Abstract
Objectives
Determine the safety, feasibility and initial efficacy of a multicomponent telerehabilitation programme for COVID-19 survivors.
Design
Pilot randomised feasibility study.
Setting
In-home telerehabilitation.
Participants
44 participants (21 female, mean age 52 years) discharged home following hospitalisation with COVID-19 (with and without intensive care unit (ICU) stay).
Interventions
Participants were block randomised 2:1 to receive 12 individual biobehaviourally informed, app-facilitated, multicomponent telerehabilitation sessions with a licenced physical therapist (n=29) or to a control group (n=15) consisting of education on exercise and COVID-19 recovery trajectory, physical activity and vitals monitoring, and weekly check-ins with study staff. Interventions were 100% remote and occurred over 12 weeks.
Primary and secondary outcome measures
The primary outcome was feasibility, including safety and session adherence. Secondary outcomes included preliminary efficacy outcomes including tests of function and balance; patient-reported outcome measures; a cognitive assessment; and average daily step count. The 30 s chair stand test was the main secondary (efficacy) outcome.
Results
No adverse events (AEs) occurred during testing or in telerehabilitation sessions; 38% (11/29) of the intervention group compared with 60% (9/15) of the control group experienced an AE (p=0.21), most of which were minor, over the course of the 12-week study. 27 of 29 participants (93%; 95% CI 77% to 99%) receiving the intervention attended ≥75% of sessions. Both groups demonstrated clinically meaningful improvement in secondary outcomes with no statistically significant differences between groups.
Conclusion
Fully remote telerehabilitation was safe, feasible, had high adherence for COVID-19 recovery, and may apply to other medically complex patients including those with barriers to access care. This pilot study was designed to evaluate feasibility; further efficacy evaluation is needed.
Trial registration number
Keywords: COVID-19, REHABILITATION MEDICINE, Rehabilitation medicine
Strengths and limitations of this study.
The findings provide among the first evidence of the safety and feasibility of a biobehaviourally informed, multicomponent telerehabilitation programme for COVID-19 survivors.
A major strength and novel aspect of the study is its fully remote delivery.
The multicomponent programme allowed several interventions to be considered but may have diminished the effects of any single intervention.
A major confounding factor was that individuals in the control group had access to the Health in Motion application, including the functional tests.
The intent of this pilot study was to test safety and feasibility; the study was not powered to rigorously compare function or patient-reported outcome measures.
Introduction
COVID-19 leads to persistent impairments across many organ systems,1 with significant long-term morbidity among even mild cases.2 While acute medical management of COVID-19 has improved substantially since the early waves of the pandemic, limited evidence exists for postacute interventions or the impact of specific rehabilitation strategies on function and recovery from COVID-19.
Many healthcare organisations made a rapid shift to telehealth services during the COVID-19 pandemic, including telerehabilitation3–10 to combat barriers to delivering in-person care10 necessitated by pandemic-related public health measures to reduce infection. Physical therapist-led telerehabilitation is effective in the context of other conditions including cardiorespiratory rehabilitation, musculoskeletal rehabilitation and neurorehabilitation.9 11 However, many authors9 11–13 focused on the rapid shift away from in-person care during the COVID-19 pandemic rather than specifically studying telerehabilitation in individuals recovering from COVID-19. While others have provided practical recommendations for implementing resistance training after COVID-1914 and recovering function and fitness after severe acute respiratory distress syndrome,15 these recommendations are not based on direct evidence in COVID-19 survivors.
As of 1 January 2022, 42 trials from around the world were registered in clinicaltrials.gov using the search terms “telerehabilitation” and “COVID-19”, yet most of these studies were still in the ‘not yet recruiting’ or ‘recruiting’ stages. Only 12 studies were listed as completed, and no results were yet posted. Furthermore, these studies largely focus on other populations and care processes affected by the COVID-19 pandemic rather than patients with COVID-19 (n=5 studies) or were very small (35 or fewer participants) pilot studies (n=5 studies). One study is evaluating respiratory muscle training compared with placebo, while another is investigating aerobic exercise reconditioning using a hybrid in-person (study initiation and evaluation) and telerehabilitation model. The effects of a multicomponent telerehabilitation programme including breathing techniques, strength and cardiovascular exercise, and physical activity education in COVID-19 survivors after hospital discharge is unknown.
The purpose of this study was to determine the safety, feasibility, and initial efficacy of a multicomponent telerehabilitation programme for COVID-19 survivors. We hypothesised that the telerehabilitation programme would be feasible, as supported by safety and adherence metrics. We also hypothesised that the intervention group would experience greater improvement in physical function and patient-reported outcome measures compared with control.
Methods
Design and setting
The study was a randomised controlled trial (pilot study). The EQUATOR-recommended Consolidated Standards of Reporting Trials for pilot studies was followed.
Telerehabilitation was delivered in the home environment to adults recently hospitalised with COVID-19 in the University of Colorado hospital system (with and without intensive care unit (ICU) stay).
Participants
We intended to enrol 45 participants in the study (30 interventions, 15 controls); however, between 2 December 2020 and 2 July 2021, only 44 adults completed baseline testing and were randomised (see Results and figure 1). Participants hospitalised with COVID-19 in the University of Colorado Health system who met the following criteria were contacted to assess interest in study participation: confirmed SARS CoV-2 infection defined by positive PCR testing, completed a hospitalisation that was at least 24 hours, enrolled within 6 weeks of hospital discharge, provided informed consent, had internet capability to access the remote therapeutic monitoring platform and virtual testing sessions and were community dwelling prior to hospitalisation. Individuals were excluded for unstable medical comorbidities that would preclude participation in exercise; receipt of more than one outpatient physical therapy session after discharge; current pregnancy; and/or anticipating concurrent additional physical therapy services during the 12-week study period. The study was also advertised to local providers within the University of Colorado system and referrals from these providers or self-referrals were screened for eligibility. Initially, participants were required to be at least 40 years of age to be eligible; however, early in the study (1 February 2021), the authors changed the minimum age to 35 years due to the severity of COVID-19 observed in some younger patients. All participants provided electronic informed consent on REDCap. The trial ended when all enrolled participants reached the 12-week mark.
Figure 1.
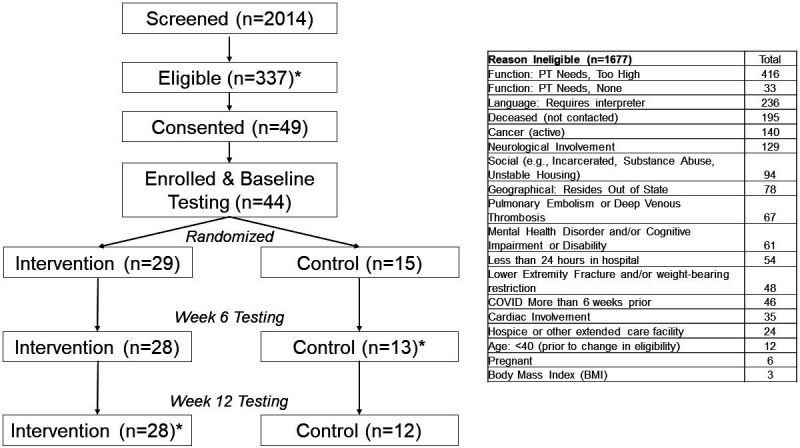
Trial flow chart reasons for ineligibility are provided in the table at the right side of the figure. If ineligibility was determined through chart review (eg, patient had active cancer or was deceased), the patient was not contacted by the study team. Five individuals signed the informed consent form but did not complete baseline testing due to already being healthy and no longer perceiving benefit (n=2), losing interest/no longer wanting to participate and being lost to follow-up. *Most common reasons for ineligibility were that the patient had physical therapy (PT) needs that were too high (n=416), the patient had language barriers (n=236), the patient was deceased (n=195), the patient had active cancer (n=140) or the patient had neurological involvement (n=129). At week 6, one participant in the control group completed patient-reported outcome measures but did not complete functional testing. At week 12, one participant in the intervention group completed patient-reported outcome measures but did not complete functional testing.
Study materials
After providing informed consent and prior to baseline evaluations and subsequent randomisation, both groups received a package of materials including an automated blood pressure cuff, pulse oximeter, Kindle Fire tablet (Amazon Inc, Seattle, Washington, USA), Fitbit Inspire 2 activity monitor (Fitbit, San Francisco, California, USA) and an equipment instruction manual. The package of materials was either shipped to the participant or delivered by study staff to the individual’s residence based on the participant’s preference and distance. The study staff preloaded the tablet with all software and unique passwords for each participant. Software included the Fitbit activity monitor portal, in which patients monitored their daily step counts and other metrics, and the Health in Motion application (Blue Marble Health, Altadena, California, USA). The Health in Motion application provided many features including physical function testing (see further), a health diary, educational lessons (prescribed to intervention only) and exercises (prescribed to intervention only).
Randomisation
Participants were block randomised to the intervention or control group using a 2:1 allocation ratio, accounting for sex, age (<55 years vs ≥55 years) and duration of mechanical ventilation (<5 days vs ≥5 days). (The 2:1 allocation ratio was selected because this was a pilot randomised trial designed primarily to assess feasibility, thus we wanted to have a higher sample size completing the intervention and greater power for feasibility (ie, safety, adherence) outcomes.) Randomisation tables were created in R V.3.6.3 (R Foundation for Statistical Computing, Vienna, Austria.) Reproducible code to generate the randomisation tables was created by MM under the direction of SM. The random allocation sequence was managed and assigned using REDCap. Elizabeth Magnan, BA, enrolled participants. Jacob J Capin, PT, DPT, PhD, MS, entered each participant’s characteristics used for randomisation (ie, age, sex and duration of mechanical ventilation) into the REDCap program designed by MM and SM, following baseline testing by MC.
Intervention and control arms
Intervention
In addition to the materials described previously, the intervention group received a second home delivery of ankle weights and resistance bands and received 12 individual sessions of telerehabilitation delivered remotely using secure, Health Insurance Portability and Accountability Act (HIPAA)-compliant video conferencing (ie, Zoom). The telerehabilitation programme incorporated breathing and clearance techniques, high-intensity strength training,16 aerobic and cardiovascular exercise, balance exercises, functional activities, stretching and biobehaviourally informed elements like lifestyle coaching and motivational interviewing (table 1). The Health in Motion application was used to facilitate the self-directed intervention outside of the supervised sessions and enable the physical therapist to monitor remotely the patient’s adherence to the app-guided exercises and education. The 12 individual, supervised telerehabilitation sessions were provided three times per week in week 1, twice per week in weeks 2–4, once per week in weeks 5–6 and a single ‘booster’ visit session during week 9 or 10. The telerehabilitation sessions were delivered by a single, licenced physical therapist (JJC) who received training in motivational interviewing by two clinical psychologists (PC and LA).
Table 1.
Multicomponent telerehabilitation programme
| Intervention category | Sample interventions | Prescription/target Intensity |
| Breathing and clearance techniques | Pursed lip, diaphragmatic, stacked, winged arm and overhead arm breathing; huffing clearance; yoga | Based on symptoms, needs and goals; often 1–5× per day for 5–15 min, incorporating into other activities as applicable |
| High-intensity strength training (8-repetition maximum) |
Sit-to-stand/squats, (single leg) heel raises (single leg) bridges, upper body rows with resistance bands, hamstring curls and side-lying hip abduction with ankle weights, etc. | 8-repetition maximum: targeting technical failure (ie, inability to complete another repetition using proper technique) on the ninth repetition (range 6–9 repetitions) |
| Aerobic/cardiovascular exercise | Walking, elliptical, cycling, rowing; includes low-intensity endurance and high-intensity interval training | Low intensity: focus on increasing duration. High intensity: focus on increasing intensity (pace, resistance) and/or number of short intervals (ranging from 10 s to 3–5 min) |
| Balance exercises | Static and dynamic balance including single leg stance, slow marching, single leg reach | Target difficulty level that achieves 50%–80% success rate |
| Functional activities | Stair climbing, return-to-work training | Based on symptoms, needs, and goals |
| Stretching | Static and dynamic stretching exercises | Based on symptoms, needs, and goals (typically 2–3 sets of 30 s per stretch) |
| Lifestyle coaching/motivational interviewing | Biobehaviourally informed programme that emphasised goal setting, self-monitoring, tailored feedback, barrier/facilitator identification, problem solving, action planning, education and encouragement; topics included physical activity, exercise, diet/nutrition, sleep and stress management | Based on symptoms, needs and goals |
The multicomponent telerehabilitation programme incorporated interventions from many different categories. The interventions that each participant received were individualised based on their impairments, functional limitations and goals.
During each telerehabilitation session, the physical therapist completed a systematic safety checklist that included assessment of vital signs (blood pressure, oxygen saturation and heart rate) and adverse events (AEs) (additional details in online supplemental appendix 1). The physical therapist also ensured the participant was wearing and syncing the Fitbit. Participants were positioned properly to promote safety, yet challenge the patient (eg, positioning the participant in a corner or by a bed during balance exercise to prevent falling to the floor). Participants were educated on vital sign monitoring, dosing exercises to the appropriate intensity (ie, high-intensity (8-repetition maximum) strength training) and other safety considerations for completing home exercises outside the individual sessions. During weeks 7–12, participants in the intervention group received weekly check-in calls from a study team research assistant who had the participant check vital signs, verified the participant was wearing and syncing the Fitbit and tracking home exercise completion and completed the AE checklist.
bmjopen-2022-061285supp001.pdf (67.1KB, pdf)
Control
The control group received no additional exercise equipment. They were provided with an educational handout describing recovery from COVID-19 including guidance on exercise precautions and safety monitoring, promotion of physical activity, sleep hygiene and cognitive health. The control group also received weekly check-in phone calls from the same research assistant. During these check-ins, the research assistant had the participant check vital signs, verified the participant was wearing and syncing the Fitbit and completed the AE checklist.
Measurements
All outcome assessments were performed by a single outcomes assessor (MC) blinded to treatment group. Individuals were tested within 6 weeks of hospital discharge (baseline), 6 weeks after baseline (week 6, primary endpoint) and 12 weeks after baseline (week 12). The primary outcome was feasibility, including safety and session adherence. Secondary outcomes included preliminary efficacy outcomes including tests of function and balance; patient-reported outcome measures; a cognitive assessment; and daily step count. The 30 s chair stand test was the main secondary (efficacy) outcome.
Primary outcome: feasibility
Feasibility was evaluated primarily by adherence and safety. Treatment fidelity and usability of the Health in Motion application were also assessed. Adherence was defined as the percentage of the 12 sessions attended. Individuals were considered adherent if they attended at least nine (75%) sessions. Adherence was measured in the intervention group only. Safety was evaluated in both groups by tracking the cumulative number of adverse events (AEs) and severe AEs from baseline through week 12. The total number of AEs, number of severe AEs and number of unique participants experiencing an AE were compared between groups. Treatment fidelity in the intervention group was assessed by video recording one treatment session for each participant. Another licenced physical therapist (AN-C) reviewed the recorded session and scored it on a comprehensive fidelity checklist. The System Usability Scale (SUS),17 a 10-item survey, was completed by only the intervention group to evaluate the Health in Motion application. Total scores range from 0 to 100, and higher scores indicate better usability. A score of 68 is considered average across a wide variety of technology-based products.18
Secondary outcomes: preliminary efficacy outcome measures
Physical function was evaluated by the 30 s chair stand test,19 the timed up-and-go test20 and the four-stage balance test. All functional tests were performed remotely and facilitated by an avatar in the Health in Motion application. A trained, blinded research assistant (MC) oversaw functional testing using secure video conferencing, verifying proper performance and recording the results. The 30 s chair stand test,19 the main secondary (efficacy) outcome measure, uses a standard height (45 cm) chair and requires participants to stand up and sit down as many times as possible in 30 s. More repetitions indicate better physical function. The timed up-and-go (TUG) test20 measures the time it takes for a person to rise from a standard height chair, walk 3 m, turn around, walk back to the chair and sit down. The faster of two, timed completions was used. Faster times indicate better physical function and lower risk of falls.21 The four-stage balance test22 measures static balance in four different standing positions (narrow base of support, semitandem, tandem and single-leg). The test requires participants to hold each position for up to 10 s. If a participant is unable to hold a position for 10 s, the next harder stage(s) is not performed. The total balance score ranges from 0 to 40 s with higher values indicating better balance and lower risk of falls.22
Patient-reported outcome measures included the Medical Research Council (MRC) Dyspnea Scale, Activities-Specific Balance Confidence (ABC) Scale,23 24 Three-Item Loneliness Scale,25 Patient Reported Outcomes Measurement and Information System (PROMIS) Short Form (SF) V.1.0 General Self-Efficacy, PROMIS Short Form (SF) Self-Efficacy for Managing Chronic Conditions, PROMIS Scale V.1.2 Global Health Measure,26 Clinical Frailty Scale (self-reported)27 and Patient Health Questionnaire 8 (PHQ8).28 The PHQ8 was added during an early amendment to the protocol (1 February 2021) due to the higher incidence of depression-like symptoms observed clinically by the study physicians (SEJ and KME).
Cognitive function was assessed by the Montreal Cognitive Assessment (MoCA)-Blind, a modified version that removed assessments requiring vision to facilitate virtual administration.29 The MoCA-Blind was administered by the same outcomes assessor (MC) at baseline and week 12. The MoCA-Blind assesses several cognitive domains: attention, concentration, memory, language, conceptual thinking, calculations and orientation. Scores range from 0 to 22, with 18 and above considered non-impaired.29
Average daily step counts were determined using data from the Fitbit activity monitors.
Statistical analysis
Descriptive statistics were used to summarise participant demographics and baseline characteristics. Differences between groups at baseline were compared using the χ2 test for categorical variables and an independent sample t-test for continuous variables. Mixed models were used to analyse changes over time for all functional and patient-reported outcome measures. In each model, the outcome was the change from baseline for the relevant assessment, and all models were adjusted for baseline characteristics that might affect performance including treatment arm, study visit, age, sex, BMI, duration of stay in the hospital and a participant’s total number of comorbidities. As this was a feasibility study with no single primary outcome, we did not adjust for multiple comparisons.30 A p value of <0.05 was considered statistically significant. We performed mixed modelling using SAS/STAT software (V.9.4 SAS Institute Inc), and we used R software (https://www.R-project.org/) for all summary statistics and graphics.
Sample size calculation
For feasibility, we estimated the proportion of participants who adhered to the intervention to be ≥50%. Assuming these rates, a sample size of 30 participants in the intervention group and a two-sided 95% CI provides a maximum margin of error of 18% for adherence. Fifteen participants were intended for the control group for a total sample of 45.
Role of the funding source
The funding sources played no role in the design of the study; the collection, analysis, interpretation or reporting of data; or in the writing of the manuscript.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research.
Results
Participants
Of 49 consented individuals, 44 completed baseline testing and were randomised (figure 1). The last enrolled participant was lost to follow-up prior to baseline testing and randomisation. Given that screening had ceased for approximately 1 month and COVID-19 numbers were very low in the region at that time, the authors decided to terminate the study with the 44 participants who completed baseline testing and were randomised.
Among 44 enrolled participants (figure 1), the mean age was 52 (SD 10) years, and 21 (48%) were females. The mean hospital stay was 6 (SD 6) days, and the mean number of comorbidities was 4 (SD 2). Ten participants (23%) were admitted to the ICU during their hospital stay for COVID-19. There were no significant differences in baseline characteristics between the intervention and control groups (table 2, restricted to participants with week 6 assessments). Compared with the 41 completing the week 6 assessments, the three participants who did not complete testing at week 6 had more comorbidities (mean 7 (SD 2) vs 3 SD (2); p=0.01), were all female (100% vs 44%; p=0.06), tended to be younger (mean 43 (SD 6) vs 53 (10); p=0.12) and had poorer ABC Scale scores (mean 56 (SD 36) vs 81 (22); p=0.07) at baseline. Two of the three individuals who did not complete week 6 testing were in the control group (p=0.22), and three of the four who did not complete week 12 testing were in the control group (p=0.07).
Table 2.
Demographic, functional testing and survey results by treatment arm for participants with available data at week 6 (primary endpoint)
| Control (n=13) | Intervention (n=28) | Total (n=41) | P value | |
| Age, years | 54 (10) | 52 (10) | 53 (10) | 0.75 |
| Sex, n (%) | 0.63 | |||
| Female | 5 (38) | 13 (46) | 18 (44) | |
| Male | 8 (62) | 15 (54) | 23 (56) | |
| BMI (kg/m2) | 36 (11) | 34 (9) | 34 (10) | 0.54 |
| Race | 0.44 | |||
| Black or African-American | 1 (8) | 5 (18) | 6 (15) | |
| White | 7 (54) | 17 (61) | 24 (59) | |
| Other or multiracial | 5 (38) | 6 (21) | 11 (27) | |
| Ethnicity, n (%) | 0.11 | |||
| Hispanic or Latino | 6 (46) | 6 (21) | 12 (29) | |
| Not Hispanic or Latino | 7 (54) | 22 (79) | 29 (71) | |
| Hospital stay (days) | 8 (9) | 5 (3) | 6 (6) | 0.10 |
| Admitted into the hospital ICU, n (%) | 0.49 | |||
| Yes | 2 (15) | 7 (25) | 9 (22) | |
| No | 11 (85) | 21 (75) | 32 (78) | |
| Functional tests | ||||
| 30 s chair stand | 11 (3) | 12 (3) | 12 (3) | 0.73 |
| Timed up-and-go (TUG) | 9 (3) | 10 (3) | 9 (3) | 0.79 |
| Total 4-stage balance | 37 (5) | 37 (5) | 37 (5) | 0.92 |
| Patient survey results | ||||
| MRC Dyspnoea Score | 3 (1) | 3 (1)* | 3 (1) | 0.43 |
| ABC Score | 76 (26) | 84 (20) | 81 (22) | 0.28 |
| 3-item Loneliness Score | 4 (1) | 5 (2) | 4 (2) | 0.51 |
| PROMIS Self-Efficacy | 17 (3) | 17 (4) | 17 (4) | 0.95 |
| PROMIS Managing Chronic Conditions |
32 (8) | 32 (7) | 32 (7) | 0.99 |
| PROMIS Global Physical Health T-Score |
41 (6) | 43 (8) | 42 (7) | 0.52 |
| Clinical Frailty Score | 3 (1) | 3 (1) | 3 (1) | 0.97 |
| PHQ8 Score | 6 (4) | 8 (6)* | 8 (5) | 0.37 |
| MoCA-Blind Score | 19 (2) | 19 (2) | 19 (2) | 0.70 |
Data are presented as n (%) or mean (SD).
*Missing data at baseline: MRC dyspnoea (intervention n=27); PHQ8 Score (intervention n=24).
ABC, Activities-Specific Balance Confidence; ICU, intensive care unit; MoCA, Montreal Cognitive Assessment; MRC, Medical Research Council; PHQ8, Patient Health Questionnaire 8; PROMIS, Patient Reported Outcomes Measurement and Information System.
Feasibility
Of the 29 participants randomised to the intervention, 24 completed all 12 telerehabilitation sessions. One individual was lost to follow-up after the baseline evaluation. The other four individuals completed 10 (83%), 10 (83%), 9 (75%) and 8 (67%) telerehabilitation sessions. Twenty-seven of 29 participants (93%, 95% CI 77%, 99%) in the intervention group met the threshold of at least 75% adherence. Treatment fidelity was 99% among 23 assessed participants.
There were no deaths or life-threatening AEs in either group. No AEs occurred during testing or any of the telerehabilitation sessions. From baseline to week 12, there was one hospitalisation (severe AE) that occurred in a control participant 5 weeks after enrolment. There were 29 total AEs (17 moderate and 12 minor) among 11 different individuals in the intervention group. In the control group, a total of 17 AEs (1 severe, 4 moderate and 12 minor) occurred in nine individuals. The proportion of individuals who experienced any AE was smaller in the intervention group compared with the control group (38% vs 60%, p=0.21).
Among 26 (93%) intervention participants who completed the SUS survey, the median (IQR) score was 72 (61, 75), which compares favourably to the industry average of 68.17 18 There were no significant differences by sex (female: mean 64 (SD 14) vs male: 71 (15); p=0.25) or by age category (<55 years: mean 67 (SD 16) versus ≥55 years: mean 69 (13); p=0.79).
Physical function, patient-reported outcomes, cognitive function and step counts
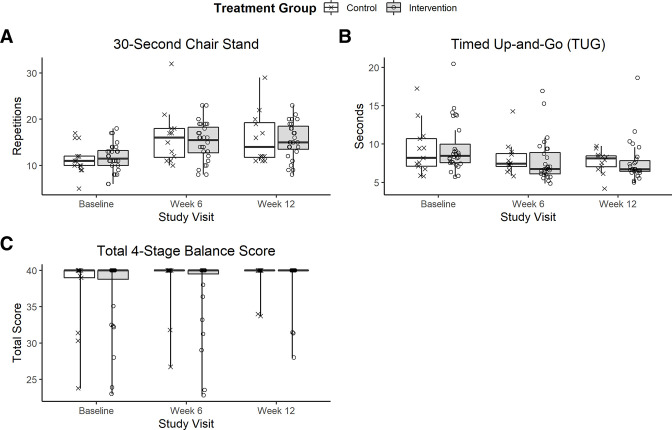
The distribution of the functional outcomes at baseline, week 6 and week 12 are shown in figure 2A-C, while mixed model results are listed in table 3. Average results on the 30 s chair stand test improved among both groups from baseline to week 6 and 12. The control group tended to have greater improvement than the intervention group with an average of 1.9 additional repetitions (p=0.06; table 3). Average times on the TUG test improved by <2 s for both groups. There were no significant differences in the TUG test changes over time between groups (p=0.21; table 3). Most (71%) participants scored perfectly on the four-stage balance test at baseline, with no significant differences in the changes over time between groups (p=0.90; table 3). On the other patient-reported outcome measures and the MoCA-Blind test, there were no significant between group differences at baseline (table 2), nor were there any significant differences in how the scores changed over time (table 3). Estimated step counts increased 56.3 (43.7, 68.8) steps/day through week 6 (p<0.001), which plateaued (p<0.001) after week 6 to an increase of 6.0 (−6.4, 18.4) steps/day. There were no significant differences in the total steps trajectory between groups (p=0.28).
Figure 2.
The distribution of the functional outcomes at baseline, week 6 and week 12 for the 30 s chair stand test (A), timed up-and-go (TUG) test (B) and four-stage balance test (C).
Table 3.
Model results showing change from baseline for functional performance and patient-reported outcomes at week 6 and week 12
| Outcome variable | Week | Estimated change from baseline* (95% CI), p value† | P value for difference between groups | |
| Intervention | Control | |||
| 30 s chair stand (repetitions) |
6 | 3.1 (1.7 to 4.5)‡ | 5.0 (3.1 to 6.9)‡ | P=0.06 |
| 12 | 3.2 (1.8 to 4.6)‡ | 5.1 (3.2 to 7.0)‡ | ||
| Timed up-and-go (seconds) |
6 | −1.7 (−2.9 to −0.5)§ | −0.6 (−2.3 to 1.0) | P=0.21 |
| 12 | −1.9 (−3.1 to −0.7)§ | −0.8 (−2.5 to 0.9) | ||
| Total four-stage balance (range: 0–40) | 6 | 1.8 (−0.1 to 3.6) | 1.6 (−0.9 to 4.1) | P=0.90 |
| 12 | 2.9 (1.1 to 4.7)§ | 2.7 (0.3 to 5.2)¶ | ||
| MRC Dyspnoea (range: 0–5) |
6 | −1.3 (−2.0 to −0.7)‡ | −1.2 (−2.1 to −0.3)§ | P=0.84 |
| 12 | −1.5 (−2.1 to −0.8)‡ | −1.4 (−2.2 to –0.5)§ | ||
| ABC Score (range: 0–100) |
6 | 7.1 (−0.2 to 14.3) | 11.3 (1.6 to 21.1)¶ | P=0.41 |
| 12 | 10.0 (2.7 to 17.3)§ | 14.2 (4.5 to 24.0)§ | ||
| Three-Item Loneliness (range 3–9) | 6 | −0.4 (−1.1 to 0.3) | 0.3 (−0.6 to 1.2) | P=0.17 |
| 12 | −0.8 (−1.5 to –0.1)¶ | −0.1 (−1.1 to 0.8) | ||
| PROMIS General Self-Efficacy (range: 4–20) |
6 | −0.3 (−1.9 to 1.3) | −0.2 (−2.3 to 1.9) | P=0.92 |
| 12 | 0.1 (−1.5 to 1.7) | 0.2 (−1.9 to 2.3) | ||
| PROMIS Self-Efficacy for Managing Chronic Conditions (range: 8–40) |
6 | 2.2 (−1.4 to 5.8) | 2.7 (−2.0 to 7.5) | P=0.82 |
| 12 | 3.9 (0.2 to 7.5)¶ | 4.4 (−0.4 to 9.2) | ||
| PROMIS Scale V.1.2 Global Health (t-score range 23–63) |
6 | 0.5 (−2.8 to 3.8) | 2.1 (−2.3 to 6.5) | P=0.50 |
| 12 | 3.0 (−0.3 to 6.3) | 4.6 (0.1 to 9.0)¶ | ||
| Clinical Frailty Scale (range: 1–9) |
6 | −0.5 (−0.8 to −0.1)¶ | −0.4 (−0.9 to 0.2) | P=0.66 |
| 12 | −0.8 (−1.2 to −0.4)‡ | −0.7 (−1.2 to −0.2)§ | ||
| Patient Health Questionnaire PHQ-8 (range: 0–24) |
6 | −3.8 (−5.8 to −1.8)‡ | −1.9 (−4.4 to 0.6) | P=0.17 |
| 12 | −5.0 (−7.0 to −2.9)‡ | −3.1 (−5.6 to −0.5)¶ | ||
| MoCA-Blind Score (range: 0–22) |
6 | Not assessed at week 6 | P=0.28 | |
| 12 | 1.1 (0.3 to 2.0)¶ | 0.5 (−0.7 to 1.6) | ||
*All models adjusted for treatment arm, visit, gender, age, BMI, duration of hospital stay and comorbidity index. The estimated change is based on the study population averages of male, age 53, BMI of 33, 5 days in the hospital and three comorbidities.
†P values: no symbol indicates p>0.05.
‡≤0.001.
§≤0.01.
¶P≤0.05.
ABC, Activities-Specific Balance Confidence; MoCA, Montreal Cognitive Assessment; MRC, Medical Research Council.
Discussion
Our findings provide among the first evidence that a biobehaviourally informed, multicomponent telerehabilitation programme for COVID-19 survivors is safe and feasible. Notably, participants in both groups improved functionally from baseline to 6 weeks and 12 weeks postintervention. Individual telerehabilitation sessions, however, may offer limited benefit beyond education on exercise and recovery from COVID-19, monitoring of vital signs and physical activity and weekly virtual check-ins.
A major strength and novel aspect of the study is its fully remote delivery including vital signs and safety monitoring, individual telerehabilitation sessions and functional data collections. Telerehabilitation has not been as widely implemented as telemedicine because of the need for hands-on interventions and concerns regarding safety during activities.31 To our knowledge, this is the first study to thoroughly describe fully remote delivery of rehabilitation to an acute, medically complex patient population. Prior work suggests that combined in-person and telehealth pulmonary rehabilitation with chronic disease is feasible and effective.32–36 However, these studies performed initial tasks in person, including the physical therapist evaluation, orientation to the programme and instruction for technology use and set-up. In-person initial evaluations were not possible during early phases of the COVID-19 pandemic due to personal quarantine requirements, fear of exposure, limited availability of personal protective equipment and closure of outpatient and other rehabilitation services, among other factors. The current study overcame these barriers with fully remote telerehabilitation services. While the intervention programme itself may benefit from refinement, the delivery of fully remote telerehabilitation has implications for patients recovering from COVID-19 and other medically complex patients lacking access to standard rehabilitative services (eg, due to distance, lack of transportation, limited availability of resources and/or poor mobility). Developing safe, feasible and effective telerehabilitation programmes as alternatives to standard rehabilitation for medically complex populations could transform the way in which acute rehabilitation and posthospital care is delivered.
Another unique element of our intervention is the biobehavioural emphasis. The use of theory-based behaviour change has improved adherence and other outcomes in patients with chronic health conditions37–40 and in pulmonary rehabilitation.41–43 While behaviour change interventions are supported by the American Thoracic Society/European Respiratory Society,44 their effect on improving adherence and quality of care in patients following COVID-19 related hospitalisation is poorly understood. This study found very high adherence among the intervention group, including 93% at the 75% threshold and 83% being 100% adherent. Pulmonary studies incorporating theory-based biobehavioural interventions have shown improved adherence to care plans and maintenance of exercise behaviours.40 45 46 Continued follow-up in the present study is needed to determine whether the biobehavioural principles lead to long-term group differences in adherence and function.
The multicomponent programme allowed several interventions to be considered and evaluated in the virtual setting in individuals after COVID-19 hospitalisation but may have diminished the effects of any single intervention. While multicomponent telerehabilitation programmes have been proposed,10 47 48 to our knowledge, no published study has tested a supervised multicomponent telerehabilitation programme versus an active control group in individuals after COVID-19. Given our wide variety of potential interventions (table 1), substantial differences in impairments following COVID-19,1 49 and the biobehavioural approach of our study valuing participants’ autonomy and goals, the treatment programmes varied substantially among participants. Investigating the use of a treatment algorithm to prioritise care based on disease severity and interventions based on impairments may also facilitate our understanding of optimising rehabilitation strategies for patients recovering from COVID-19. Notably, individuals in both treatment groups improved substantially in our study, suggesting that engaging patients in a post-COVID-19 hospitalisation programme—regardless the precise mode of delivery—may be beneficial.
There are several limitations of the study. Participants enrolled in the study were not receiving outpatient physical therapy and were not discharged to a rehabilitation facility; we may have observed a greater difference between groups in a more impaired population. The intent of this pilot study was to test safety and feasibility; the study was not powered to rigorously compare function or patient-reported outcome measures. The long-term outcomes of the biobehavioural telerehabilitation programme is unknown. The study was not designed to treat symptoms of postacute sequela of COVID-19; variability in participant outcomes may have been impacted by the presence or absence of ongoing COVID-19 related symptoms. While neither the participants nor treating therapist could be blinded to treatment group, all assessments were performed by a single, trained outcomes assessor. The remote evaluation of outcomes adds to the study’s novelty, but comparison with in-person evaluations in this patient population was not possible. Furthermore, only a fraction of participants hospitalised with COVID-19 were enrolled in the study (figure 1); thus, the generalisability may be limited. Translating the study into routine clinical care may face barriers including costs, as the equipment package alone costed approximately $250 per participant. Finally, a major confounding factor was that individuals in the control group had access to the Health in Motion application including the functional tests (eg, 30 s chair stand test). While additional exercises were not prescribed, data use records indicate that several individuals in the control group practised the functional tests repeatedly during the study. In contrast, individuals in the intervention group were specifically instructed not to practice the tests and instead use a variety of exercises for strength and power, using proper technique (ie, slow and controlled).
In conclusion, a biobehaviourally informed multicomponent telerehabilitation programme appears to be safe and feasible but may offer limited benefit over education, physical activity and vital sign monitoring, and weekly check-ins, over the course of 12 weeks following hospitalisation due to COVID-19. The delivery of fully remote rehabilitation may apply to other medically complex patient populations as well as those who have barriers to accessing in-person care. Future research is needed to test long-term effects of a biobehaviourally informed programme, compare improvement through different types of interventions and explore the unique aspects that may or may not provide benefits for patients experiencing postacute sequela of COVID-19.
Supplementary Material
Acknowledgments
The authors would like to thank clinical psychologists Paul Cook, PhD, and Laurra Aagaard, MA, MS, for their training in motivational interviewing and consultations for designing a biobehaviourally informed treatment programme. The authors also thank Larissa Pisney, MD, for her assistance with patient referral and recruitment, and Maggie Givan for her assistance with institutional review board approvals and her help with clinicaltrials.gov, and Elizabeth Magnan for her assistance with coordinating various aspects of the study. The authors are grateful for the research participants who took part in this research study. The study was funded by NIH/NIA R01 AG 054366-05S1 (KME (PI) and JES-L, Co-PI). JJC was supported by NIH/NIA F32-AG066274, an Advanced Geriatrics Fellowship from the US Department of Veterans Affairs Geriatric Research Education and Clinical Center, and an Academy of Orthopedic Physical Therapy Career Development Award. SEJ was supported by NIH/NIAAA K23 AA 026315-05. MR was supported in part by a Promotion of Doctoral Studies I Scholarship from the Foundation for Physical Therapy Research and by NIH Research Training Grant NIH/NIA T32-AG000279. This project was supported by Health Data Compass Data Warehouse (healthdatacompass.org). REDCap Database is supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535.
Footnotes
Twitter: @JacobCapin
Contributors: JJC contributed to conception and design; data acquisition, analysis, and interpretation; and drafting and revising the manuscript. SEJ contributed to conception and design, interpretation, and revising the manuscript. MM contributed to data analysis, interpretation, and drafting and revising the manuscript. MC and KH contributed to data acquisition and revising the manuscript. SM contributed to the design, data analysis, interpretation, and revising the manuscript. ANC contributed to conception and design, data acquisition, interpretation, and revising the manuscript. MR contributed to conception and design, interpretation, and revising the manuscript. SF contributed to conception and design, data acquisition, interpretation, and revising the manuscript. JESL contributed to conception and design, interpretation, and revising the manuscript. KME contributed to conception and design, interpretation, and revising the manuscript. All authors approved the final, submitted version of the manuscript and agree to be accountable for all aspects of the work. KME serves as the guarantor of the study and accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This work was supported by NIH/NIA R01 AG 054366-05S1 (KME, PI, and JES-L, Co-PI). JJC was supported by NIH/NIA F32-AG066274, Advanced Geriatrics Fellowship from the US Department of Veterans Affairs Geriatric Research Education and Clinical Center, and Academy of Orthopedic Physical Therapy Career Development Award. SEJ was supported by NIH/NIAAA K23 AA 026315-05. MR was supported in part by a Promotion of Doctoral Studies I Scholarship from the Foundation for Physical Therapy Research and by NIH Research Training Grant NIH/NIA T32-AG000279. REDCap Database is supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535.
Disclaimer: The funding sources played no role in the design of the study; the collection, analysis, interpretation or reporting of data; or in the writing of the manuscript. The contents are the authors’ sole responsibility and do not necessarily represent the official views of the funding sources.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. On written request of the study team and an established data use agreement between the University of Colorado Anschutz Medical Campus and the requestor’s institution, we will provide a deidentified dataset including a data dictionary. Deidentification of the datasets will be conducted with respect to HIPAA definitions, with add back of variables that express all dates as number of days since a milestone event, enrolment and a variable storing just the year. For example, the milestone event would be 'Day 0' in this case. A deidentified dataset is not subject to HIPAA’s minimum necessary standards, so all data can be included and shared after the primary manuscript is published. Other related study documents (eg, study protocol, statistical analysis plan, etc) will be made available on reasonable written request. The main study results will be posted in ClinicalTrials.gov, per published guidelines, and will remain there indefinitely.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Colorado Multiple Institutional Review Board: COMIRB 20-2415. Participants gave informed consent to participate in the study before taking part.
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021;27:601–15. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsend L, Dowds J, O'Brien K, et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc 2021;18:997–1003. 10.1513/AnnalsATS.202009-1175OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negrini S, Donzelli S, Negrini A, et al. Feasibility and acceptability of telemedicine to substitute outpatient rehabilitation services in the COVID-19 emergency in Italy: an observational everyday Clinical-Life study. Arch Phys Med Rehabil 2020;101:2027–32. 10.1016/j.apmr.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salawu A, Green A, Crooks MG, et al. A proposal for multidisciplinary tele-rehabilitation in the assessment and rehabilitation of COVID-19 survivors. Int J Environ Res Public Health 2020;17. 10.3390/ijerph17134890. [Epub ahead of print: 07 07 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leite VF, Rampim DB, Jorge VC, et al. Persistent symptoms and disability after COVID-19 hospitalization: data from a comprehensive telerehabilitation program. Arch Phys Med Rehabil 2021;102:1308–16. 10.1016/j.apmr.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podury A, Raefsky SM, Dodakian L, et al. Social network structure is related to functional improvement from home-based telerehabilitation after stroke. Front Neurol 2021;12:603767. 10.3389/fneur.2021.603767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis A, Knight E, Bland M, et al. Feasibility of an online platform delivery of pulmonary rehabilitation for individuals with chronic respiratory disease. BMJ Open Respir Res 2021;8:e000880. 10.1136/bmjresp-2021-000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukaino M, Tatemoto T, Kumazawa N, et al. Staying active in isolation: telerehabilitation for individuals with the severe acute respiratory syndrome coronavirus 2 infection. Am J Phys Med Rehabil 2020;99:478–9. 10.1097/PHM.0000000000001441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suso-Martí L, La Touche R, Herranz-Gómez A, et al. Effectiveness of telerehabilitation in physical therapist practice: an umbrella and mapping review with Meta-Meta-Analysis. Phys Ther 2021;101. 10.1093/ptj/pzab075. [Epub ahead of print: 04 05 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtz J, Mazariegos J, Adeyemo J, et al. Responding to an emerging need: implementing telehealth in acute hospital rehabilitation. Arch Phys Med Rehabil 2021;102:1840–7. 10.1016/j.apmr.2021.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seron P, Oliveros M-J, Gutierrez-Arias R, et al. Effectiveness of telerehabilitation in physical therapy: a rapid overview. Phys Ther 2021;101. 10.1093/ptj/pzab053. [Epub ahead of print: 01 06 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincenzo JL, Hergott C, Schrodt L, et al. Capitalizing on virtual delivery of community programs to support health and well-being of older adults. Phys Ther 2021;101. 10.1093/ptj/pzab001. [Epub ahead of print: 04 04 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turolla A, Rossettini G, Viceconti A, et al. Musculoskeletal physical therapy during the COVID-19 pandemic: is telerehabilitation the answer? Phys Ther 2020;100:1260–4. 10.1093/ptj/pzaa093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentil P, de Lira CAB, Coswig V, et al. Practical recommendations relevant to the use of resistance training for COVID-19 survivors. Front Physiol 2021;12:637590. 10.3389/fphys.2021.637590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooney S, Webster A, Paul L. Systematic review of changes and recovery in physical function and fitness after severe acute respiratory syndrome-related coronavirus infection: implications for COVID-19 rehabilitation. Phys Ther 2020;100:1717–29. 10.1093/ptj/pzaa129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustavson AM, Malone DJ, Boxer RS, et al. Application of high-intensity functional resistance training in a skilled nursing facility: an implementation study. Phys Ther 2020;100:1746–58. 10.1093/ptj/pzaa126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int J Hum Comput Interact 2008;24:574–94. 10.1080/10447310802205776 [DOI] [Google Scholar]
- 18.System usability scale (Sus), 2021. Available: https://www.usability.gov/how-to-and-tools/methods/system-usability-scale.html [Accessed 16 Nov 2021].
- 19.Jones CJ, Rikli RE, Beam WC. A 30-S chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113–9. 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- 20.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 21.Herman T, Giladi N, Hausdorff JM. Properties of the 'timed up and go' test: more than meets the eye. Gerontology 2011;57:203–10. 10.1159/000314963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phelan EA, Mahoney JE, Voit JC, et al. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015;99:281–93. 10.1016/j.mcna.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers AM, Fletcher PC, Myers AH, et al. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci 1998;53:M287–94. 10.1093/gerona/53A.4.M287 [DOI] [PubMed] [Google Scholar]
- 24.Portegijs E, Edgren J, Salpakoski A, et al. Balance confidence was associated with mobility and balance performance in older people with fall-related hip fracture: a cross-sectional study. Arch Phys Med Rehabil 2012;93:2340–6. 10.1016/j.apmr.2012.05.022 [DOI] [PubMed] [Google Scholar]
- 25.Hughes ME, Waite LJ, Hawkley LC, et al. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging 2004;26:655–72. 10.1177/0164027504268574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salsman JM, Schalet BD, Merluzzi TV, et al. Calibration and initial validation of a general self-efficacy item bank and short form for the NIH PROMIS®. Qual Life Res 2019;28:2513–23. 10.1007/s11136-019-02198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–73. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 29.Pendlebury ST, Welch SJV, Cuthbertson FC, et al. Telephone assessment of cognition after transient ischemic attack and stroke: modified telephone interview of cognitive status and telephone Montreal cognitive assessment versus face-to-face montreal cognitive assessment and neuropsychological battery. Stroke 2013;44:227–9. 10.1161/STROKEAHA.112.673384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 31.Laver KE, Adey-Wakeling Z, Crotty M, et al. Telerehabilitation services for stroke. Cochrane Database Syst Rev 2020;1:Cd010255. 10.1002/14651858.CD010255.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moss M, Nordon-Craft A, Malone D, et al. A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. Am J Respir Crit Care Med 2016;193:1101–10. 10.1164/rccm.201505-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris PE, Berry MJ, Files DC, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure. JAMA 2016;315:2694–702. 10.1001/jama.2016.7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDowell K, O'Neill B, Blackwood B, et al. Effectiveness of an exercise programme on physical function in patients discharged from hospital following critical illness: a randomised controlled trial (the revive trial). Thorax 2017;72:594.1–5. 10.1136/thoraxjnl-2016-208723 [DOI] [PubMed] [Google Scholar]
- 35.Denehy L, Skinner EH, Edbrooke L, et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care 2013;17:R156. 10.1186/cc12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh M-J, Lee W-C, Cho H-Y, et al. Recovery of pulmonary functions, exercise capacity, and quality of life after pulmonary rehabilitation in survivors of ARDS due to severe influenza A (H1N1) pneumonitis. Influenza Other Respir Viruses 2018;12:643–8. 10.1111/irv.12566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310:57–65. 10.1001/jama.2013.7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanaboni P, Hoaas H, Aarøen Lien L, et al. Long-term exercise maintenance in COPD via telerehabilitation: a two-year pilot study. J Telemed Telecare 2017;23:74–82. 10.1177/1357633X15625545 [DOI] [PubMed] [Google Scholar]
- 39.Lorig K, Ritter PL, Laurent DD, et al. Online diabetes self-management program: a randomized study. Diabetes Care 2010;33:1275–81. 10.2337/dc09-2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoaas H, Andreassen HK, Lien LA, et al. Adherence and factors affecting satisfaction in long-term telerehabilitation for patients with chronic obstructive pulmonary disease: a mixed methods study. BMC Med Inform Decis Mak 2016;16:26. 10.1186/s12911-016-0264-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabak M, Brusse-Keizer M, van der Valk P, et al. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J Chron Obstruct Pulmon Dis 2014;9:935–44. 10.2147/COPD.S60179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh 2003;35:207. 10.1111/j.1547-5069.2003.tb00001.x [DOI] [PubMed] [Google Scholar]
- 43.Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000;355:362–8. 10.1016/S0140-6736(99)07042-7 [DOI] [PubMed] [Google Scholar]
- 44.Spruit MA, Singh SJ, Garvey C, et al. An official american thoracic society/european respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13–64. 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 45.Eakin MN, Rand CS. Improving patient adherence with asthma self-management practices: what works? Ann Allergy Asthma Immunol 2012;109:90–2. 10.1016/j.anai.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benzo R, McEvoy C. Effect of health coaching delivered by a respiratory therapist or nurse on self-management abilities in severe COPD: analysis of a large randomized study. Respir Care 2019;64:1065–72. 10.4187/respcare.05927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pastora-Bernal J-M, Estebanez-Pérez M-J, Molina-Torres G, et al. Telerehabilitation intervention in patients with COVID-19 after hospital discharge to improve functional capacity and quality of life. study protocol for a multicenter randomized clinical trial. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18062924. [Epub ahead of print: 12 03 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turan Z, Topaloglu M, Ozyemisci Taskiran O. Is tele-rehabilitation superior to home exercise program in COVID-19 survivors following discharge from intensive care unit? - A study protocol of a randomized controlled trial. Physiother Res Int 2021;26:p. e1920. 10.1002/pri.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from Hospital: a cohort study. Lancet 2021;397:220–32. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061285supp001.pdf (67.1KB, pdf)
Data Availability Statement
Data are available on reasonable request. On written request of the study team and an established data use agreement between the University of Colorado Anschutz Medical Campus and the requestor’s institution, we will provide a deidentified dataset including a data dictionary. Deidentification of the datasets will be conducted with respect to HIPAA definitions, with add back of variables that express all dates as number of days since a milestone event, enrolment and a variable storing just the year. For example, the milestone event would be 'Day 0' in this case. A deidentified dataset is not subject to HIPAA’s minimum necessary standards, so all data can be included and shared after the primary manuscript is published. Other related study documents (eg, study protocol, statistical analysis plan, etc) will be made available on reasonable written request. The main study results will be posted in ClinicalTrials.gov, per published guidelines, and will remain there indefinitely.