Abstract
BACKGROUND
Primary spinal infections (PSIs) are a group of uncommon but serious infectious diseases that are characterized by inflammation of the endplate-disc unit. Pediatric spinal infection is rare and challenging to diagnose due to vague presenting symptoms. Most cases are conservatively managed with surgery rarely indicated. The authors performed a systematic review to study the baseline characteristics, clinical presentation, and outcomes of pediatric patients with PSIs who underwent surgical treatment.
OBSERVATIONS
PSI in pediatric patients might behave differently in terms of epidemiology, clinical presentation, and outcomes when compared with nonpediatric patients. Overall, PSI ultimately managed surgically in pediatric patients is associated with a high rate of localized pain, neurological compromise, and treatment failure when compared with nonsurgically managed pediatric spinal infections.
LESSONS
PSIs managed surgically in the pediatric population were found to be caused by Mycobacterium tuberculosis in 74.4% of cases and were associated with higher rates of localized pain, neurological compromise, and treatment failure than nonsurgically managed pediatric spinal infections. Thoracic involvement (71.8%) in the spinal infection was reported most commonly in our review. When omitting the cases involving M. tuberculosis infection, it was revealed that 50% of the pediatric cases involved infection in the cervical region, suggesting increased severity and disease course of cervical spinal infections in the pediatric population. Surgical treatment is indicated only in cases of severe neurological compromise and treatment failure.
Keywords: vertebral osteomyelitis, spondylodiscitis, pediatric, operative, surgical management
ABBREVIATIONS : CRP = C-reactive protein, PSI = primary spinal infection, WBC = white blood cell
Primary spinal infections (PSIs) in the pediatric population are relatively uncommon, with an estimated incidence in developed countries of 0.3 per 100,000 among individuals younger than 20 years old.1 Despite being rare in the pediatric age group, these infections can cause significant morbidity and mortality and can produce devastating consequences for patients. Thus, a timely diagnosis is vital to allow prompt treatment.2
Because of its rarity and presentation with vague clinical symptoms, such as back pain, abdominal pain, and irritability, accurate diagnosis of pediatric spinal infection is challenging. As a result, early treatment is delayed, and neurological sequelae may develop. First-line treatment for PSIs in both the adult and pediatric populations is centered on using antimicrobial sensitivity testing to guide intravenous antibiotics. In contrast to adults, there are currently no existing guidelines supported by clinical evidence addressing the management of pediatric spinal infection.3 Consequently, the development of increasingly severe symptoms is observed in many cases, many of which result in urgent spinal surgery. Indications for urgent surgery include spinal instability or neurological compromise caused by the spinal infection.4 Indications for nonurgent surgery include severe and progressive kyphosis, lack of response to antibiotic agents, or a large paraspinal abscess.5
Through our literature review, we found that no systematic review has addressed spinal infection requiring surgical management in the pediatric population. Therefore, the aim of this study was to present data on the demographic characteristics, describe the clinical course, and discuss outcomes in cases of pediatric spinal infection requiring operative management.
Illustrative Case
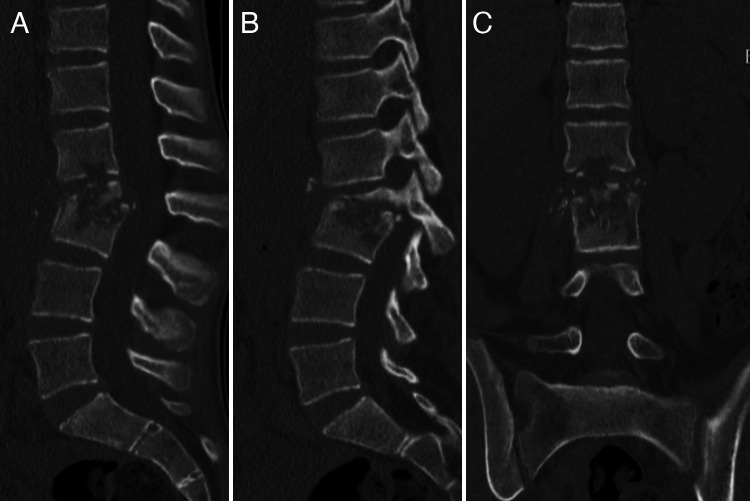
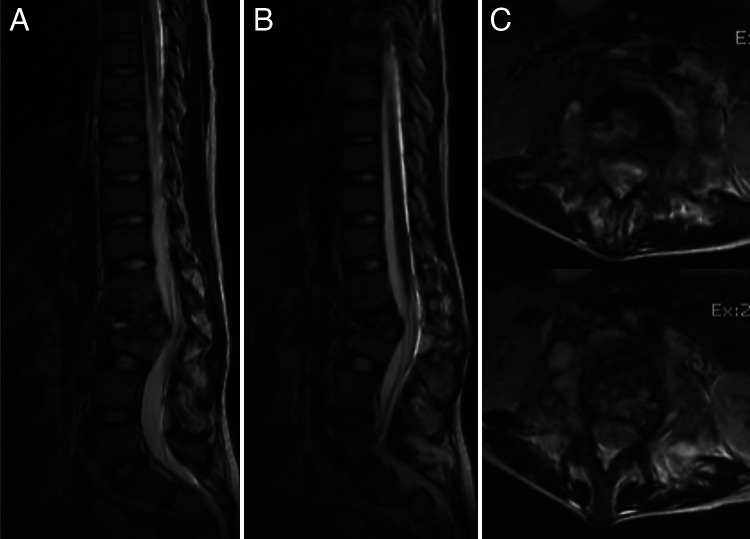
A 12-year-old girl without relevant past medical history was referred to our institution due to a 5-month history of low back pain and progressive lumbar deformity. Radiographs at the time of the visit showed a significant lumbar L2–3 kyphotic deformity (Fig. 1). Computed tomography and magnetic resonance imaging (Figs. 2 and 3) advanced compromise at the L2–3 disc unit and bilateral psoas abscesses without spinal canal involvement. The patient’s blood culture finding was negative, her white blood cell (WBC) count was 9,000 per microliter, and her C-reactive protein (CRP) level was 7 mg/dl. The patient was scheduled for surgery and T11–L4 posterior instrumented fusion, and posterior partial vertebrectomy and debridement were performed. Histopathological results were positive for Mycobacterium tuberculosis, and the patient was treated with an 8-month antituberculosis scheme, with significant improvement. The last control at 2-year follow-up showed a balanced spine and solid fusion (Fig. 4).
FIG. 1.
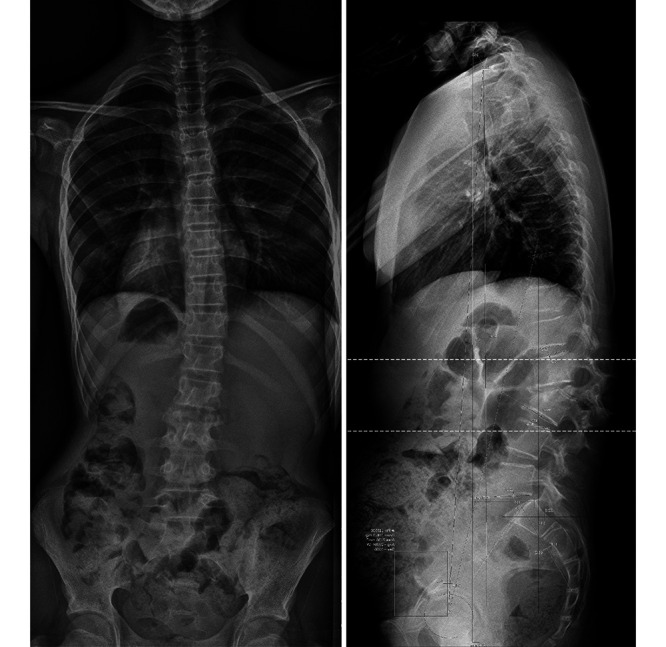
Anteroposterior (left) and sagittal (right) spine radiographs demonstrate significant kyphotic deformity due to L2–3 collapse.
FIG. 2.
Sagittal (A), parasagittal (B), and coronal (C) computed tomographic scans showing advanced bony compromise at the L2 and L3 vertebrae.
FIG. 3.
Sagittal (A), parasagittal (B), and axial (C) magnetic resonance imaging showing disc-bony compromise and bilateral psoas abscesses. No epidural involvement was observed.
FIG. 4.
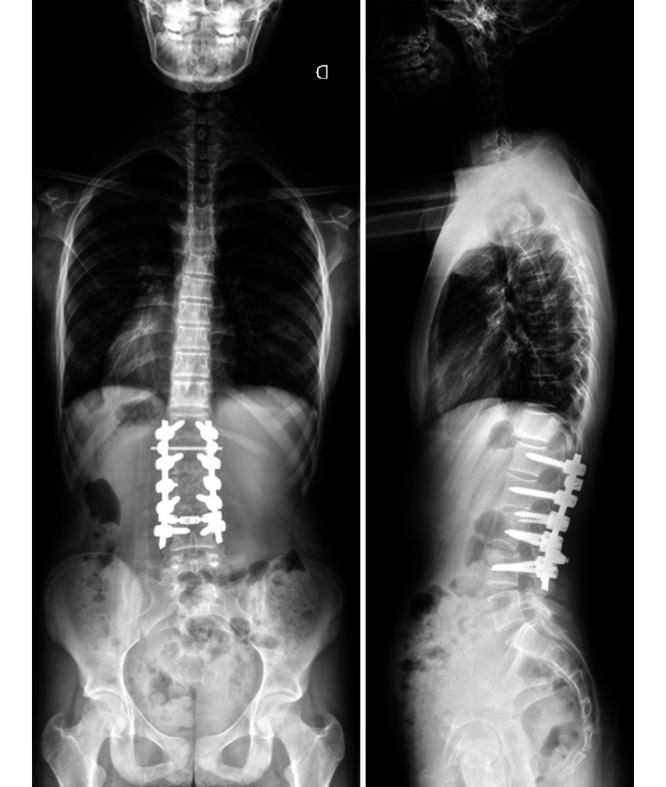
Two-year postoperative follow-up anteroposterior (left) and sagittal (right) radiographs with balanced spine and solid fusion.
Discussion
Observations
Cases of PSI managed surgically in pediatric patients might behave differently in terms of epidemiology, clinical presentation, and outcomes when compared with cases managed nonoperatively or in nonpediatric patients. A systematic literature search was performed in PubMed, Web of Science, and Google Scholar in February 2022 to identify studies reporting the outcome of spinal infection in pediatric patients who underwent surgical intervention of the spine. The search strategy was developed by one author (R.S.B.) by consulting the Peer Review of Electronic Search Strategies criteria.6 The search strategy for PubMed, Web of Science, and Google Scholar is displayed in Appendix A in the supplemental material. We performed the literature search with records filtered from 2010 to February 2022. Records identified through the searches were added to a database, and duplicates were removed. Titles and abstracts from PubMed, Web of Science, and Google Scholar were screened by one author (R.S.B.). The articles were limited to human studies published in English. Editorials, reviews, and letters to the editor were excluded. Data were collected into a table by one author (R.S.B.), and another author (A.J.F.) monitored all entries for completeness and accuracy. This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.7 Appendix B in the supplemental material shows the study selection process. Upon careful review, 12 articles published between 2011 and 2022 were selected on the basis of inclusion and exclusion criteria for the present systematic review.8–19 These included 2 case series and 10 case reports (Table 1). The sample size across all studies was 57 patients, with 39 remaining after application of inclusion and exclusion criteria. This cohort of 39 patients exhibited an age range of 1 month to 15 years. The mean age was 8 years. There were 20 males (53%) and 18 females (47%). Sex was not reported for 1 patient. Etiologies for spinal disease were reported for all 39 patients and included tuberculosis spondylodiscitis (n = 29; 74.3%), vertebral osteomyelitis (n = 5; 12.8%), spondylodiscitis (n = 3; 7.7%), discitis (n = 1; 2.6%), and spondylitis (n = 1; 2.6%).
TABLE 1.
Demographic and clinical variables of included studies
| Authors & Year | Study Design | No. of Pts | Age, Sex | Pathology | Pts w/ Pain | Pts w/ Neurological Deficit | Level of Manifestation | Main Microorganism | Surgery | Antimicrobial Treatment Duration | FU |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arockiaraj et val., 20188 |
CS |
6 |
Mean 12 yrs, 2 M, 1 F |
Tuberculosis spondylodiscitis (3) |
3 |
2 |
Thoracic (3), multifocal (1) |
Mycobacterium tuberculosis (3) |
Pst decompression (2), costotransversectomy (1) |
NA |
Mean 12 mos |
| Banerjee et al., 20119 |
CR |
1 |
4 wks, M |
C1–2 vertebral osteomyelitis |
NA |
1 |
Cervical (1) |
Staphylococcus aureus
|
Surgical drainage of RPA |
NA |
NA |
| Glotzbecker et al., 201510 |
CR |
1 |
3 mos, M |
C1–2 vertebral osteomyelitis |
NA |
1 |
Cervical (1) |
Staphylococcus aureus
|
Occiput - C2 pst instrumented fusion |
NA |
50 mos |
| Imakiire et al., 202011 |
CR |
1 |
3 yrs, F |
Bacillus Calmette-Guérin–associated cervical spondylitis |
0 |
0 |
Cervical (1) |
Mycobacterium bovis |
Extracorporeal fusion; bone transplant, on collapsed C4 vertebrae |
9 mos |
12 mos |
| Ishihama et al., 202012 |
CR |
1 |
9 yrs, F |
L5–S1 spondylodiscitis |
1 |
1 |
Lumbar (1) |
Staphylococcus aureus
|
Transforaminal discectomy |
NA |
12 mos |
| Karim et al., 202213 |
CR |
1 |
15 yr, M |
L5–S1 discitis |
1 |
0 |
Lumbar (1) |
Klebsiella aerogenes |
Abscess drainage |
6 mos |
9 mos |
| Papp et al., 201314 |
CR |
1 |
4 wks, M |
C1–2 vertebral osteomyelitis, T5–7 epidural abscess |
NA |
1 |
Cervical (1) |
Staphylococcus aureus
|
Hemisemilaminectomy; RPA & epidural abscess drainage |
6 wks |
36 mos |
| Park et al., 201715 |
CR |
1 |
6 mos, NA |
C2 odontoid osteomyelitis |
1 |
0 |
Cervical (1) |
Staphylococcus aureus
|
Surgical drainage of RPA |
3 mos |
18 mos |
| Pinto et al., 202116 |
CS |
41 |
Mean 9 yrs, 11 M, 15 F |
Tuberculosis spondylodiscitis (26) |
NA |
26 |
Cervical (2), thoracic (22), lumbar (7), multifocal (4) |
Mycobacterium tuberculosis (26) |
Pst decompression alone (6); decompression, pst instrumented fusion (8); decompression, pst instrumented fusion, ant reconstruction (12) |
NA |
Mean 31 mos |
| Romano et al., 202117 |
CR |
1 |
4 wks, M |
T12–L1 spondylodiscitis |
NA |
0 |
Thoracolumbar (1) |
Staphylococcus aureus
|
Decompression, pst instrumented T11–L2 fusion, ant reconstruction |
NA |
9 yrs |
| Tsirikos & Tome-Bermejo, 201218 |
CR |
1 |
8 wks, M |
T4–5 spondylodiscitis |
1 |
0 |
Thoracic (1) |
Staphylococcus aureus
|
T2–7 pst spinal fusion, T3–6 ant spinal fusion |
11 mos |
4 yrs |
| Vibert et al., 201819 | CR | 1 | 13 yrs, M | T11–L1 vertebral osteomyelitis | 1 | 1 | Thoracolumbar (1) | Staphylococcus aureus | T12–L2 kyphectomy, T7–S1 pst instrumentation | 3 mos | 18 mos |
CR = case report; CS = case series; FU = follow-up; NA = not applicable; pst = posterior; Pts = patients; Tx = treatment.
Clinical Presentation
Local pain, fever, and septic manifestation were reported for 9 patients, of whom 88.9% (n = 8) presented with local pain, 55.6% (n = 5) presented with fever, and 55.6% (n = 5) presented with septic manifestation. Neurological deficit was present in 33 patients (84.6%). Individual leukocyte and CRP levels were reported for 6 patients, of whom 66.7% (n = 4) presented with elevated WBC counts and 83.3% (n = 5) presented with elevated CRP levels. Level of spinal infection was reported for all 39 patients, of whom 17.9% (n = 7) presented with cervical infection, 71.8% (n = 28) presented with thoracic infection, and 28.2% (n = 11) presented with lumbar infection. Multifocal spinal infection was reported in 12.8% (n = 5) of patients. The presence of epidural abscess was reported in 12.8% (n = 5) of patients. Other abscesses were reported in 33.3% (n = 13) of patients and included prevertebral, paraspinal, and retropharyngeal abscesses.
Causative Microorganisms
M. tuberculosis was reported as the main causative microorganism in 74.4% (n = 29) of the patients. Other causative microorganisms included Staphylococcus aureus 20.5% (n = 8), Mycobacterium bovis 2.6% (n = 1), and Klebsiella aerogenes 2.6% (n = 1). A Gram-negative microorganism infected 1 patient. Antimicrobial duration after surgical intervention was reported for 6 patients, for whom the average duration of antimicrobial treatment was 5.6 months. There were no reports of antimicrobial treatment failure.
Methods of Surgical Intervention
Operative management for spinal disease was undertaken for all 39 patients. The most frequently performed procedure, performed in 14 patients (35.9%), was spinal cord decompression along with a combined anterior and posterior approach with instrumented fusion. Ten patients (25.6%) underwent spinal cord decompression with posterior instrumented fusion only. Eight patients (20.5%) underwent posterior spinal cord decompression only. Three patients (7.7%) underwent surgical drainage of an abscess only. One patient (2.6%) underwent posterior spinal cord decompression combined with abscess drainage. One patient (2.6%) was treated with transforaminal full endoscopic discectomy. One patient (2.6%) was treated with vertebral extracorporeal fusion. One patient (2.6%) was treated with costotransversectomy.
Postoperative Complications
Postoperative complications occurred in 15.4% (n = 6) of cases. Two patients’ (33.3%) postoperative complications involved wound dehiscence with either associated screw loosening (n = 1) or exposed implant (n = 1). Two patients’ (33.3%) complications involved vertebral body collapse, 1 of which presented with increased neurological deficit. One patient’s complication involved implant breakage. One patient’s complication involved progressive deformity and increasing kyphosis. Mean follow-up was reported for 38 patients, with an average of 30.3 months, ranging from 9 months to 9 years. There were no reported deaths.
Limitations
Our study has some limitations, first, all data are provided by case series and case reports; therefore, the quality of this study relies on the quality of the included articles. However, considering the relatively rare occurrence of surgical intervention for spinal disease in the pediatric population, the best and most recent evidence has been gathered in this review. The presence or absence of local pain was reported for 9 patients, of whom 8 patients had local pain. However, with regard to the 39 patients who were included in this systematic review, patient ages ranged from 1 month to 15 years. It is difficult to report on the presence or absence of local pain in the neonatal and infant populations, thus potentially altering our measurements of local pain presenting as a clinical characteristic. Furthermore, regarding the causative microorganism and diagnosis of spinal infection, we solely discuss surgical cases and held off on discussion of nonsurgical cases. Finally, we limited the search to between 2010 to 2022; even though this could represent a selection bias, we decided to provide the most recent evidence in the literature in order to summarize the standard algorithms of conservative and surgical treatment.
Lessons
In general, PSIs are classified as pyogenic and nonpyogenic infections with further subclassification according to the compromised anatomical structure. These include infection of the vertebral disc unit (spondylodiscitis), spinal canal (epidural abscess), muscle (psoas abscess), and spinal cord and nerves (intramedullary spinal cord abscess). Most often, surgery, such as laminectomy, discectomy, or abscess drainage, is secondary to initial management with an antibiotic course and supportive brace. For example, in a study by Ferri et al.20 of 340 pediatric patients, only 20 children (5.88%) underwent surgery. The resistance for electing surgery is largely due to the ever-changing nature of the pediatric skeleton; as such, the pathophysiological mechanism behind osteomyelitis in this population evolves over time. Furthermore, spine surgery is a major undertaking, especially in a pediatric patient.
The dynamic nature of the pediatric skeleton has been studied largely in long bones, where it is believed that newborns tend to have infections that spread from the metaphysis to adjacent joints due to transphyseal vessels, whereas older infants and children tend to have infections that are confined to the metaphysis and medulla due to the lack of transphyseal vessels and thicker cortices.21 Recently, studies showing the lack of pure discitis in the young infant age group has been attributed to the extensive vascular ring that is found in infants and young children that creates an anastomosis of the vascular rings at the superior and inferior vertebral levels via branches next to the posterolateral region of the disc. Similar to the theory involving long bones, it has been postulated that infectious agents are able to cross the cartilaginous vertebral plate in infants up until age 8 and thus get into the superior/inferior vertebral metaphysis and the disc space through the transphyseal vascular anastomoses.22 Currently, there is some debate on the typical age range of pediatric PSIs; some describe a triphasic distribution,23 whereas others describe a biphasic distribution.22 Our study hopes to clarify this through an extensive systematic review.
In terms of epidemiology, clinical presentation, and outcomes, PSIs in pediatric patients requiring surgical management might behave differently when compared with (1) PSIs in the nonpediatric population and (2) PSIs in the pediatric population not requiring operative management. Regarding the clinical presentation, localized pain (observed in 88.9%) was the most often reported symptom. In a similar vein, we found that neurological compromise and elevated CRP levels (observed in 84.6% and 83.3%, respectively) were highly reported as clinical symptoms of pediatric spinal infection. It is of interest to note that fever (55.6%), septic manifestation (55.6%), and elevated WBC count (66.7%) were reported as slightly less common symptoms, but they were still present in more than half of the patients for whom the clinical indication was reported. Our reports of the clinical presentations of pediatric spinal infections ultimately managed with surgical intervention conflict with observations in studies involving large series of nonoperatively managed pediatric spinal infections, suggesting different clinical presentations of spinal infection between the 2 groups. In 237 pediatric patients nonoperatively treated for spinal infection, Fernandez et al.24 observed the presence of localized pain in only 37.97% of patients. In 340 pediatric patients nonoperatively treated for spinal infection, Ferri et al.20 observed the presence of neurological manifestation in only 3.8% of patients, a stark contrast to the 84.6% neurological involvement we report in this review of surgically managed pediatric spinal disease cases. Interestingly, Ferri et al.20 observed fever as a clinical symptom in 33.23% of their 340 included patients, which is more in line with our finding of 55.6%. In a large multicenter study of 103 cases of pediatric spinal disease managed nonoperatively, Dayer et al.22 observed a mean CRP level of 19.1 mg/L and a mean WBC count of 11,020/mm3. This finding by Dayer et al. is in line with our observation of elevated CRP and WBC counts in 83.3% and 66.7% of patients, respectively. With regard to clinical manifestations of pediatric spinal disease that is eventually managed operatively and how it differs from nonoperatively managed pediatric spinal disease, it appears that there are much higher rates of localized pain and neurological compromise. It is important to note that neonates and infants lack the ability to express themselves, which could result in difficult and delayed diagnosis. Rates of elevated CRP levels and WBC counts seem to be similar between surgically and conservatively treated groups.
Invasive pathogens can infect the spine via 3 main pathways: (1) hematogenous spread, (2) contiguous spread, and (3) external inoculation. The predominant form of infection is hematogenous spread, which can be arterial or venous, via Batson’s venous plexus.25 The pediatric population differs from adults with regard to the vascularization of the disc-endplate unit. In pediatric patients, the intervertebral disc is vascularized by a rich network of vessels. This blood supply extends into the metaphyseal ring. A comprehensive understanding of the vascular physiology of the disc-endplate unit in the pediatric population and how it differs from that in the adult population is crucial for understanding the specific clinical presentations, treatments, and outcomes in pediatric spinal disease. In nonsurgically managed cases of pediatric spinal infection, the lumbar/lumbosacral spine is the most common level of involvement.22,26,27 This high level of lumbar involvement reflects the high volume of blood flow in the region and therefore increased rates of pathogens traveling in the area. In our study, we observed a high level of thoracic spinal infection (71.8%), followed by lumbar (28.2%) and cervical (17.9%) infection. The high level of thoracic involvement in pediatric spinal infections that are eventually surgically managed is explained by the high proportion of M. tuberculosis infections (74.4%) that we observed in our included patients. Previous studies likewise found M. tuberculosis to be the most common agent, though the lumbar spine was most affected in cases of spondylodiscitis. Up to 75% of pediatric spondylodiscitis patients in a comprehensive literature review were afflicted with infection of the lumbar region.28 The present study provides a layer of distinction in affected regions for surgical and nonsurgical cases, although further research must be conducted before settling on a reliable diagnosis pattern. In tuberculosis infection, major risk factors for the development of a severe kyphotic deformity and subsequent vertebral column collapse are pediatric age and thoracic involvement. Indications for surgical management of pediatric tuberculosis spinal infection include severe kyphotic deformity.29 When excluding the M. tuberculosis infections from our study, it is observed that 50% of the patients had a cervical level of spinal infection. This suggests that cervical spinal infection in pediatric patients is more involved and requires greater intervention.
It is of interest to note that multifocal infections were present in 12.8% of our included patients at the time of diagnosis. This is in line with the values previously reported in the literature for rates of multifocal involvement in spinal infection.30,31 However, no direct relationship has been found between multifocal involvement and severity of spinal infection in the literature.31
In the literature, the main documented causative microorganism of pediatric spinal infection is M. tuberculosis, with S. aureus most common among pyogenic infections.28,32 This is quite similar to our observation of 74.4% M. tuberculosis and 20.5% S. aureus infections. Referencing back to the rich vascular network of the disc-endplate unit in the pediatric population, this provides reasoning for high levels of satisfactory outcomes of treating pediatric spinal infection with intravenous antibiotics.33–35 In contrast, indications for surgical management of pediatric spinal disease include lack of response to antibiotics, progressive kyphosis for M. tuberculosis infections, or neurological compromise.35 Further reasons to pursue surgical management include the need for spinal stabilization and diagnostic findings of vertebral body deterioration.
Outcomes in nonsurgically treated pediatric patients were grossly favorable, with Ferri et al.20 reporting only 1 case (0.3%) of recurrence of infection in an observation of 340 patients. It has been said in the literature that because the posterior elements of the vertebral bodies are less vascularized, nonsurgical management may be more prone to incomplete treatment.36 In our observation of 39 surgically managed patients, we reported 6 cases (15.4%) of postoperative complications, which included 2 cases of wound dehiscence, 2 cases of vertebral body collapse, 1 case of instrumentation failure, and 1 case of increasing kyphotic deformity. The differing rates of negative outcomes in the surgically managed cases relative to the conservatively managed cases can be attributed to the fact that cases requiring surgery have more involved infections presenting with neurological involvement and complex deformities. Also, the inherent risks of undergoing surgery are important to take into consideration. Otherwise, the use of surgery improved the patient’s outcome and played a foundational part of the treatment plan when conservative management had failed. Attempts to elucidate patterns have produced signs of potential utility. This has included raised erythrocyte sedimentation rates with back pain and fever with signs of meningitis for epidural and subdural abscesses, respectively.28,37 Neurological deficits and the respective sequalae are often the primary concern with PSIs. They have presented at rates between 37% and 75% in pediatric PSIs, though such a broad range hinders their potential as a pathognomonic indicator. Overall, the coexisting tendency of spondylodiscitis and these other pathologies, in addition to the nonspecific signs, has made it difficult to make precise diagnoses, however. The literature suggests a highly suspicious approach in addressing PSIs if favoring a conservative response preceding surgical treatment.
Disclosures
Dr. Bhatia reported personal fees from Alphatec, Biomet/Zimmer, Seaspine, Spineart, Lifespine, and Aurora Spine outside the submitted work. No other disclosures were reported.
Author Contributions
Conception and design: Franklin, Hatter, Brown, Camino-Willhuber, Oh, Bhatia, Lee. Acquisition of data: Beyer, Franklin, Davies, Bhatia, Lee. Analysis and interpretation of data: Beyer, Hatter, Camino-Willhuber, Hashmi, Oh, Bhatia, Lee. Drafting the article: Beyer, Franklin, Hatter, Nguyen, Brown, Bhatia, Lee. Critically revising the article: Beyer, Hatter, Brown, Camino-Willhuber, Hashmi, Oh, Bhatia. Reviewed submitted version of manuscript: Beyer, Franklin, Hatter, Nguyen, Brown, Hashmi, Oh, Bhatia. Approved the final version of the manuscript on behalf of all authors: Beyer. Statistical analysis: Beyer. Study supervision: Brown, Camino-Willhuber, Hashmi.
Supplemental Information
Online-Only Content
Supplemental material is available with the online version of the article.
Appendices A and B. https://thejns.org/doi/suppl/10.3171/CASE22204.
References
- 1. Tyagi R. Spinal infections in children: a review. J Orthop. 2016;13(4):254–258. doi: 10.1016/j.jor.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roversi M, Mirra G, Musolino A, et al. Spondylodiscitis in children: a retrospective study and comparison with non-vertebral osteomyelitis. Front Pediatr. 2021;9:727031. doi: 10.3389/fped.2021.727031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26–e46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 4. Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56(6):401–412. doi: 10.1016/j.jinf.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 5. Huang QS, Zheng C, Hu Y, et al. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop. 2009;33(5):1385–1390. doi: 10.1007/s00264-009-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Accessed February 17, 2022. http://prisma-statement.org/
- 8. Arockiaraj J, Robert M, Rose W, Amritanand R, David KS, Krishnan V. Early detection and analysis of children with multidrug-resistant tuberculosis of the spine. Asian Spine J. 2019;13(1):77–85. doi: 10.31616/asj.2017.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banerjee J, Bhojani S, Nerminathan V. Generalised hypotonia following erosive vertebral osteomyelitis in an infant. Infant. 2011;7:16–19. [Google Scholar]
- 10. Glotzbecker MP, Wasser AM, Troy MJ, Proctor M, Emans JB. Neonatal C1 TO C2 osteomyelitis leading to instability and neurological decline: novel treatment with occiput-C1-C2 fusion and occiput to thorax growing rods. A case report. J Pediatr Orthop. 2015;35(4):379–384. doi: 10.1097/BPO.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 11. Imakiire R, Nishikawa T, Tominaga H, et al. Bacillus Calmette-Guérin-associated cervical spondylitis in a 3-year-old immunocompetent girl. Pediatr Infect Dis J. 2020;39(12):e466–e469. doi: 10.1097/INF.0000000000002893. [DOI] [PubMed] [Google Scholar]
- 12. Ishihama Y, Sakai T, Manabe H, et al. Debridement for infectious spondylodiscitis in a 9-year-old girl using full-endoscopic discectomy system: a case report and literature review. J Med Invest. 2020;67(3.4):344–351. doi: 10.2152/jmi.67.351. [DOI] [PubMed] [Google Scholar]
- 13. Karim F, Patel M, Barr LL, Maurta-Neumann PJ, Litra F. Klebsiella discitis in a 15-year-old male diagnosed with plasma microbial cell-free DNA next-generation sequencing test: a case report. Cureus. 2022;14(1):e21237. doi: 10.7759/cureus.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papp Z, Czigléczki G, Banczerowski P. Multiple abscesses with osteomyelitis and destruction of both the atlas and the axis in a 4-week-old infant. Spine (Phila Pa 1976) 2013;38(19):E1228–E1230. doi: 10.1097/BRS.0b013e31829cf0a7. [DOI] [PubMed] [Google Scholar]
- 15. Park H, Byeon HK, Kim HS, Hong JJ, Suk KS. Odontoid osteomyelitis with atlantoaxial subluxation in an infant. Eur Spine J. 2017;26(suppl 1):136–140. doi: 10.1007/s00586-016-4919-0. [DOI] [PubMed] [Google Scholar]
- 16. Pinto D, Dhawale A, Shah I, et al. Tuberculosis of the spine in children – does drug resistance affect surgical outcomes? Spine J. 2021;21(12):1973–1984. doi: 10.1016/j.spinee.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 17. Romano S, Vittoria F, Cattaruzzi E, Barbi E, Carbone M. Infectious spondylodiscitis and kyphosis correction in an infant: a case report. Ital J Pediatr. 2021;47(1):152. doi: 10.1186/s13052-021-01106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsirikos AI, Tome-Bermejo F. Spondylodiscitis in infancy: a potentially fatal condition that can lead to major spinal complications. J Bone Joint Surg Br. 2012;94(10):1399–1402. doi: 10.1302/0301-620X.94B10.29602. [DOI] [PubMed] [Google Scholar]
- 19. Vibert B, Turati M, Rabattu PY, Bigoni M, Eid A, Courvoisier A. Congenital lumbar kyphosis with skin ulceration and osteomyelitis in a myelomeningocele child: a case report. Childs Nerv Syst. 2018;34(4):771–775. doi: 10.1007/s00381-017-3598-4. [DOI] [PubMed] [Google Scholar]
- 20. Ferri I, Ristori G, Lisi C, Galli L, Chiappini E. Characteristics, management and outcomes of spondylodiscitis in children: a systematic review. Antibiotics (Basel) 2020;10(1):30. doi: 10.3390/antibiotics10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogden JA. Pediatric osteomyelitis and septic arthritis: the pathology of neonatal disease. Yale J Biol Med. 1979;52(5):423–448. [PMC free article] [PubMed] [Google Scholar]
- 22. Dayer R, Alzahrani MM, Saran N, et al. Spinal infections in children: a multicentre retrospective study. Bone Joint J. 2018;100-B(4):542–548. doi: 10.1302/0301-620X.100B4.BJJ-2017-1080.R1. [DOI] [PubMed] [Google Scholar]
- 23. Saleh ES, Vasileff CC, Omari AM, Khalil JG. The diagnosis and management of pediatric spine infections. Cureus. 2021;13(7):e16748. doi: 10.7759/cureus.16748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernandez M, Carrol CL, Baker CJ. Discitis and vertebral osteomyelitis in children: an 18-year review. Pediatrics. 2000;105(6):1299–1304. doi: 10.1542/peds.105.6.1299. [DOI] [PubMed] [Google Scholar]
- 25. Fucs PM, Meves R, Yamada HH. Spinal infections in children: a review. Int Orthop. 2012;36(2):387–395. doi: 10.1007/s00264-011-1388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chandrasenan J, Klezl Z, Bommireddy R, Calthorpe D. Spondylodiscitis in children: a retrospective series. J Bone Joint Surg Br. 2011;93(8):1122–1125. doi: 10.1302/0301-620X.93B8.25588. [DOI] [PubMed] [Google Scholar]
- 27. Henton JM, Dabis HS. Discitis and epidural abscess after dental extraction in a pediatric patient: a case report. Pediatr Emerg Care. 2009;25(12):862–864. doi: 10.1097/PEC.0b013e3181c8c60b. [DOI] [PubMed] [Google Scholar]
- 28. Mohanty CB, Fieggen G, Deopujari CE. Pediatric spinal infections-a review of non-tuberculous infections. Childs Nerv Syst. 2018;34(10):1947–1956. doi: 10.1007/s00381-018-3885-8. [DOI] [PubMed] [Google Scholar]
- 29. Issack PS, Boachie-Adjei O. Surgical correction of kyphotic deformity in spinal tuberculosis. Int Orthop. 2012;36(2):353–357. doi: 10.1007/s00264-011-1292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stangenberg M, Mende KC, Mohme M, et al. Influence of microbiological diagnosis on the clinical course of spondylodiscitis. Infection. 2021;49(5):1017–1027. doi: 10.1007/s15010-021-01642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henkelmann J, Denecke T, Pieroh P, et al. Total spine magnetic resonance imaging for detection of multifocal infection in pyogenic spondylodiscitis: a retrospective observational study. BMC Musculoskelet Disord. 2021;22(1):78. doi: 10.1186/s12891-020-03928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(suppl 3):iii11–iii24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 33. Waizy H, Heckel M, Seller K, Schroten H, Wild A. Remodeling of the spine in spondylodiscitis of children at the age of 3 years or younger. Arch Orthop Trauma Surg. 2007;127(6):403–407. doi: 10.1007/s00402-007-0316-9. [DOI] [PubMed] [Google Scholar]
- 34. Crawford AH, Kucharzyk DW, Ruda R, Smitherman HC., Jr Diskitis in children. Clin Orthop Relat Res. 1991;(266):70–79. [PubMed] [Google Scholar]
- 35. Brown R, Hussain M, McHugh K, Novelli V, Jones D. Discitis in young children. J Bone Joint Surg Br. 2001;83(1):106–111. doi: 10.1302/0301-620x.83b1.10865. [DOI] [PubMed] [Google Scholar]
- 36. Buoncristiani AM, McCullen G, Shin AY, Bathgate B, Akbarnia BA. An unusual cause of low back pain. Osteomyelitis of the spinous process. Spine (Phila Pa 1976) 1998;23(7):839–841. doi: 10.1097/00007632-199804010-00022. [DOI] [PubMed] [Google Scholar]
- 37. Sandler AL, Thompson D, Goodrich JT, et al. Infections of the spinal subdural space in children: a series of 11 contemporary cases and review of all published reports. A multinational collaborative effort. Childs Nerv Syst. 2013;29(1):105–117. doi: 10.1007/s00381-012-1916-4. [DOI] [PubMed] [Google Scholar]