Abstract
Human cerebral organoids are an exciting and novel model system emerging in the field of neurobiology. Cerebral organoids are spheres of self-organizing, neuronal lineage tissue that can be differentiated from human pluripotent stem cells and that present the possibility of on-demand human neuronal cultures that can be used for non-invasively investigating diseases affecting the brain. Compared with existing humanized cell models, they provide a more comprehensive replication of the human cerebral environment. The potential of the human cerebral organoid model is only just beginning to be elucidated, but initial studies have indicated that they could prove to be a valuable model for neurodegenerative diseases such as prion disease. The application of the cerebral organoid model to prion disease, what has been learned so far and the future potential of this model are discussed in this review.
Keywords: Prion, PrP, Cerebral organoid, Stem cells, iPSC
Introduction
Neurodegenerative diseases affecting humans have proven difficult to model in vitro. There are various reasons for this, including the difficulty of obtaining ethically sourced live human brain tissue in sufficient volumes for wide ranging investigations. The development of a new technology, human cerebral organoids, offers a model that can overcome some of the existing limitations on examining human tissue in culture. This model, and variations thereof, have been utilized for investigation of an increasing number of neurodegenerative diseases including prion diseases. Herein, we focus primarily on the human cerebral organoid protocol developed by Lancaster and Knoblich (Lancaster & Knoblich, 2014), as this is the model thus far investigated in the context of prion research, but also consider possible new research directions as these models are expanded and refined.
Prion diseases
Prion diseases or the transmissible spongiform encephalopathies are a group of protein misfolding disorders characterized by a conformational change of a native protein (PrPC) to a misfolded conformer (prions or PrPSc). PrPSc can recruit and template further misfolding of PrPC. This templated misfolding allows disease to spread through the brain and also accounts for the transmissibility of the diseases. The precursor of the misfolded isomer is the prion protein, PrPC, encoded by the prion protein gene (PRNP), which is highly expressed in the central nervous system but can be found throughout the body. It is typically found at the cell surface attached to the plasma membrane by a GPI-anchor. Although there is still considerable debate about the true function of PrPC, it has been implicated in multiple cellular roles including copper homeostasis and neuroprotection (Linden, 2017). There is currently no treatment for prion diseases, and they are universally fatal.
Prion diseases in humans have three etiologies: sporadic, genetic or acquired. Of these, sporadic disease is the most common (>85% of cases) followed by genetic disease (10–15% of cases). Acquired disease can occur through several transmission routes including from surgical instruments, human-derived products (such as blood or hormones) and ingestion of contaminated meat. Despite the notoriety of these diseases that arose following the transmission of bovine spongiform encephalopathy to humans through consumption of contaminated meat (Ironside et al., 2017), the lowest occurrence is due to acquired disease. While less prion diseases arise due to transmission, the transmissible nature of prions is advantageous for laboratory studies. As a prion is able to imprint its misfolded structure onto a normal PrPC, it can spread through tissue cultures and transmit a bona fide disease to animal models. This allows the disease development to be studied without the need for gross genetic modification of animals.
Sporadic prion diseases include sporadic Creutzfeldt–Jakob disease (sCJD), sporadic familial insomnia and variably protease-sensitive prionopathy. sCJD is the most common form of prion disease in humans with an average survival of only 6 months and a usual age of onset between 50 and 80 years (Baldwin & Correll, 2019). Biochemical hallmarks of prion disease include spongiform degeneration (vacuolation), PrPSc deposition, neuronal loss and gliosis (Geschwind, 2015). Different subtypes of sCJD have been observed that demonstrate different clinical presentations. Two factors define the different subtypes: firstly, a polymorphism at codon 129 of PRNP that can be either a methionine or a valine and, secondly, the molecular mass of the protein core of PrPSc. Careful analysis of these factors has led to two systems of disease classification where different subtypes may display similar or diverse clinical and biochemical features (Baiardi et al., 2021; Collinge et al., 1996; Hill et al., 2003; Parchi et al., 1999). The difference between the classification systems arises from the definition of subtype 1, which is further subdivided into two subtypes based upon molecular mass and disease duration in the Collinge system (Collinge et al., 1996). Diagnosing prion disease in human patients can be difficult due to the heterogeneity of the clinical presentation of the different subtypes. The primary diagnostic tools in the clinic, along with the clinical picture, are the electroencephalogram, magnetic resonance imaging and cerebro-spinal fluid (CSF) 14–3-3 analysis (Knight, 2020; Wieser et al., 2006). Recently, a highly sensitive and specific test for the presence of PrPSc in the CSF, the real-time quaking-induced conversion assay (RT-QuIC) has also become an addition to the diagnostic tools available (Cramm et al., 2016; Groveman et al., 2017; Orrú et al., 2015; Orrú et al., 2016). However, a diagnosis of subtype can only be confirmed post-mortem by looking at the electrophoretic mobility of PrPSc from autopsied brain.
Genetic prion diseases include genetic Creutzfeldt–Jakob disease (gCJD), Gerstmann–Straüssler–Scheinker syndrome (GSS) and fatal familial insomnia (FFI). Familial disease is usually caused by an autosomal dominant point mutation within PRNP; however, insertion and deletion mutations have also been identified. E200K and D178N mutations, known to cause gCJD and FFI, respectively, demonstrate the highest penetrance, with up to 100% of carriers developing the disease within their lifetime (Minikel et al., 2016). As the cause of genetic prion disease is known, aspects of these diseases can also be modeled in the laboratory by creating a system containing the mutation such as a cell line or transgenic animal.
Models of human prion disease
In vivo models
Animal models have been used extensively to model prion diseases in vivo. This has been reviewed elsewhere (Brandner & Jaunmuktane, 2017; Moreno & Telling, 2017), so we will only briefly mention the humanized models. Transgenic mice have been created expressing human PrP or chimeric human-animal PrP (Brandner & Jaunmuktane, 2017; Telling et al., 1994). Humanized mice recapitulate some of the differences in subtype pathogenicity of human prion disease, including the influence of the PRNP codon 129 genotype on disease incubation time (Asante et al., 2002; Asante et al., 2006; Asante et al., 2015; Collinge et al., 1996). Humanized mice have also been used for studying genetic disease. In these models, the primary sequence of the transgene appears to heavily influence the propensity to develop spontaneous disease. For example, a study by Asante et al. considering P102L and E200K mutations in human PrP (129MM) found no development of spontaneous disease (Asante et al., 2009). However, when using chimeric human-mouse PrP where the region between amino acids 96 to 167 of mouse PrP was replaced with the corresponding human sequence (resulting in a 9 amino acid difference from mouse PrP (Telling et al., 1994)), Friedman-Levi et al. demonstrated that the E200K mutation could cause spontaneous disease in mice (Friedman-Levi et al., 2011). Humanized mice are extremely valuable for investigating human disease, especially discerning subtype characteristics (Asante et al., 2002; Asante et al., 2015); however, prion disease in humanized mouse models progresses very slowly. This results in extremely long experimental timelines associated with significant costs.
In vitro models
Using an in vitro model system addresses several of the concerns found in using in vivo animal models such as cost, difficulty and ethical reduction of animal numbers. Many different types of cell cultures have been used over the years in attempts to model propagation of human PrPSc. Unfortunately, technical difficulties, including the substantial species barrier between the infecting human prions and the cell line and long generational times to disease possibly caused by slower conversion kinetics of huPrP, have made propagating human prions extremely difficult (for a review, see (Pineau & Sim, 2021; Priola, 2018)). Mouse adapted human prions have been propagated in cell lines including RK13 (rabbit kidney epithelium) overexpressing mouse PrP, GT1–7 (mouse hypothalamic GnRH neuronal), N2a (mouse neuroblastoma) and OBL-21 (mouse olfactory bulb) cell lines (Arjona et al., 2004; Haigh et al., 2011; Lawson et al., 2008; Lewis et al., 2009). However, while the RK13 line in particular has demonstrated permissibility to propagating different species prions (Courageot et al., 2008), no propagation is seen when human PrP is expressed within these cells (Lawson et al., 2008). Primary cell cultures offer some advantages over immortalized cell lines such as the ability to study disease pathology in different cell types and the lack of a need for serial passaging, allowing the cultures to propagate different prion strains for longer periods of time (Pineau & Sim, 2021). Cerebellar granular primary cell cultures from transgenic mice expressing human PrP with methionine at PRNP codon 129 were able to propagate human prions from a sCJD subtype 1 (the PRNP 129 genotype is not stated) with detection of protease resistant PrP at 28 days (Cronier et al., 2007). Interestingly, using primary murine glial cells from mice expressing human PrP with methionine at codon 129, Wälzlein et al. demonstrated no propagation of subtype 1 (129MM) sCJD prions but clear propagation of subtype 2 (129MM) and vCJD prions (Wälzlein et al., 2021). PrPRes was detectable from 120 days post infection but declined after 150 days, likely due to the cultures reaching the end of their natural healthy lifespan.
Organotypic slice cultures, slices of brain tissue cultured in vitro, are more difficult than monolayers to culture but offer several advantages over 2D culture as they maintain a full complement of different brain cells and the structure of the brain regions. A study from 2008 initially described the methodology of this culture system adapted for prions, termed POSCA (Prion Organotypic Slice Culture Assay), and the feasibility of its use in studying prion propagation which in this model happens at an accelerated timescale (Falsig & Aguzzi, 2008). This model also shares an advantage of primary cell culture, the ability to create organotypic slices from mice expressing human PrP (Pineau & Sim, 2020).
While all these systems have produced valuable information on disease processes, they all have one limitation in common and that is the animal cell background. To add to the understanding of human disease, a fully human culture system was desirable. However, as alluded to above, it has proved difficult to propagate human prions in human cell cultures. To date, only one study to date has successfully propagated human prions in an immortalized cell line, SH-SY5Y human neuroblastoma cells (Ladogana et al., 1995). The SH-SY5Y cell line is known to suffer consistency problems due to the mixture of different cell phenotypes (neuroblast-like and epithelial-like) and the presence of adherent and suspension cells, which often results in only the adherent cells being passaged over time (Kovalevich & Langford, 2013). The prion-SH-SY5Y model system was never adopted as a human cell model beyond the original study, likely due to the aforementioned cell line limitations.
In a landmark study, Krejciova et al. generated the first fully human model of infection since the SH-SY5Y model (Krejciova et al., 2017). The authors used human-induced pluripotent stem cells (iPSCs), which are reprogrammed from somatic cells, often dermal fibroblasts, back to an immature state that demonstrates many of the characteristics of embryonic stem cells and, most importantly, pluripotency; the capacity to differentiate into any cell type. Using the iPSCs, Krejciova et al. were able to differentiate astrocyte progenitor cells and astrocyte cultures that showed maturation stage dependent functionality (Krejciova et al., 2017). When exposed to human prions from vCJD and sCJD brain homogenates, mature astrocyte cultures, but not the astrocyte progenitor cells, demonstrated propagation of the human prions and accumulation of protease-resistant PrP at 3 and 8 days post-infection. The cultures continued to demonstrate propagation up to 28 days post-infection. Furthermore, propagation was dependent on the PRNP codon 129 genotype. In agreement with the known susceptibility of human prion genotypes to preferentially propagate specific PRNP codon 129 genotypes and subtypes (Fernández-Borges et al., 2017; Klemm et al., 2012), PRNP codon 129MM astrocytes readily propagated MM1 sCJD, VV2 sCJD and vCJD but 129VV astrocytes only propagated VV2 sCJD. The authors additionally showed that the propagated prions were infectious by passaging the infected astrocyte prions back into naïve astrocytes. This important advance demonstrated that human prions could be propagated in culture with retention of the biochemical characteristics of the infecting inoculum.
Since the cells were grown as monolayers, the astrocyte model still lacked 3D structure. Three-dimensional models of neuronal tissue are being used to more accurately model the cytoarchitecture of the brain during disease (Slanzi et al., 2020). Murine 3D models derived from undifferentiated and differentiated neural stem cells have been shown to propagate various prion strains (Collins & Haigh, 2017; Giri et al., 2006; Herva et al., 2010; Iwamaru et al., 2017; Iwamaru et al., 2013). These studies raised the possibility that human prion infections could be modeled in 3D cultures of human brain cells. The opportunity to study neurodegenerative disease in this context presented in 2013, when a study was published describing a 3D model of human brain tissue that recapitulated features of human cortical development (Lancaster et al., 2013). The authors termed the cultures ‘human cerebral organoids.’
Human cerebral organoids
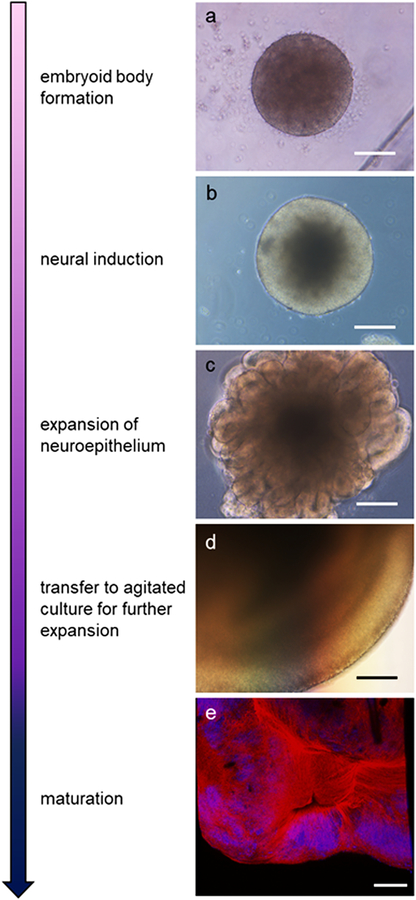
The human cerebral organoid model developed by Lancaster et al. (Lancaster et al., 2013) involved generation of self-assembling 3D structures of multiple cell types differentiated from human embryonic stem cell and human iPSCs. This is achieved in four distinct stages via the use of additives to the media, the first stage initiates the formation of aggregates of iPSCs known as embryoid bodies (Lancaster & Knoblich, 2014). The embryoid bodies (EB) are then moved to a minimal media formulation for neural induction while kept in suspension allowing formation of neural ectoderm around the surface of the EB. Organoids are then embedded within a Matrigel matrix to allow expansion of the neuroepithelium before being transferred into agitated culture for maturation and long-term maintenance (Lancaster & Knoblich, 2014). Here, they can continue to grow and develop for several months to over a year (a summary of the progression of differentiation is shown in Figure 1). Human cerebral organoids offer several benefits such as scalability and ease, like more traditional cell model systems, while retaining much of the diverse cell types and network physiology seen in in vivo models (see Table 1 and below).
Fig 1.
Organoid developmental stages and morphology. Arrow indicates increasing maturity. Bright-field images show organoid morphology at various developmental stages including; a) fully formed embryoid body beginning neural induction, b) appearance of bright neuroepithelium, c) expansion of neuroepithelial buds, and d) further expansion and structuring of the organoid. e) Detection of mature neurons. Immunofluorescent staining shows neurofilament light chain (red) and nuclei (blue; DAPI) at approximately 45 days old. Scale bar = 100 μm
Table 1.
Comparison of human/humanized models of infection
| Cerebral Organoids | Transgenic Animal | Primary Cell Culture | Organotypic Slice | |
|---|---|---|---|---|
| Cost of Generation | + | +++ | + | ++ |
| Cost of Maintenance | + | +++ | + | + |
| Difficulty of Generation | ++ | +++ | + | + |
| Difficulty of Maintenance | + | ++ | + | + |
| Cell Diversity | +++ | +++ | + | +++ |
| Experimental Timeframe | ++ | +++ | + | + |
| Time to Maturity | + | ++/+++(*) | + | + |
| Scalability | +++ | + | +++ | ++ |
| Proximity to Humans | +++ | ++ | + | ++ |
minimal
average
high
depending on the infection subtype
Organoid development recapitulates fetal human brain development in several ways. For example, they develop a similar tissue architecture and neuronal polarization. Organoids form layers during development including a ventricular zone, subventricular zone, deep layer neurons and finally upper layer neurons comprised of polarized neurons similar to a developing human brain (Chiaradia & Lancaster, 2020). Additionally, Cajal–Retzius cells may be contributing to cortical plate formation in similarity with what is seen in vivo (Chiaradia & Lancaster, 2020; Kadoshima et al., 2013; Lancaster et al., 2013). Organoids also contain the multiple cell types found in the developing human brain. Excitatory glutamatergic neurons make up the bulk of the neuronal cell types in organoids (Chiaradia & Lancaster, 2020), but inhibitory GABAergic interneurons are also present (Giandomenico et al., 2019; Velasco et al., 2019). Organoids can additionally contain astrocytes of healthy morphology, with a smaller amount of reactive astrocytes after 30 weeks in culture (Giandomenico et al., 2019; Qian et al., 2020; Sloan et al., 2017), as well as oligodendrocytes (Madhavan et al., 2018; Marton et al., 2019). This regional specificity and cellularity closely resembles what is seen in the developing brain and provides an improved experimental model of human brain tissue when compared to traditional 2D monocultures.
One of the most important stages of neuronal development is the establishment of cell–cell interactions and longer distance network formation. The organoids develop cell–cell interactions and electrophysiological signaling within the neuronal layers that is seen in the developing mammalian brain allowing for a multitude of signaling studies to be investigated using this model (Fair et al., 2020; Yakoub, 2019). For example, Watanabe et al. showed that organoids recapitulated corticogenesis and many metrics such as membrane potential, capacitance and resistance were found to be similar to what is observed in human fetal cortex (Watanabe et al., 2017). Additionally, spontaneous network activity has been recorded which further recapitulates the embryonic brain environment (Lancaster et al., 2013). Finally, organoids have also been shown to mimic the transcriptional expression of a developing brain for 24 weeks after generation at which point the maturing organoid core begins to become hypoxic and necrosis will begin (Chiaradia & Lancaster, 2020; Giandomenico et al., 2019; Tanaka et al., 2020). These properties are unique to organoid development when compared with more traditional cell models and recapitulates far more of the relevant in vivo environment from which these diseases emerge.
Organoid models and neurodegenerative disease
The value of cerebral organoids for modeling human brain diseases was quickly realized and applied to several diseases including other protein misfolding disorders. A disease phenotype that has been difficult to recapitulate in animal and cell models is amyloid deposition, tau hyperphosphorylation and neurodegeneration, which is seen in Down syndrome (DS) or Alzheimer’s disease (AD). Transgenic mice serve as the primary model for investigation into AD and have shown the ability to recapitulate several aspects of the disorder such as age-dependent amyloid plaque formation and cognitive defects. Some models, such as the knock-in of human microtubule-associated protein tau (MAPT), can mimic the widespread neuronal loss that accompanies AD in humans (Saito et al., 2019), but each of these phenotypes is largely tied to a single transgenic mutation and so recapitulating them all within a single model requires multiple knockouts followed by expression of human genes (Duyckaerts et al., 2008). Using cerebral organoids grown from patient iPSCs alleviated this barrier to an extent by providing a model utilizing all of the human genes necessary to recapitulate a disorder such as AD, and indeed, Gonzalez et al. were able to show structures similar to amyloid plaques and neurofibrillary tangles when using a cerebral organoid model (Gonzalez et al., 2018). An earlier study was able to demonstrate an effect of beta amyloid and gamma secretase inhibitors on amyloid generation and tau pathology resulting from familial AD mutations (Choi et al., 2014). Another study in 2016 using cerebral organoids showed the above pathologies as well as endosomal abnormalities to be age-dependent (Raja et al., 2016). The cellular complexity of cerebral organoids is a great opportunity to model multi-modal diseases such as AD.
Another disease that has been a subject of scrutiny in organoid models is Parkinson’s disease (PD). As PD primarily affects dopaminergic neurons of the substantia nigra pars compacta, studies using human organoids to consider mutations linked with PD (such as LRRK2 G2019S) have focused on a different organoid differentiation protocol that produces mid-brain organoids. These studies have shown that the mid-brain organoids can re-produce aspects of genetic Parkinson’s disease including, alpha-synuclein deposition, thioflavin-T positivity, and reduced tyrosine hydroxylase (Kim et al., 2019; Smits et al., 2019). The ability to differentiate organoids that correspond to different brain regions offers increased utility of the organoid model and demonstrates that organoid models can be applied across a spectrum of diseases.
Organoids in prion research
Genetic prion disease
The first studies to approach looking at prion diseases in 3D iPSC-derived cultures considered the influence of mutations within the prion gene on the development of a disease phenotype. A study using iPSCs generated from a human donor with a Y218N PRNP mutation, which is associated with the development of GSS, showed cell death and increased Tau phosphorylation without any detection of diseased isoforms of PrP (Matamoros-Angles et al., 2018). In another study, PRNP E200K and an eight-octarepeat insert mutation (both associated with development of genetic CJD) were used as negative controls for beta-amyloid, phospho-tau and neuronal morphology when comparing phenotypes with organoids generated from donors with mutations causing familial AD or DS (Gonzalez et al., 2018). The authors of this latter study did not comment on identification of any PrP-associated phenotype. We also generated organoids from two donors carrying the E200K PRNP mutation (Foliaki et al., 2020) and, likewise, found no endogenous pathology in these organoids for over a year post-differentiation, although neuroelectrophysiological dysfunction was later demonstrated (Foliaki et al., 2021). Together this indicates that PRNP mutation is not sufficient to cause a prion disease phenotype within organoids and that there is not continuous production of misfolded PrP. This instead raises the question of what might trigger PrP misfolding in the aging brain.
Prion infections
In 2019, we demonstrated that human cerebral organoids were able to propagate human prions and recapitulate some disease features (Groveman et al., 2019). This study used iPSCs that were heterozygous at PRNP codon 129 (129MV) to generate cerebral organoids that were grown for 5 months before exposure to human prions. Five months of age was selected as the time of infection because at this point organoids are populated with more mature cells including astrocytes and oligodendrocytes (Renner et al., 2017). Organoids were exposed to brain homogenate from patients who died of sCJD subtypes MV1 and MV2 for 1 week with serial dilution. At 7 days, the organoids were washed and cultured in a fresh vessel. In contrast to the astrocyte model, after removal of the infectious inoculum, organoids required 3–4 weeks to clear residual inoculum, likely due to the complexity of the 3D structure permitting residual protein to evade destruction for longer than when readily washed away in 2D culture. After approximately 5 weeks, the organoids started to accumulate de novo prions, with a greater accumulation in the organoids receiving the MV2 subtype inoculum. The accumulation in the organoids that received the MV2 inoculum was sufficient that protease-resistant PrPSc could be observed by western blotting and PrP deposits could be visualized by immunohistochemistry. The organoids exposed to the MV1 inoculum showed no protease-resistant PrPSc or tissue deposition; however, they did show seeding activity in the highly sensitive real-time quaking-induced conversion assay (RT-QuIC) (Groveman et al., 2019; Orrú et al., 2016) suggesting a low level of infection was present.
As with all cellular prion models, validation that the PrP seeds and PrPRes detected were due to de novo production rather than persistence of the initial inoculum is imperative. Production of de novo PrPSc is supported by the loss of seeding activity at 3–4 weeks followed by its increase to higher levels than detected after the initial inoculum was removed. Additionally, in organoids infected with the MV2 subtype inoculum we saw a shift to di-glycosylated dominant protease resistant species. This indicated cellular PrP within the organoid was being converted, as it is unlikely that the original, mono-glycosylated sCJD inoculum would become modified in this way. To investigate this further PrPRes production could be analyzed by the use of amino acid isotopic-labeling to show that radio labeled PrPC synthesized by the cell converts into PrPRes. The gold standard, which remains to be demonstrated, would be to show a lack of propagation in PrP knock-out organoids.
Additionally, the Groveman et al. study also demonstrated that organoids can be used to model neuronal dysfunction and death. Over the course of the experiment, the organoids were monitored weekly for changes in cellular metabolism and periodically analyzed for evidence of cell death (Groveman et al., 2019). The organoids exposed to MV1 inoculum demonstrated changes in their metabolism throughout the infection period, initially showing a decline but later increasing their metabolism over the control organoids. The MV2-inoculated organoids showed no such change in their metabolism from their baseline readings or compared with the metabolism of the control organoids (Groveman et al., 2019). Cytokine assays also showed an increase in cytokine secretion in the MV1-inoculated organoids. This started around 90 days post-infection with a large increase in chitinase 3-like-1, a cytokine that is also increased in brain tissue from people who died of MM1 and VV2 subtypes of sCJD (Llorens et al., 2017). These results showed that several changes that occur in the human brain during disease were also happening within the organoids as they developed infection and that there may be subtype-specific differences in the presentation of infection.
The potential to investigate the influence of different subtypes offers some interesting opportunities. The different sCJD subtypes show a phenomenon called neuronal selective vulnerability, wherein the different subtypes damage the cells within different brain regions disproportionately (Jackson, 2014). This results in greater lesions in some brain regions over others, which produces the lesion profiles characteristic of the subtype (Baiardi et al., 2021). Using the cerebral organoids and different infecting subtypes, it will be possible to examine which neuronal subsets are most damaged in response to which subtypes and investigate common versus disparate cell death mechanisms. This analysis is made more powerful by developments in organoid technologies that allow regional specification (Bagley et al., 2017). Comparing different regions will allow the neuronal responses to the different subtypes to be monitored over the course of infection.
Prion-infected organoids for therapeutic screening
Being able to infect organoids and monitor prion propagation over time also allows for investigation of how propagation can be slowed or halted. Prion disease therapeutics remain absent despite years of study. Many drug candidates that appeared promising in animal or traditional cell culture models failed to show efficacy when introduced to humans (Qian & Tcw, 2021). A human model was required to understand the failings in the translation of these proposed therapeutics and the organoid–prion infection model presents a new option for drug screening (Groveman et al., 2021). The study by Groveman et al. showed the capacity of the organoids to act as a drug screening model by assessing the efficacy of pentosan polysulfate (PPS), which has been shown to exhibit anti-prion activity in numerous model systems (Caughey & Raymond, 1993; Doh-ura et al., 2004; Farquhar et al., 1999; Groveman et al., 2021). Even though this compound has not been used extensively in humans due to its inability to pass the blood–brain barrier (requiring intra-cranial delivery), its potent inhibition of prion replication in animal and cell models renders it prototypical for developing new treatment systems in vitro (Groveman et al., 2021). In a treatment paradigm representing prophylactic administration, organoids were treated with PPS for 7 days before exposure to infected inoculum and for a further 14 days throughout infection and for 1 week after the infectious inocula was removed from the media. The prophylactically treated organoids showed both a reduction of RT-QuIC seeding activity and a reduction in protease-resistant PrPSc. In an alternative approach more closely representing therapeutic treatment once infection is established, organoids were infected for 63 days before treatment with PPS for 28 days (ending at 91 dpi). These organoids also showed a reduction in RT-QuIC seeding activity and PrPSc. Additionally, after PPS treatment was stopped at 91 dpi, organoids were cultured for a further month to 120 dpi and maintained the reduction in RT-QuIC seeding activity and no PrPSc detection (Groveman et al., 2021). This study illustrates the potential the organoid model to be used as a tool for investigations into therapy and even drug discovery.
The organoid model holds promise of being a versatile model for testing anti-prion therapeutics. The Groveman et al. manuscript considered only two possible therapeutic paradigms: prophylactic and therapeutic; however, many variations upon this theme could be tried. Variations might include: duration of treatment, combinations of treatments and testing toxicity (both in the context of brain cells and other tissues that can be differentiated from iPSCs such as liver or kidney organoids). A specific and powerful advantage to the prion organoid therapeutic model is that it permits study of therapeutic efficacy in different PRNP codon 129 backgrounds and with different subtypes. As only very few patients present with sCJD each year and their molecular subtyping will not be confirmed until post-mortem examination, clinical trials of new compounds are unlikely to be able to judge the efficacy of a putative therapeutic in different subtypes. However, using organoids this can be achieved in the laboratory by generating organoids from donor cells with different PRNP codon 129 genotypes and infecting these organoids with inoculums of patient brain tissue identified as different subtypes. An additional advantage to screening putative therapeutics in organoids is that they provide a means to monitor neuroelectrophysiological function. When considering the benefit of a potential therapy, the capacity to maintain or even restore neuronal function increases the likelihood of benefit for the individual. These attributes of organoids offer therapeutic screening paradigms with much greater flexibility than existing models.
Cerebral organoids could additionally allow researchers to test compounds against prion infection established within organoids generated from donors with PRNP genotypes associated with genetic disease and rapidly screen potential drug candidates with a throughput that would not be feasible in animal models. Furthermore, in families suffering genetic prion disease, organoids could provide a model for personalized medicine in which a cohort of organoids could be grown from a patient unique to their specific genetics. The feasibility of various treatments could be tested in the patient-specific organoids before administering the best candidate to the patient.
An idea that arises when considering personalized medicine is whether it is possible to produce new neurons to replenish those that have been lost and the capacity to re-grow neuronal function. While there is precedent from mouse studies looking at degeneration associated with the E200K mutation that implanting neural stem cells alone and as part of a combination therapy could delay disease and symptom onset (Frid et al., 2018; Frid et al., 2020), it is too soon to know if neurons or neuronal precursors generated within organoid cultures could be used this way. One hint of possibility comes from a case study generating personalized dopaminergic progenitors for the treatment of Parkinson’s disease. In this study, the dopaminergic precursors were grafted into the putamina of the patient who showed limited improvements in motor assessment and quality of life 24 months following the graft (Schweitzer et al., 2020). While clearly not an immediate application of organoid technology, the application to personalized medicine is certainly in interesting area to watch for future developments.
Other potential applications of the organoid model to prion disease
The use of organoids for studying prion disease is still in its foundation phase (this is summarized in Table 2). Generally, as organoids are grown in cell culture similarly to other in vitro systems, most experimental paradigms applied to investigation prion infection in cell culture are likely to be transferrable to the organoid system. This potentially permits the study of many pathways that cannot be directly interrogated in humans. For example, to date the only infection protocol published is an overlay technique, where the infectious inoculum is included in the media surrounding the organoids. However, many other protocols exist for prion infections, including steel wires and exosomes (Fevrier et al., 2004; Flechsig et al., 2001; Vella et al., 2007; Zobeley et al., 1999). The trial of these for infecting organoids may be able to provide information about the modes of cell–cell spreading as well as further avenues for preventing cell to cell spread.
Table 2.
Summary of studies using prion mutation or infections of organoids to date. N/a = not applicable.
| Organoid protocol | PRNP mutation | Prion infection | Experimental timeframe | Key findings | Ref. |
|---|---|---|---|---|---|
| Spherical neural masses | Y218N | 1Y218N and CJD2 | Differentiated for 3, 6 or 9 weeks | PRNP Y218N neurons demonstrate hyperphosphorylation of Tau and neurofibrillary degeneration without evidence of misfolded PrP | (Matamoro s-Angles et al., 2018) |
| Cerebral organoids (Lancaster & Knoblich, 2014) | E200K 8-octerepe at insertion | N/a | 110 days in culture | No pathological changes reported | (Gonzalez et al., 2018) |
| Cerebral organoids (Lancaster & Knoblich, 2014) | N/a | PRNP 129M/V organoids MV1 and MV2 subtype inoculums | 5 months from starting differentiation to infection Up to 6 months to analysis | Organoids become infected with and propagate prions. PRNP 129M/V organoids showed a preference for propagation of the MV2 subtype but greater pathological changes associated with the MV1 subtype. | (Groveman et al., 2019) |
| Cerebral organoids (Lancaster & Knoblich, 2014) | E200K | N/a | Up to and including 1 year | No pathological changes reported | (Foliaki et al., 2020) |
| Cerebral organoids (Lancaster & Knoblich, 2014) | N/a | PRNP 129M/V organoids MV2 subtype inoculum | 5 months from starting differentiation to infection Up to 4 months to analysis | Organoids could demonstrate the therapeutic efficacy of PPS3 when it was administered prophylactically, before, during and for a short time after infection, and therapeutically, administered after infection was established. | (Groveman et al., 2021) |
| Cerebral organoids (Lancaster & Knoblich, 2014) | E200K | N/a | Up to 10 months | Electrophysiological dysfunction with disturbed excitatory to inhibitory balance | (Foliaki et al., 2021) |
Forebrain neuronal cultures only were tested for uptake of infection and found negative
CJD type was not specified.
Pentosan polysulfate
Other aspects of organoid biology may be useful for the investigation of prion disease both in the context of genetic and infectious disease. A simple, but critical, aspect of organoid maintenance is that organoid culture systems are maintained in highly defined media (Lancaster & Knoblich, 2014). A strong confidence in the composition of the cellular environment provides opportunities to make precise changes to media components for investigation of their influence on prion conversion and development of infection. Many co-factors have been implicated both in the establishment of infection at the prion uptake stage and the on-going propagation of prions including glycosaminoglycan sulfation (Lawson et al., 2010), RNAs (Adler et al., 2003), and lipids (Kazlauskaite & Pinheiro, 2005). While these pathways are unlikely to offer themselves as something that could be readily manipulated to counter disease, they could provide valuable insight into pathogenic processes.
Limitations of the organoid model
When discussing the applications of the organoid models we cannot fully appreciate the value without understanding the limitations. Some limitations have already been considered, but others should also be given due consideration when interpreting experimental results. A clear variation from the human brain is that the organoid model has no vascularization or blood–brain barrier. New models, both variations of the organoid differentiation protocol and 3D-printed stand-alone models (Bose et al., 2021; Kaisar et al., 2017; Pellegrini et al., 2020; Pham et al., 2018), are attempting to create a blood–brain barrier like environment or introduce a pseudo-vasculature into the organoid. These are discussed further below. Beyond the biological improvement this also improves diffusion within the core of the organoid, which both limits its overall growth and can become necrotic once an organoid has reached a certain size (Pellegrini et al., 2020).
A second potential weakness is that there are no non-neuronally derived cells, such as immune cells. Microglia are a population of immune cells found within the brain. They have been shown to have a significant impact on the progression of prion disease; if they are prevented from proliferating, disease incubation is extended (Zhu et al., 2016), but if they are absent the disease progresses significantly faster (Carroll et al., 2018). Microglia are derived from the yolk sack, infiltrating the brain tissue during development. Therefore, organoid differentiation from neuroepithelium is naturally devoid of microglia that are derived from mesoderm. Protocols have been developed to induce microglia to develop during organoid differentiation (Ormel et al., 2018) and also to introduce microglia back into the organoid after development (Abud et al., 2017). These protocols are continuing to be developed and are also being applied to co-incubation with epithelial tissue as needed for the blood–brain barrier. It is reasonable to expect that they will become highly developed and widely available within a few years.
One of the strengths of the organoid model of prion infection is also a specific limitation. Organoids contain differentiated neuronal lineage cells that permit aspects of neuronal function and health to be monitored, but the differentiated cells within the organoid are no longer growing. This means that the culture does not keep reproducing itself in the same way as an infected immortalized cell line, which can produce generations of infected cells by passing on infection to the daughter cells. The limitation that is encountered here is inherent to all differentiated or ex vivo models, that each new infection must be established in a new batch of organoids using fresh inoculum (Krance et al., 2020). This introduces error through batch-to-batch variability. Conversely, terminal differentiation does remove the limitation that in growing cell cultures the accumulation of prions is influenced by the rate of cell division, which may alter the apparent efficacy of anti-prion therapeutics tested in such systems (Ghaemmaghami et al., 2007).
A further related consideration when producing organoids is that their cellularity and gene expression may vary across batches, within batches and across different iPSC lines. Inter- and intra-batch variations have been identified as a major consideration when modeling Alzheimer’s pathology in organoids (Hernández et al., 2021), and, for studies of gene mutations, the best consistency across iPSC lines is achieved using genetically matched, isotype controls. Single cell RNA sequencing analysis has shown that despite the variations, the cell developmental trajectories and terminal cells produced within organoids follow the complex cellular diversity of the cortex (Velasco et al., 2019). Many variations on the culture protocols are under investigation for producing greater uniformity of cultures, especially in the context of greater scale and automation for cultures (Louey et al., 2021). However, experiments should be conducted with the potential impact of this increased variability considered in the assay design and controls utilized.
A final consideration is that, like other dementias, prion diseases primarily affect older people. Human organoids are not similar to a mature or elderly brain but better represent the development of neonatal brain and, therefore, may contain cell types that respond differently to prion infection than aging brain cells. Already ways to adapt the organoid system to more accurately reflect the aging brain are underway by utilizing manipulation of known cellular factors associated with aging (reviewed in (Grenier et al., 2020)). As the model develops further, it may become possible to interrogate the influence of prion infection on aging cells.
Future developments
As we have alluded to, many refinements are being developed to address some of the potential drawbacks of the cerebral organoid model such as the addition of scaffolding, air interfaces, and vascularization for better nutrient diffusion, as well as for improving their overall usefulness. As cerebral organoid protocols have progressed, they have also specialized. Protocols now exist for producing mid-brain, ventral forebrain, dorsal forebrain, cerebellar, thalamic and hypothalamic brain organoids (Bagley et al., 2017; Huang et al., 2021; Jacob et al., 2021; Muguruma et al., 2015; Qian et al., 2016; Smits et al., 2019; Watanabe et al., 2017; Xiang et al., 2019) and this list of regions is not exhaustive. Regional specification is an exciting development for prion diseases where different subtypes and different genetic mutations preferentially attack some regions more than others. As these protocols have evolved, they have also addressed the intrinsic properties of the cells themselves and new methods can now improve neuronal myelination by enhancing the specification and survival of oligodendrocytes (Shaker et al., 2021).
Although generalized network activity and neuronal communication have been shown in cerebral organoids, steps are being taken to further recapitulate what is seen in vivo. One such study implemented an air–liquid interface to both improve organoid core survival and increase axonal outgrowth (Giandomenico et al., 2019). The authors found their culture system exhibits active neural networking, long-range axonal projection and growth cone turning in addition to expressing RNA indicating varied neuronal identity. Furthermore, most organoid models consider only one brain region, but the human brain displays circuitry between regions. Bagley et al. were able to demonstrate that organoids differentiated to represent dorsal or ventral forebrain could merge and GABAergic interneurons would migrate from the ventral forebrain organoid into the dorsal tissue (Bagley et al., 2017). The combination of these systems for improved neuronal communications with new developments in electrophysiology that permit recording of neuronal activity within the organoid 3D structure (Tasnim & Liu, 2021) offers the potential to better understand neuronal function, or disease-associated dysfunction, over long periods of time in culture.
Finally, as we have already mentioned, one of the primary difficulties with organoid cultures has been their lack of vascularization, which, aside from the lack of a biologically important system, results in less diffusion of nutrients to the organoid core. A potential solution to this is the use of an internal scaffold where channels of medium allow nutrients to reach the interior of the organoid and diffuse to the core. This has been investigated using scaffolds constructed from poly-(lactic-co-glycolic acid) (PLGA) fibers or carbon fibers to study mid-brain organoids (Tejchman et al., 2020). The scaffolds were shown to improve neuronal survival. Another approach to the problem of vascularization was demonstrated by Pham and colleagues where iPSCs from a patient were used to differentiate organoids while at the same time also performing a separate differentiation into endothelial cells. After a month, the organoids were re-embedded into Matrigel and coated with the corresponding endothelial cells. They were then either grown in vitro for 3–5 additional weeks or transferred into a mouse model for 2 weeks, resulting in vascularization of the organoid (Pham et al., 2018). Additionally, when the organoid was transplanted into a mouse model, the authors report detection of CD31-positive blood vessel within the center of the organoid. This demonstrated that organoids could be vascularized with a patient’s own tissue and provides a step over one of the largest hurdles in organoid development and one of the largest steps toward recapitulating the in vivo brain.
Summary
Human cerebral organoids present an exciting and powerful model system to study neurological disorders such as AD, PD and prion disease. They readily differentiate into neuronal cell types such as neurons, astrocytes and oligodendrocytes and form regions of specialization. Their cellularity and complexity facilitate detailed study of human neuronal function and the development of pathologies, including several diseases that have thus far been difficult to model using animal or 2D monoculture systems. Cerebral organoids are relatively simple to create and can be grown from human donors carrying genetic mutations allowing researchers to use them to study both the underlying genetics of disorders as well as the progression of infectious disease. Throughout this review, we have attempted to give a detailed account of what can be learned from organoid studies and examine the limitations to their application. The speed of developments in this area will undoubtably offer further exciting opportunities that are yet to be revealed and as the methods of organoid creation continue to be refined and expanded so will their usefulness to prion research.
Acknowledgments
This work was funded by the intramural program of the National Institute of Allergy and Infectious Diseases (National Institutes of Health). The authors would like to thank Dr Roger Moore and Dr Simote Foliaki for critical review of the manuscript.
Funding
This work was funded by the National Institute of Allergy and Infectious Diseases (NIH).
Footnotes
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Not applicable. No experiments were conducted for this review article.
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
References
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, & Blurton-Jones M (2017). iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron, 94(2), 278–293.e279. 10.1016/j.neuron.2017.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler V, Zeiler B, Kryukov V, Kascsak R, Rubenstein R, & Grossman A (2003). Small, highly structured RNAs participate in the conversion of human recombinant PrP(Sen) to PrP(Res) in vitro. J Mol Biol, 332(1), 47–57. 10.1016/s0022-2836(03)00919-7 [DOI] [PubMed] [Google Scholar]
- Arjona A, Simarro L, Islinger F, Nishida N, & Manuelidis L (2004). Two Creutzfeldt-Jakob disease agents reproduce prion protein-independent identities in cell cultures. Proc Natl Acad Sci U S A, 101(23), 8768–8773. 10.1073/pnas.0400158101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante EA, Gowland I, Grimshaw A, Linehan JM, Smidak M, Houghton R, Osiguwa O, Tomlinson A, Joiner S, Brandner S, Wadsworth JDF, & Collinge J (2009). Absence of spontaneous disease and comparative prion susceptibility of transgenic mice expressing mutant human prion proteins. J Gen Virol, 90(Pt 3), 546–558. 10.1099/vir.0.007930-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante EA, Linehan JM, Desbruslais M, Joiner S, Gowland I, Wood AL, Welch J, Hill AF, Lloyd SE, Wadsworth JD, & Collinge J (2002). BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. EMBO J, 21(23), 6358–6366. 10.1093/emboj/cdf653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante EA, Linehan JM, Gowland I, Joiner S, Fox K, Cooper S, Osiguwa O, Gorry M, Welch J, Houghton R, Desbruslais M, Brandner S, Wadsworth JD, & Collinge J (2006). Dissociation of pathological and molecular phenotype of variant Creutzfeldt-Jakob disease in transgenic human prion protein 129 heterozygous mice. Proc Natl Acad Sci U S A, 103(28), 10759–10764. 10.1073/pnas.0604292103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante EA, Smidak M, Grimshaw A, Houghton R, Tomlinson A, Jeelani A, Jakubcova T, Hamdan S, Richard-Londt A, Linehan JM, Brandner S, Alpers M, Whitfield J, Mead S, Wadsworth JD, & Collinge J (2015). A naturally occurring variant of the human prion protein completely prevents prion disease. Nature, 522(7557), 478–481. 10.1038/nature14510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley JA, Reumann D, Bian S, Lévi-Strauss J, & Knoblich JA (2017). Fused cerebral organoids model interactions between brain regions. Nat Methods, 14(7), 743–751. 10.1038/nmeth.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiardi S, Rossi M, Mammana A, Appleby BS, Barria MA, Calì I, Gambetti P, Gelpi E, Giese A, Ghetti B, Herms J, Ladogana A, Mikol J, Pal S, Ritchie DL, Ruf V, Windl O, Capellari S, & Parchi P (2021). Phenotypic diversity of genetic Creutzfeldt-Jakob disease: a histo-molecular-based classification. Acta Neuropathol. 10.1007/s00401-021-02350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin KJ, & Correll CM (2019). Prion Disease. Semin Neurol, 39(4), 428–439. 10.1055/s-0039-1687841 [DOI] [PubMed] [Google Scholar]
- Bose R, Banerjee S, & Dunbar GL (2021). Modeling Neurological Disorders in 3D Organoids Using Human-Derived Pluripotent Stem Cells. Front Cell Dev Biol, 9, 640212. 10.3389/fcell.2021.640212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner S, & Jaunmuktane Z (2017). Prion disease: experimental models and reality. Acta Neuropathol, 133(2), 197–222. 10.1007/s00401-017-1670-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JA, Race B, Williams K, Striebel J, & Chesebro B (2018). Microglia Are Critical in Host Defense against Prion Disease. J Virol, 92(15). 10.1128/jvi.00549-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B, & Raymond GJ (1993). Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J Virol, 67(2), 643–650. 10.1128/JVI.67.2.643-650.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaradia I, & Lancaster MA (2020). Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nat Neurosci, 23(12), 1496–1508. 10.1038/s41593-020-00730-3 [DOI] [PubMed] [Google Scholar]
- Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, & Kim DY (2014). A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature, 515(7526), 274–278. 10.1038/nature13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Sidle KC, Meads J, Ironside J, & Hill AF (1996). Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature, 383(6602), 685–690. 10.1038/383685a0 [DOI] [PubMed] [Google Scholar]
- Collins SJ, & Haigh CL (2017). Simplified Murine 3D Neuronal Cultures for Investigating Neuronal Activity and Neurodegeneration. Cell Biochem Biophys, 75(1), 3–13. 10.1007/s12013-016-0768-z [DOI] [PubMed] [Google Scholar]
- Courageot MP, Daude N, Nonno R, Paquet S, Di Bari MA, Le Dur A, Chapuis J, Hill AF, Agrimi U, Laude H, & Vilette D (2008). A cell line infectible by prion strains from different species. J Gen Virol, 89(Pt 1), 341–347. 10.1099/vir.0.83344-0 [DOI] [PubMed] [Google Scholar]
- Cramm M, Schmitz M, Karch A, Mitrova E, Kuhn F, Schroeder B, Raeber A, Varges D, Kim YS, Satoh K, Collins S, & Zerr I (2016). Stability and Reproducibility Underscore Utility of RT-QuIC for Diagnosis of Creutzfeldt-Jakob Disease. Mol Neurobiol, 53(3), 1896–1904. 10.1007/s12035-015-9133-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronier S, Beringue V, Bellon A, Peyrin JM, & Laude H (2007). Prion strain- and species-dependent effects of antiprion molecules in primary neuronal cultures. J Virol, 81(24), 13794–13800. 10.1128/JVI.01502-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doh-ura K, Ishikawa K, Murakami-Kubo I, Sasaki K, Mohri S, Race R, & Iwaki T (2004). Treatment of transmissible spongiform encephalopathy by intraventricular drug infusion in animal models. J Virol, 78(10), 4999–5006. 10.1128/jvi.78.10.4999-5006.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Potier MC, & Delatour B (2008). Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol, 115(1), 5–38. 10.1007/s00401-007-0312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair SR, Julian D, Hartlaub AM, Pusuluri ST, Malik G, Summerfied TL, Zhao G, Hester AB, Ackerman W. E. t., Hollingsworth EW, Ali M, McElroy CA, Buhimschi IA, Imitola J, Maitre NL, Bedrosian TA, & Hester ME (2020). Electrophysiological Maturation of Cerebral Organoids Correlates with Dynamic Morphological and Cellular Development. Stem Cell Reports, 15(4), 855–868. 10.1016/j.stemcr.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsig J, & Aguzzi A (2008). The prion organotypic slice culture assay--POSCA. Nat Protoc, 3(4), 555–562. 10.1038/nprot.2008.13 [DOI] [PubMed] [Google Scholar]
- Farquhar C, Dickinson A, & Bruce M (1999). Prophylactic potential of pentosan polysulphate in transmissible spongiform encephalopathies. Lancet, 353(9147), 117. 10.1016/S0140-6736(98)05395-1 [DOI] [PubMed] [Google Scholar]
- Fernández-Borges N, Espinosa JC, Marín-Moreno A, Aguilar-Calvo P, Asante E, Kitamoto T, Mohri S, Andréoletti O, & Torres JM (2017). Protective Effect of Val129-PrP against Bovine Spongiform Encephalopathy but not Variant Creutzfeldt-Jakob Disease. Emerging Infectious Disease journal, 23(9), 1522. 10.3201/eid2309.161948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, & Raposo G (2004). Cells release prions in association with exosomes. Proc Natl Acad Sci U S A, 101(26), 9683–9688. 10.1073/pnas.0308413101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig E, Hegyi I, Enari M, Schwarz P, Collinge J, & Weissmann C (2001). Transmission of scrapie by steel-surface-bound prions. Mol Med, 7(10), 679–684. [PMC free article] [PubMed] [Google Scholar]
- Foliaki ST, Groveman BR, Yuan J, Walters R, Zhang S, Tesar P, Zou W, & Haigh CL (2020). Pathogenic Prion Protein Isoforms Are Not Present in Cerebral Organoids Generated from Asymptomatic Donors Carrying the E200K Mutation Associated with Familial Prion Disease. Pathogens, 9(6). 10.3390/pathogens9060482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foliaki ST, Schwarz B, Groveman BR, Walters RO, Ferreira NC, Orrù CD, Smith A, Wood A, Schmit OM, Freitag P, Yuan J, Zou W, Bosio CM, Carroll JA, & Haigh CL (2021). Neuronal excitatory-to-inhibitory balance is altered in cerebral organoid models of genetic neurological diseases. Mol Brain, 14(1), 156. 10.1186/s13041-021-00864-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frid K, Binyamin O, Fainstein N, Keller G, Ben-Hur T, & Gabizon R (2018). Autologous neural progenitor cell transplantation into newborn mice modeling for E200K genetic prion disease delays disease progression. Neurobiol Aging, 65, 192–200. 10.1016/j.neurobiolaging.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Frid K, Binyamin O, Usman A, & Gabizon R (2020). Delay of gCJD aggravation in sick TgMHu2ME199K mice by combining NPC transplantation and Nano-PSO administration. Neurobiol Aging, 95, 231–239. 10.1016/j.neurobiolaging.2020.07.030 [DOI] [PubMed] [Google Scholar]
- Friedman-Levi Y, Meiner Z, Canello T, Frid K, Kovacs GG, Budka H, Avrahami D, & Gabizon R (2011). Fatal prion disease in a mouse model of genetic E200K Creutzfeldt-Jakob disease. PLoS Pathog, 7(11), e1002350. 10.1371/journal.ppat.1002350 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Geschwind MD (2015). Prion Diseases. Continuum (Minneap Minn), 21(6 Neuroinfectious Disease), 1612–1638. 10.1212/CON.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S, Phuan PW, Perkins B, Ullman J, May BC, Cohen FE, & Prusiner SB (2007). Cell division modulates prion accumulation in cultured cells. Proc Natl Acad Sci U S A, 104(46), 17971–17976. 10.1073/pnas.0708372104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, Sutcliffe M, Boulanger J, Tripodi M, Derivery E, Paulsen O, Lakatos A, & Lancaster MA (2019). Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci, 22(4), 669–679. 10.1038/s41593-019-0350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri RK, Young R, Pitstick R, DeArmond SJ, Prusiner SB, & Carlson GA (2006). Prion infection of mouse neurospheres. Proc Natl Acad Sci U S A, 103(10), 3875–3880. 10.1073/pnas.0510902103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Armijo E, Bravo-Alegria J, Becerra-Calixto A, Mays CE, & Soto C (2018). Modeling amyloid beta and tau pathology in human cerebral organoids. Mol Psychiatry, 23(12), 2363–2374. 10.1038/s41380-018-0229-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier K, Kao J, & Diamandis P (2020). Three-dimensional modeling of human neurodegeneration: brain organoids coming of age. Mol Psychiatry, 25(2), 254–274. 10.1038/s41380-019-0500-7 [DOI] [PubMed] [Google Scholar]
- Groveman BR, Ferreira NC, Foliaki ST, Walters RO, Winkler CW, Race B, Hughson AG, Zanusso G, & Haigh CL (2021). Human cerebral organoids as a therapeutic drug screening model for Creutzfeldt-Jakob disease. Sci Rep, 11(1), 5165. 10.1038/s41598-021-84689-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groveman BR, Foliaki ST, Orru CD, Zanusso G, Carroll JA, Race B, & Haigh CL (2019). Sporadic Creutzfeldt-Jakob disease prion infection of human cerebral organoids. Acta Neuropathol Commun, 7(1), 90. 10.1186/s40478-019-0742-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groveman BR, Orrú CD, Hughson AG, Bongianni M, Fiorini M, Imperiale D, Ladogana A, Pocchiari M, Zanusso G, & Caughey B (2017). Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann Clin Transl Neurol, 4(2), 139–144. 10.1002/acn3.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh CL, McGlade AR, Lewis V, Masters CL, Lawson VA, & Collins SJ (2011). Acute exposure to prion infection induces transient oxidative stress progressing to be cumulatively deleterious with chronic propagation in vitro. Free Radic Biol Med, 51(3), 594–608. 10.1016/j.freeradbiomed.2011.03.035 [DOI] [PubMed] [Google Scholar]
- Hernández D, Rooney LA, Daniszewski M, Gulluyan L, Liang HH, Cook AL, Hewitt AW, & Pébay A (2021). Culture Variabilities of Human iPSC-Derived Cerebral Organoids Are a Major Issue for the Modelling of Phenotypes Observed in Alzheimer’s Disease. Stem Cell Rev Rep. 10.1007/s12015-021-10147-5 [DOI] [PubMed] [Google Scholar]
- Herva ME, Relaño-Ginés A, Villa A, & Torres JM (2010). Prion infection of differentiated neurospheres. J Neurosci Methods, 188(2), 270–275. 10.1016/j.jneumeth.2010.02.022 [DOI] [PubMed] [Google Scholar]
- Hill AF, Joiner S, Wadsworth JDF, Sidle KCL, Bell JE, Budka H, Ironside JW, & Collinge J (2003). Molecular classification of sporadic Creutzfeldt–Jakob disease. Brain, 126(6), 1333–1346. 10.1093/brain/awg125 [DOI] [PubMed] [Google Scholar]
- Huang WK, Wong SZH, Pather SR, Nguyen PTT, Zhang F, Zhang DY, Zhang Z, Lu L, Fang W, Chen L, Fernandes A, Su Y, Song H, & Ming GL (2021). Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell. 10.1016/j.stem.2021.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside JW, Ritchie DL, & Head MW (2017). Prion diseases. Handb Clin Neurol, 145, 393–403. 10.1016/B978-0-12-802395-2.00028-6 [DOI] [PubMed] [Google Scholar]
- Iwamaru Y, Mathiason CK, Telling GC, & Hoover EA (2017). Chronic wasting disease prion infection of differentiated neurospheres. Prion, 11(4), 277–283. 10.1080/19336896.2017.1336273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamaru Y, Takenouchi T, Imamura M, Shimizu Y, Miyazawa K, Mohri S, Yokoyama T, & Kitani H (2013). Prion replication elicits cytopathic changes in differentiated neurosphere cultures. J Virol, 87(15), 8745–8755. 10.1128/jvi.00572-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WS (2014). Selective vulnerability to neurodegenerative disease: the curious case of Prion Protein. Dis Model Mech, 7(1), 21–29. 10.1242/dmm.012146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Schnoll JG, Song H, & Ming GL (2021). Building the brain from scratch: Engineering region-specific brain organoids from human stem cells to study neural development and disease. Curr Top Dev Biol, 142, 477–530. 10.1016/bs.ctdb.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, & Sasai Y (2013). Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A, 110(50), 20284–20289. 10.1073/pnas.1315710110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar MA, Sajja RK, Prasad S, Abhyankar VV, Liles T, & Cucullo L (2017). New experimental models of the blood-brain barrier for CNS drug discovery. Expert Opin Drug Discov, 12(1), 89–103. 10.1080/17460441.2017.1253676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskaite J, & Pinheiro TJ (2005). Aggregation and fibrillization of prions in lipid membranes. Biochem Soc Symp(72), 211–222. 10.1042/bss0720211 [DOI] [PubMed] [Google Scholar]
- Kim H, Park HJ, Choi H, Chang Y, Park H, Shin J, Kim J, Lengner CJ, Lee YK, & Kim J (2019). Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Reports, 12(3), 518–531. 10.1016/j.stemcr.2019.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm HMJ, Welton JM, Masters CL, Klug GM, Boyd A, Hill AF, Collins SJ, & Lawson VA (2012). The Prion Protein Preference of Sporadic Creutzfeldt-Jakob Disease Subtypes*. Journal of Biological Chemistry, 287(43), 36465–36472. 10.1074/jbc.M112.368803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R (2020). Clinical diagnosis of human prion disease. Prog Mol Biol Transl Sci, 175, 1–18. 10.1016/bs.pmbts.2020.07.006 [DOI] [PubMed] [Google Scholar]
- Kovalevich J, & Langford D (2013). Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol, 1078, 9–21. 10.1007/978-1-62703-640-5_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krance SH, Luke R, Shenouda M, Israwi AR, Colpitts SJ, Darwish L, Strauss M, & Watts JC (2020). Cellular models for discovering prion disease therapeutics: Progress and challenges. J Neurochem, 153(2), 150–172. 10.1111/jnc.14956 [DOI] [PubMed] [Google Scholar]
- Krejciova Z, Alibhai J, Zhao C, Krencik R, Rzechorzek NM, Ullian EM, Manson J, Ironside JW, Head MW, & Chandran S (2017). Human stem cell-derived astrocytes replicate human prions in a PRNP genotype-dependent manner. J Exp Med, 214(12), 3481–3495. 10.1084/jem.20161547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladogana A, Liu Q, Xi YG, & Pocchiari M (1995). Proteinase-resistant protein in human neuroblastoma cells infected with brain material from Creutzfeldt-Jakob patient. Lancet, 345(8949), 594–595. 10.1016/s0140-6736(95)90508-1 [DOI] [PubMed] [Google Scholar]
- Lancaster MA, & Knoblich JA (2014). Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc, 9(10), 2329–2340. 10.1038/nprot.2014.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, & Knoblich JA (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501(7467), 373–379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson VA, Lumicisi B, Welton J, Machalek D, Gouramanis K, Klemm HM, Stewart JD, Masters CL, Hoke DE, Collins SJ, & Hill AF (2010). Glycosaminoglycan sulphation affects the seeded misfolding of a mutant prion protein. PLoS One, 5(8), e12351. 10.1371/journal.pone.0012351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson VA, Vella LJ, Stewart JD, Sharples RA, Klemm H, Machalek DM, Masters CL, Cappai R, Collins SJ, & Hill AF (2008). Mouse-adapted sporadic human Creutzfeldt-Jakob disease prions propagate in cell culture. Int J Biochem Cell Biol, 40(12), 2793–2801. 10.1016/j.biocel.2008.05.024 [DOI] [PubMed] [Google Scholar]
- Lewis V, Hill AF, Haigh CL, Klug GM, Masters CL, Lawson VA, & Collins SJ (2009). Increased proportions of C1 truncated prion protein protect against cellular M1000 prion infection. J Neuropathol Exp Neurol, 68(10), 1125–1135. 10.1097/NEN.0b013e3181b96981 [DOI] [PubMed] [Google Scholar]
- Linden R (2017). The Biological Function of the Prion Protein: A Cell Surface Scaffold of Signaling Modules. Front Mol Neurosci, 10, 77. 10.3389/fnmol.2017.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens F, Thune K, Tahir W, Kanata E, Diaz-Lucena D, Xanthopoulos K, Kovatsi E, Pleschka C, Garcia-Esparcia P, Schmitz M, Ozbay D, Correia S, Correia A, Milosevic I, Andreoletti O, Fernandez-Borges N, Vorberg IM, Glatzel M, Sklaviadis T, Torres JM, Krasemann S, Sanchez-Valle R, Ferrer I, & Zerr I (2017). YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol Neurodegener, 12(1), 83. 10.1186/s13024-017-0226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louey A, Hernández D, Pébay A, & Daniszewski M (2021). Automation of Organoid Cultures: Current Protocols and Applications. SLAS Discov, 26(9), 1138–1147. 10.1177/24725552211024547 [DOI] [PubMed] [Google Scholar]
- Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, Jain T, Douvaras P, Fossati V, Miller RH, & Tesar PJ (2018). Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods, 15(9), 700–706. 10.1038/s41592-018-0081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, & Pasca SP (2019). Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci, 22(3), 484–491. 10.1038/s41593-018-0316-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros-Angles A, Gayosso LM, Richaud-Patin Y, di Domenico A, Vergara C, Hervera A, Sousa A, Fernandez-Borges N, Consiglio A, Gavin R, Lopez de Maturana R, Ferrer I, Lopez de Munain A, Raya A, Castilla J, Sanchez-Pernaute R, & Del Rio JA (2018). iPS Cell Cultures from a Gerstmann-Straussler-Scheinker Patient with the Y218N PRNP Mutation Recapitulate tau Pathology. Mol Neurobiol, 55(4), 3033–3048. 10.1007/s12035-017-0506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minikel EV, Vallabh SM, Lek M, Estrada K, Samocha KE, Sathirapongsasuti JF, McLean CY, Tung JY, Yu LP, Gambetti P, Blevins J, Zhang S, Cohen Y, Chen W, Yamada M, Hamaguchi T, Sanjo N, Mizusawa H, Nakamura Y, Kitamoto T, Collins SJ, Boyd A, Will RG, Knight R, Ponto C, Zerr I, Kraus TF, Eigenbrod S, Giese A, Calero M, de Pedro-Cuesta J, Haïk S, Laplanche JL, Bouaziz-Amar E, Brandel JP, Capellari S, Parchi P, Poleggi A, Ladogana A, O’Donnell-Luria AH, Karczewski KJ, Marshall JL, Boehnke M, Laakso M, Mohlke KL, Kähler A, Chambert K, McCarroll S, Sullivan PF, Hultman CM, Purcell SM, Sklar P, van der Lee SJ, Rozemuller A, Jansen C, Hofman A, Kraaij R, van Rooij JG, Ikram MA, Uitterlinden AG, van Duijn CM, Daly MJ, & MacArthur DG (2016). Quantifying prion disease penetrance using large population control cohorts. Sci Transl Med, 8(322), 322ra329. 10.1126/scitranslmed.aad5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, & Telling GC (2017). Insights into Mechanisms of Transmission and Pathogenesis from Transgenic Mouse Models of Prion Diseases. Methods Mol Biol, 1658, 219–252. 10.1007/978-1-4939-7244-9_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, & Sasai Y (2015). Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep, 10(4), 537–550. 10.1016/j.celrep.2014.12.051 [DOI] [PubMed] [Google Scholar]
- Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, Johansen LE, van Dijk RE, Scheefhals N, Berdenis van Berlekom A, Ribes Martínez E, Kling S, MacGillavry HD, van den Berg LH, Kahn RS, Hol EM, de Witte LD, & Pasterkamp RJ (2018). Microglia innately develop within cerebral organoids. Nat Commun, 9(1), 4167. 10.1038/s41467-018-06684-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, & Caughey B (2015). Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. mBio, 6(1). 10.1128/mBio.02451-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrú CD, Hughson AG, Groveman BR, Campbell KJ, Anson KJ, Manca M, Kraus A, & Caughey B (2016). Factors That Improve RT-QuIC Detection of Prion Seeding Activity. Viruses, 8(5). 10.3390/v8050140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, Zerr I, Budka H, Kopp N, Piccardo P, Poser S, Rojiani A, Streichemberger N, Julien J, Vital C, Ghetti B, Gambetti P, & Kretzschmar H (1999). Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol, 46(2), 224–233. https://www.ncbi.nlm.nih.gov/pubmed/10443888 [PubMed] [Google Scholar]
- Pellegrini L, Bonfio C, Chadwick J, Begum F, Skehel M, & Lancaster MA (2020). Human CNS barrier-forming organoids with cerebrospinal fluid production. Science, 369(6500). 10.1126/science.aaz5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, & Waldau B (2018). Generation of human vascularized brain organoids. Neuroreport, 29(7), 588–593. 10.1097/WNR.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau H, & Sim V (2020). POSCAbilities: The Application of the Prion Organotypic Slice Culture Assay to Neurodegenerative Disease Research. Biomolecules, 10(7). 10.3390/biom10071079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau H, & Sim VL (2021). From Cell Culture to Organoids-Model Systems for Investigating Prion Strain Characteristics. Biomolecules, 11(1). 10.3390/biom11010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priola SA (2018). Cell biology of prion infection. Handb Clin Neurol, 153, 45–68. 10.1016/B978-0-444-63945-5.00003-9 [DOI] [PubMed] [Google Scholar]
- Qian L, & Tcw J (2021). Human iPSC-Based Modeling of Central Nerve System Disorders for Drug Discovery. Int J Mol Sci, 22(3). 10.3390/ijms22031203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, & Ming GL (2016). Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell, 165(5), 1238–1254. 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, Su K, Li S, Lu L, Jacob F, Nguyen PTT, Huh S, Hoke A, Swinford-Jackson SE, Wen Z, Gu X, Pierce RC, Wu H, Briand LA, Chen HI, Wolf JA, Song H, & Ming GL (2020). Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell Stem Cell, 26(5), 766–781 e769. 10.1016/j.stem.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, Seo J, & Tsai LH (2016). Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes. PLoS One, 11(9), e0161969. 10.1371/journal.pone.0161969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M, Lancaster MA, Bian S, Choi H, Ku T, Peer A, Chung K, & Knoblich JA (2017). Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J, 36(10), 1316–1329. 10.15252/embj.201694700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Mihira N, Matsuba Y, Sasaguri H, Hashimoto S, Narasimhan S, Zhang B, Murayama S, Higuchi M, Lee VMY, Trojanowski JQ, & Saido TC (2019). Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J Biol Chem, 294(34), 12754–12765. 10.1074/jbc.RA119.009487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, Jeon J, Cha Y, Kim K, Li Q, Henchcliffe C, Kaplitt M, Neff C, Rapalino O, Seo H, Lee IH, Kim J, Kim T, Petsko GA, Ritz J, Cohen BM, Kong SW, Leblanc P, Carter BS, & Kim KS (2020). Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N Engl J Med, 382(20), 1926–1932. 10.1056/NEJMoa1915872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker MR, Pietrogrande G, Martin S, Lee JH, Sun W, & Wolvetang EJ (2021). Rapid and Efficient Generation of Myelinating Human Oligodendrocytes in Organoids. Front Cell Neurosci, 15, 631548. 10.3389/fncel.2021.631548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slanzi A, Iannoto G, Rossi B, Zenaro E, & Constantin G (2020). In vitro Models of Neurodegenerative Diseases. Front Cell Dev Biol, 8, 328. 10.3389/fcell.2020.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, & Pasca SP (2017). Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron, 95(4), 779–790 e776. 10.1016/j.neuron.2017.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits LM, Reinhardt L, Reinhardt P, Glatza M, Monzel AS, Stanslowsky N, Rosato-Siri MD, Zanon A, Antony PM, Bellmann J, Nicklas SM, Hemmer K, Qing X, Berger E, Kalmbach N, Ehrlich M, Bolognin S, Hicks AA, Wegner F, Sterneckert JL, & Schwamborn JC (2019). Modeling Parkinson’s disease in midbrain-like organoids. NPJ Parkinsons Dis, 5, 5. 10.1038/s41531-019-0078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Cakir B, Xiang Y, Sullivan GJ, & Park IH (2020). Synthetic Analyses of Single-Cell Transcriptomes from Multiple Brain Organoids and Fetal Brain. Cell Rep, 30(6), 1682–1689 e1683. 10.1016/j.celrep.2020.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasnim K, & Liu J (2021). Emerging Bioelectronics for Brain Organoid Electrophysiology. J Mol Biol, 167165. 10.1016/j.jmb.2021.167165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejchman A, Znoj A, Chlebanowska P, Fraczek-Szczypta A, & Majka M (2020). Carbon Fibers as a New Type of Scaffold for Midbrain Organoid Development. Int J Mol Sci, 21(17). 10.3390/ijms21175959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling GC, Scott M, Hsiao KK, Foster D, Yang SL, Torchia M, Sidle KC, Collinge J, DeArmond SJ, & Prusiner SB (1994). Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc Natl Acad Sci U S A, 91(21), 9936–9940. 10.1073/pnas.91.21.9936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, Levin JZ, & Arlotta P (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature, 570(7762), 523–527. 10.1038/s41586-019-1289-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Lawson VA, Masters CL, Cappai R, & Hill AF (2007). Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol, 211(5), 582–590. 10.1002/path.2145 [DOI] [PubMed] [Google Scholar]
- Wälzlein JH, Schwenke KA, & Beekes M (2021). Propagation of CJD Prions in Primary Murine Glia Cells Expressing Human PrP(c). Pathogens, 10(8). 10.3390/pathogens10081060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, Khakh BS, Coppola G, Pearson CA, Yamauchi K, Gong D, Dai X, Damoiseaux R, Aliyari R, Liebscher S, Schenke-Layland K, Caneda C, Huang EJ, Zhang Y, Cheng G, Geschwind DH, Golshani P, Sun R, & Novitch BG (2017). Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep, 21(2), 517–532. 10.1016/j.celrep.2017.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser HG, Schindler K, & Zumsteg D (2006). EEG in Creutzfeldt-Jakob disease. Clin Neurophysiol, 117(5), 935–951. 10.1016/j.clinph.2005.12.007 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, Kang YJ, Zhong M, Liu X, Patra P, Lee SH, Weissman SM, & Park IH (2019). hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell, 24(3), 487–497.e487. 10.1016/j.stem.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakoub AM (2019). Cerebral organoids exhibit mature neurons and astrocytes and recapitulate electrophysiological activity of the human brain. Neural Regen Res, 14(5), 757–761. 10.4103/1673-5374.249283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Herrmann US, Falsig J, Abakumova I, Nuvolone M, Schwarz P, Frauenknecht K, Rushing EJ, & Aguzzi A (2016). A neuroprotective role for microglia in prion diseases. J Exp Med, 213(6), 1047–1059. 10.1084/jem.20151000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobeley E, Flechsig E, Cozzio A, Enari M, & Weissmann C (1999). Infectivity of scrapie prions bound to a stainless steel surface. Mol Med, 5(4), 240–243. [PMC free article] [PubMed] [Google Scholar]