Abstract
Introduction
Real-world data on the epidemiology and economic burden of atopic dermatitis (AD) are limited. Here we describe the epidemiology and economic burden of AD using electronic healthcare data from Israel.
Methods
A retrospective study was performed using the Maccabi Healthcare Services database. AD incidence in 2008–2017 and point prevalence (ADprev) on 31 December 2017 were described using diagnosis codes for overall patients, and sex and age subgroups. For ADprev, severity was defined using recently dispensed treatments for AD. Annual healthcare resource utilization in AD prevalent patients was compared with non-AD matched controls using generalized linear modelling. Direct annual costs were estimated also.
Results
AD incidence was 7.0/1000 person-years; overall prevalence was 4.4% (female patients 4.5%, male patients 4.3%; age 0 to less than 6 months, 0.9%; 6 months to less than 12 years, 11.0%; 12 to less than 18 years, 5.8%; 18 years or older, 2.2%). Among ADprev (n = 94,483), mild, moderate, and severe AD comprised 57.7%, 36.2%, and 6.1% (adults 43.8%, 46.3%, 9.9%), respectively. Dermatologist and allergist visits and hospitalization rates (at least one) were 40.7%, 6.6%, and 3.8% in 2017. Compared with controls, overall and moderate-to-severe AD were associated with 36% and 52% increases in annual per-person costs (incremental costs $126 and $190).
Conclusions
AD epidemiology in Israel is comparable with other real-world database studies. AD imposes an economic burden that increases with disease severity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02120-6.
Keywords: Atopic eczema, Epidemiology, Health economics
Plain Language Summary
Occurrence and costs of atopic dermatitis in Israel
Atopic dermatitis is a disease that causes the skin to be inflamed and itchy. Atopic dermatitis is most common in children but can also occur in adolescents and adults. Using data from a large healthcare provider in Israel, this study aimed to describe how common atopic dermatitis is within the population. Costs related to the use of healthcare services (such as visits to dermatologists and creams to treat atopic dermatitis) in the year 2017 were compared between persons with versus without atopic dermatitis. For the years 2008 to 2017, approximately 7 out of 1000 people were newly diagnosed with atopic dermatitis each year (incidence). Among people alive on 31 December 2017, 4.4% had atopic dermatitis (prevalence), with 42.3% suggestive of moderate to severe disease. Patients with atopic dermatitis, particularly those with more severe disease, used healthcare services more frequently. Compared with people without atopic dermatitis, medical costs among patients with atopic dermatitis were 36% higher (corresponding to added costs of $126 per person per year). This study helps to better understand how many people have atopic dermatitis, and what healthcare resources are needed to manage this disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02120-6.
Key Summary Points
| Why carry out this study? |
| Real-world data suggest that atopic dermatitis (AD), particularly severe AD, is associated with a high comorbidity burden, healthcare resource utilization, and healthcare costs. |
| To date, limited data are available on the burden of AD in Israel. |
| This study describes the epidemiology and economic burden of AD in a large population in Israel. |
| What was learned from the study? |
| Using real-world data, we estimated an incidence of AD in Israel of 7.0/1000 person-years. Prevalence of AD was 4.4%, with 42.3% suggestive of moderate-to-severe AD. AD was associated with an added economic burden, which increased with disease severity. |
| This study may help inform decisions for appropriate healthcare resourcing. |
Introduction
Atopic dermatitis (AD) is a common, chronic, relapsing, inflammatory disease with a complex pathogenesis and significant physical, psychological, and economic burden [1–5]. AD is usually diagnosed in childhood, with approximately 85% of cases diagnosed before 5 years of age [6]. Many patients develop persistent AD [7].
The lifetime prevalence of AD is reported to be 15–30% in children and 2–10% in adults [1, 8], with an increased incidence in recent decades in industrialized countries [9]. Real-world data suggest that AD, particularly severe AD, is associated with a high comorbidity burden, healthcare resource utilization (HCRU), and healthcare costs [10–13]. However, our understanding of the burden of AD is limited by methodological differences in AD patient identification across studies [8, 14], as well as challenges in capturing patients’ HCRU in inpatient and outpatient settings. In addition, limited data are available on the burden of AD in Israel, and up-to-date epidemiological data are valuable as the AD treatment landscape evolves.
This study aimed to describe the epidemiology of AD over a 10-year period and estimate the economic burden of AD using data from a large nationally representative healthcare database in Israel.
Methods
Data Source
A retrospective database study was conducted with Maccabi Healthcare Services (MHS), a nationwide healthcare insurer/provider with more than 2.3 million members in 2017, representing approximately a quarter of the population in Israel. The MHS databases integrate routinely collected longitudinal data, computerized since 1998 (annual retention rate, greater than 98%), from the MHS central laboratory, medication prescriptions and purchases throughout the MHS pharmacy network, consultations, hospitalizations, and procedures, and sociodemographic data. The main coding systems used are the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM), Current Procedural Terminology, and Israeli medication coding system with translations to the Anatomical Therapeutic Chemical codes.
AD Case Definition
Data were collected on all inpatient and outpatient diagnoses of AD (ICD-9-CM 691.8) from 1998 to 2017. The diagnosis date was defined by the earliest diagnosis during this period. Patients diagnosed with AD were required to meet at least one of the following criteria:
Diagnosis (at least one) from a relevant specialist in dermatology or immunology/allergy
Diagnosis (at least one) given in a hospital or an MHS Medication Approval Centre (linked to medications requiring prior approval) or flagged by a physician as a chronic/recurrent (active) condition
Diagnoses (at least two separate) from a primary care physician (PCP), including pediatricians and general practitioners
Diagnosis (at least one) from a PCP or other related specialists combined with a dispensed prescription of topical calcineurin inhibitors (TCIs; indicated for patients with AD only within the Israeli national basket of health services).
Study Populations
Incidence Population
The incidence of newly diagnosed AD was described over a 10-year period (2008–2017). Incident patients had their earliest AD diagnosis date between 2008 and 2017, with at least 12 months of continuous health plan enrolment before the AD diagnosis date (except for patients diagnosed with AD before age 12 months, in order to capture newly diagnosed infants). This baseline enrolment period was intended as a “washout” period to exclude potential prevalent patients. The population was categorized into the following age groups: less than 6 months, 6 months to less than 12 years, more than 12 years to less than 18 years, and 18 years or older to investigate the incidence of AD in infants less than 6 months of age, children, adolescents, and adults in accordance with pediatric and adolescent age groups commonly used in large epidemiological studies [15].
Prevalence Population
The point prevalence of AD was described among MHS members alive on 31 December 2017 whose AD diagnosis date occurred between 1998 and 2017 and who had a diagnosis code for AD in the past 5 years (2013–2017) to classify their disease as recent or active. Continuous health plan enrolment from 1 January 2017 was required (except for infants born in 2017) in order to capture data on patient characteristics and annual HCRU. The prevalence population was categorized into the same age groups as the incident population.
Non-AD Controls
The non-AD control population was drawn from the general population of MHS members alive on 31 December 2017 who had no prior AD diagnosis and met the same enrolment criteria as the AD prevalence population. Controls were individually matched (1:1) to patients from the AD prevalence population by age (by birth year, except for infants born in 2017 who were split into 0 to less than 6 months and 6 to less than 12 months), sex, and residential area.
Study Variables and Definitions
Sociodemographic Characteristics
Patients were characterized by age, sex, residential area, and socioeconomic status (SES). SES was based on a score ranked from 1 (lowest) to 10 on an individual’s residence place (at the neighborhood level) [1, 2]. This residential SES measure was originally derived by the Israel Central Bureau of Statistics using national census data and augmented by POINTS location profiling Ltd., using aggregated data on housing prices, motorization level, education, employment, and financial resources [16, 17].
AD-Related Treatments
Data were obtained on AD-related treatments dispensed in 2013–2017 (dispensed on/after AD diagnosis date and up to 5 years before AD point prevalence assessment), including topical corticosteroids (TCS) of low/mid/high potency, TCI, systemic corticosteroids (SC), systemic immunomodulators (SI: methotrexate, azathioprine, cyclosporine, mycophenolate mofetil), and phototherapy; biologic therapy for AD was not available in Israel during the study period. TCS potency was defined according to the World Health Organization (WHO) classification, adapted to Israeli guidelines.
Severity
In the AD prevalence population, recently dispensed AD-related treatment data were used as a surrogate for disease severity: moderate AD was defined as at least two separate purchases of TCI or TCS of mid/high potency, or at least one phototherapy; severe AD as at least two SC with moderate AD criteria, or at least one SI; the remainder were defined as mild AD. The severity definition was therefore based on the maximum severity associated with dispensed treatments in the past 5 years. SC were included in the criteria for severe AD because they remain widely prescribed in the routine treatment of moderate to severe AD, despite clinical practice guidelines that largely discourage their use [18].
Healthcare Resource Utilization
Healthcare resource utilization was described for the year 2017 based on the frequency of physician (PCP and specialist [allergy/immunology, dermatology, other]) visits, emergency room visits, hospitalization, dispensed AD-related treatments, and phototherapy.
Direct Medical Costs
Direct medical costs were estimated from the health system perspective using unit costs from the Israeli Ministry of Health price list (2017). Costs were converted to 2017 US dollars, accounting for purchasing power parity [19].
Statistical Analyses
Patient Characteristics
Among the AD prevalence population (overall and by severity) and non-AD controls, descriptive statistics were presented (n, percentage; median with interquartile range [IQR]) and differences in patient characteristics across groups were evaluated using χ2 or Kruskal–Wallis tests. For comparisons of SES across groups, standardized mean difference (SMD) was calculated.
Incidence and Prevalence Rates
To calculate age/sex-specific incidence rates, the number of newly diagnosed patients with AD in each age/sex group and calendar year was divided by the sum of person-years at risk in the corresponding age/sex group and year. The denominator was based on the number of MHS members with at least 12 months of continuous enrolment (unless younger than 12 months old) and no documented diagnosis of AD. Average annual incidence rates in 2008–2017 were reported. To calculate overall and age/sex-specific prevalence rates, the AD prevalence population in each age/sex group was divided by the total population of MHS members on 31 December 2017 (with at least 12 months’ prior enrolment, except for infants born in 2017) in the corresponding age/sex group. Fisher’s 95% confidence intervals (CI) were calculated for incidence and prevalence rates. Age-standardized incidence and prevalence using the WHO world standard population was also reported [20, 21].
HCRU
Annual utilization rates were summarized as the number (percentage) of patients who used a given healthcare resource at least once in 2017. Among those who used a given resource, median (interquartile range [IQR]) quantity (e.g., number of visits) used per person was described.
Cost Analyses
In order to account for skewed distributions of costs, a generalized linear model (adjusted for age, sex, and residential area) with log-link function and gamma distribution was used to estimate mean total direct medical costs per person and compare (a) the prevalent AD population versus age- and sex-matched non-AD controls, and (b) between AD cohorts with different AD severity. Patients with extreme costs (top 1% of the distribution) were considered outliers and excluded from this analysis. If one individual in a pair was among the highest cost outliers, then both individuals were excluded.
Analyses used SPSS® v.25 and R statistical software v.3.5. This study using existing (retrospective) data/questionnaires was approved by the Maccabi Research Committee and institutional review board of Bayit Balev Hospital, Israel (0094-18-BBL). The study was conducted in accordance with the principles of the Helsinki Declaration and guidelines for medical research in humans.
Results
Incidence
Newly diagnosed patients with AD (N = 119,826) were identified between 2008 and 2017 (Supplementary Material, Fig. S1). Patients aged less than 6 months, 6 months to less than 12 years, 12 to less than 18 years, and 18 years or older accounted for 13.4%, 54.0%, 4.4%, and 28.2% of incident cases, respectively. The proportion of patients whose first diagnosis was given by a dermatologist increased with age: 24.7%, 52.9%, 75.6%, and 77.2%, respectively.
The crude incidence of AD was 7.0 (95% CI 7.0–7.0) per 1000 person-years (age-adjusted, 6.9/1000 person-years). Age-specific rates were highest in the less than 6 months group, in which rates per 1000 person-years were significantly higher among male (83.1 [95% CI 81.5–84.8]) versus female (57.3 [95% CI 55.9–58.8]) infants (Fig. 1). Incidence in children aged 0 to 5 years was 39.2/1000 person-years. Age-specific incidence rates by year are included in Supplementary Material Fig. S2.
Fig. 1.
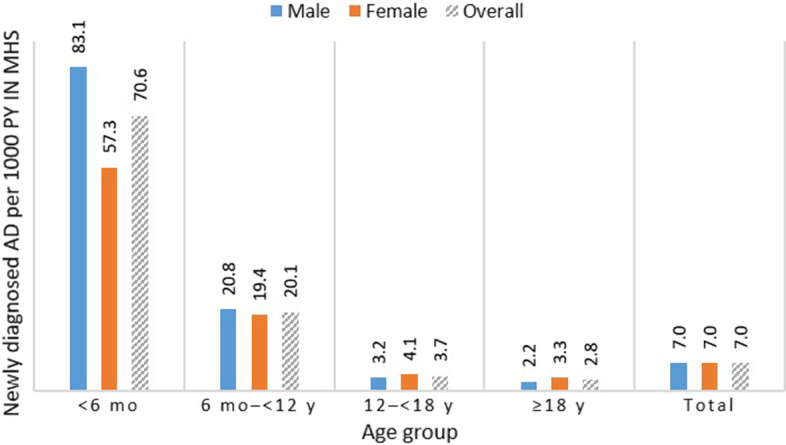
Atopic dermatitis: age- and sex-specific incidence in Israel* (2008–2017 average). AD atopic dermatitis, MHS Maccabi Healthcare Services, mo months; y years. *MHS is a nationwide healthcare insurer and provider with more than 2.3 million members in 2017
Prevalence and Severity
AD prevalent patients (n = 94,483) had a median (IQR) age of 11.2 (5.7–28.4) years (52.3% female). Patients aged less than 6 months, 6 months to less than 12 years, 12 to less than 18 years, and 18 years or older accounted for 0.2%, 52.6%, 13.7%, and 33.5% of prevalence cases, respectively (Table 1, Supplementary Material Table S1). Patients with AD had significantly higher SES compared with non-AD controls (P < 0.001); patients with severe AD had relatively lower SES (P < 0.001).
Table 1.
Characteristics of the AD prevalence population and non-AD matched controls (31 December 2017)
| Characteristics | AD vs non-AD matched controls | AD severity | |||
|---|---|---|---|---|---|
| AD overall (n = 94,483) | Non-AD controls (n = 94,483) | Mild (n = 54,525) | Moderate (n = 34,217) | Severe (n = 5741) | |
| Age,* median (IQR), years | 11.2 (5.7–28.4) | 11.2 (5.7–28.4) | 9.4 (5.1–18.3) | 14.6 (6.9–36.4) | 24.9 (6.6–53.8) |
| Sex,* % | |||||
| Female | 52.3 | 52.3 | 50.5 | 55.0 | 53.4 |
| Male | 47.7 | 47.7 | 49.5 | 45.0 | 46.6 |
| Residential area,* % | |||||
| Central | 68.1 | 68.1 | 66.9 | 71.0 | 62.9 |
| North | 17.1 | 17.1 | 17.5 | 15.9 | 19.8 |
| South | 14.8 | 14.8 | 15.6 | 13.1 | 17.4 |
| SES,† % | |||||
| Low | 17.7 | 22.6 | 18.4 | 16.3 | 19.1 |
| Medium | 33.3 | 34.4 | 33.3 | 33.2 | 34.8 |
| High | 48.9 | 42.9 | 48.2 | 50.5 | 46.0 |
| Missing | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 |
AD atopic dermatitis, IQR interquartile range, SES socioeconomic status
*Variables used for individual matching of patients with AD vs non-AD controls
†SES differed significantly between patients with AD vs non-AD controls (P < 0.001; standardized mean difference = 0.145)
The overall AD prevalence was 4.4% (95% CI 4.4–4.4%), and was significantly (P < 0.05) higher among women (4.5% [95% CI 4.4–4.5%]) versus men (4.3 [95% CI 4.2–4.3%]). Age-specific rates were 0.9%, 11.0%, 5.8%, and 2.2% for those aged less than 6 months, 6 months to less than 12 years, 12 to less than 18 years, and 18 years or older, respectively (Fig. 2). Overall prevalence remained stable (ca. 2%) after 40 years of age (Supplementary Material Fig. S3).
Fig. 2.
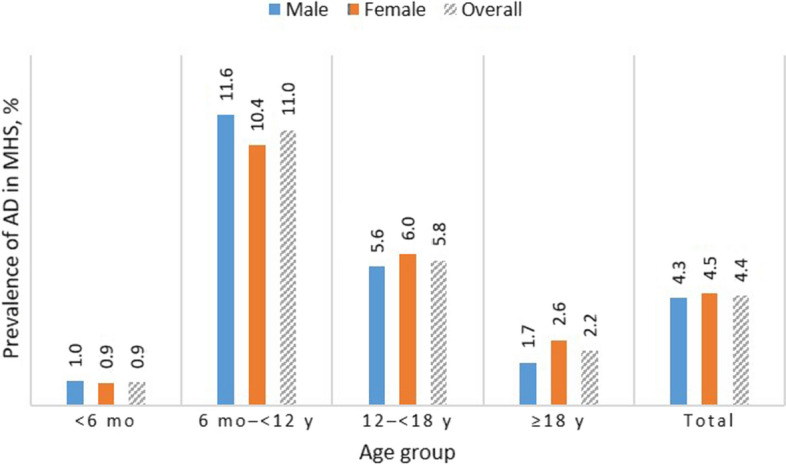
Atopic dermatitis: age-specific prevalence in Israel* by sex (31 December 2017). AD atopic dermatitis, MHS Maccabi Healthcare Services, mo months, y years. *MHS is a nationwide healthcare insurer and provider with more than 2.3 million members in 2017
Mild, moderate, and severe disease accounted for 57.7%, 36.2%, and 6.1% of the prevalence population, respectively. The corresponding percentages were 65.9%, 29.6%, and 4.4% among children aged 6 months to less than 12 years; 59.7%, 32.7%, and 3.1% among adolescents 12 to less than 18 years; and 43.8%, 46.3%, and 9.9% among adults. AD-related treatment patterns that defined severity are reported in Supplementary Material Table S1 and prevalence rates of mild, moderate, and severe AD in Supplementary Material Fig. S4.
HCRU and Costs
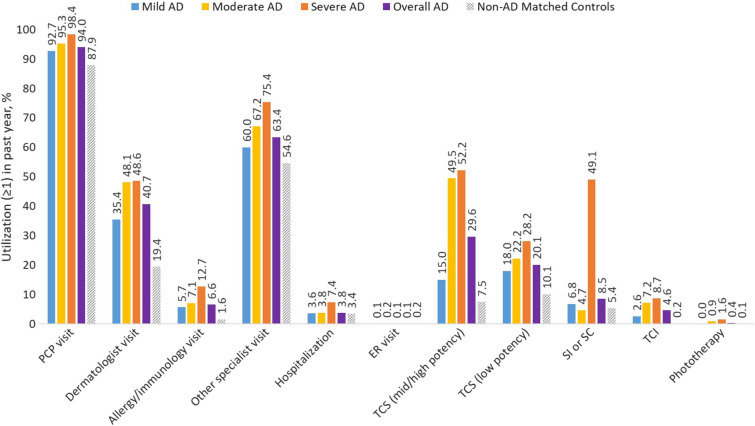
Eligible patients (n = 93,432) were included in the economic burden analysis of the prevalence population by severity. For comparisons to non-AD controls, 92,632 matched pairs were eligible after the highest cost outliers and paired counterparts were excluded. Patients with AD had higher healthcare visit rates and medication use compared with controls (Fig. 3). Overall and across age groups, patients with AD had a significantly higher frequency of PCP visits (overall patients with at least one visit, 94.0% vs 87.9%; median [IQR] number of visits, 6 [3–11] vs 5 [3–9]; P < 0.001). Dermatologist and allergist visits and hospitalization rates (at least one) were 40.7%, 6.6%, and 3.8% in 2017. HCRU rates generally increased with increasing AD severity.
Fig. 3.
Atopic dermatitis: HCRU in past year among AD prevalent patients (by severity)* and non-AD controls.† Note that high cost outliers were excluded. AD atopic dermatitis, ER emergency room, HCRU healthcare resource utilization, PCP primary care physician, SI systemic immunomodulators, SC systemic corticosteroids, TCI topical calcineurin inhibitor, TCS topical corticosteroids. *For comparison of AD by severity: n = 93,432. †For comparison of AD vs non-AD controls: n = 92,632 matched pairs
Total (unadjusted) annual direct costs were higher in patients with AD versus non-AD controls (Supplementary Material Figs. S5A, B). Compared with non-AD matched controls, overall and moderate-to-severe AD were associated with 36.3% and 52.4% increases in estimated mean per-person costs (incremental costs $125.8 and $190.4), respectively (Table 2; Supplementary Material Fig. S5B).
Table 2.
Comparisons of estimated direct healthcare costs per person per year among AD prevalence population, by severity, and vs non-AD matched controls (2017)
| Comparison | Population | Mean cost, $ (95% CI) | Difference, $ (95% CI) | Cost ratio (95% CI) |
|---|---|---|---|---|
|
Overall AD vs non-AD controls N = 92,632 matched pairs |
Non-AD controls | 346.6 (344.0–349.3) | Reference | 1 (Reference) |
| Overall AD | 472.4 (468.8–476.1) | 125.8 (121.7–129.8) | 1.4 (1.4–1.4) | |
|
Moderate-to-severe AD vs non-AD controls n = 38,847 matched pairs |
Non-AD controls | 363.2 (359.0–367.5) | Reference | 1 (Reference) |
| Moderate-to-severe AD | 553.6 (547.2–560.2) | 190.4 (183.6–197.2) | 1.5 (1.5–1.6) | |
| Moderate or severe AD vs mild AD (n = 93,432) | Mild AD (n = 54,525) | 430.6 (426.6–434.5) | Reference | 1 (Reference) |
| Moderate AD (n = 34,217) | 502.1 (496.4–507.8) | 71.6 (65.3–77.8) | 1.2 (1.2–1.2) | |
| Severe AD (n = 5741) | 757.7 (738.6–777.3) | 327.1 (307.6–346.7) | 1.8 (1.7–1.8) |
Costs are 2017 US dollars; mean per-person costs estimated in generalized linear model adjusting for age, sex, and residential area
AD atopic dermatitis
Discussion
This study provides real-world evidence of the epidemiology and economic burden of mild, moderate, and severe AD in Israel. Our findings indicate that AD incidence (7.0/1000 person-years) and prevalence (4.4%) in this Israeli population are comparable to estimates from other database studies in developed countries. Furthermore, this study indicates that patients with AD had higher HCRU rates and estimated direct healthcare costs compared with non-AD matched controls, with HCRU and costs generally increasing with disease severity.
Epidemiology of AD
In this Israeli population, we found an AD prevalence of 4.4% in the overall population and 2.2% in adults, which is comparable to other studies using routinely collected health data (median reported prevalence rate, 4.9%) [8]. Prevalence of AD was higher in boys than girls among children younger than 12 but was higher among adult women versus men, consistent with previous studies [22, 23]. In addition, incidence rates among children aged 0 to 5 years were similar to estimates from Denmark and Sweden [24] and Norway [25].
AD Severity and Patient Characteristics
The severity distribution of mild, moderate, and severe AD in our study (57.7%, 36.2%, and 6.1%; adults 43.8%, 46.3%, and 9.9%) was similar to estimates from a Spanish database study among adults with AD that also used a treatment-based severity definition (55.7%, 38.2%, and 6.1%) [26]. The proportion of moderate-to-severe disease in the current study (42.3%; adults 56.2%) was comparable to survey-based estimates from Germany, Japan, the UK [27], and the USA [28]. The relatively lower proportion of AD cases defined as moderate-to-severe in an Israeli study by Shalom et al. (4.2%) [13] likely reflects the use of more stringent treatment-based definitions, which excluded any AD-related treatments not initiated in the same month as the AD diagnosis and did not include TCS or SC.
In the present study, higher SES was associated with increased AD prevalence, whereas lower SES was associated with more severe disease. These trends have been reported elsewhere [29]. Chung and Simpson highlight potential explanations for the association between SES and prevalence: increased detection/reporting among families with higher SES (may benefit from increased awareness and/or access to care) and the hygiene hypothesis. Patients with lower SES may have decreased access to healthcare necessary to manage AD, which may contribute to exacerbating disease severity in this population. In addition to direct healthcare costs, patients with AD and their families may be burdened by indirect costs regardless of SES, including absenteeism, lack of concentration, and psychological burden that may affect learning and employment [29, 30].
Economic Burden of AD
Our findings that patients with AD had higher HCRU rates and direct healthcare costs than non-AD matched controls, generally increasing with disease severity, are consistent with recent studies from the USA [12], Singapore.[31], Spain [26], and Israel [13].
Strengths and Limitations
A key strength of this study is the ability to identify and characterize patients with AD using routine healthcare data from multiple sources, including inpatient and outpatient diagnoses and dispensed treatments. Patient-level HCRU data captured in this study allowed for estimation of direct healthcare costs to better understand the economic burden of AD. Nonetheless, several methodological limitations should be considered. As observed with other database studies, sensitivity analyses in our study underscore the challenge of comparing across studies that use different case definitions for AD identification [8, 14]. Removing the inclusion criteria for a recent diagnosis documented in the past 5 years increased the prevalence rate in our population to 7.1% (adults, 3.8%), which is closer to other published real-world estimates of lifetime prevalence [8]. In another sensitivity analysis, keeping the same time frame as in the main prevalence analysis but expanding the case definition to include at least one AD diagnosis from any source increased prevalence and incidence estimates to 6.1% (adults, 2.7%) and 9.7/1000 person-years, respectively. Previous validation studies of AD algorithms have highlighted the trade-off between sensitivity and specificity in database studies [32]. This trade-off also affects comparisons with a recent Israeli cross-sectional study by Shalom et al., who estimated an AD lifetime prevalence of 2.7% [13], based on a more specific AD algorithm in which diagnoses were given by a dermatologist or during hospitalizations in dermatology departments. Nonetheless, as discussed earlier, incidence and prevalence rates estimated in this study are in line with other database studies from developed countries. Although methodological differences limit our ability to compare estimates from database versus questionnaire studies, adult AD prevalence was also similar to estimates from Germany, Japan, and the UK (2.1–2.5%) [27]. A validation study with chart review could potentially improve AD identification methods in the future.
This study used dispensed AD-related treatments to estimate disease severity. In particular, moderate-to-severe disease may have been overestimated, given the challenges of differentiating mild from moderate disease. For example, patients with clinically mild AD could potentially have been misclassified as moderate AD based on their TCS and TCI use. In addition, AD-related medications used in other indications (e.g., SC use to treat asthma) may also have led to misclassification of severe AD. To address this limitation, we increased the rigidity of our SC-based criteria for severe AD, requiring these patients to also fulfil the criteria for moderate AD. At the same time, undiagnosed and/or undertreated patients may be underestimated.
Our estimates of economic burden did not capture out-of-pocket costs (e.g., moisturizers, over-the-counter treatments, and alternative medicines) or indirect costs due to productivity loss (e.g., time missed from work or school due to illness) [26, 33–36]. Sciattella et al. estimated that direct nonmedical and indirect costs due to productivity loss accounted for 19.9% and 60.8% of the total annual costs per patient, respectively [35]. Finally, evidence suggests that associated comorbidities may also contribute significantly to the economic burden of AD [12, 26, 37]; the impact of atopic diseases, mental health conditions, and other comorbidities will be further investigated in this study population.
Conclusions
In the current study, AD incidence was 7.0/1000 person-years, prevalence was 4.4%, with 42.3% of patients having moderate-to-severe AD, prevalence was higher among women than men, and highest prevalence was among children aged 6 months to 12 years. Patients with AD had higher HCRU rates than non-AD matched controls, which generally increased with disease severity. Moderate-to-severe AD was associated with 52% increased direct medical cost per capita compared with non-AD matched controls. While further research is needed to investigate the total costs of treating AD, consideration of the entirety of the AD care pathway may inform appropriate healthcare resourcing related to AD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
AbbVie (North Chicago, IL, USA) funded the study and the journal’s Rapid Service and Open Access Fees. AbbVie and Maccabi participated in the study design, research, interpretation of data, and reviewing and approval of the publication. Maccabi participated in the data collection and analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. No honoraria or payments were made for authorship.
Author Contributions
Conceptualization: CW, PBS, GC, VS. Data curation: CW. Formal analysis: CW. Investigation: CW, PBS, HL, HW, BMC, YAL, GC. Writing-original draft: CW. Writing-review and editing: All authors.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Lauriaselle Afanador, PhD, and Janet Matsuura, PhD (ICON plc, Blue Bell, PA, USA) and was funded by AbbVie.
Disclosures
Clara Weil, Gabriel Chodick, and Varda Shalev have no conflicts of interest to declare. Dan Ben Amitai has received consultant fees from AbbVie. Yael A. Leshem has received honoraria or fees as a consultant from AbbVie, Sanofi, and Genentech and as an advisory board member from Sanofi and Regeneron Pharmaceuticals, Pfizer, and Dexcel Pharma; and has, without personal compensation, provided investigator services for Eli Lilly, Pfizer, and AbbVie. Philip B. Sugerman is a former employee of AbbVie and may own AbbVie stock and/or stock options. His current affiliation is Genzyme, Suffolk County, MA, USA. Huifang Liang, Hongwei Wang, Brian M. Calimlim, and Ana Dorfman are full-time employees of AbbVie and may own AbbVie stock and/or stock options.
Compliance with Ethics Guidelines
This study using existing [retrospective] data was approved by the Maccabi Research Committee and institutional review board of Bayit Balev Hospital, Israel. The study was conducted in accordance with the principles of the Helsinki Declaration and guidelines for medical research in humans.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available because the data that support the findings of this study originate from Maccabi Healthcare Services and restrictions apply to the availability of these data. Because of these restrictions, these data can be accessed only by request to the authors and/or Maccabi Healthcare Services.
Prior Presentation
A portion of this research was previously presented at the virtual meeting of the American Academy of Dermatology Annual Meeting in 2020.
Footnotes
The original online version of this article was revised due to retrospective open access.
Change history
7/29/2022
A Correction to this paper has been published: 10.1007/s12325-022-02272-5
References
- 1.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74:491–498. doi: 10.1016/j.jaad.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. doi: 10.1016/S0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283–289. doi: 10.1016/j.det.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abuabara K, Yu AM, Okhovat J-P, Allen IE, Langan SM. The prevalence of atopic dermatitis beyond childhood: a systematic review and meta-analysis of longitudinal studies. Allergy. 2018;73:696–704. doi: 10.1111/all.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irvine A, Mina-Osorio P. Disease trajectories in childhood atopic dermatitis: an update and practitioner’s guide. Br J Dermatol. 2019;181:895–906. doi: 10.1111/bjd.17766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dizon M, Yu A, Singh R, et al. Systematic review of atopic dermatitis disease definition in studies using routinely collected health data. Br J Dermatol. 2018;178:1280–1287. doi: 10.1111/bjd.16340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deckers IA, McLean S, Linssen S, Mommers M, Van Schayck C, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PLoS ONE. 2012;7:e39803. doi: 10.1371/journal.pone.0039803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J Am Acad Dermatol. 2018;78:54–61 e1. doi: 10.1016/j.jaad.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743–752. doi: 10.1001/jamadermatol.2014.5432. [DOI] [PubMed] [Google Scholar]
- 12.Shrestha S, Miao R, Wang L, Chao J, Yuce H, Wei W. Burden of atopic dermatitis in the United States: analysis of healthcare claims data in the commercial, Medicare, and Medi-Cal databases. Adv Ther. 2017;34:1989–2006. doi: 10.1007/s12325-017-0582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shalom G, Babaev M, Kridin K, et al. Healthcare service utilization by 116,816 patients with atopic dermatitis in Israel. Acta Derm Venereol. 2019;99:370–374. doi: 10.2340/00015555-3117. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Haider S, Colicino S, et al. Different definitions of atopic dermatitis: impact on prevalence estimates and associated risk factors. Br J Dermatol. 2019;181:1272–1279. doi: 10.1111/bjd.17853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417–28 e2. doi: 10.1016/j.anai.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Characterization and classification of geographical units by the socio-economic level of the population 2015. https://www.cbs.gov.il/he/publications/DocLib/2019/1765_socio_economic_2015/e_print.pdf. Accessed 1 Oct 2021.
- 17.Rossman H, Shilo S, Meir T, Gorfine M, Shalit U, Segal E. COVID-19 dynamics after a national immunization program in Israel. Nat Med. 2021;27:1055–1061. doi: 10.1038/s41591-021-01337-2. [DOI] [PubMed] [Google Scholar]
- 18.Drucker AM, Eyerich K, de Bruin-Weller MS, et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol. 2018;178:768–775. doi: 10.1111/bjd.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministry of Health price list. https://www.health.gov.il/subjects/finance/taarifon/pages/pricelist.aspx. Accessed 13 Jan 2017.
- 20.Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age standardization of rates: a new WHO standard. Geneva: World Health Organization; 2001. [Google Scholar]
- 21.National Cancer Institute. World (WHO 2000–2025) standard – adjustment for SEER*Stat Standard. https://seer.cancer.gov/stdpopulations/world.who.html. Accessed 7 Aug 2020.
- 22.Liebhart J, Dobek R, Malolepszy J, et al. The prevalence of allergic diseases in Poland—the results of the PMSEAD study in relation to gender differences. Adv Clin Exp Med. 2014;23:757–762. doi: 10.17219/acem/37238. [DOI] [PubMed] [Google Scholar]
- 23.de Lusignan S, Alexander H, Broderick C, et al. The epidemiology of eczema in children and adults in England: a population-based study using primary care data. Clin Exp Allergy. 2021;51:471–482. doi: 10.1111/cea.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henriksen L, Simonsen J, Haerskjold A, et al. Incidence rates of atopic dermatitis, asthma, and allergic rhinoconjunctivitis in Danish and Swedish children. J Allergy Clin Immunol. 2015;136:360–6 e2. doi: 10.1016/j.jaci.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Mohn CH, Blix HS, Halvorsen JA, Nafstad P, Valberg M, Lagerløv P. Incidence trends of atopic dermatitis in infancy and early childhood in a nationwide prescription registry study in Norway. JAMA Netw Open. 2018;1:e184145. doi: 10.1001/jamanetworkopen.2018.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sicras-Mainar A, Navarro-Artieda R, Carrillo JC. Economic impact of atopic dermatitis in adults: a population-based study (IDEA study) Actas Dermosifiliogr (Engl Ed) 2018;109:35–46. doi: 10.1016/j.ad.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73:1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 28.Fuxench ZCC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Investig Dermatol. 2019;139:583–590. doi: 10.1016/j.jid.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:360–366. doi: 10.1016/j.anai.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Filanovsky MG, Pootongkam S, Tamburro JE, Smith MC, Ganocy SJ, Nedorost ST. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284–90 e5. doi: 10.1016/j.jpeds.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 31.Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284–293. doi: 10.1111/pde.14071. [DOI] [PubMed] [Google Scholar]
- 32.Stensballe LG, Klansø L, Jensen A, Hærskjold A, Thomsen SF, Simonsen J. The validity of register data to identify children with atopic dermatitis, asthma or allergic rhinoconjunctivitis. Pediatr Allergy Immunol. 2017;28:535–542. doi: 10.1111/pai.12743. [DOI] [PubMed] [Google Scholar]
- 33.Drucker AM. Atopic dermatitis: burden of illness, quality of life, and associated complications. Allergy Asthma Proc. 2017;38:3–8. doi: 10.2500/aap.2017.38.4005. [DOI] [PubMed] [Google Scholar]
- 34.Zink AGS, Arents B, Fink-Wagner A, et al. Out-of-pocket costs for individuals with atopic eczema: a cross-sectional study in nine European countries. Acta Derm Venereol. 2019;99:263–267. doi: 10.2340/00015555-3102. [DOI] [PubMed] [Google Scholar]
- 35.Sciattella P, Pellacani G, Pigatto PD, et al. The burden of atopic dermatitis in adults in Italy. G Ital Dermatol Venereol. 2020;155:19–23. doi: 10.23736/S0392-0488.19.06430-7. [DOI] [PubMed] [Google Scholar]
- 36.Simpson EL, Basco M, Hanifin J. A cross-sectional survey of complementary and alternative medicine use in patients with atopic dermatitis. Am J Contact Dermat. 2003;14:144–147. doi: 10.2310/6620.2003.6156. [DOI] [PubMed] [Google Scholar]
- 37.Sandhu JK, Salame N, Ehsani-Chimeh N, Armstrong AW. Economic burden of cutaneous infections in children and adults with atopic dermatitis. Pediatr Dermatol. 2019;36:303–310. doi: 10.1111/pde.13828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because the data that support the findings of this study originate from Maccabi Healthcare Services and restrictions apply to the availability of these data. Because of these restrictions, these data can be accessed only by request to the authors and/or Maccabi Healthcare Services.