Abstract
The purpose of this study is to identify and quantify the chlorogenic acids (CGAs) from the root bark of Acanthopanax gracilistylus, which is conventionally regarded as a tonic in folk Chinese Traditional medicine. The effective methods for identification and quantification analysis of CGAs were developed based on ultra high performance liquid chromatography-Q-exactive orbitrap mass spectrometry (UHPLC-Q-Orbitrap MS) in parallel reaction monitoring (PRM) and selected reaction monitoring (SIM), which showed high sensitivity and resolution for screening and quantifying compounds. The root bark of A. gracilistylus was extracted under ultrasonication with 70% methanol. Ultimately, a for total of 70 CGAs, 64 of these were tentatively identified for the first time. Moreover, a methodological study of seven kinds of CGAs was carried out. The proposed procedure was optimized and validated in terms of selectivity, linearity of analytical curves (r2 > 0.990), accuracy (recovery range from 96.7 to 105%), and repeatability (relative standard deviation <5%). Then it was applied to determine the content of the CGAs in A. gracilistylus roots from 66 of different batches. The total CGAs was quantified in a range between 2.150 and 33.51 mg/g, which could be considered as excellent source of natural bioactive compound. The result was extremely useful for understanding the bioactive substance and quality control of A. gracilistylus in depth.
1. Introduction
Acanthopanax gracilistylus W. W. Smith (AGS), belonging to the genus Araliaceae, is generally distributed in the Hubei and Anhui provinces of China as a tonic and folk medicine that plays a crucial role in treating paralysis, bone pains, arthritis, rheumatism, and liver disease.1−3 In addition, A. gracilistylus combined with several other kinds of traditional Chinese medicines (TCMs) have been made into Wujiapi liquor, which is a famous Chinese medicinal liquor that has been as a sort of TCM health food product for hundreds of years. It not only is not only a potable spirit that has a mellow taste and a long aftertaste but also has the functions of promoting blood circulation and enhancing human immunity as an ingredient of Chinese herba preparations.4−6
In addition, previous phytochemical investigations on the root bark of A. gracilistylus indicated that volatile oils, terpenoids, and phenolic acids are the primary chemical components that are responsible for its biological and pharmacological activities of anti-inflammatory, antifatigue, antiaging, and antidiabetic.7 In general, it is recorded that the biological and pharmacological functions of herbs are extremely dependent on the composition of active ingredients, offering powerful assistance on reducing the probability of many chronic diseases.8,9 In particular, chlorogenic acids (CGAs) play a vital role in the total dietary intake of phenols in the daily human diet and have been classified into a family of polyphenolic compound and esters formed between cinnamic acid derivatives (such as caffeic, ferulic, and coumaric acid) and quinic acid are also a kind of plant defense that has been shown to reduce the definite risk of type 2 diabetes, obesity, Alzheimer’s disease, eclampsia, and stroke and shown to possess the active effects of promoting cell proliferation and differentiation as well as anti-inflammatory, antineoplastic, and antioxidant properties.10−13 To our knowledge, a majority of reports draw strong attention to the pharmacological activities of A. gracilistylus, but there is no adequate relative literature that systematically illustrates the effective constituents and content of A. gracilistylus, especially CGAs. In addition, it is essential to thoroughly investigate the content difference of CGAs.14 Thus, it is necessary to develop a sensitive, effective, and rapid method for identification and quantification of CGAs from the root bark of A. gracilistylus.
A few methods have been applied to identify the structure of CGAs with the goal of discovering important information that sufficiently explains or takes advantage of available plants. With the recent progress of mass spectrometry and separation methods, liquid chromatography coupled with tandem mass spectrometry, particularly, UHPLC-Q-exactive Orbitrap mass spectrometry, has acquired considerable attraction for the qualitative and quantitative analysis of phenolic acid compounds, which reveals its remarkable high resolution and separation capability in chemical characterization and affords accurate mass measurement (<5 ppm) for providing evidence in trace analytes in complex matrices and detailed mass spectral information. It also shows higher sensitivity in full scan mode and a higher intensity range than triple quadrupole mass spectrometry and time-of-flight mass spectrometry (TOF MS).15−18 Finally, a rapid and sensitive UHPLC–MS method was considered as one of the most available detection techniques for determination of CGAs.
The aim of this study is to develop an effective UHPLC-Q-Exactive Orbitrap MS method for simultaneous determination of CGAs from the root bark of A. gracilistylus.
2. Results and Discussion
2.1. Identification of Chemical Compositions
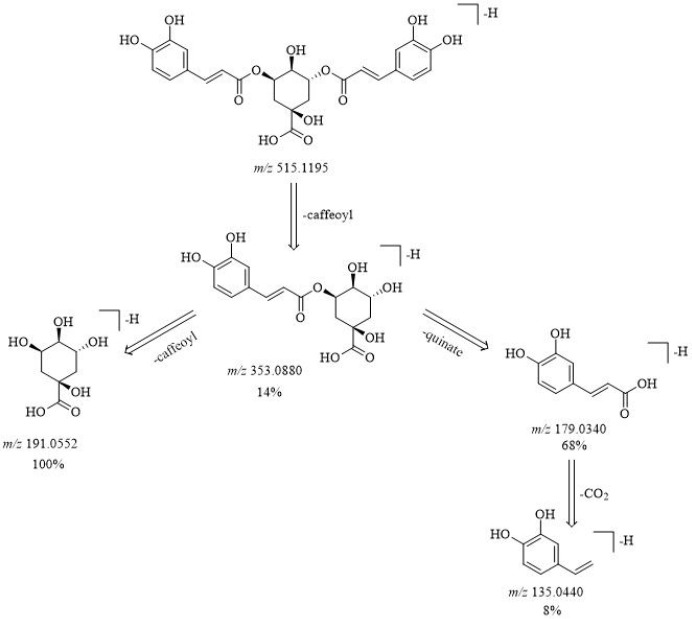
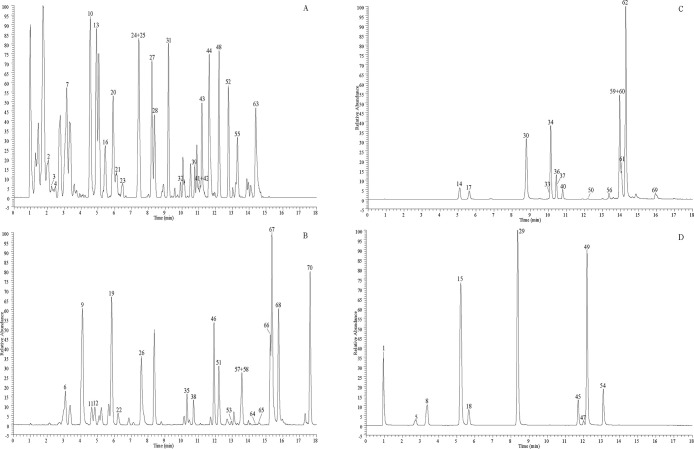
A total of 70 CGAs were explicitly identified using UHPLC-Q-exactive Orbitrap MS based on comparison of retention time and detailed mass spectrometric data in Table 1. The proposed fragmentation pathways for CGAs in the root bark of A. gracilistylus have been speculated, taking isochlorogenic acid A as an example in Figure 1. High-resolution extracted ion chromatography in negative mode is shown in Figure 2.
Table 1. Retention Time and Mass Information of CGAs in A. gracilistylus W. W. Smith. by UHPLC Q Exactive Orbitrap MS.
| peak | tR (min) | theoretical mass m/z | experimental mass m/z | error (ppm) | formula [M – H]− | MS/MS fragment | identification | abbreviation |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.95 | 191.0561 | 191.0554 | –3.61 | C7H11O6 | MS2[191]: 111.0076(100), 85.0282(48), 87.0074(38) | Quinic acid | QA |
| 2 | 2.05 | 677.1935 | 677.1943 | 1.20 | C28H37O19 | MS2[677]: 191.0554(100) | Caffeoylquinic acid-dihexoside | CQA-Dihexoside |
| 3 | 2.27 | 515.1406 | 515.1396 | –2.02 | C22H27O14 | MS2[515]: 179.0343(100), 191.0560(12), 341.0879(6) | Caffeoylquinic acid-hexoside | CQA-hexoside |
| 4 | 2.46 | 515.1406 | 515.1399 | –1.43 | C22H27O14 | MS2[515]: 179.0343(100), 191.0554(68), 341.0876(37), 323.0779(23) | Caffeoylquinic acid-hexoside | CQA-hexoside |
| 5 | 2.75 | 353.0878 | 353.0876 | –0.72 | C16H17O9 | MS2 [353]: 191.0552 (100) | 1-O-Caffeoylquinic acid | 1-CQA |
| 6 | 3.08 | 529.1563 | 529.1567 | 0.75 | C23H29O14 | MS2[529]: 193.0499(100) | Feruloylquinic acid-hexoside | FQA-hexoside |
| 7 | 3.14 | 515.1406 | 515.1403 | –0.72 | C22H27O14 | MS2 [515]: 191.0552(100), 179.0342(10), 173.0451(7), 353.0876(3) | Caffeoylquinic acid-hexoside | CQA-hexoside |
| 8a | 3.37 | 353.0878 | 353.0875 | –0.98 | C16H17O9 | MS2 [353]: 191.0552 (100),179.0339 (68), 135.0438(24) | 3-O-Caffeoylquinic acid | 3-CQA |
| 9 | 4.10 | 529.1563 | 529.1567 | 0.75 | C23H29O14 | MS2[529]: 173.0447(100), 191.0554(23), 193.0499(17) | Feruloylquinic acid-hexoside | FQA-hexoside |
| 10 | 4.55 | 515.1406 | 515.1403 | –0.72 | C22H27O14 | MS2 [515]: 191.0551(100), 323.0769(95), 161.0229(26) | Caffeoylquinic acid-hexoside | CQA-hexoside |
| 11 | 4.65 | 337.0929 | 337.0928 | –0.36 | C16H17O8 | MS2 [337]: 163.0388 (100), 119.0488(26), 191.0550(9) | 3-O-p-coumaroylquinic acid | 3-pCoQA |
| 12 | 4.83 | 529.1563 | 529.1572 | 1.68 | C23H29O14 | MS2[529]: 173.0447(100), 193.0500(22) | Feruloylquinic acid-hexoside | FQA-hexoside |
| 13 | 4.91 | 341.0878 | 341.0880 | 0.68 | C15H17O9 | MS2 [341]: 179.0342(100), 191.0554(33) | Caffeic acid-hexoside | CA-hexoside |
| 14 | 5.09 | 367.1035 | 367.1030 | –1.16 | C17H19O9 | MS2 [367]: 193.0497(100), 191.0550(65),173.0444(43), 134.0361(16) | 1-O-Feruloylquinic acid | 1-FQA |
| 15a | 5.22 | 353.0878 | 353.0873 | –1.32 | C16H17O9 | MS2 [353]: 191.0552 (100), 179.0339 (2) | 5-O-Caffeoylquinic acid | 5-CQA |
| 16 | 5.43 | 371.0984 | 371.0987 | 0.94 | C16H19O10 | MS2 [371]: 191.0554(100), 173.0449(20) | 3-O-Hydroxydihydrocaffeoylquinic acid | 3-O-HydroxydihydroCQA |
| 17 | 5.62 | 367.1035 | 367.1029 | –1.49 | C17H19O9 | MS2 [367]: 193.0496(100), 134.0360(20), 173.0444(5), 191.0554(4) | 3-O-Feruloylquinic acid | 3-FQA |
| 18a | 5.67 | 353.0878 | 353.0874 | –1.06 | C16H17O9 | MS2 [353]: 173.0440(100), 191.0551(93), 179.0339(75), 135.0439(33) | 4-O-Caffeoylquinic acid | 4-CQA |
| 19a | 5.84 | 179.0350 | 179.0343 | –3.64 | C9H7O4 | MS2[179]: 135.0441(100) | Caffeic acid | CA |
| 20 | 5.90 | 371.0984 | 371.0987 | 0.86 | C16H19O10 | MS2 [371]: 173.0448(100) | 4-O-Hydroxydihydrocaffeoylquinic acid | 4-O-HydroxydihydroCQA |
| 21 | 6.12 | 677.1723 | 677.1726 | 0.42 | C31H33O17 | MS2 [677]: 179.0342(100), 353.0883(44), 191.0550(43), 335.0780(13) | Dicaffeoylquinic acid-hexoside | DiCQA-hexoside |
| 22 | 6.21 | 529.1563 | 529.1572 | 1.68 | C23H29O14 | MS2[529]: 173.0449(100), 367.1034(31), 193.0501(15) | Feruloylquinic acid-hexoside | FQA-hexoside |
| 23 | 6.46 | 677.1723 | 677.1715 | –1.20 | C31H33O17 | MS2 [677]: 179.0342(100), 353.0887(15) | Dicaffeoylquinic acid-hexoside | DiCQA-hexoside |
| 24 | 7.42 | 691.1880 | 691.1888 | 1.16 | C32H35O17 | MS2 [691]: 179.0342(100), 191.0553(32), 353.0880(27), 135.0441(18), 335.0778(10) | Caffeoylferuloylquinic acid-hexoside | CFQA-hexoside |
| 25 | 7.45 | 677.1723 | 677.1713 | –1.47 | C31H33O17 | MS2 [677]: 179.0339(100), 341.0871(42), 191.0550(19), 515.1405(16), 323.0774(10), 353.0875(9) | Dicaffeoylquinic acid-hexoside | DiCQA-hexoside |
| 26 | 7.60 | 337.0929 | 337.0927 | –0.54 | C16H17O8 | MS2 [337]: 191.0551(100), 173.0444(11), 163.0388 (10) | 5-O-p-coumaroylquinic acid | 5-pCoQA |
| 27 | 8.21 | 677.1723 | 677.1717 | –0.93 | C31H33O17 | MS2 [677]: 179.0340(100), 191.0550(75), 353.0875(28), 323.0774(15), 161.0234(14), 135.0438(13), 341.0874(10) | Dicaffeoylquinic acid-hexoside | DiCQA-hexoside |
| 28 | 8.37 | 335.0772 | 335.0765 | –2.18 | C13H21O11 | MS2 [335]: 179.0341(100), 161.0235(52), 135.0440(40), 173.0446(12) | 5-O-caffeoylshikimic acid | 5-CSA |
| 29a | 8.37 | 515.1195 | 515.1188 | –1.88 | C25H23O12 | MS2[515]: 191.0551(100), 179.0339(87), 353.0875(14), 135.0439(13) | 1,3-O-Dicaffeoylquinic acid | 1,3-DiCQA |
| 30 | 8.79 | 367.1035 | 367.1031 | –1.08 | C17H19O9 | MS2 [367]: 191.0555(100), 173.0448(18), 193.0499(8) | 5-O-Feruloylquinic acid | 5-FQA |
| 31 | 9.20 | 691.1880 | 691.1893 | 1.96 | C32H35O17 | MS2 [691]: 179.0342(100), 341.0876(23), 335.0771(11) | Caffeoylferuloylquinic acid-hexoside | CFQA-hexoside |
| 32 | 9.91 | 677.1723 | 677.1731 | 1.15 | C31H33O17 | MS2 [677]: 179.0343(100), 191.0555(70), 335.0773(13) | Dicaffeoylquinic acid-hexoside | DiCQA-hexoside |
| 33 | 10.05 | 677.1512 | 677.1493 | –2.81 | C34H29O15 | MS2 [677]: 179.0343(100), 191.0550(58), 135.0441(30) | Tricaffeoylquinic acid | TriCQA |
| 34 | 10.14 | 529.1352 | 529.1348 | –0.72 | C26H25O12 | MS2[529]: 173.0445(100), 179.0340(86), 203.0341(39), 191.0553(28), 135.0439(15), 353.0873(14) | 3-O-feruloyl-4-O-caffeoylquinic acid | 3F,4CQA |
| 35 | 10.31 | 499.1246 | 499.1245 | –0.21 | C25H23O11 | MS2[499]: 163.0389(100), 191.0551(6), 337.0930(5), 173.0445(5) | 5-O-Caffeoyl-3-O-p-coumaroylquinic acid | 5C,3pCoQA |
| 36 | 10.44 | 529.1352 | 529.1348 | –0.72 | C26H25O12 | MS2[529]: 173.0445(100), 193.0497(17), 367.1031(4) | 3-O-caffeoyl-4-O-feruloylquinic acid | 3C,4FQA |
| 37 | 10.62 | 677.1512 | 677.1527 | 2.24 | C34H29O15 | MS2 [677]: 179.0343(100), 191.0555(81), 341.0672(23) | Tricaffeoylquinic acid | TriCQA |
| 38 | 10.70 | 499.1246 | 499.1243 | –0.63 | C25H23O11 | MS2[499]: 173.0447(100), 179.0342(93), 191.0551(40), 203.0345(25), 353.0884(20), 135.0442(18) | 4-O-Caffeoyl-3-O-p-coumaroylquinic acid | 4C,3pCoQA |
| 39 | 10.76 | 691.1880 | 691.1905 | 3.64 | C32H35O17 | MS2 [691]: 193.0500(100), 173.0446(11) | Caffeoylferuloylquinic acid-hexoside | CFQA-hexoside |
| 40 | 10.80 | 529.1352 | 529.1350 | –0.26 | C26H25O12 | MS2[529]: 191.0552(100), 193.0496(89), 173.0444(72), 143.0341(20), 179.0341(15) | 3-O-feruloyl-5-O-caffeoylquinic acid | 3F,5CQA |
| 41 | 10.98 | 677.1723 | 677.1078 | –1.69 | C31H33O17 | MS2 [677]: 191.0555(100), 179.0342(73), 353.0881(32), 323.0769(22) | Dicaffeoylquinic acid-hexoside | DiCQA-hexoside |
| 42 | 11.09 | 677.1723 | 677.1707 | –2.37 | C31H33O17 | MS2 [677]: 191.0555(100), 179.0343(74), 323.0781(61), 161.0238(22) | Dicaffeoylquinic acid-hexoside | DiCQA-hexoside |
| 43 | 11.19 | 677.1723 | 677.1718 | –0.76 | C31H33O17 | MS2 [677]: 191.0552(100), 323.0769(94), 179.0342(23), 161.0229(10), 341.0864(9), 515.1417(7) | Dicaffeoylquinic acid-hexoside | DiCQA-hexoside |
| 44 | 11.62 | 691.1880 | 691.1896 | 2.32 | C32H35O17 | MS2 [691]: 173.0447(100), 193.0499(16) | Caffeoylferuloylquinic acid-hexoside | CFQA-hexoside |
| 45a | 11.71 | 515.1195 | 515.1190 | –1.05 | C25H23O12 | MS2[515]: 173.0444(100), 179.0339(80), 191.0552(30), 353.0874(18), 135.0440(13) | 1,5-O-Dicaffeoylquinic acid | 1,5-DiCQA |
| 46 | 11.91 | 543.1508 | 543.1510 | 0.36 | C27H27 O12 | MS2[543]: 193.0500(100), 134.0363(13), 173.0448(10) | Caffeoyl-O-dimethoxycinnamoylquinic acid | C,dimethoxyCiQA |
| 47 | 12.01 | 515.1195 | 515.1192 | –0.58 | C25H23O12 | MS2[515]: 173.0445(100), 179.0339(92), 191.0552(78), 353.0872(17), 161.0232(16), 135.0439(15), 335.0777(10) | 3,4-O-Dicaffeoylquinic acid (Isochlorogenic acid B) | 3,4-DiCQA |
| 48 | 12.20 | 335.0772 | 335.0771 | –0.45 | C13H21O11 | MS2 [335]: 179.0342(100), 173.0448(54), 135.0442(37), 161.0100(28) | 4-O-Caffeoylshikimic acid | 4-CSA |
| 49a | 12.20 | 515.1195 | 515.1190 | –1.05 | C25H23O12 | MS2[515]: 191.0552(100), 179.0340(68), 353.0880(14), 135.0440(8) | 3,5-O-Dicaffeoylquinic acid (Isochlorogenic acid A) | 3,5-DiCQA |
| 50 | 12.21 | 677.1512 | 677.1521 | 1.34 | C34H29O15 | MS2 [677]: 191.0555(100), 335.0772(20), 179.0341(19) | Tricaffeoylquinic acid | TriCQA |
| 51 | 12.23 | 543.1508 | 543.1518 | 1.82 | C27H27 O12 | MS2[543]: 193.0500(100), 134.0364(8), 173.0448(8) | Caffeoyl-O-dimethoxycinnamoylquinic acid | C,dimethoxyCiQA |
| 52 | 12.76 | 691.1880 | 691.1895 | 2.13 | C32H35O17 | MS2 [691]: 179.0341(100), 323.0769(57), 191.0554(50), 173.0446(42), 335.0771(22) | Caffeoylferuloylquinic acid-hexoside | CFQA-hexoside |
| 53 | 12.95 | 499.1246 | 499.1253 | 1.51 | C25H23O11 | MS2[499]: 173.0448(100), 163.0391(14) | 3-O-Caffeoyl-4-O-p-coumaroylquinic acid | 3C,4pCoQA |
| 54a | 13.09 | 515.1195 | 515.1190 | –0.93 | C25H23O12 | MS2[515]: 173.0444(100), 179.0339(72), 191.0552(28), 353.0875(22), 135.0439(11) | 4,5-O-Dicaffeoylquinic acid (Isochlorogenic acid C) | 4,5-DiCQA |
| 55 | 13.30 | 559.1457 | 559.1460 | 0.51 | C27H27O13 | MS2[559]: 223.0607(100), 173.0447(73), 179.0342(55), 161.0235(24), 335.0776(17) | Caffeoylsinapoylquinic acids | SCQA |
| 56 | 13.37 | 529.1352 | 529.1348 | –0.72 | C26H25O12 | MS2[529]: 191.0552(100), 173.0445(14) | 3-O-Caffeoyl-5-O-feruloylquinic acid | 3C,5FQA |
| 57 | 13.57 | 337.0929 | 337.0931 | 0.74 | C16H17O8 | MS2 [337]: 191.0555(100), 173.0447(13) | 1-O-p-coumaroylquinic acid | 1-pCoQA |
| 58 | 13.57 | 499.1246 | 499.1244 | –0.33 | C25H23O11 | MS2[499]: 191.0551(100), 179.0340(16), 173.0445(10), 337.0940(4) | 3-O-Caffeoyl-5-O-p-coumaroylquinic acid | 3C,5pCoQA |
| 59 | 13.96 | 529.1352 | 529.1346 | –1.08 | C26H25O12 | MS2[529]: 173.0445(100), 193.0495(18), 367.1033(5) | 4-O-feruloyl-5-O-caffeoylquinic acid | 4F,5CQA |
| 60 | 13.96 | 677.1512 | 677.1509 | –0.37 | C34H29O15 | MS2 [677]: 191.0551(100), 353.0877(91), 179.0339(72),335.0769(29), 161.0231(18), 135.0439(10) | Tricaffeoylquinic acid | TriCQA |
| 61 | 14.07 | 529.1352 | 529.1348 | –0.60 | C26H25O12 | MS2[529]: 173.0445(100), 179.0340(46), 191.0553(36), 353.0874(9),135.0444(8) | 4-O-Caffeoyl-5-O-feruloylquinic acid | 4C,5FQA |
| 62 | 14.30 | 677.1512 | 677.1509 | –0.46 | C34H29O15 | MS2 [677]: 179.0340(100), 173.0445(91), 353.0876(73), 161.0233(58), 191.0553(27), 255.0657(27),335.0766(22) | Tricaffeoylquinic acid | TriCQA |
| 63 | 14.39 | 559.1457 | 559.1462 | 0.83 | C27H27O13 | MS2[559]: 173.0446(100), 223.0605(17) | Caffeoylsinapoylquinic acids | SCQA |
| 64 | 14.41 | 499.1246 | 499.1248 | 0.41 | C25H23O11 | MS2[499]: 173.0448(100), 163.0393(12) | 5-O-Caffeoyl-4-O-p-coumaroylquinic acid | 5C,4pCoQA |
| 65 | 14.56 | 499.1246 | 499.1238 | –1.61 | C25H23O11 | MS2[499]: 173.0449(100), 179.0343(72), 191.0554(40), 353.0881(28), 135.0442(9) | 4-O-Caffeoyl-5-O-p-coumaroylquinic acid | 4C,5pCoQA |
| 66 | 15.26 | 691.1668 | 691.1675 | 1.01 | C35H31O15 | MS2 [691]: 179.0341(100), 191.0341(83), 353.0877(67), 335.0770(16) | Dicaffeoylferuloylquinic acids | DiCFQA |
| 67 | 15.35 | 691.1668 | 691.1676 | 1.09 | C35H31O15 | MS2 [691]: 179.0342(100), 161.0235(67), 353.0880(55), 193.0499(49) | Dicaffeoylferuloylquinic acids | DiCFQA |
| 68 | 15.75 | 691.1668 | 691.1676 | 1.09 | C35H31O15 | MS2 [691]: 173.0447(100), 179.0342(55) | Dicaffeoylferuloylquinic acids | DiCFQA |
| 69 | 15.94 | 677.1512 | 677.1514 | 0.35 | C34H29O15 | MS2 [677]: 173.0445(100), 179.0340(89), 353.0876(83), 191.0554(25), 161.0235(23), 255.0657(14), 135.0440(10), 335.0781(5) | 3,4,5-Tricaffeoylquinic acid | 3,4,5-TriCQA |
| 70 | 17.62 | 193.0506 | 193.0489 | –8.86 | C10H9O4 | MS2[193]: 134.0362(100), 178.0263(99), 149.0598(34), 137.0233(21) | Ferulic acid | FA |
Identified by comparing with reference standards.
Figure 1.
Proposed fragmentation patterns of the main fragment ions in negative-ion mode for isochlorogenic acid A in the root bark of A. gracilistylus.
Figure 2.
High-resolution extracted ion chromatogram (HREIC) for multiple compounds in A. gracilistylus W. W. Smith. (A) m/z 335.0772, 341.0878, 371.0983, 515.1406, 559.1457, 677.1723, 677.1934, 691.1879; (B) m/z 179.0349, 193.0506, 337.0928, 499.1245, 529.1562, 543.1507, 691.1668; (C) m/z 367.1034, 529.1351, 677.1511; (D) m/z 191.0561, 353.087, 515.1195.
2.1.1. Identification of Chlorogenic Acid Moieties
Compound 19 with a precursor ion [M – H]− at m/z 179.0349 (C9H7O4) was identified as caffeic acid, which yielded a product ion at m/z 135.044 [caffeic acid–H–CO2]− corresponding to the public data.19 Compound 1 was identified as quinic acid, which gave an ion at m/z 191.0561 (C7H11O6) and produced fragment ions at m/z 111.007, 85.028, and 87.007 in keeping with ref (20). Compound 70 showed an [M–H]− ion at m/z 193.0506 (C10H9O4) and yielded a product ion at m/z 178.026 [M–H–CH3]−· by the loss of a methyl radical and then loss of a CO2 to obtain an ion at m/z 134.036 [M–H–CH3–CO2]−· in line with the literature,21 so it was identified as ferulic acid. Compound 13 with a precursor ion at m/z 341.0878 (C15H17O9) showed a product ion at m/z 179.034 (C9H7O4) [caffeic acid–H]−, which indicated subsequent loss of the hexose, so it was annotated as CA-hexoside.
2.1.2. Identification of Caffeoylquinic Acids
Compounds 5, 8, 15, and 18 with retention times (tR) of 2.75, 3.37, 5.22, 5.67 min gave the identical [M–H]− ion at m/z 353.0878 (C16H17O9) [caffeoyquinic acid–H]− and a similar MS2 product ion m/z 191.055 (C7H11O6) [quinic acid–H]−. Compound 8 was identified as 3-CQA by the presence of the distinctive ion with a peak at m/z 135.043 (C8H7O2) [caffeic acid–H–CO2]− in the MS2 spectra of the targeted ion, distinguished with compound 15 as 5-CQA. Compound 18 was identified as 4-CQA possessing extraordinary and intense ion at m/z 173.044 (C7H9O5) [quinate-H2O]−, and compounds 8, 15, and 18 were matched with those of the authentic standards by comparing their chromatography retention times, accurate mass measurement, and fragment pattern with those data. Respectively, 1-CQA (peak 5) and 5-CQA have remarkably similar product ion so as to difficultly recognize them except standards comparison and the consideration of their chromatographic behaviors on C18 columns.22,23
Compounds 16 and 20 were attributed to hydroxydihydrocaffeoylquinic acid, as these produced fragment ions at m/z 191.055 (C7H11O6) [quinate]− and 173.044 (C7H9O5) [quinate–H2O]−; compound 16 yielded a base peak product ion at m/z 191.055 (C7H11O6) [quinate]−, identified as 3-O-Hydroxydihydrocaffeoylquinic acid; and compound 20 yielded a base peak product ion at m/z 173.044 (C7H9O5) [quinate–H2O]−, identified as 4-O-hydroxydihydrocaffeoylquinic acid.
Compounds 29, 45, 47, 49, and 54 were respectively identified as 1,3-DiCQA, 1,5-DiCQA, 3,4-DiCQA, 3,5-DiCQA, and 4,5-DiCQA based on comparison of the retention time and MS patterns with those reference standards.
Compounds 3, 4, 7, and 10 presented the same [M–H]− ion at m/z 515.1406 (C22H27O14), their MS2 spectra gave the expected MS2 ions at m/z 179.034 (C9H7O4) [caffeic acid–H]−, 191.055 (C7H11O6) [quinate]−, 173.044 (C7H9O5) [quinate–H2O]−, and 353.0878 (C16H17O9) [caffeoyquinic acid-H]−, which indicated loss of a hexose. As far as we know, such compounds have not previously been characterized unequivocally; therefore, they were considered as CQA-hexoside isomers.24
Compounds 33, 37, 50, 60, 62, and 69 showed an MS base peak at m/z 677.1511 (C34H29O15) [tricaffeoylquinic acid–H]− and MS2 base peak at m/z 179.034 (C9H7O4) [caffeic acid–H]− in addition to compound 69 an also yielded other major product ions at m/z 191.055 (C7H11O6) [quinate]−, 353.0878 (C16H17O9) [caffeoyquinic acid–H]−, and m/z 173.044 (C7H9O5) [quinate–H2O]−; therefore, compounds 33, 37, 50, 60, and 62 were defined as TriCQA isomers, while compound 69 was identified as 3,4,5-TriCQA by a comparison of fragmentation pattern with those of 3,4,5-tri-O-caffeoylquinic acid presented in the published data.25
Compounds 21, 23, 25, 27, 32, 41, 42, and 43 all displayed a precursor ion at m/z 677.1723 (C31H33O17) and produced MS2 product ions characteristic of a quinic acid residue and a caffeic acid residue at m/z 179.034 (C9H7O4) [caffeic acid–H]−, 191.055 (C7H11O6) [quinate]−, 353.0878 (C16H17O9) [caffeoyquinic acid–H]−. As far as we know, such compounds have not previously been characterized definitely, and it is possible that these compounds were regarded as isomeric DiCQA-hexosides.25
Compound 2 with the same precursor ion at m/z 677.1934 was identified as CQA-dihexoside base on he fragmentation pattern at m/z 191.055 (C7H11O6) [caffeoyquinic acid–H–caffeoy–H2O]−, which indicated loss of two hexose.
2.1.3. Identification of Caffeoylshikimic Acids
Compounds 28 and 48 produced the identical precursor ion [M–H]− at m/z 335.0772 (C13H21O11) and product ions at m/z 179.034 (C9H7O4) [caffeic acid–H]− and 135.044 (C8H7O2) [caffeic acid–H–CO2]−, respectively, identified as 5-CSA, 4-CSA according to the retention behavior of the C18 columns and the literature.12
2.1.4. Identification of Coumaroylquinic Acids
Three compounds 11, 26, and 57 with the same precursor ion at m/z 337.0928 (C16H17O8) were, respectively, identified as 3-p-coumaroylquinic acid (3-pCoQA), 5-p-coumaroylquinic acid (5-pCoQA), and 1-p-coumaroylquinic acid (1-pCoQA). It is an essential distinction based on the different base peak ion in MS2 spectrum to distinguish the compounds 11 and 26 that MS2 base peak of 3-pCoQA at m/z 163.038 (C9H7O3) [coumaric acid–H]− while 5-pCoQA at m/z 191.055 (C7H11O6) [quinic acid–H]−. According to the chromatographic behavior of the eluted sequence on C18 columns, compound 57 was identified as 1-p-coumaroylquinic acid (1-pCoQA).26,27
2.1.5. Identification of Feruloylquinic Acids
Compounds 14, 17, and 30 were eluted at 5.09, 5.62, and 8.79 min, and all displayed precursor ion with peaks at m/z 367.1034 (C17H19O9) [feruloylquinic acid–H]−. Their MS2 spectra gave common ions at m/z 193.049 (C10H9O4) [ferulic acid–H]−, 191.055 (C7H11O6) [quinic acid–H]−, and 173.044 (C7H9O5) [quinate–H2O]−. In MS2 spectra, compound 30 was identified as 5-FQA and produced a strong base ion at m/z 191.055 (C7H11O6) [quinate]−, whereas 5-FQA (peak 10) yield a characterized MS2 product ion at m/z 193.049 (C10H9O4) [ferulate]−. Additionally, 1-FQA (peak 14) displayed spectroscopic data similar to those of 3-FQA, which were distinguished by the relative intensity of the secondary ion at m/z 191.055 (C7H11O6).28,29
Compounds 6, 9, 12, and 22 with a precursor ion [M–H]− at m/z 529.1562 (C23H29O14) were observed. They were annotated as FQA-hexoside as these compounds fragmented to produce product ions at m/z 367.103 [feruloylquinic acid–H]−, which indicated loss of a hexose and m/z 193.049 (C10H9O4) [ferulic acid–H]−, 173.044 (C7H9O5) [quinate–H2O]−, and 191.055 (C7H11O6) [quinate]−.12
2.1.6. Identification of Caffeoyl-O-p-coumaroylquinic Acids
Compounds 35, 38, 53, 58, 64, and 65 were regarded as six caffeoyl-p-coumaroylquinic acid isomers and presented the same [M–H]− ion at m/z 499.1245 (C25H23O11), respectively, as 5-caffeoyl-3-p-coumaroylquinic acid, 4-caffeoyl-3-p-coumaroylquinic acid, 3-caffeoyl-4-p-coumaroylquinic acid, 3-caffeoyl-5-p-coumaroylquinic acid, 5-caffeoyl-4-p-coumaroylquinic acid, and 4-caffeoyl-5-p-coumaroylquinic acid. Compound 35 produced the MS2 base peak at m/z 163.038 (C9H7O3) [p-hydroxycinnamic acid–H]− and also lost a caffeoyl residue at m/z 337.093 (C16H17O8) [p-coumaroylquinic acid–H]−. Compound 38 yielded the MS2 base peak at m/z 173.044 (C7H9O5) [quinate–H2O]−, which were similar to compounds 53, 64, and 65, respectively; compound 53 was given as m/z 163.039 (C9H7O3) [p-hydroxycinnamic acid–H]−, identified as 3C,4pCoQA the same as compound 64 (5C,4pCoQA) according to the published data.30 Compound 65 produced the MS2 product ions at m/z 179.034 (C9H7O4) [caffeic acid–H]−, 191.055 (C7H11O6) [quinate]−, 353.088 (C16H17O9) [caffeoyquinic acid–H]−, 135.044 (C8H7O2) [caffeic acid–H]− while compound 58 yielded the MS2 base peak ion at m/z 191.055 (C7H11O6) [quinate]−, identified as 3C,5pCoQA.12
2.1.7. Identification of Feruloylcaffeoylquinic Acids
Compounds 34, 36, 40, 56, 59, and 61 all showed the precursor ion with a peak at m/z 529.1351 (C26H25O12) [caffeoyl–feruloylquinic acids–H]−; compound 34 was identified as 3F,4CQA due to the MS2 base peak at m/z 173.044 (C7H9O5) [M–H–2feruloyl]−, the secondary product ion at m/z 179.034 (C9H7O4) [caffeic acid–H]−, and other ion at m/z 353.087 (C16H17O9) [M–H–feruloyl−]−; while 3C,4FQA (compound 36) showed as the MS2 base peak of the product ion at m/z 173.044 (C7H9O5) [M–H–2feruloyl]− and the secondary product ion at m/z 193.049 (C10H9O4) [ferulic acid–H]− and spectra of the characteristic ions with peak at m/z 367.103 (C17H19O9) [M–H–caffeoyl]− based on the literature;26 compound 40 was characterized as 3F,5CQA that yielded a MS2 base peak at m/z 191.055 (C7H11O6) [M–H–2caffeoyl]−, and the secondary product ion at m/z 193.049 (C10H9O4) [ferulic acid–H]− distinguished from 3C,5FQA (compound 56) produced a secondary product ion at m/z 173.044 (C7H9O5) [M–H–-2feruloyl]−; 4F,5CQA (compound 59) generated a base peak product ion at m/z 173.044 (C7H9O5) [M–H–2feruloyl]−, and the secondary product ion at m/z 193.049 (C10H9O4) [ferulic acid–H]− differentiated from the 4C,5FQA (compound 61) by the presence of the MS2 secondary product ion at m/z 179.034 (C9H7O4) [caffeic acid–H]− according to the public data.25,28,31
Compounds 66, 67, and 68 with a precursor ion [M–H]− at m/z 691.1668 were attributed to DiCFQA, based on the product ion at m/z 179.034 (C9H7O4) [caffeic acid–H]−, 173.044 (C7H9O5) [quinate–H2O]−.12
Compounds 24, 31, 39, 44, and 52 were followed in the identification of CFQA-glycoside which were identified by their precursor ion [M–H]− at m/z 691.1879 and based on their product ions at m/z 179.034 (C9H7O4) [caffeic acid–H]−, 191.055 (C7H11O6) [quinate]−, 193.050 (C10H9O4) [ferulic acid–H]−.
2.1.8. Identification of Caffeoyl-O-dimethoxycinnamoylquinic Acids
Compounds 46 and 51 with the same precursor ion at m/z 543.1507 (C27H27O12) as these produced product ions at m/z 193.050 (C10H9O4) [ferulic acid–H]− and 173.044 (C7H9O5) [quinate–H2O]− and were annotated as caffeoyl-O-dimethoxycinnamoylquinic acids.32
2.1.9. Identification of Caffeoylsinapoylquinic Acids
Compounds 55 and 63 were identified as SCQA, which yielded a precursor ion [M–H]− at m/z 559.1457 (C27H27O13), and based on their fragmentation patterns at m/z 173.044 (C7H9O5) [quinate–H2O]−, 179.034 (C9H7O4) [caffeic acid–H]−.12
2.2. Method Performance
In this study, quantification of individual compounds was carried out by an external calibration method, and the CGA concentration of 66 batches was calculated by plotting the area response versus the analytes concentration using 1/x weighted calibration curves. Seven kinds of standard working mixture solutions of CGAs analogue were completely separated using the delicate gradient program by UHPLC-Q-Orbitrap-MS. Linearity equations were obtained by plotting corresponding peak areas versus different concentrations. All the linearity equations exhibited excellent linearity, and the values of linear ranges (r2) of CGAs calculated from analytical curves both were >0.990 and linearity equations were listed in Table 2. RSDs of the repeatability test of seven kinds of CGA ranged from 2.10% to 3.32%. In addition, the accuracy of the proposed method was assessed in which 0.25 g of the A. gracilistylus roots powder was mixed with a known amount of seven CGAs reference substances and then extracted by the “4.2 Sample preparation” method. Ultimately, the results indicated that the UHPLC-Q-orbitrap method possessed good accuracy with recoveries ranging from 96.7% to 105%, while all of the RSDs were less than 5% (Table 2).
Table 2. Method Validation.
| stability RSD (%) | recovery | |||||||
|---|---|---|---|---|---|---|---|---|
| compd | analytical curve | range (μg/mL) | linearity (r2) | repeatability RSD (%) | rt for 4 h | autosampler at 10 °C for 24 h | mean (%) | RSD (%) |
| Isochlorogenic acid C | y = 2E+07x – 1E+07 | 0.428–42.8 | 0.9987 | 3.32 | 3.24 | 3.56 | 103.39 | 4.23 |
| Isochlorogenic acid A | y = 3E+07x – 3E+06 | 0.224–22.4 | 0.9926 | 2.62 | 2.38 | 2.94 | 103.44 | 3.27 |
| 1,5-Dicaffeoylquinic acid | y = 2E+07x – 3E+07 | 1.08–108 | 0.9958 | 3.14 | 2.86 | 3.18 | 96.92 | 2.94 |
| 1,3-Dicaffeoylquinic acid | y = 2E+07x – 2E+07 | 1.14–114 | 0.9987 | 2.21 | 2.28 | 2.96 | 104.97 | 4.09 |
| Cryptochlorogenic acid | y = 2E+07x – 6E+06 | 0.236–23.6 | 0.9988 | 2.13 | 2.36 | 2.74 | 96.74 | 3.83 |
| Chlorogenic acid | y = 1E+07x – 2E+07 | 1.47–147 | 0.9983 | 3.04 | 2.84 | 3.12 | 104.43 | 4.03 |
| Neochlorogenic acid | y = 3E+07x – 1E+07 | 0.246–24.6 | 0.9999 | 2.10 | 2.32 | 2.56 | 104.45 | 2.98 |
2.3. Quantification of the CGAs from the Root Bark of A. gracilistylus from Different Batches
Sixty-six different batches of A. gracilistylus root were extracted by the “4.2” method, and four batches were parallel. The optimized and validated UHPLC-Q-Orbitrap method was used for analysis. The contents and total contents of seven kinds of CGAS in different batches of the root bark of A. gracilistylus are shown in Table 3. The results are expressed as the average content, which range from 2.150 to 33.51 mg/g.
Table 3. Content (mg/g) of Seven Compounds in 66 Batches of A. gracilistylus.
| sample | isochlorogenic acid C | isochlorogenic acid A | 1,5-dicaffeoylquinic acid | 1,3-dicaffeoylquinic acid | cryptochlorogenic acid | chlorogenic acid | neochlorogenic acid | total of 7 CGAs |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.4394 | 0.1156 | 1.2204 | 1.2144 | 0.1335 | 2.4537 | 0.0928 | 5.6698 |
| 2 | 0.3302 | 0.0723 | 0.7922 | 1.5293 | 0.1713 | 2.4025 | 0.1181 | 5.4160 |
| 3 | 0.4783 | 0.1211 | 1.0640 | 2.2070 | 0.2161 | 2.9812 | 0.1385 | 7.2062 |
| 4 | 0.8879 | 0.1831 | 2.1499 | 3.4529 | 0.3040 | 4.9937 | 0.1909 | 12.1625 |
| 5 | 1.7865 | 0.3828 | 4.2989 | 5.4546 | 0.5336 | 8.5707 | 0.2919 | 21.3191 |
| 6 | 0.1348 | 0.0439 | 0.3245 | 0.3728 | 0.0850 | 0.8272 | 0.0655 | 1.8537 |
| 7 | 2.6670 | 0.3573 | 6.8101 | 8.8480 | 1.5234 | 12.5397 | 0.7607 | 33.5061 |
| 8 | 1.5031 | 0.2500 | 2.9831 | 6.0364 | 0.8261 | 8.9139 | 0.4276 | 20.9404 |
| 9 | 1.0828 | 0.2200 | 2.3473 | 3.7173 | 0.2765 | 4.5276 | 0.1694 | 12.3408 |
| 10 | 1.2616 | 0.2490 | 2.9858 | 4.9309 | 0.4935 | 7.0510 | 0.2784 | 17.2503 |
| 11 | 0.9332 | 0.2488 | 2.3801 | 4.6309 | 0.5019 | 7.3602 | 0.2976 | 16.3527 |
| 12 | 0.3664 | 0.0680 | 0.9773 | 1.7046 | 0.1973 | 2.5470 | 0.1190 | 5.9797 |
| 13 | 0.2234 | 0.0526 | 0.6306 | 1.2095 | 0.1781 | 2.2868 | 0.1087 | 4.6897 |
| 14 | 0.6201 | 0.0841 | 1.4992 | 4.3618 | 0.5675 | 5.3342 | 0.2909 | 12.7578 |
| 15 | 0.3553 | 0.1088 | 0.8689 | 1.0065 | 0.1315 | 1.8223 | 0.0942 | 4.3876 |
| 16 | 0.4121 | 0.0625 | 0.2259 | 0.1524 | 1.1007 | 6.7135 | 0.5096 | 9.1767 |
| 17 | 1.1915 | 0.0927 | 0.2257 | 0.1526 | 1.6890 | 14.9070 | 0.7693 | 19.0279 |
| 18 | 0.5806 | 0.0731 | 0.2257 | 0.1526 | 1.2095 | 7.8349 | 0.5514 | 10.6277 |
| 19 | 0.7340 | 0.0827 | 0.2259 | 0.1523 | 1.0969 | 9.4249 | 0.4960 | 12.2126 |
| 20 | 0.8554 | 0.0827 | 0.2255 | 0.1513 | 1.1499 | 9.1684 | 0.4255 | 12.0586 |
| 21 | 1.1278 | 0.2224 | 2.7329 | 5.6454 | 0.6323 | 7.5803 | 0.3197 | 18.2609 |
| 22 | 0.4302 | 0.0916 | 1.3544 | 2.4938 | 0.2246 | 3.3550 | 0.1297 | 8.0792 |
| 23 | 1.0061 | 0.1402 | 3.2950 | 6.6471 | 0.7794 | 8.8358 | 0.4052 | 21.1088 |
| 24 | 0.6241 | 0.0762 | 0.2278 | 0.1769 | 0.8244 | 8.9469 | 0.3895 | 11.2657 |
| 25 | 0.2393 | 0.0612 | 0.5896 | 0.8275 | 0.1180 | 1.2718 | 0.0847 | 3.1921 |
| 26 | 0.4483 | 0.1396 | 0.9919 | 1.4076 | 0.1474 | 2.3734 | 0.1060 | 5.6143 |
| 27 | 0.8515 | 0.1768 | 1.4544 | 3.4910 | 0.3786 | 4.3833 | 0.1974 | 10.9331 |
| 28 | 0.7188 | 0.1479 | 2.2285 | 3.6455 | 0.3429 | 4.4520 | 0.1780 | 11.7136 |
| 29 | 1.1125 | 0.2819 | 2.4977 | 4.0027 | 0.3419 | 5.1007 | 0.1967 | 13.5340 |
| 30 | 1.1743 | 0.1437 | 2.8828 | 6.4459 | 0.6489 | 8.2812 | 0.3792 | 19.9560 |
| 31 | 1.3846 | 0.1471 | 4.6142 | 7.3481 | 0.8489 | 10.0207 | 0.4282 | 24.7919 |
| 32 | 0.2127 | 0.0636 | 0.6896 | 1.3044 | 0.1212 | 1.8306 | 0.0844 | 4.3066 |
| 33 | 0.3465 | 0.0671 | 0.8200 | 2.2290 | 0.2445 | 3.6460 | 0.1385 | 7.4915 |
| 34 | 0.3144 | 0.0621 | 0.7011 | 1.1032 | 0.1548 | 2.0547 | 0.0989 | 4.4893 |
| 35 | 0.1374 | 0.0457 | 0.3529 | 0.4788 | 0.0917 | 0.9693 | 0.0696 | 2.1454 |
| 36 | 1.7910 | 0.2386 | 4.8744 | 7.6217 | 1.1431 | 10.5136 | 0.6422 | 26.8246 |
| 37 | 0.4089 | 0.0911 | 0.6533 | 0.9748 | 0.1576 | 1.6972 | 0.0986 | 4.0815 |
| 38 | 0.5105 | 0.1255 | 0.9811 | 2.3708 | 0.2258 | 2.6069 | 0.1374 | 6.9579 |
| 39 | 0.5768 | 0.1840 | 1.3572 | 1.9262 | 0.1943 | 2.9179 | 0.1252 | 7.2816 |
| 40 | 0.4451 | 0.1234 | 1.0176 | 2.2658 | 0.2048 | 2.6311 | 0.1284 | 6.8161 |
| 41 | 0.6479 | 0.1544 | 1.6070 | 2.6093 | 0.3415 | 4.8076 | 0.2038 | 10.3715 |
| 42 | 0.3011 | 0.0647 | 0.5976 | 0.8859 | 0.1568 | 2.0606 | 0.1052 | 4.1718 |
| 43 | 0.6872 | 0.1326 | 1.3026 | 2.8249 | 0.3069 | 3.5877 | 0.1679 | 9.0099 |
| 44 | 1.5833 | 0.2408 | 3.0702 | 5.0738 | 0.4881 | 5.8988 | 0.2702 | 16.6251 |
| 45 | 1.3846 | 0.2267 | 3.7062 | 6.1550 | 0.8085 | 8.3969 | 0.4244 | 21.1023 |
| 46 | 1.0668 | 0.2360 | 3.3036 | 5.2315 | 0.4652 | 7.1653 | 0.2811 | 17.7495 |
| 47 | 0.6570 | 0.1411 | 2.3286 | 3.6160 | 0.3659 | 6.2115 | 0.2039 | 13.5239 |
| 48 | 1.1735 | 0.1799 | 4.1459 | 6.5087 | 0.7401 | 8.6935 | 0.3826 | 21.8242 |
| 49 | 0.6935 | 0.0982 | 2.0010 | 4.2946 | 0.4486 | 6.3842 | 0.2603 | 14.1804 |
| 50 | 0.3733 | 0.3548 | 1.9426 | 2.1792 | 0.1693 | 7.3236 | 0.1119 | 12.4548 |
| 51 | 0.4652 | 0.1078 | 1.1403 | 2.5015 | 0.2238 | 3.2971 | 0.1443 | 7.8799 |
| 52 | 0.9932 | 0.1649 | 2.0831 | 4.0205 | 0.4474 | 5.4517 | 0.2260 | 13.3868 |
| 53 | 0.8968 | 0.1742 | 3.1494 | 5.6577 | 0.6011 | 8.3693 | 0.3357 | 19.1842 |
| 54 | 0.3330 | 0.0742 | 0.9370 | 1.7401 | 0.1698 | 2.3906 | 0.1079 | 5.7525 |
| 55 | 2.4113 | 0.3664 | 5.9124 | 7.9553 | 1.3870 | 12.2521 | 0.6290 | 30.9134 |
| 56 | 1.5661 | 0.3768 | 7.0236 | 6.1277 | 0.4254 | 10.8771 | 0.2593 | 26.6560 |
| 57 | 0.3396 | 0.0626 | 1.1852 | 2.2231 | 0.3003 | 4.1459 | 0.1656 | 8.4223 |
| 58 | 1.2344 | 0.2578 | 3.7820 | 5.4153 | 0.7176 | 8.7960 | 0.4072 | 20.6103 |
| 59 | 0.2732 | 0.0791 | 0.6962 | 1.2328 | 0.1721 | 2.1886 | 0.1102 | 4.7522 |
| 60 | 0.9107 | 0.2500 | 2.1171 | 3.1238 | 0.2688 | 4.9800 | 0.1682 | 11.8187 |
| 61 | 1.2000 | 0.2217 | 2.4788 | 5.3140 | 0.5450 | 6.1195 | 0.2213 | 16.1002 |
| 62 | 0.8603 | 0.2128 | 1.2014 | 2.1413 | 0.2124 | 2.1439 | 0.1318 | 6.9039 |
| 63 | 0.3572 | 0.0623 | 0.8102 | 1.2717 | 0.1470 | 1.4440 | 0.0929 | 4.1854 |
| 64 | 0.7848 | 0.1841 | 1.4553 | 2.7977 | 0.3560 | 4.0524 | 0.2156 | 9.8459 |
| 65 | 0.8741 | 0.2677 | 2.0449 | 2.8329 | 0.2643 | 5.0967 | 0.1666 | 11.5472 |
| 66 | 0.3533 | 0.0876 | 0.9504 | 1.4404 | 0.1500 | 2.3428 | 0.1118 | 5.4364 |
3. Conclusions
In conclusion, the qualitative and quantitative methods using UHPLC-Q-Exactive Orbitrap MS combined with PRM mode and SIM mode were successfully established in this study. Finally, a total of 70 CGAs (64 of them for the first time) and 7 CGAs were identified and quantified from the root bark of A. gracilistylus, which suggested that A. gracilistylus is an excellent source of CGAs. Meantime, this result is very useful for the further investigation of A. gracilistylus including bioactive chemical and quality control.
4. Materials and Methods
4.1. Materials and Reagents
A. gracilistylus sample was authenticated in line with the Chinese Pharmacopoeia (edition 2020, volume 1) by Associate Professor Jian-Bo Yang. The root sample of A. gracilistylus has been deposited at the Research and Inspection Center of Traditional Chinese Medicine and Ethnomedicine, National Institutes for Food and Drug Control, State Food and Drug Administration, Beijing, China. Reference standards of trans-3-caffeoylquinic acid (trans-3-CQA, nechlorogenic acid, ≥98%, L-007-171216), trans-4-caffeoylquinic acid (trans-4-CQA, cryptochlorogenic acid, ≥98%, Y-067-180320), trans-5-caffeoylquinic acid (trans-5-CQA, chlorogenic acid, ≥98%, X-014-170309), 3,5-dicaffeoylquinic acid (3,5-DiCQA, isochlorogenic acid A, ≥98%, Y-068-170903), 4,5-dicaffeoylquinic acid (4,5-DiCQA, isochlorogenic acid C, ≥98%, Y-070-170515) were provided by Chengdu Herbpurify Co., Ltd. (Chengdu, China); 1,3-dicaffeoylquinic acid (1,3-DiCQA, ≥98%, MUST-16022610) and 1,5-dicaffeoylquinic acid (1,5-DiCQA, ≥98%, MUST-15080115) were provided by Chengdu Must Biological Technology Co., Ltd. (Chengdu China); caffeic acid (≥98%, C108306) was purchased by Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai China). HPLC-grade acetonitrile and methanol were obtained from Fisher scientific (New Jersey), LC–MS grade formic acid was supplied by Thermo Fisher Scientific China, water used as the LC mobile phase, and aqueous solvents were prepared by watsons water. Other reagents were of analytical grade.
4.2. Sample Preparation
A stock standard solution of each standard at 1 mg/mL was prepared by accurately weighing solid standards and being dissolved in methanol. The individual solutions of 7 reference standards were mixed and diluted in methanol at 10 μg/mL to prepare standard working mixture solutions.
The root bark of A. gracilistylus samples was ground into powder, accurately weighed to 0.5 g, and extracted under ultrasonication with about 5 and 75 mL of 70% methanol for 1 h, respectively; afterward, the extracted solution was filtered by a 0.45 μm microfiltration membrane. All working solutions were stored at (4 °C) until qualitative and quantitative analysis.
4.3. Instruments and Conditions
4.3.1. Identification of CGAs in A. gracilistylus
Chromatographic analysis was performed on a Thermo Scientific Dionex Ultimate 3000 RS (Thermo Fisher Scientific, CA) composed of an online degasser, pump, autoinjector, column heater, and UV detector. Sample separations were carried out on a HYPERSIL GOLD C18 column (100 × 2.1 mm, 1.9 μm) from (Thermo Scientific) using gradient elution at 45 °C. The mobile phases were made up of solvent A (0.1% formic acid water, v/v) and solvent B (100% acetonitrile). at a flow rate of 0.3 mL/min. The gradient conditions of the mobile phases were optimized as follows: 0–2 min, 95–92% A; 2–5 min, 92–90% A; 5–20 min, 90–60% A; 20–24 min, 60–5% A; 24–26 min, 5% A; 26–27 min, 5–95% A; 27–30 min, 95% A. The injection volume was 2 μL.
MS analysis was performed on a Thermo Scientific Q-Exactive Focus Orbitrap MS (Thermo Electron, Bremen, Germany) operated with a heated electrospray ionization (HESI) in negative ion mode. The mass spectra were acquired with full MS mode in a mass range from m/z 100–1200 at a resolution of 70000, combined with the data dependent scan (dd-MS2) at a resolution of 35000 and isolation window at m/z 3.0. Other Q-Exactive general parameters were nebulizer pressure at 10 arb, sheath gas and auxiliary gas at the flow rate of 30 arb, capillary temperature at 320 °C, auxiliary gas heater temperature at 350 °C, spray voltage at 3.2 kV, and S-lens level at 50.
4.3.2. Quantification of CGAs in A. gracilistylus
LC–MS analysis was performed on the same machine as described in section 4.3.1. Agilent-XDB-C18 (100 mm × 2.1 mm, 1.8 μm) was applied for chromatographic separation with a column temperature of 40 °C. The mobile phase consisted of solvent A (0.1% formic acid water, v/v) and solvent B (100% acetonitrile). The gradient elution condition was as follows: 0–2 min, 95–89% A; 2–3 min, 89–78% A; 3–4 min, 78–80% A; 4–4.5 min, 80–88% A; 4–4.5 min, 80–88% A; 4.5–5.5 min, 80–50% A; 5.5–6.5 min, 50–75% A; 6.5–7.0 min, 75–20% A; 7.0–8.0 min, 20–20% A; 8–8.1 min, 20–95% A; and 8.1–11 min, 95–95% A. The samples were injected in 1 μL with constant flow rates of 0.28 mL/min. The MS scan mode was detected in selected reaction monitoring (SIM) mode at a resolution of 35000. The major MS parameters used were identical with the condition of identification.
4.4. Method Validation
The method for quantitative analysis of CGAs was validated with regard to its selectivity, linearity, sensibility, accuracy, and precision following the 2020 edition of Chinese Pharmacopoeia guidance document on analytical quality control and method validation procedures. The selectivity of the method was ascertained by analyzing the standards of seven kinds of CGAs and the samples. The peaks for the studied compounds in the samples were confirmed by comparing the retention times of the peaks with those of standards as well as by recognizing both the full MS precursor and product ions MS2 with an mass error below 5 ppm. The linearity of the methods of isochlorogenic acid C, isochlorogenic acid A, 1,5-dicaffeoylquinic acid, 1,3-dicaffeoylquinic acid, cryptochlorogenic acid, neochlorogenic acid, and chlorogenic acid were assessed using six concentration ranges, respectively. Repeatability is a measure of repeatability of the analytical method in the normal operating conditions and expressed as the percentage relative standard deviation (%RSD). The accuracy is based on recovery studies in the present work. Stability studies of the method including short-term stability (room temperature, 4 h), and postpreparative stability (storage in the autosampler, 10 °C, 24 h) were achieved by the test of sample with six replicates.
4.5. Data Processing and Analysis
Xcalibur 4.1 (Thermo Scientific, CA) was applied in the acquisition of raw data in full-scan/dd-MS2 mode. Compound Discoverer version 3.0 (Thermo Scientific, CA) was used to dispose the data which passed the workflow templates to predict some expected compounds.
The data were input into Excel for statistical analysis. Seven kinds of chlorogenic acids were determined from 66 different producing areas of the root bark of A. gracilistylus. All analyses were conducted in triplicate. The data is presented as a mean.
Acknowledgments
This work was financially supported by the Scientific Research Fund of Hunan Provincial Education Department (no. 19A353).
Glossary
Abbreviations
- CGAs
chlorogenic acids
- UHPLC-Q-Orbitrap MS
ultra high performance liquid chromatography-Q-exactive orbitrap mass spectrometry
- PRM
parallel reaction monitoring
- SIM
selected reaction monitoring
- TOF-MS
time-of-flight mass spectrometry
- HESI
heated electrospray ionization
- dd-MS2
data-dependent MS2 scan
- QA
quinic acid
- CQA-dihexoside
caffeoylquinic acid-dihexoside
- CQA-hexoside
caffeoylquinic acid-hexoside
- FQA-hexoside
feruloylquinic acid-hexoside
- 3-CQA
3-O-caffeoylquinic acid
- 3-pCoQA
3-O-p-coumaroylquinic acid
- CA-hexoside
caffeic acid-hexoside
- 1-FQA
1-O-feruloylquinic acid
- 5-CQA
5-O-caffeoylquinic acid
- 3-O-hydroxydihydroCQA
3-O-hydroxydihydrocaffeoylquinic acid
- 3-FQA
3-O-feruloylquinic acid
- 4-CQA
4-O-faffeoylquinic acid
- CA
caffeic acid
- 4-O-hydroxydihydroCQA
4-O-hydroxydihydrocaffeoylquinic acid
- DiCQA-hexoside
dicaffeoylquinic acid-hexoside
- CFQA-hexoside
caffeoylferuloylquinic acid-hexoside
- 5-pCoQA
5-O-p-coumaroylquinic acid
- 1,3-DiCQA
1,3-O-dicaffeoylquinic acid
- 5-FQA
5-O-feruloylquinic acid
- CFQA-hexoside
caffeoylferuloylquinic acid-hexoside
- TriCQA
tricaffeoylquinic acid
- 3F,4CQA
3-O-feruloyl-4-O-caffeoylquinic acid
- 5C,3pCoQA
5-O-caffeoyl-3-O-p-coumaroylquinic acid
- 3C,4FQA
3-O-caffeoyl-4-O-feruloylquinic acid
- 4C,3pCoQA
4-O-caffeoyl-3-O-p-coumaroylquinic acid
- 3F,5CQA
3-O-feruloyl-5-O-caffeoylquinic acid
- CFQA-hexoside
caffeoylferuloylquinic acid-hexoside
- 1,5-DiCQA
1,5-O-dicaffeoylquinic acid
- C,DimethoxyCiQA
caffeoyl-O-dimethoxycinnamoylquinic acid
- 3,4-DiCQA
3,4-O-dicaffeoylquinic acid (isochlorogenic acid B)
- 4-CSA
4-O-caffeoylshikimic acid
- 3,5-DiCQA
3,5-O-dicaffeoylquinic acid (isochlorogenic acid A)
- 3C,4pCoQA
3-O-caffeoyl-4-O-p-coumaroylquinic acid
- 4,5-DiCQA
4,5-O-dicaffeoylquinic acid (isochlorogenic acid C)
- SCQA
caffeoylsinapoylquinic acids
- 3C,5FQA
3-O-caffeoyl-5-O-feruloylquinic acid
- 1-pCoQA
1-O-p-coumaroylquinic acid
- 3C,5pCoQA
3-O-caffeoyl-5-O-p-coumaroylquinic acid
- 4F,5CQA
4-O-feruloyl-5-O-caffeoylquinic acid
- 4C,5FQA
4-O-caffeoyl-5-O-feruloylquinic acid
- 5C,4pCoQA
5-O-caffeoyl-4-O-p-coumaroylquinic acid
- 4C,5pCoQA
4-O-caffeoyl-5-O-p-coumaroylquinic acid
- DiCFQA
dicaffeoylferuloylquinic acids
- 3,4,5-TriCQA
3,4,5-tricaffeoylquinic acid
- FA
ferulic acid
Author Contributions
§ J.Y. and L.Y. contributed equally to the manuscript.
The authors declare no competing financial interest.
References
- Wu Z. Y.; Zhang Y. B.; Zhu K. K.; Luo C.; Zhang J. X.; Cheng C. R. Anti-inflammatory diterpenoids from the root bark of Acanthopanax gracilistylus. J. Nat. Prod. 2014, 77, 2342–2351. 10.1021/np500125x. [DOI] [PubMed] [Google Scholar]
- Xu H. B.; Yang T. H.; Xie P.; Tang Z. S.; Song X.; Xu H. L. LC-MS guided isolation of gracilistones A and B, a pair of diastereomeric sesquiterpenoids with an unusual tetrahydrofuran-fused tricyclic skeleton from Acanthopanax gracilistylus and their potential anti-inflammatory activities. Fitoterapia. 2018, 130, 265–271. 10.1016/j.fitote.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Zou Q. P.; Liu X. Q.; Huang J. J.; Yook C. S.; Whang W. K.; Lee H. K. Inhibitory effects of lupane-type triterpenoid saponins from the leaves of Acanthopanax gracilistylus on lipopolysaccharide-induced TNF-α, IL-1βand high-mobility group box 1 release in macrophages. Mol. Med. Rep. 2017, 16, 9149–9156. 10.3892/mmr.2017.7767. [DOI] [PubMed] [Google Scholar]
- Ma L.; Gao W.; Chen F.; Meng Q. HS-SPME and SDE combined with GC-MS and GC-O for characterization of flavor compounds in Zhizhonghe Wujiapi medicinal liquor. Food Res. Int. 2020, 137, 109590. 10.1016/j.foodres.2020.109590. [DOI] [PubMed] [Google Scholar]
- Ma L. H.; Chen F.; Meng Q. R. Characterization of Volatile Components in Zhizhonghe Wujiapi Medicinal Liquor by HS-SPME/GC-MS/GC-O. Liquor-making Sci. Technol. 2021, 01, 92–101. 10.1016/j.foodchem.2017.04.103. [DOI] [Google Scholar]
- Wang J. G. Formula, preparation method, efficacy and historical origin of Acanthopanax senticosus wine in different periods. Chin Brewing. 2008, 18, 103–104. [Google Scholar]
- Yang J. B.; Cai W.; Li M. H. Review on the chemical constituents and pharmacological activities of Acanthopanax alba. Chin Mod. Chin Mater. Med. 2020, 22, 652–662. [Google Scholar]
- Lepiniec L.; Debeaujon I.; Routaboul J. M.; Baudry A.; Pourcel L.; Nesi N.; Caboche M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. 10.1146/annurev.arplant.57.032905.105252. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- Stalmach A.; Steiling H.; Williamson G.; Crozier A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. 10.1016/j.abb.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Wang J.; Ballevre O.; Luo H.; Zhang W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens Res. 2012, 35, 370–374. 10.1038/hr.2011.195. [DOI] [PubMed] [Google Scholar]
- Cai W.; Li K. L.; Xiong P.; Gong K. Y.; Zhu L.; Yang J. B.; Wu W. H. A systematic strategy for rapid identification of chlorogenic acids derivatives in Duhaldea nervosa using UHPLC-Q-Exactive Orbitrap mass spectrometry. Arab J. Chem. 2020, 13, 3751–3761. 10.1016/j.arabjc.2020.01.007. [DOI] [Google Scholar]
- Gismondi A.; Serio M.; Canuti L.; Canini A. Biochemical, antioxidant and antineoplastic properties of Italian saffron (Crocus sativus L.). Am. J. Plant Sci. 2012, 3, 1573. 10.4236/ajps.2012.311190. [DOI] [Google Scholar]
- Bajpai V.; Kumar S.; Singh A.; Bano N.; Patha M.; Kumar N.; Kuma B. Metabolic fingerprinting of dioecious Tinospora cordifolia (Thunb) Miers stem using DART TOF MS and differential pharmacological efficacy of its male and female plants. Ind. Crops Prod. 2017, 101, 46–53. 10.1016/j.indcrop.2017.02.037. [DOI] [Google Scholar]
- Bai Y. L.; Hong Z. D.; Zhang T. Y.; Cai B. D.; Zhang Y. Z.; Feng Y. Q. A Method for Simultaneous Determination of 14 Carbonyl-Steroid Hormones in Human Serum by Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Anal Test. 2020, 4, 1–12. 10.1007/s41664-020-00120-5. [DOI] [Google Scholar]
- Hogenboom A. C.; Van L. J. A.; De V. P. Accurate mass screening and identification of emerging contaminants in environmental samples by liquid chromatography-hybrid linear ion trap Orbitrap mass spectrometry. J. Chromatogr A 2009, 1216, 510–519. 10.1016/j.chroma.2008.08.053. [DOI] [PubMed] [Google Scholar]
- Shi T.; Yao Z.; Qin Z.; Ding B.; Dai Y.; Yao X. Identification of absorbed constituents and metabolites in rat plasma after oral administration of Shen-Song-Yang-Xin using ultra-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry. Biomed Chromatogr. 2015, 29, 1440–1452. 10.1002/bmc.3443. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Zuo L.; Sun T.; Tang J.; Ding D.; Zhou L.; Zhang X. Chemical profiling and quantification of XueBiJing injection, a systematic quality control strategy using UHPLC-Q Exactive hybrid quadrupole-orbitrap high-resolution mass spectrometry. Sci. Rep. 2017, 7, 1–15. 10.1038/s41598-017-17170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Li W.; Ma X.; Chu Y.; Li S.; Guo J.; Liu C. Simultaneous determination of caffeic acid and its major pharmacologically active metabolites in rat plasma by LC-MS/MS and its application in pharmacokinetic study. Biomed Chromatogr. 2015, 29, 552–559. 10.1002/bmc.3313. [DOI] [PubMed] [Google Scholar]
- Joo Y. H.; Nam M. H.; Chung N.; Lee Y. K. UPLC-QTOF-MS/MS screening and identification of bioactive compounds in fresh, aged, and browned Magnolia denudata flower extracts. Food Res. Int. 2020, 133, 109192. 10.1016/j.foodres.2020.109192. [DOI] [PubMed] [Google Scholar]
- Khallouki F.; Ricarte I.; Breuer A.; Owen R. W. Characterization of phenolic compounds in mature Moroccan Medjool date palm fruits (Phoenix dactylifera) by HPLC-DAD-ESI-MS. J. Food Compost Anal. 2018, 70, 63–71. 10.1016/j.jfca.2018.03.005. [DOI] [Google Scholar]
- Chen J.; Mangelinckx S.; Lü H.; Wang Z. T.; Li W. L.; De K. N. Profiling and elucidation of the phenolic compounds in the aerial parts of Gynura bicolor and G. divaricata collected from different Chinese origins. Chem. Biodivers. 2015, 12, 96–115. 10.1002/cbdv.201400134. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Li H.; Hu J.; Li J.; Fan Y. W.; Liu X. R.; Deng Z. Y. Qualitative and quantitative analysis of phenolics in Tetrastigma hemsleyanum and their antioxidant and antiproliferative activities. J. Agric. Food Chem. 2013, 61, 10507–10515. 10.1021/jf4037547. [DOI] [PubMed] [Google Scholar]
- Zhong J.; Chen N.; Huang S.; Fan X.; Zhang Y.; Ren D.; Yi L. Chemical profiling and discrimination of green tea and Pu-erh raw tea based on UPLC-Q-Orbitrap-MS/MS and chemometrics. Food Chem. 2020, 326, 126760. 10.1016/j.foodchem.2020.126760. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y.; Zhang Q.; Li N.; Wang Z. J.; Lu J. Q.; Qiao Y. J. Diagnostic fragment-ion-based and extension strategy coupled to DFIs intensity analysis for identification of chlorogenic acids isomers in Flos Lonicerae Japonicae by HPLC-ESI-MSn. Talanta. 2013, 104, 1–9. 10.1016/j.talanta.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Clifford M. N.; Johnston K. L.; Knight S.; Kuhnert N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- Zhao T.; He J.; Wang X.; Ma B.; Wang X.; Zhang L.; Zhang X. Rapid detection and characterization of major phenolic compounds in Radix Actinidia chinensis Planch by ultra-performance liquid chromatography tandem mass spectrometry. J. Pharm. Biomed Anal. 2014, 98, 311–320. 10.1016/j.jpba.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. P.; Bragagnolo N. Identification and quantification of bioactive compounds in coffee brews by HPLC-DAD-MSn. J. Food Compost Anal. 2013, 32, 105–115. 10.1016/j.jfca.2013.09.002. [DOI] [Google Scholar]
- Parveen I.; Threadgil M. D.; Hauck B.; Donnison I.; Winters A. Isolation, identification and quantitation of hydroxycinnamic acid conjugates, potential platform chemicals, in the leaves and stems of Miscanthus × giganteus using LC-ESI-MSn. Phytochemistry. 2011, 72, 2376–2384. 10.1016/j.phytochem.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Jaiswal R.; Deshpande S.; Kuhnert N. Profiling the chlorogenic acids of Rudbeckia hirta, Helianthus tuberosus, Carlina acaulis and Symphyotrichum novae-angliae leaves by LC-MSn. Phytochem Anal. 2011, 22, 432–441. 10.1002/pca.1299. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y.; Cai W.; Li Y.; Liu R.; Wang Z.; Qiao Y. Rapid characterization of chlorogenic acids analogues in Artemisia younghusbandii using HPLC/LTQ-Orbitrap MSn coupled with MDF data mining technology. J. Chin Mass Spectrom Soc. 2015, 36, 321–327. [Google Scholar]
- Jaiswal R.; Matei M. F.; Golon A.; Witt M.; Kuhnert N. Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies. Food Funct. 2012, 3, 976–984. 10.1039/c2fo10260a. [DOI] [PubMed] [Google Scholar]