADPKD trials that have been performed, most notably the TEMPO 3:4 and REPRISE trials [25, 26], were vastly overpowered. As stated in the power calculation for the TEMPO 3:4 trial [27], the adopted α, β and estimated treatment effect were more stringent than usual (0.045, 0.15, and 20%, respectively). Even with these figures, only 504 patients were calculated to be necessary. However, it was stated in the power calculation that this number was doubled to enroll 1200–1500 patients to provide a higher than usual degree of statistical significance for the primary endpoint and to be able to evaluate a plethora of secondary endpoints [27]. After completion of the TEMPO 3:4 trial, it appeared that all p-values for the primary and most secondary endpoints were extremely small, even in subgroups, indicating that the trial was indeed overpowered. Unfortunately, despite being of great importance to the field of ADPKD, this trial can lead to the unjustified assumption that ADPKD trials should typically include this large number of patients. As reasoned above, conventional power analyses show that considerably lower numbers should be sufficient.
Currently Available Treatments
Increasing knowledge of the underlying pathophysiology of the disease has laid the basis for the development of new potential therapeutic targets. Table 1 depicts the landmark clinical trials performed in ADPKD so far. Unfortunately, many of these therapeutic targets are involved not only in cyst formation, but also in numerous physiological processes throughout the body that are important for cellular proliferation, growth, and repair. Drugs that target these processes may therefore lead to severe adverse effects that limit their utility. An example of this is the mammalian target of rapamycin (mTOR) pathway. For the mTOR pathway, two landmark trials were published in 2010 that investigated inhibition of this pathway with everolimus and sirolimus in early- [28] and later-stage [29] ADPKD. While animal studies were promising, both clinical studies produced disappointing results, probably because side effects, such as mucositis and diarrhea, limited the use of higher dosages that are needed to achieve effect. In a similar fashion, the somatostatin analogues lanreotide and octreotide have shown efficacy in animal studies. However, several clinical studies that investigated somatostatin analogues reported conflicting results in ADPKD patients [22, 30, 31]. For instance, the ALADIN trial [30] investigated the effect of octreotide long-acting release (LAR) versus placebo in 79 ADPKD patients (eGFR ≥ 40 mL/min/1.73 m2) with change in TKV as primary endpoint. Octreotide LAR significantly reduced TKV growth after 1 year but not at 3 years. The effects on kidney function are more complex to interpret. The decline in measured GFR from baseline to year 3 was not significantly different in the octreotide LAR group compared to placebo, but it was significant when measured from year 1 to 3. Unfortunately, despite careful randomization, patients in the placebo group appeared to have more severe disease, making it complicated to draw conclusions. The ALADIN 2 trial [31] investigated octreotide LAR versus placebo in 100 ADPKD patients with later-stage disease (eGFR 15–40 mL/min/1.73 m2), with TKV growth and measured GFR decline as primary endpoints. In this study, octreotide LAR significantly reduced TKV growth at 1 and 3 years, but there was no significant effect on measured GFR decline (neither when measured as slope from baseline to year 3, nor as slope from year 1 to 3). Despite the lack of effect on measured GFR decline, patients treated with octreotide LAR progressed less frequently to a composite endpoint of doubling of serum creatinine or ESKD compared to placebo (17.6% vs. 42.9%, respectively). This composite endpoint was not a priori defined [32]. These data have led to registration of octreotide LAR as treatment for ADPKD in Italy. Later, a larger study that randomized 309 ADPKD patients to the somatostatin analogue lanreotide or standard treatment (DIPAK 1) [22] found no significant effect of lanreotide on the primary outcome rate of eGFR decline compared to placebo (− 3.53 mL/min/1.73 m2 per year vs. -3.46 mL/min/1.73 m2, respectively), nor on worsening of kidney function (defined as a 30% eGFR decrease or start of dialysis). However, similar to earlier trials, this study also demonstrated that the rate of TKV growth was significantly reduced by a somatostatin analogue. Lastly, the LIPS study [33] also investigated lanreotide using renal function as primary outcome in 159 ADPKD patients. It was completed in 2019, but publication of the results is still awaited. A recently published systematic review concluded that somatostatin analogues as class have no significant effect on (e)GFR decline or on progression to ESKD [34]. However, the same study demonstrated that they do have an effect on change in total liver volume and possibly also kidney volume, at the expense of side effects. It cannot be excluded that there are differences in efficacy within the class of somatostatin analogues between octreotide LAR and lanreotide, because differences in affinity for the various somatostatin receptors have been described [35]. Although somatostatin analogues do not have clear renoprotective effects, there may be a role for these agents in reducing volume-related complaints in ADPKD, especially in polycystic liver disease [36]. This topic falls beyond the scope of this review.
Table 1.
Overview of landmark clinical trials that investigated disease-modifying drugs in autosomal dominant polycystic kidney disease (ADPKD)
| Agent | Trial design | Main inclusion criteria | No. of patients | Follow-up duration | Status | Main potential adverse events | Main results |
|---|---|---|---|---|---|---|---|
| Vaptans | |||||||
| Tolvaptan |
TEMPO 3:4 [25] Phase 3, double-blind placebo-controlled |
Age 18–50 years, CrCL ≥ 60 mL/min and TKV ≥ 750 mL | 1445 | 36 months | Published | Aquaresis and hepatotoxicity |
Reduction of TKV growth (2.8% per year for tolvaptan vs 5.5 for placebo) and eGFR decline (−2.72 mL/min/1.73m2 per year for tolvaptan vs −3.70 for placebo) over the 3 year study period |
|
REPRISE [26] Phase 3, double-blind placebo-controlled |
Age 18 - 65 years, eGFR 25 - 65 mL/min/1.73m2 (eGFR 25–44 mL/min/1.73m2 and progressive disease for patients aged 56–65 years) | 1370 | 12 months | Published | Reduction of eGFR decline (−2.34 mL/min/1.73m2 per year for tolvaptan vs -3.61 for placebo), but no effect on eGFR decline in patients aged > 55 years | ||
| Somatostatin analogues | |||||||
| Octreotide LAR |
ALADIN 1 [30] Phase 3, single-blind placebo-controlled |
Age > 18 years, eGFR ≥ 40 mL/min/1.73m2 | 79 | 36 months | Published | Cholelithiasis, acute cholecystitis and gastrointestinal side effects | Significant reduction of TKV growth after 1 year, but not after 3 years. Slower GFR decline from year 1 to 3 in the octreotide group vs placebo (-2.28 mL/min/1.73m2 per year vs -4.32, respectively). No significant differences in GFR decline over the 3 year study period |
|
ALADIN 2 [31] Phase 3, double-blind placebo-controlled |
Age > 18 years, eGFR 15 − 40 mL/min/1.73m2 | 100 | 36 months | Published | Reduction of TKV growth after 1 and 3 years, but no significant differences in GFR decline between groups. Less frequent progression to a composite endpoint of doubling of serum creatinine or ESKD in the octreotide LAR group vs placebo (17.6% vs 42.9, respectively) | ||
| Lanreotide |
DIPAK 1 [22] Phase 3, open label |
Age 18 - 60 years, eGFR 30–60 mL/min/1.73m2 | 309 | 30 months | Published | Hepatic cyst infection, cholelithiasis and related problems, and gastrointestinal side effects | Reduction of TKV growth (4.15% per year for lanreotide vs 5.56 for placebo), but no significant effect on eGFR decline (−3.53 ml/min/1.73m2 per year vs −3.46 for lanreotide and placebo, respectively) |
| mTOR inhibitors | |||||||
| Everolimus | Phase 3, double-blind, placebo-controlled [29] | eGFR > 30 mL/min/1.73m2 and TKV > 1000 mL | 433 | 24 months | Published | Mucositis, diarrhea, acne, increased proteinuria and reduced hematopoiesis. Angioedema when combined with ACE-inhibitors | Reduction of TKV growth after 1 year (102 mL vs 157 for everolimus and placebo, respectively). Trend towards reduction of TKV growth after 2 years (230 mL for everolimus vs 301 for placebo, p=0.06). No significant differences in eGFR decline between groups |
| Sirolimus | Phase 3, open label [28] | Age 18–40 years, eGFR ≥ 70 mL/min/1.73m2 | 100 | 18 months | Published | Mucositis, diarrhea, acne and peripheral edema | No significant effects on TKV growth (7.8% per year for sirolimus vs 6.8 for placebo). No differences in eGFR decline between groups. Higher urinary excretion rates in the sirolimus group compared to placebo |
CrCL creatinine clearance, eGFR estimated glomerular filtration rate, LAR long-acting repeatable, mTOR mammalian target of rapamycin, TKV total kidney volume
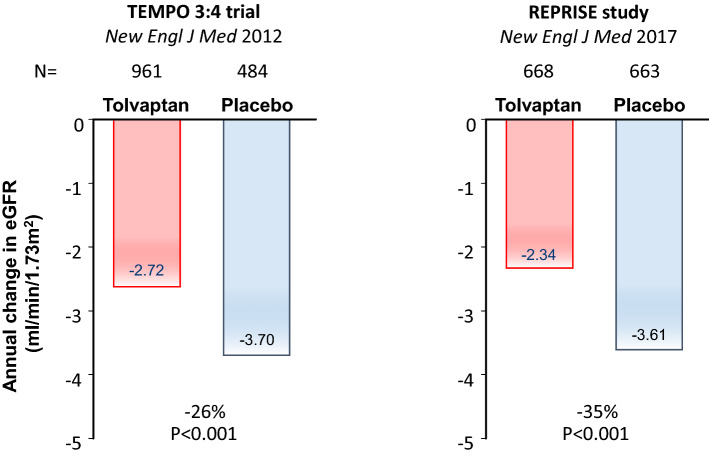
A more kidney-specific target for therapeutic intervention is the vasopressin 2 receptor (V2R), located on the basolateral membrane of collecting duct cells. Animal experiments showed a beneficial effect of vasopressin 2 receptor antagonists (V2RA) by limiting the generation of cAMP [37, 38]. In 2012, the landmark clinical trial with a V2RA (TEMPO 3:4 trial) was published [25]. In this multicenter trial, 1445 ADPKD patients were included with relatively early stage disease (defined as an estimated creatinine clearance of more than 60 mL/min), but who were at high risk of rapid disease progression (defined as a TKV of more than 750 mL). In these patients, the V2RA tolvaptan (90/30 mg split dose) decreased TKV growth by 49% and rate of eGFR decline on treatment by 26% (Fig. 2). After this trial, 871 patients continued in a 2-year open-label follow-up study, showing that the beneficial effect of tolvaptan on eGFR was sustained [39]. In later stage disease [26] (REPRISE trial, Fig. 2) and in a real-world setting [40], tolvaptan was also proven effective with a decrease in rate of eGFR decline of 38%. These results led to a marketing authorization for tolvaptan by, among others, the FDA and the EMA for the indication of ADPKD with a high likelihood of rapid disease progression. Despite that disease-modifying treatment is now available for clinical use, which is an important breakthrough in ADPKD, the moderate efficacy, limited tolerability (due to aquaretic events), and risk of hepatic toxicity warrant the development of additional treatment options.
Fig. 2.
Treatment effect of tolvaptan on annual rate of estimated glomerular filtration rate (eGFR) decline in the TEMPO 3:4 and REPRISE trials [25, 26]. Tolvaptan reduced the annual eGFR decline by 26% and 35% in the TEMPO 3:4 and REPRISE studies, respectively
Improving Currently Available Therapeutic Options
Overcoming Aquaretic Side Effects of Vasopressin Receptor Blockade
As stated in the Introduction, treatment with tolvaptan preserves kidney function in ADPKD, but aquaretic side effects such as polyuria, nycturia, and thirst limit its use. Many ADPKD patients opt not to be treated with tolvaptan because of these side effects, and so reducing these effects would improve the therapeutic potential of this drug. Tolvaptan blocks the vasopressin V2 receptor and thus results in a state that resembles diabetes insipidus. It therefore seems reasonable to apply strategies during tolvaptan use that have been proven to limit aquaresis in other forms of diabetes insipidus. The first strategy could be to lower the excretion of urinary osmoles by dietary interventions [41]. Since tolvaptan impairs the urinary concentrating capacity, osmolar excretion drives the amount of aquaresis. A decrease in urinary osmole excretion will correspondingly result in a decrease in urine volume. Whether this can be achieved by limiting protein as well as by limiting salt intake is the subject of clinical investigation [42]. Experimental evidence suggests that co-prescription with metformin also reduces polyuria during tolvaptan treatment [43], possibly by a vasopressin-independent upregulation of AQP-2 channels in the collecting duct [44]. However, in a small-scale cross-over study, the tolerability of metformin appeared less than that of hydrochlorothiazide due to gastrointestinal side effects [45], and consequently, metformin did not improve quality of life [43]. A third method might be to co-prescribe a thiazide diuretic, such as hydrochlorothiazide. Although seemingly paradoxical, co-treatment with a thiazide diuretic may lead to (considerably) less 24-h urine production. The mechanism is not fully understood, but it is assumed that blockage of the NCC channel with a thiazide diuretic leads to a state of mild volume depletion. The compensatory increase in salt and water reabsorption in the proximal tubule then results in a decreased urinary volume. In two small-scale clinical trials, this strategy resulted in a significant decrease in urinary volume and an improved quality of life in patients on tolvaptan [43, 46]. This was corroborated in an experimental study that suggested that co-treatment with a thiazide diuretic may also increase the renoprotective efficacy of tolvaptan [43]. Whether such a strategy also has longer-term effects in human ADPKD is going to be investigated in a large-scale clinical trial that is due to start in 2022 [24].
Overcoming Systemic Adverse Events; Targeted Treatment
Since molecular targets that are involved in cyst growth are also involved in other important biological processes in the body (such as cellular growth, proliferation, and repair), targeting these pathways may cause important systemic adverse events. In line, several potential therapies in ADPKD were very effective in animal models, but clinical studies were disappointing because systemic adverse events and toxicity limited adequate dosing. Given that chronic treatment is necessary for ADPKD (in contrast to, e.g., cancer treatment), adverse events should be kept to a minimum.
As mentioned above, an example of the inability to reach therapeutic dosage due to adverse effects is mTOR inhibition. For mTOR inhibition, clear attenuation of renal cystic disease was shown in several rodent models [47]. However, two clinical trials with mTOR inhibitors produced disappointing results, probably because side effects such as mucositis and diarrhea led to suboptimal dosing [28, 29]. Tissue targeting of these drugs to renal cysts, thereby minimizing drug exposition in extrarenal tissues, may be a potential way around this problem.
Targeting Drugs to Renal Cysts Via the Folate Receptor
In cancer, many tumors express the folate receptor-α (FRα). Using this receptor, folate-conjugated compounds can be taken up by receptor-mediated endocytosis [48]. Since cyst-lining cells also express the folate receptor-α (FRα), this enables the delivery of drugs specifically to these cells [49]. Folate-conjugated sirolimus (an mTOR inhibitor) is an engineered compound with a cleavable linker that allows the intracellular release of sirolimus in cyst-lining cells. An experimental study showed that this mechanism works in mice. The renoprotective effect of folate-conjugated sirolimus was comparable to that of unconjugated sirolimus, whereas systemic effects as weight loss and cell cycling in the thymus were absent [50]. This therapeutic strategy may also be used for other small molecules that have shown promising results in experimental studies, but whose therapeutic effect may be limited by adverse events, for example epidermal growth factor receptor (EGFR) inhibition (as described in section 5.8, Other Agents).
Targeting Drugs to Renal Cysts Via the Polymeric Immunoglobin Receptor
Drugs may also be targeted to renal cysts by coupling to monoclonal antibodies (mAb) that bind receptors on the basolateral membrane of the renal tubular cell, after which the drug:mAb complex is internalized and the drug is released. By reducing the drug exposition of extrarenal tissues, fewer off-target adverse effects are expected. A major obstacle, however, is that most available monoclonal antibodies are of the IgG type, which are not capable of crossing the epithelial barrier of renal cysts [51]. Some drug targets, like EGFR, are localized on the apical membrane of renal tubular cells in ADPKD [52]. In these cases, even though an IgG-bound drug may target a component of the basolateral membrane of renal tubular cells, the drug would still have to be transported through the cytoplasm and across the apical cell membrane to exert an effect.
It was recently found that the polymeric immunoglobin receptor (pIgR) is highly expressed on the basolateral membrane of cyst-lining cells in ADPKD [51]. This transmembrane protein is capable of binding polymeric immunoglobulins (pIg) of the IgA and IgM subtypes. The pIgR:pIg complex is then transcytosed through the cytoplasm to the apical membrane, where proteolytic cleavage releases the immunoglobulin into the cyst lumen (in complex with an extracellular portion of the pIgR) [51]. The same study demonstrated that in a murine ADPKD model, pIgR-mediated transport of dimeric IgA (dIgA) is unilateral and leads to an accumulation of dIgA in the renal cyst lumen. This opens up the idea of reformatting antagonistic monoclonal antibodies against growth factors or receptors implicated in ADPKD to the dIgA subtype for delivery to the cyst lumen by this mechanism. An example is the EGF receptor. Although EGFR antibodies that are currently in clinical use are of the IgG isotype, anti-EGFR antibodies of the IgA isotype have previously been engineered for use in oncological immunotherapy [53]. It is important to note that pIgR is also expressed in other epithelial tissues, like bronchial and gastrointestinal mucosa [54]. While these tissues may have less potential for drug accumulation than renal cysts because of their secretory nature, they may still be exposed to the drug to some extent which could lead to adverse events.
Drugs Under Investigation in Clinical Trials
The main emerging renoprotective therapies that are currently being investigated in clinical trials are discussed in detail below. Figure 3 gives a simplified overview of potential treatment targets in a renal tubular cell and Table 2 summarizes the ongoing or recently completed clinical trials that have been performed in ADPKD.
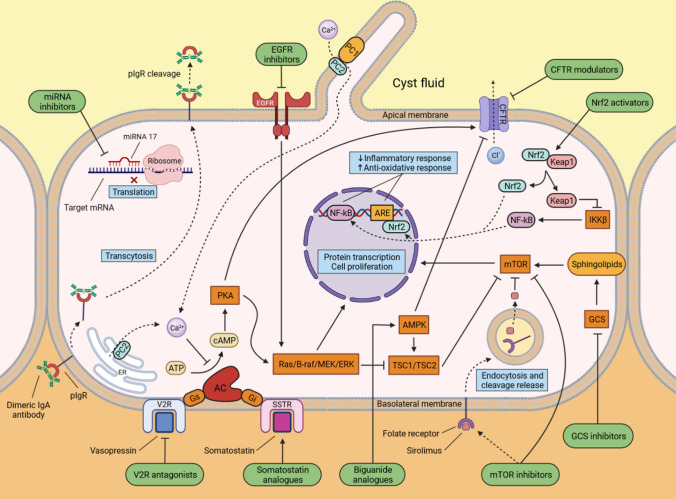
Fig. 3.
Illustration of the principal mechanisms of autosomal dominant polycystic kidney disease (ADPKD) pathogenesis and main targets of potential treatments. Dysfunction of polycystin 1 and 2 leads to abnormal ciliary function and a decrease in the intracellular calcium concentration, resulting in increased cAMP generation and mTOR activity which subsequently promote protein transcription and cell proliferation. Current and potential treatment options are depicted in green. AC adenylyl cyclase, ARE antioxidant response element, ATP adenosine triphosphate, AMPK 5’ AMP-activated protein kinase, B-raf serine/threonine-protein kinase B-Raf, cAMP cyclic adenosine monophosphate, CFTR cystic fibrosis transmembrane conductance regulator, EGFR epidermal growth factor receptor, ER endoplasmatic reticulum, ERK extracellular signal-regulated kinase, GCS glucosylceramide synthase, Gi inhibitory G protein of adenylyl cyclase, Gs stimulatory G protein of adenylyl cyclase, IKKβ I-kappa-B kinase unit beta, Keap1 Kelch-like ECH-associated protein 1, MEK mitogen-activated protein kinase kinase, miRNA 17 micro RNA 17, mTOR mammalian target of rapamycin, NF-κB nuclear factor kappa B, Nrf2 nuclear factor erythroid 2-related factor 2, PC1 polycystin 1, PC2 polycystin 2, PKA protein kinase A, pIgR polymeric immunoglobulin receptor, SSTR somatostatin receptor, TSC1/TSC2 tuberous sclerosis complex subunit 1/2, V2R vasopressin receptor 2
Table 2.
Overview of the most important ongoing or recently completed clinical trials investigating disease-modifying drugs in ADPKD
| Agent | Trial design | Main inclusion criteria | No. of patients | Follow-up duration (mo) | Status | Main potential adverse events | Primary outcome/main results |
|---|---|---|---|---|---|---|---|
| Vaptans | |||||||
| Lixivaptan |
ALERT [63] Phase 3, open label |
Age 18–65 years, eGFR ≥ 20 mL/min/1.73 m2, contra-indication to treatment with tolvaptan due to hepatocellular toxicity | 50 | 16.8 | Active, recruiting | Aquaresis | Hepatic safety (indicated by serum ALT levels) |
|
ACTION [62] Phase 3, double-blind placebo-controlled, followed by 1 year open label phase |
Age 18–60 years, eGFR 25–90 mL/min/1.73m2, Mayo class 1C, 1D or 1E | 1200 | 24 | Active, recruiting | Annualized change in eGFR during the first study year (placebo-controlled phase) | ||
| Somatostatin analogues | |||||||
| Lanreotide |
LIPS [33] Phase 3, double-blind, placebo-controlled |
Age > 18 years, mGFR 30 − 89 mL/min/1.73m2 | 159 | 36 | Completed, unpublished | Hepatic cyst infection, cholelithiasis and related problems, and gastrointestinal side effects | Changes in measured GFR |
| Octreotide LAR plus tolvaptan vs tolvaptan |
TOOL [170] Phase 2, double-blind, placebo-controlled |
Age > 18 years, mGFR > 80 mL/min/1.73m2 and stable renal function, TKV 1000–2000 mL | 20 | 4 | Active, not recruiting | Cholelithiasis, acute cholecystitis and gastrointestinal side effects | Changes in measured GFR |
| GCS inhibitors | |||||||
| Venglustat |
STAGED-PKD [23] Phase 2-3, double-blind placebo-controlled |
Age 18–55 years, eGFR > 30 mL/min/1.73m2 and < 90 mL/min/1.73m2, Mayo class 1C, 1D or 1E | 478 | 26 | Terminated, unpublished | Gastrointestinal side effects, possibly also ocular lens degeneration and depression | Terminated for futility based on interim analyses. Primary outcomes were changes in TKV, the results are not yet available |
| AL01211 | Phase 1, double-blind placebo-controlled [67] | Age 18–55 years, eGFR 30–89 mL/min/1.73m2 for ADPKD patients | 98 subjects (18 ADPKD patients) | 3 | Active, recruiting | Unknown | Safety and tolerability (based on incidence of (serious) adverse events) |
| Nrf2 activators | |||||||
| Bardoxolone |
FALCON [82] Phase 3, double-blind placebo-controlled |
Age 18–70 years, eGFR 30–90 or 30–44 mL/min/1.73m2 depending on age. Patients with eGFR ≥ 60 mL/min/1.73m2 or age ≥ 56 years must have progressive disease (eGFR decline of ≥ 2.0 mL/min/1.73m2 per year). No evidence of cardiac disease | 550 | 24 | Active, recruiting | Congestive heart failure and possibly tubuloglomerular damage secondary to glomerular hyperfiltration | Changes in eGFR between baseline and week 108 (off-treatment), and safety |
| CFTR modulators | |||||||
| GLPG2737 | Phase 2, double-blind, placebo-controlled followed by 1 year open label phase [96] | Age 18–50 years, eGFR 30–90 or 30–60 mL/min/1.73m2 depending on age, TKV >750 mL and Mayo class 1C, 1D or 1E | 66 | 24 | Active, not recruiting | Possibly nasopharyngitis, headache, pulmonary and gastrointestinal side effects | Changes in htTKV and safety |
| Biguanide analogues | |||||||
| Metformin | Phase 2, double-blind, placebo-controlled [45] | Age 30–60 years, eGFR 50–80 mL/min/1.73m2, non-diabetic | 51 | 12 | Published | Gastrointestinal side effects, vitamin B12 deficiency and lactic acidosis | 82% of patients tolerated a metformin dose of ≥ 1000 mg/day. No significant differences in changes of htTKV or eGFR between metformin and placebo groups (exploratory outcomes) |
|
TAME [110] Phase 2, double-blind, placebo-controlled |
Age 18–60 years, eGFR > 50 mL/min/1.73m2, non-diabetic | 97 | 26 | Published | 67% of metformin treated patients tolerated the target dose of 2000mg/day. No significant differences in changes of htTKV or eGFR between metformin and placebo groups (exploratory outcomes) | ||
| Metformin extended release |
IMPEDE-PKD [111] Phase 3, double-blind, placebo-controlled |
Age 18–70 years, eGFR 45–90 mL/min/1.73m2 and (risk of) rapidly progressive disease (based on volumetric criteria, PROPKD score or eGFR decline), non-diabetic | 1164 | 24 | Active, not yet recruiting | Changes in eGFR | |
| miRNA inhibitors | |||||||
| RGLS4326 | Phase 1, open label [123] | Age 18–70 years, eGFR 30–90 mL/min/1.73m2, Mayo class 1C, 1D or 1E | 19 | 2.3 | Completed, unpublished | Unknown | Changes in polycystin 1 and -2 levels in urinary exosomes |
| Other agents | |||||||
| Pravastatin | Phase 4, double-blind, placebo-controlled [148] | Age 25–60 years, eGFR ≥ 60 mL/min/1.73m2, TKV > 500 mL | 200 | 24 | Active, recruiting | Muscle pain, headache, gastrointestinal side effects | Changes in TKV |
| Phase 3, double-blind, placebo-controlled [171] | Age 8–22 years, eGFR ≥ 80 mL/min/1.73m2 | 110 | 36 | Completed, published | Reduced increase of several proinflammatory and oxidative stress markers in pravastatin treated patients compared to placebo | ||
| Pravastatin plus sodium citrate |
ADPKD-SAT [149] Phase 2, open label |
Age > 18 years, eGFR ≥ 30 mL/min/1.73m2 and evidence of metabolic acidosis | 30 | 12 | Active, recruiting | Changes in kidney function, liver function, blood pressure and incidence of muscle tenderness/rhabdomyolysis | |
| Pioglitazone |
PIOPKD [151] Phase 1b, double-blind, placebo-controlled |
Age 18–55 years, eGFR ≥ 50 mL/min/1.73m2 and (risk of) rapidly progressive disease (based on volumetric criteria), non-diabetic | 18 | 24 | Completed, published | Hypoglycemia | Low-dose pioglitazone appeared safe. No effect on TKV or eGFR |
| Tesevatinib | Phase 1-2, open label [172] | Age 18–62 years, eGFR ≥ 35 mL/min/1.73m2, htTKV ≥ 1000 mL | 74 | 24 | Completed, unpublished | QT-prolongation, diarrhea and acne | Safety, pharmacokinetics, maximum tolerated dose and changes in eGFR |
| Phase 2, double-blind, placebo-controlled [152] | Age 18–60 years, eGFR 25–90 mL/min/1.73m2, htTKV ≥ 500 mL, ≥ 750 mL or ≥ 900 mL depending on age | 80 | 24 | Active, not recruiting | Changes in htTKV | ||
| Curcumin | Phase 4, double-blind, placebo-controlled [164] | Age 6–25 years, eGFR > 80 mL/min/1.73m2 | 68 | 12 | Completed, published | Nausea and diarrhea (at high doses) | No differences between surrogate markers of vascular endothelial dysfunction and arterial stiffness in curcumin treated patients vs placebo. No significant differences in htTKV or eGFR (exploratory outcomes) |
ADPKD autosomal dominant polycystic kidney disease, ALT alanine aminotransferase, eGFR estimated glomerular filtration rate, mo months, mGFR measured GFR, TKV total kidney volume, htTKV height-adjusted TKV
Lixivaptan
Tolvaptan potentially causes severe hepatotoxic reactions, and carries a Black Box Warning in the USA [55] and EU [56] that cautions against the risk of liver injury. The mechanism that causes hepatocellular damage is still unknown. Regular monitoring of liver function tests is therefore warranted; monthly during the first 18 months of treatment and every 3 months thereafter. Clear decision rules have been defined on when to temporarily withhold or stop treatment in case of liver function test abnormalities [55, 56]. Severe hepatotoxic reactions to tolvaptan are extremely rare when such precautions are taken. Lixivaptan is an alternative V2RA that is hypothesized to carry less risk of hepatocellular toxicity. This is based upon quantitative systems toxicology modeling, that correctly predicted hepatotoxicity of tolvaptan [57]. Like tolvaptan, lixivaptan prevents the insertion of aquaporin channels into the collecting duct by competitively binding and antagonizing V2 receptors, leading to decreased cAMP production and subsequently, reduced cyst proliferation. It also leads to increased solute-free water excretion. Lixivaptan has a higher affinity for the V2 receptor than other VRAs, and is metabolized in the liver with a half-life of 7–10 h [58]. It was investigated for treatment of hyponatremia in congestive heart failure [59, 60] and liver cirrhosis [61], where lixivaptan was effective in increasing serum sodium concentrations. In these studies, significant hepatotoxic reactions in response to lixivaptan did not occur.
At the end of 2021, a large phase 3 placebo-controlled, randomized clinical trial (ACTION) was started to investigate the efficacy and safety of lixivaptan in participants with ADPKD [62]. This trial is designed to demonstrate the efficacy of lixivaptan in slowing the decline in renal function as measured by the difference in eGFR between the lixivaptan-treated and placebo-treated participants during 1 year, followed by an open-label extension period. The primary endpoint is the annualized change in eGFR from baseline to follow-up. Secondary endpoints are, amongst others, hepatocellular toxicity (defined as an increase of serum ALT levels >3 x ULN) and changes in TKV. The design of this trial strongly resembles that of the landmark REPRISE study [26], which has proven the efficacy of tolvaptan in later-stage disease. In view of their similar effects on hyponatremia and the high affinity of lixivaptan for the V2 receptor, it is expected that lixivaptan will have a similar renoprotective efficacy to tolvaptan. In that respect, it may be problematic to include patients in this study where they might receive a placebo, when tolvaptan is available for clinical use. Perhaps it is more appropriate to first demonstrate the safety of lixivaptan in patients with contraindications to treatment with tolvaptan due to hepatocellular toxicity. A phase 3, open-label study (ALERT) will administer lixivaptan to 50 patients with previous hepatotoxic reactions to tolvaptan and follow them for 12 months [63]. The primary endpoint is hepatic safety and the estimated study completion date is November 2022.
Glucosylceramide Synthase (GCS) Inhibitors
Another class of drugs that are momentarily under investigation in clinical trials as renoprotective agents in ADPKD are glucosylceramide synthase (GCS) inhibitors, among which is venglustat. These drugs decrease the synthesis of glucosylceramide (GL-1), a central building block for more complex glycosphingolipids (GSLs). GSLs are part of the cell membrane and thus necessary for normal physiological processes. However, in some diseases including lysosomal storage diseases (such as Fabry and Gauchier disease), an increased amount of GL-1 and other GSLs is found. Surprisingly, and not predicted based on knowledge of the pathophysiology of the disease, murine and human ADPKD were found to be also accompanied by increased GCS activity, leading to a pathogenic accumulation of GSLs such as GL-1, lactosylceramide (GL-2), and GM3 [64, 65]. Subsequently, it was shown that treatment with a GCS inhibitor significantly reduced cyst growth and preserved renal function in three different polycystic kidney disease (PKD) animal models [66]. In October 2018, the STAGED-PKD study [23] was therefore initiated to examine the effect of venglustat in ADPKD patients at risk for progressive disease based on Mayo classification (class 1C-E) and renal function (eGFR 30–90 mL/min/1.73 m2). Unfortunately, this study was terminated prematurely in August 2021, after an interim analysis showed lack of efficacy. Publication of the results of this trial is awaited.
Currently, another GCS inhibitor, AL01211, is being investigated in a phase 1 clinical study [67]. This study is currently recruiting and aims to include 80 healthy subjects and 18 ADPKD patients. The estimated study completion date is June 2022.
Nuclear Factor Erythroid-2 Related Factor 2 (Nrf2) Activators
Oxidative stress has emerged as a significant contributor to disease progression in ADPKD [68]. Nuclear factor erythroid-2 related factor 2 (Nrf2) is an important transcription factor for regulating the defensive response against oxidative stress [69]. Under normal circumstances, Nrf2 is bound to one of three ubiquitin ligase complexes, most importantly keap1-cullin 3-Ring box 1 (Keap1-CUL3-RBX1) [70], and is consequently targeted for rapid ubiquitin-proteasomal degradation. Oxidative stress induces conformational changes in the Keap1 molecule, releasing Nrf2 from the Keap1-CUL3-RBX1 complex and allowing it to translocate to the nucleus. Here, Nrf2 binds to the antioxidant response element to induce the transcription of numerous target genes and thereby reduce oxidative stress, among other cytoprotective effects [71]. In an orthologous ADPKD mouse model, Nrf2 deletion increased ROS generation and promoted cyst growth, while pharmacological Nrf2 induction reduced ROS production and cystogenesis [68].
Bardoxolone is a potent activator of Nrf2 [72, 73] that was originally developed as an antineoplastic drug. In addition to the activation of Nrf2, bardoxolone inhibits the nuclear factor κB (NF-κB) inflammatory pathway [74]. Bardoxolone was found to have renoprotective effects in a phase 1 clinical trial that included patients with advanced solid tumors and lymphomas [75] and was then investigated in diabetic kidney disease (DKD) [76, 77]. The BEAM trial was a randomized controlled trial involving 227 patients with type 2 diabetes and advanced chronic kidney disease (eGFR 20–45 mL/min/1.73 m2) who were assigned to placebo or varying doses of bardoxolone methyl [78]. At 52 weeks, treatment with bardoxolone was associated with an increased eGFR compared to placebo in all dose groups. The most common adverse event in this study were muscle spasms. After this pilot study, a large-scale follow-up study (BEACON trial) was designed to test the effect of bardoxolone in 2,185 type 2 diabetics with stage 4 CKD (eGFR 15–29 mL/min/1.73 m2). This study also found a significant increase in eGFR in bardoxolone treated patients. In addition, an increase in urinary albumin to creatinine ratio (ACR) was reported, which may point towards drug-induced glomerular hyperfiltration. However, the study was discontinued because of higher rates of cardiovascular events and heart failure in the bardoxolone group [79]. Post hoc analyses showed that elevated B-type natriuretic peptide and previous hospitalizations for heart failure were predictive of these adverse events [80]. In a more recent trial that also studied the effects of bardoxolone in DKD, and that excluded patients at risk for heart failure, no episodes of heart failure were observed [81]. These data suggest that bardoxolone might be beneficial in progressive kidney diseases characterized by oxidative stress and a low risk of heart failure, such as ADPKD. However, the increase in albuminuria in bardoxolone-treated patients, which may reflect drug-induced glomerular hyperfiltration, could be detrimental to renal function during long-term treatment.
Unpublished data from the PHOENIX study, an open label phase 2 study, suggested an improvement in kidney function in 31 ADPKD patients treated with bardoxolone (mean eGFR improvement of 6.6 mL/min/1.73 m2 at Week 4 (p < 0.0001), increasing to 12.0 mL/min/1.73 m2 at Week 12 (p < 0.0001) from a mean baseline eGFR of 47.7 mL/min/1.73 m2. Data on albuminuria from this study are not available. A follow-up phase 3 placebo-controlled study (FALCON) [82] is currently recruiting to investigate the safety, tolerability, and efficacy of bardoxolone in ADPKD patients without evidence of cardiac disease. The primary efficacy endpoint is the change in eGFR from baseline to 4 weeks after withdrawal in bardoxolone versus placebo-treated patients. This study aims to enroll 550 patients with an eGFR of 30–90 mL/min/1.73 m2 for patients 18–55 years of age, or 30–45 mL/min/1.73 m2 for patients aged 55–70 years. In addition, when the eGFR is between 60 and 90 mL/min/1.73 m2 or when age is between 55 and 70 years, patients must have evidence of ADPKD progression, i.e., a historical annual rate of eGFR decline ≥ 2.0 mL/min/1.73 m2. Given the average eGFR decline of approximately 3.5 mL/min/1.73 m2 per year in placebo-treated patients from previous ADPKD trials [25, 26], it is uncertain whether these inclusion criteria sufficiently select patients at risk for rapid disease progression. As such, the effects of bardoxolone in this study may be obscured. Additionally, as mentioned above, the increase of albuminuria in previous studies possibly indicates glomerular hyperfiltration. In view of the potential harmful effects of long-standing glomerular hyperfiltration, this study will address the question whether long-term treatment with bardoxolone is safe and effective. The estimated completion date of this study is December 2023.
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulators
Transepithelial chloride secretion is a key pathogenetic mechanism behind cystogenesis and is mediated by cystic fibrosis transmembrane conductance regulator (CFTR). This Cl- channel is primarily located in the apical membrane of cyst lining cells and stimulates the secretion of chloride ions into cyst fluid [83, 84]. The resulting negative ion gradient is followed by transepithelial movement of Na+ ions and subsequently by water molecules (respectively termed “electric” and “osmotic coupling”), leading to cyst expansion [85]. The activity of CFTR is in part regulated by protein kinase A (PKA) and AMP-activated protein kinase (AMPK), and therefore depends on intracellular cAMP levels [86, 87]. The observation that some individuals with ADPKD and concurrent homozygous CFTR mutations (leading to cystic fibrosis; CF) exhibit an attenuated ADPKD phenotype may suggest a role of CFTR in cystogenesis [88, 89]. However, another similar study did not find such an association [90].
It was subsequently shown that two CFTR inhibitors of the thiazolidinone and glycine hydrazide groups slow cyst formation in a murine embryonic kidney cyst model. In the same study, treatment of kidney-specific PKD1-knockout mice with either compound reduced cyst growth and preserved renal function [91]. VX-809 (Lumacaftor), one of the available CFTR modulators originally designed to treat CF, restores the folding and cellular trafficking of mutant CFTR proteins [92]. Treatment of PKD1-knockout mice with VX-809 induced the localization of CFTR proteins to the basolateral membrane of cyst lining cells. In addition, the translocation of Na+/H+ exchanger 3 and epithelial sodium channels to the apical membrane were increased. Together, these effects were hypothesized to promote net resorption of cyst fluid and thereby reduce cyst growth [93, 94]. A follow-up study by the same authors produced similar results on cyst growth, while also preserving renal function in VX-809 treated PKD1RC/RC mice [95].
Given these promising results, a phase 2, placebo-controlled, randomized, controlled trial (RCT) was started to investigate the safety and tolerability of the CFTR modulator GLPG2737 in 66 ADPKD patients at risk for rapidly progressive disease [96]. The treatment duration is 12 months, followed by an optional open label extension period of another 12 months. The estimated study completion date is February 2024. Seeing as this drug influences CFTR activity, which is altered in CF, one of the issues to be addressed will be the adverse event profile given the possibility of pulmonary and gastrointestinal side effects (corresponding to the clinical features of CF).
Biguanide Analogues
The biguanide analogue metformin is a well-known first line treatment for type 2 diabetes mellitus. It also has beneficial effects in other diseases such as polycystic ovary syndrome and non-alcoholic fatty liver disease [97, 98]. Metformin has been prescribed for decades and generally has a good safety profile, although there are dose-dependent gastrointestinal adverse events, such as nausea, abdominal pain and diarrhea. Moreover, lactic acidosis is a significant potential risk, particularly in patients with impaired renal function [99].
Abnormal polycystin signaling in renal tubular PKD cells is accompanied with a metabolic shift to glycolysis (similar to the Warburg effect in cancer cells) and excessive ATP production [100]. AMPK serves as a cellular energy-sensing molecule that inhibits mTOR signaling and CFTR activity during energy depletion [87, 101, 102]. The enhanced metabolic rate in PKD cells inhibits AMP-activated protein kinase (AMPK), thus leading to increased mTOR signaling and CFTR activity, which respectively stimulate the proliferative and secretory aspects of cyst formation [103]. Metformin activates AMPK and could reduce ADPKD disease progression through the abovementioned mechanisms [103].
Several preclinical in vivo studies have found beneficial effects of metformin on ADPKD disease parameters such as cystic index [103–106], with some also describing an improved renal function [105, 106]. However, a recently conducted study demonstrated no effect on cystic index or renal function in a murine ADPKD model [107], while another study reported a paradoxical increase in cystic index and worsened renal function in metformin-treated mice [108]. These discrepant results could be explained by heterogeneity in terms of animal models, the timing of treatment relative to cyst formation, the method of metformin administration (some studies delivered metformin by intraperitoneal injection, thereby circumventing first pass effects) and treatment duration. Of note, some authors drew attention to the dose of metformin that was given in the various experimental models, with only models in which high dosages were given reaching positive results [109]. By extrapolating these data, they reasoned that metformin should be given in a dose of at least 2000 mg/day to patients with ADPKD to achieve beneficial effects.
To date, two placebo-controlled small-scale phase 2 trials have been performed in non-diabetic ADPKD patients with varying degrees of renal insufficiency [45, 110]. Although metformin treatment appeared to be safe in these patients, limited tolerability due to gastrointestinal side effects could impact its therapeutic potential. For example, Brosnahan et al. reported that only 50% of 51 metformin-treated patients tolerated the target dose of 2000 mg/day [45]. Exploratory analyses showed no effects on height adjusted TKV (htTKV) growth or renal function decline, although these studies were not powered to detect such differences and they were not enriched for inclusion of patients with rapidly progressive disease [45, 110]. Additional studies are therefore needed.
Recently, a phase 3 study to investigate the effect of metformin on disease progression has been announced (IMPEDE-PKD) [111]. This placebo-controlled RCT will include 1164 ADPKD patients with CKD stages 2–3A (eGFR 45–90 mL/min/1.73 m2) and risk of rapid disease progression (based on kidney volume or previous rate of renal function decline), for a total treatment duration of 24 months. The primary endpoint will be change in eGFR, and metformin will be dosed at 1000–2000 mg/day. The use of an extended-release metformin formulation in this study could enhance its tolerability and thus prevent the need to down-titrate, consequently optimizing the potential therapeutic effect.
Of note, the gastrointestinal side effects of metformin could influence dietary patterns. Salt intake has been shown to be associated with the rate of kidney function decline in ADPKD [112], possibly because a higher intake of osmoles increases vasopressin secretion and, subsequently, cAMP production in renal tubular cells. We therefore suggest monitoring dietary osmole intake by measuring sodium and protein intake in interventional studies that investigate metformin to exclude any confounding effects.
miRNA Inhibitors
Micro RNA inhibitors or anti-micro RNAs (anti-MiRs) are a new class of drugs that are being investigated for use in ADPKD. Micro RNAs (miRNAs or miRs) are short, noncoding RNA fragments that function as sequence-specific, posttranscriptional inhibitors of gene expression. The binding of miRNAs to target mRNA transcripts results in translational repression and, ultimately, to degradation of the targeted mRNA sequence [113]. Anti-MiRs are modified nucleotides designed to sterically hinder miRNAs, thus leading to de-repression of the target mRNA sequence and increased expression of encoded proteins [114]. Multiple miRNAs are abnormally expressed in ADPKD, and assumed to be causally related to the rate of disease progression [115]. Currently, miR-17 is the only miRNA that is targeted in clinical trials in ADPKD. We will therefore briefly discuss the role of miR-17 in ADPKD pathogenesis. Other miRNAs and their possible role in ADPKD are reviewed elsewhere [115].
miR-17 is part of a polycistronic cluster (miR-17~92) that produces six individual miRNAs (miR-17, -18, -19a, -20a, -19b-1 and -92-1) [116]. In both murine and human ADPKD, the miR-17~92 cluster is vastly overexpressed and genetic deletion of the cluster mitigates disease progression in ADPKD mouse models [117, 118]. Transgenic upregulation of c-Myc, a transcription factor that controls transcription of miR-17~92, promotes cyst formation in murine kidney tubules [119]. Additionally, cyst growth is reduced when PKD1-knockout mice are treated with anti-miR-17 [120], which supports the notion that miR-17 is an important driver of disease progression in ADPKD.
Numerous mRNA transcripts are subject to miR-17 binding and therefore, miR-17 controls the expression of many different target genes (both directly and indirectly). This includes major regulatory genes involved in proliferative signaling pathways such as PPARA and mTOR119, which could explain the effect of miR-17 on cyst growth and disease progression. In addition, miR-17 seems capable of directly binding and repressing the mRNA transcripts of PKD1, PKD2 and HNF-1β [118, 121]. Conversely, anti-miR-17 treatment increases PKD1 and PKD2 expression [122]. This is especially important in case of hypomorphic mutations, where cyst growth is promoted because PKD1 and PKD2 activity falls below a critical threshold. Therefore, the effects of anti-miR-17 treatment are likely mediated through several signaling pathways and possibly also by a direct effect on PKD1 and PKD2 expression.
RGLS4326 and RGLS8429 were developed as anti-miR-17 oligonucleotides that preferentially target the kidney. RGLS4326 inhibits cyst growth in multiple PKD mouse models and human in vitro ADPKD models [122]. A dose-escalating phase 1b study was recently performed to assess the short-term safety and tolerability of RGLS4326 [123], but its results are not yet available. Recently, a phase 1b clinical study with RGLS8429 was also announced. Although anti-miR-17 treatment is an intriguing potential ADPKD treatment option, it is currently unclear whether long-term treatment is safe, especially in view of its effects on proliferative signaling pathways that are ubiquitous throughout the body.
Uric Acid-Lowering Therapy
Both in healthy subjects and in patients with various forms of chronic kidney disease, high serum uric acid concentrations have been associated with the development and progression of renal disease [124–128]. In ADPKD, several studies also point towards an association between high serum uric acid and disease progression [129, 130], although this association was not evident in other studies [131, 132]. In addition, hyperuricemic ADPKD patients with preserved renal function seem to display higher rates of endothelial dysfunction [133], which is an early indicator of cardiovascular disease [134].
Since uric acid is primarily excreted by the kidney [135], a loss of glomerular filtration rate is expected to cause a rise of serum uric acid levels. Hyperuricemia may therefore simply be viewed as a marker of impaired renal function in patients with chronic kidney disease. Nevertheless, an increasing body of preclinical evidence suggests a causal role of uric acid in the development of renal dysfunction through several mechanisms, including the induction of oxidative stress, proliferation of vascular smooth muscle cells and promotion of epithelial-to-mesenchymal transition in renal tubular cells (as reviewed elsewhere [136]). Such effects could also influence renal outcomes in ADPKD. Despite reports of an increased prevalence of renal cysts in patients with gout, in type 2 diabetics with hyperuricemia and in some hereditary syndromes associated with hyperuricemia [137–139], experimental evidence of the ability of uric acid to directly promote renal cyst formation is lacking.
Xanthine oxidase inhibitors (e.g., allopurinol, oxypurinol, febuxostat) are the preferred treatment for hyperuricemia because of their effectiveness and generally favorable safety profile, even in patients with CKD [140, 141]. Uricosuric agents such as benzbromarone are also available to reduce serum uric acid levels. The effect of uric acid-lowering therapy on disease progression in ADPKD was retrospectively analyzed in two studies. Han et al. describe that, while hyperuricemia was not an independent predictor of renal function decline in their cohort, the initiation of uric acid-lowering therapy appeared to mitigate annual eGFR decline in 53 ADPKD patients with mild renal insufficiency [132]. The majority of these patients received benzbromarone. It is unclear whether this effect can be attributed to reduced serum uric acid levels since benzbromarone itself may directly suppress cyst growth by inhibiting the Ca2+ activated Cl− channel TMEM16A, resulting in decreased transepithelial chloride secretion [142]. In contrast, a Korean cohort study that included 364 ADPKD patients reported that the use of febuxostat, but not allopurinol or benzbromarone, was associated with higher baseline eGFR [143]. The results of this study are difficult to interpret due to its cross-sectional nature. In addition to these retrospective studies, a phase 2/3 trial was recently announced to evaluate the effect of oxypurinol in ADPKD patients [144].
Other Agents
Several other agents are being investigated in clinical trials for use in ADPKD. In addition to their cholesterol-lowering effects, statins have other properties that could benefit patients with chronic renal disease, such as anti-oxidative and anti-inflammatory effects [145, 146]. Recently, a systematic review examined the effect of statin therapy on ADPKD progression and did not find a significant effect on annual TKV growth or eGFR decline, although urinary protein excretion and serum low-density lipoprotein levels were reduced [147]. A phase 4 placebo-controlled RCT is currently recruiting to examine the effect of pravastatin on TKV148. In addition, a phase 2 open-label study is evaluating the effect of pravastatin in combination with sodium citrate in 30 ADPKD patients with evidence of metabolic acidosis [149]. Primary endpoints of this study are changes in kidney function, liver function, and safety.
Pioglitazone is a thiazolidinedione and acts as a peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist. This nuclear receptor forms a heterodimer with retinoic acid receptor A to control the transcription of multiple target genes. In addition to its insulin-sensitizing effects, pioglitazone may inhibit cystogenesis in animal models through several mechanisms, including the inhibition of CFTR expression and downregulation of proliferative pathways [150]. The PIOPKD study was a phase 1b placebo-controlled cross-over study that evaluated the safety of low-dose pioglitazone treatment in 18 non-diabetic ADPKD patients [151]. In this pilot study, pioglitazone at a dose of 15 mg/day for 1 year appeared safe. Treatment with pioglitazone was followed by 1-year double-blind placebo treatment (or vice versa). No effects of pioglitazone on TKV or renal function were seen compared to placebo treatment, although the study was not sufficiently powered to detect such effects.
Tesevatinib (KD019) belongs to the class of tyrosine kinase inhibitors and is currently being investigated in a placebo-controlled phase 2 clinical trial [152]. It is a multi-kinase inhibitor that decreases the phosphorylation of c-Src, EGFR, and Erb2, and thereby reduces cellular proliferation in polycystic mice [153]. By targeting multiple pathways that are important to the pathophysiology of ADPKD, tesevatinib may be an effective therapeutic strategy. However, phase 1 data presented at the American Society of Nephrology 2015 annual meeting reported frequent adverse events such as QT-prolongation, and side effects that are typically associated with EGFR inhibition like diarrhea and an acneiform rash [154]. The primary endpoint of this phase 2 trial are changes in htTKV. The study was completed in January 2022, and its results have not yet been published.
Bosutinib (SKI-606) is a dual Src/Bcr-Abl tyrosine kinase inhibitor and was approved for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia. Preclinical data indicated that inhibition of c-Src with bosutinib ameliorates renal cyst formation in two animal models [155]. A randomized, placebo-controlled clinical trial in 172 ADPKD patients showed a reduction of kidney growth in patients treated with bosutinib at 200 mg/day versus placebo, but unfortunately no significant effect on annual eGFR decline was found [156]. Adverse events were frequently encountered and led to a protocol amendment that reduced the bosutinib dose in 24 patients who were initially randomized to receive 400 mg/day. Treatment-related adverse events were responsible for 94% of study discontinuations in bosutinib 400 mg/day (n = 17 of 18) versus 60% in bosutinib 200 mg/day (n = 9 of 15), compared to 43% for placebo (n = 3 of 7). The most frequent adverse events were diarrhea and hepatocellular toxicity. Since then, new studies to investigate the effect of bosutinib in ADPKD have not been announced.
Curcumin is a naturally occurring polyphenol with anti-oxidative, anti-inflammatory, and anti-proliferative properties that have been extensively studied in multiple diseases [157]. These effects are exerted by its influence on various cellular signaling pathways, some of which are also dysregulated in ADPKD, including mTOR, NF-κB, MAPK, and Wnt signaling [158–160]. In tamoxifen-inducible PKD1-knockout mice, treatment with curcumin reduced cystogenesis and delayed renal failure [161]. Two additional animal studies have demonstrated beneficial effects of curcumin on ADPKD disease progression [162, 163]. Recently, a randomized, placebo-controlled clinical trial in 68 children and young adults with ADPKD was performed to examine the effect of daily oral curcumin supplementation (25 mg/kg per day) for 12 months on surrogate markers of vascular endothelial dysfunction and arterial stiffness [164]. This study failed to show any benefit on flow-mediated dilation of the brachial artery (p = 0.48), nor on pulse-wave velocity (p = 0.67) or total kidney volume. Despite the use of a formulation with enhanced bioavailability, the discrepant results between experimental studies and this clinical study may be explained by the fact that curcumin has very low oral bioavailability with extensive first-pass metabolism [165]. In experimental studies, high oral doses of curcumin were utilized (curcumin was given in a dose equal to 1% of food intake in a previous experimental study [161]), but it is questionable whether such high oral dosing or parenteral administration are feasible and tolerable in humans.
Extrapolating Treatment Efficacy from Animal Models
Animal models of ADPKD offer valuable insights into the pathogenesis of ADPKD and are an essential part of the development of new treatment options for humans. Multiple rodent ADPKD models have been developed for preclinical testing, including models without PKD1/PKD2 mutations, models with reduced PKD1/PKD2 expression or missense mutations, and models with germline or conditional PKD1/PKD2 gene knockouts. While these models seek to recreate human ADPKD, which itself is already a clinically and genetically heterogeneous disease, differences in key aspects such as lifespan, metabolism, renal anatomy, involved nephron segments, and genetics mean that rodent PKD models only partially resemble human ADPKD [166]. As such, novel therapies like mTOR inhibitors, somatostatin analogues, or curcumin may produce promising results in rodent models but subsequently fail to demonstrate meaningful effects in human ADPKD [22, 28–31].
Conversely, a single preclinical study with a novel medicament that suggests harmful effects in experimental ADPKD can potentially lead to the unjustified exclusion of ADPKD patients from large clinical trials. Examples are the DAPA-CKD and EMPA-KIDNEY studies [167, 168]. The DAPA-CKD found a promising attenuated rate of kidney function decline with the SGLT2 inhibitor dapagliflozin in subjects with chronic kidney disease and an elevated ACR. Unfortunately, these trials excluded subjects with polycystic kidney disease, because an experimental study suggested that dapagliflozin may aggravate polycystic kidney disease in PCK rats [169]. This precludes a post hoc analysis of the effect of SGTL2 inhibitors in ADPKD.
Given the many and sometimes fundamental differences between human ADPKD and animal disease models, the exclusion of ADPKD patients from large clinical trials in chronic kidney disease based solely on a single animal study is, in our opinion, unjustified. To do so, harmful effects would have to be demonstrated in several different experimental models. In addition, when taking into account the essential differences between the available rodent models, the results of a single negative study are also not sufficient to stop developing a drug, nor should the results of a single positive study suffice to move drug development from the preclinical to the clinical phase. The question then arises which and how many rodent PKD models should be tested before proceeding to human trials. Although this question has no definitive answer, the use of at least two different models, preferably a model with germline hypomorphic or missense mutations in combination with an inducible conditional knockout model, has been suggested [166].
Conclusion
As we have described above, several drugs have been or are being developed as renoprotective treatment in ADPKD. Since ADPKD is a complex disease with a high degree of genetic heterogeneity, and the mechanisms implicated in cyst growth also have important functions in various physiological processes throughout the body, it may be difficult to design new interventions that effectively target cyst growth and preserve kidney function without causing major adverse events. Since lifelong treatment is required in ADPKD, only a minimal degree of adverse events is acceptable. Options to overcome these difficulties are additional interventions to decrease adverse events, for instance in case of vasopressin 2 receptor antagonists, or to design drugs that can be selectively transported into renal cysts. Advancements in the knowledge of the pathophysiology of the disease, as well as better insight in the process of drug development, will hopefully bring new treatments to prevent the need for kidney replacement therapy in ADPKD patients.
Declarations
Funding
No financial support was received for the preparation of this article.
Conflicts of interest
EM received consultancy fees from Otsuka, research funding from Sanofi and the Dutch kidney foundation. RG received consultancy fees and/or research grants from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Galapagos, Ipsen, Mironid, Otsuka and Sanofi. TB has no conflicts of interest to declare. All money was paid to the institution.
Ethics approval
Not applicable.
Consent (for participation and publication)
Not applicable.
Author contributions
TB and EM conceived the design of the article. RG critically revised the work. All authors contributed to the literature search and editing of the manuscript. All authors have read and approved the final version of the manuscript.
Data and code availability
Not applicable.
References
- 1.Chebib FT, Torres VE. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis. 2016;67:792–810. doi: 10.1053/j.ajkd.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willey CJ, et al. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant. 2017;32:1356–1363. doi: 10.1093/ndt/gfw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spithoven EM, et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival—an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014;29(Suppl 4):15–25. doi: 10.1093/ndt/gfu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheong B, Muthupillai R, Rubin MF, Flamm SD. Normal values for renal length and volume as measured by magnetic resonance imaging. Clin J Am Soc Nephrol. 2007;2:38–45. doi: 10.2215/CJN.00930306. [DOI] [PubMed] [Google Scholar]
- 5.Reeders ST, et al. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 2021;317:542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- 6.Peters DJ, et al. Chromosome 4 localization of a second gene for autosomal dominant polycystic kidney disease. Nat Genet. 1993;5:359–362. doi: 10.1038/ng1293-359. [DOI] [PubMed] [Google Scholar]
- 7.Hopp K, et al. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest. 2012;122:4257–4273. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leeuwen ISL, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13:3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 9.Porath B, et al. Mutations in GANAB, encoding the glucosidase iiα subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet. 2016;98:1193–1207. doi: 10.1016/j.ajhg.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornec-Le Gall E, et al. Monoallelic mutations to DNAJB11 cause atypical autosomal-dominant polycystic kidney disease. Am J Hum Genet. 2018;102:832–844. doi: 10.1016/j.ajhg.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gall EC-L, Torres VE, Harris PC. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol. 2017;29:13–23. doi: 10.1681/ASN.2017050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, Harris PC. Regulation of polycystin expression, maturation and trafficking. Cell Signal. 2020;72:109630. doi: 10.1016/j.cellsig.2020.109630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z-Y, Wang Z-M, Huang Y. Polycystic liver disease: classification, diagnosis, treatment process, and clinical management. World J Hepatol. 2020;12:72–83. doi: 10.4254/wjh.v12.i3.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meijer E, Gansevoort RT. Emerging non-pharmacological interventions in ADPKD: an update on dietary advices for clinical practice. Curr Opin Nephrol Hypertens. 2021;30:482–492. doi: 10.1097/MNH.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 15.Gall EC-L, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019;393:919–935. doi: 10.1016/S0140-6736(18)32782-X. [DOI] [PubMed] [Google Scholar]
- 16.Chebib FT, Torres VE. Assessing risk of rapid progression in autosomal dominant polycystic kidney disease and special considerations for disease-modifying therapy. Am J Kidney Dis. 2021;78:282–292. doi: 10.1053/j.ajkd.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Müller R-U, et al. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA working group on inherited kidney disorders, the European rare kidney disease reference network and polycystic kidney disease international. Nephrol Dial Transplant. 2022;37:825–839. doi: 10.1093/ndt/gfab312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavu S, et al. The value of genotypic and imaging information to predict functional and structural outcomes in ADPKD. JCI Insight. 2020;5:13. doi: 10.1172/jci.insight.138724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gansevoort RT, et al. Plasma copeptin levels predict disease progression and tolvaptan efficacy in autosomal dominant polycystic kidney disease. Kidney Int. 2019;96:159–169. doi: 10.1016/j.kint.2018.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messchendorp AL, et al. Rapid progression of autosomal dominant polycystic kidney disease: urinary biomarkers as predictors. Am J Nephrol. 2019;50:375–385. doi: 10.1159/000502999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irazabal MV, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–172. doi: 10.1681/ASN.2013101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meijer E, et al. Effect of Lanreotide on kidney function in patients with autosomal dominant polycystic kidney disease: the DIPAK 1 randomized clinical trial. JAMA. 2018;320:2010–2019. doi: 10.1001/jama.2018.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Library of Medicine (U.S.). A medical research study designed to determine if Venglustat can be a future treatment for ADPKD patients (STAGED-PKD). https://clinicaltrials.gov/ct2/show/NCT03523728 (2018).
- 24.National Library of Medicine (U.S.). HYDROchlorothiazide to PROTECT polycystic kidney disease patients and improve their quality of life (HYDRO-PROTECT). https://clinicaltrials.gov/ct2/show/NCT05373264 (2022).
- 25.Torres VE, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres VE, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377:1930–1942. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 27.Torres VE, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3–4 Study. Am J Kidney Dis. 2011;57:692–699. doi: 10.1053/j.ajkd.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 28.Serra AL, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:1. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 29.Walz G, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 30.Caroli A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382:1485–1495. doi: 10.1016/S0140-6736(13)61407-5. [DOI] [PubMed] [Google Scholar]
- 31.Perico N, et al. Octreotide-LAR in later-stage autosomal dominant polycystic kidney disease (ALADIN 2): a randomized, double-blind, placebo-controlled, multicenter trial. PLoS Med. 2019;16:e1002777. doi: 10.1371/journal.pmed.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Library of Medicine (U.S.). Somatostatin in polycystic kidney: a long-term three year follow up study (ALADIN). https://www.clinicaltrials.gov/ct2/show/NCT00309283 (2006).
- 33.National Library of Medicine (U.S.). Lanreotide in polycystic kidney disease study (LIPS). https://clinicaltrials.gov/ct2/show/NCT02127437 (2014).
- 34.Griffiths J, Mills MT, Ong AC. Long-acting somatostatin analogue treatments in autosomal dominant polycystic kidney disease and polycystic liver disease: a systematic review and meta-analysis. BMJ Open. 2020;10:e032620. doi: 10.1136/bmjopen-2019-032620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messchendorp AL, Casteleijn NF, Meijer E, Gansevoort RT. Somatostatin in renal physiology and autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2020;35:1306–1316. doi: 10.1093/ndt/gfz054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suwabe T, Barrera FJ, Rodriguez-Gutierrez R, Ubara Y, Hogan MC. Somatostatin analog therapy effectiveness on the progression of polycystic kidney and liver disease: a systematic review and meta-analysis of randomized clinical trials. PLoS ONE. 2021;16:e0257606. doi: 10.1371/journal.pone.0257606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres VE, et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 38.Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 39.Torres VE, et al. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 Trial. Nephrol Dial Transplant. 2017;33:477–489. doi: 10.1093/ndt/gfx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards ME, et al. Long-term administration of Tolvaptan in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2018;13:1153–1161. doi: 10.2215/CJN.01520218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kramers BJ, van Gastel MDA, Boertien WE, Meijer E, Gansevoort RT. Determinants of urine volume in ADPKD patients using the vasopressin V2 receptor antagonist Tolvaptan. Am J Kidney Dis. 2019;73:354. doi: 10.1053/j.ajkd.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 42.National Library of Medicine (U.S.). Wishing to decrease aquaresis in ADPKD patients treated with a V2Ra; the effect of regulating protein and salt (WATER). https://clinicaltrials.gov/ct2/show/NCT04310319 (2020).
- 43.Kramers BJ, et al. Effects of hydrochlorothiazide and metformin on aquaresis and nephroprotection by a vasopressin V2 receptor antagonist in ADPKD: a randomized crossover trial. Clin J Am Soc Nephrol. 2022 doi: 10.2215/CJN.11260821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Efe O, Klein JD, LaRocque LM, Ren H, Sands JM. Metformin improves urine concentration in rodents with nephrogenic diabetes insipidus. JCI Insight. 2016;1:11. doi: 10.1172/jci.insight.88409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brosnahan GM, et al. Metformin therapy in autosomal dominant polycystic kidney disease: a feasibility study. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchiyama K, Kitayama C, Yanai A, Ishibashi Y. The effect of trichlormethiazide in autosomal dominant polycystic kidney disease patients receiving tolvaptan: a randomized crossover controlled trial. Sci Rep. 2021;11:17666. doi: 10.1038/s41598-021-97113-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shillingford JM, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Update. 2021;17:89. doi: 10.1016/j.drup.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Shillingford JM, Leamon CP, Vlahov IR, Weimbs T. Folate-conjugated rapamycin slows progression of polycystic kidney disease. J Am Soc Nephrol. 2012;23:1674. doi: 10.1681/ASN.2012040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kipp KR, et al. Comparison of folate-conjugated rapamycin versus unconjugated rapamycin in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol. 2018;315:395. doi: 10.1152/ajprenal.00057.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olsan EE, Matsushita T, Rezaei M, Weimbs T. Exploitation of the polymeric immunoglobulin receptor for antibody targeting to renal cyst lumens in polycystic kidney disease. J Biol Chem. 2015;290:15679. doi: 10.1074/jbc.M114.607929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orellana SA, Sweeney WE, Neff CD, Avner ED. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int. 1995;47:490. doi: 10.1038/ki.1995.62. [DOI] [PubMed] [Google Scholar]
- 53.Lohse S, et al. An anti-EGFR IgA that displays improved pharmacokinetics and myeloid effector cell engagement in vivo. Cancer Res. 2016;76:403–417. doi: 10.1158/0008-5472.CAN-15-1232. [DOI] [PubMed] [Google Scholar]
- 54.Wei H, Wang J-Y. Role of polymeric immunoglobulin receptor in IgA and IgM transcytosis. Int J Mol Sci. 2021;22:2284. doi: 10.3390/ijms22052284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Center for Drug Evaluation and Research (U.S.). Drug approval package: Jynarque (tolvaptan). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/204441Orig1s000Lbl.pdf (2009).
- 56.European Medicines Agency. Tolvaptan summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/jinarc-epar-product-information_en.pdf (2015).
- 57.Woodhead JL, Pellegrini L, Shoda LKM, Howell BA. Comparison of the hepatotoxic potential of two treatments for autosomal-dominant polycystic kidney disease using quantitative systems toxicology modeling. Pharmaceut Res. 2020;37:1–2. doi: 10.1007/s11095-019-2726-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.di Mise A, et al. Lixivaptan, a new generation diuretic, counteracts vasopressin-induced aquaporin-2 trafficking and function in renal collecting duct cells. Int J Mol Sci. 2019;21:183. doi: 10.3390/ijms21010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finley JJ, Konstam MA, Udelson JE. Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia. Circulation. 2008;118:410–421. doi: 10.1161/CIRCULATIONAHA.108.765289. [DOI] [PubMed] [Google Scholar]
- 60.Abraham WT, et al. Oral lixivaptan effectively increases serum sodium concentrations in outpatients with euvolemic hyponatremia. Kidney Int. 2012;82:1215–1222. doi: 10.1038/ki.2012.274. [DOI] [PubMed] [Google Scholar]
- 61.Gerbes AL, et al. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: A randomized double-blind multicenter trial. Gastroenterology. 2003;124:933–939. doi: 10.1053/gast.2003.50143. [DOI] [PubMed] [Google Scholar]
- 62.National Library of Medicine (U.S.). Efficacy and safety of lixivaptan in the treatment of autosomal dominant polycystic kidney disease (ACTION). https://clinicaltrials.gov/ct2/show/NCT04064346 (2019).
- 63.National Library of Medicine (U.S.). Safety of lixivaptan in subjects previously treated with tolvaptan for autosomal dominant polycystic kidney disease (ALERT). https://clinicaltrials.gov/ct2/show/NCT04152837 (2019).
- 64.Deshmukh GD, Radin NS, Gattone VH, Shayman JA. Abnormalities of glycosphingolipid, sulfatide, and ceramide in the polycystic (cpk/cpk) mouse. J Lipid Res. 1994;35:1611. doi: 10.1016/S0022-2275(20)41159-9. [DOI] [PubMed] [Google Scholar]
- 65.Chatterjee S, Shi WY, Wilson P, Mazumdar A. Role of lactosylceramide and MAP kinase in the proliferation of proximal tubular cells in human polycystic kidney disease. J Lipid Res. 1996;37:1344. doi: 10.1016/S0022-2275(20)39163-X. [DOI] [PubMed] [Google Scholar]
- 66.Natoli TA, et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med. 2010;16:788. doi: 10.1038/nm.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National Library of Medicine (U.S.). To evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of oral AL01211 in healthy volunteers and autosomal dominant polycystic kidney disease subjects. https://clinicaltrials.gov/ct2/show/NCT04908462 (2021).
- 68.Lu Y, et al. Activation of NRF2 ameliorates oxidative stress and cystogenesis in autosomal dominant polycystic kidney disease. Sci Transl Med. 2020;12:3613. doi: 10.1126/scitranslmed.aba3613. [DOI] [PubMed] [Google Scholar]
- 69.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98:1169. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito M, Tanaka T, Nangaku M. Nuclear factor erythroid 2-related factor 2 as a treatment target of kidney diseases. Curr Opin Nephrol Hypertens. 2020;29:128. doi: 10.1097/MNH.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 72.Yates MS, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 73.Rojas-Rivera J, Ortiz A, Egido J. Antioxidants in kidney diseases: the impact of bardoxolone methyl. Int J Nephrol. 2012;2012:2012. doi: 10.1155/2012/321714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:357674. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 75.Hong DS, et al. A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin Cancer Res. 2012;18:3396. doi: 10.1158/1078-0432.CCR-11-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Østergaard JA, Cooper ME, Jandeleit-Dahm KAM. Targeting oxidative stress and anti-oxidant defence in diabetic kidney disease. J Nephrol. 2020;33:917. doi: 10.1007/s40620-020-00749-6. [DOI] [PubMed] [Google Scholar]
- 77.Jha JC, Banal C, Chow BSM, Cooper ME, Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25:657. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pergola, P. E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med365, (2011). [DOI] [PubMed]
- 79.de Zeeuw D, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:327. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chin MP, et al. Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J Card Fail. 2014;20:953. doi: 10.1016/j.cardfail.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 81.Nangaku M, et al. Randomized clinical trial on the effect of bardoxolone methyl on GFR in diabetic kidney disease patients (TSUBAKI study) Kidney Int Rep. 2020;5:879. doi: 10.1016/j.ekir.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.National Library of Medicine (U.S.). A trial of bardoxolone methyl in patients with ADPKD-FALCON (FALCON). https://clinicaltrials.gov/ct2/show/NCT03918447 (2019).
- 83.Hanaoka K, Devuyst O, Schwiebert EM, Wilson PD, Guggino WB. A role for CFTR in human autosomal dominant polycystic kidney disease. Am J Physiol. 1996;270:C389–C399. doi: 10.1152/ajpcell.1996.270.1.C389. [DOI] [PubMed] [Google Scholar]
- 84.Brill SR, et al. Immunolocalization of ion transport proteins in human autosomal dominant polycystic kidney epithelial cells. Proc Natl Acad Sci U S A. 1996;93:10206–10211. doi: 10.1073/pnas.93.19.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jouret F, Devuyst O. Targeting chloride transport in autosomal dominant polycystic kidney disease. Cell Signal. 2020;73:109703. doi: 10.1016/j.cellsig.2020.109703. [DOI] [PubMed] [Google Scholar]
- 86.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 87.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest. 2000;105:1711–1721. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Sullivan DA, et al. Cystic fibrosis and the phenotypic expression of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1998;32:976–983. doi: 10.1016/S0272-6386(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 89.Xu N, et al. Autosomal dominant polycystic kidney disease coexisting with cystic fibrosis. J Nephrol. 2021;19:529–534. [PubMed] [Google Scholar]
- 90.Persu A, et al. CF gene and cystic fibrosis transmembrane conductance regulator expression in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11:2285–2296. doi: 10.1681/ASN.V11122285. [DOI] [PubMed] [Google Scholar]
- 91.Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:1300–1310. doi: 10.1681/ASN.2007070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laselva O, et al. Small-molecule drugs for cystic fibrosis: where are we now? Pulm Pharmacol Ther. 2021;72:102098. doi: 10.1016/j.pupt.2021.102098. [DOI] [PubMed] [Google Scholar]
- 93.Yanda MK, Cha B, Cebotaru CV, Cebotaru L. Pharmacological reversal of renal cysts from secretion to absorption suggests a potential therapeutic strategy for managing autosomal dominant polycystic kidney disease. J Biol Chem. 2019;294:17090–17104. doi: 10.1074/jbc.RA119.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yanda MK, Liu Q, Cebotaru L. A potential strategy for reducing cysts in autosomal dominant polycystic kidney disease with a CFTR corrector. J Biol Chem. 2018;293:11513–11526. doi: 10.1074/jbc.RA118.001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yanda MK, Cebotaru L. VX-809 mitigates disease in a mouse model of autosomal dominant polycystic kidney disease bearing the R3277C human mutation. FASEB J. 2021;35:e21987. doi: 10.1096/fj.202101315R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.National Library of Medicine (U.S.). A study to evaluate the effects of GLPG2737 in participants with autosomal dominant polycystic kidney disease (ADPKD). https://clinicaltrials.gov/ct2/show/NCT04578548 (2020).
- 97.Notaro ALG, Neto FTL. The use of metformin in women with polycystic ovary syndrome: an updated review. J Assist Reprod Genet. 2022 doi: 10.1007/s10815-022-02429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jalali M, et al. The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: an up-to date systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2020;159:104799. doi: 10.1016/j.phrs.2020.104799. [DOI] [PubMed] [Google Scholar]
- 99.Lazarus B, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med. 2018;178:903–910. doi: 10.1001/jamainternmed.2018.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rowe I, et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med. 2013;19:488–493. doi: 10.1038/nm.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.King JD, et al. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am J Physiol Cell Physiol. 2009;297:C94–101. doi: 10.1152/ajpcell.00677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takiar V, et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci U S A. 2011;108:2462–2467. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang M-Y, et al. Metformin inhibits cyst formation in a zebrafish model of polycystin-2 deficiency. Sci Rep. 2017;7:7161. doi: 10.1038/s41598-017-07300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pastor-Soler NM, et al. Metformin improves relevant disease parameters in an autosomal dominant polycystic kidney disease mouse model. Am J Physiol Renal Physiol. 2022;322:F27–F41. doi: 10.1152/ajprenal.00298.2021. [DOI] [PubMed] [Google Scholar]
- 106.Lian X, et al. The combination of metformin and 2-deoxyglucose significantly inhibits cyst formation in miniature pigs with polycystic kidney disease. Br J Pharmacol. 2019;176:711–724. doi: 10.1111/bph.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leonhard WN, et al. Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease. EBioMedicine. 2019;47:436–445. doi: 10.1016/j.ebiom.2019.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang M-Y, et al. Metformin induces lactate accumulation and accelerates renal cyst progression in Pkd1-deficient mice. Hum Mol Genet. 2021 doi: 10.1093/hmg/ddab340. [DOI] [PubMed] [Google Scholar]
- 109.Ong ACM, Gansevoort RT. TAMEing ADPKD with metformin: safe and effective? Kidney Int. 2021;100:513–515. doi: 10.1016/j.kint.2021.07.021. [DOI] [PubMed] [Google Scholar]
- 110.Perrone RD, et al. Primary results of the randomized trial of metformin administration in polycystic kidney disease (TAME PKD) Kidney Int. 2021;100:684–696. doi: 10.1016/j.kint.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.National Library of Medicine (U.S.). Implementation of Metformin theraPy to Ease Decline of Kidney Function in Polycystic Kidney Disease (IMPEDE-PKD). https://clinicaltrials.gov/ct2/show/NCT04939935 (2021).
- 112.Kramers BJ, et al. Salt, but not protein intake, is associated with accelerated disease progression in autosomal dominant polycystic kidney disease. Kidney Int. 2020;98:989–998. doi: 10.1016/j.kint.2020.04.053. [DOI] [PubMed] [Google Scholar]
- 113.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S. Inhibition of microRNA function by antimiR oligonucleotides. Silence. 2012;3:1–17. doi: 10.1186/1758-907X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramalingam H, Yheskel M, Patel V. Modulation of polycystic kidney disease by non-coding RNAs. Cell Signal. 2020;71:109548. doi: 10.1016/j.cellsig.2020.109548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Concepcion CP, Bonetti C, Ventura A. The microRNA-17-92 family of microRNA clusters in development and disease. Cancer J. 2021;18:262. doi: 10.1097/PPO.0b013e318258b60a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hajarnis S, et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat Commun. 2017;8:1–15. doi: 10.1038/ncomms14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel V, et al. miR-17~92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc Natl Acad Sci U S A. 2013;110:10765. doi: 10.1073/pnas.1301693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trudel M, D’Agati V, Costantini F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991;39:665. doi: 10.1038/ki.1991.80. [DOI] [PubMed] [Google Scholar]
- 120.Yheskel M, Lakhia R, Cobo-Stark P, Flaten A, Patel V. Anti-microRNA screen uncovers miR-17 family within miR-17~92 cluster as the primary driver of kidney cyst growth. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-38566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun H, et al. MicroRNA-17 post-transcriptionally regulates polycystic kidney disease-2 gene and promotes cell proliferation. Mol Biol Rep. 2010;37:2951–2958. doi: 10.1007/s11033-009-9861-3. [DOI] [PubMed] [Google Scholar]
- 122.Lee EC, et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat Commun. 2019;10:4148. doi: 10.1038/s41467-019-11918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.National Library of Medicine (U.S.). A study of RGLS4326 in patients with autosomal dominant polycystic kidney disease. https://clinicaltrials.gov/ct2/show/NCT04536688 (2020).
- 124.Tomita M, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10:403. doi: 10.2188/jea.10.403. [DOI] [PubMed] [Google Scholar]
- 125.Bellomo G, et al. Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis. 2010;56:264. doi: 10.1053/j.ajkd.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 126.Iseki K, et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642. doi: 10.1016/S0272-6386(04)00934-5. [DOI] [PubMed] [Google Scholar]
- 127.Weiner DE, et al. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Srivastava A, Kaze AD, McMullan CJ, Isakova T, Waikar SS. Uric acid and the risks of kidney failure and death in individuals with cKD. Am J Kidney Dis. 2018;71:362. doi: 10.1053/j.ajkd.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Helal I, et al. Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant. 2013;28:380. doi: 10.1093/ndt/gfs417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Uchiyama K, et al. Factors predicting decline in renal function and kidney volume growth in autosomal dominant polycystic kidney disease: a prospective cohort study (Japanese Polycystic Kidney Disease registry: J-PKD) Clin Exp Nephrol. 2021;25:970. doi: 10.1007/s10157-021-02068-x. [DOI] [PubMed] [Google Scholar]
- 131.Brosnahan GM, You Z, Wang W, Gitomer BY, Chonchol M. Serum uric acid and progression of autosomal dominant polycystic kidney disease: results from the HALT PKD Trials. Curr Hypertens Rev. 2020 doi: 10.2174/1573402116666200817113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han M, et al. Hyperuricemia and deterioration of renal function in autosomal dominant polycystic kidney disease. BMC Nephrol. 2014;15:1–8. doi: 10.1186/1471-2369-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kocyigit I, et al. Serum uric acid levels and endothelial dysfunction in patients with autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2013;123:157. doi: 10.1159/000353730. [DOI] [PubMed] [Google Scholar]
- 134.Incalza MA, et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 135.Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19:358. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumagai T, et al. Time to target uric acid to retard CKD progression. Clin Exp Nephrol. 2017;21:182. doi: 10.1007/s10157-016-1288-2. [DOI] [PubMed] [Google Scholar]
- 137.Hasegawa EM, Fuller R, Chammas MC, de Mello FM, Goldenstein-Schainberg C. Increased prevalence of simple renal cysts in patients with gout. Rheumatol Int. 2013;33:413. doi: 10.1007/s00296-012-2380-x. [DOI] [PubMed] [Google Scholar]
- 138.Han Y, et al. Hyperuricemia and overexcretion of uric acid increase the risk of simple renal cysts in type 2 diabetes. Sci Rep. 2017;7:3802. doi: 10.1038/s41598-017-04036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eckardt K-U, et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—a KDIGO consensus report. Kidney Int. 2015;88:676. doi: 10.1038/ki.2015.28. [DOI] [PubMed] [Google Scholar]
- 140.Thurston MM, Phillips BB, Bourg CA. Safety and efficacy of allopurinol in chronic kidney disease. Ann Pharmacother. 2013;47:1507. doi: 10.1177/1060028013504740. [DOI] [PubMed] [Google Scholar]
- 141.Cicero AFG, Fogacci F, Cincione RI, Tocci G, Borghi C. Clinical effects of xanthine oxidase inhibitors in hyperuricemic patients. Med Princ Pract. 2021;30:122. doi: 10.1159/000512178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cabrita I, et al. Cyst growth in ADPKD is prevented by pharmacological and genetic inhibition of TMEM16A in vivo. Nat Commun. 2020;11:1–13. doi: 10.1038/s41467-020-18104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim H, et al. Baseline characteristics of the autosomal-dominant polycystic kidney disease sub-cohort of the KoreaN cohort study for outcomes in patients with chronic kidney disease. Nephrology (Carlton) 2019;24:422. doi: 10.1111/nep.13407. [DOI] [PubMed] [Google Scholar]
- 144.Springer Nature Switzerland AG. https://adisinsight.springer.com/trials/700301041 (Adis International Ltd, 2021).
- 145.Beltowski J. Statins and modulation of oxidative stress. Toxicol Mech Methods. 2005;15:61–92. doi: 10.1080/15376520590918766. [DOI] [PubMed] [Google Scholar]
- 146.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 147.Xue C, Zhang L-M, Zhou C, Mei C-L, Yu S-Q. Effect of statins on renal function and total kidney volume in autosomal dominant polycystic kidney disease. Kidney Dis (Basel) 2020;6:407–413. doi: 10.1159/000509087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.National Library of Medicine (U.S.). Statin therapy in patients with early stage ADPKD. https://clinicaltrials.gov/ct2/show/NCT03273413 (2017).
- 149.National Library of Medicine (U.S.). Pravastatin and alkali therapy in patients with autosomal dominant polycystic kidney disease (ADPKD-SAT). https://clinicaltrials.gov/ct2/show/NCT04284657 (2020).
- 150.Saini AK, Saini R, Singh S. Autosomal dominant polycystic kidney disease and pioglitazone for its therapy: a comprehensive review with an emphasis on the molecular pathogenesis and pharmacological aspects. Mol Med. 2020;26:128. doi: 10.1186/s10020-020-00246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blazer-Yost BL, et al. A randomized phase 1b cross-over study of the safety of low-dose pioglitazone for treatment of autosomal dominant polycystic kidney disease. Clin Kidney J. 2021;14:1738–1746. doi: 10.1093/ckj/sfaa232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.National Library of Medicine (U.S.). Study of the efficacy and safety of tesevatinib in subjects with ADPKD. https://clinicaltrials.gov/ct2/show/NCT03203642 (2017).
- 153.Sweeney WE, Frost P, Avner ED. Tesevatinib ameliorates progression of polycystic kidney disease in rodent models of autosomal recessive polycystic kidney disease. World J Nephrol. 2017;6:188–200. doi: 10.5527/wjn.v6.i4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Parker MI, Nikonova AS, Sun D, Golemis EA. Proliferative signaling by ERBB proteins and RAF/MEK/ERK effectors in polycystic kidney disease. Cell Signal. 2020;67:109497. doi: 10.1016/j.cellsig.2019.109497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sweeney WE, von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol. 2008;19:1331–1341. doi: 10.1681/ASN.2007060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tesar V, et al. Bosutinib versus Placebo for Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2017;28:3404. doi: 10.1681/ASN.2016111232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ghafouri-Fard S, et al. Nrf2-Related Therapeutic Effects of Curcumin in Different Disorders. Biomolecules. 2022;12:82. doi: 10.3390/biom12010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Beevers CS, Zhou H, Huang S. Hitting the golden TORget: curcumin’s effects on mTOR signaling. Anticancer Agents Med Chem. 2013;13:988–994. doi: 10.2174/1871520611313070004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kahkhaie KR, et al. Curcumin: a modulator of inflammatory signaling pathways in the immune system. Inflammopharmacology. 2019;27:885–900. doi: 10.1007/s10787-019-00607-3. [DOI] [PubMed] [Google Scholar]
- 160.Reiterová J, Tesař V. Autosomal dominant polycystic kidney disease: from pathophysiology of cystogenesis to advances in the treatment. Int J Mol Sci. 2022;23:3317. doi: 10.3390/ijms23063317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leonhard WN, et al. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol. 2011;300:F1193–F1202. doi: 10.1152/ajprenal.00419.2010. [DOI] [PubMed] [Google Scholar]
- 162.Li Y, et al. Combination of curcumin and ginkgolide B inhibits cystogenesis by regulating multiple signaling pathways. Mol Med Rep. 2021;23:1. doi: 10.3892/mmr.2021.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Patera F, Cudzich-Madry A, Huang Z, Fragiadaki M. Renal expression of JAK2 is high in polycystic kidney disease and its inhibition reduces cystogenesis. Sci Rep. 2019;9:4491. doi: 10.1038/s41598-019-41106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.National Library of Medicine (U.S.). Curcumin therapy to treat vascular dysfunction in children and young adults with ADPKD. https://clinicaltrials.gov/ct2/show/NCT02494141 (2015).
- 165.Dei Cas M, Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential. Nutrients. 2019;11:2147. doi: 10.3390/nu11092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Happé H, Peters DJM. Translational research in ADPKD: lessons from animal models. Nat Rev Nephrol. 2014;10:587–601. doi: 10.1038/nrneph.2014.137. [DOI] [PubMed] [Google Scholar]
- 167.EMPA-KIDNEY Collaborative Group Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant. 2022 doi: 10.1093/ndt/gfac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Heerspink HJL, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 169.Kapoor S, et al. Effect of sodium-glucose cotransport inhibition on polycystic kidney disease progression in PCK rats. PLoS ONE. 2015;10:e0125603. doi: 10.1371/journal.pone.0125603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.National Library of Medicine (U.S.). Tolvaptan-octreotide LAR combination in ADPKD (TOOL). https://clinicaltrials.gov/ct2/show/NCT03541447 (2018).
- 171.Klawitter J, et al. Pravastatin therapy and biomarker changes in children and young adults with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2015;10:1534–1541. doi: 10.2215/CJN.11331114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.National Library of Medicine (U.S.). A safety, pharmacokinetic & dose-escalation study of KD019 in subjects with autosomal dominant polycystic kidney disease. https://clinicaltrials.gov/ct2/show/NCT01559363 (2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.