Abstract
Background:
Ofatumumab is approved for the treatment of relapsing multiple sclerosis (RMS). Ongoing safety reporting is crucial to understand its long-term benefit–risk profile.
Objective:
Report the safety and tolerability of ofatumumab in RMS after extended treatment up to 3.5 years.
Methods:
Patients completing ASCLEPIOS I/II (phase 3), APLIOS, or APOLITOS (phase 2) trials could enter ALITHIOS, a phase 3b, open-label, long-term safety study. We analyzed cumulative data of continuous ofatumumab treatment and of patients newly switched from teriflunomide.
Results:
The safety population had 1969 patients: 1292 continuously treated with ofatumumab (median time-at-risk 35.5 months, 3253 patient-years) and 677 newly switched (median time-at-risk 18.3 months, 986 patient-years). A total of 1650 patients (83.8%) had ⩾1 adverse events and 191 (9.7%) had ⩾1 serious adverse events. No opportunistic infections or progressive multifocal leukoencephalopathy events were identified; the risk of malignancies was low. Mean serum immunoglobulin (Ig) G levels remained stable. Mean IgM levels decreased but remained above the lower limit of normal in most. Serious infection incidence was low; decreased Ig levels were not associated with serious infections.
Conclusion:
In patients with up to 3.5 years’ exposure, ofatumumab was well tolerated, with no new safety risks identified. These findings, with its established effectiveness, support a favorable benefit–risk profile of ofatumumab in RMS.
Keywords: Ofatumumab, multiple sclerosis, antibodies, monoclonal, relapsing multiple sclerosis, safety
Introduction
Ofatumumab, the first fully human anti-CD20 monoclonal antibody (mAb), 1 is a highly effective disease-modifying therapy (DMT) 2 for the treatment of relapsing multiple sclerosis (RMS).3,4 Fully human mAbs are derived from human gene sequences alone and are generally considered to have a low immunogenic potential. 5 Ofatumumab binds to two distinct, noncontinuous regions on a unique conformational epitope of the CD20 receptor, including the small and large extracellular loops, 6 resulting in a slower off-rate and greater binding affinity than other anti-CD20 mAbs.1,6- 9 Ofatumumab induces B-cell lysis primarily through pronounced complement-dependent cytotoxicity.6,8,9 Owing to its high potency, distinct mode of action,6,8,9 and subcutaneous delivery, ofatumumab is effective when administered at a low dose, 10 and can be self-administered after the initial dose has been given under medical supervision. 7
To produce rapid and sustained B-cell depletion while minimizing B-cell repletion between doses, ofatumumab is administered by subcutaneous injection in 20 mg initial loading doses at weeks 0, 1, and 2, then followed by 20 mg monthly (q4w) dosing starting at week 4. 10 The efficacy and tolerability profile of ofatumumab in RMS was investigated in the pivotal, randomized, double-blind, phase 3 ASCLEPIOS I (NCT02792218) and ASCLEPIOS II (NCT02792231) trials of up to 30 months’ duration 7 ; in the 12 week, randomized, phase 2 bioequivalence APLIOS (NCT03560739) trial 11 ; and in the 24 week, randomized, phase 2 APOLITOS trial, with an additional open-label extension phase (NCT03249714). 12 Ofatumumab is currently approved in many countries including European Union countries, the USA, Japan, the United Kingdom, and Australia.
Following participation in the core parts of ASCLEPIOS I/II, APLIOS, or the open-label extension part of APOLITOS, patients could enter ALITHIOS (NCT03650114), which is an open-label, phase 3b trial. 13 The aims of ALITHIOS are to assess the long-term safety of ofatumumab and to examine its safety, tolerability, and effectiveness in a population of patients with RMS.13,14 Here, we provide an update on the cumulative safety experience with extended treatment of ofatumumab following exposure for up to 3.5 years, with data from initiation of ofatumumab treatment in the core/extension studies.
Methods
Trial designs and patients
The ongoing, phase 3b, open-label, single-arm, ALITHIOS umbrella extension trial of ofatumumab 20 mg subcutaneously q4w in RMS includes patients who were randomized to ofatumumab in the ASCLEPIOS I/II and APLIOS trials and who continued ofatumumab treatment in ALITHIOS. In addition, ALITHIOS includes patients randomized to teriflunomide in ASCLEPIOS I/II who switched to ofatumumab in ALITHIOS. ALITHIOS also includes patients who were randomized to ofatumumab or placebo during the 24-week double-blind core part of APOLITOS and who subsequently received ofatumumab during the APOLITOS open-label extension part before entering ALITHIOS. The trial designs and eligibility criteria of ASCLEPIOS I/II, APLIOS, and APOLITOS are described elsewhere.7,11,12 The key eligibility criteria are described in Supplementary Appendix 1.
ALITHIOS started on 22 November 2018, and is expected to complete in 2029. 13 Patients will be treated with ofatumumab in ALITHIOS for a maximum period of 5 years from joining this trial. The present interim analysis provides up to 3.5 years of cumulative safety data, from initiation of ofatumumab treatment in the core/extension studies to the cut-off date of 29 January 2021. ALITHIOS trial oversight information is described in Supplementary Appendix 2.
Analysis populations
The safety analysis set consisted of all patients who received at least one dose of ofatumumab in the “overall safety population,” which comprised of “continuous ofatumumab group” and “newly switched ofatumumab group.” The continuous ofatumumab group includes all patients randomized to ofatumumab in ASCLEPIOS I/II (up to 30 months/120 weeks), APLIOS (12 weeks), and APOLITOS (up to 72 weeks; including patients who switched from placebo to ofatumumab in the APOLITOS open-label extension) and who then continued in ALITHIOS; the group also includes patients who completed/discontinued ofatumumab during one of the four trials and continued in the safety follow-up of their respective trials, but did not enter ALITHIOS. The safety follow-up period lasted at least 9 months after the last dose administration, and all safety data from the first dose of ofatumumab until up to 100 days after the last dose were considered to be treatment-emergent (i.e. this period was taken as a patient’s time at risk, as described in Supplementary Appendix 3). The newly switched ofatumumab group included patients who were randomized to teriflunomide in ASCLEPIOS I/II and switched to ofatumumab on entering ALITHIOS.
Safety and laboratory assessments
Treatment-emergent adverse event (AE) data were collected for the safety analysis set to determine the number of patients who experienced an AE (including grade and severity), with a particular focus on injection-related reactions (IRRs), infections, and malignancies. Safety data were summarized for all patients in the continuous ofatumumab group, the newly switched ofatumumab group, and the overall safety population. Immunoglobulin G (IgG) and immunoglobulin M (IgM) levels were assessed at baseline, week 4, week 12, and every 12 weeks thereafter in the ASCEPLIOS studies; in the ALITHIOS study, data were collected every 12 weeks up to week 48, and every 24 weeks thereafter until the end of the study; data were analyzed up to the cut-off date of 29 January 2021. The pregnancy outcomes in women inadvertently exposed to ofatumumab were also recorded up to a cut-off date of 21 April 2021.
Statistical analyses
Occurrences of AEs were summarized as binary endpoints and presented as the percentage of patients with at least one AE, AEs of grade 3 or 4 (combined) severity, AEs leading to ofatumumab discontinuation, serious AEs (SAEs), AEs occurring in at least 5% of patients in the overall safety population, IRRs, infections, malignancies, and pregnancies. Exposure-adjusted incidence rates (EAIRs) per 100 patient-years and 95% confidence intervals (CIs) were estimated for AEs, infections, serious infections, and malignancies overall and by year (Supplementary Appendices 4 and 5) and for the entire analysis period using a Poisson model. Cumulative incidence function (CIF) estimates were reported yearly to adjust for competing risks (treatment discontinuation) and variable follow-up duration. The percentage of patients with IRRs associated with each of the first five doses of ofatumumab was summarized for the overall, continuously treated, and newly switched groups.
Absolute serum IgG and IgM levels and change (absolute and percentage) from baseline in IgG and IgM levels were summarized over time for patients in the continuous ofatumumab group and in the newly switched group. The percentages of patients who had IgG or IgM levels below the lower limit of normal (LLN) at least once post-baseline were also summarized (Supplementary Appendix 6). Absolute IgG and IgM values, absolute change from baseline, and percentage change from baseline in IgG and IgM levels were analyzed over time by subgroups of baseline quartiles through week 168 in the continuous ofatumumab group. Serious infections occurring within 1 month prior to and until 1 month after low IgG/IgM levels below the LLN were also assessed, as were events of low IgG or IgM levels leading to treatment interruption or discontinuations.
Results
Patient demographics
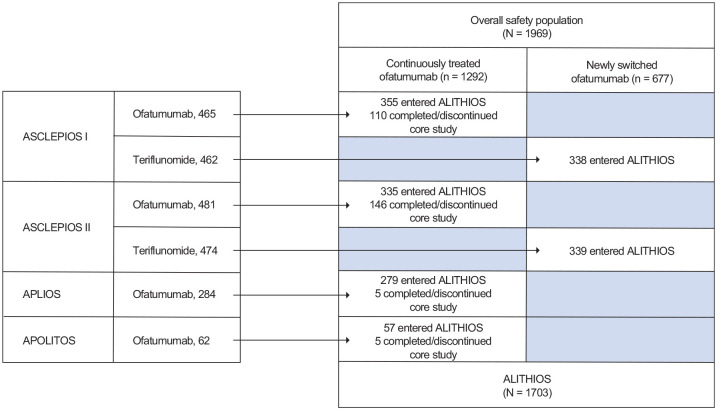
The overall safety population included 1969 patients, of whom 1292 were in the continuous ofatumumab group and 677 in the newly switched ofatumumab group. Baseline patient demographics and disease characteristics have been reported for the phase 3 ASCLEPIOS I/II trials 7 and the phase 2 APLIOS and APOLITOS trials.11,12 Baseline demographics for the patient groups described in this analysis are summarized in Table 1. In total, 86.5% of patients (n = 1703) who completed the ASCLEPIOS I/II, APLIOS, and APOLITOS trials entered ALITHIOS (Figure 1). Mean (standard deviation (SD)) age of patients in the overall safety population was 38.7 (9.2) years, and time since diagnosis was 6.4 (6.2) years.
Table 1.
Demographics and disease characteristics (safety analysis set).
| Overall safety population (N = 1969) | Continuous ofatumumab group (n = 1292) | Newly switched ofatumumab group (n = 677) | |
|---|---|---|---|
| Age, years, mean (SD) | 38.7 (9.2) | 38.0 (9.1) | 40.1 (9.2) |
| Female, no. (%) | 1345 (68.3) | 889 (68.8) | 456 (67.4) |
| Type of MS, no. (%) | |||
| Relapsing–remitting | 1869 (94.9) | 1223 (94.7) | 646 (95.4) |
| Secondary progressive | 100 (5.1) | 69 (5.3) | 31 (4.6) |
| Time since MS symptom onset (years), mean (SD) | 9.0 (7.3) | 8.5 (7.3) | 9.9 (7.2) |
| Time since MS diagnosis, years, mean (SD) | 6.4 (6.2) | 5.9 (6.3) | 7.3 (6.0) |
| EDSS score, mean (SD) | 2.9 (1.4) | 2.9 (1.3) | 2.8 (1.5) |
MS: multiple sclerosis; EDSS: Expanded Disability Status Scale; SD: standard deviation. The data in the table are baseline values corresponding to the last non-missing value before first administration of ofatumumab. For patients in the newly switched group, baseline refers to the time prior to the first administration of ofatumumab in the ALITHIOS extension study. The EDSS scores range from 0.0 to 10.0, with higher scores indicating worse disability.
Figure 1.
Schematic summary of the patient flow in the analysis groups.
APOLITOS had two phases: a core phase of 0–24 weeks and an extension phase of 24–48 weeks.
Exposure to ofatumumab, expressed as time at risk, is summarized in Table 2 and Supplementary Figure 1 for the overall safety population, continuous ofatumumab group, and newly switched ofatumumab group. Median (range) time at risk and total patient-years at risk were 21.0 (0.0–51.8) months and 4239 patient-years in the overall safety population, 35.5 (0.0–51.8) months and 3253 patient-years in the continuous ofatumumab group, and 18.3 (1.2–22.7) months and 986 patient-years in the newly switched ofatumumab group. Of the patients who entered ALITHIOS, 1569 (92.1%) were still receiving ofatumumab treatment at the time of data cut-off.
Table 2.
Time at risk with ofatumumab (safety analysis set).
| Overall safety population (N = 1969) | Continuous ofatumumab group (n = 1292) | Newly switched ofatumumab group (n = 677) | |
|---|---|---|---|
| Time at risk, n (%) | |||
| <48 weeks (1 year) | 131 (6.7) | 108 (8.4) | 23 (3.4) |
| 48–96 weeks (1–2 years) | 910 (46.2) | 257 (19.9) | 653 (96.5) |
| 96–144 weeks (2–3 years) | 265 (13.5) | 264 (20.4) | 1 (0.1) |
| 144–192 weeks (3–4 years) | 525 (26.7) | 525 (40.6) | 0 |
| >192 weeks (4 years) | 138 (7.0) | 138 (10.7) | 0 |
| Time at risk, months, median (range) | 21.0 (0.0–51.8) | 35.5 (0.0–51.8) | 18.3 (1.2–22.7) |
| Patient-years | 4239 | 3253 | 986 |
Time at risk defined as the time from first dose of ofatumumab until 100 days after last dose.
Summary of overall AEs
In total, 1650 patients (83.8%) in the overall safety population had at least one AE (continuous ofatumumab group, 1128 (87.3%); newly switched ofatumumab group, 522 (77.1%); Table 3). Most AEs were mild to moderate in severity, with 11.0% of grade 3/4 AEs in the continuous group and 5.3% in the newly switched group. In the continuous group, EAIRs for total AEs were higher in the first year and approximately constant through years 2, 3, and 4 (Supplementary Table 1). The nature and frequency of the most common AEs were comparable with those reported in ASCLEPIOS I/II, 7 and no new safety signals were identified (Table 3).
Table 3.
Summary of AEs with ofatumumab (safety analysis set).
| Patients with ⩾1 event, n (%) (EAIR (95% CI)) | Overall safety population (N = 1969) | Continuous ofatumumab group (n = 1292) | Newly switched ofatumumab group (n = 677) |
|---|---|---|---|
| Patients with at least one AE | 1650 (83.8) (148.7 (141.7–156.1)) | 1128 (87.3) (153.4 (144.7–162.6)) | 522 (77.1) (139.6 (128.1–152.1)) |
| Grade 3/4 AEs | 178 (9.0) | 142 (11.0) | 36 (5.3) |
| Serious AEs | 191 (9.7) (4.8 (4.1–5.5)) | 155 (12.0) (5.1 (4.4–6.0)) | 36 (5.3) (3.7 (2.7–5.2)) |
| AEs leading to ofatumumab discontinuation | 115 (5.8) | 89 (6.9) | 26 (3.8) |
| AEs occurring in ⩾5% of the overall group | |||
| IRRs | |||
| Injection-related systemic reaction | 489 (24.8) (15.0 (13.7–16.4)) | 336 (26.0) (13.5 (12.1–15.0)) | 153 (22.6) (19.8 (16.9–23.2)) |
| Injection-site reaction | 227 (11.5) (5.9 (5.2–6.8)) | 171 (13.2) (5.9 (5.1–6.9)) | 56 (8.3) (6.1 (4.7–7.9)) |
| Infections | |||
| All infections reported as AEs | 1070 (54.3) (44.1 (41.5–46.8)) | 761 (58.9) (43.8 (40.8–47.0)) | 309 (45.6) (44.8 (40.1–50.1)) |
| Nasopharyngitis | 331 (16.8) (9.1 (8.2–10.2)) | 257 (19.9) (9.4 (8.3–10.7)) | 74 (10.9) (8.2 (6.5–10.3)) |
| Upper respiratory tract infection | 203 (10.3) (5.2 (4.5–6.0)) | 148 (11.5) (5.0 (4.2–5.9)) | 55 (8.1) (5.9 (4.5–7.7)) |
| Urinary tract infection | 192 (9.8) (4.9 (4.2–5.6)) | 156 (12.1) (5.2 (4.5–6.1)) | 36 (5.3) (3.8 (2.7–5.2)) |
| COVID-19 a | 114 (5.8) (2.7 (2.3–3.3)) | 70 (5.4) (2.2 (1.7–2.7)) | 44 (6.5) (4.5 (3.4–6.1)) |
| Other AEs | |||
| Headache | 238 (12.1) (6.2 (5.5–7.1)) | 197 (15.2) (6.9 (6.0–7.9)) | 41 (6.1) (4.3 (3.2–5.9)) |
| IgM decreased | 215 (10.9) (5.3 (4.7–6.1)) | 153 (11.8) (4.9 (4.2–5.8)) | 62 (9.2) (6.6 (5.1–8.4)) |
| Back pain | 161 (8.2) (4.0 (3.5–4.7)) | 135 (10.4) (4.5 (3.8–5.3)) | 26 (3.8) (2.7 (1.8–4.0)) |
| Fatigue | 135 (6.9) (3.4 (2.8–4.0)) | 112 (8.7) (3.7 (3.1–4.4)) | 23 (3.4) (2.4 (1.6–3.6)) |
| Arthralgia | 133 (6.8) (3.3 (2.8–3.9)) | 102 (7.9) (3.3 (2.7–4.0)) | 31 (4.6) (3.2 (2.3–4.6)) |
| Diarrhea | 108 (5.5) (2.7 (2.2–3.2)) | 94 (7.3) (3.0 (2.5–3.7)) | 14 (2.1) (1.4 (0.9–2.4)) |
| Other AEs of interest occurring in <5% of the overall group | |||
| Malignancies occurring in more than one patient b | |||
| All malignancies | 11 (0.6) (0.3 (0.1–0.5)) | 8 (0.6) (0.3 (0.1–0.5)) | 3 (0.4) (0.3 (0.1–0.9)) |
| Basal cell carcinoma | 4 (0.2) (0.1 (0.0–0.3)) | 3 (0.2) (0.1 (0.0–0.3)) | 1 (0.1) (0.1 (0.0–0.7)) |
| Invasive breast carcinoma | 2 (0.1) (0.1 (0.0–0.2)) | 1 (0.1) (0.0 (0.0–0.2)) | 1 (0.1) (0.1 (0.0–0.7)) |
| SAEs of interest | |||
| Serious infections occurring in more than one patient | |||
| All serious infections | 58 (2.9) (1.4 (1.1–1.8)) | 48 (3.7) (1.5 (1.1–2.0)) | 10 (1.5) (1.0 (0.6–1.9)) |
| Appendicitis | 12 (0.6) (0.3 (0.2–0.5)) | 11 (0.9) (0.3 (0.2–0.6)) | 1 (0.1) (0.1 (0.0–0.7)) |
| Pneumonia | 9 (0.5) (0.2 (0.1–0.4)) | 7 (0.5) (0.2 (0.1–0.5)) | 2 (0.3) (0.2 (0.1–0.8)) |
| COVID-19 pneumonia a | 7 (0.4) (0.2 (0.1–0.4)) | 6 (0.5) (0.2 (0.1–0.4)) | 1 (0.1) (0.1 (0.0–0.7)) |
| Urinary tract infection | 6 (0.3) (0.1 (0.1–0.3)) | 4 (0.3) (0.1 (0.1–0.3)) | 2 (0.3) (0.2 (0.1–0.8)) |
| COVID-19 a | 4 (0.2) (0.1 (0.0–0.3)) | 3 (0.2) (0.1 (0.0–0.3)) | 1 (0.1) (0.1 (0.0–0.7)) |
| Gastroenteritis | 3 (0.2) (0.1 (0.0–0.2)) | 3 (0.2) (0.1 (0.0–0.3)) | 0 |
| Urosepsis | 3 (0.2) (0.1 (0.0–0.2)) | 3 (0.2) (0.1 (0.0–0.3)) | 0 |
| Influenza | 2 (0.1) (0.1 (0.0–0.2)) | 2 (0.2) (0.1 (0.0–0.3)) | 0 |
| Osteomyelitis | 2 (0.1) (0.1 (0.0–0.2)) | 1 (0.1) (0.0 (0.0–0.2)) | 1 (0.1) (0.1 (0.0–0.7)) |
AE: adverse event; CI: confidence interval; COVID-19: coronavirus disease 2019; EAIR: exposure-adjusted incidence rate per 100 patient-years; Ig: immunoglobulin; IRR: injection-related reaction; SAE: serious AE. AEs leading to trial drug discontinuation are based on the entries on the AE electronic case report form (eCRF) page with “action taken with trial treatment” selected as “Drug Withdrawn.”
Incidence of “COVID-19” refers to confirmed SARS-CoV-2 infections, and “COVID-19 pneumonia” refers to cases of COVID-19 pneumonia as reported by the investigator. Investigators may report an AE both as “COVID-19” and “COVID-19 pneumonia”.
Incidence of malignancies includes malignancies that occurred during the core trials (n = 5): two cases of basal cell carcinoma from ASCLEPIOS II and one case each of malignant melanoma in situ, recurrent non-Hodgkin’s lymphoma, and invasive breast carcinoma from ASCLEPIOS I.
In the overall safety population, 191 patients (9.7%) had at least one SAE (continuous ofatumumab group, 155 (12.0%); newly switched ofatumumab group, 36 (5.3%)); the corresponding EAIRs per 100 patient-years (95% CIs) in each group were 4.8 (4.1–5.5), 5.1 (4.4–6.0), and 3.7 (2.7–5.2), respectively (Table 3). In the continuous group, EAIRs did not substantially change year-by-year (Supplementary Table 1). The most common SAEs in the overall safety population were infections: appendicitis (n = 12; 0.6%), pneumonia (n = 9; 0.5%), COVID-19 pneumonia (n = 7; 0.4%), and urinary tract infection (n = 6; 0.3%).
Two deaths were reported, both in the continuous ofatumumab group, and neither were reported as related to ofatumumab: one patient had COVID-19 pneumonia and another patient committed suicide.
AEs leading to ofatumumab discontinuation occurred in 115 patients (5.8%) in the overall safety population, with 89 (6.9%) in the continuously treated group and 26 (3.8%) in the newly switched group. The most common AEs leading to discontinuation were reduced IgM levels (n = 53, 46.1%) and other reduced immunoglobulin levels (n = 11, 9.6%); the study protocol mandated ofatumumab treatment interruption based on notably low immunoglobulin values.
IRRs
IRRs included systemic (occurring within 24 hours after injection) and injection-site reactions. Systemic IRRs occurred in 24.8% of the overall safety population, 26.0% of the continuous ofatumumab group, and 22.6% of the newly switched ofatumumab group (Table 3). In the newly switched group, systemic IRRs were mild-to-moderate (all grade 1/2), none were serious, and they rarely led to withdrawal of ofatumumab (four patients discontinued treatment, two of which were after the first injection). Of the 123 patients who reported systemic IRRs after their first injection in the newly switched group, 61% used premedication for the first injection.. Systemic IRRs occurred mostly after the first injection of ofatumumab, with 18.2% of patients in the newly switched group experiencing systemic IRRs after the first dose of ofatumumab. This rate dropped markedly for subsequent injections (Figure 2 and Supplementary Table 2). These results are consistent with those reported in ASCLEPIOS I/II. 7
Figure 2.
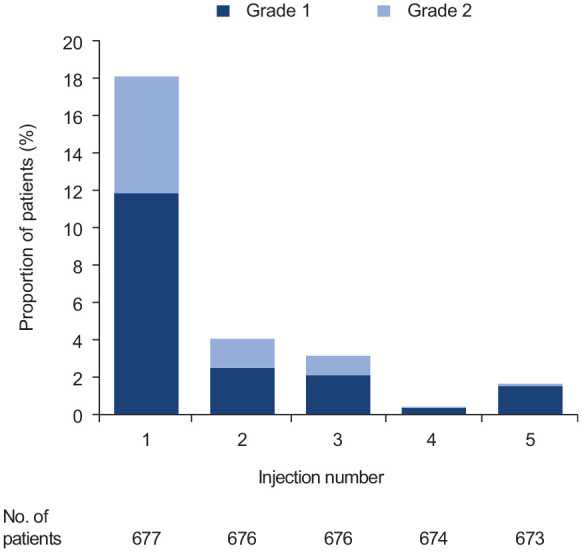
Injection-related systemic reactions by injection number in the newly switched group (first five injections).
Injection-site reactions occurred in 11.5% of the overall safety population, 13.2% of the continuous ofatumumab group, and 8.3% of the newly switched ofatumumab group (Table 3). In the newly switched group, reactions were all mild-to-moderate (all grade 1/2) and non-serious, with one patient discontinuing treatment owing to an injection-site reaction.
Infections
In the overall safety population, 1070 patients (54.3%) had infections reported, an incidence similar to the ofatumumab group in the ASCLEPIOS I/II studies (51.6%). 7 The EAIR per 100 patient-years (95% CIs) for infections was 44.1 (41.5–46.8) (Table 3). The most frequent infections were nasopharyngitis, upper respiratory tract infection, urinary tract infection, and COVID-19. There was no increase in the EAIR for infections with extended exposure to ofatumumab; the exception being upper respiratory tract infection, which became more frequent by year 3 potentially owing to the emergence of COVID-19 (Supplementary Table 1). Overall incidence of infections remained stable over time and did not increase (Supplementary Figures 2 and 3). There were no opportunistic infections, hepatitis B reactivation, or progressive multifocal leukoencephalopathy events identified. Serious infections were reported in 58 patients (2.9%) in the overall safety population (Table 3), a rate consistent with those observed in the ofatumumab groups of ASCLEPIOS I (2.6%) and ASCLEPIOS II (2.5%). 7 The EAIR per 100 patient-years (95% CIs) for serious infections was 1.4 (1.1–1.8; Table 3). The serious infections occurring in more than one patient in the overall safety population were appendicitis, pneumonia, COVID-19 pneumonia, and urinary tract infection. The EAIR (95% CI) for serious infections was 1.4 (1.1–1.8; Table 3) overall and did not increase with long-term exposure to ofatumumab (Supplementary Table 1).
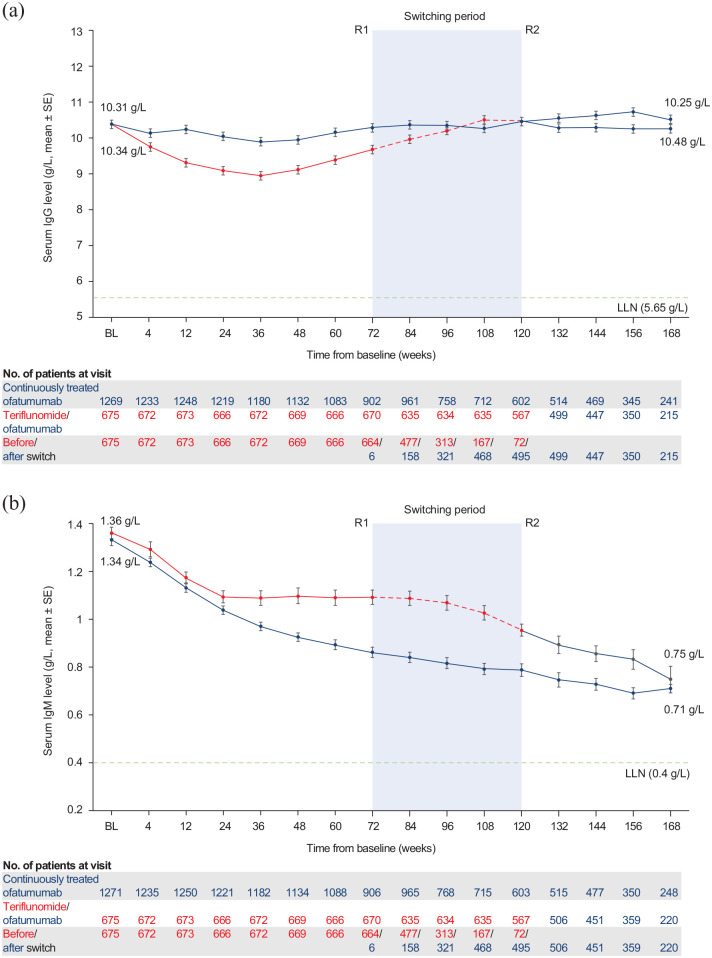
Figure 3.
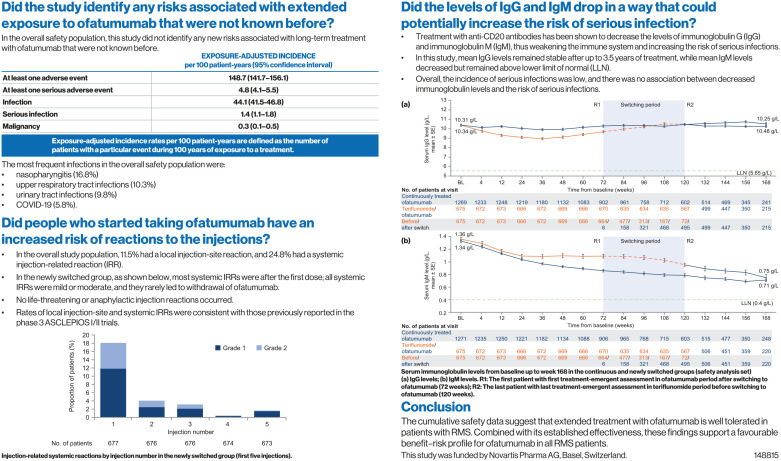
Serum immunoglobulin levels from baseline up to week 168 in the continuous and newly switched groups (safety analysis set): (a) IgG levels and (b) IgM levels.
R1: The first patient with first treatment-emergent assessment in ofatumumab period after switching to ofatumumab (72 weeks); R2: The last patient with last treatment-emergent assessment in teriflunomide period before switching to ofatumumab (120 weeks); BL: baseline; Ig: immunoglobin; LLN: lower limit of normal; SE: standard error.
COVID-19 infections
In the overall population, 139 patients (7.1%) had a COVID-19-related AE, including 115 confirmed (with positive laboratory confirmation as reported by the investigator) and 24 suspected COVID-19 cases. Of these confirmed and suspected COVID-19 cases, investigators also reported an AE of COVID-19 pneumonia in 13 patients, and 10 patients were hospitalized due to COVID-19. The majority of patients with a COVID-19-related AE recovered without sequelae (n = 128; 92.1%). One patient was hospitalized and died due to confirmed COVID-19 with COVID-19 pneumonia. No other patients discontinued treatment owing to COVID-19.
Malignancies
In total, malignancy AEs were reported in 11 patients (0.6%) in the overall safety population, with EAIRs (95% CIs) of 0.3 (0.1–0.5) (Table 3). In the overall population, malignancies reported by more than one patient were basal cell carcinoma (n = 4) and invasive breast carcinoma (n = 2). EAIRs for malignancies did not increase over time (Supplementary Figure 2). Details of malignancy type, time to onset, and causality in relation to treatment, as assessed by the investigator are given in Supplementary Table 3.
Pregnancy
Overall, there were four women in ASCLEPIOS I/II and 10 women in ALITHIOS who were exposed to ofatumumab during the first trimester of pregnancy. Of these pregnancies, six resulted in early termination, one resulted in early intrauterine fetal demise at approximately 8.5 weeks’ gestation, three resulted in live birth, and four pregnancies were ongoing at data cut-off. Among the three live births, all were full-term deliveries with no birth defects, congenital anomalies, hematological abnormalities, or serious infections in the newborns reported up to the cut-off date of 21 April 2021.
Immunoglobulin levels
Mean serum levels of IgG remained stable throughout the entire treatment period in both the continuously treated and newly switched groups (Figure 3) and, in the majority of patients, IgG levels remained above the LLN (5.65 g/L). In the continuously treated group, the percent change in IgG levels from baseline was +1.1% at week 168. Mean serum levels of IgM decreased in both groups (Figure 3), and in the majority of patients, IgM levels remained above the LLN (0.40 g/L). In the continuously treated group, the percent change in IgM levels from baseline was −47% at week 168, with the majority of the reduction occurring by week 48. In the overall safety population, 30 patients (1.5%) had IgG levels below LLN and 454 patients (23.1%) had IgM levels below the LLN at least once post-baseline (Table 4).
Table 4.
Low serum levels (below the LLN at least once) for IgG and IgM (safety analysis set).
| Overall safety population (N = 1969) | Continuous ofatumumab group (n = 1292) | Newly switched ofatumumab group (n = 677) | |
|---|---|---|---|
| Low IgG, n (%) | |||
| <5.65 g/L | 30 (1.5) | 22 (1.7) | 8 (1.2) |
| Low IgM, n (%) | |||
| <0.40 g/L | 454 (23.1) | 324 (25.1) | 130 (19.2) |
LLN for IgG = 5.65 g/L; LLN for IgM = 0.40 g/L. Ig: immunoglobulin; LLN: lower limit of normal.
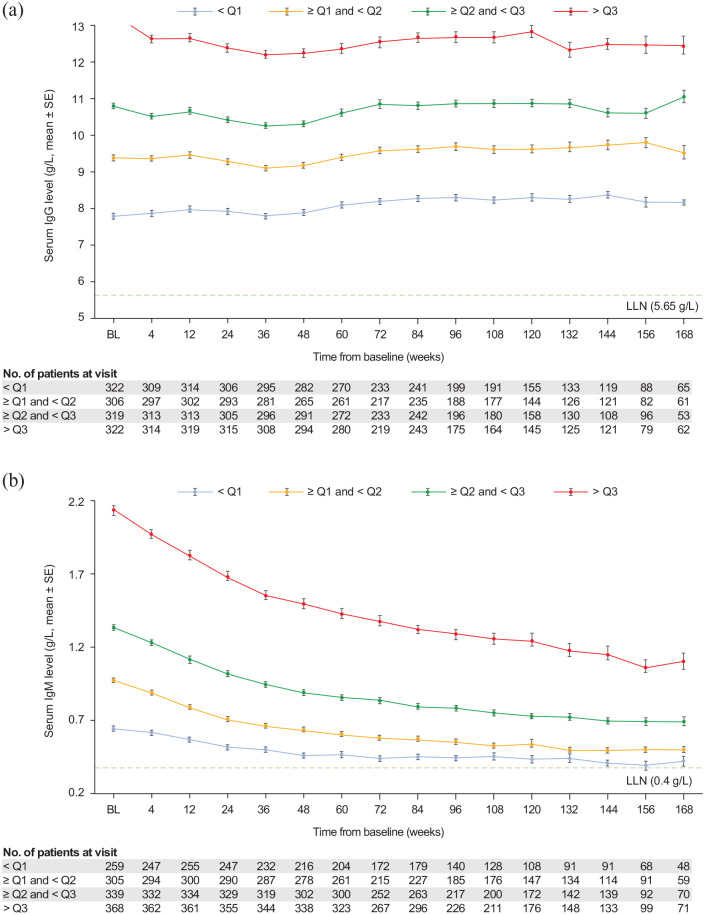
In the continuously treated group, mean (SD) IgG and IgM values were analyzed over time by baseline quartile of IgG and IgM levels, respectively, from baseline to week 168 (Figure 4). Mean IgG levels were relatively stable in each quartile from baseline to week 168. Mean IgM levels in each baseline quartile decreased over time but stayed above the LLN for all quartiles from baseline to week 168. Overall, 179 patients (9.1%) interrupted treatment per protocol because of notably low IgM levels (>10% below the LLN) that were reported as AEs, with 65 patients (3.3%) permanently discontinuing treatment (Supplementary Table 4). Treatment interruptions and permanent discontinuations due to low IgG levels occurred in two patients (0.1%) and four patients (0.2%), respectively (Supplementary Table 4).
Figure 4.
Serum immunoglobulin levels up to week 168 in the continuous ofatumumab group, by baseline quartile value (safety analysis set): (a) IgG levels and (b) IgM levels.
IgG quartiles (Q): Q1, <8.57 g/L; Q2, ⩾8.57 and <10.07 g/L; Q3, ⩾10.07 and <11.51 g/L; Q4, ⩾11.51 g/L. IgM quartiles (Q): Q1, <0.81 g/L; Q2, ⩾0.81 and <1.14 g/L; Q3, ⩾1.14 and <1.57 g/L; Q4, ⩾1.57 g/L. BL, baseline; Ig: immunoglobin; SE: standard error.
In general, the number of serious infections was low in ALITHIOS. In the overall population, the proportion (number) of patients with serious infection SAEs within 1 month before or after any decline in IgG was 3.3% (1/30) in patients who had IgG levels below the LLN (5.65 g/L) at any time post-baseline and 2.8% (55/1936) in those whose levels remained equal to or above the LLN. Corresponding values for IgM were 0.7% (3/454) in patients with IgM levels below the LLN (0.40 g/L) at any time post-baseline and 2.9% (44/1512) in patients whose IgM levels remained equal to or above the LLN (Table 5). One patient whose IgG levels were below the LLN had a serious infection SAE (pneumonia). Three patients whose IgM levels were below the LLN at any time had a serious infection SAE (herpes zoster; upper respiratory tract infection; urinary tract infection). Further information about these patients is provided in Supplementary Table 5.
Table 5.
Association of serious infections with IgG/IgM decrease in ofatumumab-treated patients (overall group).
| Overall |
IgM |
IgG |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 1969 | <LLN a (n = 454) | ⩾LLN (n = 1512) | <LLN (n = 30) | ⩾LLN (n = 1936) | ||||||
| n (%) | EAIR | n (%) | EAIR | n (%) | EAIR | n (%) | EAIR | n (%) | EAIR | |
| Patients with at least 1 serious infection | 58 (2.9) | 1.39 | 3 (0.7) | 0.80 | 44 (2.9) | 1.38 | 1 (3.3) | 7.02 | 55 (2.8) | 1.34 |
| Herpes zoster | 1 (0.1) | 0.02 | 1 (0.2) | 0.27 | 0 | 0 | 0 | 0 | 1 (0.1) | 0.02 |
| Upper respiratory tract infection | 1 (0.1) | 0.02 | 1 (0.2) | 0.27 | 0 | 0 | 0 | 0 | 1 (0.1) | 0.02 |
| Urinary tract infection | 6 (0.3) | 0.14 | 1 (0.2) | 0.27 | 3 (0.2) | 0.09 | 0 | 0 | 6 (0.3) | 0.14 |
| Pneumonia | 9 (0.5) | 0.21 | 0 | 0 | 8 (0.5) | 0.25 | 1 (3.3) | 7.02 | 8 (0.4) | 0.19 |
EAIR: exposure-adjusted incidence rate; Ig: immunoglobulin; LLN: lower limit of normal. For all pooled analyses, a fixed value of LLN (using ALITHIOS study reference) was used. LLN for IgG: 5.65 g/L; IgM: 0.4 g/L.
Patients with ⩾1 serious infection within 1 month prior to and until 1 month (defined as episodes) after any series of drop in IgG/IgM levels < LLN. Time at risk for this group of patients is defined as the sum of duration of such episodes until the onset of serious infection.
Discussion
This analysis provides a robust picture of extended safety data for ofatumumab, with a larger population size (approximately 2000 patients with RMS) exposed for a longer period (up to 3.5 years and 4238.5 patient-years) than those reported in previous studies.7,11,12 The proportions of patients with AEs, SAEs, grade 3/4 AEs, and AEs leading to discontinuation were consistent with those observed in the core ASCLEPIOS I/II trials,7,11,12 and EAIRs for AEs and SAEs did not increase over time. The risks of serious infections or malignancies, few of which were previously reported, 7 did not increase with additional ofatumumab exposure.
The incidence of systemic IRRs after first injection in patients who newly switched to ofatumumab in ALITHIOS (18.2%) was similar to that observed in those who initiated ofatumumab in the ASCLEPIOS trials (20.2%) and not substantially different from the rate after the first placebo injections in the teriflunomide arm in ASCLEPIOS (15.0%). 7 The low incidence of systemic IRRs with ofatumumab after the initial dose is possibly attributable to a limited recovery of circulating B-cells between doses with the approved dosing regimen. 15 All of the IRRs (site and systemic) in the newly switched group were non-serious and mild-to-moderate, and none required treatment discontinuation. When compared to other anti-CD20 therapies, ofatumumab was associated with fewer and less severe systemic IRRs.7,16,17 The rate of serious infections did not increase over time and was also similar to that observed in ASCLEPIOS. Overall, there were no opportunistic infections and no hepatitis B reactivation or progressive multifocal leukoencephalopathy events. In addition, B-cell repletion after treatment cessation is faster with ofatumumab (approximately 24 weeks after cessation) 18 than with other anti-CD20 therapies (72 weeks after cessation). 19
Low serum immunoglobulin levels, which can occur with anti-CD20 therapies, 20 - 24 have been linked with an apparent risk of infection. 25 - 27 In patients treated with ofatumumab, including the group that received continuous treatment of up to 3.5 years, mean IgG levels remained similar to baseline values. Mean IgM levels decreased over time; however, in the majority of patients, IgM levels remained above the LLN throughout. The overall incidence of serious infections was low, and no association was observed between decreased immunoglobulin levels and the risk of serious infections. These results were consistent with the 96-week phase 3 ASCLEPIOS I/II trial data. 6
The observed incidence of COVID-19 infections and related outcomes in ofatumumab-treated patients show no evidence of an increase in the incidence or severe outcomes of COVID-19 infections compared to the general population 28 and that the majority of COVID-19 infections in ofatumumab-treated patients are self-limited. Although reassuring, these observations are still preliminary and it would be premature to draw firm conclusions at this time about potential effects of ofatumumab treatment on the incidence or severity of COVID-19 infection.
The mean age of patients in this analysis was 38.7 years at baseline, and people living with MS up to the age of 55 at baseline were included in this study. It would be important to understand the benefit and safety profile of ofatumumab treatment in older patients or in those with greater degrees of disability, who may be more vulnerable to certain AEs.
In the small cohort analysis of 14 pregnant women exposed to ofatumumab, there were no birth defects, congenital anomalies, or serious infections reported to date. Future registry studies will provide additional data on maternal exposure to ofatumumab.
Compared with other injectable therapies for MS, monthly ofatumumab subcutaneous injections are less burdensome for patients 11 than the more frequent injections required with interferon-beta or glatiramer acetate. Ofatumumab was rarely associated with flu-like symptoms, which occur frequently with interferon-beta. These factors provide a plausible explanation for the high adherence observed with ofatumumab treatment (only 2/1882 (0.2%) of patients discontinued from the treatment epoch owing to non-compliance). 29 Another advantage of ofatumumab’s subcutaneous route of administration over DMTs that require intravenous infusion is the convenience of self-administration at home. Without the need for clinic time and infusion costs, there are also potential cost-effectiveness advantages associated with ofatumumab. 30
One limitation of these new analyses is the lack of a comparator cohort. However, this is a common limitation of long-term studies and does not undermine the utility of data reported specifically for long-term safety follow-up.
The safety profile with continued exposure and additional patients who switched to ofatumumab did not change from that reported during the phase 3 ASCLEPIOS I/II trials, 7 and no new safety signals were detected. The ALITHIOS trial is expected to continue until 2029, and post-marketing surveillance is ongoing to identify any new safety signals.
In conclusion, the cumulative safety data suggest that extended treatment with ofatumumab up to 3.5 years is well tolerated in patients with RMS, and, in conjunction with its established effectiveness, are supportive of a favorable benefit–risk profile of ofatumumab for patients with RMS, including patients with early MS.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-2-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-3-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-4-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-5-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-6-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-7-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-8-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-9-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-pdf-10-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-11-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Acknowledgments
The authors thank the patients for their participation in and commitment to the ASCEPLIOS I/II and ALITHIOS trials, and the clinical study team for the conduct of the study. The authors are grateful to Joana Osório and Akhil Bansal (Oxford PharmaGenesis Ltd, Oxford, UK) for providing medical writing support. These services were sponsored by Novartis Pharma AG.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.L.H. has received personal compensation from Accure, Alector, Annexon Biosciences, and Neurona Therapeutics; he has also received travel reimbursement from F. Hoffmann-La Roche and Novartis for CD20-related meetings and presentations. A.H.C. has consulted for Biogen, Celgene, EMD Serono, Genentech/Roche, Greenwich Biosciences, Janssen Pharmaceuticals, Novartis, and TG Therapeutics. L.K.’s institution (University Hospital Basel) received in the last 3 years and used exclusively for research support: steering committee, advisory board, and consultancy fees (Actelion, Bayer HealthCare, Biogen, BMS, Genzyme, Janssen, Japan Tobacco, Merck, Novartis, Roche, Sanofi, Santhera, Shionogi, and TG Therapeutics); speaker fees (Bayer HealthCare, Biogen, Merck, Novartis, Roche, and Sanofi); support of educational activities (Allergan, Bayer HealthCare, Biogen, CSL Behring, Desitin, Genzyme, Merck, Novartis, Roche, Pfizer, Sanofi, Shire, and Teva); license fees for Neurostatus products; and grants (Bayer HealthCare, Biogen, European Union, InnoSwiss, Merck, Novartis, Roche, Swiss MS Society, and Swiss National Research Foundation). P.S.G. has received honoraria for speaking, consulting, or advisory board participation from Actelion, Alexion, Biogen Idec, Bristol Myers Squibb, Celgene, EMD Serono, F. Hoffmann-La Roche, Genzyme-Sanofi, Innodem Neurosciences, Novartis, Pendopharm, and Teva Innovation Canada. D.A.H., R.W., and B.C.K. are employees of Novartis Pharma AG, Basel, Switzerland. R.P. and W.S. are employees of Novartis Pharmaceutical Corporation, East Hanover, NJ, USA. F.S. received public speaking honoraria from Alexion, Biogen, Mylan, Novartis, Roche, Sanofi, and Teva; he also received compensation for advisory boards or consultation fees from Alexion, Almirall, Argenx, Avexis, Biogen, Forward Pharma, Lexeo Therapeutics, Merck, Novartis, Novatek, Pomona, Roche, Sanofi, and Takeda. R.Z. is an employee of Novartis Pharma BV, Amsterdam, the Netherlands.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Novartis Pharma AG (Basel, Switzerland).
ORCIDs: Stephen L Hauser  https://orcid.org/0000-0002-4932-4001
https://orcid.org/0000-0002-4932-4001
Anne H Cross  https://orcid.org/0000-0003-0829-7569
https://orcid.org/0000-0003-0829-7569
Heinz Wiendl  https://orcid.org/0000-0003-4310-3432
https://orcid.org/0000-0003-4310-3432
Paul S Giacomini  https://orcid.org/0000-0002-1346-3042
https://orcid.org/0000-0002-1346-3042
Francesco Saccà  https://orcid.org/0000-0002-1323-6317
https://orcid.org/0000-0002-1323-6317
Roman Willi  https://orcid.org/0000-0001-9612-9877
https://orcid.org/0000-0001-9612-9877
Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Stephen L Hauser, UCSF Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
Anne H Cross, Washington University School of Medicine, St Louis, MO, USA.
Kevin Winthrop, Oregon Health & Sciences University, Portland, OR, USA.
Heinz Wiendl, Department of Neurology with Institute of Translational Neurology, University of Münster, Münster, Germany.
Jacqueline Nicholas, OhioHealth Multiple Sclerosis Center, Riverside Methodist Hospital, Columbus, OH, USA.
Sven G Meuth, Department of Neurology, University Hospital Düsseldorf, Düsseldorf, Germany.
Paul S Giacomini, Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montreal, QC, Canada.
Francesco Saccà, Department of Neurosciences, Odontostomatological and Reproductive Sciences, University Federico II, Naples, Italy.
Linda Mancione, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Ronald Zielman, Novartis Pharma BV, Amsterdam, the Netherlands.
Morten Bagger, Novartis Pharma AG, Basel, Switzerland.
Ayan Das Gupta, Novartis Healthcare Pvt. Ltd, Hyderabad, Telangana, India.
Dieter A Häring, Novartis Pharma AG, Basel, Switzerland.
Valentine Jehl, Novartis Pharma AG, Basel, Switzerland.
Bernd C Kieseier, Novartis Pharma AG, Basel, Switzerland and Department of Neurology, Medical Faculty, Heinrich-Heine University, Düsseldorf, Germany.
Ratnakar Pingili, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Dee Stoneman, Novartis Pharma AG, Basel, Switzerland.
Wendy Su, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Roman Willi, Novartis Pharma AG, Basel, Switzerland.
Ludwig Kappos, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB) and MS Center, Departments of Head, Spine and Neuromedicine, Clinical Research, Biomedicine, Biomedical Engineering, University Hospital and University of Basel, Basel, Switzerland.
References
- 1. Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 2004; 104: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 2. Samjoo IA, Worthington E, Drudge C, et al. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: a network meta-analysis. J Comp Eff Res 2020; 9: 1255–1274. [DOI] [PubMed] [Google Scholar]
- 3. Novartis Pharmaceuticals Corporation. Kesimpta: Prescribing information (updated August 2020), https://www.novartis.us/sites/www.novartis.us/files/kesimpta.pdf (2020, accessed 9 June 2021).
- 4. Novartis Ireland Ltd. Kesimpta: Summary of product characteristics (updated 2 June 2021), https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar-product-information_en.pdf (2021, accessed 9 June 2021).
- 5. Harding FA, Stickler MM, Razo J, et al. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. Mabs 2010; 2(3): 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin TS. Ofatumumab: A novel monoclonal anti-CD20 antibody. Pharmgenomics Pers Med 2010; 3: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 2020; 383: 546–557. [DOI] [PubMed] [Google Scholar]
- 8. Engelberts PJ, Voorhorst M, Schuurman J, et al. Type I CD20 antibodies recruit the B cell receptor for complement-dependent lysis of malignant B cells. J Immunol 2016; 197: 4829–4837. [DOI] [PubMed] [Google Scholar]
- 9. Semple KM, Gonzalez CM, Zarr M, et al. Evaluation of the ability of immune humanized mice to demonstrate CD20-specific cytotoxicity induced by ofatumumab. Clin Transl Sci 2019; 12(3): 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Novarts Pharmaceuticals Corporation. Kesimpta: Prescribing information, 2020, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf
- 11. Bar-Or A, Wiendl H, Montalban X, et al. Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase 2 study. Mult Scler. Epub ahead of print 4 October 2021. DOI: 10.1177/13524585211044479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kira J, Nakahara J, Sazonov DV, et al. Effect of ofatumumab versus placebo in relapsing multiple sclerosis patients from Japan and Russia: Phase 2 APOLITOS study. Mult Scler. Epub ahead of print 17 November 2021. DOI: 10.1177/13524585211055934. [DOI] [PubMed] [Google Scholar]
- 13. Novartis Pharmaceuticals Corporation. Long-term safety, tolerability and effectiveness study of ofatumumab in patients with relapsing MS (ALITHIOS). ClinicalTrials.gov Identifier: NCT03650114 (updated 11 May 2021), https://clinicaltrials.gov/ct2/show/NCT03650114?term=ALITHIOS&draw=2&rank=1 (2021, accessed 9 June 2021).
- 14. Cross AH, Fox EJ, de Seze J, et al. Safety experience with extended exposure to ofatumumab in patients with relapsing multiple sclerosis from phase 2 and 3 clinical trials. In: Eposter (P0234) presented at the 8th Joint ACTRIMS-ECTRIMS meeting, Msvirtual, 1–13 September 2020. [Google Scholar]
- 15. Mayer L, Kappos L, Racke MK, et al. Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: Results from the phase 3 randomized OPERA I, OPERA II, and ORATORIO studies. Mult Scler Relat Disord 2019; 30: 236–243. [DOI] [PubMed] [Google Scholar]
- 16. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. [DOI] [PubMed] [Google Scholar]
- 17. Hauser SL, Kappos L, Arnold DL, et al. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology 2020; 95: e1854–e1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bar-Or A, Grove RA, Austin DJ, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study. Neurology 2018; 90: e1805–e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baker D, Pryce G, James LK, et al. The ocrelizumab phase II extension trial suggests the potential to improve the risk: Benefit balance in multiple sclerosis. Mult Scler Relat Disord 2020; 44: 102279. [DOI] [PubMed] [Google Scholar]
- 20. Shortt J, Spencer A. Adjuvant rituximab causes prolonged hypogammaglobulinaemia following autologous stem cell transplant for non-Hodgkin’s lymphoma. Bone Marrow Transplant 2006; 38(6): 433–436. [DOI] [PubMed] [Google Scholar]
- 21. Besada E, Koldingsnes W, Nossent JC. Serum immunoglobulin levels and risk factors for hypogammaglobulinaemia during long-term maintenance therapy with rituximab in patients with granulomatosis with polyangiitis. Rheumatology 2014; 53(10): 1818–1824. [DOI] [PubMed] [Google Scholar]
- 22. Marcinno A, Marnetto F, Valentino P, et al. Rituximab-induced hypogammaglobulinemia in patients with neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm 2018; 5(6): e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evertsson B, Hoyt T, Christensen A, et al. A comparative study of tolerability and effects on immunoglobulin levels and CD19 cell counts with ocrelizumab vs low dose of rituximab in multiple sclerosis. Mult Scler J Exp Transl Clin 2020; 6(4): DOI: 10.1177/2055217320964505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ottaviano G, Marinoni M, Graziani S, et al. Rituximab unveils hypogammaglobulinemia and immunodeficiency in children with autoimmune cytopenia. J Allergy Clin Immunol Pract 2020; 8(1): 273–282. [DOI] [PubMed] [Google Scholar]
- 25. Md Yusof MY, Vital EM, McElvenny DM, et al. Predicting severe infection and effects of hypogammaglobulinemia during therapy with rituximab in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2019; 71(11): 1812–1823. [DOI] [PubMed] [Google Scholar]
- 26. Barmettler S, Ong MS, Farmer JR, et al. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open 2018; 1: e184169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Derfuss T, Weber MS, Hughes R, et al. Serum immunoglobulin levels and risk of serious infections in the pivotal phase III trials of ocrelizumab in multiple sclerosis and their open label extensions. In: Presentation (P65) at the 35th ECTRIMS meeting, Stockholm, 11–13 September 2019. [Google Scholar]
- 28. Cross AH, Delgado S, Habek M, et al. Outcomes of COVID-19 in patients with relapsing multiple sclerosis receiving ofatumumab: Data from the ALITHIOS study and post marketing surveillance. Poster (LB-ECTRIMS-2021-01573) Presented at the 37th ECTRIMS meeting, Vienna, 13–15 October 2021. [Google Scholar]
- 29. Tolley K, Hutchinson M, You X, et al. A network meta-analysis of efficacy and evaluation of safety of subcutaneous pegylated interferon beta-1a versus other injectable therapies for the treatment of relapsing multiple sclerosis. PLoS ONE 2015; 10(6): e0127960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stoner KL, Harder H, Fallowfield LJ, et al. Intravenous versus subcutaneous drug administration: Which do patients prefer? A systematic review. Patient. Epub ahead of print 12 July 2014. DOI: 10.1007/s40271-014-0075-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-2-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-3-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-4-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-5-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-6-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-7-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-8-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-9-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-pdf-10-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal
Supplemental material, sj-docx-11-msj-10.1177_13524585221079731 for Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years by Stephen L Hauser, Anne H Cross, Kevin Winthrop, Heinz Wiendl, Jacqueline Nicholas, Sven G Meuth, Paul S Giacomini, Francesco Saccà, Linda Mancione, Ronald Zielman, Morten Bagger, Ayan Das Gupta, Dieter A Häring, Valentine Jehl, Bernd C Kieseier, Ratnakar Pingili, Dee Stoneman, Wendy Su, Roman Willi and Ludwig Kappos in Multiple Sclerosis Journal