Abstract
Production of new cells as a result of progression through the cell division cycle is a fundamental biological process for the perpetuation of both unicellular and multicellular organisms. In the case of plants, their developmental strategies and their largely sessile nature has imposed a series of evolutionary trends. Studies of the plant cell division cycle began with cytological and physiological approaches in the 1950s and 1960s. The decade of 1990 marked a turn point with the increasing development of novel cellular and molecular protocols combined with advances in genetics and, later, genomics, leading to an exponential growth of the field. In this article, I review the current status of plant cell cycle studies but also discuss early studies and the relevance of a multidisciplinary background as a source of innovative questions and answers. In addition to advances in a deeper understanding of the plant cell cycle machinery, current studies focus on the intimate interaction of cell cycle components with almost every aspect of plant biology.
Keywords: cell cycle, cell division, retinoblastoma, DNA replication, geminivirus, Arabidopsis, plant
1. Introduction
The iterative mode of plant organogenesis during postembryonic growth requires a continuous supply of new cells. Therefore, the cell division cycle is not only of primary relevance but also a process strictly coordinated with morphogenesis. It is generally accepted that the cell division cycle results from the occurrence of processes, highly regulated in time and space, leading to the production of two daughter cells. In all cases, genome duplication occurs during S-phase and genome (chromosome) segregation during mitosis (M), that includes cytokinesis to separate the two division products. S and M are separated by the pre-replicative G1 and the post-replicative G2 phases. Progression through S and M is dictated by the activity of cyclin-dependent kinases (CDKs)/cyclin complexes, which is regulated mainly at the quantitative level [1]. CDK activity also depends on the nature of cyclins and their availability, the presence of CDK inhibitors, and the activation/inhibition of the CDK moiety by protein phosphorylation and phosphatase balance.
Advances in gene discovery clearly supported the conclusion that the eukaryotic cell cycle machinery has been highly conserved throughout evolution from the unicellular ancestors to the current variety of multicellular forms of life. The Arabidopsis genome encodes for ~40 cyclins, a variety of CDK inhibitors (KRPs, SIM and SMR) and various CDK activating kinases and phosphatases.
In addition, there are other cyclic events coordinated with those occurring throughout these phases, such as the CDK/cyclin activity peaks, frequently in mid/late G1 and late G2, cytokinesis, centromere maturation, chromatin remodeling, among others. All of them are highly regulated to function in an unidirectional manner [1,2], as a consequence of several redundant or complementary pathways, e.g., gene expression, subcellular localization, post-translational modifications or targeted proteolysis. In the case of plants, the cell cycle is coordinated with morphogenesis by the direct interaction of cell cycle regulators with plant-specific cell fate pathways. However, there are a number of features that are universal to all eukaryotic cell cycles.
2. Where Do We Come From?
Why are we interested in plant cell cycle, DNA replication and chromatin? Understanding the reasons to launch a research project to study various aspects of cell proliferation in plants in 1993 requires a few considerations. It was not the result of a Eureka moment in the middle of a night dream, as advances in science are frequently presented. The main reason to choose a model and a project with a long-term projection was the consequence of a previous background in apparently disparate topics, several in our case. These go deep into several years before launching the project, when I had a direct experience with different research interests and model systems, something that with the perspective of time, I see as a very useful and important source of freedom to tackle scientific questions.
During my PhD, I was trained in a plant cell biology laboratory, then I worked on DNA repair in plants (see also below) and wanted to gain experience in molecular biology in mammalian cells. In the mid-1980s, I was studying the molecular basis of eukaryotic DNA replication, in particular the initiation steps, using animal oncoviruses, mainly SV40 as a model, in the laboratory of Mel DePamphilis (https://www.nichd.nih.gov/research/atNICHD/Investigators/depamphilis; accessed on 20 July 2022). The SV40 genome is a small double-stranded DNA (dsDNA) circular molecule that replicates in the nucleus of the infected mammalian cells, where it associates with host nuclear histones to form nucleosomes. Therefore, at that time, SV40 as well as other oncoviruses such as adenoviruses and polyomaviruses were considered the most suitable and amenable model systems to study eukaryotic DNA replication. Initiation of SV40 DNA replication requires a single virally encoded protein, the T-antigen (T-ag), that also regulates transcription of other viral genes [3,4]. Using reconstitution experiments, various laboratories established in vitro DNA replication assays, which were crucial for the identification of many cellular components involved in viral DNA replication such as several DNA polymerases and accessory factors, PCNA, RFC, RPA, among others. We focused on the interaction of various forms of T-ag with the SV40 DNA replication origin [5], studies that later were instrumental in the initial stages of our laboratory. The SV40 T-ag was also well-known as a key viral protein that stimulates proliferation of the infected cell, and establishes a host cell environment full of cellular factors suitable for efficiently complete the viral replication cycle.
While still working in this project, J. M. Sogo (now retired from the Institute of Cell Biology in Zürich) spent a sabbatical stay in the DePamphilis laboratory. I joined him in a project using psoralen crosslinking and electron microscopy strategies, which were developed by him, to study how the SV40 DNA molecule associates with histones to form nucleosomes [6]. These studies deeply influenced our interest not only in chromatin dynamics but also in DNA-protein complexes during my time in the laboratory of Margarita Salas, back in Madrid [7,8]. This provided a useful background in protein-DNA interactions and electron microscopy, also very relevant at the initial stages of our laboratory. In fact, acquiring a background in different disciplines is beneficial not only to approach question from complementary perspectives but also to become more versatile in the daily laboratory life. Regarding the relevance and advantage of having a multidisciplinary background, I would like to quote Ardem Patapoutian, awarded the Nobel Prize in Physiology or Medicine in 2021: “Perhaps the most important decision for scientists is to decide which questions to ask. I’ve found it helpful to talk to people outside my immediate field. Explaining the impact of your research to a broader audience is a strong litmus test”.
3. Early Studies
The decision to choose a topic to launch a long-term independent project takes time for reflection, and certainly it is permeated and enriched by various inputs. I remember trying to identify a model system where I could apply my previous background and enter a field with sufficient freedom to ask questions from a different, sometimes unusual, perspective. I thought of combining approaches to integrate DNA-protein complexes, DNA replication and cell proliferation, ideally in a viral system that looked relatively simple, where I could establish a suitable scientific niche. It was not trivial, but in the early 1990s, I started to learn about geminiviruses, a family of relatively poorly known plant viruses of small ssDNA genomes that encode only a few proteins and replicate in the host cell nucleus [9] through dsDNA intermediates that could form nucleosomes [10]. My impression was that these plant viruses really looked like SV40!
I attended a seminar in 1992 delivered by Philip Mullineaux (https://www.essex.ac.uk/people/mulli06704/philip-mullineaux; accessed 20 July 2022), who at that time worked at the John Innes Centre in Norwich, in which he reported studies with the wheat dwarf geminivirus (WDV). It was a pleasure and very enlightening to have a long discussion with him about geminivirus biology. I soon recognized the potential of geminiviruses as excellent models to tackle the kind of questions I had in mind to define mechanisms of initiation of DNA replication and also how the viral proteins functionally interacted with the host machinery. This was a convincing set of arguments to justify launching a project based on geminivirus biology. A short stay in the Mullineaux’s laboratory and his generosity to provide wheat cell cultures, viral vectors and other materials facilitated enormously the initial experiments on two complementary roads: one focused on the mechanisms of viral DNA replication and another on virus–host cell interactions, in particular, with the host cell proliferation machinery.
Regarding the mechanism of WDV genome replication at the dsDNA stage, we applied our SV40 background to identify minimal sequences required for initiation of DNA replication, their DNA sequence-based structural properties, the protein complexes formed by the early proteins RepA and Rep, and the host cell proteins loaded after the initial step of Rep-mediated nicking [11,12,13,14]. Further studies on various members of the geminivirus family have been reviewed elsewhere [15,16,17]. The early days in our laboratory were also marked by a close relationship with Eduardo Rodriguez-Bejarano (https://www.ihsm.uma-csic.es/investigadores/27; accessed 20 July 2022), who organized the 1st International Symposium on Geminivirus (Almeria, 1994) and was very kind to invite us to participate, present our work and introduce our new lab to the geminivirus world.
The field of DNA replication initiation complexes in eukaryotic viruses and cells was hot at that time, and I recall a key presentation in a CSHL meeting where the Stillman’s laboratory presented the identification of the Origin Recognition Complex (ORC), the putative initiator complex, functionally equivalent to the SV40 T-ag [18]. Everyone thought that our understanding of how eukaryotic cells initiate DNA replication was solved. It was certainly a seminal work, although it has taken several decades to obtain a more detailed account of its complexity and how far we still are from fully understanding how the interaction of ORC and DNA replication origins work, in the context of a replicating chromatin molecule [19,20].
In line with this discovery and our interest in viral and cellular DNA replication, we initiated a long-term project to identify components of the initiation of DNA replication in plant cells. It is worth keeping in mind that this was years before any plant genome sequences were available, and therefore, the effort relied on screening EST (expression sequence tags) collections and cDNA libraries by Southern blots and PCR amplification based on DNA sequence information of putative plant homologs. This led us to isolate cDNA clones encoding several proteins involved in the formation of the pre-replication complexes (pre-RC), such as CDC6, CDT1 and ORC1 [21,22,23] (see also below).
The other laboratory project was aimed at understanding the molecular basis of the functional interaction of geminiviruses with the host cell cycle machinery. This was virtually a black box, although it was known that replicative forms of geminiviruses were abundant in S-phase cells [24]. This side of the original project had deep roots into (i) my early work of cell proliferation kinetics and DNA repair in Allium cepa meristems [25,26,27,28,29], and (ii) my previous experience with SV40 T-ag interactions with the host cell. The identification in 1987 of the human homolog of the yeast p34Cdc2 protein [30], the main cyclin-dependent kinase (CDK) active during the cell cycle triggered the interest in several laboratories to ask whether plant cells would have a similar protein. This led to the original finding of Cdc2 kinase homolog and its putative cyclin partners of the A- and B-types [31,32,33,34,35,36].
The accumulated evidence at that time strongly supported the prevailing view that plants, as evolutionary old organisms, might have a cell cycle control similar to that of yeasts. Our studies of the different domains and amino acid motifs of the WDV RepA early protein pointed to a very different direction. I acknowledge my frequent and highly illuminating discussions with Manuel Serrano (https://www.irbbarcelona.org/en/research/manuel-serrano; accessed 20 July 2022; https://altoslabs.com/team/principal-investigators-cambridge/manuel-serrano/; accessed 20 July 2022), who was then a postdoc with David Beach (CSHL), where he identified p16, the first CDK inhibitor identified in human cells [37]. These discussions on how the cell cycle is triggered by oncoviruses were instrumental to identify a short amino acid motif (LxCxE) in the WDV RepA protein. Remarkably, this motif was also present in the early proteins of animal oncoviruses, responsible for their interaction with the human tumor suppressor retinoblastoma (RB) protein and other so-called “pocket” proteins (p107 and p130). That was highly suggestive that the LxCxE motif of the geminivirus RepA might well be functionally equivalent to that of SV40 Tag and that it might mediate interaction with a putative plant retinoblastoma homolog, a totally unexpected view. It was demonstrated that point mutations in the LxCxE motif of WDV RepA reduced the efficiency of viral DNA replication in cultured wheat cells and, strikingly, expression of human Rb in plant cells was also detrimental for WDV DNA replication [38].
Two other publications in 1995 produced an excitement in our laboratory difficult to express with words. One was the identification of D-type cyclins, which share with their animal counterparts an LxCxE motif [39,40]. Another was that geminiviruses triggered an unscheduled S-phase in infected cells [41]. These findings led us to hypothesize that plant cell cycle regulation, in particular the G1 progression, might have evolved early in eukaryotic evolution from an ancient RB-related protein, then maintained and expanded in animal lineages [42,43]. With this conceptual framework and through a collaboration with Greg Hannon (https://www.hannonlab.org/; accessed 20 July 2022), who had access to one of the few maize cDNA libraries, we sought to identify a cDNA clone encoding a plant retinoblastoma-related (RBR), a homolog of human Rb protein [44]. This also paved the way to the identification of the first plant E2F transcription factors [45,46]. I am indebted to Denes Duddits (BRC, Szeged), who invited me to attend an EMBO Workshop that he organized in Szeged on the “Control of cell division in higher plants”, which was key to introducing us to the incipient plant cell cycle community. RBR proteins were later found in other plant species, including unicellular algae, and shown to be essential [42,43,47,48,49,50]. Likewise, E2F family members were characterized [51,52], including the atypical members E2F/DEL, later identified in mammalian cells [53,54,55], as well as their genome-wide targets [56,57].
4. Where Are We Now?
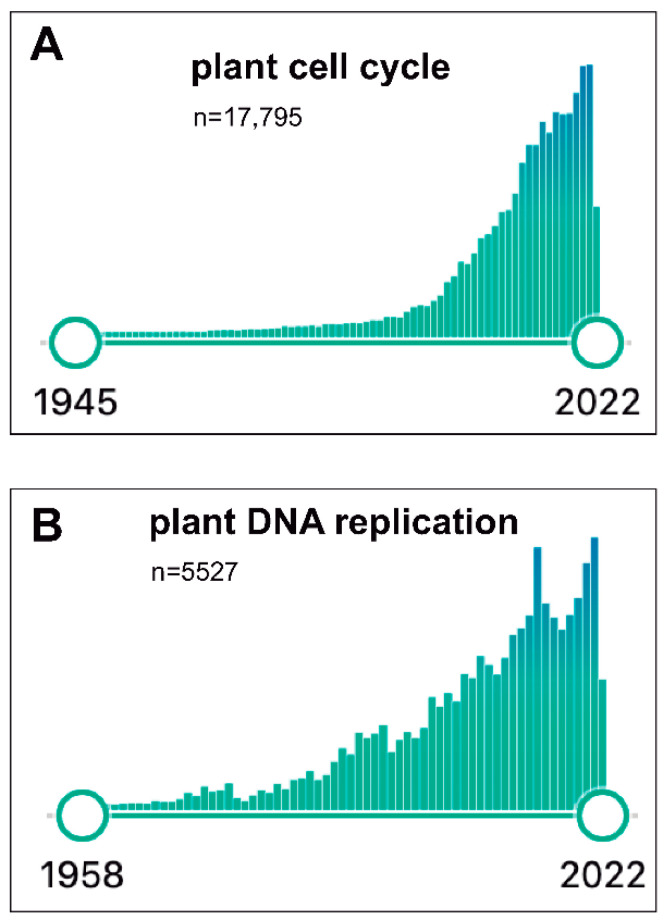
Since the mid-1990s, the plant community had been expectant with the possibility of having access to the full genome sequence of Arabidopsis thaliana, which finally came to be true [58]. This led our laboratory to leave the geminivirus projects and move fully to Arabidopsis, focusing on several cell cycle aspects. In fact, during the last two decades, an impressive advance has occurred in our understanding of the basic components of the cell cycle machinery, not only in Arabidopsis but also in other plant species. Figure 1A illustrates the exponential interest in plant cell cycle research moving from ~75 publications in 1993 up to >1370 during 2021, with a total of >17,750 publications since 1945. Likewise, the plant DNA replication field has also followed a similar growing pattern, steadily increasing from ~100 publications in 1995 up to ~330 in 2021 (with a total of >5500; Figure 1B). Several laboratories, including ours (where the participation of many outstanding PhD candidates, postdocs and visitors has been crucial), have joined efforts to identify and define the role of cell cycle factors that participate in various stages and how they work coordinately to regulate cell division. I will not present here a detailed account of our current knowledge of mechanistic aspects of plant cell cycle. The interested reader is directed to several comprehensive reviews published recently [43,59,60,61,62], although I would also recommend to have a look at the earlier reviews where the evolution of this field can be easily grasped [63,64,65,66,67,68,69,70].
Figure 1.
Timeline results of publications recorded in PubMed (June 2022) for the terms “plant cell cycle” (A) and “plant DNA replication” (B).
Advances in scientific research are intimately associated not only with new conceptual contributions but also with novel technological approaches and tools. In our case, they have come from two major lines. One consists of tools to assess cell cycle progression by live-imaging, which has been mastered in our laboratory by Bénédicte Desvoyes. By collecting results of different projects dealing with the dynamics of DNA replication proteins and histones during S-phase, a multiple expression cassette was designed containing three markers labeled with different fluorescent tags (CDT1a-CFP, H3.1-mCherry and CYCB1;1-YFP) that allows the identification of all cell cycle phases in different plant organs [71,72]. The PlaCCI marker line was originally developed for Arabidopsis (PlaCCI), but a similar strategy is being transferred to other plant species by different laboratories. The use of PlaCCI in several developmental contexts is confirming its usefulness for cell proliferation studies [73,74,75].
Another came from advances in chromatin biology with the development of highly specific and sensitive tools to identify various chromatin features both genome wide and at specific genomic locations, and related to initiation of DNA replication and transcriptional activity [76,77]. The combination of these tools together with other cell and molecular biology, genetic and genomic strategies constitutes the basis on which our current research projects are supported to tackle several complementary objectives.
4.1. Cell Cycle Control during Organ Development
The continuous production of new organs in plant during its postembryonic growth (vegetative and reproductive) requires a sustained supply of new cells that will eventually give rise to all different cell types of a particular organ. These cells are exclusively produced in the meristems, unique plant locations where all the cell proliferation occurs. We have focused on the root apical meristem (RAM), which is located at the root apex, where three major compartments can be distinguished. One is the quiescent center (QC), a group of few cells that divide rarely or in response to organ damage after root stress, and that constitutes an organizing center for the stem cell niche. Surrounding the QC cells are the stem cells that divide asymmetrically to produce one daughter cell that remains in contact with the QC cells and is perpetuated as a stem cell, and another daughter that has a limited proliferation potential. Division of these stem cell daughters during several cell division cycles produces a cohort of cells that constitutes a transit-amplifying compartment, the RAM proper [78].
Cell proliferation kinetics in the RAM has been assumed to be homogeneous with cells dividing at a fast rate, compared with the slow dividing rate of the stem cells. All different cell types located along the RAM have been also considered to develop cell cycles of similar length, following the same exponential kinetics (see discussion in [79]), a view that nonetheless had been challenged long time ago [80]. It is also known that the stem cell cycle is slow [81] and that cells located in the more proximal half of the RAM (in contact with the RAM boundary) possess a smaller probability to divide. This together with direct experimental data [82] gave support to distinguish two domains within the RAM: the proliferation domain (PD), located in the more distal half, and the transition domain (TD), in contact with the RAM boundary [79,83].
In an effort to define cell proliferation kinetics during organogenesis, it was asked whether chromatin dynamics could provide new insights from a different perspective [84]. Thus, by using live-imaging of roots, the incorporation of the canonical histone H3.1-GFP during S-phase and its replacement by the histone variant H3.3-mRFP was followed [85]. A subpopulation of RAM cells, which corresponded to cells undergoing their last cell cycle within the RAM, was identified by its reduced H3.1 level. A massive replacement of H3.1 occurs during the last G2 phase before entering the endocycle program. It was also found that this G2 phase has a ~30% longer duration than the G2 phase of earlier stem cell derivatives, particularly in the two epidermal cell types. There are various possibilities currently being tested to define the mechanism responsible for the G2 lengthening during the last cell cycle in the RAM. It is worth noting that a similar phenomenon has been reported for the last cell cycle in Drosophila embryos [86].
One possible control stage is the accumulation dynamics of CYCB1s and CDKBs, active during G2 that can be modified depending on the position of a cell within the RAM. The analysis of the appearance of EdU-labeled mitosis after an EdU pulse, a read-out of G2 progression, should shed light onto this question. Additionally, a control mechanism could depend on the DREAM complex, originally identified in Drosophila but widespread in plants [87], where it plays different roles in connection with RBR [43,88]. A recent report showed that specific CDK inhibitors act downstream of DREAM in G2 to control cell size [89]. Thus, a reasonable hypothesis is that DREAM complexes might participate in the regulatory network controlling G2 duration along the proximal–distal axis of the RAM.
A detailed analysis of live-imaging recordings has revealed significant differences in cell cycle duration along the RAM axis, mainly dictated by differences in the G1 duration. The long G1 phase is not restricted exclusively to stem cells. Rather, a gradient of G1 durations is established ranging from >20 h of stem cells and their derivatives up to ~1/3 of the RAM to only 1–2 h in cells close to the RAM boundary [90]. This G1 duration gradient conforms to an incoherent feed-forward loop (IFFL) where a driver possesses two opposite functions: one confers cell proliferation potential and another activates the expression of a negative regulator of G1 progression, thus affecting the cell cycle progression rate. One of the components of the “driver” seems to be the PLETHORA (PLT) proteins, since the G1 gradient is abolished in the plt1, plt2 double mutant.
There are several questions that remain unanswered. One is whether there are other genetic pathways that coordinate upstream regulation of PLT function [91] with G1 progression control. Analysis of transcription factors expressed in the RAM revealed a subset that has an expression pattern similar to that of PLT proteins. Another key component of the pathway is the RBR1 protein, since it is known that the unphosphorylated (or underphosphorylated) forms of RBR1 restrict G1 progression. Consistent with this, rbr1 mutants develop very rapid G1 phases along the entire RAM without any signs of a G1 duration gradient [90]. Therefore, an important question to answer in the future will be to identify how the phosphorylation state of RBR1 affects its repressive function and the phosphorylation sites responsible for restricting G1 progression. Successful development of these studies will constitute a major advance in our understanding of the developmental zonation of the root apical meristem. Differences in cell cycle duration in the proximal–distal axis of the RAM occur in all cell types, that is, across the radial axis. However, we do not know yet how a given cell at a given location in the RAM senses the proliferation status in coordination with its neighbors to produce a G1 duration gradient, something that is being currently explored in our laboratory.
4.2. Chromatin Dynamics and Genome Replication during S-Phase
Deposition and/or maintenance of chromatin features, that is, cytosine methylation, histone variants and their post-translational modifications, is strictly regulated throughout the cell cycle. Some of them are related to the transcriptional waves characteristic of G1 and G2, responsible for the synthesis of products required for S-phase and mitosis, respectively. However, others play a function in other cellular processes. The chromatin landscape associated with initiation of DNA replication during S-phase and how they regulate eu- and heterochromatin organization has been a long-standing interest of our laboratory.
All eukaryotic cells initiate replication of their genomes at multiple locations known as DNA replication origins (ORIs). Their location is dictated by the assembly of the pre-replication complexes (pre-RC), a process that occurs early in G1 [19]. Pre-RCs consist of the heterohexameric origin recognition complex (ORC), CDC6, CDT1 and the heterohexameric minichromosome maintenance (MCM) complex. Pre-RC homologs have been now identified in several plant species [21,22,23,92,93,94,95]. Pre-RC assembly is known as licensing of chromatin for replication, but only a subset of licensed chromatin locations marked with assembled pre-RCs are later activated at the G1/S transition by disassembly of the pre-RC and further addition of other replication proteins, thus defining the location of active ORIs. Efforts in different laboratories have identified the genomic location of ORIs in several eukaryotic cell types, including plant cells [76,77,96,97,98,99,100,101,102].
A long-standing question is whether and how the chromatin landscape at and around active ORIs serves to define ORI location and/or their potential to be activated [103]. A detailed map of nine chromatin states (CS) of Arabidopsis has been reported based on the combination of 16 different chromatin features, including DNA base composition, histone types and their post-translational modifications [99]. These chromatin states are highly correlated with the major genomic features: CS1, CS2 and CS3, enriched in H3K4me3 and histone variants H3.3 and H2A.Z, colocalize with TSS, proximal promoters and 5′ end of genes, respectively; CS4 and CS5, both enriched in H3K27me3, mainly correspond to long intergenic regions and Polycomb-regulated genes; CS6 and CS7 are associated with 3′ end of genes and long genes, respectively; finally, CS8 and CS9, enriched in C methylation, H3K9me2 and H3K27me1, correspond to the two types of heterochromatin, AT-rich and GC-rich, respectively [104].
Detailed analysis of ORI location in developing Arabidopsis seedlings in relation to these chromatin states revealed that they can occur in any CS [77]. However, a high preference for CS1-2-3 is detected, whereas ORIs tend to be less represented in CS4, CS5, CS8 and CS9. These results suggested that ORIs have a preference for regions of open and accessible chromatin, as it occurs in other eukaryotes [105]. Studies using a different approach have mapped ORIs in AT-rich genomic locations [106]. A future challenge is to identify the molecular determinants defining ORI activity. Since ORIs have been detected in genomic regions with very different chromatin landscape, it is conceivable that a single chromatin mark, as originally hypothesized, is not sufficient to confer ORI activity, although it might be necessary. It is also conceivable that ORI located in closed chromatin regions, e.g., heterochromatin, might prefer some regions with a relatively less compact chromatin feature. This has been tested by analyzing ORIs located in Arabidopsis transposon elements (TEs). It has been found that active ORIs associated with TEs tend to be located in GC-regions, where Gypsy family TEs are located and, among them, tend to have a higher GC content [107].
It is well established that embryonic cells develop very rapid S-phases because they possess a larger set of functional ORIs than differentiated cells [108]. One attractive aspect to explore in the future is whether modifying the active ORI set has an impact on cell growth performance and cell proliferation potential. Could changes in the ORI location pattern have an impact at the cellular and developmental level? In the case of plants, this could be particularly relevant given their regeneration capacity and the challenges faced by the changing environmental conditions, an aspect that is actively studied in our laboratory.
A particularly case interesting is that of the ORC1 proteins encoded by two genes, ORC1a and ORC1b, in Arabidopsis. By studying the phenotypes of single orc1a-2 and orc1b-2 mutants, we have found that they play distinct functions. The knockout orc1b-2 mutant is viable under normal growth conditions but is severely impaired after a treatment with the DNA polymerase inhibitor aphidicolin, likely due to its deficiency in the amount of pre-RC complexes assembled. On the contrary, the orc1a-2 mutant shows a wild-type phenotype with and without aphidicolin and apparently normal heterochromatic chromocenters. However, they show defects in deposition of the typical heterochromatic mark H3K27me1, while maintaining normal levels of H3K9me2 [109]. H3K27me1 is deposited by the histone methyltransferases ATXR5 and ATXR6 [110,111], cell cycle-regulated enzymes with a peak of expression in S-phase [112]. Establishment and maintenance of heterochromatin is crucial for genome integrity during development [113,114] and ATXR5 and ATXR6 function redundantly to suppress over-replication of heterochromatin [110]. It has been reported that mutations in these two genes cause a reduction of ~20% in H3K27me1 levels, in addition to heterochromatin decondensation and upregulation of TE expression [110,115]. Therefore, it is likely that ORC1a, but not ORC1b, somehow facilitates ATXR5/6 activity, although the precise mechanism is yet to be defined.
4.3. Perspectives on Other Cell Cycle Topics
The general strategies of plant cell cycle and the cellular machinery are much more similar to the animal cell cycle than to yeast. However, one of the more striking features of the plant cell cycle is the very large amount of core cell cycle components. Thus, in Arabidopsis 8–9 CDKs, >40 cyclins, >20 CDK inhibitors or 4 RBR proteins in many monocots, to cite some examples, have been identified (https://www.araport.org/; https://www.arabidopsis.org/; accessed 20 July 2022). Initial genome-wide transcriptomic studies of cell cycle genes indicated that many of them were expressed in Arabidopsis cultured cells [116], which was initially considered as a case of functional redundancy. Later studies revealed, however, that different sets or components of the cell cycle machinery had a cell type-, growth- and/or developmental stage-specific expression pattern. As a consequence, cell cycle regulators have been identified now as targets of a plethora of signaling pathways controlling many aspects of plant physiology [62,117].
Plant growth under normal and stress conditions is the result of a complex network of signaling pathways. Abiotic stress is associated with specific changes in cell proliferation and gene expression as well as heterochromatin disorganization and transposon reactivation, all having a significant impact on plant genomes and growth [118,119]. A current focus is on histone dynamics in both euchromatin and heterochromatin maintenance in relation to environmental factors [120]. An attractive hypothesis is that, in the long term, deposition of various histone forms and the activity of chromatin modifying enzymes at specific genomic locations could be targeted to improve plant tolerance to abiotic stress, genome stability and cell division potential.
Organogenesis requires not only the production of new cells of a given type but also the occurrence of formative cell divisions to produce daughter cells that eventually will take different cell fates and differentiation pathways. Thus, cell fate decisions are needed in many plant locations during the entire life of the plant, including embryogenesis [74]. Control of formative divisions, frequently through asymmetric cell division (ACD), directly involves specific cell cycle regulatory factors. Thus, Aurora (AUR) kinases possess RBR1 binding motifs, highlighting a novel possible role of RBR1 in formative division and the machinery involved in cell plate orientation are examples of the importance during formative cell divisions [121,122,123].
Once the newborn cells are produced, they must decide whether they maintain the same fate as their mothers or acquire a new one. They possess a window during the cell cycle to acquire a new fate, a process that is not fully understood regarding how this decision occurs during the cell cycle. The end of mitosis and early stages of G1 seems appropriate, since changes in chromatin accessibility have been reported, for example, in the trichoblast/atrichoblast decision in the root epidermis [124]. Therefore, much research is needed to identify the cell cycle regulatory factors and parameters that affect cell fate decisions at different developmental stages. In the context of a growing organ, it is of primary relevance to understand the control of cell division potential [125,126] and the positional dependence of cell cycle phase duration [85,87,90,91,127].
The average cell size varies among different cell types, normally associated with the cell fate decisions taken, yet they follow similar rules for cell division. Cell size control during the cell cycle is intimately linked to cell cycle progression, whereby specific cell cycle components are involved, e.g., KRP4 used as a cell size monitoring molecule [73]. A topic of special relevance is cell size control during the endocycle [128,129], when cells duplicate their genome without an intervening mitosis, since the presence of endoreplicated cells is frequent in many plant species.
The high capacity to regenerate organs is one of the most striking features of many plants. This depends on the presence of long-lived stem cells, the capacity to reprogram somatic cells into pluripotent cells and the action of hormonal signals [126]. The response to hormonal signals is also directly linked to the activity of specific cell cycle regulatory factors, e.g., RBR1 in the auxin/cytokinin balance in the root [130,131], the ethylene response through ERF115 [132,133], the brassinosteroid signaling through BRAVO and CCS52A2 [134,135,136]. The interplay of hormonal signals with nutrient availability and metabolism is another field that should benefit from being analyzed from a cell proliferation perspective [137,138].
As an insider witness of plant cell cycle research over the past four decades, I can confidently confirm that the combination of cellular, molecular, developmental, genetic and genomic approaches has led to a brilliant growth of this field. Now the cell cycle components are no longer considered only part of a machine to produce two daughter cells but as true hubs that network with a large variety of processes crucial for the life of plants. As the scope of plant cell cycle research expands, we can only see new exciting discoveries. These should range from detailed mechanistic aspects of how cell cycle components act to cytoplasmic and nuclear signaling cascades, translational control, chromatin dynamics involved in replication and transcription, novel interactions with plant developmental processes and interaction with the environment.
Acknowledgments
The author is deeply and warmly indebted to the many students, postdocs, technicians and visitors who have contributed to the laboratory projects throughout all these years.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
Research in the author’s laboratory is currently funded by Ministerio de Ciencia e Innovación and Fondo Europeo de Desarrollo Regional FEDER grant RTI2018-094793-B-I00, by H2020 European Research Council grant ERC-2018-AdG_833617, and by institutional grants from Banco de Santander and Fundación Ramon Areces to the Centro de Biología Molecular Severo Ochoa.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Basu S., Greenwood J., Jones A.W., Nurse P. Core Control Principles of the Eukaryotic Cell Cycle. Nature. 2022;607:381–386. doi: 10.1038/s41586-022-04798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coudreuse D., Nurse P. Driving the Cell Cycle with a Minimal CDK Control Network. Nature. 2010;468:1074–1079. doi: 10.1038/nature09543. [DOI] [PubMed] [Google Scholar]
- 3.DePamphilis M.L., editor. DNA Replication in Eukaryotic Cells. Cold Spring Harbor Laboratory Press; Long Island, NY, USA: 1996. [Google Scholar]
- 4.DePamphilis M.L., editor. DNA Replication and Human Disease. Cold Spring Harbor Laboratory Press; Long Island, NY, USA: 2006. [Google Scholar]
- 5.Gutierrez C., Guo Z.S., Roberts J., DePamphilis M.L. Simian Virus 40 Origin Auxiliary Sequences Weakly Facilitate T-Antigen Binding but Strongly Facilitate DNA Unwinding. Mol. Cell. Biol. 1990;10:1719–1728. doi: 10.1128/mcb.10.4.1719-1728.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruss C., Gutierrez C., Burhans W.C., DePamphilis M.L., Koller T., Sogo J.M. Nucleosome Assembly in Mammalian Cell Extracts before and after DNA Replication. EMBO J. 1990;9:2911–2922. doi: 10.1002/j.1460-2075.1990.tb07482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutiérrez C., Sogo J.M., Salas M. Analysis of Replicative Intermediates Produced during Bacteriophage Phi 29 DNA Replication In Vitro. J. Mol. Biol. 1991;222:983–994. doi: 10.1016/0022-2836(91)90589-X. [DOI] [PubMed] [Google Scholar]
- 8.Gutiérrez C., Freire R., Salas M., Hermoso J.M. Assembly of Phage Phi 29 Genome with Viral Protein P6 into a Compact Complex. EMBO J. 1994;13:269–276. doi: 10.1002/j.1460-2075.1994.tb06257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies J.W., Stanley J. Geminivirus Genes and Vectors. Trends Genet. 1989;5:77–81. doi: 10.1016/0168-9525(89)90030-9. [DOI] [PubMed] [Google Scholar]
- 10.Pilartz M., Jeske H. Abutilon Mosaic Geminivirus Double-Stranded DNA Is Packed into Minichromosomes. Virology. 1992;189:800–802. doi: 10.1016/0042-6822(92)90610-2. [DOI] [PubMed] [Google Scholar]
- 11.Suárez-López P., Gutiérrez C. DNA Replication of Wheat Dwarf Geminivirus Vectors: Effects of Origin Structure and Size. Virology. 1997;227:389–399. doi: 10.1006/viro.1996.8353. [DOI] [PubMed] [Google Scholar]
- 12.Sanz-Burgos A.P., Gutiérrez C. Organization of the Cis-Acting Element Required for Wheat Dwarf Geminivirus DNA Replication and Visualization of a Rep Protein-DNA Complex. Virology. 1998;243:119–129. doi: 10.1006/viro.1998.9037. [DOI] [PubMed] [Google Scholar]
- 13.Castellano M.M., Sanz-Burgos A.P., Gutiérrez C. Initiation of DNA Replication in a Eukaryotic Rolling-Circle Replicon: Identification of Multiple DNA-Protein Complexes at the Geminivirus Origin. J. Mol. Biol. 1999;290:639–652. doi: 10.1006/jmbi.1999.2916. [DOI] [PubMed] [Google Scholar]
- 14.Luque A., Sanz-Burgos A.P., Ramirez-Parra E., Castellano M.M., Gutierrez C. Interaction of Geminivirus Rep Protein with Replication Factor C and Its Potential Role during Geminivirus DNA Replication. Virology. 2002;302:83–94. doi: 10.1006/viro.2002.1599. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez C. Geminivirus DNA Replication. Cell. Mol. Life Sci. 1999;56:313–329. doi: 10.1007/s000180050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez C. DNA Replication and Cell Cycle in Plants: Learning from Geminiviruses. EMBO J. 2000;19:792–799. doi: 10.1093/emboj/19.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanley-Bowdoin L., Bejarano E.R., Robertson D., Mansoor S. Geminiviruses: Masters at Redirecting and Reprogramming Plant Processes. Nat. Rev. Microbiol. 2013;11:777–788. doi: 10.1038/nrmicro3117. [DOI] [PubMed] [Google Scholar]
- 18.Bell S.P., Kobayashi R., Stillman B. Yeast Origin Recognition Complex Functions in Transcription Silencing and DNA Replication. Science. 1993;262:1844–1849. doi: 10.1126/science.8266072. [DOI] [PubMed] [Google Scholar]
- 19.Costa A., Diffley J.F.X. The Initiation of Eukaryotic DNA Replication. Annu. Rev. Biochem. 2022;91:107–131. doi: 10.1146/annurev-biochem-072321-110228. [DOI] [PubMed] [Google Scholar]
- 20.Lewis J.S., Gross M.H., Sousa J., Henrikus S.S., Greiwe J.F., Nans A., Diffley J.F.X., Costa A. Mechanism of Replication Origin Melting Nucleated by CMG Helicase Assembly. Nature. 2022;606:1007–1014. doi: 10.1038/s41586-022-04829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellano M.M., del Pozo J.C., Ramirez-Parra E., Brown S., Gutierrez C. Expression and Stability of Arabidopsis CDC6 Are Associated with Endoreplication. Plant Cell. 2001;13:2671–2686. doi: 10.1105/tpc.010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellano M.M., Boniotti M.B., Caro E., Schnittger A., Gutierrez C. DNA Replication Licensing Affects Cell Proliferation or Endoreplication in a Cell Type-Specific Manner. Plant Cell. 2004;16:2380–2393. doi: 10.1105/tpc.104.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz-Trivino S., Castellano M.M., Sanchez M.P., Ramirez-Parra E., Desvoyes B., Gutierrez C. The Genes Encoding Arabidopsis ORC Subunits Are E2F Targets and the Two ORC1 Genes Are Differently Expressed in Proliferating and Endoreplicating Cells. Nucleic Acids Res. 2005;33:5404–5414. doi: 10.1093/nar/gki854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accotto G.P., Mullineaux P.M., Brown S.C., Marie D. Digitaria Streak Geminivirus Replicative Forms Are Abundant in S-Phase Nuclei of Infected Cells. Virology. 1993;195:257–259. doi: 10.1006/viro.1993.1369. [DOI] [PubMed] [Google Scholar]
- 25.Schvartzman J.B., Gutierrez C. The Relationship between the Cell Time Available for Repair and the Effectiveness of a Damaging Treatment in Provoking the Formation of Sister-Chromatid Exchanges. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1980;72:483–489. doi: 10.1016/0027-5107(80)90120-7. [DOI] [Google Scholar]
- 26.López-Sáez J.F., Calvo A., Cruz J.L., Gutierrez C., Carmona M.J., García-Herdugo G. Cell Proliferation in File Meristems: A General Theory and Its Analysis by Computer Simulation. Environ. Exp. Bot. 1983;23:59–69. doi: 10.1016/0098-8472(83)90021-7. [DOI] [Google Scholar]
- 27.Hernandez P., Gutierrez C. Caffeine Induces Sister-Chromatid Exchanges during the Whole S-Phase of the Cell Cycle. Chromosoma. 1985;92:214–217. doi: 10.1007/BF00348696. [DOI] [PubMed] [Google Scholar]
- 28.Pardo E.G., Hernández P., Gutiérrez C. The Incorporation of Deoxyuridine Monophosphate into DNA Increases the Sister-Chromatid Exchange Yield. Exp. Cell Res. 1987;168:507–517. doi: 10.1016/0014-4827(87)90023-1. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez C. Excision Repair of Uracil in Higher Plant Cells: Uracil-DNA Glycosylase and Sister-Chromatid Exchanges. Mutat. Res. 1987;181:111–126. doi: 10.1016/0027-5107(87)90293-4. [DOI] [Google Scholar]
- 30.Lee M.G., Nurse P. Complementation Used to Clone a Human Homologue of the Fission Yeast Cell Cycle Control Gene Cdc2. Nature. 1987;327:31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- 31.John P.C., Sek F.J., Lee M.G. A Homolog of the Cell Cycle Control Protein P34cdc2 Participates in the Division Cycle of Chlamydomonas, and a Similar Protein Is Detectable in Higher Plants and Remote Taxa. Plant Cell. 1989;1:1185–1193. doi: 10.1105/tpc.1.12.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feiler H.S., Jacobs T.W. Cell Division in Higher Plants: A Cdc2 Gene, Its 34-KDa Product, and Histone H1 Kinase Activity in Pea. Proc. Natl. Acad. Sci. USA. 1990;87:5397–5401. doi: 10.1073/pnas.87.14.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira P.C., Hemerly A.S., Villarroel R., Van Montagu M., Inze D. The Arabidopsis Functional Homolog of the P34cdc2 Protein Kinase. Plant Cell. 1991;3:531–540. doi: 10.1105/tpc.3.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hata S., Kouchi H., Suzuka I., Ishii T. Isolation and Characterization of CDNA Clones for Plant Cyclins. EMBO J. 1991;10:2681–2688. doi: 10.1002/j.1460-2075.1991.tb07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemerly A., Bergounioux C., Van Montagu M., Inze D., Ferreira P. Genes Regulating the Plant Cell Cycle: Isolation of a Mitotic-like Cyclin from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 1992;89:3295–3299. doi: 10.1073/pnas.89.8.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirt H., Mink M., Pfosser M., Bogre L., Gyorgyey J., Jonak C., Gartner A., Dudits D., Heberle-Bors E. Alfalfa Cyclins: Differential Expression during the Cell Cycle and in Plant Organs. Plant Cell. 1992;4:1531–1538. doi: 10.1105/tpc.4.12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serrano M., Hannon G.J., Beach D. A New Regulatory Motif in Cell-Cycle Control Causing Specific Inhibition of Cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 38.Xie Q., Suarez-Lopez P., Gutierrez C. Identification and Analysis of a Retinoblastoma Binding Motif in the Replication Protein of a Plant DNA Virus: Requirement for Efficient Viral DNA Replication. EMBO J. 1995;14:4073–4082. doi: 10.1002/j.1460-2075.1995.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soni R., Carmichael J.P., Shah Z.H., Murray J.A. A Family of Cyclin D Homologs from Plants Differentially Controlled by Growth Regulators and Containing the Conserved Retinoblastoma Protein Interaction Motif. Plant Cell. 1995;7:85–103. doi: 10.1105/tpc.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahl M., Meskiene I., Bogre L., Ha D.T., Swoboda I., Hubmann R., Hirt H., Heberle-Bors E. The D-Type Alfalfa Cyclin Gene CycMs4 Complements G1 Cyclin-Deficient Yeast and Is Induced in the G1 Phase of the Cell Cycle. Plant Cell. 1995;7:1847–1857. doi: 10.1105/tpc.7.11.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagar S., Pedersen T.J., Carrick K.M., Hanley-Bowdoin L., Robertson D. A Geminivirus Induces Expression of a Host DNA Synthesis Protein in Terminally Differentiated Plant Cells. Plant Cell. 1995;7:705–719. doi: 10.1105/tpc.7.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desvoyes B., Mendoza A.D., Ruiz-Trillo I., Gutierrez C. Novel Roles of Plant RETINOBLASTOMA-RELATED (RBR) Protein in Cell Proliferation and Asymmetric Cell Division. J. Exp. Bot. 2014;65:2657–2666. doi: 10.1093/jxb/ert411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desvoyes B., Gutierrez C. Roles of Plant Retinoblastoma Protein: Cell Cycle and Beyond. EMBO J. 2020;39:e105802. doi: 10.15252/embj.2020105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Q., Sanz-Burgos A.P., Hannon G.J., Gutierrez C. Plant Cells Contain a Novel Member of the Retinoblastoma Family of Growth Regulatory Proteins. EMBO J. 1996;15:4900–4908. doi: 10.1002/j.1460-2075.1996.tb00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez-Parra E., Xie Q., Boniotti M.B., Gutierrez C. The Cloning of Plant E2F, a Retinoblastoma-Binding Protein, Reveals Unique and Conserved Features with Animal G(1)/S Regulators. Nucleic Acids Res. 1999;27:3527–3533. doi: 10.1093/nar/27.17.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sekine M., Ito M., Uemukai K., Maeda Y., Nakagami H., Shinmyo A. Isolation and Characterization of the E2F-like Gene in Plants. FEBS Lett. 1999;460:117–122. doi: 10.1016/S0014-5793(99)01296-X. [DOI] [PubMed] [Google Scholar]
- 47.Ach R.A., Taranto P., Gruissem W. A Conserved Family of WD-40 Proteins Binds to the Retinoblastoma Protein in Both Plants and Animals. Plant Cell. 1997;9:1595–1606. doi: 10.1105/tpc.9.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umen J.G., Goodenough U.W. Control of Cell Division by a Retinoblastoma Protein Homolog in Chlamydomonas. Genes Dev. 2001;15:1652–1661. doi: 10.1101/gad.892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong L.J., Orozco B.M., Roe J.L., Nagar S., Ou S., Feiler H.S., Durfee T., Miller A.B., Gruissem W., Robertson D., et al. A Geminivirus Replication Protein Interacts with the Retinoblastoma Protein through a Novel Domain to Determine Symptoms and Tissue Specificity of Infection in Plants. EMBO J. 2000;19:3485–3495. doi: 10.1093/emboj/19.13.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebel C., Mariconti L., Gruissem W. Plant Retinoblastoma Homologues Control Nuclear Proliferation in the Female Gametophyte. Nature. 2004;429:776–780. doi: 10.1038/nature02637. [DOI] [PubMed] [Google Scholar]
- 51.Mariconti L., Pellegrini B., Cantoni R., Stevens R., Bergounioux C., Cella R., Albani D. The E2F Family of Transcription Factors from Arabidopsis thaliana. Novel and Conserved Components of the Retinoblastoma/E2F Pathway in Plants. J. Biol. Chem. 2002;277:9911–9919. doi: 10.1074/jbc.M110616200. [DOI] [PubMed] [Google Scholar]
- 52.Kosugi S., Ohashi Y. E2Ls, E2F-like Repressors of Arabidopsis that Bind to E2F Sites in a Monomeric Form. J. Biol. Chem. 2002;277:16553–116558. doi: 10.1074/jbc.M200913200. [DOI] [PubMed] [Google Scholar]
- 53.de Bruin A., Maiti B., Jakoi L., Timmers C., Buerki R., Leone G. Identification and Characterization of E2F7, a Novel Mammalian E2F Family Member Capable of Blocking Cellular Proliferation. J. Biol. Chem. 2003;278:42041–42049. doi: 10.1074/jbc.M308105200. [DOI] [PubMed] [Google Scholar]
- 54.Di Stefano L., Jensen M.R., Helin K. E2F7, a Novel E2F Featuring DP-Independent Repression of a Subset of E2F-Regulated Genes. EMBO J. 2003;22:6289–6298. doi: 10.1093/emboj/cdg613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Logan N., Delavaine L., Graham A., Reilly C., Wilson J., Brummelkamp T.R., Hijmans E.M., Bernards R., La Thangue N.B. E2F-7: A Distinctive E2F Family Member with an Unusual Organization of DNA-Binding Domains. Oncogene. 2004;23:5138–5150. doi: 10.1038/sj.onc.1207649. [DOI] [PubMed] [Google Scholar]
- 56.Ramirez-Parra E., Frundt C., Gutierrez C. A Genome-Wide Identification of E2F-Regulated Genes in Arabidopsis. Plant J. 2003;33:801–811. doi: 10.1046/j.1365-313X.2003.01662.x. [DOI] [PubMed] [Google Scholar]
- 57.Vandepoele K., Vlieghe K., Florquin K., Hennig L., Beemster G.T., Gruissem W., Van de Peer Y., Inze D., De Veylder L. Genome-Wide Identification of Potential Plant E2F Target Genes. Plant Physiol. 2005;139:316–328. doi: 10.1104/pp.105.066290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.AGI Analysis of the Genome Sequence of the Flowering Plant Arabidopsis Thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 59.Lang L., Schnittger A. Endoreplication—A Means to an End in Cell Growth and Stress Response. Curr. Opin. Plant Biol. 2020;54:85–92. doi: 10.1016/j.pbi.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Shimotohno A., Aki S.S., Takahashi N., Umeda M. Regulation of the Plant Cell Cycle in Response to Hormones and the Environment. Annu. Rev. Plant Biol. 2021;72:273–296. doi: 10.1146/annurev-arplant-080720-103739. [DOI] [PubMed] [Google Scholar]
- 61.Pedroza-Garcia J.A., Mazubert C., Del Olmo I., Bourge M., Domenichini S., Bounon R., Tariq Z., Delannoy E., Pineiro M., Jarillo J.A., et al. Function of the Plant DNA Polymerase Epsilon in Replicative Stress Sensing, a Genetic Analysis. Plant Physiol. 2017;173:1735–1749. doi: 10.1104/pp.17.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sablowski R., Gutierrez C. Cycling in a Crowd: Coordination of Plant Cell Division, Growth, and Cell Fate. Plant Cell. 2022;34:193–208. doi: 10.1093/plcell/koab222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Potuschak T., Doerner P. Cell Cycle Controls: Genome-Wide Analysis in Arabidopsis. Curr. Opin. Plant Biol. 2001;4:501–506. doi: 10.1016/S1369-5266(00)00207-7. [DOI] [PubMed] [Google Scholar]
- 64.Gutierrez C., Ramirez-Parra E., Castellano M.M., del Pozo J.C. G(1) to S Transition: More than a Cell Cycle Engine Switch. Curr. Opin. Plant Biol. 2002;5:480–486. doi: 10.1016/S1369-5266(02)00301-1. [DOI] [PubMed] [Google Scholar]
- 65.Dewitte W., Murray J.A. The Plant Cell Cycle. Annu. Rev. Plant Biol. 2003;54:235–264. doi: 10.1146/annurev.arplant.54.031902.134836. [DOI] [PubMed] [Google Scholar]
- 66.Doonan J.H. The Plant Cell Cycle: An Overview. Methods Mol. Biol. 2005;296:31–50. doi: 10.1385/1-59259-857-9:031. [DOI] [PubMed] [Google Scholar]
- 67.Inze D., De Veylder L. Cell Cycle Regulation in Plant Development. Annu. Rev. Genet. 2006;40:77–105. doi: 10.1146/annurev.genet.40.110405.090431. [DOI] [PubMed] [Google Scholar]
- 68.Francis D. The Plant Cell Cycle—15 Years On. New Phytol. 2007;174:261–278. doi: 10.1111/j.1469-8137.2007.02038.x. [DOI] [PubMed] [Google Scholar]
- 69.Bryant J.A., Francis D. The Plant Cell Cycle. Ann. Bot. 2011;107:1063. doi: 10.1093/aob/mcr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scofield S., Jones A., Murray J.A.H. The Plant Cell Cycle in Context. J. Exp. Bot. 2014;65:2557–2562. doi: 10.1093/jxb/eru188. [DOI] [PubMed] [Google Scholar]
- 71.Desvoyes B., Arana-Echarri A., Barea M.D., Gutierrez C. A Comprehensive Fluorescent Sensor for Spatiotemporal Cell Cycle Analysis in Arabidopsis. Nat. Plants. 2020;6:1330–1334. doi: 10.1038/s41477-020-00770-4. [DOI] [PubMed] [Google Scholar]
- 72.Echevarría C., Gutierrez C., Desvoyes B. Tools for Assessing Cell Cycle Progression in Plants. Plant Cell Physiol. 2021;62:1231–1238. doi: 10.1093/pcp/pcab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D’Ario M., Tavares R., Schiessl K., Desvoyes B., Gutierrez C., Howard M., Sablowski R. Cell Size Controlled in Plants Using DNA Content as an Internal Scale. Science. 2021;372:1176–1181. doi: 10.1126/science.abb4348. [DOI] [PubMed] [Google Scholar]
- 74.Simonini S., Bemer M., Bencivenga S., Gagliardini V., Pires N.D., Desvoyes B., van der Graaff E., Gutierrez C., Grossniklaus U. The Polycomb Group Protein MEDEA Controls Cell Proliferation and Embryonic Patterning in Arabidopsis. Dev. Cell. 2021;56:1945–1960.e7. doi: 10.1016/j.devcel.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han S.-K., Herrmann A., Yang J., Iwasaki R., Sakamoto T., Desvoyes B., Kimura S., Gutierrez C., Kim E.-D., Torii K.U. Deceleration of the Cell Cycle Underpins a Switch from Proliferative to Terminal Divisions in Plant Stomatal Lineage. Dev. Cell. 2022;57:569–582.e6. doi: 10.1016/j.devcel.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costas C., Sanchez M.P., Stroud H., Yu Y., Oliveros J.C., Feng S., Benguria A., Lopez-Vidriero I., Zhang X., Solano R., et al. Genome-Wide Mapping of Arabidopsis Origins of DNA Replication and Their Associated Epigenetic Marks. Nat. Struct. Mol. Biol. 2011;18:395–400. doi: 10.1038/nsmb.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sequeira-Mendes J., Araguez I., Peiro R., Mendez-Giraldez R., Zhang X., Jacobsen S.E., Bastolla U., Gutierrez C. The Functional Topography of the Arabidopsis Genome Is Organized in a Reduced Number of Linear Motifs of Chromatin States. Plant Cell. 2014;26:2351–2366. doi: 10.1105/tpc.114.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scheres B. Stem-Cell Niches: Nursery Rhymes across Kingdoms. Nat. Rev. 2007;8:345–354. doi: 10.1038/nrm2164. [DOI] [PubMed] [Google Scholar]
- 79.Desvoyes B., Echevarria C., Gutierrez C. A perspective on cell proliferation kinetics in the root apical meristem. J. Exp. Bot. 2021;72:6708–6715. doi: 10.1093/jxb/erab303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clowes F.A.L. The duration of the G1 phase of the mitotic cycle and its relation to radiosensitivity. New Phytol. 1965;64:355–359. doi: 10.1111/j.1469-8137.1965.tb07544.x. [DOI] [Google Scholar]
- 81.Rahni R., Birnbaum K.D. Week-Long Imaging of Cell Divisions in the Arabidopsis Root Meristem. Plant Methods. 2019;15:30. doi: 10.1186/s13007-019-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pacheco-Escobedo M.A., Ivanov V.B., Ransom-Rodríguez I., Arriaga-Mejía G., Avila H., Baklanov I.A., Pimentel A., Corkidi G., Doerner P., Dubrovsky J.G., et al. Longitudinal Zonation Pattern in Arabidopsis Root Tip Defined by Multiple Structural Change Algorithm. Ann. Bot. 2016;118:763–776. doi: 10.1093/aob/mcw101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ivanov V.B., Dubrovsky J.G. Longitudinal Zonation Pattern in Plant Roots: Conflicts and Solutions. Trends Plant Sci. 2013;18:237–243. doi: 10.1016/j.tplants.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Desvoyes B., Fernandez-Marcos M., Sequeira-Mendes J., Otero S., Vergara Z., Gutierrez C. Looking at Plant Cell Cycle from the Chromatin Window. Front. Plant Sci. 2014;5:369. doi: 10.3389/fpls.2014.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Otero S., Desvoyes B., Peiro R., Gutierrez C. Histone H3 Dynamics Uncovers Domains with Distinct Proliferation Potential in the Arabidopsis Root. Plant Cell. 2016;28:1361–1371. doi: 10.1105/tpc.15.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blythe S.A., Wieschaus E.F. Zygotic Genome Activation Triggers the DNA Replication Checkpoint at the Midblastula Transition. Cell. 2015;160:1169–1181. doi: 10.1016/j.cell.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi K., Suzuki T., Iwata E., Nakamichi N., Suzuki T., Chen P., Ohtani M., Ishida T., Hosoya H., Muller S., et al. Transcriptional Repression by MYB3R Proteins Regulates Plant Organ Growth. EMBO J. 2015;34:1992–2007. doi: 10.15252/embj.201490899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Magyar Z., Bögre L., Ito M. DREAMs Make Plant Cells to Cycle or to Become Quiescent. Curr. Opin. Plant Biol. 2016;34:100–106. doi: 10.1016/j.pbi.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Nomoto Y., Takatsuka H., Yamada K., Suzuki T., Suzuki T., Huang Y., Latrasse D., An J., Gombos M., Breuer C., et al. A Hierarchical Transcriptional Network Activates Specific CDK Inhibitors That Regulate G2 to Control Cell Size and Number in Arabidopsis. Nat. Commun. 2022;13:1660. doi: 10.1038/s41467-022-29316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Echevarria C., Desvoyes B., Marconi M., Franco-Zorrila J.M., Lee L., Sablowski R., Birnbaum K., Wabnik K., Gutierrez C. Stem Cell Regulators Control a G1 Duration Gradient in the Pant Root Meristem. BioRxiv. 2022 doi: 10.1101/2022.03.09.483577. [DOI] [Google Scholar]
- 91.Ercoli M.F., Ferela A., Debernardi J.M., Perrone A.P., Rodriguez R.E., Palatnik J.F. GIF Transcriptional Coregulators Control Root Meristem Homeostasis. Plant Cell. 2018;30:347–359. doi: 10.1105/tpc.17.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shultz R.W., Tatineni V.M., Hanley-Bowdoin L., Thompson W.F. Genome-Wide Analysis of the Core DNA Replication Machinery in the Higher Plants Arabidopsis and Rice. Plant Physiol. 2007;144:1697–1714. doi: 10.1104/pp.107.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shultz R.W., Lee T.J., Allen G.C., Thompson W.F., Hanley-Bowdoin L. Dynamic Localization of the DNA Replication Proteins MCM5 and MCM7 in Plants. Plant Physiol. 2009;150:658–669. doi: 10.1104/pp.109.136614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Masuda H.P., Ramos G.B., de Almeida-Engler J., Cabral L.M., Coqueiro V.M., Macrini C.M., Ferreira P.C., Hemerly A.S. Genome Based Identification and Analysis of the Pre-Replicative Complex of Arabidopsis thaliana. FEBS Lett. 2004;574:192–202. doi: 10.1016/j.febslet.2004.07.088. [DOI] [PubMed] [Google Scholar]
- 95.Sanchez M.P., Costas C., Sequeira-Mendes J., Gutierrez C. Regulating DNA Replication in Plants. Cold Spring Harb. Perspect. Biol. 2012;4:a010140. doi: 10.1101/cshperspect.a010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macalpine H.K., Gordan R., Powell S.K., Hartemink A.J., Macalpine D.M. Drosophila ORC Localizes to Open Chromatin and Marks Sites of Cohesin Complex Loading. Genome Res. 2010;20:201–211. doi: 10.1101/gr.097873.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cayrou C., Ballester B., Peiffer I., Fenouil R., Coulombe P., Andrau J.C., van Helden J., Mechali M. The Chromatin Environment Shapes DNA Replication Origin Organization and Defines Origin Classes. Genome Res. 2015;25:1873–1885. doi: 10.1101/gr.192799.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Comoglio F., Schlumpf T., Schmid V., Rohs R., Beisel C., Paro R. High-Resolution Profiling of Drosophila Replication Start Sites Reveals a DNA Shape and Chromatin Signature of Metazoan Origins. Cell Rep. 2015;11:821–834. doi: 10.1016/j.celrep.2015.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pourkarimi E., Bellush J.M., Whitehouse I. Spatiotemporal Coupling and Decoupling of Gene Transcription with DNA Replication Origins during Embryogenesis in C. elegans. eLife. 2016;5:e21728. doi: 10.7554/eLife.21728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sequeira-Mendes J., Diaz-Uriarte R., Apedaile A., Huntley D., Brockdorff N., Gomez M. Transcription Initiation Activity Sets Replication Origin Efficiency in Mammalian Cells. PLoS Genet. 2009;5:e1000446. doi: 10.1371/journal.pgen.1000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodriguez-Martinez M., Pinzon N., Ghommidh C., Beyne E., Seitz H., Cayrou C., Mechali M. The Gastrula Transition Reorganizes Replication-Origin Selection in Caenorhabditis elegans. Nat. Struct. Mol. Biol. 2017;24:290–299. doi: 10.1038/nsmb.3363. [DOI] [PubMed] [Google Scholar]
- 102.Sequeira-Mendes J., Vergara Z., Peiro R., Morata J., Araguez I., Costas C., Mendez-Giraldez R., Casacuberta J.M., Bastolla U., Gutierrez C. Differences in Firing Efficiency, Chromatin and Transcription Underlie the Developmental Plasticity of Arabidopsis Originome. Genome Res. 2019;29:784–797. doi: 10.1101/gr.240986.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mechali M. Eukaryotic DNA Replication Origins: Many Choices for Appropriate Answers. Nat. Rev. 2010;11:728–738. doi: 10.1038/nrm2976. [DOI] [PubMed] [Google Scholar]
- 104.Vergara Z., Gutierrez C. Emerging Roles of Chromatin in the Maintenance of Genome Organization and Function in Plants. Genome Biol. 2017;18:96. doi: 10.1186/s13059-017-1236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sequeira-Mendes J., Gutierrez C. Links between Genome Replication and Chromatin Landscapes. Plant J. 2015;83:38–51. doi: 10.1111/tpj.12847. [DOI] [PubMed] [Google Scholar]
- 106.Wheeler E., Brooks A.M., Concia L., Vera D.L., Wear E.E., LeBlanc C., Ramu U., Vaughn M.W., Bass H.W., Martienssen R.A., et al. Arabidopsis DNA Replication Initiates in Intergenic, AT-Rich Open Chromatin. Plant Physiol. 2020;183:206–220. doi: 10.1104/pp.19.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vergara Z., Sequeira-Mendes J., Morata J., Peiro R., Henaff E., Costas C., Casacuberta J.M., Gutierrez C. Retrotransposons Are Specified as DNA Replication Origins in the Gene-Poor Regions of Arabidopsis Heterochromatin. Nucleic Acids Res. 2017;45:8358–8368. doi: 10.1093/nar/gkx524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duronio R.J. Developing S-Phase Control. Genes Dev. 2012;26:746–750. doi: 10.1101/gad.191171.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vergara Z., Sequeira-Mendes J., Masoud K., Costas C., Noir S., Caro E., Mora-Gil V., Genschik P., Gutierrez C. Distinct Roles of Arabidopsis ORC1 Proteins in DNA Replication and Heterochromatin Maintenance. Nat. Commun. p. 2022. submitted . [DOI] [PMC free article] [PubMed]
- 110.Jacob Y., Stroud H., Leblanc C., Feng S., Zhuo L., Caro E., Hassel C., Gutierrez C., Michaels S.D., Jacobsen S.E. Regulation of Heterochromatic DNA Replication by Histone H3 Lysine 27 Methyltransferases. Nature. 2010;466:987–991. doi: 10.1038/nature09290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hale C.J., Potok M.E., Lopez J., Do T., Liu A., Gallego-Bartolome J., Michaels S.D., Jacobsen S.E. Identification of Multiple Proteins Coupling Transcriptional Gene Silencing to Genome Stability in Arabidopsis thaliana. PLoS Genet. 2016;12:e1006092. doi: 10.1371/journal.pgen.1006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raynaud C., Sozzani R., Glab N., Domenichini S., Perennes C., Cella R., Kondorosi E., Bergounioux C. Two Cell-Cycle Regulated SET-Domain Proteins Interact with Proliferating Cell Nuclear Antigen (PCNA) in Arabidopsis. Plant J. 2006;47:395–407. doi: 10.1111/j.1365-313X.2006.02799.x. [DOI] [PubMed] [Google Scholar]
- 113.Fransz P., ten Hoopen R., Tessadori F. Composition and Formation of Heterochromatin in Arabidopsis thaliana. Chromosome Res. 2006;14:71–82. doi: 10.1007/s10577-005-1022-5. [DOI] [PubMed] [Google Scholar]
- 114.Benoit M., Simon L., Desset S., Duc C., Cotterell S., Poulet A., Le Goff S., Tatout C., Probst A.V. Replication-Coupled Histone H3.1 Deposition Determines Nucleosome Composition and Heterochromatin Dynamics during Arabidopsis Seedling Development. New Phytol. 2018;221:385–398. doi: 10.1111/nph.15248. [DOI] [PubMed] [Google Scholar]
- 115.Jacob Y., Feng S., LeBlanc C.A., Bernatavichute Y.V., Stroud H., Cokus S., Johnson L.M., Pellegrini M., Jacobsen S.E., Michaels S.D. ATXR5 and ATXR6 Are H3K27 Monomethyltransferases Required for Chromatin Structure and Gene Silencing. Nat. Struct. Mol. Biol. 2009;16:763–768. doi: 10.1038/nsmb.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Menges M., de Jager S.M., Gruissem W., Murray J.A. Global Analysis of the Core Cell Cycle Regulators of Arabidopsis Identifies Novel Genes, Reveals Multiple and Highly Specific Profiles of Expression and Provides a Coherent Model for Plant Cell Cycle Control. Plant J. 2005;41:546–566. doi: 10.1111/j.1365-313X.2004.02319.x. [DOI] [PubMed] [Google Scholar]
- 117.Gutierrez C. 25 Years of Cell Cycle Research: What’s Ahead? Trends Plant Sci. 2016;21:823–833. doi: 10.1016/j.tplants.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 118.Anca I.A., Fromentin J., Bui Q.T., Mhiri C., Grandbastien M.A., Simon-Plas F. Different Tobacco Retrotransposons Are Specifically Modulated by the Elicitor Cryptogein and Reactive Oxygen Species. J. Plant Physiol. 2014;171:1533–1540. doi: 10.1016/j.jplph.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Makarevitch I., Waters A.J., West P.T., Stitzer M., Hirsch C.N., Ross-Ibarra J., Springer N.M. Transposable Elements Contribute to Activation of Maize Genes in Response to Abiotic Stress. PLoS Genet. 2015;11:e1004915. doi: 10.1371/journal.pgen.1004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bourbousse C., Mestiri I., Zabulon G., Bourge M., Formiggini F., Koini M.A., Brown S.C., Fransz P., Bowler C., Barneche F. Light Signaling Controls Nuclear Architecture Reorganization during Seedling Establishment. Proc. Natl. Acad. Sci. USA. 2015;112:E2836–E2844. doi: 10.1073/pnas.1503512112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Van Damme D., De Rybel B., Gudesblat G., Demidov D., Grunewald W., De Smet I., Houben A., Beeckman T., Russinova E. Arabidopsis Alpha Aurora Kinases Function in Formative Cell Division Plane Orientation. Plant Cell. 2011;23:4013–4024. doi: 10.1105/tpc.111.089565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weimer A.K., Nowack M.K., Bouyer D., Zhao X., Harashima H., Naseer S., De Winter F., Dissmeyer N., Geldner N., Schnittger A. Retinoblastoma Related1 Regulates Asymmetric Cell Divisions in Arabidopsis. Plant Cell. 2012;24:4083–4095. doi: 10.1105/tpc.112.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petrovska B., Cenklova V., Pochylova Z., Kourova H., Doskocilova A., Plihal O., Binarova L., Binarova P. Plant Aurora Kinases Play a Role in Maintenance of Primary Meristems and Control of Endoreduplication. New Phytol. 2012;193:590–604. doi: 10.1111/j.1469-8137.2011.03989.x. [DOI] [PubMed] [Google Scholar]
- 124.Costa S., Shaw P. Chromatin Organization and Cell Fate Switch Respond to Positional Information in Arabidopsis. Nature. 2006;439:493–496. doi: 10.1038/nature04269. [DOI] [PubMed] [Google Scholar]
- 125.Desvoyes B., Ramirez-Parra E., Xie Q., Chua N.H., Gutierrez C. Cell Type-Specific Role of the Retinoblastoma/E2F Pathway during Arabidopsis Leaf Development. Plant Physiol. 2006;140:67–80. doi: 10.1104/pp.105.071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ikeuchi M., Iwase A., Sugimoto K. Control of Plant Cell Differentiation by Histone Modification and DNA Methylation. Curr. Opin. Plant Biol. 2015;28:60–67. doi: 10.1016/j.pbi.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 127.Schommer C., Debernardi J.M., Bresso E.G., Rodriguez R.E., Palatnik J.F. Repression of Cell Proliferation by MiR319-Regulated TCP4. Mol. Plant. 2014;7:1533–1544. doi: 10.1093/mp/ssu084. [DOI] [PubMed] [Google Scholar]
- 128.Bhosale R., Boudolf V., Cuevas F., Lu R., Eekhout T., Hu Z., van Isterdael G., Lambert G., Xu F., Nowack M.K., et al. A Spatiotemporal DNA Endoploidy Map of the Arabidopsis Root Reveals Roles for the Endocycle in Root Development and Stress Adaptation. Plant Cell. 2018;30:2330–2351. doi: 10.1105/tpc.17.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bhosale R., Maere S., De Veylder L. Endoreplication as a Potential Driver of Cell Wall Modifications. Curr. Opin. Plant Biol. 2019;51:58–65. doi: 10.1016/j.pbi.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 130.Harashima H., Sugimoto K. Integration of Developmental and Environmental Signals into Cell Proliferation and Differentiation through RETINOBLASTOMA-RELATED 1. Curr. Opin. Plant Biol. 2016;29:95–103. doi: 10.1016/j.pbi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 131.Perilli S., Perez-Perez J.M., Di Mambro R., Peris C.L., Diaz-Trivino S., Del Bianco M., Pierdonati E., Moubayidin L., Cruz-Ramirez A., Costantino P., et al. RETINOBLASTOMA-RELATED Protein Stimulates Cell Differentiation in the Arabidopsis Root Meristem by Interacting with Cytokinin Signaling. Plant Cell. 2013;25:4469–4478. doi: 10.1105/tpc.113.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heyman J., Cools T., Vandenbussche F., Heyndrickx K.S., Van Leene J., Vercauteren I., Vanderauwera S., Vandepoele K., De Jaeger G., Van Der Straeten D., et al. ERF115 Controls Root Quiescent Center Cell Division and Stem Cell Replenishment. Science. 2013;342:860–863. doi: 10.1126/science.1240667. [DOI] [PubMed] [Google Scholar]
- 133.Cruz-Ramirez A., Diaz-Trivino S., Wachsman G., Du Y., Arteaga-Vazquez M., Zhang H., Benjamins R., Blilou I., Neef A.B., Chandler V., et al. A SCARECROW-RETINOBLASTOMA Protein Network Controls Protective Quiescence in the Arabidopsis Root Stem Cell Organizer. PLoS Biol. 2013;11:e1001724. doi: 10.1371/journal.pbio.1001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vilarrasa-Blasi J., Gonzalez-Garcia M.P., Frigola D., Fabregas N., Alexiou K.G., Lopez-Bigas N., Rivas S., Jauneau A., Lohmann J.U., Benfey P.N., et al. Regulation of Plant Stem Cell Quiescence by a Brassinosteroid Signaling Module. Dev. Cell. 2014;30:36–47. doi: 10.1016/j.devcel.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 135.Gonzalez-Garcia M.P., Pavelescu I., Canela A., Sevillano X., Leehy K.A., Nelson A.D., Ibanes M., Shippen D.E., Blasco M.A., Cano-Delgado A.I. Single-Cell Telomere-Length Quantification Couples Telomere Length to Meristem Activity and Stem Cell Development in Arabidopsis. Cell Rep. 2015;11:977–989. doi: 10.1016/j.celrep.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Betegón-Putze I., Mercadal J., Bosch N., Planas-Riverola A., Marquès-Bueno M., Vilarrasa-Blasi J., Frigola D., Burkart R.C., Martínez C., Conesa A., et al. Precise Transcriptional Control of Cellular Quiescence by BRAVO/WOX5 Complex in Arabidopsis Roots. Mol. Syst. Biol. 2021;17:e9864. doi: 10.15252/msb.20209864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xiong Y., McCormack M., Li L., Hall Q., Xiang C., Sheen J. Glucose-TOR Signalling Reprograms the Transcriptome and Activates Meristems. Nature. 2013;496:181–186. doi: 10.1038/nature12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Belda-Palazón B., Adamo M., Valerio C., Ferreira L.J., Confraria A., Reis-Barata D., Rodrigues A., Meyer C., Rodriguez P.L., Baena-González E. A Dual Function of SnRK2 Kinases in the Regulation of SnRK1 and Plant Growth. Nat. Plants. 2020;6:1345–1353. doi: 10.1038/s41477-020-00778-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.