Abstract
Purpose
In Eritrea, a 6-month isoniazid preventive therapy (IPT) was introduced in Eritrea in 2014 to prevent/reduce risk of incident tuberculosis in people living with HIV (PLHIV). The global and local uptake of IPT in newly enrolled PLHIV was reported to be low. Anecdotal reports showed that there was resistance from clinicians against its implementation. This study was therefore conducted to explore the factors that affect implementation of IPT in Eritrea from the perspectives of healthcare professionals.
Materials and Methods
An exploratory qualitative study that used a framework content analysis using inductive approach was employed. Data were collected from a sample of HIV care clinic prescribers from regional and national referral hospitals through in-depth interviews. Senior program officers were also interviewed as key informants. A conceptual framework model was developed using a root cause analysis.
Results
Overall, five themes and 13 sub-themes emerged from the in-depth interviews with healthcare professionals and key informants. Several multi-level causes/factors related to the healthcare system, HIV control program, healthcare professionals, patients and the product were identified as barriers to the implementation of IPT. Information gap on IPT and fear of isoniazid-induced liver injury were identified as the main reasons for the reluctance in administering IPT. It was observed that healthcare professionals had significant information gap that resulted in rumors and doubts on the benefits and risks of IPT, which ultimately caused reluctance on its implementation. Inadequate planning and operationalization during the introduction of IPT and inadequate laboratory setups were found to be the possible root causes for the aforementioned central problems.
Conclusion
The root causes/factors for the limited implementation of IPT in Eritrea were mainly related to the HIV control program and the healthcare system. Adequate planning, operationalization and capacitation of the existing laboratory setups are recommended for a successful implementation of IPT.
Keywords: isoniazid preventive therapy, tuberculosis preventive therapy, people living with HIV, implementation, limiting factors, barriers
Introduction
Successful implementation of healthcare innovations is not an easy-going process. It was shown that only a small fraction of such innovations are ever put into practice.1 Innovation can be an idea, a health intervention, technology, or practice that is perceived as new to an existing system.2 The diffusion or implementation process of an innovation encompasses a complex chain of processes that require non-stop advocacy programs, sensitization workshops, trainings and other systematic approaches to persuade users and facilitators to ensure their acceptability.2,3 Once the innovation is accepted by the implementors and users, it is then easier to ensure its successful implementation. Acceptability is an important outcome measure used to assess implementation success of an innovation.4,5 Healthcare providers’ acceptability of healthcare interventions is known to be key to success.6 Acceptability is defined as “a perception among implementation shareholders that a given treatment, service, practice or innovation is agreeable, palatable, or satisfactory”.7
Isoniazid Preventive Therapy (IPT) is one of the healthcare innovations recommended by the World Health Organization (WHO) to reduce the development of active tuberculosis (TB) in people living with HIV (PLHIV).8 As per the recommendation, eligible countries are expected to achieve at least 90% IPT coverage to ensure successful protection. Despite efforts made by member states, the global uptake of IPT in newly enrolled patients at HIV clinics was reported to be very low, ie 46%.9 Different studies conducted in different parts of the world reflect that roll out of IPT have been affected by multifaceted problems such as health system-related factors (inadequate political support, increased workload, stock-outs of IPT, inaccuracy of TB screening tools to exclude active TB, and shortage of isoniazid and tuberculin skin tests); HIV control programs-related factors (limited engagement of healthcare providers during introduction of IPT, poor integration of IPT-related services including TB/HIV collaborative activities, unclear IPT guidelines and standard operating procedures, lack of national consensus on IPT-related services, limited training, and weak monitoring and supervision); provider-related factors (perceived fear of isoniazid resistance, peer influence, negative perception on the benefits/risks of IPT, rumors and misconception about IPT); product-related factors (long duration of IPT, adverse effects); and patient-related factors (non-adherence, pill burden).10–15
Eritrea has a TB incidence rate of 67 cases per 100,000 people16 and HIV prevalence of 0.93% in the general population17 and 0.65% of pregnant women attending antenatal care services.18 When the Ministry of Health decided to programmatically introduce IPT, guidelines on its roll out was incorporated in the national “Comprehensive HIV/AIDS Care Manual”.19 Despite efforts made by the communicable disease control (CDC) program to maximize IPT uptake, the overall average implementation coverage was insufficient, 75% (range: 12–85%)20 even though it was better than the global average.9 Anecdotal reports show that there was resistance from healthcare professionals on the implementation of IPT which might be among the barriers to implementation. However, no documented information is available on the healthcare providers’ perception, acceptability and the possible influencing factors on IPT implementation. This qualitative study was, thus, conducted to explore the perspectives of healthcare providers and professionals working in the CDC program on the factors that caused limited implementation of IPT in Eritrea.
Materials and Methods
Study Design
This was an exploratory qualitative study that used a framework content analysis to explore the factors limiting the implementation of IPT in Eritrea. An in-depth interview with senior HIV care clinic prescribers were carried out between October 13, 2020 and February 22, 2021. Key informants’ interview (KII) with senior staff of the CDC division was also conducted on November 10–11, 2021.
Study Setting
This study was conducted in HIV care clinics in all Eritrea’s regional referral hospitals, namely: Hazhaz, Keren, Barentu, Assab, and Mendeferra, and Massawa hospitals and one national referral hospital, ie, Halibet national referral hospital. These clinics serve for about 65% of all PLHIV in Eritrea. All interviews were conducted in a quiet place, and there were no other participants present during the interview.
Study Participants
Purposively selected senior physicians and nurse practitioners working in the HIV care clinics at national and regional referral hospitals were enrolled for in-depth interviews. HIV care clinic prescribers (physicians and registered degree nurses) who had the longest years of working experience were included in the study. Though these were the pre-set criteria for selection, all prescribers working in the HIV care clinics of the regional referral hospitals were included as they were few in number. From Halibet National Referral Hospital, one of the two prescribers was purposively selected and interviewed. Eritrea has two national referral hospitals that have HIV care clinics, Hablibet and Orotta. Prescribers at Orotta National Referral Hospital, which had good uptake of IPT and served both adults and pediatrics, were not interviewed as we had already reached a theoretical data saturation point. Nurse practitioners and counselors who were involved only in counseling and refill of medicines were excluded from the study as they had no decision-making power in prescribing medicines. Anecdotal reports showed that implementation of IPT was poor in the majority of regional referral hospitals and that was later confirmed by a recent study.20 For this reason, priority was given to the prescribers from the regional referral hospitals. The large number of PLHIV served by regional referral hospitals and their prescribers’ potential influence on other prescribers working in the lower-level health facilities were also additional reasons. The sample size was determined by a theoretical data saturation at which no new information was gathered by additional interviews. Senior staff of the CDC of whom it was expected that they could provide adequate information on the planning and implementation efforts of IPT were also purposively selected as key informants.
Research Team and Reflexivity
MR is a pharmacist, pharmacoepidemiologist and pharmacovigilance specialist and was a PhD student during the data collection period. He works for the National Medicines and Food Administration (NMFA) of Eritrea and is a trained and experienced professional in designing, conducting and reporting qualitative studies. Though the interviewer had no affiliation with the study sites, he had a work-related relationship with some of the participants. The participants were well aware that the researcher and his colleagues were conducting the study to explore how people feel about the value of IPT in Eritrea, and to identify factors limiting implementation of IPT and possible solutions. The other authors had no direct role in the data collection process or interviews. DYBJ is an epidemiologist with experience in mixed-method research, and he is a PhD student at the Erasmus Medical Centre, the Netherlands. ST, a pharmacist working for the NMFA, is an experienced researcher in qualitative studies. BHS and KV, who supervised the research project, are pharmacoepidemiologists working for the Erasmus Medical Centre as professor and associate professor, respectively.
Data Collection Approach
A semi-structured interview guide (S1) adapted to the local context from a similar study conducted previously10 was used for the in-depth interviews. The interview guide aimed to explore the overall impression of prescribers towards the implementation of IPT, multi-level factors that could affect successful implementation of IPT, and areas of improvements.
All interviews were done by one of the researchers (MR), and all except two in-depth interviews were conducted face-to-face by following COVID-19 prevention protocols. Data from the two respondents who were not interviewed face-to-face was collected using telephone interviews as one respondent was not available during the data collection visit and the other was working about 800 kilometers away from the interviewer’s address. Prior to each interview, the interviewer introduced himself and the objectives of the study, and provided information with regard to a consent. Consent for all the interviewees was taken verbally and was audio-taped for audit trail. Appointments were made in advance with every interviewee at his/her convenience and the timing of the interview was recorded. All interviews were audio-tapped, upon consent, for full documentation and to allow quote verbatim statements or phrases. Probing questions were included in the interviewer’s guide, but additional follow-up questions were asked as appropriate. Based on the objective of the study, the questions including probes were focused on exploring the factors limiting the IPT implementation. Facial expressions, sense of confusion and reluctance of interviewees, if any, were documented by the interviewer. All interviews were successful at the first attempt and thus, there was no need for repeated interviews. Data coding was performed in parallel to the interviews. All interviews were conducted within the study sites in a local language, Tigrigna. The average time taken for each interview was about 19 minutes long (range: 10–26 minutes).
A semi-structured KII guide was self-developed based on the preliminary findings of the in-depth interviews of the prescribers. Key informants were senior professionals working at the CDC division, including HIV and TB control programs. The KII guide was mainly designed in a way to confirm claims made by the prescribers, understand the key informants’ assumption on possible causes of the problem, assess the extent of planning and efforts made in implementing IPT as well as what they would do differently to avoid problems in the future (S2). Interviews were carried out in their workplace with no other participants. All interviews were successful at the first attempt. Other procedures of data collection such as audio-taping, consent, record of the interview duration, medium of interview [language], and use of probes and follow-up questions were the same as what was followed for the in-depth interview of the prescribers.
Data Analysis
All audio records were transcribed verbatim and translated into English by nine research assistants, all pharmacists. Each transcribed and translated interview was again validated by another research assistant. Finally, each transcription and translation was approved by the lead investigator (MR). After repeated reads of the verbatim translation, data was coded and charted by MR and DBYJ. During data familiarization, important phrases or words that were related to the topic of interest were identified and coded by selecting a significant word or phrase from the interviewee’s statement. No software was used to code or manage the data. Codes were revisited several times, merged, split and modified as appropriate. Codes along with their summary notes were then charted in an Excel spreadsheet to look at patterns and ensure consistencies of data coding. Similar codes were aggregated and classified into meaningful categories. The major categories were labelled as themes and named as appropriate. Sub-categories of every theme were considered as sub-themes. To illustrate the themes and sub-themes, selected quotations are presented and each quotation is accompanied by a coded participant number. Prescribers are coded as HP1, HP2, etc., while key informants are coded as KI1, KI2, and KI3.
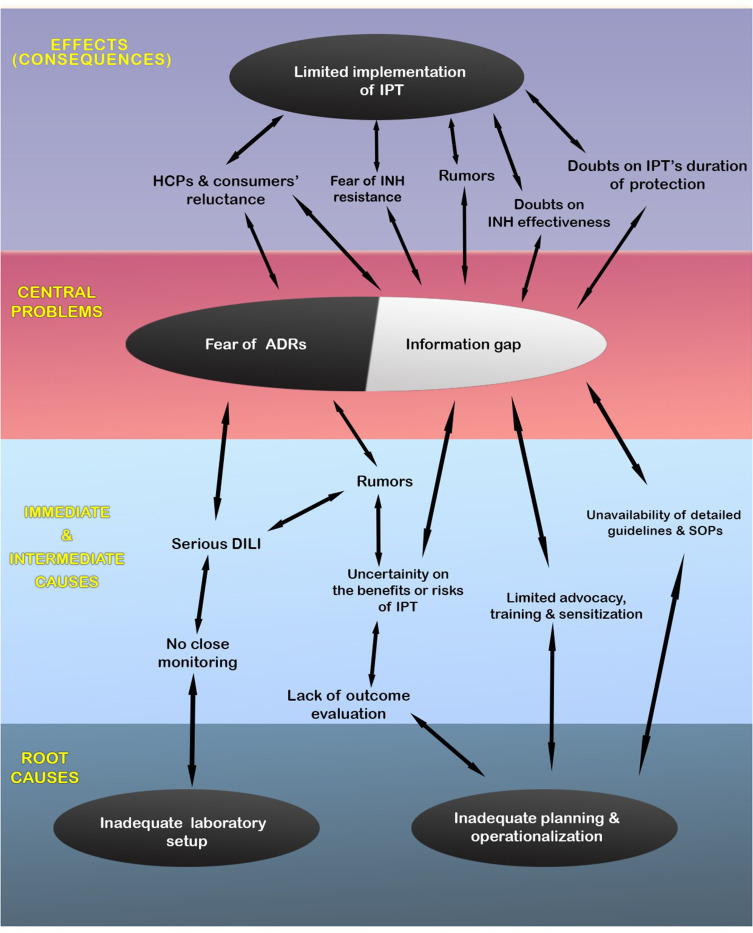
To better understand the problem, a conceptual framework model was developed using a problem tree analysis or root cause analysis.21 To do this, the existing problems were first identified from the generated themes and sub-themes. A core or central problem of the overall problem, which is expected to be the trunk of the problem tree, was identified. At the end, causes and effects (consequences) of the central problem were identified. The themes and sub-themes were identified to be the causes and/or effects of the central problem(s). Finally, a conceptual framework model was developed and designed as a problem tree diagram for better visualization (Figure 1).
Figure 1.
Conceptual framework model describing the factors that affect or limit the implementation of isoniazid preventive therapy (IPT) in Eritrea, 2021.
Abbreviations: ADRs, Adverse drug reactions; DILI, Drug-induced liver injury; HCPs, Healthcare professionals; INH, Isoniazid; SOPs, Standard Operating Procedures.
The study was reported according to the Standards for Reporting Qualitative Research (SRQR) and Consolidated Criteria for Reporting Qualitative research (COREQ).22,23 The manuscript was shared with study participants to check its appropriateness.
Results
Sociodemographic Characteristics
Overall, in-depth interviews with 11 prescribers working in HIV care clinics and three senior key informants working in the CDC Division of the Department of Public Health of the Ministry of Health were conducted. None of the approached prescribers and key informants refused to take part in the study. The demographic details of the study participants are depicted in Table 1. Theoretical data saturation was reached at the 10th prescriber and confirmed after another prescriber was interviewed, and no new information was gathered.
Table 1.
Demographic Characteristics of the Study Participants Involved in Interviews on Factors Limiting Implementation of Isoniazid Preventive Therapy in People Living with HIV in Eritrea
| Variable | Category | Frequency |
|---|---|---|
| Sex | ||
| Male | 12 | |
| Female | 2 | |
| Profession | ||
| Medical Doctors | 10 | |
| Registered Nurses | 4 | |
| Age (in years) Median: 42.5 (range: 30–67) | ||
| 30–40 | 7 | |
| 41–50 | 1 | |
| >50 | 6 | |
| Overall work experience (in years), Median: 12 (range: 3–47) | ||
| <5 | 1 | |
| 5–10 | 5 | |
| >10 | 8 | |
| Work experience in HIV care (in years), Median: 7 (range: 2–34) | ||
| <5 | 4 | |
| 5–10 | 5 | |
| >10 | 5 | |
Factors Limiting Implementation of Isoniazid Preventive Therapy in Eritrea
In an effort to identify the factors that limit implementation of IPT in PLHIV in Eritrea, five themes and 13 sub-themes emerged from the in-depth interviews and KIIs. The main themes and sub-themes are summarized in a thematic framework analysis (Figure 1). Based on the perspectives of the healthcare professionals and key informants, five multi-level factors that are related to (1) health systems, (2) the National HIV Control Program, (3) healthcare professionals, (4) the product itself, and (5) patients were reported.
Health System Related Factors
This domain captures system-related elements that are mainly associated with the then existing laboratory setup and its challenges.
Inadequacy of Laboratory Setups
Unavailability of chemistry laboratory and shortage of laboratory supplies, especially, in the regional hospitals, were among some of the frequently reported challenges for implementation of IPT. Even though the national HIV guideline did not require routine liver function tests for initiating IPT in PLHIV, due to fear of drug-induced liver injury, some of the clinicians reported that unavailability/inadequacy of chemistry laboratory test was one of the major challenges for IPT implementation. Complete dependence on clinical monitoring was also reported to be impractical, as some patients without clinical signs were found to have markedly elevated liver enzymes. Initiating IPT without having baseline information was reported to be challenging. This was reported by one of the HIV care clinic prescribers as follows:
Here we have no chemistry lab and we usually do not dare to start IPT without baseline data. Incidence of hepatitis in our zone is high and thus, it is difficult to start IPT without having baseline data. [HP1]
Another respondent from another health facility also raised the laboratory-related issue as a central problem for the poor uptake of IPT in their setting and stated it as:
… There is a need to take baseline [lab] tests. Hepatitis B & C testing and LFT are a must and you cannot put someone on IPT without those tests. But this has been a constant problem for us. I mean, hepatitis B & C testing has been unavailable for several months. … The biggest issue therefore is the laboratory. Other constraints are subjective and are not as important. If lab tests were available, there wouldn’t have been problems because we would be able to detect and explain scenarios. [HP3]
In situations where chemistry laboratory was available, intermittent stock-outs of laboratory supplies for testing hepatitis B and C, as well as liver function tests, were reported as a challenge. Accordingly, for patients that were suspected or at risk of developing isoniazid-induced liver injury, blood specimens were sent to the National Health Laboratory which was reported as “discouraging” due to delays in obtaining results.
Stock-Out of INH
Few prescribers reported that stock-outs of INH, though infrequent, as a setback in implementing IPT. Although this problem is categorized under the system-related factors, it can also be program-related as the procurement and supply chain management of the CDC have been playing a role in ensuring the availability of INH. One prescriber reported:
Our main reason for not starting IPT was mainly the unavailability of INH. Otherwise, we have no problem to start INH. [HP7]
Program-Related Factors
Using inductive approach, several limiting factors, related to the National HIV Control Program, such as inadequacy of information, lack of collaboration and ownership, inadequate preparation, and lack of outcome evaluation, were identified and labeled as “program-related factors”.
Inadequate Preparation
Implementation of the IPT without adequate planning, advocacy, engagement of healthcare professionals (HCPs), working documents such as registers, guidelines and standard operating procedures (SOPs), awareness raising activities, regular follow-ups, and so on were reported as major challenges for successful implementation of IPT. Inadequate preparation was one of the most often reported problems by prescribers. From their perspectives, all the aforementioned issues should be addressed prior to the introduction of IPT. A key informant acknowledged this as follows:
We should have prepared a good guideline before starting to implement the IPT [six months isoniazid]. … We should have collected cohort based-data until patients complete their six-month INH regimen and analyzed it as appropriate. Besides, continuous training should have been given to healthcare professionals and the consumers of IPT. Campaigns targeting consumers on the consequence/risks of TB on PLHIV and the benefits of the IPT should be have been carried out by their [PLHIV’s] association. [KI1]
The key informants also disclosed that the program did not take adequate measures in planning and implementation of the intervention. The above KI also reported the inadequate preparation as follows:
I do not believe that adequate measures were taken [by the program]. Advocacy and sensitization were not as it should be. There was not even a register and also no SOPs. The guideline was not in-depth. The program was only following whether it [isoniazid] was started; monitoring tools that can measure final outcomes were not in place. Meaning, there was no follow-up approaches. [KI1]
Information Gap
Deployment of IPT without provision of adequate information to healthcare professionals on benefits/effectiveness of INH, its risks, how a six-month IPT intake could prevent TB for several years, risk of resistance, adverse drug reaction monitoring, etc., were reported among the major factors for hesitancy. The ART clinic physicians asserted that the intervention was introduced without adequate training/sensitization to healthcare professionals.
One of the prescribers disclosed that they had been administering INH to prevent incidents of TB in children having contact with TB patients with full confidence as they had enough information on it. As for the INH preventive therapy in PLHIV, due to limited information on its clinical benefits, potential risks and how the mechanism works, the importance of its implementation was reported as doubtful. The significance of inadequate information in limiting the implementation of IPT was explained by an ART clinic physician, who was not in favor of it, as:
… If I could get someone who can tell me all the benefits and risks of implementing IPT in detail and found it convincing, I would definitely initiate it to my patients. [HP5]
The clinician also added:
This has always been in my agenda. We have pressure [from the program] to start implementing it [INH] but with the existing rumors and the limited knowledge we have, we could not dare to start IPT. If the study you have been conducting comes up with concrete answers/information that favors its implementation, we will use it. [HP5]
Lack of Collaboration and Ownership
There were claims that there was lack of coordination, collaboration, and stakeholders’ engagement and that policy-making decisions related to the introduction of IPT were made behind closed doors. Some of the stakeholders were dissatisfied with communication gaps because they were unable to get involved and did not consider themselves as part of the process. The HIV/TB collaboration was reported to be very weak and the two programs had no sense of collaboration in implementing IPT. This was considered as one of the contributory factors. One prescriber said:
… It has been forgotten by all parties. Currently, everyone does not know how to move forward as there is no unified approach. [HP6]
Some of the HIV care clinic prescribers claimed that they were not part of the planning process when IPT was introduced. It was reported that there was a rush from the program and HIV care clinics were simply instructed to implement IPT without clear guidelines and adequate orientation. One of the key informants acknowledged the prescribers claim as follows:
Most of them have no good impression on it [IPT]. Usually, as program managers, we insist on implementing IPT. We tell them that it is important for patients. When I see its acceptability even by the counselors, it seems low. They usually say, ‘if we are instructed to do so we will do’. … Thus, instead of just ordering to implement the TB preventive therapy, it would be good to involve senior ART clinic physicians prior to introduction. It is not good to simply order or impose without providing adequate information and reaching to consensus. [KI2]
Lack of Outcome Evaluation
Unavailability of evidence to evaluate the impact of the intervention in Eritrea, despite its use for many years, was also reported as a limiting factor. Some prescribers reported that the unavailability of local data on the benefits and risks of the intervention was discouraging. They had the expectation that the program could regularly evaluate the impact of the intervention so that they could build confidence in it. One of the key informants reported the limitations as follows:
In the past, except the number of people who started isoniazid, we had no information on how many people have completed the preventive therapy, how many of them experienced ADRs, discontinued treatment, experienced hepatotoxicity, and died. The information we have so far is mainly from the studies conducted recently [by the authors]. [KI1]
Healthcare Professionals’ Related Factors
Fear of ADRs, peer influence/rumors, doubts on duration of protection of IPT and effectiveness of IPT in settings with high latent TB infection, as well as fear of INH resistance and genetic polymorphism were some of the prescriber-related factors that were reported to affect the implementation of IPT.
Fear of Adverse Drug Reactions
Some healthcare professionals were not encouraged to administer INH due to fear of potential risks of ADRs. Though they personally had no patients with serious adverse effects, they had the understanding that INH could cause serious hepatic injury especially when there are no laboratory monitoring strategies. Accordingly, several prescribers disclosed that they were sometimes asking their patients if they would like to start IPT, but they had no courage to motivate them. One prescriber said:
We simply ask patients to take IPT, but we don’t motivate them because of the risks of ADRs. [HP4]
The interviews indicate that a few fatal experiences of hepatic injury had tremendous impact in creating hesitancy in several prescribers. Two prescribers reported their source of hesitancy as follows:
At this time, we all [our staff] have fear of starting IPT. We had a patient who had good adherence to his medications. One day one of our staff, who had close contact with him, met him in town and she up-front informed him about the new TB preventive therapy being introduced [isoniazid] and encouraged him to come and start it. He completely agreed and came the next day. Within one month following initiation of isoniazid, he developed severe hepatotoxicity and died within a short period of time. As a consequence, the condition was traumatizing, though it was a single experience. [HP2]
One patient died with complications of hepatic injury, yet; other patients begun to question the drug and even healthcare professionals started to have some sort of fear on deciding to whether they should put their patients on INH. This was the biggest problem in patients as well as health workers. It even influenced me. This was a big set-back to be frank. [HP3]
On the other hand, a few prescribers mentioned that INH is tolerable and incidence of hepatic injury was minimal.
Peer Influence/Rumors
A fast-spreading rumor related to a few fatal hepatic injuries was reported to be influential in intensifying healthcare professionals’ and consumers’ hesitancy on the IPT. For this reason, some prescribers reported that they had no confidence to initiate IPT with their patients and were waiting for further evidence that would help them to take informed decision. Two prescribers stated the impact of the rumors as follows:
Even recently we have been instructed by the program to start IPT but we are not encouraged with the existing rumors. [HP5]
… we heard from the HIV focal persons that there is a controversy surrounding it [INH] that put us in a limbo. So, we had to wait for further data and thus, we haven’t started with any new patients [HP6]
Another prescriber also reported that the rumor was spreading fast and reached out to patients and the Bdeho (HIV patients’) association that triggered discussions with the program.
Doubts on Duration of Protection of INH
For some prescribers, it was not clear how a six-month IPT could prevent incident TB for several years. Based on the national guidelines, some prescribers had the assumption that a six-month IPT could protect all their patients from TB for about five years. In clinical practice, observing a short-lived protection of TB in some patients was reported as discouraging/conflicting. Moreover, the fact that different studies show inconsistent lengths of duration of protection of IPT in PLHIV was described as confusing.
Doubts on IPT Effectiveness in Settings with High Latent TB Infection
Two prescribers argued that administering IPT in settings where latent TB infection is common could not be effective. They had the assumption that it might work in the developed world but not in their settings (Eritrea) as almost every HIV patient could have latent TB due to environmental contamination and poor standard of living. One prescriber stated this as follows:
There are arguments that latent TB is common in all our setting and thus, administering TPT will not be effective; maybe in developed countries. Hence, there are many healthcare professionals who do not accept it. [HP9]
Fear of INH Resistance and Genetic Polymorphism
While some of the key informants had the assumption that adverse effects of INH could be the main source of hesitancy, some prescribers voiced that the risk of antibiotic [INH] resistance was a concern. Unavailability of information on INH acetylation profile of the Eritrean population was also reported as a concern by one prescriber.
Patient-Related Factors
Reluctance of Patients and Their Association
It was reported that following observation of serious hepatic injuries in some patients, with fast spreading rumors, others started to get concerned of the adverse effects of the intervention. Pill burden was also another issue mentioned as a barrier to implementing IPT. One prescriber mentioned this as follows:
In the beginning, patients had full acceptance over the TB preventive therapy. Later, when some patients start to experience ADRs, they communicated with each other and some of them started to reject it. [HP4]
Product-Related Factors
Seriousness of ADRs /Liver Injury/Death
Serious INH-induced liver injuries, although rarely encountered, were of concern to several prescribers and they were not confident to administer INH without baseline and follow-up liver function tests and tests for hepatitis B and C markers. Some prescribers were administering INH without baseline and follow-up liver function tests, but when they observed a few life-threatening hepatic injuries including death, they were forced to discontinue the treatment in several patients. One prescriber reported:
In the beginning, in the absence of laboratory tests we used to put patients on the regimen but with the occurrence of adverse drug reactions [drug-induced liver injury] we started to worry and we realized that we could not start it without having baseline and follow-up tests. [HP2]
One of the key informants also reported that many of the HIV care clinic physicians were against the tuberculosis preventive therapy as they started to observe many previously stable patients deteriorating following initiation of INH.
Discussion
Several multi-level - immediate, intermediate, and root - causes/factors related to the healthcare system, HIV control program, healthcare professionals, patients and the product were identified as barriers to the successful implementation of IPT in Eritrea (Figure 1). Information gap on IPT and fear of drug-induced liver injury were found to be the central problems for the limited implementation of IPT in PLHIV. This study revealed that healthcare professionals had lots of information gaps that resulted in rumors and doubts on the benefits and risks of IPT which ultimately caused reluctance on its implementation.
Inadequate advocacy, unavailability of detailed working documents such as registers, guidelines and SOPs, inadequate sensitization and training, lack of regular outcome evaluation of the intervention were among the immediate/intermediate causes/factors for the information gap created which in turn limited the implementation of IPT. Failing to involve key stakeholders including prescribers in the planning and introduction of IPT could also be a source of hesitancies. The thematic analysis reflects that there were program-related problems in convincing the implementors and users to successfully implement the intervention. Taking the multi-level causes into consideration, successful implementation of IPT seems to be a complex process that requires a well-thought-out plan and operationalization. Provision of adequate knowledge on the intervention and persuasion of providers and users to help them decide on the implementation of IPT is important.2 Once an intervention is accepted by the providers and users, it is then easier to ensure its wider diffusion in the healthcare system. Identifying early adopters of the intervention such as senior clinicians/experts and working with them could also facilitate the implementation process. Overall, one of the root causes for the poor acceptability and limited implementation of IPT in Eritrea was found to be inadequate planning and preparation of the National HIV Control Program towards deployment of IPT [Figure 1]. As reported by one of the key informants, one reason for the inadequate planning/preparation was downplaying of the risks of hepatic injury related to IPT in the WHO information note on adverse events of IPT.24 The information note emphasized that INH is generally safe and routine baseline liver function tests are not recommended “given the paucity of data on its role, rarity of adverse events and absence of evidence on any real predictor for future toxicity”.
A considerably reported cause/factor that forced prescribers to be reluctant on the implementation of IPT was fear of INH-induced liver injuries. This was based on real (rarely encountered but fatal cases of hepatic injuries), perceived risks, and/or rumors. During the early days of introduction, some prescribers reported that they had some level of confidence to prescribe a six-month INH to PLHIV without taking baseline and follow-up laboratory tests. Later, when some clinicians started to experience life-threatening adverse effects that caused death in a few patients, the majority of them became reluctant to administer INH without close laboratory monitoring. Another root cause of hesitancy in administering INH was the inadequacy of laboratory setups, especially in the regional hospitals. This might be the probable reason why about 50% of PLHIV attending regional referral hospitals were not exposed to IPT as compared to the 14.3% in the Central region.20 Acknowledging the existing practicality challenges to carry out regular laboratory monitoring for every patient on IPT, strengthening laboratory setups would help clinicians to at least closely monitor at-risk patients as clinical monitoring only might not be adequate. This could ultimately build confidence on the prescribers to administer IPT and accordingly facilitate its implementation.
Studies, conducted elsewhere, that assessed the general barriers to IPT implementation reported that inaccuracy of TB screening tools to exclude active TB, shortage of isoniazid and tuberculin skin tests, lack of knowledge on the benefits of IPT, pill burden, perceived fear of isoniazid resistance due to poor adherence, poor monitoring and lack of high-level supervision of the IPT implementation, lack of coordination between TB and HIV activities, and heavy workload were among the factors that deter the roll out of IPT.10–15 Although the methods of problem analysis differ among studies, the barriers to implementing IPT identified in this study are more or less similar to what has been reported elsewhere. One of the aforementioned factors, lack/inadequate collaboration between TB/HIV activities, needs to be highlighted as it is one of the bottlenecks. Coordinated inputs such as logistical, technical, and operational would create fertile grounds for a successful implementation of IPT in Eritrea as they were doing for forecasting, procurement, and supply chain management of INH.
The findings of the study have the following important programmatic/policy implications: In future, before the introduction of any form of TB preventive therapy, non-stop advocacies, provision of adequate information through regular trainings and campaigns, as well as preparation of adequate working documents should be considered. Also, the involvement of civil societies such as PLHIV’s association and other professional societies, HIV care clinic prescribers, the national pharmacovigilance center and National TB Control Program in the planning and operationalization of the intervention would be required for a successful implementation and to ensure ownership of stakeholders. Jointly with stakeholders, setting national and regional indicators, coordination and implementation mechanisms, and a monitoring and evaluation framework are critical factors for success. Another implication is that, once a new intervention is introduced, there should be a requirement to collect cohort-based data for regular evaluation of the benefits and risks of the intervention and elucidate uncertainties by effectively disseminating findings among stakeholders. Moreover, the laboratory setups in HIV care clinics need to be capacitated in order to ensure improved uptake of TB preventive therapy. In Eritrea, the HIV control program is one of the strongest public health programs in the country that has achieved several success stories in planning, advocacy, public campaigns, implementation of new therapeutic agents, program evaluation, and fund acquisition/mobilization. Considering the program’s capacity and capability, the afore-mentioned recommendations could be achievable.
Conducting in-depth interviews with programmers and prescribers working in all regional hospitals and a national referral hospital that serve for about 60% of the PLHIV in Eritrea helped us to critically analyze and comprehensively understand the perceived central problems and root causes for the limited implementation of IPT. The study, however, was not without limitations. It was only aimed to identify implementation problems of IPT from perspectives of HCPs as resistance to implementation the TB preventive therapy was mainly reported by prescribers. Considering the perspectives of consumers would, however, be important. Besides, thoughts of the HCPs were collected after things have happened, and we have seen that some people were enthusiastic with IPT before changing their minds and others were, however, already skeptical. Thus, we are not sure of the prescribers’ perspective on IPT during its first introduction. Another limitation was that the findings of this study would not necessarily represent the situation for other countries. Moreover, the fact that the interviews were conducted by staff of the Ministry of Health of Eritrea might have led to bias in responding to some questions. Further input that could analyze the challenges of the HIV control program in addressing the issue would be important.
Conclusion
The reasons for the limited implementation of IPT in Eritrea were complex multi-level factors/causes and the core problems were mainly related to the HIV control program and the healthcare system. The authors therefore recommend ensuring adequate planning and operationalization on provision of adequate working documents, organizing regular advocacies, strengthening TB/HIV collaborative activities, sensitization and educational programs prior to deployment of a new TB preventive therapy and evaluation of the impact of the intervention. Where it is practical, ensuring the availability of biochemical tests for close laboratory monitoring of at-risk patients would minimize the fear of ADRs that warrants capacitating the laboratory setups. The program is also recommended to take immediate actions to fully understand such problems and strategically restore any confidence gap that may arise during the implementation process as appropriate.
Acknowledgments
The authors sincerely thank the study participants for their time and willingness. The authors would also like to acknowledge the staff of the National Medicines and Food Administration of Eritrea, namely: Azania Werede, Feven Ghebreberhan, Liya Abraham, Merhawi Bahta, Merhawi Debesai, Meron Tesfagaber, Michael Habteslassie, Natnael Araya, and Yodit Fitsum who participated in the transcription and translation of the interviews and for their review comments. Finally, the authors sincerely thank the Communicable Disease Control (CDC) division of the Ministry of Health of the State of Eritrea for their immense cooperation, comments and partial funding of the study.
Funding Statement
This work was funded, in part, by the CDC Division of the Ministry of Health of the State of Eritrea (Global Fund HIV Grant – activity code: 9.8) and the funding agency had no role in the design, interpretation and write-up of the article.
Abbreviations
ADRs, adverse drug reactions; ART, antiretroviral therapy; CDC, Communicable Disease Control; HCPs, healthcare professionals; INH, isoniazid; IPT, isoniazid preventive therapy; KI, key informant; KII, key informant interview; LFT, Liver function tests; NMFA, National Medicines and Food Administration; PLHIV, people living with HIV; SOPs, standard operating procedures; TB, tuberculosis; WHO, World Health Organization.
Data Sharing Statement
All information gathered is included in the manuscript and the codebook can be requested from the corresponding author (satiswt@gmail.com or m.russomghebremedhin@erasmusmc.nl).
Ethical Considerations
Ethical clearance to conduct the study was obtained from the Health Research Ethics and Protocol Review Committee of the Ministry of Health of the State of Eritrea (reference number: 7-18/2020). As obtaining written informed consent might create insecurities on some respondents and some interviews were carried out via telephone, the researchers opted to take verbal informed consent that was approved by the ethics committee. Thus, audiotaped verbal consent was taken, and all ethical and professional considerations were followed, particularly with confidentiality of the reports; only anonymized information is reported.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Katia Verhamme reports grants from Chiesi, GSK, UCB, Amgen, Pfizer-Boehringer Ingelheim, and EMA, outside the submitted work. The authors report no other potential conflicts of interest in relation to this work.
References
- 1.Haines A, Kuruvilla S, Matthias B. Bridging the implementation gap between knowledge and action for health. Bull World Health Organ. 2004;82:724–731. [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers EM. Diffusion of Innovations. 5th ed. New York: Free Press; 2003. [Google Scholar]
- 3.Dearing JW. Applying diffusion of innovation theory to intervention development. Res Soc Work Pract. 2009;19(5):503–518. doi: 10.1177/1049731509335569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters DH, Adam T, Alonge O, et al. Implementation research: what it is and how to do it. BMJ. 2013;347:f6753. doi: 10.1136/bmj.f6753 [DOI] [PubMed] [Google Scholar]
- 5.Proctor EK, Landsverk J, Aarons G, et al. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health. 2009;36:24–34. doi: 10.1007/s10488-008-0197-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17:88. doi: 10.1186/s12913-017-2031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65–76. doi: 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Guidelines for Intensified Tuberculosis Case‐finding and Isoniazid Preventive Therapy for People Living with HIV in Resource‐constrained Settings. Switzerland: WHO Geneva; 2011. [Google Scholar]
- 9.WHO Global TB Report, 2017. UNAIDS Database; 2017.
- 10.Wambiya EOA, Atela M, Eboreime E, et al. Factors affecting the acceptability of isoniazid preventive therapy among healthcare providers in selected HIV clinics in Nairobi County, Kenya: a qualitative study. BMJ Open. 2018;8:e024286. doi: 10.1136/bmjopen-2018-024286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teklay G, Teklu T, Legesse B, Tedla K, Klinkenberg E. Barriers in the implementation of isoniazid preventive therapy for people living with HIV in Northern Ethiopia: a mixed quantitative and qualitative study. BMC Public Health. 2016;16(1):840. doi: 10.1186/s12889-016-3525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makoni A, Chemhuru M, Tshimanga M, et al. Evaluation of the isoniazid preventive therapy (IPT) program in Shurugwi District, Midlands Province, Zimbabwe, January 2013 to August 2014. BMC Res Notes. 2015;8:476. doi: 10.1186/s13104-015-1451-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churchyard GJ, Chaisson RE, Maartens G, Getahun H. Tuberculosis preventive therapy: an underutilized strategy to reduce individual risk of TB and contribute to TB control. S Afr Med J. 2014;104(5):339–343. doi: 10.7196/samj.8290 [DOI] [PubMed] [Google Scholar]
- 14.Lester R, Hamilton R, Charalambous S, et al. Barriers to implementation of isoniazid preventive therapy in HIV clinics: a qualitative study. AIDS. 2010;24(Suppl 5):S45–S48. doi: 10.1097/01.aids.0000391021.18284.12 [DOI] [PubMed] [Google Scholar]
- 15.Aït-Khaled N, Alarcon E, Bissell K, et al. Isoniazid preventive therapy for people living with HIV: public health challenges and implementation issues. Int J Tuberc Lung Dis. 2009;13:927–935. [PubMed] [Google Scholar]
- 16.World Health Organization. Global tuberculosis report; 2018. Available from: https://apps.who.int/iris/handle/10665/274453. Accessed July 20, 2022.
- 17.National Statistics Office and Fafo Institute for Applied International Studies. Eritrea Population and Health Survey 2010. Asmara, Eritrea: National Statistics Office and Fafo Institute for AIS; 2013. Available from: https://www.unicef.org/eritrea/ECO_resources_populationhealthsurvey2010.pdf. Accessed July 15, 2022. [Google Scholar]
- 18.Internet. HIV/AIDS prevalence rates in Eritrea the lowest in the sub-Saharan Africa: UNAIDS; 2014. Available from: https://www.tesfanews.net/eritrea-lowest-hiv-aids-prevalence-in-sub-saharan-africa/. Accessed July 15, 2022.
- 19.Ministry of Health of Eritrea. Comprehensive HIV/AIDS Care Manual. Ministry of Health, Department of Public Health; 2017. [Google Scholar]
- 20.Russom M, Woldu GH, Berhane A, et al. Effectiveness of a 6-month isoniazid on prevention of incident tuberculosis among people living with HIV in Eritrea: a retrospective cohort study. Infect Dis Ther. 2022;11:559–579. doi: 10.1007/s40121-022-00589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Internet. Problem tree analysis. Available from: https://cio-wiki.org/wiki/Problem_Tree_Analysis. Accessed July 15, 2022.
- 22.O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245–1251. doi: 10.1097/ACM.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 23.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Adverse events associated with the use of isoniazid preventive therapy among people living with HIV. Geneva, Switzerland. Information note; 2015. Available from: https://www.who.int/tb/challenges/hiv/info_note_ipt_adverse_events.pdf?ua=1. Accessed July 15, 2022. [Google Scholar]