Abstract

Many anticancer drugs are reported to have low physicochemical stability after dilution; therefore, producers impose short times from reconstitution, dilution, and the end of administration. The precariousness of cancer patients’ health in real-life experience within cancer hospitals often forces delays in the drug administration with respect to the standard treatment schedule timing, because of acute toxicities or the need to postpone a control analysis before administration. The public health costs for discarded anticancer drugs due to administration interruptions can be avoided, thanks to independent analytical studies, which integrate the producer’s data reported in the technical sheet, referring to the real conditions of preparation in a sterile atmosphere under a cabin in a laboratory dedicated to handling cytotoxic drugs in controlled conditions of temperature, pressure, and particulate contamination. Decitabine is apparently an unstable molecule, whose reported stability is only 3 h at 2–8 °C when diluted, while the mother solution must be immediately used or, otherwise, discarded. This study has investigated the physicochemical stability of decitabine both in diluted infusion bags and in sterile water reconstituted syringes at 4 °C for 0, 24, 48, and 72 h. In all performed studies, the stability-indicating method involves, for the first time, the use of liquid chromatography–tandem mass spectrometry analysis. Unexpectedly, both diluted and reconstituted solutions of decitabine are more stable than previously reported data, with a 48 h-long physicochemical stability at 2–8 °C and protected from light.
1. Introduction
Oncologic and onco-hematologic diseases represent a great burden on worldwide health systems, involving, at different levels, patients and caregivers in their multiple paths of care, physicians that must choose the right therapy for the right patient for the best possible quality of life, different professional operators involved in each passage of care and, finally, the whole hospital organization in which the patients are confident in.
Target therapies, immunotherapy, early access programs to innovative medicines, investigational medicinal drugs (IMPs) within clinical trials are only the last conquests of hospital pharmacists’ management upon pharmaceutical market.
The precariousness of cancer patients’ health often provokes delays in the drug administration with respect to the standard treatment schedule timing, because of acute toxicities or the need to postpone a control analysis before administration. Sometimes, the physician’s warning to postpone therapies is transmitted to hospital pharmacies after preparation; for this reason, it is important to know the real stability of the drugs diluted in infusion bags, syringes, and elastomeric devices according to the producer’s indication reported in the technical sheet. Many drugs are reported as not having sufficient physicochemical stability after dilution and producers impose short times from reconstitution, dilution, and the end of administration. Decitabine (Dacogen) is an anticancer drug belonging to the nucleic acid synthesis inhibitors (cytidine analogue) class and is indicated in clinics for myelodysplastic syndromes and acute myeloid leukemia at a dose of 20 mg/m2 of body surface for 5 consecutive days every four weeks. No reduction or adjustment dose is prescribed in the case of toxicities, but temporary or definitive suspension of the treatment. Decitabine has very low nominal stability according to its technical sheet: the reconstituted powder with water for injectables at 5 mg/mL must be furtherly diluted within 15 min or, otherwise, must be discarded, while the diluted preparations in cold sodium chloride 0.9% at 0.15–1 mg/mL concentrations are stable for 3 h if stored at 2–8 °C.1 After this refrigeration time, the diluted solutions are stable for 1 h at room temperature before administration, which lasts for 1 h.
In the literature, only two independent studies have already investigated decitabine stability in reconstituted and diluted solutions.2,3 In Kim’s study, analysis was performed by a validated stability-indicating reversed-phase high-performance liquid chromatography (HPLC) assay with photodiode array detection. The results report that reconstituted decitabine solution with cold sterile water is physicochemical stable in glass vials for 12 h at 2–8 °C and in 1 mL PC/PP (PC is polycarbonate, and PP is polypropylene) syringes for at least 28 days at −25 °C, while diluted decitabine solution in cold normal saline (NS) infusion bags stored under refrigeration is stable for approximately 24 h. In Fernàndez-Ginés’s study, 5 mg/mL decitabine solutions in water for injection were packaged in polyethylene syringes and stored in a refrigerator at 4 ± 2 °C for 24 h. Chemical stability was assessed by means of 1H NMR spectroscopy, by acquiring consecutive spectra every hour during a 24 h period. Signals obtained in these experiments were compared with those of the reference compound. For clinical aims, in both studies, information on the stability of decitabine solutions was insufficient to our real-life experience. In particular, the 5 mg/mL mother solution stability was in both studies enlarged to 12 and 24 h under refrigeration, respectively, but only one result for 24 h stability was determined for the diluted solution in NS.
Our study has investigated the physicochemical stability of decitabine both in NS diluted infusion bags and in sterile water reconstituted syringes at storage periods and temperatures similar to those the drug is usually prepared and stored in hospitals pharmacies and for administration to patients. In particular, decitabine diluted solution in NS has been studied in infusion bag, while the reconstituted solution in water has been studied in the syringe. The studies were carried out at 4 °C for 0, 24, 48, and 72 h. In all performed studies, the stability-indicating method involves the use of liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis allowing an unambiguous assessment of drug purity, stability, and compatibility with medical devices.4 Our results show unexpectedly that both diluted and reconstituted solutions of decitabine are more stable than previously reported data, with 48 h—long physicochemical stability at 2–8 °C and protected from light. With respect to previous analyses, we have found by means of a new experimental method of LC–MS/MS analysis that reconstituted and diluted solutions of decitabine, prepared under controlled conditions of temperature and sterile handling inside biological cabins in a hospital pharmacy, are chemically and physically stable and in accord with medical needs of the hospital organization.
2. Experimental Section
2.1. Chemical and Reagents
Decitabine was commercially available as Dacogen 50 mg (Janssen-Cilag).
Acetonitrile and water were HPLC grade and were obtained from Honeywell Burdick & Jackson (MI, USA). Formic acid was purchased from Fisher Chemicals (LE, UK).
2.2. LC–MS/MS Apparatus
LC–MS/MS analyses were performed on a Shimadzu LC–MS 8040 Trip Quad MS equipped with the system controller CBM-20A and a Trip Quad mass spectrometer detector and controlled by LabSolutions WS Software (Neonatal Solution version 2.0 part-number 225-24442-91). The employed column was a Luna Omega 5 μm PS C18 100 Å (column dimensions 150 × 4.6 mm, from Phenomenex QC MIX 870). LC–MS/MS operating conditions such as flow, mobile phase, injection volume, retention time, column temperature, and condition of detection, are reported in the Operating Conditions Section.
2.3. HPLC Chromatographic and Mass Spectrometric Conditions
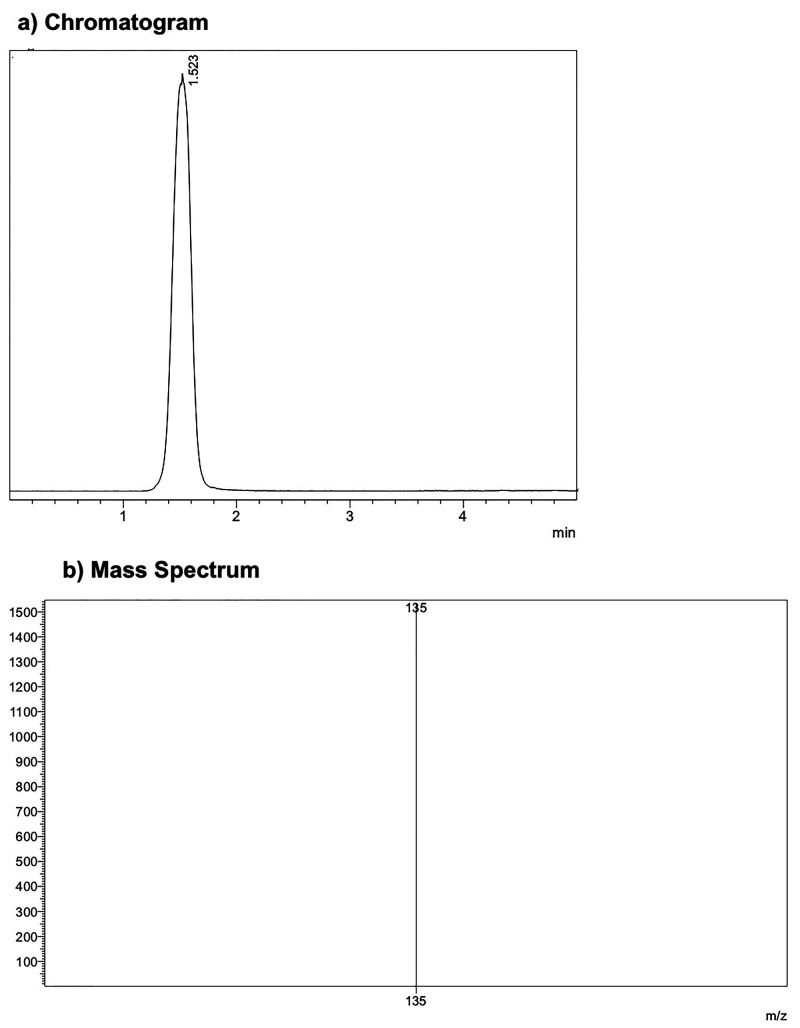
Decitabine is separated on a Luna Omega 5 μm PS C18 100 Å (column dimensions 150 × 4.6 mm, from Phenomenex QC MIX 870), using the mobile phase consisting of 30% methanol with 0.1% formic acid at the flow rate of 0.3 mL/min. The LC eluted is introduced into the electrospray ionization (ESI) source at 15 mL/min after a 95:5 (LC/MS) split. The mass spectrometer (Shimadzu 8040) operated under ESI. The positive ion multiple reaction monitoring (MRM) mode analysis was performed using nitrogen as the collision gas. The curtain gas (nitrogen) and the nebulizer gas (nitrogen) flow rates were set at 3 and 15 L/min, respectively. The LC eluent was introduced to the mass spectrometer using the ESI interface in the positive ion mode. The ion spray voltage, turbo heater, temperature, and decluttering potential were set at 2500 V, 400 °C, and 60 V, respectively. In order to monitor precursor/product, we used the following ion pair of m/z 251.15/135.00 for decitabine. The optimized collision energy was at 11 eV for decitabine. The retention time was about 1.5 min.
2.4. Stability Criterion
The qualitative and quantitative results have been compared with the data reported in the literature about substance identification, drug shelf-life, safety dose, and degradation products of decitabine. The studied compound is considered stable when its concentration is ≥95% with respect to the starting concentration at reconstitution/dilution, and other peaks in the chromatograms are not reported.
2.5. Sample Preparation
Decitabine was recovered from commercially available Dacogen 50 mg (Janssen-Cilag). The powder in its commercial vial was reconstituted at 5 mg/mL with cold water for injectables. After reconstitution, the decitabine solution for the infusion bag was then diluted in 100 mL cold sodium chloride solution of 0.9%.
Water and normal saline solution were refrigerated for one night at 2–8 °C before use.
The manipulation and preparation of the mother solutions were performed in accordance with international standard guidelines on injectables by nurses with expertise in handling chemotherapeutics within U.Ma.C.A. Laboratory in IRCCS IstitutoTumori “Giovanni Paolo II” in Bari.
The samples for analysis were collected and analyzed by Biofordrug operators.
The collection was carried out directly from the terminal part of the infusion bag in a tube and then transferred into vials for LC–MS/MS analysis. The collected solutions were diluted in water for the LC–MS/MS analysis.
All chemicals and reagents had the highest purity. All solvents were ultra performance liquid chromatography grade quality, and all chemicals were purchased from Honeywell Riedel-de-Haën.
3. Results and Discussion
After the routine calibration curves with the standard solutions, in the analysis samples, the concentrations were 4.81 mg/mL (instead of theoretical 5 mg/mL) and 0.334 mg/mL (instead of theoretical 0.37 mg/mL) for the reconstituted and diluted solutions, respectively. The infusion bag and the syringe have been stored at 2–8 °C for 0, 24, and 48 h and then at 72 h, and determinations are reported in Table 1. The diagram of the calibration curve for the standard analysis solutions is reported in Figure 1.
Table 1. Operating Conditions and Determinations for LC–MS Analysis of Reconstituted and Diluted Decitabine Solutions.
| analysis parameters | reconstituted solution | diluted solution | ||||
|---|---|---|---|---|---|---|
| concentration | 4.81 mg/mL (syringe) | 0.334 mg/mL (infusionbag) | ||||
| diluent | water for injectables | normal saline | ||||
| storage conditions | 2–8 °C | 2–8 °C | ||||
| determinations | 0 h | 24 h | 48 h | 0 h | 24 h | 48 h |
| average concentration | 4.81 ± 0.02 | 4.77 ± 0.1 | 4.81 ± 0.05 | 0.334 ± 0.01 | 0.327 ± 0.1 | 0.343 ± 0.2 |
| % average concentration change | –0.8 | 0 | –2.1 | +2.7 | ||
Figure 1.
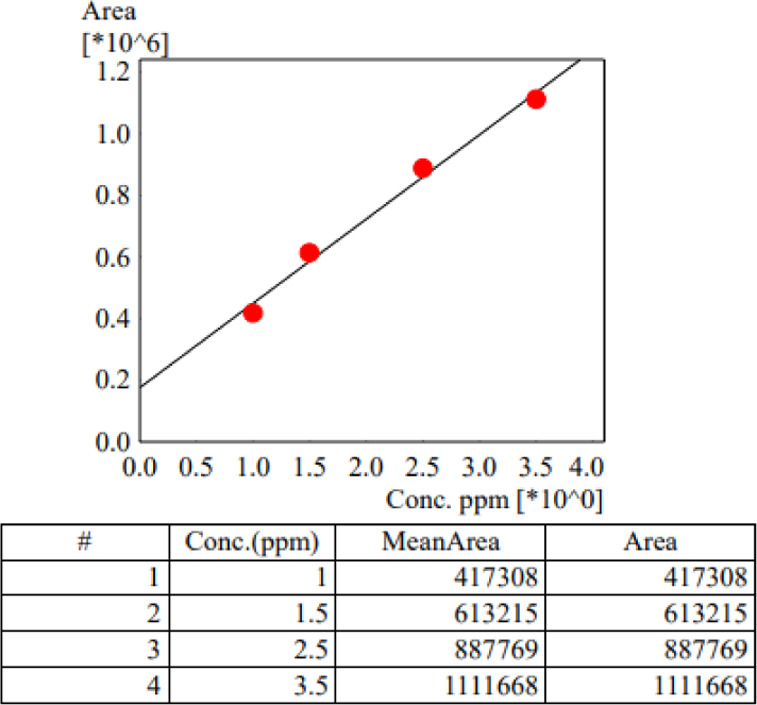
Decitabine calibration curve with standard samples.
The representative chromatograms obtained by LC–MS/MS analysis for standard decitabine samples is depicted in Figure 2.
Figure 2.
Representative chromatograms obtained by LC–MS/MS analysis for standard decitabine samples.
Moreover, in Figure 3, the chromatograms of decitabine reconstituted solution after 0, 24, and 48 h in refrigerated storage conditions are reported chronologically.
Figure 3.
Chromatograms of the reconstituted solution samples after 0, 24, and 48 h (from left to right), respectively, of refrigerated storage conditions.
Analogous stability has been reported also for the diluted decitabine solution in the NaCl infusion bag and the relative chromatograms are reported in Figure 4. In this study, the experimental method involved LC–MS/MS analysis. Decitabine standard solutions were prepared and used to implement the method and the calibration curve. The samples were taken all along the studied period and submitted to LC–MS/MS analysis allowing an unambiguous assessment of drug purity, stability, and compatibility. The establishment of drug purity and stability was conducted by comparing the LC–MS/MS results with those deduced from the standard solutions.
Figure 4.
Chromatograms of the diluted solution samples after 0, 24, and 48 h (from left to right), respectively, in refrigerated storage conditions.
In particular, to appreciate its physicochemical stability, the quantitative evaluation was carried on by comparing the areas of the decitabine peak at different analysis times and the peak of reference solutions. Final quantitative results are reported in terms of “average concentration” and “% concentration” of samples considering the starting concentration measured at t = 0 and calculated after 24 and 48 and 72 h. Moreover, particular attention was given to the monitoring of color and clarity of the solution in the device and in the taken samples. The mixture was transparent.
The slight difference between theoretical concentration data of the reconstituted (4.81 mg/mL instead of 5 mg/mL) and diluted (0.334 instead of 0.37 mg/mL) solutions of analysis was probably due to expanded volume solution in the vial during reconstitution and to overfill volume in the NaCl 0.9% infusion bag, as declared by the producer, respectively.
The same LC–MS/MS analysis after 48 h has been performed for both examined solutions. At 72 h from preparation, the peak area is halved confirming the degradation of the compound (Figure 5).
Figure 5.
The chromatograms of the diluted solution samples after 72 h of refrigerated storage conditions. The reduction of peak area confirms the degradation of the compound.
The average mass spectrum as acquired under positive ion ESI exhibited one major ion at m/z 251.15 (Figure 4). The protonated molecule m/z 229 was mostly used as the precursor ion in previously published methods.4 In our study, the average mass spectrum as acquired under positive ion ESI exhibited one major ion at m/z 251.15 (Figure 4), and decitabine formed predominantly sodium adducts. Moreover, sodium adducts are still predominant at the highest formic acid concentrations and other protonated ions in Q1 have been not observed. These ions correspond to (M – Na+). Decitabine sodium adducts were observed, with formic acid as eluent. MRM spectrum of the decitabine at m/z 251.15 exhibited a base fragment ion at m/z 135.00, corresponding to the sodium adducts formed by glycosidic cleavage of decitabine sodium adducts. Therefore, the ion transition of m/z 251.15–135.00 was selected for monitoring decitabine (Figure 6).6
Figure 6.
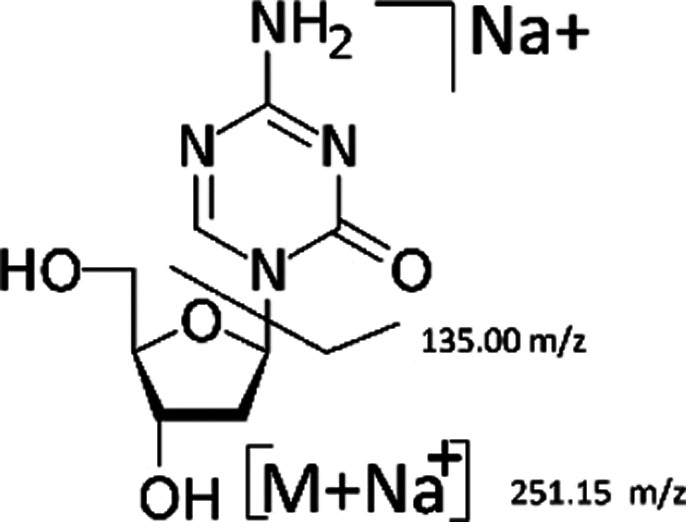
Average mass spectrums of 5-methyl-2′-deoxycytidine (1 mg/mL) and its fragmentation, sodium adduct molecular ion.
3.1. Experimental Methods
3.1.1. Linearity
The correlation coefficient of R2 = 0.9977 proved linearity over the concentration range. The equation of the calibration curve is y = 265 571x + 199 692.
3.1.2. Accuracy
Accuracy was evaluated at two different concentration levels (1 and 2.5 mg/mL) and with 9-fold injection. The accuracy was 97.8 ± 0.1% for 1 mg/mL and 98.3 ± 0.1% for 2.5 mg/mL of decitabine solutions.
3.1.3. Intra and Interday Precision
The intraday precision of the assay method was evaluated by carrying out 9 independent assays of decitabine solution prepared with cold (4 °C) buffer solution pH = 7.4 at two concentration levels (1 and 2.5 mg/mL).
For interday precision at each concentration level, a single injection of diluted decitabine solution and cold (4 °C) buffer solution pH = 7.4 was assayed daily for three consecutive days.
The % relative standard deviation of the intra and interday assays were 0.5% for 1 mg/mL and 0.7% for 2.5 mg/mL as well as 9.5% for 1 mg/mL and 8.7% for 2.5 mg/mL decitabine, respectively,.
The limit of detection and the limit of quantification were calculated by the equation LOD = 3.3 SD/s and LOQ = 10 SD/s, respectively. In the equation, s represents the slope of the calibration curve, and SD, the standard deviation of the peak area.
The LOD and the LOQ amounted to 0.012 and 0.039 mg/mL of decitabine, respectively.
4. Conclusions
In conclusion, both mother and diluted decitabine solutions have been demonstrated to be stable at 2–8 °C for 48 h long. The study of percentage variation of concentration has been employed to appreciate the stability of decitabine during the period of observation (24 and 48 h) at 2–8 °C, and as reported in Table 1, variability of concentration is acceptable (<5%).
The importance of these data is reflected in the activity of cancer hospitals, where the precariousness of patients’ health, the frequent emergency management of adverse events during the administration of chemotherapeutics, and the occasional need to postpone treatments, can also put in trouble different health professionals’ organization, hospital pharmacists in particular to prepare a new therapy.5
The higher stability of decitabine in refrigerated conditions that we have demonstrated integrates both the 15 min and the 3 h stability of the reconstituted and diluted decitabine reported in the technical sheet while using the frequent dosage range in clinics (32–40 mg, corresponding to 0.30–0.37 mg/mL). The 48 h stability is an acceptable time for all clinical occurrences that can provoke the postponement of therapy infusion.6,7
This result also translates into an economic advantage for the public health system, avoiding any drug discard and excessive consumption of drug commercial vials, besides permitting a redistribution of economic forces in other important sections of the hospital assistance.8
The authors declare no competing financial interest.
References
- Décitabine (Dacogen)—Summary of Product Characteristics; Janssen Pharmaceuticals, 2019.
- Kim S. H.; Heeb R. M.; Krämer I. Physicochemical Stability of Reconstituted Decitabine (Dacogen) solutions and ready-to-administer infusion bags when stored refrigerated or frozen. Pharm. Technol. Hosp. Pharm. 2017, 2, 145–157. 10.1515/pthp-2017-0025. [DOI] [Google Scholar]
- Fernández-Ginés F. D.; Garcia-Muñoz S.; Rodríguez-Cuadros T. B.; Sierra-Garcia F.; Molina Cuadrado E. Stability of intraveous injection of decitabine stored in polyethylene syringes. Eur. J. Hosp. Pharm. 2017, 24, A205–A207. 10.1136/ejhpharm-2017-000640.456. [DOI] [Google Scholar]
- Kanneti R.; Rajesh R.; Aravinda Raj J. R.; Bhatt P. A. An LC–MS–MS method for the simultaneous quantitation of acyclovir and valacyclovir in human plasma. Chromatographia 2009, 70, 407–414. 10.1365/s10337-009-1171-3. [DOI] [Google Scholar]
- Lin K.-t.; Momparler R. L.; Rivard G. E. Sample preparation for the determination of 5-aza-2′-deoxycytidine in plasma by high-performance liquid chromatography. J. Chromatogr. 1985, 345, 162–167. 10.1016/0378-4347(85)80148-1. [DOI] [PubMed] [Google Scholar]
- Hua W.; Ierardi T.; Lesslie M.; Hoffman B. T.; Mulvana D. Development and validation of a HILIC–MS/MS method for quantification of decitabine in human plasma by using lithium adduct detection. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2014, 969, 117–122. 10.1016/j.jchromb.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Marcucci G.; Byrd J. C.; Grever M.; Xiao J.; Chan K. K. Characterization of decomposition products and preclinical and low dose clinical pharmacokinetics of decitabine (5-aza-2′-deoxycytidine) by a new liquid chromatography/tandem mass spectrometry quantification method. Rapid Commun. Mass Spectrom. 2006, 20, 1117–1126. 10.1002/rcm.2423. [DOI] [PubMed] [Google Scholar]
- Briot T.; Roger E.; Lautram N.; Verger A.; Clavreul A.; Lagarce F. Development and in vitro evaluations of new decitabine nanocarriers for the treatment of acute myeloid leukemia. Int. J. Nanomed. 2017, 12, 8427–8442. 10.2147/ijn.s147659. [DOI] [PMC free article] [PubMed] [Google Scholar]