Abstract
Gardeniae fructus (GF), the fruit from Gardenia jasminoides Ellis, is a traditional Chinese medicine used for the treatment of nonalcoholic fatty liver disease (NAFLD) in the clinic. To explore the hepatoprotective mechanism of GF for the treatment of NAFLD, we proposed a novel strategy that integrated in vivo efficacy evaluation, network pharmacology analysis, molecular docking, and experimental validation. A NAFLD animal model induced by high fat diet (HFD) feed was established, then orally administrated with or without GF. The results showed that GF significantly decreased the levels of serum total cholesterol (TC), lipoprotein cholesterol, triglyceride (TG), alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, free fatty acids, glucose, and insulin and the levels of liver TG, TC, and malondialdehyde compared with the nontreated HFD group. Network pharmacology studies showed that quercetin, oleanolic acid, kaempferol, and geniposide were the main biocompounds in GF that targeted the PPARα and PPARγ genes through regulating the PPAR and AMPK signal pathways to protect against NAFLD. The interactions between bioactive compounds and their corresponding target proteins were analyzed by molecular docking and subsequently confirmed using the qRT-PCR assay. Collectively, GF was a therapeutic drug for the treatment of NAFLD.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD), a prevalent hepatic manifestation of metabolic syndrome disorder, is characterized by ectopic accumulations of triglycerides in the absence of excessive alcohol consumption.1 NAFLD causes severe and prolonged damage to liver tissue, which may progress into fibrosis and cirrhosis and eventually lead to hepatocellular carcinoma. About 30% of adults in the Chinese population have fatty liver disease, and the incidence of NAFLD increases in the world every year. However, understand of the precise mechanisms of NAFLD and an effective treatment strategy continue to lag behind, and there are no effective drugs approved for the treatment of NAFLD.2 Thus, the development of novel strategies for preventing and treating such diseases is urgently needed. Recently, natural products were found to exert potential efficacy in the prevention or treatment of NAFLD.3−5
Gardeniae Fructus (GF), derived from the dried fruits of Gardenia jasminoides Ellis, is widely used as a common traditional Chinese medicine (TCM). GF was recorded in the Chinese Pharmacopoeia in 2020. It had been also used for the treatment of acute or chronic hepatic diseases,6 icteric hepatitis, diabetes,7 depression, viruses,8 cancer,9 and ischemic stroke.10 A chemical investigation suggested that GF contained iridoid glycosides, diterpenes, triterpenes, flavonoids, and other chemical components.11 Network pharmacology emerged as a new field that integrated chemistry, pharmacology, bioinformatics, and genomics to construct a model based on as system-level network analysis and shed new light on the complicated mechanisms of TCM.12 Molecular docking, an increasingly important tool for structural molecular biology, is widely used to identify the binding modes of mechanisms between small molecules and target proteins.13 However, to the best of our knowledge, no reports had been published on the therapeutic effects and mechanisms of GF for the treatment of NAFLD up to now. Thus, we attempted to evaluate the potential efficiency of GF to attenuate the development of NAFLD induced by the oral administration of a high fat diet (HFD) in rats as a model animal and explored the mechanism of action using network pharmacology, molecular docking analysis, and experiment validation.
2. Results
2.1. Quility Control of GF
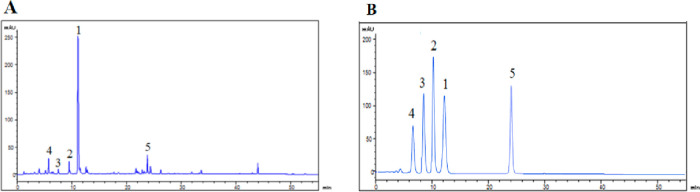
GF was purchased from Shanghai Kang Qiao Herbal Pieces Co. Ltd. (Shanghai, China). HPLC was used to identify the active components of GF. Additionally, the compound was finally confirmed by the comparison with the authentic compound. The chromatographic separation was carried out on a Diamonsil C18 column (150 × 4.6 mm i.d., 5 mm) at 25 °C. The mobile phase consisted of acetonitrile (solvent A) and water with 0.1% formic acid (solvent B). The optimized elution condition was applied as follows: 0–60 min, 5–95% A. The solvent flow rate and injection volume were kept as 0.5 mL/min and 5 μL, respectively. The detection wavelength was 254 nm. As shown by the results in Figure 1, compounds 1–5 were identified as geniposide, genipin 1-gentiobioside, 6α-hydroxygeniposide, gardenoside, and corcin I through comparing their chromatographic profiles with standards, and their contents were qualified as 32.32 ± 2.13, 1.59 ± 0.12, 0.36 ± 0.09, 0.21 ± 0.05, and 3.87 ± 0.56 mg/mL, respectively.
Figure 1.
(A) HPLC chromatograph of GF. (B) HPLC chromatograph of compounds 1–5.
2.2. GF Reduced the Body Weight, Liver Weight Index, and Visceral Weight Index in the HFD-Fed Rat
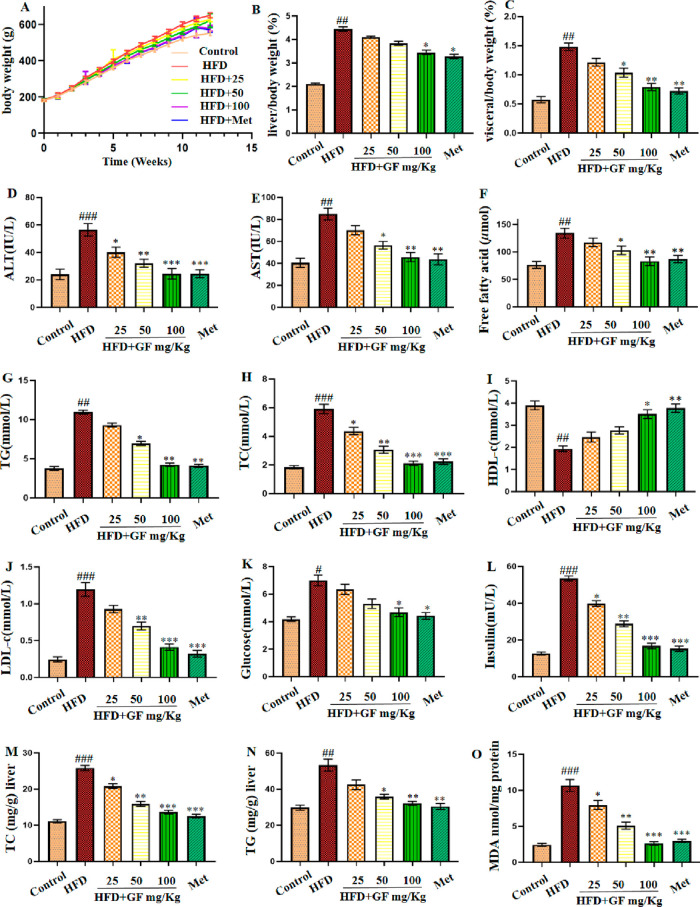
As exhibited in Figure 2A, there were significant differences in body weight between the control group and the HFD group treated with high fat food for 12 weeks. However, the GF-treated group exhibited significantly reduced body weight compared with the HFD group. Additionally, as shown in Figure 2B and C, the liver and visceral index (g/body weight) in the HFD group were significantly higher than those in the control group, suggesting that the hepatic steatosis induced by HFD-modeled NAFLD was successful. Similarly, the GF treatment reversed these changes. Moreover, GF administration decreased body weight, liver weight index, and visceral weight index in a dose-dependent manner.
Figure 2.
Effect of the GF treatment on (A) body weight, (B) liver index/body weight (%), (C) visceral index/body weight (%), (D) ALT (IU/L), (E) AST (IU/L), (F) free fatty acid (μmol), (G) TG (mmol/L), (H) TC (mmol/L), (I) HDL-c (mmol/L), (J) LDL-c (mmol/L), (K) glucose (mmol/L), and (L) insulin (mU/L) in serum and (M) TC (mg/g) liver, (N) TG (mg/g) liver, and (O) MDA nmol/mg protein in the liver. Results are expressed as mean ± SEM (n = 6). #p < 0.05, ##p < 0.01, and ###p < 0.001 vs the control group and *p < 0.05, **p < 0.01, and ***p < 0.001 vs the HFD group.
2.3. GF Improved Hepatic Steatosis and Liver Function in HFD-Fed rats
To further explore the preventive effects of GF on HFD-induced NAFLD, we measured the serum and liver biochemical indices in different groups of rats. As exhibited in Figure 2, the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), free fatty acid, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), triglyceride (TG), glucose, and insulin and liver levels of TC, TG, and malondialdehyde (MDA) were significantly increased in the HFD group compared with the corresponding indices in the control group. In contract, when the HFD-fed rats were orally administrated with GF, the corresponding biochemical features in those rats decreased significantly in a dose-dependent manner. The serum HDL-C levels exhibited the opposite trends. On the other hand, the histological changes in the liver were evaluated by hematoxylin and eosin (H&E) staining. As shown in Figure 3, the liver samples obviously increased in terms of hepatocytic lipid vacuoles and hepatocyte ballooning in the HFD-fed rats compared to those without any treatment, while these features were reduced significantly following the administration of the GF supplement to HFD-fed rats. Moreover, the liver sections were stained with Oil Red O, as shown in Figure S1. Very strong Oil Red O staining was observed in the liver issues of the NAFLD model group, which suggested the hepatocytes were filled with a large amount of lipid. However, the intensity decreased with the higher dosage of GF. Therefore, the data suggested that GF treatment on HFD-fed rats exerted protective effects against NAFLD.
Figure 3.
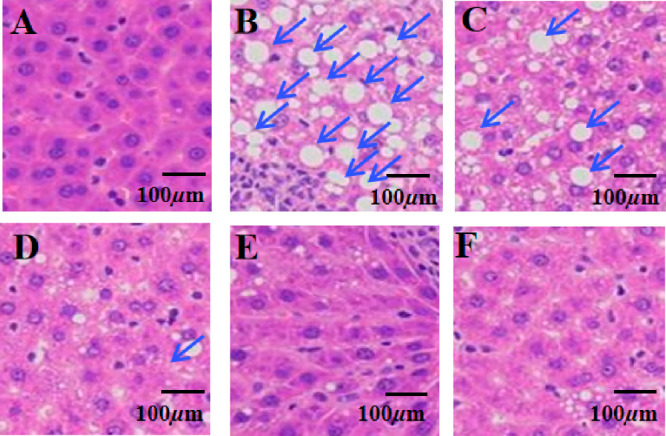
Liver tissue stained with H&E. (A) Control group, (B) HFD-fed group, (C) HFD and 25 mg/kg GF, (D) HFD and 50 mg/kg GF, (E) HFD and 100 mg/kg GF, and (F) HFD and metformin.
2.4. In Silico Network Analysis and Prediction of Target Genes and Pathways Related to NAFLD
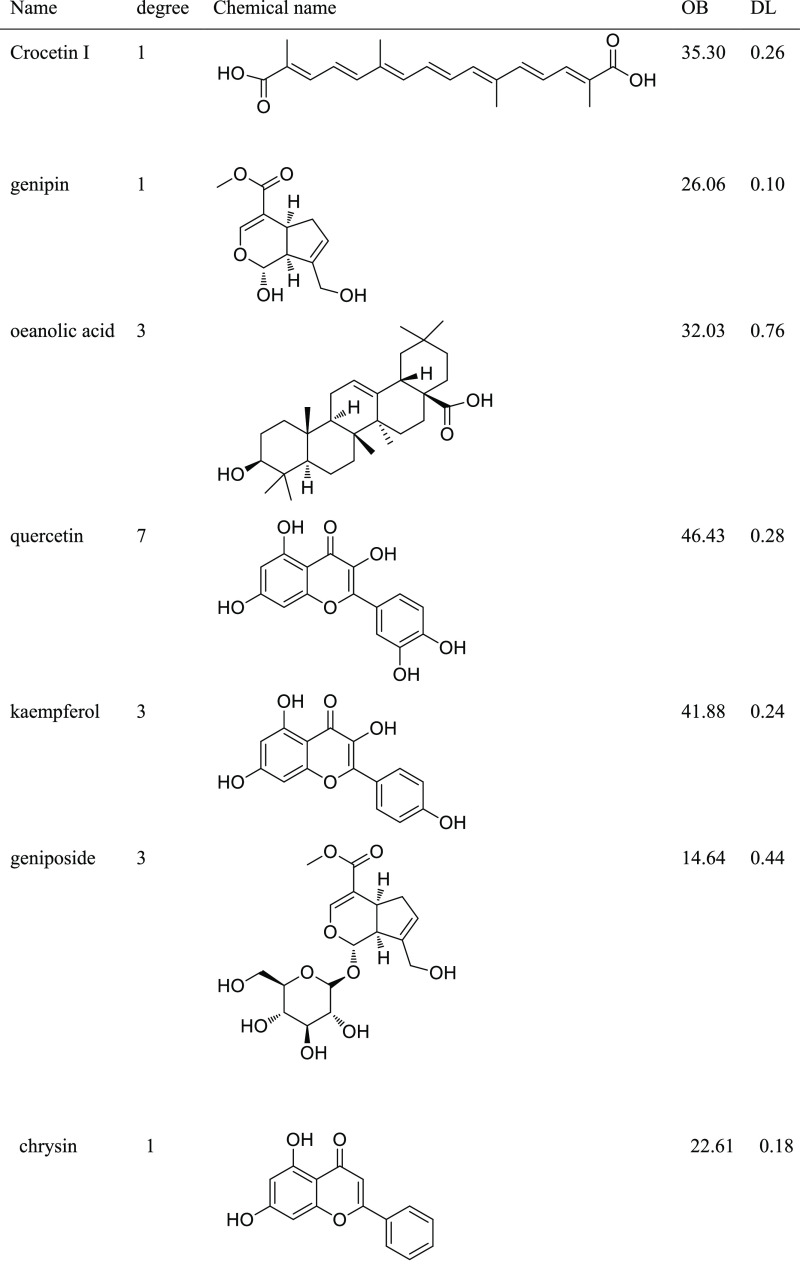
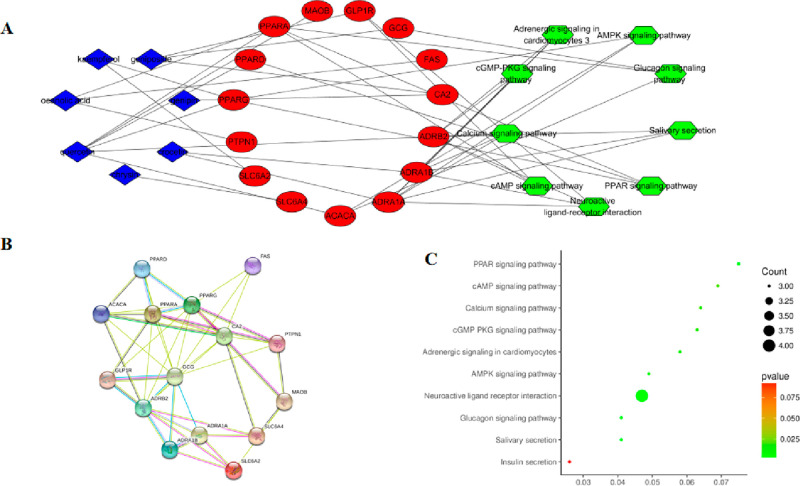
Several compounds in GF may act in a synergistic manner to protect against NAFLD. Network pharmacology analysis was carried out to further illustrate the mechanism of action of GF-induced hepatoprotective activity. Seven compounds (Table 1) with oral bioavailability (OB) values ≥30% and drug-like (DL) values ≥0.18 were selected as potential candidates. On the other hand, 15 overlapping targets (Supporting Information) derived from the target database of compounds and NAFLD were considered as potential candidates. Their interactions were constructed using Cytoscape. As showed in Figure 4A, quercetin,7 oleanolic acid,3 kaempferol,3 and geniposide3 were linked to three or more target genes with more degrees. In addition, the core target genes included peroxisome proliferator-activated receptor-α (PPARα) regulated by oleanolic acid and quercetin and peroxisome proliferator-activated receptor-γ (PPARγ) targeted by quercetin and kaempferol. Overall, the above compounds and their corresponding target genes might play myriad roles in the development and progression of NAFLD regulated by GF.
Table 1. A List of the Final Compounds in GF Selected for Network Analysis.
Figure 4.
Network pharmacology of GF inhibition against NAFLD. (A) Network “compound–target–pathway” diagram. (B) The PPI network diagram. (C) KEGG enrichment analysis map of the potential treatment pathways of GF for NAFLD.
2.5. Network Analysis of Protein–Protein Interactions (PPI)
The interactions among the core 15 target genes were illustrated in the STRING database, where nodes represent proteins and edges stand for protein–protein interactions. The result was downloaded and is shown in Figure 4B. PPARA possessed four degrees, and PPARG and ADRB2 possessed five degrees. These proteins featured more degrees than others, which indicated that they might play important roles in GF against NAFLD. Interestingly, this was consistent with the conclusion from the above network analysis.
To understand the hepatoprotective mechanism of action of GF against NFALD, we performed the functional enrichment analysis (as present in Figure 4C) of the target genes of bioactive compounds from GF using DAVID software and the KEGG database. Potential target genes were functionally associated with various signal transduction pathways such as PPAR, the glucagon signaling pathway, the AMP-activated protein kinase (AMPK) signaling pathway, and the cGMP-PKG signaling pathway (cGMP-PKG) (Table 2). Interestingly, the potential target genes appeared to be connected to the PPAR and AMPK signaling pathways. Subsequently, GF protected against NAFLD by regulating multiple pathways concentrated on the PPAR and AMPK signaling pathways.
Table 2. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways and Target Genes of Compounds in the GF Extract (GFE) Potentially Responsible for the Therapeutic Activities against NAFLD.
| pathway classification | pathway ID | term | target gene |
|---|---|---|---|
| signal transduction | hsa03320 | PPAR signaling pathway | PPARA, PPARD, PPARG |
| signal transduction | hsa04970 | salivary secretion | ADRB2, ADRA1B, ADRA1A |
| signal transduction | hsa04080 | neuroactive ligand–receptor interaction | ADRB2, ADRA1B, ADRA1A, GLP1R |
| signal transduction | hsa04922 | glucagon signaling pathway | GCG, PPARA, ACACA |
| signal transduction | hsa04152 | AMPK signaling pathway | PPARG, ACACA, ADRA1A |
| signal transduction | hsa04261 | adrenergic signaling in cardiomyocytes | ADRB2, ADRA1B, ADRA1A |
| signal transduction | hsa04022 | cGMP-PKG signaling pathway | ADRB2, ADRA1B, ADRA1A |
| signal transduction | hsa04020 | calcium signaling pathway | ADRB2, ADRA1B, ADRA1A |
| signal transduction | hsa04024 | cAMP signaling pathway | PPARA, ADRB2, GLP1R |
2.6. Docking Exercises of Binding the Main Ingredients and the Protein
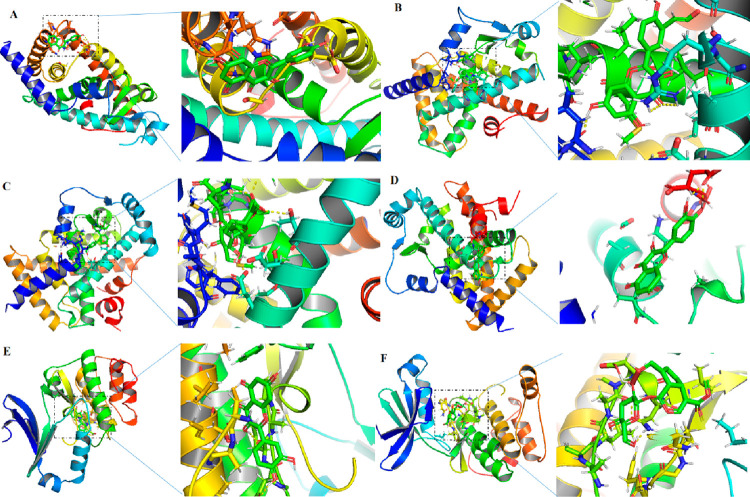
Computational docking exercises were conducted to explore the characteristics of the ingredient–target binding mode (Figure 5). The results (Table 3) showed that quercetin and kaempferol formed strong interactions with PPARγ, geniposide and quercetin formed strong interactions with PPARα, and quercetin and geniposide formed strong interactions with AMPK, which featured a hydrogen bond and an other covalent bond with a docking energy less than −2.53 kcal/moL. The molecular docking results were consistent with the network analysis, for example, quercetin and kaempferol wre linked with PPARγ while geniposide and quercetin were linked with PPARα. Therefore, GF protected against NAFLD via multiple compounds acting on multiple targets. In detail, quercetin and kaempferol targeted PPARA and quercetin and kaempferol targeted PPARγ through regulating AMPK signal pathways.
Figure 5.
Molecular docking analyses of the molecular interactions and binding modes of compounds with the active sites of target genes. (A) PPARγ–quercetin, (B) PPARγ–kaempferol, (C) PPAR–-geniposide, (D) PPARα–quercetin, (E) AMPK–quercetin, and (F) AMPK–geniposide.
Table 3. Docking Analysis of Target Genes and Compounds.
2.7. Network Pharmacology and Molecular Docking Analysis Results Confirmed by qRT-PCR
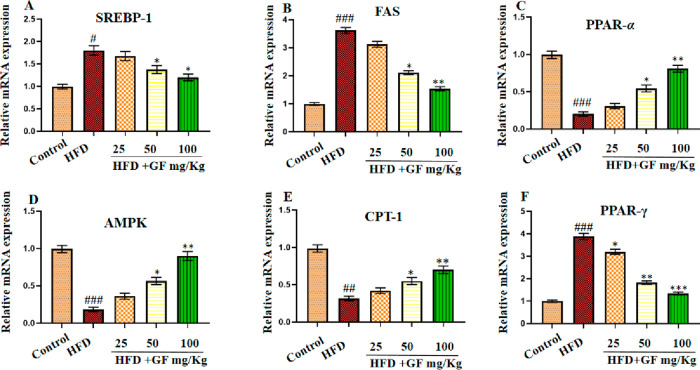
We next searched for evidence of the mechanism of GF against NAFLD deduced by network pharmacology. Although molecular docking had clarified the interplay mechanism between bioactive compounds and targets, the evidence of molecular events was still deficient, so a qRT-PCR assay was performed using the potential target genes regulated by GF. The mRNA expression levels of the core target genes PPARα and PPARγ exhibited by the network pharmacology network diagram, their related lipogenesis factors (SREBP-1c, FAS, and CPT-1), and the main signal pathways PPAR and AMPK analyzed by PPI were measured in the liver. As shown in Figure 6, the mRNA expression levels of SREBP-1c, FAS, and PPARγ were remarkably reduced in the HFD-fed rats supplemented with GF compared with those in the nontreated HFD-fed rats. On the other hand, the mRNA expression levels of PPARα, CPT-1, and AMPK were significantly higher in HFD-fed rats supplemented with GF compared to those in the nontreat HFD-fed rats. The data suggested that GF protected against NAFLD by regulating the mRNA expression of lipogenesis (SREBP-1c, FAS, PPARγ, PPARα, CPT-1, and AMPK) in liver tissue.
Figure 6.
Effect of the GF treatment on mRNA expression levels of (A) SREAP-1, (B) FAS, (C) PPAR-α, (D) AMPK, (E) CPT-1, and (F) PPAR-γ. Results are expressed as the mean ± SEM (n = 6). #p < 0.05, ##p < 0.01, and ###p < 0.001 vs the control group and *p < 0.05, **p < 0.01, and ***p < 0.001 vs the HFD group.
3. Discussion
The progression and development of NAFLD are complicated processes involving multiple factors that alter the homeostasis of liver tissue. On the other hand, TCM contains of tens or thousands of compounds that can act on multiple target proteins by regulating multiple pathways to exert their synergism or exert compatible pharmacological effects on complex diseases.14 Thus, TCM may have myriad advantages in the treatment of NAFLD.15 In this paper, we proposed a strategy that integrated pharmaceutical a efficacy evaluation in an animal model, network pharmacology, molecular docking, and experimental verification to explore the potential efficacy and mechanism of action of GF for the treatment of NAFLD.
After the HFD-induced rat model of NAFLD was established and then treated with GF for 12 weeks, the results of the histopathological analysis of the liver, the serum and liver biochemical indices, the body weight and the liver weight index showed that GF exerted potential protective effects against NAFLD. In detail, we observed significant changes in serum glucose, insulin, lipid, and liver function enzyme (ALT and AST) levels in serum and the mRNA expression of lipogenesis in liver tissue among different groups of rats. ALT, AST, and LDH-c are the important biochemical indicators of liver function and are widely used in clinical diagnosis. Moreover, serum glucose, insulin resistance, dyslipidemia, and metabolic syndromes have played important roles in NAFLD. For example, insulin resistance inhibited the antilipolytic activity of insulin in the adipose tissue and increased free fatty acids (FFAs) in the serum and liver, leading to mitochondrial dysfunction and cardiac fat accumulation.16 As expected, the serum glucose, TC, LDL-c, TG , and insulin levels were lower in HFD-fed rats supplemented with GF compared to those in untreated rats. Furthermore, oxidative stress is an essential risk factor for NAFLD, as it promotes the production of reactive oxygen species (ROS) that stimulate an inflammatory process in hepatic tissues.17 Our research showed that the MDA levels in liver were lower in the HFD-induced rats treated with GF compared to those without any supplement. Moreover, the higher dosage (100 mg/kg) of GF showed effects similar to those of the positive control drug metformin in the treatment of rats with NAFLD. Consequently, GF may play an important role in preventing or slowing the progression of NAFLD by regulating multiple factors, such serum glucose and liver enzyme levels, insulin resistance, oxidative stress, free fatty acids.
According to a network pharmacology analysis, the main bioactive compounds in GF, including quercetin, oleanolic acid, kaempferol, and geniposide, played key roles in the treatment of NAFLD by targeting PPARα and PPARγ. Moreover, these main protein genes exerted their effects on the PPAR and AMPK signal pathways. Next, we will discuss their roles in the process of NAFLD from three molecular levels, namely,compounds, protein genes, and pathways.
First, quercetin and kaempferol are natural flavonoids that are widely distributed in herbal medicine, vegetables, and edible fruits. They feature a variety of biological functions and are studied primarily for their potential roles in combating oxidative and inflammatory processes. They showed potential for the treatment of fatty liver disease. Previous studies showed that quercetin both alone and in herbal medicine could ameliorate NAFLD in HFD-induced mice.18 Moreover, mice treated with up to 3000 mg/kg quercetin did not show any toxic effects.19 Kaempferol, which has chemical structure analogous to that of quercetin with one less hydroxyl, also exhibited a hepatoprotective effect,20 suppressed hepatic gluconegeonesis, and inhibited hepatocellular carcinoma cell. Geniposide, a major characteristic constituent in GF, exerted protective effects against hepatic steatosis in rats fed with HFD; the underlying mechanism might be associated with its antioxidant actions or the regulation of adipocytokine release and the expression of PPARα.21 finally, oleanolic acid attenuated the subsequent development of high fructose diet-induced NAFLD in rats.22
Second, we identified PPARα and PPARγ as potential target genes using network pharmacology. PPARγ is a regulator of adipocyte differentiation. Additionally, it has been implicated in the pathology of numerous diseases including obesity, diabetes, atherosclerosis, and cancer.23 Previous study showed that PPARα could inhibit fatty liver disease by activating the periostin-dependent JNK signaling pathway and modulating fatty acid oxidation.24 PPARs are binder-activated nuclear receptors that are involved in the transcriptional regulation of lipid metabolism, energy balance, inflammation, and atherosclerosis.25
Third, the functions of potential target genes identified from the KEGG enrichment analysis were associated with multiple signal transduction pathways. Among these, the PPAR and AMPK pathways played the most important roles with highest degrees, which was consistent with the results from the PPI analysis. Recent research showed that PPARs were closely related to metabolic syndrome and its relevant complications. Additionally, PPARs had protective effects on the NAFLD development because they could regulate the lipid metabolism.25 Moreover, a large number of studies suggested that activation of AMPK could ameliorate NAFLD, and AMPK was a potential therapeutic target for the treatment of NAFLD.26 On the other hand, previous reports suggested that the main bioactive compounds from GF regulated these pathways, examples of which are as follows: Quercetin attenuated NAFLD by improving lipid metabolism through modulating the AMPK/PPAR pathway and gut microbiome.27 Kaempferol protected against NAFLD by regulating hepatic PPARα levels.28 Geniposide alleviated NAFLD by blocking Nrf2/AMPK/mTOR signaling pathways.29 Therefore, our results suggested that these signal pathways might be coordinated during the NAFLD progression, and the effects of GF could be mediated through the PPAR and AMPK signaling pathways.
Fourth, computational docking exercises showed that the main ingredients from GF formed comparative interactions with their corresponding predicted proteins. The regulatory mechanisms of the processes are likely valid targets for modulating lipid metabolism and inflammation in the treatment of NAFLD. According to the network pharmacology and PPI analysis, the main bioactive compounds quercetin, kaempferol, and geniposide interacted with their corresponding targets PPARγ and PPARα through the AMPK signal pathways.
Finally, the mRNA expression levels of the core target genes and pathways analyzed by network pharmacology were measured using qRT-PCR. AMPK is a master energy sensor that monitors metabolic homeostasis, and the repression of its activity might lead to metabolic disorders, such as NAFLD and diabetes.30 In the liver, AMPK plays an important role in hepatic lipid metabolism through inhibiting lipogenesis and activating fatty acid oxidation. In detail, PPARα is mainly restricted to the liver issue,31 and promotes the uptake and oxidation of hepatic fatty acids; carnitine palmitoyltransferase-1 (CPT-1) also has a similar function. PPARγ and PPARα are two PPAR isotypes, but they have different functions. PPARγ is a master transcription factor in adipogenesis that participates in the process of lipogenesis, lipid droplet formation, and TG synthesis.32 FAS is a transcription lipogenesis factor that uses acetyl-CoA or malonyl-CoA to synthesize fatty acids. In this study, GF protected against NAFLD by activating AMPK signal pathway and then inhibiting AMPK adipogenesis and lipogenesis by decreasing the mRNA expression levels of PPARγ, FAS, C/EBPα, and SREBP1; however, it promoted fatty acid oxidation by increasing mRNA expression levels of PPARα and CPT-1.
4. Materials and Methods
4.1. Chemical Reagents
Gardenoside, geniposide, genipin 1-gentiobioside, 6α-hydroxygeniposide, and crocetin I were purchased from EFEBIO (Shanghai, China). Acetonitrile, ethanol, and formic acid of HPLC grade were purchased from Merck KGaA (Darmstadt, Germany). Deionized water purified by a Milli-Q water purification system (Millipore, Billerica, MA) was applied to prepare and extract plasma samples. Other reagents and chemicals were all of analytical grade.
4.2. Preparation of Gardeniae Fructus Extract (GFE)
GF was purchased from Shanghai Kang Qiao Herbal Pieces Co. Ltd. (Shanghai, China). Morphological and microscopic authentications, thin layer chromatography, and HPLC were performed in accordance with Chinese Pharmacopoeia (2020) by author Dr. Zhengxiang Xia. GFE was prepared as follows: The herbal materials (200 g) were crushed into small pieces and mixed/ The mixture was then soaked in 70% ethanol for 0.5 h before decocted for 1 h. The filtrates were collected, and the residues were then refluxed in water (1:5, w/v) for 1 h. GFE (equal to 1.0 g/mL GF) could be obtained by mixing the two-stage filtrates and concentrating the volume to 200 mL. The chemical profile was characterized by HPLC (Milford, MA).
4.3. Animal Experiment
Five-week-old male Sprague–Dawley (SD) rats weighing 180–220 g were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). The scheme for the animal experimental method is shown in Figure 7. After a one-week acclimation period, the rats were divided into six groups (n = 6 each). The control group was fed a normal diet (Con, 10 kcal % fat, D12450B, Research Diet, Inc., New Brunswick, NJ), and the others were fed with a HFD (60 kcal % fat, D12492). After six weeks, the NAFLD model was established, and four groups were supplemented with GFE (25, 50, and 100 mg/kg, daily oral administration) and metformin (100 mg/kg, daily oral administration), respectively, for six weeks. At the end of the 13-week treatment period, the rats were sacrificed with 10% chloral hydrate. Blood was collected from the portal vein and then centrifuged at 4 °C and 500 × g for 20 min. Plasma was collected and stored at −80 °C in a centrifuge tube prior to the biochemical analysis. The separated liver was weighed, and the left lobe fixed in neutral formaldehyde for histopathological analysis. The rest of liver was snap frozen in liquid nitrogen and stored at −80 °C for subsequent analysis. The visceral adipose tissues were collected and weighed, and body fat rates were calculated. The ethical aspects of animal experimentation studies were approved by the Ethics Committee on Animal Research in School and Hosipital of Stomatology, Tongji University. All surgeries were performed under 10% chloral hydrate, and all efforts were made to minimize suffering. Body weight and food intake were surveyed every week.
Figure 7.
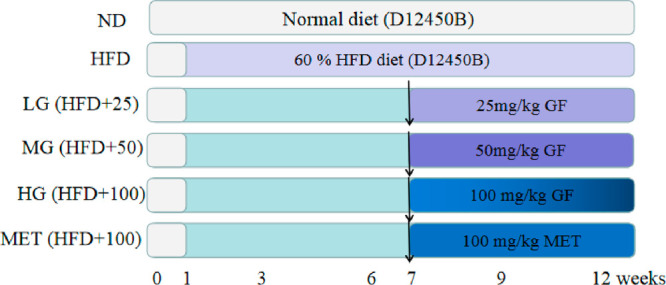
Schematic overview of the experimental design.
4.4. Blood and Liver Biochemical Assays
Detection kits for triglycerides (TG), total cholesterol (TC), free fatty acid, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), and aspartate transaminase (AST) in serum and TG, TC, and MDA in the liver were purchased from Jiangsu Jiancheng Company (Nanjing, China) and were performed according to the manufacturer’s protocol.
4.5. Tests for Glucose and Insulin Metabolic Parameters
When the blood was obtained from each rat, it was quickly measured using an ACCU-CHEK blood glucose meter (Roche Diagnostics Ltd. Company, Shanghai, China). Insulin levels were measured using an insulin ELISA kit (Jiangsu Jiancheng Company, Nanjing, China).
4.6. Determination of Lipid Peroxidation and TG and TC Contents in the Liver
TG, TC, and MDA contents in the liver were determined using commercial kits (Jiancheng Company, Nanjing, China) according to the manufacturer’s protocol.
4.7. Histopathological Analysis
The liver tissue specimens were fixed in 10% formalin, embedded in paraffin, and serially sectioned. H&E staining was used to visualize liver cells and matrices. Histological changes were observed using light microscopy (Olympus CX31/BX51, Olympus Optical Co., Tokyo, Japan) and photographed (Olympus DP70).
4.8. Oil Red O Staining
The liver sections of the rats were stained with Oil Red O (ORO) to assess hepatic steatosis. The tissues were cut into 5 μm slices, and rinsed with 60% isopropyl alcohol. To the tissues was then added the Oil Red O staining solution (Jiangsu Jiancheng, Nanjing, China) according to the manufacturer’s instructions. The images were observed using light microscopy (Olympus CX31/BX51, Olympus Optical Co., Tokyo, Japan) and photographed (Olympus DP70).
4.9. Molecular Docking
The crystal structure of PPARγ was obtained from the Protein Data Bank (PDB ID 3K8S). The crystal structure of PPARα was also obtained from the Protein Data Bank (PDB ID 3ET1). The crystal structure of AMPK was also obtained from the Protein Data Bank (PDB ID 6BX6). The docking exercise was conducted using AutoDock sofware following the procedure from previous literature.33,34
4.10. qRT-PCR Analysis
Total RNA liver tissues were isolated from by TRIzol reagent (Beyotime, Shanghai, China) according to the manufacturer’s method, and the assay was subsequently performed as previously report.35 Primer sequences are shown in Table 4.
Table 4. Primer Sequences Used in Quantitative Real-Time PCRa.
| gene | primers | sequence (5′–3′) |
|---|---|---|
| SREBP-1c | forward | CCCTGCGAAGTGCTCACAA |
| reverse | GCGTTTCTACCACTTCAGGTTTCA | |
| FAS | forward | GCTGCTACAAACAGGACCATCAC |
| reverse | TCTTGCTGGCCTCCACTGAC | |
| PPAR-α | forward | TTCGGAAACTGCAGACCT |
| reverse | TTAGGAACTCTCGGGTGAT | |
| PPAR-γ | forward | GGACGCTGAAGAAGAGACCTG |
| reverse | CCGGGTCCTGTCTGAGTATG | |
| AMPK | forward | TCTCGGGGTGGTTCGGTG |
| reverse | GGGGACAGGATTTTCGGATT | |
| CPT-1 | forward | CCATCTCTTCTGCCTCTATGT |
| reverse | GTCAGGGTTTTTCTCAAAGTC | |
| β-action | forward | CTCTTCCAGCCTTCCTTCCT |
| reverse | AGCACTGTGTTGGCGTACAG |
SREBP-1c, sterol regulatory element binding protein 1c; FAS, fatty acid synthase; PPAR-α, peroxisomeproliferator-activated receptor-α; PPAR-γ, peroxisomeproliferator-activated receptor-γ; AMPK, Adenosine monophosphate-activated kinase; CPT-1, carnitine palmitoyltransferase 1.
4.11. Statistical Analysis
All results were shown as the mean ± SEM. Statistical analysis was performed using one-way analysis of variance with Dunnett’s multiple comparisons test for multiple comparisons, and p < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism Software ver. 8.0 for Windows (GraphPad Software, La Jolla, CA).
4.12. Construction and Screening of the Active Components in GF
To obtain active components from GF, Gardenia jasminoides was first investigated using the literature and public databases, such as PubMed, Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) (available online at http://lsp.nwsusaf.edu.cn/), and TCM Database@Taiwan. Ninety-eight compounds were collected. Second, the OB and DL values were screened using absorption, distribution, metabolism, and excretion (ADME) models provided by TCMSP. The threshold values for these screening models were set to OB ≥ 30% and DL ≥ 0.18. Finally, seven compounds were selected as candidates for subsequent analysis.
4.13. Target Fishing
To obtain target information on the active compounds from GF for the treatment of NAFLD, a comprehensive method was applied using chemoinformatics and a text-mining database. First, the information obtained from the search tool for interactions of chemicals and proteins in databases such as STITCH (http://stitch.embl.de/), TCMSP (http://lsp.nwu.edu.cn/tcmsp.php), and PharmMapper (http://lilab.ecust.edu.cn/pharmmapper/index.php) were used (Table S2). After the removal of duplicates, 112 target genes were collected. Second, keywords including nonalcoholic fatty liver disease, obesity, and lipid metabolism on NAFLD-associated target genes were searched in the therapeutic targets database (TTD, http://bidd.nus.edu.sg/BIDD-Databases/TTD/TTD.asp, updated January 11, 2018), Pubmed (https://www.ncbi.nlm.nih.gov/pubmed/), and DRUGBANK (https://www.drugbank.ca/). Third, the overlapping targets that came from the above two methods were considered to be potential targets in GF for the treatment of NAFLD and were analyzed using http://jvenn.toulouse.inra.fr/app/example.html. Finally, 15 target genes (Table S1) were obtained as candidates for subsequent analysis.
4.14. Construction of the Target Protein–Protein Interaction (PPI) Network
To explore the relationships among these target proteins, the core target proteins of the active compounds were enriched using STRING (http://string-db.org). The species was limited to Homo sapiens, the minimum required interaction score was set to 0.400, and the dependent target protein nodes were shown.
4.15. Network Construction and Analysis
To facilitate the visualization of bioactive compounds from GF and their potential target genes related to NAFLD, networks were constructed using software Cytoscape ver. 3.2.1. This software was used to visualize compounds, biological pathways, and molecular interaction networks to explore the pharmacological mechanism of action. In networks, nodes represented compounds, target genes, or pathway, and edges indicated compound–target or compound–pathway interactions. To illustrate the mechanisms of action of the active compounds from GF and their roles in signal transduction, the Database for Annotation, Visualization, and Integrated Discovery (DAVID) was used to analyze the KEGG pathway enrichment of the target protein genes. The cellular components involved in the target protein and the pathways were also described.
5. Conclusion
In conclusion, our studies revealed that GF exerted protective effects against NAFLD in the HFD-induced rats and restored triglycerides by alleviating histopathological features and suppressing the levels of ALT, AST, free fatty acid, TC, LDL-C, TG, glucose, and insulin in serum in addition to TC, TG, and MDA in the liver. GF exhibited an effect against NAFLD comparable to that of the positive control metformin. Our subsequent network pharmacology analysis suggested that quercetin, oleanolic acid, and geniposide from GF treated NAFLD by targeting PPAR and PPARα through various signaling pathways, which were concentrated on the AMPK and PPAR signaling pathways. Molecular docking studies and mRNA expression in the qRT-PCR assay further confirmed the above results. Therefore, the pharmaceutical mechanism of GF exerting inhibitory effects on the process of NAFLD was as follows: GF first activated the AMPK signal. The activated AMPK inhibited adipogenesis and lipogenesis by decreasing the mRNA expression levels of PPARγ, FAS, and SREBP1; however, it promoted fatty acid oxidation by increasing mRNA expression levels of PPAR-α and CPT-1. To our knowledge, this is first report on the effects and mechanism of GF against NAFLD that is guided by network pharmacology, molecular docking, and experimental verification. The results not only provide evidence for the therapeutic mechanism of action of GF against NAFLD but also shed new light on the molecular mechanism analysis of other complex drugs.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02629.
Liver tissues stained with hematoxylin and eosin (H&E) for all groups in this study; the target information on active compounds from GF via STITCH, TCMSP, and PharmMapper; and the relationship among compounds, targets, and signal pathways (PDF)
Author Contributions
Z.Y.T., L.L., and Z.X.X. contributed to conceptualization, methodology, validation, formal analysis, and investigation. Z.Y.T. and Z.X.X. contributed to the original draft preparation. Z.X.X. contributed to review, editing, and supervision. All authors have read and agreed to the published version of the manuscript. Z.Y.T. and L.L. contributed equally.
The authors declare no competing financial interest.
Notes
All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the U.S. National Institutes of Health and approved by the Institutional Animal Care and Use Committee guideline of Hospital of Stomatology affiliated with Tongji University (TJKQ-2019–053).
Notes
All study data are presented in the main text. Raw data and experimental materials used in this study are available upon reasonable request to the corresponding author.
Supplementary Material
References
- Huang D. Q.; El-Serag H. B.; Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A. M.; Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J. Med. 2017, 377, 2063–72. 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- Yan T.; Yan N.; Wang P.; Xia Y.; Hao H.; Wang G.; Gonzalez F. J. Herbal drug discovery for the treatment of nonalcoholic fatty liver disease. Acta Pharm. Sin. B 2020, 10 (1), 3–18. 10.1016/j.apsb.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Y.; Meng T.; Zuo L.; Bei Y.; Zhang Q. H.; Su Z. J.; Huang Y. D.; Pang J. Y.; Xiang Q.; Yang H. T. Xyloketal B attenuates fatty acid-induced lipid accumulation via the SREBP-1c pathway in NAFLD models. Mar Drugs 2017, 15 (6), 163. 10.3390/md15060163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q.; Liu H.; Zhang Y. Q.; Qiao C. Q.; Ge S. Q. Lipidomics revealed alteration of the sphingolipid metabolism in the liver of nonalcoholic steatohepatitis mice treated with scoparone. ACS Omega 2022, 7, 14121–14127. 10.1021/acsomega.2c00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M. R.; Lee J. A.; Kim M.; Lee S.; Oh M.; Moon J.; Nam J. W.; Choi H.; Mun Y. J.; Roh S. S. Gardeniae Fructus attenuates thioacetamide-induced liver fibrosis in mice via both AMPK/SIRT1/NF-κB pathway and Nrf2 signaling. Antioxidants (Basel). 2021, 10 (11), 1837. 10.3390/antiox10111837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L. H.; Li J.; Deng Y. X.; Zhang X. J.; Chen R.; Lv Y. Comparative investigation on the pharmacokinetics of geniposide in type 2 diabetic and normal rats after oral administration of Fructus Gradeniae extract. J. Chromatogr B 2016, 1033, 180–186. 10.1016/j.jchromb.2016.08.030. [DOI] [PubMed] [Google Scholar]
- Guo S.; Bao L.; Li C.; Sun J.; Zhao R.; Cui X. Antiviral activity of iridoid glycosides extracted from Gardeniae Fructus against influenza A virus by PACT-dependent suppression of viral RNA replication. Sci. Rep. 2020, 10, 1897. 10.1038/s41598-020-58443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira H.; Cai X. S.; Zhang Q.; deFreitas V.; Mateus N.; He J. R.; Fernandes I. Gastrointestinal absorption, antiproliferative and anti-inflammatory effect of the major carotenoids of Gardenia jasminoides Ellis on cancer cells. Food Funct. 2017, 8 (4), 1672–1679. 10.1039/C7FO00091J. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li P. L.; Zhang X.; Li L. Y.; Liu M. J.; Li X. Q.; Dai Y. J.; Zhang C.; Li S. J. Mitochondrial-respiration-improving effects of three different Gardeniae Fructus preparations and their components. ACS Omega 2021, 6, 34229–34241. 10.1021/acsomega.1c03265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J.; Qin S.; Han J.; Meng J.; Liang A. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). J. Ethnopharmacol. 2022, 289, 114984. 10.1016/j.jep.2022.114984. [DOI] [PubMed] [Google Scholar]
- Kazybay B.; Sun Q. L.; Dukenbayev K.; Nurkesh A. A.; Xu N.; Kutzhanova A.; Razbekova M.; Kabylda A.; Yang Q.; Wang Q.; Ma C. Q.; Xie Y. Q. Network Pharmacology with experimental investigation of the mechanisms of Rhizoma Polygonati against prostate cancer with additional herbzymatic activity. ACS Omega 2022, 7 (17), 14465–14477. 10.1021/acsomega.1c03018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.; Zaheer M. R.; Iqbal S.; Roohi; Ahmad A.; Alshammari M. B. Photodegradation and in silico molecular docking study of a diuretic drug: Clopamide. ACS Omega 2022, 7 (16), 13870–13877. 10.1021/acsomega.2c00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras G.; Chassagne F.; Lyles J. T.; Marquez L.; Dettweiler M.; Salam A. M.; Samarakoon T.; Shabih S.; Farrokhi D. R.; Quave C. L. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem. Rev. 2021, 121 (6), 3495–3560. 10.1021/acs.chemrev.0c00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. J.; Lin H.; Cheng W. L.; Huang Z. R.; Zhang W. J. Protective effect and mechanism of plant-based monoterpenoids in non-alcoholic fatty liver diseases. J. Agric. Food Chem. 2022, 70 (16), 4839–4859. 10.1021/acs.jafc.2c00744. [DOI] [PubMed] [Google Scholar]
- Vuppalanchi R.; Noureddin M.; Alkhouri N.; Sanyal A. J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nature Reviews Gastroenterology & Hepatology 2021, 18, 373–392. 10.1038/s41575-020-00408-y. [DOI] [PubMed] [Google Scholar]
- Borrelli A.; Bonelli P.; Tuccillo F. M.; Goldfine I. D.; Evans J. L.; Buonaguro F. M.; Mancini A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol. 2018, 15, 467–479. 10.1016/j.redox.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnoni A.; Di Chiara Stanca B.; Giannotti L.; Gnoni G. V.; Siculella L.; Damiano F. Quercetin reduces lipid accumulation in a cell model of NAFLD by inhibiting de novo fatty acid synthesis through the acetyl-coA carboxylase 1/AMPK/PP2A axis. Int. J. Mol. Sci. 2022, 23 (3), 1044. 10.3390/ijms23031044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M. J.; Fernández M.; Picó Y.; Mañes J.; Asensi M.; Carda C.; Asensio G.; Estrela J. M. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. 10.1021/jf803579e. [DOI] [PubMed] [Google Scholar]
- Tie F.; Ding J.; Hu N.; Dong Q.; Chen Z.; Wang H. Kaempferol and kaempferide attenuate oleic acid-induced lipid accumulation and oxidative stress in HepG2 Cells. Int. J. Mol. Sci. 2021, 22 (16), 8847. 10.3390/ijms22168847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X.; Lin L.; Cui B.; Zhao T.; Mao L.; Song Y.; Wang X.; Feng H.; Yu Q.; Zhang J.; Jiang K.; Cao X.; Wang B.; Sun C. Therapeutic potential of genipin in various acute liver injury, fulminant hepatitis, NAFLD and other non-cancer liver diseases: More friend than foe. Pharmacol. Res. 2020, 159, 104945. 10.1016/j.phrs.2020.104945. [DOI] [PubMed] [Google Scholar]
- Xue C.; Li Y.; Lv H.; Zhang L.; Bi C.; Dong N.; Shan A.; Wang J. Oleanolic acid targets the gut-liver axis to alleviate metabolic disorders and hepatic steatosis. J. Agric. Food Chem. 2021, 69 (28), 7884–7897. 10.1021/acs.jafc.1c02257. [DOI] [PubMed] [Google Scholar]
- Barroso I.; Gurnell M.; Crowley V. E.; Agostini M.; Schwabe J. W.; Soos M. A.; Maslen G. L.; Williams T. D.; Lewis H.; Schafer A. J.; et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999, 402, 880–883. 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- Batatinha H. A.P.; Lima E. A.; Teixeira A. A.S.; Souza C. O.; Biondo L. A.; Silveira L. S.; Lira F. S.; Rosa Neto J. C. Association Between Aerobic Exercise and Rosiglitazone Avoided the NAFLD and Liver Inflammation Exacerbated in PPAR-α Knockout Mice. Journal of Cellular Physiology 2017, 232, 1008–1019. 10.1002/jcp.25440. [DOI] [PubMed] [Google Scholar]
- Kersten S.; Desvergne B.; Wahli W. Roles of PPARs in health and disease. Nature 2000, 405, 421–424. 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- Garcia D.; Hellberg K.; Chaix A.; Wallace M.; Herzig S.; Badur M. G.; Lin T.; Shokhirev M. N.; Pinto A. F. M.; Ross D. S.; Saghatelian A.; Panda S.; Dow L. E.; Metallo C. M.; Shaw R. J. Genetic liver-specific AMPK activation protects against diet-induced obesity and NAFLD. Cell Rep. 2019, 26, 192–208. 10.1016/j.celrep.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Wang B.; Wang S. S.; Lu H.; Wu H.; Ding M. Y.; Ying L. L.; Mao Y. J.; Li Y. Effect of Quercetin on Lipids Metabolism Through Modulating the Gut Microbial and AMPK/PPAR Signaling Pathway in Broilers. Front Cell Dev Biol. 2021, 9, 616219. 10.3389/fcell.2021.616219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. J.; Tzeng T. F.; Liou S. S.; Chang Y. S.; Liu I. M. Kaempferol regulates the lipid-profile in high-fat diet-fed rats through an increase in hepatic PPARα levels. Planta Med. 2011, 77 (17), 1876–82. 10.1055/s-0031-1279992. [DOI] [PubMed] [Google Scholar]
- Shen B.; Feng H.; Cheng J.; Li Z.; Jin M.; Zhao L.; Wang Q.; Qin H.; Liu G. Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J. Cell Mol. Med. 2020, 24 (9), 5097–5108. 10.1111/jcmm.15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G. R.; Carling D. AMP-activated protein kinase: the current landscape for drug development. Nat. Rev. Drug Discovery 2019, 18, 527–551. 10.1038/s41573-019-0019-2. [DOI] [PubMed] [Google Scholar]
- Tahri-Joutey M.; Andreoletti P.; Surapureddi S.; Nasser B.; Cherkaoui-Malki M.; Latruffe N. Mechanisms mediating the regulation of peroxisomal fatty acid beta-oxidation by PPARα. Int. J. Mol. Sci. 2021, 22 (16), 8969. 10.3390/ijms22168969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skat-Rordam J.; Ipsen D. H.; Lykkesfeldt J.; Tveden-Nyborg P. A role of peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. Basic Clin. Pharmacol. Toxicol. 2019, 124, 528–537. 10.1111/bcpt.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z. Y.; Lu L. H.; Xia Z. X. Anti-Tumor xanthones from Garcinia nujiangensis suppress proliferation, and induce apoptosis via PARP, PI3K/AKT/mTOR, and MAPK/ERK signaling pathways in human ovarian cancers cells. Drug Des Devel Ther. 2020, 14, 3965–3976. 10.2147/DDDT.S258811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z. X.; Tang Z. Y. Network pharmacology analysis and experimental pharmacology study explore the mechanism of Gambogic acid against endometrial cancer. ACS Omega. 2021, 6 (16), 10944–10952. 10.1021/acsomega.1c00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z. Y.; Lu L. H.; Zhou X. Y.; Shen J.; Song W. P.; Tang Y. D.; Xia Z. X. A new cytotoxic polycyclic polyprenylated acylphloroglucinol from Garcinia nujiangensis screened by the LC-PDA and LC-MS. Nat. Prod Res. 2020, 34 (17), 2448–2455. 10.1080/14786419.2018.1539983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.