Abstract
Purpose:
BRCA1/2 pathogenic variant (PV) mutations confer radiation sensitivity preclinically, but there are limited data regarding breast cancer outcomes after radiation therapy (RT) among patients with documented BRCA1/2 PV mutations versus no PV mutations.
Methods and Materials:
This retrospective cohort study included women with clinical stage I-III breast cancer who received definitive surgery and RT and underwent BRCA1/2 genetic evaluation at the The University of Texas MD Anderson Cancer Center. Rates of locoregional recurrence (LRR), disease-specific death (DSD), toxicities, and second cancers were compared by BRCA1/2 PV status.
Results:
Of the 2213 women who underwent BRCA1/2 testing, 63% self-reported their race as White, 13.6% as Black/African American, 17.6% as Hispanic, and 5.8% as Asian/American Indian/Alaska Native; 124 had BRCA1 and 100 had BRCA2 mutations; and 1394 (63%) received regional nodal RT. The median follow-up time for all patients was 7.4 years (95% confidence interval [CI], 7.1–7.7 years). No differences were found between the groups with and without BRCA1/2 PV mutations in 10-year cumulative incidences of LRR (with mutations: 11.6% [95% CI, 7.0%−17.6%]; without mutations: 6.6% [95% CI, 5.3%−8.0%]; P = .466) and DSD (with mutations: 12.3% [95% CI, 8.0%−17.7%]; without mutations: 13.8% [95% CI, 12.0%−15.8%]; P = .716). On multivariable analysis, BRCA1/2 status was not associated with LRR or DSD, but Black/African American patients (P = .036) and Asians/American Indians/Alaska Native patients (P = .002) were at higher risk of LRR compared with White patients, and Black/African American patients were at higher risk of DSD versus White patients (P = .004). No in-field, nonbreast second cancers were observed in the BRCA1/2 PV group. Rates of acute and late grade ≥3 radiation-related toxicity in the BCRA1/2 PV group were 5.4% (n = 12) and 0.4% (n = 1), respectively.
Conclusions:
Oncologic outcomes in a diverse cohort of patients with breast cancer who had a germline BRCA1/2 PV mutation and were treated with RT were similar to those of patients with no mutation, supporting the use of RT according to standard indications in patients with a germline BRCA1/2 PV mutation.
Introduction
As knowledge about the link between germline mutations and the development of cancers has evolved, guidelines have expanded regarding which patients with breast cancer should be evaluated for pathogenic mutations.1–5 Patients with germline BRCA1 or BRCA2 (BRCA1/2) pathogenic variant (PV) mutations may be offered alternative definitive pharmacologic and surgical treatment options, distinct from the recommendations for non-BRCA1/2-associated breast cancers.4,6 Preclinical literature suggests that BRCA1/2-mutated tumors may be more radiosensitive,7 although this has not been universally found8; whether this is relevant in patients and if this should affect clinical decisions regarding radiation therapy (RT) for patients with breast cancer and germline BRCA1/2 mutations remains uncertain. There have been several attempts to create guidelines and reach consensus on how to best deliver RT for such patients,4,9,10 but additional data on clinical outcomes are needed to guide decisions.
Ionizing radiation induces both single- and double-strand breaks (DSBs) in DNA, with the latter being particularly lethal via compromising the integrity of both DNA strands simultaneously.11 DSBs may be repaired by homologous recombination, which has high fidelity, or by nonhomologous end joining, which is relatively more error-prone. Both BRCA1 and BRCA2 are important to the canonical homologous recombination pathway.12 Consequently, RT-induced DSBs in tumors with a BRCA1/2 mutation can result in increased chromosomal rearrangements, genomic instability, and eventual cell death owing to deficiency in homologous recombination.11 Several preclinical and clinical models suggest that a BRCA1/2 PV mutation may render a tumor more sensitive to RT.13–16 Although this has been hypothesized to improve tumor control, concerns have been expressed that the normal tissues of patients with BRCA1/2 germline mutations may also be more radiosensitive and thus that such patients may experience greater toxicities and secondary radiation-induced malignancies compared with patients without such mutations.
Real-world evidence is needed to support appropriate practice in this area. A recent population-based cohort study showed that women with germline pathogenic mutations in breast cancer−associated genes were less likely to receive RT after breast-conserving surgery for early-stage, hormone receptor−positive cancer.17 Prior studies compared outcomes for patients with early-stage breast cancer who had BRCA1/2 PV mutations with outcomes for patients thought to have sporadic breast cancer,18–21 but the sporadic controls had not undergone genetic testing, and BRCA1/2 pathogenic mutation status was not confirmed.20,22,23 Reports of outcomes among patients with BRCA1/2 mutations and locally advanced breast cancer, for whom regional nodal irradiation (RNI) is recommended, are limited. Notably, RNI delivered in the treatment of locally advanced breast cancer exposes significantly more of the thoracic contents (including the heart and lungs) to radiation than does RT targeting only the breast (ie, for early-stage disease). For this reason, any pathogenic normal-tissue effects could be more likely to appear among patients treated with RNI. Because the clinical indications for RNI have increased,24,25 questions about the safety and efficacy of RT for women with locally advanced breast cancer and BRCA1/2 PV mutations are particularly relevant.
The purpose of this study was to evaluate oncologic outcomes and RT-related toxicity in a group of patients with breast cancer who underwent BRCA1/2 germline testing via a large genetic screening program that serves a diverse patient population.
Methods and Materials
Patients
With institutional review board approval, patient databases at The University of Texas MD Anderson Cancer Center Departments of Radiation Oncology and Breast Medical Oncology were retrospectively queried and cross-referenced to identify women ≥18 years old with a diagnosis of clinical stage I−III invasive breast cancer through the year 2017 who were treated with definitive surgery and adjuvant external-beam RT, underwent BRCA1/2 germline mutation testing, were evaluated by a breast medical oncologist at our institution, and were seen in follow-up. We included patients evaluated for a second primary cancer who underwent germline mutation testing, and information about the primary breast cancer was included for analysis. BRCA1/2 mutations were classified as either a PV or no mutation; the latter consisted of variants of unknown significance (VUS) or no identifiable BRCA1/2 PV. Referrals for genetic testing were based on patients’ personal and family medical histories, contemporaneous National Comprehensive Cancer Network guidelines, and shared decision-making between the patient and her health care providers.
Clinicopathologic features, treatment details, and follow-up information were abstracted from the electronic medical record. Disease was staged in all cases according to the American Joint Committee on Cancer seventh edition staging manual.26 Among women with synchronous, bilateral breast cancers, the cancer with higher clinical stage was included as the index primary breast cancer. The Common Terminology Criteria for Adverse Events, version 5.0, were used to record RT-related toxicities for the BRCA1/2 PV cohort. Acute toxicities were those observed within 3 months of RT treatment completion, and late toxicities were those observed afterward. Evaluation of subsequent in-field nonbreast cancers included tumors of nonbreast histology that arose in the breast/chest wall, thorax, axilla, or neck.
Statistical analysis
Patient, tumor, and treatment characteristics were compared by χ2 or Fisher exact tests for categorical variables; t tests were used to compare continuous variables. All time intervals were calculated from the date of definitive surgery for the first diagnosed breast cancer. Locoregional recurrence (LRR) was defined as clinically or pathologically confirmed disease recurrence in the ipsilateral breast/chest wall or axillary, internal mammary chain, or supraclavicular fossa nodal basins. Because local recurrences could not be distinguished from new ipsilateral primary breast cancers, both were considered to be LRR events. For disease-specific death (DSD), breast cancer−related death was scored as an event, with patients otherwise censored at last follow-up; death from other causes was considered a competing risk. Only patients with at least 1 year of follow-up after breast surgery were included in the DSD and LRR analyses. Rates of LRR and DSD were estimated by the method of cumulative incidence; outcomes based on BRCA1/2 PV mutational status were compared by using the Gray test.27 Death was considered a competing risk for both LRR and DSD. The actuarial probabilities of overall survival by BRCA1/2 status were estimated with the Kaplan-Meier method; differences were assessed with log-rank tests.
Univariate and multivariable proportional hazards models described by Fine and Gray, based on the competing risk Cox proportional hazards regression model, were used to assess the effect of potential prognostic factors on LRR and DSD.28 Corresponding hazard ratios and 95% confidence intervals (CIs) are reported. Statistical tests were based on a 2-sided significance level. A P value ≤.05 was considered statistically significant in all analyses. Toxicity data were summarized by descriptive statistics such as counts and percentages. Statistical analyses were performed with SAS, version 9.4 (SAS Institute Inc, Cary, NC) and Splus, version 8.2 (TIBCO Software Inc, Palo Alto, CA) or R, version 2.15.1 (R Project for Statistical Computing).29
Results
Patients
Clinicopathologic features at the time of breast cancer diagnosis of the 2213 women who met the inclusion criteria and received a diagnosis between 1977 and 2017 are shown in Table 1. The population included 37% women who self-reported their race as non-White (13.6% Black/African American, 17.6% Hispanic, and 6% Asian American or American Indian). BRCA1/2 PV mutations were identified in 224 women (10%), 124 with BRCA1 and 100 with BRCA2 PV mutations. A total of 73 patients (3.2% of the entire cohort) harbored a BRCA1/2 VUS without a PV mutation (16 BRCA1, 54 BRCA2, and 3 both).
Table 1.
Clinicopathologic patient characteristics
| Patients, no. (%) |
|||
|---|---|---|---|
| Characteristic | BRCA1/2 PV (n = 224) | No mutation (n = 1989) | P value |
| Age, y | |||
| Median (range) | 41 (23–84) | 46 (19–83) | <.001 |
| ≤40 | 108 (48.2) | 577 (29) | <.001 |
| >40 | 116(51.8) | 1412 (71) | |
| Race | |||
| Black /African American | 35 (15.6) | 266 (13.4) | .075 |
| Hispanic/Latino | 47 (21) | 342 (17.2) | |
| White | 124 (55.4) | 1271 (63.9) | |
| Asian or American Indian/Alaska Native | 18(8) | 110 (5.5) | |
| Menopausal status | |||
| Premenopausal | 62 (27.7) | 760 (38.2) | .002 |
| Postmenopausal* | 162 (72.3) | 1229 (61.8) | |
| Clinical T stage | |||
| Unknown† | 4(1.8) | 35 (1.8) | .036 |
| T0/Tis | 1 (0.5) | 11 (0.6) | |
| T1 | 57 (25.9) | 704 (36) | |
| T2 | 110(50) | 807 (41.3) | |
| T3 | 35 (15.9) | 266 (13.6) | |
| T4 | 17 (7.7) | 166 (8.5) | |
| Clinical nodal status | |||
| Unknown† | 4(1.8) | 35 (1.8) | <.001 |
| N0 | 143(65) | 993 (50.8) | |
| N+ | 77 (35) | 961 (49.2) | |
| Overall clinical stage | |||
| I | 40 (17.9) | 554 (27.9) | .005 |
| II | 110(49.1) | 890 (44.7) | |
| III | 74 (33) | 545 (27.4) | |
| Pathologic T stage | |||
| T0/Tis | 57 (25.4) | 356 (17.9) | .037 |
| T1 | 86 (38.4) | 866 (43.5) | |
| T2 | 60 (26.8) | 504 (25.3) | |
| T3 | 17 (7.6) | 227 (11.4) | |
| T4 | 4(1.8) | 36 (1.8) | |
| Pathologic nodal status | |||
| N0 | 119 (53.1) | 1056 (53.1) | .993 |
| N+ | 105 (46.9) | 933 (46.9) | |
| Overall pathologic stage | |||
| 0 | 46 (20.5) | 292 (14.7) | .063 |
| I | 55 (24.6) | 605 (30.4) | |
| II | 80 (35.7) | 676 (34) | |
| III | 43 (19.2) | 416 (20.9) | |
| Hormone receptor status | |||
| Unknown† | 7(3.1) | 10 (0.5) | <.001 |
| Negative | 93 (42.9) | 517 (26.1) | |
| Positive | 124 (57.1) | 1462 (73.9) | |
| Her2-neu status | |||
| Unknown† | 17 (7.6) | 65 (3.3) | <.001 |
| Negative | 192 (92.8) | 1557 (80.9) | |
| Positive | 15 (7.2) | 367 (19.1) | |
| TNBC status | |||
| Unknown† | 13 (5.8) | 22 (1.1) | <.001 |
| Non-TNBC | 126 (59.7) | 1605 (81.6) | |
| TNBC | 85 (40.3) | 362 (18.4) | |
| Nuclear grade Unknown† | 7 (3.1) | 18 (0.9) | <.001 |
| I-II | 54 (24.9) | 903 (45.8) | |
| III | 163 (75.1) | 1068 (54.2) | |
| LVSI | |||
| Unknown† | 2 (0.9) | 22 (1.1) | .770 |
| Yes | 63 (28.4) | 540 (27.5) | |
| No | 159 (71.6) | 1427 (72.5) | |
| Year of surgery ≤2000 | 25 11.2) | 139 (7) | .038 |
| 2001–2010 | 89 39.7) | 745 (37.5) | |
| 2011–2017 | 110 (49.1) | 1105 (55.6) | |
| Type of definitive surgery | |||
| Breast conserving surgery | 72 (32.1) | 1077 (54.1) | <.001 |
| Mastectomy | 152 (67.9) | 912 (45.9) | |
| Positive nodes, no. | |||
| <10 | 211 (94.2) | 1856 (93.3) | .614 |
| ≥10 | 13 (5.8) | 133 (6.7) | |
| Nodes removed, no. | |||
| Unknown* | 1 (0.5) | 3 (0.2) | .014 |
| <10 | 75 (33.6) | 837 (42.1) | |
| ≥10 | 148 (66.4) | 1149 (57.9) | |
| Radiation type | |||
| Breast | 54 (24.1) | 748 (37.6) | <.001 |
| Breast + RNI | 18 (8.0) | 329 (16.5) | |
| CW only | 5 (2.2) | 12 (0.6) | |
| CW + RNI | 147 (65.6) | 900 (45.2) | |
| Neoadjuvant chemotherapy | |||
| No | 72 (32.1) | 930 (46.8) | <.001 |
| Yes | 152 (67.9) | 1059 (53.2) | |
| Any chemotherapy | |||
| Yes | 202 (90.2) | 1667 (83.8) | .013 |
| No | 22 (9.8) | 322 (16.2) | |
| Adjuvant hormone therapy | |||
| Unknown† | 1 (0.5) | 0 | <.001 |
| Yes | 105 (47.1) | 1372 (69.0) | |
| No | 118 (52.9) | 617 (31.0) | |
| BSO | |||
| Yes | 64 (28.6) | 228 (11.5) | <.001 |
| No or > 1 y from breast surgery | 160 (71.4) | 1761 (88.5) | |
| Synchronous contralateral breast cancer | |||
| Yes | 19 (8.5) | 78 (3.9) | .002 |
| No | 205 (91.5) | 1911 (96.1) | |
Abbreviations: BSO = bilateral salpingo-oophorectomy; CW = chest wall; LVSI = lymphovascular space invasion; PV = pathogenic variant; RNI = regional nodal irradiation; TNBC = triple negative breast cancer.
Patients who had a prophylactic or therapeutic BSO at the time of diagnosis were considered postmenopausal.
Patients with unknown status were omitted from statistical analyses.
Patients in the BRCA1/2 PV group were younger, with a median age of 41 years (P < .001), and were more likely to have tumors of a higher clinical stage, triple-negative phenotype, high grade, and synchronous contralateral breast cancers (all P < .005) than the no-mutation group, but no significant differences were found by race between groups (P = .075). The BRCA1/2 PV mutation group more often received mastectomy than breast-conserving surgery, radiation to the chest wall and RNI versus other RT targets, and chemotherapy versus no chemotherapy (all P <.05) and underwent bilateral salpingo-oophorectomy and risk-reducing contralateral prophylactic mastectomy within 1 year of definitive breast cancer surgery.
Survival and LRR outcomes
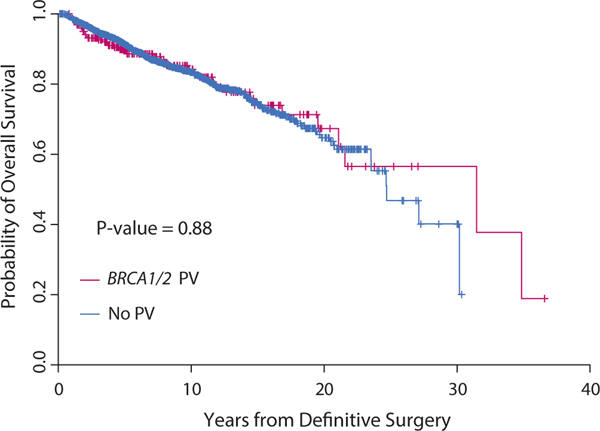
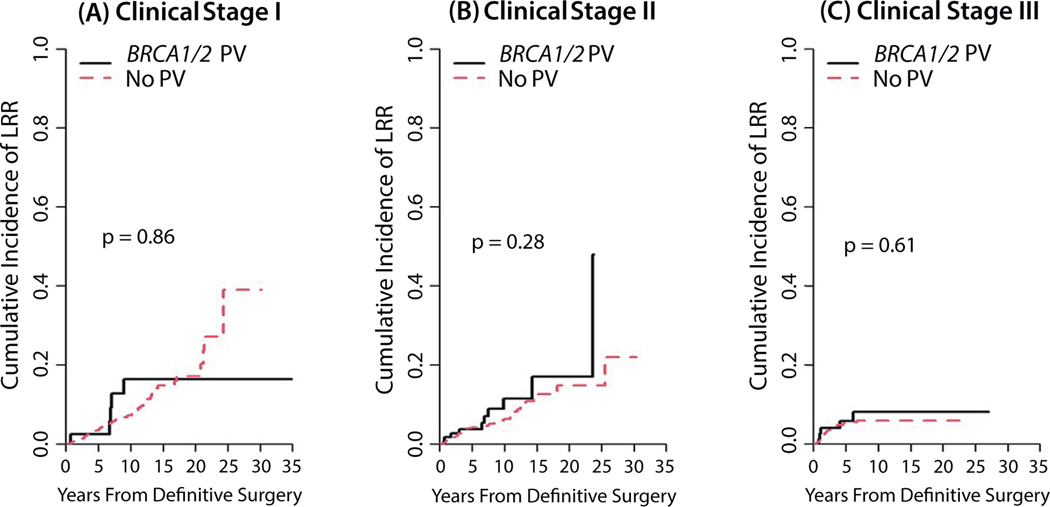
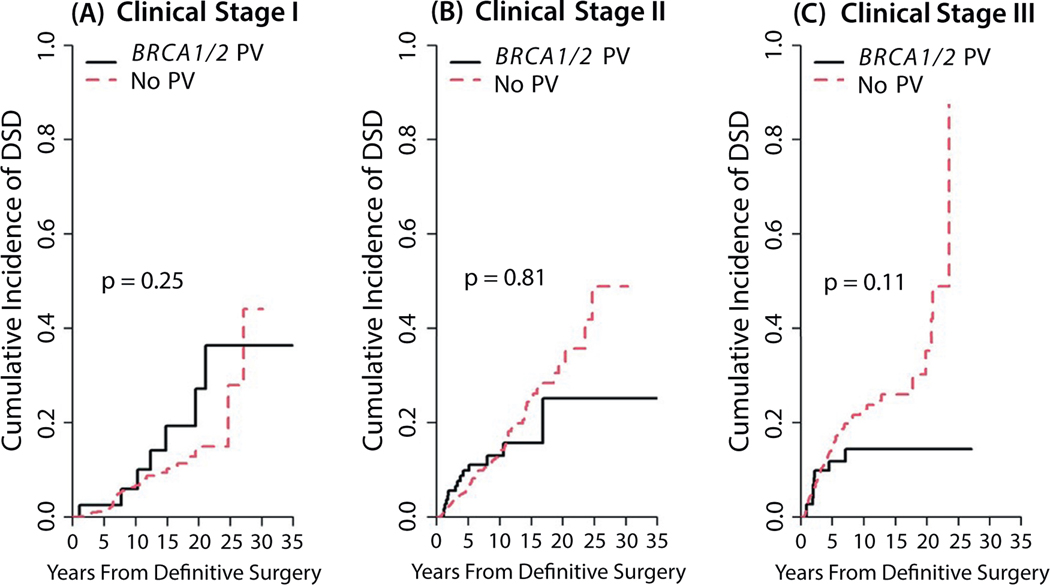
The median follow-up time for this analysis was 7.4 years (95% CI, 7.1–7.7 years). Ten-year overall survival rates were comparable for the BRCA1/2 PV and no-mutation groups (P = .875; Fig. 1). The 10-year cumulative incidence of LRR was 11.6% (95% CI, 7.0%−17.6%) in the BRCA1/2 PV group and 6.6% (95% CI, 5.3%−8.0%) in the no-mutation group (P = .466). Similarly, the 10-year DSD rate was 12.3% (95% CI, 8.0%−17.7%) in the BRCA1/2 PV group versus 13.8% (95% CI, 12.0%−15.8%) in the no-mutation group (P = .716). No differences were found in LRR or DSD rates by BRCA1/2 status when analyzed by clinical disease stage (Fig. 2 and 3). Patients who received RNI had a lower risk of LRR than those who did not (10-year cumulative incidence rates of LRR, 5.9% [95% CI, 4.6%−7.5%] vs 8.9% [95% CI, 6.7%−11.5%]; P = .004) but higher risk of DSD (16.6% [95% CI, 14.2%−19.1%] vs 8.7% [95% CI, 6.5%−11.5%]; P < .001). The LRR rates also did not differ among women who underwent bilateral salpingo-oophorectomy before or within 1 year of their breast surgery compared with women who did not, with death considered a competing risk (P > .05). Univariate analyses of factors found to be associated with LRR and DSD are shown in Tables E1 and E2.
Fig. 1.
Overall survival by BRCA1/2 status.
Fig. 2.
Locoregional recurrence rates by BRCA1/2 status according to clinical disease stage I (A), II (B), or III (C).
Fig. 3.
Disease-specific death rates by BRCA1/2 status according to clinical disease stage I (A), II (B), or III (C).
Multivariable analyses
On multivariable analysis, age ≤40 and higher pathologic disease stage retained significance for associations with both LRR and DSD (Table 2). In the LRR model, being Black/African American or Asian and American Indian/Alaska Native was associated with higher rates of LRR compared with being White, whereas receipt of adjuvant hormone therapy was associated with lower rates of LRR. Factors associated with higher rates of DSD were Black/African American race compared with White race, clinical stage III versus stage I, high nuclear grade, and increased nodal burden. Although mastectomy was associated with lower rates of LRR (hazard ratio [HR], 0.360 [95% CI, 0.219–0.593]; P ≤ .001), it correlated with higher rates of DSD (HR, 1.555 [95% CI, 1.130–2.141]; P = .007). Notably, BRCA1/2 status was not an independent predictor of LRR (HR, 0.873 [95% CI, 0.496–1.536]; P = .640). Despite inclusion of an interaction term for TNBC and BRCA1/2 PV in the DSD multivariable analysis model, BRCA1/2 status was not statistically significantly associated with DSD in either the non-TNBC group (HR, 0.697 [95% CI, 0.376–1.293]; P = .250) or the TNBC group (HR, 0.574 [95% CI, 0.308–1.073]; P = .082).
Table 2.
Multivariable analyses for factors associated with locoregional recurrence and disease-specific death
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| Multivariable analysis for factors associated with locoregional recurrence | |||
| Age, y | |||
| ≤40 vs >40 | 1.669 | 1.167–2.385 | .005 |
| Race | |||
| Black/African American vs White | 1.601 | 1.032–2.483 | .036 |
| Hispanic/Latino vs White | 1.091 | 0.671–1.776 | .730 |
| Asian or American Indian/Alaska Native vs White | 2.329 | 1.353–4.009 | .002 |
| Overall pathologic stage | |||
| I vs 0 | 4.447 | 1.734–11.406 | .002 |
| II vs 0 | 5.026 | 1.974–12.799 | <.001 |
| III vs 0 | 6.833 | 2.547–18.332 | <.001 |
| Nuclear grade | |||
| III vs I-II | 1.225 | 0.840–1.788 | .290 |
| LVSI | |||
| Yes vs no | 1.657 | 1.110–2.474 | .014 |
| Type of definitive surgery | |||
| Mastectomy vs breast-conserving surgery | 0.360 | 0.219–0.593 | <.001 |
| Year of surgery | |||
| ≤2000 vs 2011–2017 | 1.347 | 0.849–2.137 | .210 |
| 2001–2010 vs 2011–2017 | 0.871 | 0.596–1.274 | .480 |
| Regional nodal irradiation | |||
| Yes vs no | 0.985 | 0.584–1.661 | .950 |
| Adjuvant hormone therapy | |||
| Yes vs no | 0.444 | 0.304–0.648 | <.001 |
| BRCA1/2 status | |||
| PV vs no mutation | 0.873 | 0.496–1.536 | .640 |
| Multivariable analysis for factors associated wtih disease-specific death | |||
| Age, y | |||
| ≤40 vs >40 | 1.532 | 1.179–1.991 | .001 |
| Race | |||
| Black/African American vs White | 1.679 | 1.195–2.360 | .003 |
| Hispanic/Latino vs White | 1.064 | 0.751–1.506 | .730 |
| Asian, American Indian/Alaska native vs White | 0.804 | 0.429–1.509 | .500 |
| Overall clinical stage | |||
| II vs I | 1.359 | 0.874–2.115 | .170 |
| III vs I | 1.707 | 1.056–2.758 | .029 |
| Overall pathologic stage | |||
| I vs 0 | 2.867 | 1.548–5.310 | .001 |
| II vs 0 | 3.363 | 1.894–5.972 | <.001 |
| III vs 0 | 5.317 | 2.950–9.582 | <.001 |
| Nuclear grade | |||
| III vs I-II | 1.697 | 1.246–2.310 | .001 |
| LVSI | |||
| Yes vs no | 1.234 | 0.926–1.645 | .150 |
| Type of definitive surgery | |||
| Mastectomy vs breast-conserving surgery | 1.555 | 1.130–2.141 | .007 |
| Year of surgery | |||
| ≤2000 vs 2011–2017 | 0.469 | 0.289–0.761 | .002 |
| 2001–2010 vs 2011–2017 | 0.780 | 0.586–1.039 | .090 |
| Nodes positive, no. | |||
| ≥10 vs <10 | 1.512 | 1.015–2.253 | .042 |
| Triple-negative and BRCA1/2 status | |||
| Non-TNBC: PV vs no mutation | 0.697 | 0.376–1.293 | .250 |
| TNBC: PV vs no mutation | 0.574 | 0.308–1.073 | .082 |
| PV: TNBC vs non-TNBC | 2.713 | 1.907–3.860 | <.001 |
| No mutation: TNBC vs non-TNBC | 2.236 | 0.973–5.136 | .058 |
Abbreviations: CI = confidence interval; HR = hazard ratio; LVSI = lymphovascular space invasion; PV = pathogenic variant; TNBC = triple-negative breast cancer.
Second nonbreast cancers in the radiation treatment field
No in-field nonbreast secondary tumors were observed in the BRCA1/2 PV group. Thirteen women without BRCA1/2 PV mutations experienced a second nonbreast primary tumor within the radiation fields (median time after surgery, 4.4 years [range, 4 months to 20 years]). Six of these women developed thyroid cancer; all 6 had received RNI that included targeting of the supraclavicular fossa. The other 7 women developed a radiation-associated sarcoma; 4 of these were spindle cell sarcomas and none were angiosarcomas in the breast, chest wall, or intrathoracic region.
Toxicity in BRCA1/2 PV group
Acute and late toxicities among the BRCA1/2 PV cohort are shown in Table 3. Overall, grade 3 toxicities were minimal, and no grade 4–5 toxicities were noted. Twelve women (5.4%) experienced any acute grade 3 toxicities, most of which were adverse skin effects such as dermatitis, erythema, desquamation, or hyperpigmentation. One patient developed grade 3 herpetic neuralgia of the untreated, contralateral chest wall and arm during the course of RT. No acute lung or cardiac toxicities were observed. One patient developed grade 1, asymptomatic radiation pneumonitis diagnosed on follow-up computed tomography (CT) at 3 months, and no late grade ≥2 pulmonary or cardiac toxicities were noted.
Table 3.
Toxicities
| Toxicity | Grade | No. (%) |
|---|---|---|
| Acute | ||
| Any acute* | <3 | 212 (94.6) |
| 3 | 12 (5.4) | |
| Acute skin | <3 | 213 (95.1) |
| 3 | 11 (4.9) | |
| Acute breast pain, atrophy, or edema | <3 | 224 (100) |
| 3 | 0 (0) | |
| Acute other | <3 | 223 (99.6) |
| 3 | 1 (0.4) | |
| Late | ||
| Any late† | <3 | 223 (99.6) |
| 3 | 1 (0.4) | |
| Late skin | <3 | 223 (99.6) |
| 3 | 1 (0.4) | |
| Late breast pain, atrophy, or edema | <3 | 224 (100) |
| 3 | 0(0) | |
| Late other | <3 | 224 (100) |
| 0 | 0 (0) | |
No lung acute toxic effects were noted.
One late grade-1 lung toxic effect of asymptomatic radiation pneumonitis was detected on follow-up imaging.
Discussion
To our knowledge, this study is one of the largest to directly compare oncologic outcomes after surgery and adjuvant RT between BRCA1/2 PV mutation carriers and testing-confirmed noncarriers. With more than 35% of patients self-identifying as non-White, our findings represent the racially diverse demographic of the United States. Other large-scale efforts to evaluate clinical outcomes after RT in patients with a BRCA1/2 PV mutation have focused primarily on patients with Ashkenazi Jewish ancestry and founder mutations,30–32 although reports of other ethnic groups with BRCA 1/2 PV mutations have also been published from Korea, France, and the Netherlands.33–35 Strikingly, in our multivariate models of outcomes of LRR and DSD, neither BRCA 1/2 PV mutation status was significant, whereas race was.
We demonstrated that overall survival, LRR, and DSD rates were similar between patients with and without a BRCA1/2 PV mutation. No in-field secondary nonbreast cancers were observed in the BRCA1/2 PV mutation group. Toxicities in the BRCA1/2 PV mutation group were low overall. Most earlier studies comparing clinical outcomes based on BRCA1/2 PV status did not have documentation confirming which patients did not have a BRCA1/2 mutation and instead relied primarily on a negative family history to define that cohort,18–20,22 whereas testing was performed for every patient in our cohort, strengthening our results. Collectively, these findings do not suggest that women with germline BRCA1/2 mutations have more radiosensitive disease or have a different clinical response to RT than do BRCA1/2 PV noncarriers.
Preclinical studies have shown that tumors with a heterozygous BRCA1/2 mutation are more radiosensitive and more likely to have homologous recombination deficiencies and G2/M checkpoint defects.7 This heightened radiosensitivity has been found to affect not only tumor cells but also lymphocytes and other benign tissues.15,36,37 Increased radiosensitivity among BRCA1/2 PV carriers is thought to act as a double-edged sword that may increase tumor control but simultaneously increase secondary tumors and toxicity.16,38–40
Second primary cancers after RT have been documented in large, contemporary, population-based breast cancer data sets, albeit at low frequencies.41–44 Low rates of second cancers are, in part, attributable to the use of modern radiation techniques, including CT-based simulation, image guidance, and newer linear accelerators relative to historical experiences with orthovoltage and Cobalt-60 machines. Sixty-three percent of the patients in our study (1394 women) received RNI, which exposes more of the intrathoracic contents to low-dose RT relative to whole- or partial-breast RT. In contrast, many earlier studies evaluating clinical outcomes for patients with breast cancer based on BRCA1/2 status included only patients who received whole-breast irradiation.18–20,22 No in-field second cancers were observed in the 214 patients in the BRCA1/2 PV mutation group, similar to the <0.5% rate of second primary in-field tumors detected in BRCA1/2 carriers reported by Schlosser et al.31 Given these low frequencies, we propose that the benefits of RT, when indicated, outweigh the risk of second malignancies for women with BRCA1/2 PV mutations.
Prior studies of toxicities among BRCA1/2 PV mutation carriers did not find significant increases in toxicities,18,40,45 but these studies were limited largely to women with early-stage disease requiring RT to the breast alone, a treatment with a toxicity profile distinct from that of RNI. Our rates of acute and late grade 3 dermatitis of 4.9% and 0.4% are comparable to those in the randomized NCIC MA.20 trial (3.7% and 0.7%, respectively) for patients with unknown BRCA1/2 status.25 Three studies have shown equivalent rates of RT-related toxicity among BRCA1/2 carriers and sporadic, untested controls.18,33,40 To our knowledge, no demonstrable evidence has been published to date of increased radiosensitivity among BRCA1/2 carriers.
With expanding recommendations, germline mutations will undoubtedly be detected in a larger number of patients with breast cancer than before. Currently, BRCA1 and BRCA2 germline mutations are associated with 5% to 7% of all breast cancer cases, disproportionately affecting younger women and portending a 50% to 80% lifetime risk of developing breast cancer.11 The clinical conundrum for many physicians is the recommendation for subsequent surgery, RT, and systemic therapy for these patients. Women with early-stage breast cancer and PVs in BRCA1/2 and other breast cancer−associated genes are reportedly less likely to be treated with guideline-concordant modalities, including omission of standard RT, than are their counterparts without PV mutation.17 To address this issue, the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology evaluated the current literature and issued a consensus statement specifically for patients with germline PV mutations; that statement declared that adjuvant RT is appropriate and carries no increased risk of toxicity in BRCA1/2 PV mutation carriers versus noncarriers.4 The present study adds further evidence that RT can be used safely among patients with a BRCA1/2 PV, including a racially diverse population and those requiring RNI, groups previously understudied in this context.
Although this study generated insights into the consequences of adjuvant RT for BRCA1/2 PV mutation carriers, certain limitations must be acknowledged. First, BRCA1/2 PV patients in our cohort had biologically higher-risk disease, with TNBC presenting at a younger age, and were more likely to undergo neoadjuvant chemotherapy and mastectomy. This skew was expected, because many BRCA1/2 PV carriers with early-stage disease opt for mastectomy and do not require adjuvant RT and thus were not eligible for evaluation. In addition, even with comparable pathologic stage disease, patients with a BRCA1/2 PV were more likely to receive RNI, likely because of some of these underlying biologically aggressive features of their tumors. However, even with more aggressive stage and pathologic features, survival and locoregional control rates were comparable between groups. A second limitation is that a large majority of patients included in the study were treated before the use of Poly(ADP-ribose)polymerase-1 inhibitors was incorporated into the treatment of patients with BRCA1/2 PV-associated breast cancer, which is now standard. A third limitation is the challenges inherent in a retrospective study of this kind, specifically in the collection of toxicity data, with indisputable selection, follow-up, and survival biases, and the difficulty discerning between an LRR and a new primary on the ipsilateral side. Toxicities were recorded only for the 224 patients with a BRCA1/2 PV because there are data from prospective phase 3 clinical trials with long-term follow-up documenting standard toxicities seen after RT in other cohorts. Fourth, we recorded the index primary breast cancer for all included patients. However, these patients often presented to our tertiary care cancer center after diagnosis of a second breast cancer, at which time germline testing was conducted, concordant with current National Comprehensive Cancer Network guidelines.1 We considered VUS to be in the “no mutation” comparison group. Although 3% of BRCA1/2 gene perturbations detected are VUS, up to 20% are estimated to be pathogenic mutations,46,47 and the implications of this for use of RT remains uncertain. We also acknowledge that follow-up time for assessing second in-field cancers remains limited, and additional long-term screening of such patients is warranted. Nonetheless, major strengths of this series include the large number and racial diversity of patients who underwent germline mutation testing and had a known BRCA1/2 PV mutation status.
Conclusions
This single-institution study showed that among 2213 racially diverse women who underwent germline BRCA1/2 testing, surgery, and adjuvant RT, oncologic outcomes were similar for patients with and without PV mutations. Possessing a germline BRCA1/2 mutation does not seem to translate to increased radiosensitivity in the clinical setting. Our findings support the delivery of guideline-concordant care for patients with breast cancer and a BRCA1/2 mutation.
Supplementary Material
Acknowledgments—
We thank Christine F. Wogan, MS, ELS, from MD Anderson’s Division of Radiation Oncology, for editorial assistance.
This research has been supported in part by the Assessment, Intervention and Measurement shared resource through a Cancer Center support grant (P30 CA016672; principal investigator [PI], P. Pisters, MD Anderson Cancer Center) and a Biostatistics Research Group Cancer Center support grant (5P30CA016672-44; PI, J. Lee, MD Anderson Cancer Center) from the National Cancer Institute, National Institutes of Health.
Disclosures:
G.O.S. reports research support from the National Institutes of Health (grant R21CA252411), the Cancer Prevention and Research Institute of Texas, the Emerson Collective Foundation, Alpha Tau, and Artios Pharma and a research grant from TAE Life Sciences. S.J.B. reports a research grant from TAE Life Sciences. B.J.D. reports a research grant from Varian Medical Systems and royalty and equity interest in Oncora Medical. K.E.F. reports a research grant from Varian Medical Systems. S.F.S. reports a research grant from TAE Life Sciences; research support from the National Institutes of Health (grant R21CA252411), the Emerson Collective Foundation, Alpha Tau, and Artios Pharma; and contracted research support from Exact Sciences. J.K.L. is involved with research supported by Pfizer/medivation, Genentech, AstraZeneca, Merck, Jounce, and EMD Serono; she also serves on guideline committees for ASCO, NCCN and SITC. S.R.S. reports non-financial support from Myriad Genetics. W. A.W. has received fees from Exact Sciences and Epic Sciences.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijrobp.2021.09.033.
References
- 1.Daly MB, Reiser G, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. JNCCN 2021;77–102. [DOI] [PubMed]
- 2.Nelson HD, Pappas M, Cantor A, Haney E, Holmes R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019;322:666. [DOI] [PubMed] [Google Scholar]
- 3.Manahan ER, Kuerer HM, Sebastian M, et al. Consensus guidelines on genetic testing for hereditary breast cancer from the American Society of Breast Surgeons. Ann Surg Oncol 2019;26:3025–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tung NM, Boughey JC, Pierce LJ, et al. Management of hereditary breast cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology guideline. JCO 2020;38:2080–2106. [DOI] [PubMed] [Google Scholar]
- 5.Pujol P, Barberis M, Beer P, et al. Clinical practice guidelines for BRCA1 and BRCA2 genetic testing. Eur J Cancer 2021;146:30–47. [DOI] [PubMed] [Google Scholar]
- 6.Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med 2021;384:2394–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foray N, Randrianarison V, Marot D, Perricaudet M, Lenoir G, Feunteun J. Gamma-rays-induced death of human cells carrying mutations of BRCA1 or BRCA2. Oncogene 1999;18:7334–7342. [DOI] [PubMed] [Google Scholar]
- 8.Bright SJ, Flint DB, Chakraborty S, et al. Nonhomologous end joining is more important than proton linear energy transfer in dictating cell death. Int J Radiat Oncol Biol Phys 2019;105:1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall WA, Bergom C, Thompson RF, et al. Precision oncology and genomically guided radiation therapy: A report from the American Society for Radiation Oncology/American Association of Physicists in Medicine/National Cancer Institute Precision Medicine Conference. Int J Radiat Oncol Biol Phys 2018;101:274–284. [DOI] [PubMed] [Google Scholar]
- 10.Bergom C, West CM, Higginson DS, et al. The implications of genetic testing on radiation therapy decisions: A guide for radiation oncologists. Int J Radiat Oncol Biol Phys 2019;105:698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat Rev Cancer 2012;12:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasin M. Homologous repair of DNA damage and tumorigenesis: The BRCA connection. Oncogene 2002;21:8981–8993. [DOI] [PubMed] [Google Scholar]
- 13.Sharan SK, Morimatsu M, Albrecht U, et al. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature 1997;386:804–810. [DOI] [PubMed] [Google Scholar]
- 14.Buchholz TA, Wu X, Hussain A, et al. Evidence of haplotype insufficiency in human cells containing a germline mutation in BRCA1 or BRCA2. Int J Cancer 2002;97:557–561. [DOI] [PubMed] [Google Scholar]
- 15.Ernestos B, Nikolaos P, Koulis G, et al. Increased chromosomal radiosensitivity in women carrying BRCA1/BRCA2 mutations assessed with the G2 assay. Int J Radiat Oncol Biol Phys 2010;76:1199–1205. [DOI] [PubMed] [Google Scholar]
- 16.Bernier J, Poortmans P. Clinical relevance of normal and tumor cell radiosensitivity in BRCA1/BRCA2 mutation carriers: A review. The Breast 2015;24:100–106. [DOI] [PubMed] [Google Scholar]
- 17.Kurian AW, Ward KC, Abrahamse P, et al. Association of germline genetic testing results with locoregional and systemic therapy in patients with breast cancer. JAMA Oncol 2020;6:e196400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce LJ, Strawderman M, Narod SA, et al. Effect of radiotherapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J Clin Oncol 2000;18:3360–3369. [DOI] [PubMed] [Google Scholar]
- 19.Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol 2006;24:2437–2443. [DOI] [PubMed] [Google Scholar]
- 20.Kirova YM, Savignoni A, Sigal-Zafrani B, et al. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat 2010;120:119–126. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin PJ, Phillips K-A, West DW, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: An international prospective breast cancer family registry population-based cohort study. J Clin Oncol 2012;30:19–26. [DOI] [PubMed] [Google Scholar]
- 22.Haffty BG, Harrold E, Khan AJ, et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. The Lancet 2002;359(9316):1471–1477. [DOI] [PubMed] [Google Scholar]
- 23.Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: Differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 2004;6:R8–R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 2015;373:317–327. [DOI] [PubMed] [Google Scholar]
- 25.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early stage breast cancer. N Engl J Med 2015;373:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag; 2010. [Google Scholar]
- 27.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–1154. [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94(446):496–509. [Google Scholar]
- 29.R: The R Project for Statistical Computing. Available at https://www.rproject.org/. Accessed December 25, 2020.
- 30.Evron E, Ben-David AM, Goldberg H, et al. Prophylactic irradiation to the contralateral breast for BRCA mutation carriers with early-stage breast cancer. Ann Oncol 2019;30:412–417. [DOI] [PubMed] [Google Scholar]
- 31.Schlosser S, Rabinovitch R, Shatz Z, et al. Radiation-associated secondary malignancies in BRCA mutation carriers treated for breast cancer. Int J Radiat Oncol Biol Phys 2020;107:353–359. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein-Molho R, Laitman Y, Galper S, et al. Locoregional treatments and ipsilateral breast cancer recurrence rates in BRCA1/2 mutation carriers. Int J Radiat Oncol Biol Phys 2021;109:1332–1340. [DOI] [PubMed] [Google Scholar]
- 33.Park H, Choi DH, Noh JM, et al. Acute skin toxicity in Korean breast cancer patients carrying BRCA mutations. Int J Radiat Biol 2014;90:90–94. [DOI] [PubMed] [Google Scholar]
- 34.Kirova YM, Stoppa-Lyonnet D, Savignoni A, Sigal-Zafrani B, Fabre N, Fourquet A. Risk of breast cancer recurrence and contralateral breast cancer in relation to BRCA1 and BRCA2 mutation status following breast-conserving surgery and radiotherapy. Eur J Cancer 2005;41:2304–2311. [DOI] [PubMed] [Google Scholar]
- 35.Seynaeve C, Verhoog LC, van de Bosch LMC, et al. Ipsilateral breast tumour recurrence in hereditary breast cancer following breast-conserving therapy. Eur J Cancer 2004;40:1150–1158. [DOI] [PubMed] [Google Scholar]
- 36.Febrer E, Mestres M, Rosa Caballín M, et al. Mitotic delay in lymphocytes from BRCA1 heterozygotes unable to reduce the radiation-induced chromosomal damage. DNA Repair 2008;7:1907–1911. [DOI] [PubMed] [Google Scholar]
- 37.Kote-Jarai Z, Salmon A, Mengitsu T, et al. Increased level of chromosomal damage after irradiation of lymphocytes from BRCA1 mutation carriers. Br J Cancer 2006;94:308–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kan C, Zhang J. BRCA1 mutation: A predictive marker for radiation therapy? Int J Radiat Oncol Biol Phys 2015;93:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baeyens A, Thierens H, Claes K, Poppe B, de Ridder L, Vral A. Chromosomal radiosensitivity in BRCA1 and BRCA2 mutation carriers. Int J Radiat Biol 2004;80:745–756. [DOI] [PubMed] [Google Scholar]
- 40.Shanley S, McReynolds K, Ardern-Jones A, et al. Late toxicity is not increased in BRCA1/BRCA2 mutation carriers undergoing breast radiotherapy in the United Kingdom. Clin Cancer Res 2006;12:7025–7032. [DOI] [PubMed] [Google Scholar]
- 41.Burt LM, Ying J, Poppe MM, Suneja G, Gaffney DK. Risk of secondary malignancies after radiation therapy for breast cancer: Comprehensive results. Breast 2017;35:122–129. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y-J, Huang T-W, Lin F-H, Chung C-H, Tsao C-H, Chien W-C. Radiation therapy for invasive breast cancer increases the risk of second primary lung cancer: A nationwide population-based cohort analysis. J Thorac Oncol 2017;12:782–790. [DOI] [PubMed] [Google Scholar]
- 43.Grantzau T, Mellemkjær L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: A national population based study under the Danish Breast Cancer Cooperative Group (DBCG). Radiat Ther Oncol 2013;106:42–49. [DOI] [PubMed] [Google Scholar]
- 44.Berrington de Gonzalez A, Curtis RE, Gilbert E, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer 2010;102:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huszno J, Budryk M, Koɫosza Z, Nowara E. The influence of BRCA1/BRCA2 mutations on toxicity related to chemotherapy and radiotherapy in early breast cancer patients. OCL 2013;85: 278–282. [DOI] [PubMed] [Google Scholar]
- 46.Voelker R. Quick uptakes: Taking the uncertainty out of interpreting BRCA variants. JAMA 2019;321:1340–1341. [DOI] [PubMed] [Google Scholar]
- 47.Riaz N, Blecua P, Lim RS, et al. Pan-cancer analysis of bi-allelic alterations in homologous recombination DNA repair genes. Nat Comm 2017;8:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.