Abstract
Medium-chain-length polyhydroxyalkanoates (PHAs) are polyesters having properties of biodegradable thermoplastics and elastomers that are naturally produced by a variety of pseudomonads. Saccharomyces cerevisiae was transformed with the Pseudomonas aeruginosa PHAC1 synthase modified for peroxisome targeting by the addition of the carboxyl 34 amino acids from the Brassica napus isocitrate lyase. The PHAC1 gene was put under the control of the promoter of the catalase A gene. PHA synthase expression and PHA accumulation were found in recombinant S. cerevisiae growing in media containing fatty acids. PHA containing even-chain monomers from 6 to 14 carbons was found in recombinant yeast grown on oleic acid, while odd-chain monomers from 5 to 15 carbons were found in PHA from yeast grown on heptadecenoic acid. The maximum amount of PHA accumulated was 0.45% of the dry weight. Transmission electron microscopy of recombinant yeast grown on oleic acid revealed the presence of numerous PHA inclusions found within membrane-bound organelles. Together, these data show that S. cerevisiae expressing a peroxisomal PHA synthase produces PHA in the peroxisome using the 3-hydroxyacyl coenzyme A intermediates of the β-oxidation of fatty acids present in the media. S. cerevisiae can thus be used as a powerful model system to learn how fatty acid metabolism can be modified in order to synthesize high amounts of PHA in eukaryotes, including plants.
Polyhydroxyalkanoate (PHA) is a family of polyesters composed primarily of R-3-hydroxyalkanoic acids (2, 30, 40, 41). PHA is synthesized as intracellular inclusions by a wide variety of bacteria, including gram-positive and gram-negative species as well as some phototrophic bacteria, and is used as a carbon and electron sink. PHAs have been broadly defined in two main classes, namely, short-chain-length PHA (SCL-PHA) and medium-chain-length PHA (MCL-PHA). Whereas SCL-PHA typically harbors monomers of 3-hydroxy acids from 3 to 5 carbons, MCL-PHA contains 3-hydroxy acids from 6 to 16 carbons in length (2, 40). The best-characterized SCL-PHA is polyhydroxybutyrate (PHB), a homopolymer of 3-hydroxybutyric acid. In Ralstonia eutropha, PHB accumulates up to 80% of the dry weight (dwt), with inclusions being typically 0.2 to 1 μm in diameter. MCL-PHAs are typically synthesized by pseudomonads, such as Pseudomonas oleovorans and Pseudomonas aeruginosa.
Industrial interest in PHAs arises largely from their properties as thermoplastics and elastomers (2, 30, 40). Furthermore, numerous bacteria and fungi can hydrolyze PHAs to monomers and oligomers, which are further metabolized as a carbon source (2). PHAs are thus attractive as a source of renewable and biodegradable polyesters. PHB is a highly crystalline polymer with rather poor physical properties, being relatively stiff and brittle and degrading at temperatures slightly above its melting point (5). Incorporation of longer-chain monomers into the polymer, to form the copolymer poly(hydroxybutyrate-cohydroxyvalerate) [P(HB-HV)], reduces the crystallinity and melting point of the polymer, leading to improvement in the flexibility, strength, and processing of the polymer (5).
In contrast to SCL-PHAs, which are regarded as thermoplastics, MCL-PHAs have low crystallinity and melting points and are generally regarded as elastomers (15). There exist two main pathways for the synthesis of MCL-PHAs in bacteria. In bacteria such as P. oleovorans, MCL-PHA accumulates when cells are grown on alkanoic acids as the carbon source (2, 40, 41). The nature of the PHA produced is related to the substrate used for growth and is typically composed of monomers that are 2n (n ≥ 0) carbons shorter than the substrate. For example, growth of P. oleovorans on octanoate generates a PHA polymer containing 89 mol% 3-hydroxyoctanoic acid and 11 mol% 3-hydroxyhexanoic acid, whereas growth on dodecanoate generates PHA containing 31 mol% 3-hydroxydodecanoic acid, 36 mol% 3-hydroxydecanoic acid, 31 mol% 3-hydroxyoctanoic acid, and 2 mol% 3-hydroxyhexanoic acid (22). These studies have indicated that MCL-PHAs are synthesized by the PHA synthase from 3-hydroxyacyl coenzyme A (CoA) intermediates generated by the β-oxidation of alkanoic acids. A second pathway exists in some bacteria, such as Pseudomonas putida, that can synthesize MCL-PHA from glucose using intermediates of fatty acid biosynthesis (16, 20, 35, 43). The phaG gene that encodes a 3-hydroxyacyl-acyl carrier protein–CoA transferase provides the metabolic link between fatty acid biosynthesis and MCL-PHA synthesis (9, 32).
The main limitation for the use of PHAs as a commodity polymer is the high cost of producing PHA by bacterial fermentation relative to the cost of petroleum-derived plastics (27, 30). In this perspective, synthesis of PHAs in crop plants has been seen as an attractive alternative for the commercial production of large amounts of PHA at low cost (27, 28). Synthesis of SCL-PHAs has been demonstrated in a number of plants (28). Synthesis of PHB from acetyl-CoA was first shown in the cytoplasm and plastids of Arabidopsis thaliana cells (26, 29) and later in the peroxisomes of maize culture cells (14). Accumulation of PHB up to 40% dwt has recently been demonstrated in A. thaliana (4) as well as the synthesis of the copolymer P(HB-HV) in the seed leukoplasts of A. thaliana and Brassica napus (39). Furthermore, synthesis of PHB has also been demonstrated in the cytoplasm of S. cerevisiae (23) and in transgenic insect cells (45) expressing the PHB synthase from R. eutropha.
Synthesis of MCL-PHA in plants has been demonstrated in transgenic A. thaliana expressing the PHA synthase from P. aeruginosa in the peroxisome (24, 25). In these plants, MCL-PHAs containing saturated and unsaturated 3-hydroxyalkanoic acids ranging from 6 to 16 carbons were synthesized using intermediates of the β-oxidation of fatty acid (24, 25). The maximal amount of PHA synthesized in this system is low, at approximately 0.4 to 0.6% dwt, indicating the need for further modifications of fatty acid metabolic pathways before plants can be used for commercial production of MCL-PHAs.
We have sought to develop a more amenable eukaryotic system to understand how to manipulate the pathways involved in the degradation of fatty acids to increase the amount of MCL-PHA synthesized in peroxisomes. We now report the development of Saccharomyces cerevisiae for the synthesis of MCL-PHA in peroxisomes using intermediates of the β-oxidation of fatty acids.
MATERIALS AND METHODS
Strains and culture conditions.
Plasmids were maintained and propagated in Escherichia coli DH5α according to Sambrook et al. (36). S. cerevisiae diploid strain INVSc1 (his3D1 leu2 trp1-289 ura3-52) was obtained from Invitrogen (Groningen, The Netherlands). S. cerevisiae harboring the PHA synthase gene was maintained in leucine-deficient media (0.67% yeast nitrogen base without amino acids [Difco, Detroit, Mich.], 0.5% ammonium sulfate, 2% glucose, and 0.4 g of leucine dropout supplement [Clontech, Palo Alto, Calif.]/liter). For PHA production, a stationary-phase culture was harvested by centrifugation and cells were washed once in water and resuspended at a 1:10 dilution in fresh leucine-deficient media containing 0.1% or no glucose as well as detergent (either Tween 80 or Pluronic-127 [Sigma, St. Louis, Mo.]) and fatty acid (oleic acid or heptadecenoic acid). Cells were grown for an additional 1 to 6 days before harvest of the cells for PHA analysis. The pH of the growth media with or without fatty acids was 6.0.
DNA constructs.
The plasmid pYE352-PHA was constructed from the plasmid pYE352-CTA1 containing the S. cerevisiae catalase gene with its promoter and terminator sequences (10). The catalase-coding region of pYE352-CTA1 was removed by a SacI-XhoI digestion, and the vector was made blunt ended by using T4 DNA polymerase. The PHA synthase from P. aeruginosa modified for peroxisomal targeting by the addition, at the carboxy end, of the last 34 amino acids of the B. napus isocitrate lyase, was obtained from the plasmid pART7-PhaC1-ICL (25). The plasmid pART7-PhaC1-ICL was digested by EcoRI-XbaI, and the fragment harboring the modified PHA synthase was made blunt ended by T4 DNA polymerase and was ligated to the blunt-ended pYE352-CTA1 vector to create pYE352-PHA. The gene expression cassette containing the CTA1 promoter-PHA synthase-CTA1 terminator was excised from pYE352-PHA by a partial EcoRI digestion and was cloned into the EcoRI site of the integrative shuttle vector Yiplac128 (12), giving the plasmid Yiplac128-PHA. This plasmid was linearized by digestion with ClaI and transferred into the S. cerevisiae strain INVSc1 by the lithium acetate procedure. Transformants were recovered on media without leucine.
Western blot analysis.
The equivalent of an optical density at 600 nm of 0.2 of S. cerevisiae cells was harvested by centrifugation in a 1.5-ml tube and was suspended in 6 μl of 2 N NaOH–5% (vol/vol) β-mercaptoethanol. The suspension was put on ice for 10 min before adding 7.8 μl of 10% (wt/vol) sodium dodecyl sulfate. The mixture was briefly vortexed and was left at room temperature for 20 min with occasional mixing. The resulting crude protein extract was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and was blotted onto nitrocellulose membranes using a Trans-Blot electrophoretic cell (Bio-Rad, Richmond, Calif.). Free binding sites were saturated by incubation in blocking buffer (0.4 M NaCl, 2 mM KCl, 20 mM Tris-HCl, pH 7.4, 1% [vol/vol] Tween 40, and 5% [wt/vol] milk powder) for 1 h. The membranes were incubated for 2 h at room temperature in blocking buffer with anti-PHA synthase antibody. The antigen-antibody complexes were visualized with horseradish peroxidase-coupled goat anti-rabbit antibodies using the enhanced-chemiluminescence method (Amersham, Little Chalfont, United Kingdom).
Analysis of fatty acids and PHA.
Fatty acids present in the growth media were determined by analysis of fatty acid methyl-esters by gas chromatography (GC). Briefly, media were harvested and cleared of cells by centrifugation at 5,000 × g for 10 min. The cleared solution was transferred to a fresh tube and lyophilized. The dried residues were suspended in a methanol solution containing 1 N HCl and were heated at 80°C for 2 h. The fatty acid methyl-esters were extracted with 0.5 to 1 ml of hexane and 1 ml of 0.9% (wt/vol) NaCl, and the organic phase was transferred to autoinjector vials. GC analysis was performed using a Hewlett Packard 5890 gas chromatograph that was equipped with a Supelco SP2330 glass capillary column and coupled to a flame ionization detector.
For PHA analysis, cells were harvested by centrifugation, washed twice in water, and lyophilized. The dried material was then weighed (approximately 15 to 30 mg) and transferred to a glass tube. The material was extracted four or five times with warm (65°C) methanol to remove lipids, free fatty acids, and acyl-CoA, including 3-hydroxyacyl–CoA, while PHA, which is insoluble in methanol, remains associated with the cells. After centrifugation and removal of the residual methanol, the material was suspended in 0.5 ml of chloroform to which 0.5 ml of methanol containing 3% sulfuric acid was added. The mixture was heated at 95°C for 4 h and cooled down on ice. One milliliter of 0.1% NaCl was added to each tube, and the mixture was vortexed vigorously and centrifuged at 5,000 × g for 5 min. The chloroform phase was harvested and dried over anhydrous MgCl2. The methyl-esters of 3-hydroxy acids were identified and quantified by GC-mass spectrometry (GC-MS) using a Hewlett-Packard 5890 gas chromatograph (HP-5MS column) coupled to a Hewlett-Packard 5972 mass spectrometer. Analysis by GC-MS was made utilizing the ion-selective mode (mass-to-charge ratio of 103). Identification of monomers present in plant PHA was facilitated by the use of commercial 3-hydroxy acid standards and purified bacterial PHAs. In one experiment, lyophilized cells were extracted with methanol in a Soxhlet apparatus for 24 h followed by PHA extraction with chloroform for 24 h. The PHA-containing chloroform was concentrated using a Rotovapor and extracted once with water to remove residual solid particles. PHA was precipitated by the addition of 10 volumes of cold methanol and was subsequently washed by two cycles of chloroform solubilization and methanol precipitation. PHA dissolved in chloroform was analyzed by GC-MS as described above. The composition of the PHA isolated after Soxhlet extraction was similar to the PHA analyzed following esterification of the methanol-washed cells in chloroform.
Electron microscopy.
Cells were fixed for 4 h at room temperature in 4% (vol/vol) glutaraldehyde in 0.1 M sodium cacodylate, pH 7.2, and 0.1% (wt/vol) Brij 35, followed by an overnight treatment in the same solution without Brij 35. The cells were then rinsed several times with 0.1 M sodium cacodylate, pH 7.2, transferred to 2% osmium tetroxide for either 8 h at room temperature or 16 h at 4°C, and were then transferred to 2% uranyl acetate in 10% ethanol for 40 min. Cells were dehydrated through a graded series of ethanol with a final treatment in propylene oxide. Cells were embedded in Epon/Araldite resin and were polymerized for 3 days at 70°C. The blocks were cut with a Diatome diamond knife on a Reichret ultracut S microtome. Fine sections of 50 nm were placed on Formvar-coated copper grids, contrasted with a 2% aqueous solution of uranyl acetate for 6 min followed by lead citrate for 6 min. The grids were examined with a Philips Biotwin CM100 (Lab6 filament) transmission electron microscope.
RESULTS
Expression of the P. aeruginosa PHA synthase in recombinant yeast.
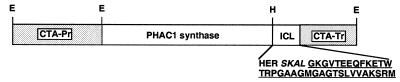
Previous studies had shown that the PHA synthase from P. aeruginosa could be targeted to plant peroxisomes by modifying the carboxy end of the bacterial protein by the addition of the last 34 amino acids of the peroxisomal protein isocitrate lyase from B. napus (25). This protein harbors the carboxy-terminal tripeptide ARM, a peroxisomal signal that was shown to be effective in targeting foreign proteins to peroxisomes in both plants and yeast (1, 44). The same modified PHA synthase was thus expressed in S. cerevisiae. The structure of the gene expression cassette is shown in Fig. 1. The modified PHA synthase was put under the control of the promoter and transcription terminator of the yeast catalase gene CTA1 (10). This promoter allows strong expression of genes in yeast grown in media containing fatty acids as the carbon source (19, 38). The expression cassette was cloned into the integrative yeast shuttle vector Yiplac128 for transformation into S. cerevisiae.
FIG. 1.
DNA construct used to express the PHAC1 synthase of P. aeruginosa in S. cerevisiae. Only the portion of the construct containing the gene (open box) and regulatory elements (shaded box) is shown. The partial amino acid sequence of the C terminus of the PHA synthase-isocitrate lyase (ICL) fusion is indicated using the one-letter symbols, with the amino acids derived from the PHAC1 protein given in plain letters, novel amino acids created at the fusion junction italicized, and the last 34 amino acids derived from the B. napus isocitrate lyase underlined. CTA-Pr, CTA1 promoter; CTA-Tr, CTA1 terminator; E, EcoRI; H, HindIII.
Western blot analysis of recombinant PHA synthase.
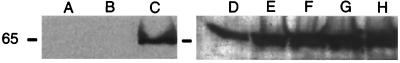
Recombinant yeast transformed with Yiplac128-PHA was tested for expression of the PHA synthase by Western blot analysis using an anti-PHA synthase serum (Fig. 2). In these experiments, yeast grown to stationary phase in media containing 2% glucose was washed in water and resuspended at a 1:10 dilution in leucine-deficient media supplemented with glucose, oleic acid, and/or the detergent Pluronic-127 or Tween 80. While Pluronic-127 is a polyoxyethylene polymer containing no fatty acids, Tween 80 is a detergent containing approximately 20% oleic acid by weight that is esterified to the polyoxyethylenesorbitan backbone. These detergents were used to insure solubilization of the free oleic acid added to the media. While no expression of the PHA synthase was detected in transformed cells grown for 24 h in media supplemented with 1% glucose, expression of the 65-kDa PHA synthase was readily detected in cells grown for 72 h in media supplemented with only 0.1% glucose or in media containing 0.1% glucose and either 0.5% Tween 80, 2% Pluronic-127, 0.5% Tween 80 with 0.1% oleic acid, or 2% Pluronic-127 with 0.1% oleic acid (Fig. 2).
FIG. 2.
Western blot analysis of PHA synthase expression in S. cerevisiae. Wild-type (A) or recombinant yeast transformed with the PHA synthase (B to H) was grown for 24 h in media containing 1% glucose (B) or 0.1% glucose, 2% Pluronic-127, and 0.1% oleic acid (A and C) or for 72 h in media containing 0.1% glucose (D); 0.1% glucose and 0.5% Tween 80 (E); 0.1% glucose and 2% Pluronic-127 (F); 0.1% glucose, 0.5% Tween 80, and 0.1% oleic acid (G); or 0.1% glucose, 2% Pluronic-127, and 0.1% oleic acid (H). Molecular mass marker (in kilodaltons) is indicated on the left.
Production of MCL-PHA.
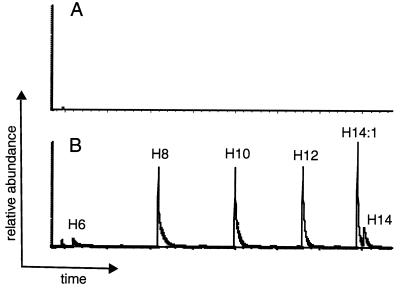
Cells grown to stationary phase in media containing 2% glucose were shifted to media containing various fatty acids and detergents with or without 0.1% glucose and were grown for an additional 4 days before being harvested for PHA analysis (Table 1). Yeast grown on 0.5% Tween 80 produced only 0.02% (dwt) PHA, while addition of 0.1% oleic acid to media containing 0.5% Tween 80 led to a 10-fold increase of PHA to 0.2% (dwt). Since the PHA synthase is well expressed in yeast grown in media containing either Tween 80 alone or Tween 80 supplemented with free oleic acid (Fig. 2), these data indicate that free oleic acid is a better substrate for PHA synthesis in yeast than is the esterified oleic acid found in Tween 80. Addition of 0.1% glucose to media containing 0.5% Tween 80 and 0.1% oleic acid led to an increase of PHA to 0.35% (dwt). Recombinant yeast grown in media containing 0.1% glucose and 2% Pluronic-127 shows less than 0.01% PHA (Table 1), similar to cells grown in media containing only 0.1% or 2% glucose (data not shown). In contrast, cells grown in media supplemented with 0.1% glucose, 2% Pluronic-127, and 0.1% oleic acid produced 0.31% (dwt) PHA. No PHA could be detected in cells that were transformed with the control vector pYE352-CTA1 (which harbors the peroxisomal catalase gene) and were grown in media containing oleic acid (Fig. 3). Together, these data show that PHA synthesis in recombinant yeast is dependent on the presence of both a PHA synthase and an external source of fatty acids.
TABLE 1.
Synthesis of PHA in recombinant S. cerevisiae grown in various media
| Treatment | PHA % (wt/dwt) |
|---|---|
| 0.5% Tween 80 | 0.020 ± 0.004 |
| 0.5% Tween 80–0.1% oleic acid | 0.20 ± 0.03 |
| 0.5% Tween 80–0.1% oleic acid–0.1% glucose | 0.35 ± 0.03 |
| 2% Pluronic-127 | <0.01 |
| 2% Pluronic-127–0.1% oleic acid–0.1% glucose | 0.31 ± 0.03 |
FIG. 3.
GC-MS analysis of PHA produced in transgenic yeast expressing the PHA synthase. Yeast cells transformed with the control vector pYE352-CTA1 (A) or the plasmid pYE352-PHA harboring the PHA synthase gene (B) were grown for 3 days in media containing 2% Pluronic-127, 0.1% glucose, and 0.1% oleic acid, and the PHA was analyzed as described in Materials and Methods. Only ions with a mass-to-charge ratio of 103 are shown. The various 3-hydroxy acids are identified with the prefix H. The y axes in panels A and B are on the same scale.
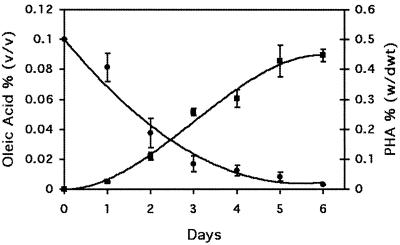
The amount of PHA in cells, as well as concentrations of oleic acid and glucose in the media, were monitored over 6 days after the shift of cells to media containing 0.1% glucose, 2% Pluronic-127, and 0.1% oleic acid (Fig. 4). The amount of glucose in the media was below detection (<0.002%) after 24 h, while oleic acid decreased only slightly to 0.09%. PHA accumulated in cells until day 5, in parallel with the decrease in the amount of free oleic acid in the media. At days 5 and 6, PHA was found in S. cerevisiae at 0.43 to 0.45% (dwt).
FIG. 4.
Time course of the accumulation of PHA in S. cerevisiae. Recombinant yeast was used to inoculate media containing 0.1% glucose, 2% Pluronic-127, and 0.1% oleic acid. PHA content (▪) in cells and the concentration of oleic acid (●) present in the media were monitored over 6 days. Values represent the mean and standard deviation of four measurements. w/dwt, weight/dwt; v/v, vol/vol.
PHA inclusions detected by electron microscopy.
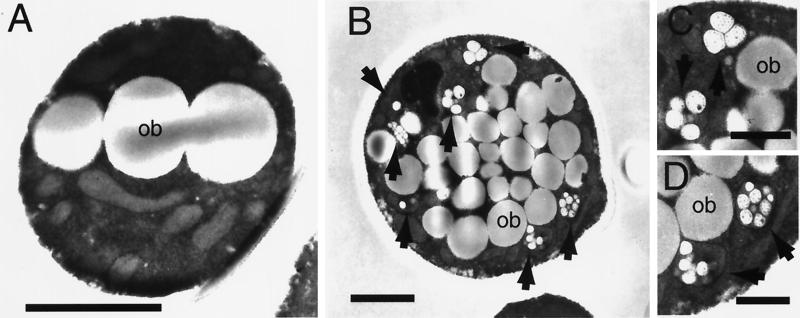
Wild-type and recombinant cells grown for 3 days in media containing oleic acid were analyzed by transmission electron microscopy (TEM). While both types of cells showed the presence of numerous oil bodies (Fig. 5A and B), only recombinant cells producing PHA showed the presence of small electron-lucent inclusions within membrane-bound organelles (Fig. 5B to D). The apparent size of these inclusions is in the range of 0.1 to 0.2 μm in diameter. Both the size and general appearance of these inclusions, as seen by TEM, are very similar to PHA granules found in bacteria (2, 30) as well as in transgenic plants (25, 29), indicating that, similar to PHA synthesized in these other hosts, PHA produced in yeast accumulates in the form of inclusions.
FIG. 5.
Analysis of PHA inclusions in S. cerevisiae. The wild-type (A) or recombinant yeast expressing the PHA synthase gene (B to D) was used to inoculate media containing 0.1% glucose, 2% Pluronic-127, and 0.1% oleic acid and was grown for 4 days before being processed for TEM. Panels C and D are close-up views of panel B. Arrows indicate the presence of PHA inclusions within membrane-bound organelles. ob, oil body. Bars indicate 1 μm (A and B) and 0.5 μm (C and D).
Composition of MCL-PHA produced in yeast.
In order to determine the influence of the carbon source on PHA monomer composition, recombinant yeast was grown for 4 days in media containing 0.1% glucose, 2% Pluronic-127, and 0.1% oleic acid or 0.1% heptadecenoic acid (17:1 Δ10cis). Table 2 shows that the PHA monomer composition is dependent on the nature of the external fatty acids and on the range of 3-hydroxyacyl–CoA intermediates generated by the degradation of the external fatty acid by the peroxisomal β-oxidation pathway. PHA synthesized in yeast growing on oleic acid (18:1 Δ9cis) contains even-chain 3-hydroxy acid monomers from 6 to 14 carbons in length. The presence of both 3-hydroxytetradecanoic acid and 3-hydroxytetradecenoic acid agrees with the generation of the corresponding acyl-CoA by the β-oxidation of fatty acids having a cis-unsaturated bond at an odd-numbered carbon (13, 17). Similarly, growth of recombinant yeast in media containing 17:1 Δ10cis gave a PHA containing odd-chain monomers ranging from 5 to 15 carbons, with two monomers being unsaturated (Table 2). Together, these data show that external fatty acids are imported into cells and degraded via the peroxisomal β-oxidation cycle and that 3-hydroxyacyl–CoAs generated by the β-oxidation cycle are used for the synthesis of MCL-PHA.
TABLE 2.
Monomer composition of PHA synthesized in S. cerevisiae
| Fatty acidb | PHA monomer composition (mol%)a for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H5 | H6 | H7 | H8 | H9 | H10 | H11 | H12 | H13:1 | H14:1 | H14 | H15:1 | |
| 18:1 Δ9cis | 21 | 46 | 19 | 10 | 3.8 | 0.9 | ||||||
| 17:1 Δ10cis | 9.4 | 40 | 22 | 16 | 11 | 1.7 | ||||||
3-Hydroxy acid monomers are denoted by the prefix H.
Cells were grown in a leucine-deficient medium supplemented with 0.1% glucose, 2% Pluronic-127, and 0.1% fatty acids.
DISCUSSION
Expression in S. cerevisiae of a PHA synthase from P. aeruginosa, modified at the carboxy end by the addition of a peroxisome targeting signal, was shown to lead to MCL-PHA synthesis in yeast growing in media containing fatty acid. Expression of MCL-PHA was dependent on the promoter of the CTA1 gene encoding the peroxisomal catalase A. This gene was shown previously to be repressed by glucose and activated by fatty acids (19, 38). In consequence, the PHA synthase was found to be expressed in cell cultures that had depleted the glucose initially present in the media, as well as in cells growing in media containing fatty acids.
Feeding experiments with oleic acid or heptadecenoic acid added to the growth media clearly indicate that the monomers used in the synthesis of MCL-PHA in yeast are derived from the β-oxidation of the external fatty acids. Thus, addition of heptadecenoic acid leads to the synthesis of MCL-PHA containing odd-chain monomers, while addition of oleic acid leads to the synthesis of a PHA containing even-chain monomers. Furthermore, for each of these fatty acids, the monomers found in PHA correspond to the 3-hydroxyacyl–CoAs generated by the degradation of the external fatty acid through peroxisomal β-oxidation. Thus, degradation of the fatty acid 17:1 Δ10cis, according to the pathway for the degradation of fatty acids having a cis double bond at an even-numbered carbon (13), is expected to lead to the production of the three unsaturated 3-hydroxyacyl–CoAs, namely, H17:1 (the prefix H refers to the 3-hydroxy moiety), H15:1, and H13:1, and of five saturated 3-hydroxyacyl–CoAs, namely, H11, H9, H7, H5, and H3. All these 3-hydroxy acids are found in the PHA synthesized in S. cerevisiae grown in 17:1 Δ10cis, except for H17:1 and H3, which fall outside the range of monomers generally accepted by the P. aeruginosa PHA synthase. Incorporation of 3-hydroxypentanoic acid in the yeast PHA is interesting, since PHA synthesized by P. aeruginosa, as well as other by pseudomonads producing MCL-PHAs, typically includes only monomers between 6 and 16 carbons (40). It has been previously reported that expression of a PHA synthase in a heterologous host may lead to the incorporation into PHA of a range of monomers broader than that normally found in the polymer of the native host (3, 6, 21). For example, while Chromobacterium violaceum growing on fatty acids produces a PHA containing only 3- or 4-carbon monomers, expression of the C. violaceum PHA synthase in R. eutropha leads to the synthesis of PHA containing in addition a 6-carbon monomer (21). These studies demonstrate that the PHA monomer composition is not only dependent on the substrate specificity of the PHA synthase but also on the metabolic environment of the host organism. Thus, in contrast to P. aeruginosa, the β-oxidation cycle of yeast allows the inclusion of 3-hydroxypentanoic acid into PHA.
Growth of recombinant yeast on oleic acid leads to the synthesis of PHA containing even-chain monomers from 6 to 14 carbons that are generated by the degradation of this fatty acid via the peroxisomal β-oxidation cycle, namely, H14:1, H14, H12, H10, H8, and H6. No 16-carbon 3-hydroxy acids were detectable in the yeast PHA, even though expression of the same PHA synthase in the peroxisome of the plant A. thaliana led to the incorporation of saturated and unsaturated 16-carbon monomers in the PHA (25). It is again likely that differences in the in vivo concentration or availability of the 3-hydroxyhexadecanoyl–CoA intermediates between S. cerevisiae and A. thaliana may influence the incorporation of 16-carbon monomers into PHA.
PHA production in yeast is accompanied by the appearance of electron-lucent inclusions within membrane-bound organelles. The size and general appearance of these inclusions are very similar to PHA granules found in bacteria or plants accumulating PHA. TEM, by itself, cannot unambiguously identify the organelle containing the PHA inclusions as being peroxisomes. However, all results obtained strongly support the notion that PHA granules are found within the peroxisomes. First, PHA synthesized in yeast is clearly derived from intermediates of β-oxidation of the fatty acids added to the media. Furthermore, we have recently found that expression of the modified PHA synthase in the fox1 mutant, deficient in the peroxisomal β-oxidation enzyme acyl-CoA oxidase, does not produce MCL-PHA (data not shown). These data show that MCL-PHA synthesis depends directly on substrates generated by the peroxisomal β-oxidation cycle. Second, the PHA synthase contains a peroxisomal targeting sequence that has been clearly shown to direct foreign proteins to the peroxisome (1, 44). Finally, β-oxidation is found to occur only in the peroxisome in S. cerevisiae.
In bacteria and plants, PHAs are composed of the R isomer of 3-hydroxyalkanoic acids due to the stereospecificity of the PHA synthase, which accepts only R-3-hydroxyacyl–CoAs (15). Since the core β-oxidation cycle of bacteria, mammals, and plants generates mainly the S isomer of 3-hydroxyacyl–CoAs from the hydration of enoyl-CoAs by the enoyl-CoA hydratase I (17, 37), synthesis of MCL-PHAs in these organisms implicates the presence of enzymes which can convert intermediates of β-oxidation to R-3-hydroxyacyl–CoAs. In several bacteria synthesizing MCL-PHAs from alkanoic acids, an R-specific enoyl-CoA hydratase II has been identified which converts the β-oxidation intermediate trans-2-enoyl–CoAs to R-3-hydroxyacyl–CoAs (11, 33). Furthermore, a 3-ketoacyl–ACP reductase has also been identified in E. coli and P. aeruginosa which can contribute to the synthesis of R-3-hydroxyacyl–CoAs from 3-ketoacyl–CoA (34, 42). In plants, a range of R-3-hydroxyacyl–CoAs is thought to be generated by either a monofunctional enoyl-CoA hydratase II or the 3-hydroxyacyl–CoA epimerase activity found within the β-oxidation multifunctional protein, which also harbors an enoyl-CoA hydratase I and a 3-hydroxyacyl–CoA dehydrogenase (8, 31).
In contrast to bacteria, plants, and animals, many fungi, including S. cerevisiae and Candida tropicalis, have a β-oxidation cycle that normally occurs through R-3-hydroxyacyl–CoA intermediates. This is because these organisms have only an R-specific enoyl-CoA hydratase II instead of an S-specific enoyl-CoA hydratase I (18). It was thus expected that S. cerevisiae would be a good host for the synthesis of MCL-PHAs from the intermediates of fatty acid β-oxidation. However, PHA synthesis in yeast is 1 or 2 orders of magnitude lower then MCL-PHA production in several pseudomonads. For example, growth of P. putida in media containing oleic acid gives an accumulation of 37% MCL-PHA (7), while in this study recombinant S. cerevisiae accumulates a maximum of 0.45% PHA. Although one should be cautious about comparing PHA synthesis in bacteria grown in fermentors and yeast cultures grown in shake flasks, these studies nevertheless indicate that PHA synthesis in yeast is suboptimal compared to bacterial production. This raises the interesting question of the biochemical basis of this difference.
Interestingly, although the isomers of 3-hydroxyacyl–CoA generated by the β-oxidation cycle in yeast and plants are different, the levels of PHA synthesized in the peroxisome of these two organisms are similar at 0.4% (dwt) (24, 25). These results indicate that the stereospecificity of the substrate generated by the core β-oxidation cycle may not be the only factor influencing PHA synthesis from β-oxidation intermediates. Although it is possible that some of the factors limiting MCL-PHA synthesis in yeast and plants may be different, the pathways of degradation of fatty acids is sufficiently similar between these organisms to consider using the power of yeast genetics as a tool to understand how more intermediates from the peroxisomal β-oxidation cycle can be channeled towards PHA and to apply this knowledge to the production of MCL-PHA in plants.
ACKNOWLEDGMENTS
This research was funded by the Etat de Vaud.
We thank Silvia Marchesini for help with the figures and Kalervo Hiltunen for providing the plasmid pYE352-CTA1.
REFERENCES
- 1.Aitchison J D, Nuttley W M, Szilard R K, Brade A M, Glover J R, Rachubinski R A. Peroxisome biogenesis in yeast. Mol Microbiol. 1992;6:3455–3460. doi: 10.1111/j.1365-2958.1992.tb01780.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson A J, Dawes E A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonio R V, Steinbüchel A, Rehm B H A. Analysis of in vivo substrates specificity of the PHA synthase from Ralstonia eutropha: formation of novel copolyesters in recombinant Escherichia coli. FEMS Microbiol Lett. 2000;182:111–117. doi: 10.1111/j.1574-6968.2000.tb08883.x. [DOI] [PubMed] [Google Scholar]
- 4.Bohmert K, Balbo I, Kopka J, Mittendorf V, Nawrath C, Poirier Y, Tischendorf G, Trethewey R N, Willmitzer L. Transgenic Arabidopsis plants can accumulate polyhydroxybutyrate to up to 4% of their fresh weight. Planta. 2000;211:841–845. doi: 10.1007/s004250000350. [DOI] [PubMed] [Google Scholar]
- 5.de Koning G. Physical properties of bacterial poly[(R)3-hydroxyalkanoates] Can J Microbiol. 1995;41(Suppl.):303–309. [Google Scholar]
- 6.Dennis D, McCoy M, Stangl A, Valentin H E, Wu Z. Formation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by PHA synthase from Ralstonia eutropha. J Biotechnol. 1998;64:177–186. doi: 10.1016/s0168-1656(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 7.de Waard P, van der Wal H, Huijberts G N M, Eggink G. Heteronuclear NMR analysis of unsaturated fatty acids in poly(3-hydroxybutyrate). Study of β-oxidation in Pseudomonas putida. J Biol Chem. 1993;268:315–319. [PubMed] [Google Scholar]
- 8.Engeland K, Kindl H. Evidence for a peroxisomal fatty acid β-oxidation involving D-3-hydroxyacyl-CoAs: characterisation of two forms of hydro-lyase that convert D-(-)-3-hydroxyacyl-CoA. Eur J Biochem. 1991;200:171–178. doi: 10.1111/j.1432-1033.1991.tb21064.x. [DOI] [PubMed] [Google Scholar]
- 9.Fiedler S, Steinbüchel A, Rehm B H A. PhaG-mediated synthesis of poly(3-hydroxyalkanoates) consisting of medium-chain-length constituents from nonrelated carbon sources in recombinant Pseudomonas fragi. Appl Environ Microbiol. 2000;66:2117–2124. doi: 10.1128/aem.66.5.2117-2124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filppula S A, Sormunen R T, Hartig A, Kunau W-H, Hiltunen J K. Changing the stereochemistry for a metabolic pathway in vivo. Experiments with the peroxisomal β-oxidation in yeast. J Biol Chem. 1995;270:27453–27457. doi: 10.1074/jbc.270.46.27453. [DOI] [PubMed] [Google Scholar]
- 11.Fukui T, Shiomi N, Doi Y. Expression and characterization of (R)-specific enoyl coenzyme A hydratase involved in polyhydroxyalkanoate biosynthesis by Aeromonas caviae. J Bacteriol. 1998;180:667–673. doi: 10.1128/jb.180.3.667-673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 13.Gurvitz A, Mursula A M, Yagi A I, Hartig A, Ruis H, Rottensteiner H, Hiltunen J K. Alternatives to the isomerase-dependent pathway for the β-oxidation of oleic acid are dispensable in Saccharomyces cerevisiae. J Biol Chem. 1999;274:24514–24521. doi: 10.1074/jbc.274.35.24514. [DOI] [PubMed] [Google Scholar]
- 14.Hahn J J, Eschenlauer A C, Sleytr U B, Somers D A, Srienc F. Peroxisomes as sites for synthesis of polyhydroxyalkanoates in transgenic plants. Biotechnol Progr. 1999;15:1053–1057. doi: 10.1021/bp990118n. [DOI] [PubMed] [Google Scholar]
- 15.Haywood G W, Anderson A J, Dawes E A. The importance of PHB-synthase substrate specificity in polyhydroxyalkanoate synthesis by Alcaligenes eutrophus. FEMS Microbiol Lett. 1989;57:1–6. [Google Scholar]
- 16.Haywood G W, Anderson A J, Ewing D F, Dawes E A. Accumulation of a polyhydroxyalkanoate containing primarily 3-hydroxydecanoate from simple carbohydrate substrates by Pseudomonas sp. strain NCIMB 40135. Appl Environ Microbiol. 1990;56:3354–3359. doi: 10.1128/aem.56.11.3354-3359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiltunen J K, Filppula S A, Koivuranta K T, Siivari K, Qin Y-M, Häyrinen H-M. Peroxisomal β-oxidation and polyunsaturated fatty acids. Ann N Y Acad Sci. 1996;804:116–128. doi: 10.1111/j.1749-6632.1996.tb18612.x. [DOI] [PubMed] [Google Scholar]
- 18.Hiltunen J K, Wenzel B, Beyer A, Erdmann R, Fosså A, Kunau W-H. Peroxisomal multifunctional β-oxidation protein of Saccharomyces cerevisiae. Molecular analysis of the FOX2 gene and gene product. J Biol Chem. 1992;267:6646–6653. [PubMed] [Google Scholar]
- 19.Hortner H, Ammerer G, Hartter E, Hamilton B, Rytka J, Bilinski T, Ruis H. Regulation of synthesis of catalases and iso-cytochrome c in Saccharomyces cerevisiae by glucose, oxygen and heme. Eur J Biochem. 1982;128:179–184. doi: 10.1111/j.1432-1033.1982.tb06949.x. [DOI] [PubMed] [Google Scholar]
- 20.Huijberts G N M, Eggink G, de Waard P, Huisman G W, Witholt B. Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl Environ Microbiol. 1992;58:536–544. doi: 10.1128/aem.58.2.536-544.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolibachuk D, Miller A, Dennis D. Cloning, molecular analysis, and expression of the polyhydroxyalkanoic acid synthase (phaC) gene from Chromobacterium violaceum. Appl Environ Microbiol. 1999;65:3561–3565. doi: 10.1128/aem.65.8.3561-3565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lageveen R G, Huisman G W, Preusting H, Ketelaar P, Eggink G, Witholt B. Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol. 1988;54:2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leaf T A, Peterson M S, Stoup S K, Somers D, Srienc F. Sacchoromyces cerevisiae expressing bacterial PHB synthase produces poly-3-hydroxybutyrate. Microbiology. 1996;142:1169–1180. doi: 10.1099/13500872-142-5-1169. [DOI] [PubMed] [Google Scholar]
- 24.Mittendorf V, Bongcam V, Allenbach L, Coullerez G, Martini N, Poirier Y. Polyhydroxyalkanoate synthesis in transgenic plants as a new tool to study carbon flow through β-oxidation. Plant J. 1999;20:45–55. doi: 10.1046/j.1365-313x.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 25.Mittendorf V, Robertson E J, Leech R M, Krüger N, Steinbüchel A, Poirier Y. Synthesis of medium-chain-length polyhydroxyalkanoates in Arabidopsis thaliana using intermediates of peroxisomal fatty acid β-oxidation. Proc Natl Acad Sci USA. 1998;95:13397–13402. doi: 10.1073/pnas.95.23.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawrath C, Poirier Y, Somerville C R. Targeting of the polyhydroxybutyrate biosynthetic pathway to the plastids of Arabidopsis thaliana results in high-levels of polymer accumulation. Proc Natl Acad Sci USA. 1994;91:12760–12764. doi: 10.1073/pnas.91.26.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poirier Y. Production of new polymeric compounds in plants. Curr Opin Biotechnol. 1999;10:181–185. doi: 10.1016/s0958-1669(99)80032-9. [DOI] [PubMed] [Google Scholar]
- 28.Poirier Y. Production of poylesters in transgenic plants. In: Babel W, Steinbüchel A, editors. Biopolyesters. Berlin, Germany: Springer-Verlag; 2001. pp. 209–240. [Google Scholar]
- 29.Poirier Y, Dennis D E, Klomparens K, Somerville C. Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science. 1992;256:520–523. doi: 10.1126/science.256.5056.520. [DOI] [PubMed] [Google Scholar]
- 30.Poirier Y, Nawrath C, Somerville C. Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Bio/Technology. 1995;13:142–150. doi: 10.1038/nbt0295-142. [DOI] [PubMed] [Google Scholar]
- 31.Presig-Müller R, Gühnemann-Schäfer K, Kindl H. Domains of the tetrafunctional protein acting in glyoxysomal fatty acid β-oxidation: demonstration of epimerase and isomerase activities on a peptide lacking hydratase activity. J Biol Chem. 1994;269:20475–20481. [PubMed] [Google Scholar]
- 32.Rehm B H A, Krüger N, Steinbüchel A. A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. The phaG gene from Pseudomonas putida KT2440 encodes a 3-hydroxyacyl-acyl carrier protein-coenzyme a transferase. J Biol Chem. 1998;273:24044–24051. doi: 10.1074/jbc.273.37.24044. [DOI] [PubMed] [Google Scholar]
- 33.Reiser S E, Mitsky T A, Gruys K J. Characterization and cloning of an (R)-specific trans-2,3-enoylacyl-CoA hydratase from Rhodospirillum rubrum and use of this enzyme for PHA production in Escherichia coli. Appl Microbiol Biotechnol. 2000;53:209–218. doi: 10.1007/s002530050010. [DOI] [PubMed] [Google Scholar]
- 34.Ren Q, Sierro N, Witholt B, Kessler B. FabG, an NADPH-dependent 3-ketoacyl reductase of Pseudomonas aeruginosa, provides precursors for medium-chain-length poly-3-hydroxyalkanoate biosynthesis in Escherichia coli. J Bacteriol. 2000;182:2978–2981. doi: 10.1128/jb.182.10.2978-2981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito Y, Doi Y. Biosynthesis of poly(3-hydroxy-alkanoates) in Pseudomonas aeruginosa AO-232 from 13C-labeled acetate and propionate. Int J Biol Macromol. 1993;15:287–292. doi: 10.1016/0141-8130(93)90028-k. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Schulz H. Beta oxidation of fatty acids. Biochim Biophys Acta. 1991;1081:109–120. doi: 10.1016/0005-2760(91)90015-a. [DOI] [PubMed] [Google Scholar]
- 38.Skoneczny M, Chelstowska A, Rytka J. Study of the coinduction by fatty acids of catalase A and acyl-CoA oxidase in standard and mutant Saccharomyces cerevisiae strains. Eur J Biochem. 1988;174:297–302. doi: 10.1111/j.1432-1033.1988.tb14097.x. [DOI] [PubMed] [Google Scholar]
- 39.Slater S, Mitsky T A, Houmiel K L, Hao M, Reiser S E, Taylor N B, Tran M, Valentin H E, Rodriguez D J, Stone D A, Padgette S R, Kishore G, Gruys K J. Metabolic engineering of Arabidopsis and Brassica for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production. Nat Biotechnol. 1999;17:1011–1016. doi: 10.1038/13711. [DOI] [PubMed] [Google Scholar]
- 40.Steinbüchel A. Polyhydroxyalkanoic acids. In: Byrom D, editor. Novel biomaterials from biological sources. New York, N.Y: Macmillan; 1991. pp. 123–216. [Google Scholar]
- 41.Steinbüchel A, Füchtenbusch B. Bacterial and other biological systems for polyester production. Trends Biotechnol. 1998;16:419–427. doi: 10.1016/s0167-7799(98)01194-9. [DOI] [PubMed] [Google Scholar]
- 42.Taguchi K, Aoyagi Y, Matsusaki H, Fukui T, Doi Y. Co-expression of 3-ketoacyl-ACP reductase and polyhydroxyalkanoate synthase genes induces PHA production in Escherichia coli HB101 strain. FEMS Microbiol Lett. 1999;176:183–190. doi: 10.1111/j.1574-6968.1999.tb13660.x. [DOI] [PubMed] [Google Scholar]
- 43.Timm A, Steinbüchel A. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl Environ Microbiol. 1990;56:3360–3367. doi: 10.1128/aem.56.11.3360-3367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volokita M. The carboxy-terminal end of glycolate oxidase directs a foreign protein into tobacco leaf peroxisomes. Plant J. 1991;1:361–366. doi: 10.1046/j.1365-313x.1991.t01-4-00999.x. [DOI] [PubMed] [Google Scholar]
- 45.Williams M D, Rahn J A, Sherman D H. Production of a polyhydroxyalkanoate biopolymer in insect cells with a modified eucaryotic fatty acid synthase. Appl Environ Microbiol. 1996;62:2540–2546. doi: 10.1128/aem.62.7.2540-2546.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]