Abstract
Transient focal ischemia induces a sustained downregulation of miR-7 leading to derepression of its target α-synuclein (α-Syn), which promotes neuronal death. We previously showed that treatment with miR-7 mimic prevents α-Syn induction and protects brain after stroke in rodents irrespective of age and sex. To further decipher the role of miR-7, we currently studied infarction and motor function in miR-7 double knockout mice (lack both miR-7a and miR-7b) subjected to focal ischemia. Adult miR-7−/− mice showed similar motor and cognitive functions to miR-7+/+ mice. However, when subjected to even a mild focal ischemia, the miR-7−/− mice showed exacerbated brain damage and worsened motor function compared with the miR-7+/+ mice. Replenishing miR-7 in miR-7−/− mice (IV injection of miR-7 mimic) restored miR-7 mediated neuroprotection and motor recovery, potentially by preventing α-Syn protein induction. Thus, we show that miR-7 is an essential miRNA in the brain that prevents α-Syn translation and the ensuing brain damage after stroke.
Keywords: Ischemia, noncoding RNA, brain damage, motor function, poststroke recovery
INTRODUCTION
The microRNAs (miRNAs) are short (21- to 23 nt) noncoding RNAs that are highly abundant in the brain [1]. Stroke was shown to alter the expression of many miRNAs and several of them modulate the poststroke functional outcome [2–5]. We recently reported that the levels of miR-7 were reduced in the brain following transient focal ischemia in rodents and its replenishment promotes poststroke recovery [3]. α-Synuclein (α-syn), the protein that plays a significant role in neuronal death in Parkinson’s disease (PD), is a major target of miR-7 [6]. We showed that focal ischemia significantly induces α-syn protein expression in rodent brain regardless of age or sex, and preventing it by treatment with a miR-7 mimic promotes smaller infarcts and functional recovery [3, 7].
In both humans and mice, pri-miR-7a and pri-miR-7b that release mature miR-7a and miR-7b are transcribed from 2 distinct loci. The miR-7a and miR-7b share 100% sequence homology in humans and 95% sequence homology in mice (only 1 nucleotide is different). Importantly, these 2 share the seed sequence and can be detected using the same set of primers. Hence, we used miR-7 double knockout mice (DKO) that lack both the primary miR-7 gene loci [8]. These mice were created by injecting Cas9 RNA and sgRNAs to excise the DNA encoding pre-miRNA hairpin loci. The miR-7 DKO mice were born at the expected Mendelian frequency and displayed no gross physical abnormalities [8]. To further prove the essential role of miR-7 in protecting the brain, we presently evaluated poststroke brain damage and α-syn protein expression in these mice.
MATERIALS AND METHODS
Focal ischemia, miRNA mimic treatment, outcome analysis, and immunostaining
Supplementary Information shows detailed methods. Animal procedures were approved by the University of Wisconsin Research Animal Resources and Care Committee. Animals were randomly assigned, and a blinded investigator performed data analyses. Focal ischemia was induced in male miR-7+/+ and miR-7−/− mice (C57BL/6J background; 12 weeks, 27±2 g) by intraluminal middle cerebral artery occlusion (MCAO; 45 min) under isoflurane anesthesia [3, 9–12]. Sham-operated mice served as control. Only male mice were used as poststroke miR-7 downregulation and miR-7 mimic mediated neuroprotection are independent of age and sex [3]. The miR-7 mimic or control non-targeting mimic was injected (IV; retro-orbital sinus) with PEG-Liposome In Vivo Transfection at 30 min of reperfusion [3]. Motor function was analyzed with rotarod test and beam-walk test between days 1 to 7 of reperfusion [3, 12, 13]. Ischemic brain damage was assessed at 7 days of reperfusion with T2-MRI [10]. The volume was computed by numeric integration of data from serial coronal sections to the sectional interval and corrected for edema [3, 7, 12, 13]. The effect of miR-7 supplementation on α-Syn levels was examined by immunostaining brain sections with anti-α-Syn and anti-NeuN antibodies [3, 13].
RESULTS
miR-7 deletion worsened poststroke functional outcome
In miR-7+/+mice, a shorter duration transient MCAO (45 min) induced mild motor dysfunction at 1 day that resolved by 3 days of reperfusion and a small infarct (9 mm3) (Fig. 1A and B). Whereas, miR-7−/− mice subjected to 45 min transient MCAO showed a significant motor dysfunction by day 1 that was still impaired even at day 7 of reperfusion compared with the miR-7+/+ cohort (Fig. 1A). The miR-7−/− mice also showed a significantly bigger infarct (6.1 fold) compared with the miR-7+/+ mice (Fig. 1B). This indicates that miR-7 is critical for neuronal survival during stressful conditions like stroke.
Fig. 1: miR-7 deficiency worsened ischemic brain injury.
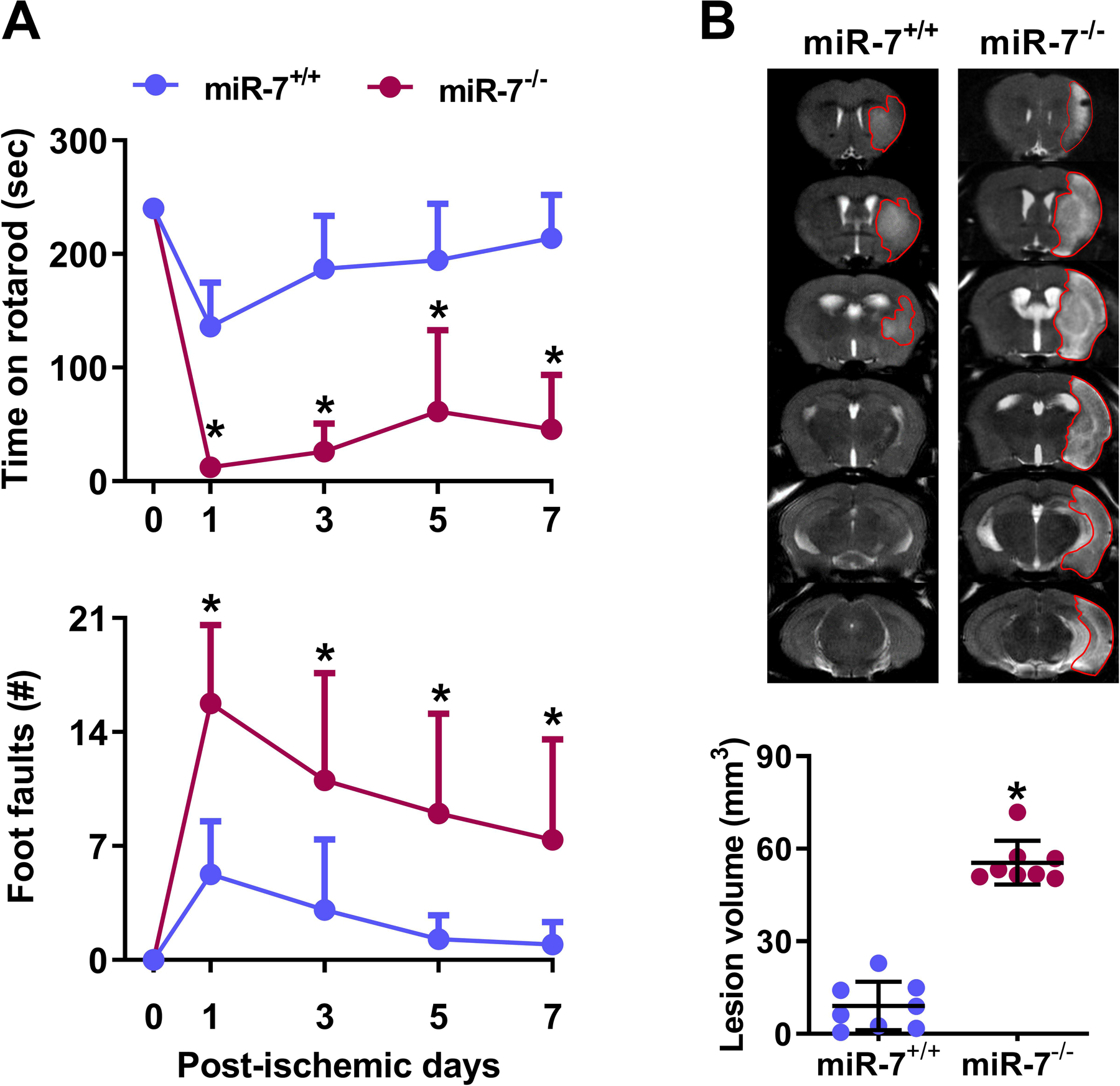
miR-7−/− mice showed significantly lower motor function recovery on days 1 to 7 following transient MCAO compared with the miR-7+/+ cohort (A). *p<0.05 compared with miR-7+/+ cohort by repeated-measures ANOVA followed by Sidak’s multiple comparisons posttest. miR-7−/− mice also showed bigger infarcts compared with the miR-7+/+ cohort on day 7 of reperfusion following transient MCAO (T2-MRI; B). *P<0.05 compared with miR-7+/+ cohort by Mann-Whitney U test. Values are mean±SD (n=8/group).
miR-7 supplementation decreased brain damage in miR-7−/− mice
To further establish the neuroprotective ability of miR-7 after stroke, we restored miR-7 levels in miR-7−/− mice by injecting a miR-7 mimic at 30 min of reperfusion following transient MCAO. The miR-7 mimic treated cohort showed a significantly improved motor function recovery between days 3 and 7 of reperfusion compared with the control mimic treated cohort (Fig. 2A). The miR-7 mimic treated cohort also showed a significantly smaller infarct volume (by 2.6 fold) compared with the control mimic treated cohort (Fig. 2B).
Fig. 2: miR-7 supplementation decreased ischemic brain injury in miR-7−/− mice.
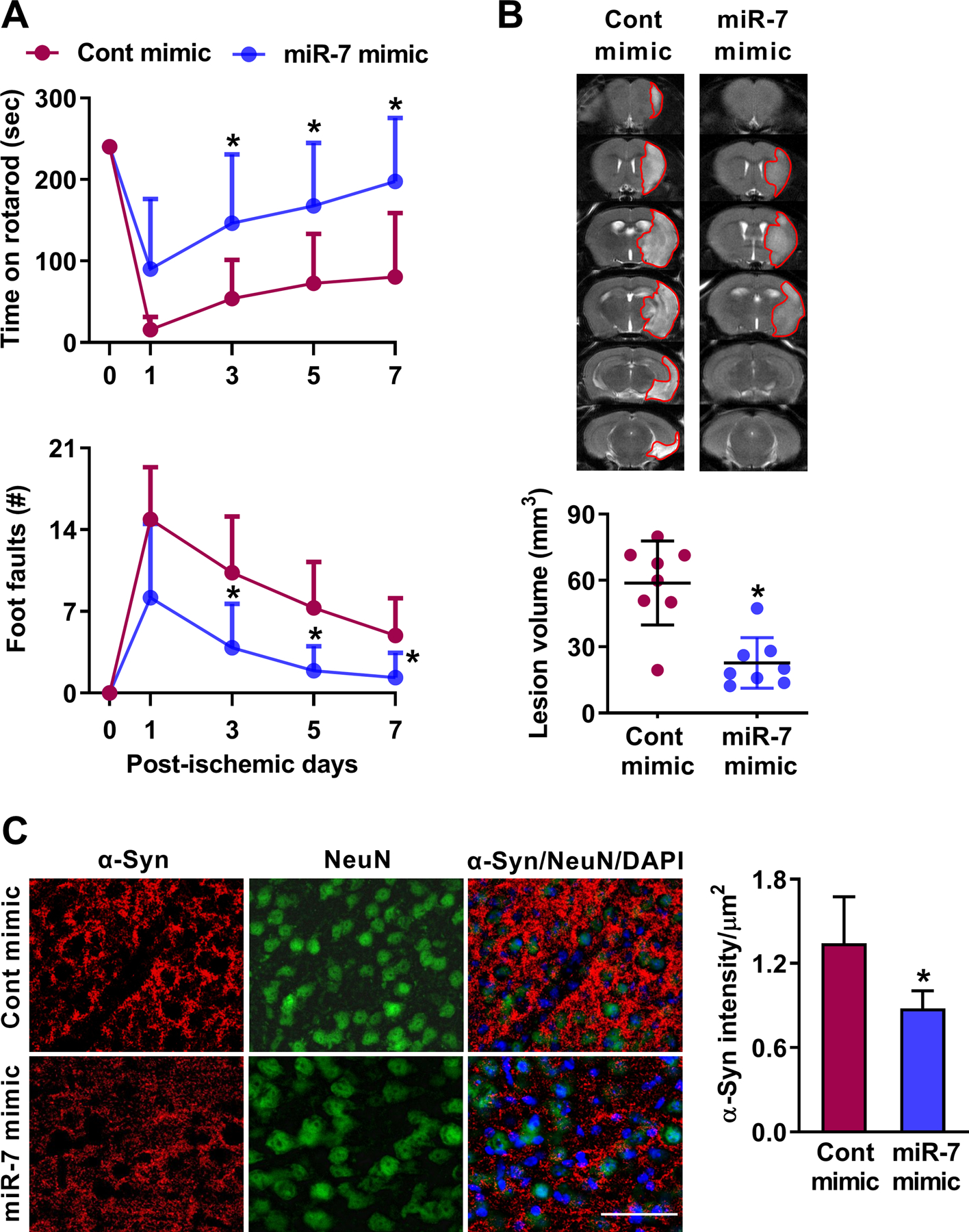
IV injection of miR-7 mimic (20 nmol at 30 min of reperfusion following transient MCAO) in miR-7−/− mice promoted better motor function recovery (A) and decreased lesion volume (B) compared with control mimic treated cohort. Values are mean±SD (n=8/group). *p<0.05 compared with control mimic by repeated-measures ANOVA followed by Sidak’s multiple comparisons posttest (A) and Mann-Whitney U test (B). Following transient MCAO and 1 day of reperfusion, control mimic injected miR-7−/− mice showed increased abundance of α-Syn immunostaining, which was reduced by miR-7 mimic administration (C) (n=4/group). Bar=20 μm.
miR-7 supplementation suppressed α-Syn expression
Transient focal ischemia significantly induces α-Syn expression in rodent brain and treatment with miR-7 mimic curtails this, and poststroke neuroprotection induced by miR-7 mimic will be abrogated in α-Syn−/− mice [3]. This indicates that miR-7 is instrumental in protecting the brain after stroke, specifically by preventing α-Syn [3]. We presently extend this by showing that in miR-7−/− mice, miR-7 mimic treatment significantly curtails poststroke induction of α-Syn protein compared with control mimic treated cohort at 1 day of reperfusion following transient MCAO (Fig. 2C).
DISCUSSION
Many miRNAs altered after stroke are reported to modulate neuronal death and/or survival [4, 14]. The miR-7 is a highly abundant miRNA downregulated rapidly in rodent brain following transient focal ischemia, and importantly replenishing its levels using a mimic promoted functional recovery and decreased infarction [3]. Recently, we reported that stroke in humans and focal ischemia in rodents significantly increases α-Syn protein levels that form proteinase-K insoluble oligomers [7]. Furthermore, α-Syn siRNA significantly protected the poststroke brain [7]. Specifically, α-Syn is a conserved target of miR-7, and the neuroprotective potential of miR-7 mimic is due to its ability to repress α-Syn after stroke [3].
CNS contains a high abundance of the monomeric α-Syn that is localized in neurons [7]. Its physiological significance is not clear, but the pathologic increase in α-Syn protein, its oligomerization and tangle/plaque formation is neurotoxic and the major pathologic mechanism of PD [15]. Of the 3 miRNAs (miR-7a/b, miR-673 and miR-153) that were found to target α-Syn with high affinity by all 4 bioinformatic algorithms (microRNA.org, TargetScan, miRDB and miRanda), only miR-7a was downregulated in rodent brain after transient MCAO [2]. We also observed that of the top 5% predicted targets of miR-7a, α-Syn is the only common conserved target identified by all 4 algorithms (data not shown). Luciferase assays confirmed that premiR-7a prevents α-Syn 3’UTR vector expression in PC12 cells [3]. Thus, there is an inverse relation between α-Syn and miR-7 levels. The high expression of miR-7 might be a natural adaptation to prevent unusual induction of α-Syn under pathologic conditions. However, conditions like stroke lead to an acute downregulation of miR-7 in a sustained manner leading to derepression of α-Syn expression and thus pathologic changes [3].
We presently extended the above concept by using miR-7−/− mice. When these mice were subjected to even a milder transient MCAO, they showed a significant functional impairment and exacerbated infarction compared with the miR-7+/+ mice. This indicates the essential role of miR-7 in protecting the brain after stroke. We further showed that supplementation of miR-7 with a miR-7 mimic protects miR-7−/− mice following transient MCAO. We also showed extensive induction of α-Syn protein in miR-7−/− mice subjected to focal ischemia, which was significantly curtailed by miR-7 mimic. This evidence strongly suggests that miR-7 plays a prosurvival role in the brain after a stroke and its downregulation promotes neurological dysfunction, probably by derepressing α-Syn protein expression. miR-7−/− mice also showed exacerbated infiltration of leukocytes and increased levels of pro-inflammatory factors such IL-1β, IL-6, and TNF-α following focal ischemia [16]. As α-Syn induction also promotes inflammation, miR-7 might be essential to control these pathologic changes after an injury [7, 15]. Notably, miR-7 was also shown to facilitate the clearance of α-Syn aggregates by promoting autophagy [17]. Overall, we show that miR-7 has significant therapeutic potential in protecting the brain following stroke.
Supplementary Material
acknowledgements:
We thank Dr. Benjamin Kleaveland, MD PhD, Weill Cornell Medicine, NY, for providing the miR-7 knockout mice.
Funding:
Partially supported by NIH grants NS099531, NS109459, and NS101960, and the Department of Neurological Surgery, UW-Madison. Dr. Vemuganti is the recipient of a Research Career Scientist award (# IK6BX005690) from the US Department of Veterans Affairs.
Footnotes
Conflict of Interest: None.
Compliance with Ethical Standards
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants.
Availability of data and material: All data generated or analyzed during this study are included in this article (and its supplementary information file).
References
- 1.Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64(3):303–9. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29(4):675–87. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim T, Mehta SL, Morris-Blanco KC, Chokkalla AK, Chelluboina B, Lopez M, et al. The microRNA miR-7a-5p ameliorates ischemic brain damage by repressing alpha-synuclein. Science signaling. 2018;11(560). doi: 10.1126/scisignal.aat4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G, Morris-Blanco KC, Lopez MS, Yang T, Zhao H, Vemuganti R, et al. Impact of microRNAs on ischemic stroke: From pre- to post-disease. Prog Neurobiol. 2018;163–164:59–78. doi: 10.1016/j.pneurobio.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Vemuganti R The MicroRNAs and Stroke: No Need to be Coded to be Counted. Translational stroke research. 2010;1(3):158–60. doi: 10.1007/s12975-010-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A. 2009;106(31):13052–7. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim T, Mehta SL, Kaimal B, Lyons K, Dempsey RJ, Vemuganti R. Poststroke Induction of alpha-Synuclein Mediates Ischemic Brain Damage. J Neurosci. 2016;36(26):7055–65. doi: 10.1523/jneurosci.1241-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleaveland B, Shi CY, Stefano J, Bartel DP. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell. 2018;174(2):350–62 e17. doi: 10.1016/j.cell.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta SL, Pandi G, Vemuganti R. Circular RNA Expression Profiles Alter Significantly in Mouse Brain After Transient Focal Ischemia. Stroke. 2017;48(9):2541–8. doi: 10.1161/strokeaha.117.017469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chelluboina B, Chokkalla AK, Mehta SL, Morris-Blanco KC, Bathula S, Sankar S, et al. Tenascin-C induction exacerbates post-stroke brain damage. J Cereb Blood Flow Metab. 2021:271678×211056392. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chokkalla AK, Mehta SL, Kim T, Chelluboina B, Kim J, Vemuganti R. Transient Focal Ischemia Significantly Alters the m(6)A Epitranscriptomic Tagging of RNAs in the Brain. Stroke. 2019;50(10):2912–21. doi: 10.1161/strokeaha.119.026433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris-Blanco KC, Kim T, Lopez MS, Bertogliat MJ, Chelluboina B, Vemuganti R. Induction of DNA Hydroxymethylation Protects the Brain After Stroke. Stroke. 2019;50(9):2513–21. doi: 10.1161/strokeaha.119.025665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta SL, Chokkalla AK, Kim T, Bathula S, Chelluboina B, Morris-Blanco KC, et al. Long Noncoding RNA Fos Downstream Transcript Is Developmentally Dispensable but Vital for Shaping the Poststroke Functional Outcome. Stroke. 2021:Strokeaha120033547. doi: 10.1161/strokeaha.120.033547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun P, Liu DZ, Jickling GC, Sharp FR, Yin KJ. MicroRNA-based therapeutics in central nervous system injuries. J Cereb Blood Flow Metab. 2018;38(7):1125–48. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nature reviews Neuroscience. 2013;14(1):38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yue D, Zhao J, Chen H, Guo M, Chen C, Zhou Y, et al. MicroRNA-7, synergizes with RORα, negatively controls the pathology of brain tissue inflammation. Journal of neuroinflammation. 2020;17(1):28. doi: 10.1186/s12974-020-1710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi DC, Yoo M, Kabaria S, Junn E. MicroRNA-7 facilitates the degradation of alpha-synuclein and its aggregates by promoting autophagy. Neurosci Lett. 2018;678:118–23. doi: 10.1016/j.neulet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


