Abstract

This study presents the isolation, characterization, and kinetic analyses of cellulose nanocrystals (CNCs) from date palm waste in the United Arab Emirates. After bleaching date palm stem waste with acidified NaClO2 and delignification via NaOH treatments, cellulose was extracted. Mineral acid hydrolysis (62 wt % H2SO4) was performed at 45 °C for 45 min to produce crystalline nanocellulose. Fourier transform infrared (FTIR) and chemical composition analysis confirmed the removal of noncellulosic constituents. The crystallinity index increased gradually with chemical treatments, according to the obtained X-ray diffraction (XRD) results. Thermogravimetric analysis and differential scanning calorimetry results revealed that the CNC has high thermal stability. The Coats–Redfern method was used to determine the kinetic parameters. The kinetic analysis confirmed that CNC has more activation energy than cellulose and thus confirms its compact and resistive crystalline structure. This has been attributable to the stronger hydrogen bonding in CNC crystalline domains than that in cellulose crystalline domains. Scanning electron microscopy revealed that lignin and hemicellulose were eliminated after chemical pretreatments, and CNC with a rodlike shape was obtained after hydrolysis. Moreover, transmission electron microscopy confirmed the nanoscale of crystalline cellulose. ζ potential analysis indicated that the CNC afforded a stable suspension (−29.27 mV), which is less prone to flocculation. Kinetic analyses of cellulose and cellulose nanocrystals isolated from date palm waste are useful for making composites and designing selective pyrolysis reactors.
1. Introduction
For economic success and environmental persistence, the “Bioeconomy” model of the 21st century promotes the utilization of renewable resources instead of the mere use of nonrenewables.1 With an approximate yearly production of 7.5 × 1010 tons, cellulose is perhaps the most plentiful natural biopolymer on earth. Cellulose is a high-molecular-weight homopolymer made up of β-d-glucopyranosyl repeating units linked by (1–4) glycoside linkages that can be extracted from a variety of biomasses.2 There are both crystalline and amorphous regions in cellulose. Cellulose nanocrystals (CNCs) are crystalline particles formed after eliminating the amorphous area using acid hydrolysis treatment.3 CNC offers unique properties such as lower cost, nontoxicity, superior thermal stability, optical transparency, and biodegradability.4 However, CNC is widely applied as a reinforcing reagent in polymer composites owing to its extraordinary thermal and mechanical characteristics.5 CNC considerably improves the physicochemical, thermal, and insulating properties of various biodegradable polymers that would otherwise be unsuitable for many applications.6 Additionally, CNC’s large specific surface area (hundreds of m2/g),7 high elasticity modulus (approximately 150 GPa),8 ultralightweight (1.6 g/cm3),9 biodegradability,10 and biocompatibility11 are the main factors that encourage its use as a reinforcement agent in composite manufacturing. Additionally, CNC can be used to prepare barrier films,12 shape-memory polymers,13 bionanocomposites,14 drug-delivery materials,15 photonic crystals,16 biomedical devices,17 filaments,18 aerogels,19 hydrogels,20 fuel cells,21 and three-dimensional (3D) printing22 as well as for wastewater treatment23 and producing agricultural products,24 adsorbents,25 and materials for cultural heritage.26 The surface chemistry of cellulose derivates can further be modified to use in many other applications.27
CNCs generally produced from agricultural waste biomass and forest industry wastes such as cotton stalks, corncobs, wheat straw, coconut husks, maize straws, and pea hull fibers.28,29 As per the United Nations’ Food and Agriculture Organization report, the annual production of dates in 2018 was approximately 8.7 million tons, with the majority of the products originating from the Middle East and North Africa region.30 Annually, the United Arab Emirates (UAE) accounts for 14% of global total date fruit production. It is estimated that there will be over 40 million date palm trees in the UAE.31 Hence, an enormous amount of lignocellulosic biomass waste material is available in the form of leaves, fibers, date pits/stones, and stem waste.32 Furthermore, it contains a plentiful amount of cellulose (30–45%), which is an advantage to isolate good yields of CNCs.33,34
To date, there has been a lack of studies on the isolation of CNCs from date palm stems. Othman et al.35 isolated the cellulose nanocrystals from date fruit seeds using sulfuric acid hydrolysis. Semispherical-shaped cellulose nanocrystals with a size range of 20–100 nm were reported through scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis. CNCs had good thermal stability with an initial degradation temperature of 200 °C and a higher crystallinity index (72.49%). Alothman et al.36 isolated cellulose nanocrystals from date palm surface fibers using sulfuric/acetic acid hydrolysis. Rod-shaped cellulose nanocrystals having a length of 146–215 nm and a width of 8–13 nm were observed through field emission SEM (FESEM) and TEM analsyis. The thermal stability and crystallinity index of CNCs isolated using sulfuric/acetic acid in a 30/70 ratio were 330 °C and 84.2%, respectively. Alhamzani et al.37 isolated cellulose nanocrystals from date palm leaflets using sulfuric acid hydrolysis. Cellulose nanocrystals with an average diameter of less than 50 nm were observed through SEM images. The cellulose nanocrystals had higher thermal stability with an initial decomposition temperature of 300 °C. Shaikh et al.38 isolated cellulose nanocrystals from date palm trunk using sulfuric acid hydrolysis. Irregular-shaped particles with sizes ranging from 26 to 61 nm were detected through SEM images. Cellulose nanocrystals had a higher thermal stability of 290 °C and a crystallinity of 68%. Each variety of waste types offers different morphologies as well as physicochemical and thermal properties. The morphological and physicochemical properties of CNCs are considered to have an impact on their performance. The morphology, physiochemical properties, thermal stability, and yield of CNCs are known to be reliant on the material used for cellulose extraction. The isolation of CNCs from various materials is appropriate and necessary to present their potential use as a feedstock for cellulose nanocrystal production.39,40 The significance of this study lies in the utilization of the date palm stem material from the UAE for the isolation and characterization of CNCs. The aim is to bring knowledge on the chemical composition, thermal profiles, morphology, and kinetics of cellulose and nanocellulose isolated from date palm stem waste.
Moreover, this is the first study to estimate the activation energy (Ea) of cellulose nanocrystals isolated from any date palm waste material. This analysis not only helps to understand the extent of the change in the crystalline structure but also better estimates the thermal stability of CNC-based composites. CNC has found extensive use in the development of biodegradable composites. Prior knowledge of kinetic parameters is advantageous. The knowledge of the kinetic parameters is critical in the context of pyrolysis of lignocellulosic materials to perform selective pyrolysis. Cellulose is primarily responsible for bio-oil production.41 Information on the kinetic parameters of cellulose isolated from date palm waste helps design pyrolysis reactors for enhanced bio-oil production.
2. Materials and Methods
2.1. Materials
Date palm stem waste (Lulu palm tree) was obtained from the UAE University’s Al-Foah experimental farm. The woody biomass was first ground with a shredder and then using a mechanical grinder to a mesh size of 160 μm. Sigma-Aldrich provided sodium chlorite, sodium hydroxide (bioXtra 98%), acetic acid glacial (100%), sulfuric acid (95–97%), and dialysis tubing (14 000 Da).
2.2. Isolation of Cellulose from Palm Waste
Raw date fiber (R-DW) was bleached with an acidified 4% w/v NaClO2 solution. Before bleaching, the pH of the NaClO2 solution was brought to 3.5–4 by adding glacial acetic acid solution (10% v/v). At 80 °C and after 1 h of stirring, the fiber-to-NaClO2 solution ratio was 1:50 (10 g of raw fibers into 500 mL of NaClO2 solution). Vacuum-filtered bleached fiber (B-DW) was washed several times with fresh water until the pH of the filtrate reached 6.5–7. For 24 h, B-DW was placed in an air-circulating oven at 105 °C.
B-DW was delignified using a 4% w/v NaOH solution at 25–30 °C and stirring for 30 min, with a fiber-to-NaOH solution ratio of 1:50 (10 g of B-DW into 500 mL of NaOH solution). The delignified sample (D-DW) was vacuum-filtered and rinsed with fresh water several times until the pH of the filtrate reached 6.5–7. D-DW was also stored for 24 h in an air-circulating oven at 105 °C.
2.3. Isolation of Cellulose Nanocrystals
The method used for CNC extraction was modified from previous studies.42,43 CNCs were extracted from cellulose (D-DW) using mineral acid hydrolysis (62 wt % H2SO4) conducted at 45 °C with a reaction time of 45 min and an acid-to-fiber ratio of 1:20. The hydrolysis was terminated by diluting the solution 20 times with deionized water (4 °C). The suspension was centrifuged for 20 min at 8000 rpm to remove the residual sulfuric acid. The fibers were washed again in deionized water and centrifuged seven times until the supernatant pH was 4.5–6. The resulting nanofiber suspension was dialyzed against distilled water for 5 days, with a change of water every 24 h, until a consistent pH of 6.5–7 was obtained. Ultrasonication was then performed for 30 min to homogenize the nanofiber suspension. The cellulose nanocrystal suspension was then freeze-dried under vacuum at −85 °C to obtain CNCs and then kept in a refrigerator for further analysis. An overview of the CNC isolation process is presented in Figure 1.
Figure 1.

Isolation process of cellulose nanocrystals.
2.4. Characterization of Cellulose Nanocrystals
2.4.1. Thermogravimetric Analysis (TGA)
The thermal property profiles of R-DW, B-DW, D-DW, and CNC were measured using a thermogravimetric analyzer. Test samples with a preset weight of 5–10 mg were analyzed using thermogravimetric analysis (TGA, Q 500 series, TA Instruments). The thermal profiles of the four samples were analyzed at a constant heating rate of 10 °C/min up to 800 °C in a nitrogen environment (60 mL/min).
2.4.2. Differential Scanning Calorimetry (DSC)
The thermomolecular characteristics were investigated using differential scanning calorimetry from TA equipment (DSC25). The differential scanning calorimetric (DSC) analysis of a 5 mg CNC sample was performed at temperatures ranging from 30 to 350 °C. The heating rate was 10 °C/min in a N2 atmosphere (50 mL/min).
2.4.3. X-ray Diffraction (XRD)
Cu K radiation was used to examine the sample with the following working lamp parameters: V = 40 kV, I = 30 mA, and receiving slit = 0.15 mm. With a scan range of 10–80 and a scan speed of 2°/min, the intensity of the reflections was measured. The crystallinity index of the samples was calculated using the Segal equation, as illustrated in eq 1.44 Scherrer equation was used to calculate the average crystallite size, as illustrated in eq 2.45
| 1 |
where Crl is the crystallinity index, I200 denotes the diffracted intensity at the highest crystalline peak, and Iam denotes the amorphous region’s diffraction intensity.
| 2 |
where L denotes the size of the crystal in nanometers. Constant k has a value of 0.89, h is the X-ray wavelength, and β is the full width at half-maximum (FWHM) height of the primary diffraction peak in radians. The FWHM of the samples’ X-ray diffraction (XRD) data was calculated using OriginPro software. h is the wavelength of X-ray sources (0.15406 nm). θ is the Bragg angle in radians equal to half of the 2θ.
2.4.4. Fourier Transform Infrared (FTIR) Analysis
A Fourier transform infrared (FTIR) spectrometer was used to perform functional chemistry studies (Shimadzu, Kyoto, Japan). The functional group variations at different wavelengths were investigated. The FTIR spectra were obtained using an attenuated total reflection FTIR (ATR-FTIR) spectrograph with an average of 34 scans and a spectral resolution of 4 cm–1 over a range of 500–4000 cm–1.
2.4.5. ζ Potential and Average Particle Diameter
Dynamic light scattering (DLS) was used to determine the average hydrodynamic diameter and particle size distribution of cellulose nanocrystals suspension (Otsuka Electronics, Japan). At 25 °C, particle size and ζ potential were measured in a fully automated mode. The CNC was diluted 100 times with deionized water and sonicated for 30 min before analysis.
2.4.6. Kinetic Analysis
The kinetics of extracted cellulose and cellulose nanocrystals from date palm waste were calculated using the Coats–Redfern method, which is a model-fitting approach. The samples’ activation energy (Ea) was estimated using a fundamental Coats–Redfern equation, as illustrated in eq 3.46
| 3 |
2RT/Ea will be far less than 1. As a result, this expression has been eliminated.47,48 The kinetic analyses are thus conducted using eq 4.
| 4 |
Here, g(α) signifies the model that represents the reaction mechanism, β is the thermal ramp rate (°C/min), R stands for universal gas constant (0.008321 kJ/mol), T is the reaction temperature (K), and A is pre-exponential or frequency factor (m–1).
α is the degree of transformation, which is calculated using eq 5.49
| 5 |
where mo is the original mass of the respective sample at t = 0, mi is the instantaneous mass of the respective sample at any time t, mf is the final mass of the respective sample.
Plotting the left
side of eq 4 versus 1/T will
yield
both Ea and A. The resultant
straight line’s slope will be −Ea/R and its intercept will be
versus 1/T will
yield
both Ea and A. The resultant
straight line’s slope will be −Ea/R and its intercept will be 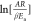 . Using the appropriate reaction mechanism
model g(α), Ea/R and A are found from the data taken from
the slope and intercept of the regression lines. Reaction model mechanisms
for solids have been considered using g(α),
as shown in Table 1.
. Using the appropriate reaction mechanism
model g(α), Ea/R and A are found from the data taken from
the slope and intercept of the regression lines. Reaction model mechanisms
for solids have been considered using g(α),
as shown in Table 1.
Table 1. Reaction Mechanism Models g(α) of the Coats–Redfern Methoda.
| symbol | function models | g(α) |
|---|---|---|
| Chemical Reaction | ||
| CRO 1 | first order | –ln(1 – α) |
| CRO 2 | second order | [1/(1 – α)] – 1 |
| Diffusion Reaction | ||
| DM1 | one-way transport | α2 |
| DM2 | two-way transport | (1 – α) – ln(1 – α) + α |
| DM3 | three-way transport | [[−ln(1 – α)]1/3]2 |
| DM4 | Valensi equation | α + (1 – α)ln(1 – α) |
| DM5 | Ginstling–Brounshtein equation | (1 – 2α/3) – (1 – α)2/3 |
| DM6 | Zhuravlev equation | [(1 – α)−1/3 – 1]2 |
| DM7 | Jander equation | [1 – (1 – α)1/3]2 |
| DM8 | Ginstling equation | 1 – (0.67α) – (1 – α)0.67 |
| Geometric Reaction | ||
| GM1 | cylindrical shape | 1 – (1 – α)1/2 |
| GM2 | sphere shape | 1 – (1 – α)1/3 |
| Nucleation Reaction | ||
| NM1 | 1/2 Avrami–Erofeev equation | [−ln(1 – α)]1/2 |
| NM2 | 1/3 Avrami–Erofeev equation | [−ln(1 – α)]1/3 |
Adapted in part with permission from ref (50). Copyright 2022 Elsevier.
2.4.7. Morphological Analysis
The morphologies of R-DW, B-DW, D-DW, and CNC were observed using a scanning electron microscope (SEM) at a certain spatial resolution. A JEOL/EO SEM operating at 10 kV was used to examine the surface morphology of the samples. The samples were gold-coated prior to analysis to avoid electrostatic charge during the test. The material was dispersed in ethanol and sonicated for 30 min prior to analysis using a Tecnai transmission electron microscope (JEM-3000F, JEOL, Japan) at 200 kV. The nanoparticles at different spatial resolutions were observed via transmission electron microscopy (TEM).
2.4.8. Chemical Composition Analysis
Chemical composition analysis was performed to calculate the amounts of holocellulose, cellulose, and lignin in R-DW, B-DW, D-DW, and CNC. Hemicellulose was calculated by subtracting the amount of cellulose from that of holocellulose. To determine the extractives in R-DW, the Technical Association of Pulp and Paper Industry (TAPPI) standard method T 204-cm-97 is used. A 10 g sample was placed in a Soxhlet thimble and fitted to a 2000 mL Soxhlet extractor apparatus. The extraction was performed using 1000 mL of the ethanol–benzene mixture (ethanol/benzene = 1:2) for 12 h. The percent of extractives was considered based on the original weight of the sample. Holocellulose was calculated using the method presented by Wise;51 5 g of the extracted sample was combined with 160 mL of distilled water, 0.5 mL of acetic acid, and 1.5 g of NaClO2 in a solution. The mixture was heated to 70 °C for 1 h (the beaker was shaken every 5 min). Every hour, 0.5 mL of acetic acid and 1.5 g of NaClO2 were added to the solution (a total of four additions of acetic acid and sodium chlorite). The solution mixture was vacuum-filtered to obtain holocellulose as a filter cake and washed with acetone and then with hot water until pH 6.5–7 was achieved. Finally, it was dried in an oven at 105 °C until it reached a steady weight. A percent of holocellulose was calculated based on the original mass of the sample. α-Cellulose was calculated based on a method presented by Hastuti et al.52 Two grams of the extracted sample was added to a 50 mL 17.5% w/v NaOH solution and allowed to sit for 30 min at 25 °C. Then, 50 mL of DI water was added and mixed for 1 min before being set aside for 5 min. The weight of the dry filter paper was measured before filtration. The sample was vacuum-filtered three times—once with 1200 mL of DI water, then with 80 mL of acetic acid (10% v/v), and finally with 2 L of boiled water. The filter cake (α-cellulose) was dried to a constant weight at 105 °C. α-Cellulose is the increase in the weight of filter paper. Lignin was measured using the TAPPI standard method. 15 g of 72.5% sulfuric acid (cooled to 15 °C) was slowly added to 2 g of the extractive-free sample. To avoid overheating, sulfuric acid was added to the solution in an ice bath. For 5 min, the solution was stirred with a stirring rod. Then, the solution was stirred every 2 h. Following that, the mixture was gradually transferred to a beaker containing 400 mL of DI water. The solution was allowed to settle and precipitate lignin for 24 h. Filtered lignin was dried at 105 °C until it reached a constant weight. The original weight of the sample was used to calculate the percentage yield. Equation 6 was used to calculate the yield of final cellulose nanocrystals.
| 6 |
3. Results and Discussion
3.1. Thermogravimetric Analysis
The degradation characteristics of R-DW, B-DW, D-DW, and CNC were determined using TGA, as shown in Figure 2a. TGA was useful for determining the thermal stability of the samples, whereas differential thermogravimetric (DTG) analysis, as shown in Figure 2b, determined the maximum weight loss rate of the sample. TGA curves of all four samples began to change after some mass loss from room temperature to 115 °C. The evaporation of weakly bonded moisture was responsible for the initial mass loss of the samples. Table 2 summarizes the initial degradation temperature (°C), moisture content (%), weight loss (%), the weight of residue (%), and temperature at the peak degradation rate (°C) of the four samples. The degradation of the R-DW sample is a two-step mechanism, indicating the decomposition of multiple components. Because of the different weight percentages of lignin, hemicellulose, and cellulose, thermal degradation occurred in several steps. At various temperatures, these biopolymers decomposed into amorphous and crystalline phases.53 R-initial DW’s degradation temperature was 227 °C. As bleaching and delignification treatments were applied, the degradation temperature increased to 239 °C for B-DW and 242 °C for D-DW, successively. The removal of hemicellulose and lignin during the bleaching and delignification pretreatment processes increased thermal stability. After pretreatment, the lignocellulosic feedstock (R-DW) became dense and compact, increasing the onset temperature of degradation.54 The thermal degradation temperature of CNC reaches 249.5 °C with a mass loss of only 6.9%. The superior thermal stability of CNC can be attributed to acid hydrolysis, which removes amorphous regions from the cellulose pulp, leaving only a crystalline structure.55 At thermal degradation temperature, the mass loss of the four samples—R-DW, B-DW, D-DW, and CNC—decreased from 12.8 to 6.9%. The total mass loss of the samples also followed the same pattern, with the highest mass loss for R-DW and the minimum for CNC. The degradation of CNC was a single-step mechanism, unlike the behavior of R-DW degradation. Because lignin and hemicellulose no longer existed in CNC, the major degradation region corresponded to cellulose nanocrystals’ weight loss. According to Zhao et al.,56 lignin contained a variety of aromatic rings with numerous branches. Hence, lignin started to degrade over a wider temperature range of 215–585 °C, resulting in a broader range of chemical bond activity.
Figure 2.
(a) Thermogravimetric analysis (TGA) curves and (b) derivative TGA curves for R-DW, B-DW, D-DW, and CNC.
Table 2. Thermal Data of Raw Date Fiber (R-DW), Bleached Fiber (B-DW), Delignified Sample (D-DW), and CNCa.
| TGA |
DTG
peak (°C) |
||||||
|---|---|---|---|---|---|---|---|
| sample | Tost (°C) | W@Tost | MH2O (%) | Wlos (%) | Wrsd (%) | Tmax1 | Tmax2 |
| R-DW | 227.7 | 87.2 | 12.8 | 85.2 | 2.0 | 190 | 342 |
| B-DW | 239.7 | 90.3 | 9.7 | 84.0 | 6.3 | 332 | |
| D-DW | 242.1 | 91.9 | 8.1 | 84.3 | 7.5 | 315 | |
| CNC | 249.5 | 93.1 | 6.9 | 83.6 | 9.5 | 330 | |
Tost = initial degradation temperature, W@Tost = weight of the sample at Tost, MH2O = moisture content, Wlos = weight loss of the sample, Wrsd = weight of the sample residue, and Tmax1 and Tmax2 = DTG peaks.
The rate of degradation reached its peak value at 342 °C for R-DW, 332 °C for B-DW, 315 °C for D-DW, and 330 °C for CNC, as revealed by the DTG curves. The residues at the end of degradation were 2.0, 6.3, 7.5, and 9.5 wt % for R-DW, B-DW, D-DW, and CNC, respectively. Char formation occurred due to the decomposition of lignocellulosic materials into a solid residue. Char was produced due to the presence of lignin, hemicellulose, inorganic minerals, and stable oxides in raw biomass sources.57 B-DW and D-DW had nearly identical char residues. Lignin was responsible for solid yields in lignocellulosic materials.58 Therefore, the solid char at the end of the main mass-loss region differed for each of the four samples.
3.2. Differential Scanning Calorimetry
Figure 3 presents the DSC curve for the thermomolecular analysis of the produced CNC. In a studied temperature range of 30–350 °C, two distinctive endothermic curves were obtained. The first endotherm, which appeared between 100 and 210 °C, indicated moisture loss due to evaporation and other thermally unstable chemicals. The TGA of CNC identified the same region. The terminating temperature of this endotherm was very similar to the CNC onset temperature. The second endotherm was in a very narrow temperature range (290–297 °C). This corresponded to the course of fusion due to the decomposition of CNC crystallites. However, for cellulose (D-DW), this endotherm appears at a much lesser temperature (190 °C). This is due to the presence of amorphous cellulose, which fuses at lower temperatures than cellulose nanocrystals (CNC). Mandal and Chakrabarty54 discovered similar endotherms for moisture loss and melting point for CNC prepared from waste sugarcane bagasse as a raw material. The fusion or melting temperature of the produced CNC was also comparable to that prepared from corn stalk, as reported by Huang et al.,59 and that prepared from corn stalk, as reported by Maiti et al.60
Figure 3.
Differential scanning calorimetry curve of CNC.
3.3. Kinetic Analysis
The activation energy of cellulose (D-DW) and CNC has been determined using the Coats–Redfern approach using several solid-state reaction models. Table 3 shows the activation energy (Ea), frequency factor (A), and corresponding correlation factor (R2). The criteria of model selection are based on the best linear fitting of a kinetic reaction model (g(x)) and the linear regression coefficient (R2). When performing kinetic analysis, the primary degradation zone was considered. Figure 4 depicts the mass loss of cellulose and CNC at 230–360 °C. This region is also known as the active mass-loss region or the active pyrolysis zone.
Table 3. Activation Energy Analysis of D-DW and CNC.
| model code | Ea (kJ/mol) | A (1/min) | R2 |
|---|---|---|---|
| cellulose (D-DW) | |||
| DM1 | 89.2 | 8.9 × 103 | 0.99 |
| DM4 | 102.0 | 97.8 × 103 | 0.99 |
| DM5 | 107.5 | 81.5 × 103 | 0.99 |
| DM7 | 120.7 | 1.8 × 106 | 0.99 |
| DM8 | 107.3 | 87.5 × 103 | 0.99 |
| GM1 | 51.0 | 2.3 | 0.99 |
| GM2 | 55.6 | 4.9 | 0.99 |
| average value | 90.5 | 299.4 × 103 | 0.99 |
| CNC | |||
| DM1 | 117.0 | 1.39 × 109 | 0.99 |
| DM4 | 124.9 | 8.6 × 106 | 0.99 |
| DM5 | 129.9 | 6.4 × 106 | 0.99 |
| DM8 | 130.7 | 7.4 × 106 | 0.99 |
| average value | 125.6 | 353.1 × 106 | 0.99 |
Figure 4.
TGA curves of D-DW and CNC.
Table 3 shows that there are seven reaction models for cellulose that have a regression coefficient value of 0.99. There are five diffusion models—parabolic law (DM1), 2D diffusion (DM4), anti Jander equation (DM5), 3D diffusion (DM7), and four-dimensional (4D) diffusion (DM8)—and two geometric models—cylindrical symmetry (GM1) and spherical symmetry (GM2). The average value of activation energy (Ea) for cellulose pulp was 90.5 kJ/mol. The average activation energy for CNC was 125.6 kJ/mol (Table 3). Four diffusion models with a regression coefficient of 0.9 were used to calculate the activation energy of CNC. Thermal stability is a major factor in limiting the properties and application possibilities of polymer/CNC composites. The physical and chemical structures of CNCs affect their thermal stability. Because of the different orientation of the cellulose chains and the pattern of hydrogen bonding in cellulose I and cellulose II, different crystalline arrangements of cellulose influenced their thermal stability, resulting in an increase in the activation energy for cellulose II and a slight decrease in the activation energy for cellulose I.61 Huang et al.59 found a higher value of Ea for corn stalk-isolated CNC, when compared to its cellulose pulp, and confirmed the presence of stronger hydrogen bonding in the crystalline domains of CNC than that in cellulose. A similar relationship between activation energy and resistive crystalline structure of cellulose or CNC was presented by Morgado et al.62
The Ea value was significant because it assisted in the prediction of the thermal stability characteristics of CNC/polymer composites. The best-fitting diffusion models in both samples were DM1, DM4, DM5, and DM8, and their linear fitting with experimental data is shown in Figures 5 and 6. This topic is covered in greater depth in the Supporting Information.
Figure 5.
Best-fitted diffusion models ((a) DM1, (b) DM4, (c) DM5, and (d) DM8) (cellulose).
Figure 6.
Best-fitted diffusion models ((a) DM1, (b) DM4, (c) DM5, and (d) DM8) (CNC).
3.4. X-ray Diffraction
In the powder form, the size of the crystallite is the smallest single crystal. Therefore, the nanoparticle, under no conditions, can reach below the crystallite size. The Scherrer equation, described in Section 2, was used to determine the average cross-sectional dimension of crystallites in the four samples. Data are summarized in Table 4. The Scherrer equation is mainly suitable for materials with high crystallinity and less broadening of peaks. So, we mainly analyzed the step just before the cellulose nanocrystal synthesis. The crystallite size increases from 2.6 to 2.7 nm during the hydrolysis of D-DW into CNC. Cellulosic crystals form due to the elimination of cellulose amorphous regions. The cellulosic polymeric chain was depolymerized and downscaled efficiently, resulting in the formation of cellulose nanocrystals as discrete crystallites. Flauzino Neto et al.63 discovered a comparable value for the CNC crystallite (2.7 nm) prepared using soy hulls via acid hydrolysis.
Table 4. X-ray Diffraction Analysis of R-DW, B-DW, D-DW, and CNC.
| amorphous |
crystalline |
||||||
|---|---|---|---|---|---|---|---|
| sample | FWHM | crystallite size (nm) | 2θ | Iam | 2θ | I200 | Crl (%) |
| R-DW | 2.7449 | 2.9 | 18.2 | 735.0 | 21.8 | 1059.0 | 30.6 |
| B-DW | 2.9811 | 2.7 | 18.6 | 806.9 | 21.8 | 1268.3 | 36.4 |
| D-DW | 3.0344 | 2.6 | 18.4 | 684.8 | 22.0 | 1275.5 | 46.3 |
| CNC | 2.9264 | 2.7 | 18.4 | 620.1 | 22.4 | 2010.1 | 69.2 |
The crystalline structure of date palm waste was evaluated via XRD analysis after each chemical treatment. Unlike lignin and hemicellulose, which are purely amorphous structures, cellulose comprised both crystalline and amorphous sections. The crystallinity index (Crl) in a sample is the ratio of cellulose-related diffraction to total diffraction.43Figure 7 depicts the diffractograms of R-DW, B-DW, D-DW, and CNC. Table 4 shows the results of the crystallinity index. The raw date palm waste material contained structural lignin and hemicellulose and had a minimum crystallinity index of 30.6%. Because hemicellulose and some lignin were removed from the raw sample during the bleaching treatment, the crystallinity index improved to 36.4%. When the bleached sample was further delignified, the crystallinity index increased to 46.31%, indicating that lignin has been removed. After hydrolyzing cellulose pulp with sulfuric acid, the crystallinity index of CNC increased to 69.1%. The elimination of the amorphous cellulose structure accounted for this significant increase in crystallinity. A higher crystallinity index was related to the rigidity of cellulose nanocrystals. As a result, when high cellulose nanocrystals were used as a reinforcement agent, the composite materials exhibited improved mechanical properties (tensile strength).64 The CNC crystallinity index produced in this study was comparable to those produced from date seeds (70%),65 coffee silverskin (72%),66 and groundnut shells (74%).67
Figure 7.
X-ray diffractions of (a) R-DW, (b) B-DW, (c) D-DW, and (d) CNC.
Moreover, all samples exhibited diffraction peaks at approximately 2θ = 16, 22.5, and 34.5°, which corresponded to 110, 200, and 004 crystal planes of the typical structure of cellulose Iβ, respectively. The occurrences of these peaks established that the crystalline structure of cellulose Iβ was still conserved after the acid-assisted extraction of cellulose nanocrystals from raw date palms.42,68−71
3.5. Fourier Transform Infrared Microscopy Analysis
Chemical treatments of R-DW were done to produce CNC by removing lignin and hemicellulose. FTIR analyses were performed to examine the removal of these impurities. Figure 8 depicts the FTIR spectra of the four samples (R-DW, B-DW, D-DW, and CNC). Date palm waste is a lignocellulosic material primarily comprising cellulose, lignin, and hemicellulose, which means it contains esters, ketones, alkanes, aromatics, and alcohols with various oxygenated functional groups.72
Figure 8.
Fourier transform infrared spectroscopy of (a) R-DW, (b) B-DW, (c) D-DW, and (d) CNC.
Because of the presence of C–H and C–O stretching peaks for cellulose linkage, absorption bands in the 800–900 cm–1 area were identified in the four samples.73 The C=O stretching of the uronic ester and acetyl groups of hemicellulose or the ester linkage of carboxylic groups of ferulic and p-coumaric acids of lignin is attributed to the band seen at 1728 cm–1.57,74−77 The absence of this band in the FTIR spectra of CNC confirms the removal of lignin and hemicellulose. Because of the removal of lignin, the relative strength of the absorption band at 1615 cm–1 was slightly reduced, which could be attributed to the fluctuation of the —C=O stretch of conjugated p-substituted aryl ketones.78 The bands at 1235 cm–1, corresponding to the elongation of the ether C—O—C linkage, and 1425 cm–1, corresponding to aromatic ring vibrations or CH3 of the acetyl group, are also linked to lignin.79 The characteristic peaks for cellulose appear at 665, 893, and 1026 cm–1.80,8180,81 C—H rocking vibrations are responsible for the absorption band at 893 cm–1.82,83 Stretching the —OH (hydrogen-bonded) groups results in a broad absorption band with peaks at approximately 3300 cm–1, indicating the hydrophilic nature of the fibers,84 whereas peaks at 2900 cm–1 are associated with C—H stretching vibrations.85 After bleaching, delignification, and acid hydrolysis, FTIR results confirmed that lignin and hemicellulose have been removed without altering the cellulose structure.
3.6. ζ Potential and Average Particle Diameter
The ζ potential was used for determining the surface charge of nanoparticles. It aids in comprehending the physical stability of nanoscale suspensions.86 A high ζ potential (either negative or positive) indicates good physical stability due to electrostatic repulsion between the nanoparticles. A suspension with a ζ potential value greater than +30 mV or less than −30 mV is thought to have sufficient repulsive forces to manage better stability. Suspensions with extremely low ζ potential, on the contrary, are unstable and prone to flocculation and aggregation.87 For cellulose nanocrystals, agglomeration will occur if the ζ potential is between +15 and −15 mV.88 The ζ potential value for CNC in this study is −29.3 ± 2.0 mV. This value is well below the agglomeration range (−15 mV) and close to the ζ potential value known for better stability (−30 mV). Guo et al.89 used sulfuric acid hydrolysis to create cellulose nanocrystals, produced using agricultural waste tea stalks, with a ζ potential of −33.4 mV.
The Brownian motion of the nanoparticles was used in dynamic light scattering (DLS) measurements. To calculate the value, the radius of a sphere with the same diffusion coefficient as the rodlike-shaped CNC particles was used. The method was suitable for calculating the CNC average particle size. Other authors have also identified CNC as having a rodlike structure; however, they discovered that the DLS technique is appropriate.90 Many other authors35,76,91 have used the DLS technique for CNC particle size distribution. The average hydrodynamic diameter of CNC in this study is represented by two main groups: 90% of the cellulose nanocrystals have a size range of less than 78.4 nm and 10% of the cellulose nanocrystals have a size range of less than 45 nm. The polydispersity index (PDI) was 0.27. The PDI had a size-based measure of a sample’s heterogeneity. Polydispersity occurred due to the sample’s size dispersion or agglomeration during isolation. Hence, a lower PDI value indicated a narrow particle size distribution, also known as homogeneous particle size. A PDI value of 0.2 or lower is preferable for polymer-based nanomaterials.92 In addition, DLS reports for ζ potential and particle size distribution are also available in the Supporting Information.
3.7. Morphological Analysis
Figure 9 presents the morphological structure of R-DW, B-DW, D-DW, and CNC at a spatial resolution of 50 μm. Understanding the structural properties of composite materials relies heavily on fiber morphology.93Figure 9 shows that each treatment process results in significant changes in the morphology of natural fibers. The morphological structure of R-DW is depicted in Figure 9a. The fibrils of the lignocellulosic material are rough and irregular. Amorphous regions of hemicellulose, lignin, pectin, waxy materials, and other noncellulosic materials that act as a protective surface bind the fibrils. In lignocellulosic materials, hemicelluloses and lignin are deposited between the cellulosic fibrils. The removal of these undesired components is accomplished using bleaching, delignification, and hydrolysis treatments. Figure 9b shows that after bleaching, the surface morphology of B-DW becomes cleaner and more porous than that of R-DW. This occurs due to the removal of impurities, extractives, and a portion of lignin/hemicellulose. Bleaching extracts cellulose fibrils more visibly than R-DW. D-DW as presented in Figure 9c shows that the morphology of cellulose fibrils becomes almost independent of noncellulosic materials. Delignification removes the lignin and any remaining hemicellulose. Both bleaching and delignification aid in opening cellulose fiber bundles. Furthermore, hydrolysis of D-DW with sulfuric acid removes the amorphous regions of cellulose, yielding highly CNC. The needle-like cellulose nanocrystals are depicted in Figure 9d. The findings are also supported by XRD analysis, which revealed a gradual increase in crystallinity after each treatment process, confirming an increase in cellulose content. A similar needle-shaped CNC was also reported by Aguayo et al.94 by utilizing the rejected fibers from the kraft pulping process and Khan et al.95 using conocarpus fibers. TEM images of CNC at different spatial resolutions show the nanodimensions of crystalline cellulose (Figure 10). The presence of nanoscale cellulose suggests that the synthesis process used for date palm waste is capable of producing CNC with nanosize dimensions. The fibrils in an orderly arrangement represent the crystalline region of the resulting CNC (dotted rectangle region). TEM images also show that the produced CNC are nonagglomerated, indicating a departure from the natural nature of cellulose.
Figure 9.
Scanning electron microscopic images of (a) R-DW, (b) B-DW, (c) D-DW, and (d) CNC.
Figure 10.
Transmission electron microscopic images of CNC.
3.8. Chemical Composition Analysis
Table 5 presents the chemical composition analysis of R-DW, B-DW, D-DW, and CNC. With the elimination of hemicellulose, lignin, and other extractives, such as waxes or pectins, the chemical treatments of R-DW improve the cellulose content. Bleaching treatment significantly increases the cellulose content, which is 39.8% in R-DW to a much higher value of 74.9% in B-BW. This occurs due to the solubilization of hemicellulose and lignin in a bleaching solvent. With additional delignification of B-DW, the amounts of lignin and hemicellulose decreased further, while the amount of cellulose increased to 87.2%. Lignin and hemicellulose were still present in trace amounts in D-DW. This can be due to the durable binding nature of the lignocellulosic component of the date palm. The strong hydronium ion attack removed the remaining hemicellulose and lignin from D-DW, yielding a CNC of 94.3%. The remaining 5.7% of CNC will most likely be chemical impurities such as sodium, hydroxide, sulfate, and traces of various minerals.96 The ultimate yield of CNC by considering raw date waste (R-DW 20 g) as estimated from eq 6 was found to be 21.0 wt %. R-DW contains 39.8% cellulose, hence 20 g has 8.0 g of total cellulose. From 20 g of R-DW, we produced 6.7 g of D-DW (cellulose), which is approximately 84% recovery, since total cellulose was 8.0 g. From 6.6 g of (D-DW/cellulose), we produced 4.2 g of CNC on a dry mass basis. This is approximately 64% recovery; the losses are due to losing cellulose amorphous regions. More research is needed to investigate possible routes to prevent cellulose loss. Alothman et al.36 calculated a CNC yield of 17.3 wt % from date palm fibers hydrolyzed with sulfuric acid/acetic acid.
Table 5. Chemical Composition Analysis of R-DW, B-DW, D-DW, and CNC.
| samples | extractive | holocellulose | α-cellulose | hemicellulose | lignin | yield % |
|---|---|---|---|---|---|---|
| R-DW | 10.4 | 70.4 | 39.8 | 30.6 | 17.1 | |
| B-BW | 85.8 | 74.9 | 10.9 | 12.2 | 51 ± 0.35 | |
| D-DW | 93.7 | 87.2 | 6.5 | 3.8 | 33.3 ± 0.30 | |
| CNC | 94.3 | 21.2 ± 0.5 |
4. Conclusions
Cellulose nanocrystals were successfully isolated from date palm waste, which is the most abundant biomass available in the UAE. CNC was produced through the following steps: bleaching, delignification, and acid hydrolysis. The removal of lignin and hemicellulose after bleaching and delignification was confirmed via chemical composition analysis and FTIR spectra. After defibrillation of cellulose from the noncellulosic matrix, SEM analysis revealed a rodlike-shaped CNC. TGA results showed that CNC had a high thermal degradation temperature (249.5 °C). Similarly, the high thermal stability of CNC was confirmed through DSC analysis (290–297 °C). CNC had higher activation energy than cellulose pulp (125.6 kJ/mol). This supports the presence of stronger hydrogen bonds in the crystalline domains of cellulose nanocrystals and confirms the removal of the cellulose amorphous region after acid hydrolysis. XRD analysis shows a high value of crystallinity index (69.2%) for CNC. CNC had a ζ potential value of −29.3 mV, which corresponds to good physical stability. DLS results exhibited a very narrow particle size distribution (PDI = 0.274), with d90 and d10 values of 78.4 and 45 nm, respectively. As a result, CNC from date palm waste is a promising candidate in several applications such as the production of polymer composites, food packaging films, biomedical equipment, and thermal resistive materials.
Acknowledgments
The authors gratefully acknowledge the funding support (Project Nos. 31R272 and 12R014) from the National Water and Energy Centre at United Arab Emirates University. The authors are also thankful to the chemical and petroleum engineering department at United Arab Emirates University for providing all necessary facilities to conduct the experimental study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02333.
Average particle size analysis with dynamic light scattering (DLS) and ζ potential analysis; further detailed information on Section 2.4.6: kinetic analysis; activation energy (Ea), frequency factor (A), and regression coefficient (R2) are provided for all 14 kinetic models used; and model fitting versus experimental data graphs are provided for all models with R2 ≥ 0.99 (PDF)
Author Contributions
M.R.: investigation, formal analysis, experimental work, and writing–original draft. B.A.-J.: conceptualization, formal analysis, methodology, funding acquisition, supervision, project administration, and writing–review, and editing. F.B.: conceptualization, writing, review, and editing. A.H.A.-M.: cosupervision, conceptualization, writing, review, and editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Hassan S. S.; Williams G. A.; Jaiswal A. K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. 10.1016/j.biortech.2018.04.099. [DOI] [PubMed] [Google Scholar]
- Makarov I. S.; Golova L. K.; Bondarenko G. N.; Anokhina T. S.; Dmitrieva E. S.; Levin I. S.; Makhatova V. E.; Galimova N. Z.; Shambilova G. K. Structure, morphology, and permeability of cellulose films. Membranes 2022, 12, 297 10.3390/membranes12030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neenu K.; Dominic C. M.; Begum P. S.; Parameswaranpillai J.; Kanoth B. P.; David D. A.; Sajadi S. M.; Dhanyasree P.; Ajithkumar T.; Badawi M. Effect of oxalic acid and sulphuric acid hydrolysis on the preparation and properties of pineapple pomace derived cellulose nanofibers and nanopapers. Int. J. Biol. Macromol. 2022, 209, 1745 10.1016/j.ijbiomac.2022.04.138. [DOI] [PubMed] [Google Scholar]
- Bagde P.; Nadanathangam V. Mechanical, antibacterial and biodegradable properties of starch film containing bacteriocin immobilized crystalline nanocellulose. Carbohydr. Polym. 2019, 222, 115021 10.1016/j.carbpol.2019.115021. [DOI] [PubMed] [Google Scholar]
- Li W.; Shen Y.; Liu H.; Huang X.; Xu B.; Zhong C.; Jia S. Bioconversion of lignocellulosic biomass into bacterial nanocellulose: Challenges and perspectives. Green Chem. Eng. 2022, (In press) 10.1016/j.gce.2022.04.007. [DOI] [Google Scholar]
- Kian L.; Saba N.; Jawaid M.; Sultan M. A review on processing techniques of bast fibers nanocellulose and its polylactic acid (PLA) nanocomposites. Int. J. Biol. Macromol. 2019, 121, 1314–1328. 10.1016/j.ijbiomac.2018.09.040. [DOI] [PubMed] [Google Scholar]
- Osorio M.; Fernández-Morales P.; Gañán P.; Zuluaga R.; Kerguelen H.; Ortiz I.; Castro C. Development of novel three-dimensional scaffolds based on bacterial nanocellulose for tissue engineering and regenerative medicine: Effect of processing methods, pore size, and surface area. J. Biomed. Mater. Res., Part A 2019, 107, 348–359. 10.1002/jbm.a.36532. [DOI] [PubMed] [Google Scholar]
- Amara C.; El Mahdi A.; Medimagh R.; Khwaldia K. Nanocellulose-based composites for packaging applications. Curr. Opin. Green Sustainable Chem. 2021, 31, 100512 10.1016/j.cogsc.2021.100512. [DOI] [Google Scholar]
- Daicho K.; Kobayashi K.; Fujisawa S.; Saito T. Crystallinity-independent yet modification-dependent true density of nanocellulose. Biomacromolecules 2020, 21, 939–945. 10.1021/acs.biomac.9b01584. [DOI] [PubMed] [Google Scholar]
- Kargarzadeh H.; Huang J.; Lin N.; Ahmad I.; Mariano M.; Dufresne A.; Thomas S.; Gałęski A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. 10.1016/j.progpolymsci.2018.07.008. [DOI] [Google Scholar]
- Huang S.; Zhao Z.; Feng C.; Mayes E.; Yang J. Nanocellulose reinforced P (AAm-co-AAc) hydrogels with improved mechanical properties and biocompatibility. Composites, Part A 2018, 112, 395–404. 10.1016/j.compositesa.2018.06.028. [DOI] [Google Scholar]
- Ahankari S. S.; Subhedar A. R.; Bhadauria S. S.; Dufresne A. Nanocellulose in food packaging: A review. Carbohydr. Polym. 2021, 255, 117479 10.1016/j.carbpol.2020.117479. [DOI] [PubMed] [Google Scholar]
- Cao L.; Huang J.; Fan J.; Gong Z.; Xu C.; Chen Y. Nanocellulose-A Sustainable and Efficient Nanofiller for Rubber Nanocomposites: From Reinforcement to Smart Soft Materials. Polym. Rev. 2021, 549. 10.1080/15583724.2021.2001004. [DOI] [Google Scholar]
- Hachaichi A.; Kouini B.; Kian L. K.; Asim M.; Fouad H.; Jawaid M.; Sain M. Nanocrystalline cellulose from microcrystalline cellulose of date palm fibers as a promising candidate for bio-nanocomposites: isolation and characterization. Materials 2021, 14, 5313 10.3390/ma14185313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghav N.; Sharma M. R.; Kennedy J. F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031 10.1016/j.carpta.2020.100031. [DOI] [Google Scholar]
- Kelly J. A.; Shukaliak A. M.; Cheung C. C.; Shopsowitz K. E.; Hamad W. Y.; MacLachlan M. J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angew. Chem., Int. Ed. 2013, 52, 8912–8916. 10.1002/anie.201302687. [DOI] [PubMed] [Google Scholar]
- Capadona J. R.; Tyler D. J.; Zorman C. A.; Rowan S. J.; Weder C. Mechanically adaptive nanocomposites for neural interfacing. MRS Bull. 2012, 37, 581–589. 10.1557/mrs.2012.97. [DOI] [Google Scholar]
- Bagis F. H.; Setiadi Nanocellulose filament fabrication from Sugarcane Bagasse through wet spinning method. AIP Conf. Proc. 2020, 040005 10.1063/5.0014780. [DOI] [Google Scholar]
- Nemoto J.; Saito T.; Isogai A. Simple freeze-drying procedure for producing nanocellulose aerogel-containing, high-performance air filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. 10.1021/acsami.5b05841. [DOI] [PubMed] [Google Scholar]
- Qin Y.; Mo J.; Liu Y.; Zhang S.; Wang J.; Fu Q.; Wang S.; Nie S. Stretchable Triboelectric Self-Powered Sweat Sensor Fabricated from Self-Healing Nanocellulose Hydrogels. Adv. Funct. Mater. 2022, 2201846 10.1002/adfm.202201846. [DOI] [Google Scholar]
- Bayer T.; Cunning B. V.; Selyanchyn R.; Nishihara M.; Fujikawa S.; Sasaki K.; Lyth S. M. High temperature proton conduction in nanocellulose membranes: paper fuel cells. Chem. Mater. 2016, 28, 4805–4814. 10.1021/acs.chemmater.6b01990. [DOI] [Google Scholar]
- Li V. C.-F.; Dunn C. K.; Zhang Z.; Deng Y.; Qi H. J. Direct ink write (DIW) 3D printed cellulose nanocrystal aerogel structures. Sci. Rep. 2017, 7, 8018 10.1038/s41598-017-07771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moharrami P.; Motamedi E. Application of cellulose nanocrystals prepared from agricultural wastes for synthesis of starch-based hydrogel nanocomposites: Efficient and selective nanoadsorbent for removal of cationic dyes from water. Bioresour. Technol. 2020, 313, 123661 10.1016/j.biortech.2020.123661. [DOI] [PubMed] [Google Scholar]
- Li S.; Chen G. Agricultural waste-derived superabsorbent hydrogels: Preparation, performance, and socioeconomic impacts. J. Cleaner Prod. 2020, 251, 119669 10.1016/j.jclepro.2019.119669. [DOI] [Google Scholar]
- Sun C.; Ni J.; Zhao C.; Du J.; Zhou C.; Wang S.; Xu C. Preparation of a cellulosic adsorbent by functionalization with pyridone diacid for removal of Pb (II) and Co (II) from aqueous solutions. Cellulose 2017, 24, 5615–5624. 10.1007/s10570-017-1519-z. [DOI] [Google Scholar]
- Hamed S. A. A. K. M.; Hassan M. L. A new mixture of hydroxypropyl cellulose and nanocellulose for wood consolidation. J. Cult. Heritage 2019, 35, 140–144. 10.1016/j.culher.2018.07.001. [DOI] [Google Scholar]
- Liu Y.; Fu Q.; Mo J.; Lu Y.; Cai C.; Luo B.; Nie S. Chemically tailored molecular surface modification of cellulose nanofibrils for manipulating the charge density of triboelectric nanogenerators. Nano Energy 2021, 89, 106369 10.1016/j.nanoen.2021.106369. [DOI] [Google Scholar]
- Raza M.; Abu-Jdayil B. Cellulose nanocrystals from lignocellulosic feedstock: a review of production technology and surface chemistry modification. Cellulose 2022, 29, 685–722. 10.1007/s10570-021-04371-y. [DOI] [Google Scholar]
- Xiong C.; Li B.; Duan C.; Dai L.; Nie S.; Qin C.; Xu Y.; Ni Y. Carbonized wood cell chamber-reduced graphene oxide@ PVA flexible conductive material for supercapacitor, strain sensing and moisture-electric generation applications. Chem. Eng. J. 2021, 418, 129518 10.1016/j.cej.2021.129518. [DOI] [Google Scholar]
- FAO Food and Agriculture Organization of the United Nations (FAO). https://www.fao.org/home/en.
- AlMahmoud T.; Elhanan M.; Abu-Zidan F. M. Eye injuries caused by date palm thorns and leaves. Saudi J. Ophthalmol. 2020, 34, 13. 10.4103/1319-4534.301296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanny S.; Ibrahim H.; Darwish L.; Farag M.; El-Habbak A.-H. M.; El-Kashif E.. Effect of Environmental Conditions on Date Palm Fiber Composites. Date Palm Fiber Composites; Springer, 2020; pp 287–320. [Google Scholar]
- Galiwango E.; Rahman N. S. A.; Al-Marzouqi A. H.; Abu-Omar M. M.; Khaleel A. A. Isolation and characterization of cellulose and α-cellulose from date palm biomass waste. Heliyon 2019, 5, e02937 10.1016/j.heliyon.2019.e02937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza M.; Abu-Jdayil B.; Al-Marzouqi A. H.; Inayat A. Kinetic and thermodynamic analyses of date palm surface fibers pyrolysis using Coats-Redfern method. Renewable Energy 2022, 183, 67–77. 10.1016/j.renene.2021.10.065. [DOI] [Google Scholar]
- Othman I.; Pal P.; Abu Haija M.; Hassan S. W.; Abu-Jdayil B.; AlKhateeb B.; Banat F. Extraction of crystalline nanocellulose from palm tree date seeds (Phoenix dactylifera L.). Chem. Eng. Commun. 2021, 1–13. 10.1080/00986445.2021.2001458. [DOI] [Google Scholar]
- Alothman O. Y.; Kian L. K.; Saba N.; Jawaid M.; Khiari R. Cellulose nanocrystal extracted from date palm fibre: Morphological, structural and thermal properties. Ind. Crops Prod. 2021, 159, 113075 10.1016/j.indcrop.2020.113075. [DOI] [Google Scholar]
- Alhamzani A. G.; Habib M. A. preparation of cellulose nanocrystals from date palm tree leaflets (phoenix dactylifera l.) Via repeated chemical treatments. Cellul. Chem. Technol. 2021, 55, 33–39. 10.35812/CelluloseChemTechnol.2021.55.04. [DOI] [Google Scholar]
- Shaikh H. M.; Anis A.; Poulose A. M.; Al-Zahrani S. M.; Madhar N. A.; Alhamidi A.; Alam M. A. Isolation and characterization of alpha and nanocrystalline cellulose from date palm (Phoenix dactylifera L.) trunk mesh. Polymers 2021, 13, 1893 10.3390/polym13111893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro K. B.; Migliorini F. L.; Facure M. H.; Correa D. S. Conductive electrospun nanofibers containing cellulose nanowhiskers and reduced graphene oxide for the electrochemical detection of mercury (II). Carbohydr. Polym. 2019, 207, 747–754. 10.1016/j.carbpol.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Oun A. A.; Rhim J.-W. Preparation of multifunctional chitin nanowhiskers/ZnO-Ag NPs and their effect on the properties of carboxymethyl cellulose-based nanocomposite film. Carbohydr. Polym. 2017, 169, 467–479. 10.1016/j.carbpol.2017.04.042. [DOI] [PubMed] [Google Scholar]
- Chen X.; Chen Y.; Yang H.; Wang X.; Che Q.; Chen W.; Chen H. Catalytic fast pyrolysis of biomass: selective deoxygenation to balance the quality and yield of bio-oil. Bioresour. Technol. 2019, 273, 153–158. 10.1016/j.biortech.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Kian L.; Saba N.; Jawaid M.; Alothman O.; Fouad H. Properties and characteristics of nanocrystalline cellulose isolated from olive fiber. Carbohydr. Polym. 2020, 241, 116423 10.1016/j.carbpol.2020.116423. [DOI] [PubMed] [Google Scholar]
- Abdul Rahman N. H.; Chieng B. W.; Ibrahim N. A.; Abdul Rahman N. Extraction and characterization of cellulose nanocrystals from tea leaf waste fibers. Polymers 2017, 9, 588 10.3390/polym9110588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Zhao X.; Zhu J. Kinetics of strong acid hydrolysis of a bleached kraft pulp for producing cellulose nanocrystals (CNCs). Ind. Eng. Chem. Res. 2014, 53, 11007–11014. 10.1021/ie501672m. [DOI] [Google Scholar]
- Ahmed-Haras M. R.; Kao N.; Ward L. Single-step heterogeneous catalysis production of highly monodisperse spherical nanocrystalline cellulose. Int. J. Biol. Macromol. 2020, 154, 246–255. 10.1016/j.ijbiomac.2020.02.298. [DOI] [PubMed] [Google Scholar]
- Gayathri K.; Rajesh K.; Krishnan P.; Anandan K.; Swadhi R.; Devaraj A. R.; Anbalagan G. A study on kinetic properties of brucinium hydrogen (s) malate pentahydrate single crystal by Coats Redfern method. AIP Conf. Proc. 2020, 030425 10.1063/5.0017481. [DOI] [Google Scholar]
- Sait H. H.; Hussain A.; Salema A. A.; Ani F. N. Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour. Technol. 2012, 118, 382–389. 10.1016/j.biortech.2012.04.081. [DOI] [PubMed] [Google Scholar]
- Elmay Y.; Jeguirim M.; Trouvé G.; Said R. Kinetic analysis of thermal decomposition of date palm residues using Coats–Redfern method. Energy Sources, Part A 2016, 38, 1117–1124. 10.1080/15567036.2013.821547. [DOI] [Google Scholar]
- Naqvi S. R.; Uemura Y.; Osman N.; Yusup S. Kinetic study of the catalytic pyrolysis of paddy husk by use of thermogravimetric data and the Coats–Redfern model. Res. Chem. Intermed. 2015, 41, 9743–9755. 10.1007/s11164-015-1962-0. [DOI] [Google Scholar]
- Raza M.; Abu-Jdayil B.; Al-Marzouqi A. H.; Inayat A. Kinetic and thermodynamic analyses of date palm surface fibers pyrolysis using Coats-Redfern method. Renewable Energy 2021, 67. 10.1016/j.renene.2021.10.065. [DOI] [Google Scholar]
- Wise L. E. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Pap. Trade J. 1946, 122, 35–43. [Google Scholar]
- Hastuti N.; Kanomata K.; Kitaoka T. Hydrochloric acid hydrolysis of pulps from oil palm empty fruit bunches to produce cellulose nanocrystals. J. Polym. Environ. 2018, 26, 3698–3709. 10.1007/s10924-018-1248-x. [DOI] [Google Scholar]
- Zianor Azrina Z.; Beg M. D. H.; Rosli M.; Ramli R.; Junadi N.; Alam A. M. Spherical nanocrystalline cellulose (NCC) from oil palm empty fruit bunch pulp via ultrasound assisted hydrolysis. Carbohydr. Polym. 2017, 162, 115–120. 10.1016/j.carbpol.2017.01.035. [DOI] [PubMed] [Google Scholar]
- Mandal A.; Chakrabarty D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. 10.1016/j.carbpol.2011.06.030. [DOI] [Google Scholar]
- Wulandari W.; Rochliadi A.; Arcana I. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser.: Mater. Sci. Eng. 2016, 012045 10.1088/1757-899X/107/1/012045. [DOI] [Google Scholar]
- Zhao C.; Jiang E.; Chen A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J. Energy Inst. 2017, 90, 902–913. 10.1016/j.joei.2016.08.004. [DOI] [Google Scholar]
- Ilyas R.; Sapuan S.; Ishak M. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. 10.1016/j.carbpol.2017.11.045. [DOI] [PubMed] [Google Scholar]
- Stefanidis S. D.; Kalogiannis K. G.; Iliopoulou E. F.; Michailof C. M.; Pilavachi P. A.; Lappas A. A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. 10.1016/j.jaap.2013.10.013. [DOI] [Google Scholar]
- Huang S.; Zhou L.; Li M.-C.; Wu Q.; Zhou D. Cellulose nanocrystals (CNCs) from corn stalk: Activation energy analysis. Materials 2017, 10, 80 10.3390/ma10010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti S.; Jayaramudu J.; Das K.; Reddy S. M.; Sadiku R.; Ray S. S.; Liu D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567. 10.1016/j.carbpol.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Henrique M. A.; Neto W. P. F.; Silvério H. A.; Martins D. F.; Gurgel L. V. A.; da Silva Barud H.; de Morais L. C.; Pasquini D. Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crops Prod. 2015, 76, 128–140. 10.1016/j.indcrop.2015.06.048. [DOI] [Google Scholar]
- Morgado D. L.; Frollini E. Thermal decomposition of mercerized linter cellulose and its acetates obtained from a homogeneous reaction. Polímeros 2011, 21, 111–117. 10.1590/S0104-14282011005000025. [DOI] [Google Scholar]
- Flauzino Neto W. P.; Silvério H. A.; Dantas N. O.; Pasquini D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue–Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. 10.1016/j.indcrop.2012.06.041. [DOI] [Google Scholar]
- Bhatnagar A.; Sain M. Processing of cellulose nanofiber-reinforced composites. J. Reinf. Plast. Compos. 2005, 24, 1259–1268. 10.1177/0731684405049864. [DOI] [Google Scholar]
- Abu-Thabit N. Y.; Judeh A. A.; Hakeem A. S.; Ul-Hamid A.; Umar Y.; Ahmad A. Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2020, 155, 730–739. 10.1016/j.ijbiomac.2020.03.255. [DOI] [PubMed] [Google Scholar]
- Sung S. H.; Chang Y.; Han J. Development of polylactic acid nanocomposite films reinforced with cellulose nanocrystals derived from coffee silverskin. Carbohydr. Polym. 2017, 169, 495–503. 10.1016/j.carbpol.2017.04.037. [DOI] [PubMed] [Google Scholar]
- Mohamed M. A.; Salleh W.; Jaafar J.; Ismail A.; Abd Mutalib M.; Mohamad A. B.; Zain M.; Awang N. A.; Hir Z. A. M. Physicochemical characterization of cellulose nanocrystal and nanoporous self-assembled CNC membrane derived from Ceiba pentandra. Carbohydr. Polym. 2017, 157, 1892–1902. 10.1016/j.carbpol.2016.11.078. [DOI] [PubMed] [Google Scholar]
- Lu P.; Hsieh Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. 10.1016/j.carbpol.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Macías-Almazán A.; Lois-Correa J.; Domínguez-Crespo M.; López-Oyama A.; Torres-Huerta A.; Brachetti-Sibaja S.; Rodríguez-Salazar A. Influence of operating conditions on proton conductivity of nanocellulose films using two agroindustrial wastes: Sugarcane bagasse and pinewood sawdust. Carbohydr. Polym. 2020, 238, 116171 10.1016/j.carbpol.2020.116171. [DOI] [PubMed] [Google Scholar]
- Zheng D.; Zhang Y.; Guo Y.; Yue J. Isolation and characterization of nanocellulose with a novel shape from walnut (Juglans regia L.) shell agricultural waste. Polymers 2019, 11, 1130 10.3390/polym11071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Chen Y.; Wang S.; Ma L.; Yu Y.; Dai H.; Zhang Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180 10.1016/j.carbpol.2020.116180. [DOI] [PubMed] [Google Scholar]
- Lamaming J.; Hashim R.; Leh C. P.; Sulaiman O.; Sugimoto T.; Nasir M. Isolation and characterization of cellulose nanocrystals from parenchyma and vascular bundle of oil palm trunk (Elaeis guineensis). Carbohydr. Polym. 2015, 134, 534–540. 10.1016/j.carbpol.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Adel A. M.; Abd El-Wahab Z. H.; Ibrahim A. A.; Al–Shemy M. T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour. Technol. 2010, 101, 4446–4455. 10.1016/j.biortech.2010.01.047. [DOI] [PubMed] [Google Scholar]
- Matuana L. M.; Balatinecz J.; Sodhi R.; Park C. Surface characterization of esterified cellulosic fibers by XPS and FTIR spectroscopy. Wood Sci. Technol. 2001, 35, 191–201. 10.1007/s002260100097. [DOI] [Google Scholar]
- Segal L.; Creely J. J.; Martin A. Jr.; Conrad C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. 10.1177/004051755902901003. [DOI] [Google Scholar]
- Kalita E.; Nath B.; Agan F.; More V.; Deb P. Isolation and characterization of crystalline, autofluorescent, cellulose nanocrystals from saw dust wastes. Ind. Crops Prod. 2015, 65, 550–555. 10.1016/j.indcrop.2014.10.004. [DOI] [Google Scholar]
- Johar N.; Ahmad I.; Dufresne A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. 10.1016/j.indcrop.2011.12.016. [DOI] [Google Scholar]
- Himmelsbach D. S.; Khalili S.; Akin D. E. The use of FT-IR microspectroscopic mapping to study the effects of enzymatic retting of flax (Linum usitatissimum L) stems. J. Sci. Food Agric. 2002, 82, 685–696. 10.1002/jsfa.1090. [DOI] [Google Scholar]
- Bykov I.Characterization of Natural and Technical Lignins Using FTIR Spectroscopy. Thesis, Luleå University of Technology, 2008. http://www.diva-portal.org/smash/get/diva2:1016107/FULLTEXT01.pdf. [Google Scholar]
- Adel A. M.; Abd El-Wahab Z. H.; Ibrahim A. A.; Al-Shemy M. T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part II: Physicochemical properties. Carbohydr. Polym. 2011, 83, 676–687. 10.1016/j.carbpol.2010.08.039. [DOI] [Google Scholar]
- Alemdar A.; Sain M. Isolation and characterization of nanofibers from agricultural residues–Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. 10.1016/j.biortech.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Mohamad Haafiz M.; Eichhorn S.; Hassan A.; Jawaid M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. 10.1016/j.carbpol.2013.01.035. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Kim H.-J. Fourier transform infrared spectroscopy (FT-IR) and simple algorithm analysis for rapid and non-destructive assessment of developmental cotton fibers. Sensors 2017, 17, 1469 10.3390/s17071469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Yao Z.; Zhou J.; He M.; Jiang Q.; Li S.; Ma Y.; Liu M.; Luo S. Isolation and characterization of cellulose nanocrystals from pueraria root residue. Int. J. Biol. Macromol. 2019, 129, 1081–1089. 10.1016/j.ijbiomac.2018.07.055. [DOI] [PubMed] [Google Scholar]
- Oun A. A.; Rhim J.-W. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2015, 127, 101–109. 10.1016/j.carbpol.2015.03.073. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Oberdörster G.; Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. 10.1007/s11051-008-9446-4. [DOI] [Google Scholar]
- Mehta M.; Shah J.; Khakhkhar T.; Shah R.; Hemavathi K. Anticonvulsant hypersensitivity syndrome associated with carbamazepine administration: case series. J. Pharmacol. Pharmacother. 2014, 5, 59. 10.4103/0976-500X.124428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJ N.; Balaji A.; Ramanujam N. Isolation and characterization of cellulose nanocrystals from Saharan aloe vera cactus fibers. Int. J. Polym. Anal. Charact. 2020, 25, 51–64. 10.1080/1023666X.2018.1478366. [DOI] [Google Scholar]
- Guo Y.; Zhang Y.; Zheng D.; Li M.; Yue J. Isolation and characterization of nanocellulose crystals via acid hydrolysis from agricultural waste-tea stalk. Int. J. Biol. Macromol. 2020, 163, 927–933. 10.1016/j.ijbiomac.2020.07.009. [DOI] [PubMed] [Google Scholar]
- Kunaver M.; Anžlovar A.; Žagar E. The fast and effective isolation of nanocellulose from selected cellulosic feedstocks. Carbohydr. Polym. 2016, 148, 251–258. 10.1016/j.carbpol.2016.04.076. [DOI] [PubMed] [Google Scholar]
- Karakehya N.; Bilgic C. Preparation of nanocrystalline cellulose from tomato stem and commercial microcrystalline cellulose: a comparison between two starting materials. Cellul. Chem. Technol. 2019, 53, 993–1000. 10.35812/CelluloseChemTechnol.2019.53.97. [DOI] [Google Scholar]
- Danaei M.; Dehghankhold M.; Ataei S.; Hasanzadeh Davarani F.; Javanmard R.; Dokhani A.; Khorasani S.; Mozafari M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira E. d. M.; Bondancia T. J.; Teodoro K. B. R.; Corrêa A. C.; Marconcini J. M.; Mattoso L. H. C. Sugarcane bagasse whiskers: extraction and characterizations. Ind. Crops Prod. 2011, 33, 63–66. 10.1016/j.indcrop.2010.08.009. [DOI] [Google Scholar]
- Aguayo M. G.; Fernández Pérez A.; Reyes G.; Oviedo C.; Gacitúa W.; Gonzalez R.; Uyarte O. Isolation and characterization of cellulose nanocrystals from rejected fibers originated in the kraft pulping process. Polymers 2018, 10, 1145 10.3390/polym10101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.; Jawaid M.; Kian L. K.; Khan A. A. P.; Asiri A. M. Isolation and production of nanocrystalline cellulose from conocarpus fiber. Polymers 2021, 13, 1835 10.3390/polym13111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. S.; Kao N.; Bhattacharya S. N.; Gupta R.; Choi H. J. Potential aspect of rice husk biomass in Australia for nanocrystalline cellulose production. Chin. J. Chem. Eng. 2018, 26, 465–476. 10.1016/j.cjche.2017.07.004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.











