This cohort study examines the association between early left ventricular ejection fraction (LVEF) improvement and 5-year clinical outcomes after transfemoral transcatheter aortic valve replacement (TAVR) in high- or intermediate-risk patients with symptomatic severe aortic stenosis and LVEF less than 50%.
Key Points
Question
What is the prognostic significance of left ventricular ejection fraction (LVEF) improvement after transcatheter aortic valve replacement (TAVR)?
Findings
In this cohort study of 659 high- or intermediate-risk patients with symptomatic severe aortic stenosis and LVEF less than 50% undergoing transfemoral TAVR, 1 in 3 experienced early LVEF improvement (defined as ≥10 percentage points increase over baseline LVEF at 30 days). Early LVEF improvement after TAVR was associated with lower 5-year all-cause death and cardiac death.
Meaning
Further studies are needed to understand the pathophysiologic mechanism(s) mediating reduced mortality in patients with early LVEF recovery after TAVR.
Abstract
Importance
In patients with severe aortic stenosis and left ventricular ejection fraction (LVEF) less than 50%, early LVEF improvement after transcatheter aortic valve replacement (TAVR) is associated with improved 1-year mortality; however, its association with long-term clinical outcomes is not known.
Objective
To examine the association between early LVEF improvement after TAVR and 5-year outcomes.
Design, Setting, and Participants
This cohort study analyzed patients enrolled in the Placement of Aortic Transcatheter Valves (PARTNER) 1, 2, and S3 trials and registries between July 2007 and April 2015. High- and intermediate-risk patients with baseline LVEF less than 50% who underwent transfemoral TAVR were included in the current study. Data were analyzed from August 2020 to May 2021.
Exposures
Early LVEF improvement, defined as increase of 10 percentage points or more at 30 days and also as a continuous variable (ΔLVEF between baseline and 30 days).
Main Outcomes and Measures
All-cause death at 5 years.
Results
Among 659 included patients with LVEF less than 50%, 468 (71.0%) were male, and the mean (SD) age was 82.4 (7.7) years. LVEF improvement within 30 days following transfemoral TAVR occurred in 216 patients (32.8%) (mean [SD] ΔLVEF, 16.4 [5.7%]). Prior myocardial infarction, diabetes, cancer, higher baseline LVEF, larger left ventricular end-diastolic diameter, and larger aortic valve area were independently associated with lower likelihood of LVEF improvement. Patients with vs without early LVEF improvement after TAVR had lower 5-year all-cause death (102 [50.0%; 95% CI, 43.3-57.1] vs 246 [58.4%; 95% CI, 53.6-63.2]; P = .04) and cardiac death (52 [29.5%; 95% CI, 23.2-37.1] vs 135 [38.1%; 95% CI, 33.1-43.6]; P = .05). In multivariable analyses, early improvement in LVEF (modeled as a continuous variable) was associated with lower 5-year all-cause death (adjusted hazard ratio per 5% increase in LVEF, 0.94 [95% CI, 0.88-1.00]; P = .04) and cardiac death (adjusted hazard ratio per 5% increase in LVEF, 0.90 [95% CI, 0.82-0.98]; P = .02) after TAVR. Restricted cubic spline analysis demonstrated a visual inflection point at ΔLVEF of 10% beyond which there was a steep decline in all-cause mortality with increasing degree of LVEF improvement. There were no statistically significant differences in rehospitalization, New York Heart Association functional class, or Kansas City Cardiomyopathy Questionnaire Overall Summary score at 5 years in patients with vs without early LVEF improvement. In subgroup analysis, the association between early LVEF improvement and 5-year all-cause death was consistent regardless of the presence or absence of coronary artery disease or prior myocardial infarction.
Conclusions and Relevance
In patients with severe aortic stenosis and LVEF less than 50%, 1 in 3 experience LVEF improvement within 1 month after TAVR. Early LVEF improvement is associated with lower 5-year all-cause and cardiac death.
Introduction
Transcatheter aortic valve replacement (TAVR) is a safe and effective treatment option in patients with symptomatic severe aortic stenosis (AS) across the entire spectrum of surgical risk, including in those with left ventricular (LV) systolic dysfunction.1 Prior studies have shown that in patients with severe AS and LV dysfunction (LV ejection fraction [LVEF], <40%-50%), approximately half experience an improvement in LVEF (defined as an increase of ≥10% points) within 30 days after TAVR.2,3,4,5,6 Although the association of baseline LV dysfunction with outcomes following TAVR is controversial, among such patients, failure to improve LVEF portends an increased risk of 1-year mortality and rehospitalization after TAVR.2,3,7,8 However, prior studies examining the association between LVEF improvement and outcomes are limited by small sample size, inclusion of extreme- or high-risk patients only, inclusion of transapical TAVR, which can adversely affect LVEF improvement after TAVR, and short-term (1-year) follow-up.2,3,4 Thus, the primary objective of this study was to examine the association between early LVEF improvement and 5-year clinical outcomes after transfemoral TAVR in high- or intermediate-risk patients with symptomatic severe AS and LVEF less than 50%.
Methods
Study Population
The study population was drawn from patients at high or intermediate surgical risk included in the Placement of Aortic Transcatheter Valves (PARTNER) 1, 2, and S3 trials and registries and who underwent transfemoral TAVR from July 2007 and April 2015. Patients who underwent transapical TAVR or surgical aortic valve replacement were excluded from this analysis. The design, inclusion and exclusion criteria, and primary results from these trials and registries have been published previously.9,10,11 In all cohorts, patients had severe AS, defined as an aortic valve area (AVA) less than 0.8 cm2 (or indexed AVA <0.5 cm2/m2) and either a resting or inducible mean gradient more than 40 mm Hg or peak jet velocity more than 4 m/s. All patients were symptomatic with New York Heart Association (NYHA) functional class II or higher. For this analysis, we included only patients who underwent transfemoral TAVR, had baseline LVEF less than 50%, and had 30-day follow-up LVEF assessment by echocardiography. Race and ethnicity data were self-reported. The protocols were approved by the institutional review boards of each participating site, and all patients provided written informed consent. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline to report the findings of this study.
Echocardiographic Assessment
Echocardiograms were analyzed by 5 core laboratories using standardized methodology. LVEF was measured using the biplane Simpson volumetric method combining apical 4- and 2-chamber views. LVEF was also determined by visual estimation (in 5-point increments), and when the definition of the LV endocardial border was not adequate for biplane tracing, was substituted to provide a single combined LVEF determination in all patients. For the present analysis, LV dysfunction was defined as an LVEF less than 50%. Improvement in LVEF was assessed as a dichotomous variable (defined as an increase of ≥10 percentage points over baseline LVEF at 30 days) as well as a continuous variable (defined as degree of LVEF improvement [ΔLVEF] between baseline and 30 days). Because 30 days may be early in the course of recovery of LVEF function following TAVR, we performed a secondary analysis using LVEF improvement at 1 year (increase of ≥10 percentage points over baseline LVEF at 1 year, as well as ΔLVEF between baseline and 1 year). The core laboratories followed the American Society of Echocardiography/European Association for Echocardiography guideline for assessing the severity of native valvular stenosis and regurgitation, LV stroke volume index (SVi), and LV mass index.
Study End Points
The primary end point for this study was all-cause death at 5 years. Secondary end points included cardiac death, noncardiac death, rehospitalization, all-cause death or rehospitalization, cardiac death or rehospitalization, NYHA functional class, and Kansas City Cardiomyopathy Questionnaire Overall Summary score at 5 years. Mortality and rehospitalization end points were adjudicated by independent clinical events committees. Definitions of end points are identical to those reported previously. Rehospitalization was defined as hospital readmission for symptoms of AS and/or complications of the valve procedure.
Statistical Analysis
Baseline characteristics were compared between patients with vs without LVEF improvement. Categorical variables are presented as frequency and percentages and compared using Fisher exact test. Continuous variables are presented as mean (SD) and compared using the t test.
We used multivariable logistic and linear regression models to identify independent predictors of LVEF improvement of 10% points or more and ΔLVEF, respectively, at 30 days following TAVR. The following candidate variables were selected a priori for inclusion in the full multivariable models: age, sex, body mass index (BMI), Society of Thoracic Surgeons (STS) score, NYHA functional class, coronary artery disease (CAD), prior myocardial infarction (MI), prior coronary artery bypass grafting, prior percutaneous coronary intervention, prior balloon aortic valvuloplasty, cerebrovascular disease, peripheral artery disease, hypertension, diabetes, chronic obstructive pulmonary disease, oxygen-dependent lung disease, chronic kidney disease (serum creatinine >2 mg/dL; to convert to micromoles per liter, multiply by 88.4), cancer, hemoglobin, baseline LVEF, LV mass index, LV end-diastolic diameter (LVEDD), SVi, AVA, moderate or severe aortic regurgitation, moderate or severe mitral regurgitation, β-blockers at discharge, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker at discharge, post-TAVR aortic valve (AV) mean gradient (at discharge or 30 days), at least moderate paravalvular leak, need for permanent pacemaker, and new atrial fibrillation. Backward variable selection method was used to derive parsimonious multivariable models, keeping only covariates with P value less than .10 in the final models.
Time-to-event end points are presented as Kaplan-Meier estimates and were compared using log-rank test. Outcomes were assessed between 30 days and 5 years to avoid immortal time bias (ie, inclusion of patients who died within 30 days in the no LVEF improvement group). Multivariable Cox proportional hazards regression models were used to compare outcomes of patients with vs without LVEF improvement (improvement of ≥10% points as well as ΔLVEF). The multivariable models adjusted for variables that were statistically significant on univariate analysis (sex, BMI, STS score, diabetes, prior MI, cancer, and baseline LVEF). Restricted cubic spline curve with 3 knots was used to examine the association between ΔLVEF and all-cause death through 5 years. Association between LVEF improvement of 10% points or more and 5-year all-cause death was examined in the overall TAVR cohort as well as the following prespecified subgroups: age (<80 and ≥80 years), sex (male and female), CAD (yes/no), prior MI (yes/no), STS score (<4, 4-8, and >8), baseline LVEF (≤35% and >35%), baseline AVA (<0.6 and ≥0.6 cm2), baseline aortic valve mean gradient (<50 and ≥50 mm Hg), baseline aortic regurgitation (none/mild and ≥moderate to severe), and baseline mitral regurgitation (none/mild and ≥moderate to severe). Interactions between the above prespecified subgroups and LVEF improvement were examined in the multivariable Cox regression models, adjusting for significant covariates. Secondary analysis was performed to determine the association between LVEF improvement at 1 year post-TAVR and 5-year outcomes. For secondary analyses, outcomes were assessed between 1 and 5 years.
Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute) and R version 3.6.1 (R Foundation). A 2-sided P value less than .05 was considered statistically significant. No multiplicity adjustment was performed. Analysis took place between August 2020 to May 2021.
Results
Baseline Characteristics
The study included 659 patients with symptomatic severe AS at high or intermediate surgical risk and baseline LVEF less than 50% who underwent transfemoral TAVR. A total of 468 patients (71.0%) were male and 191 (29.0%) were female; 4 (0.6%) were Asian, 23 (3.5%) were Black, 12 (1.8%) were Hispanic, 591 (89.7%) were White, and 29 (4.4%) were of other race; and the mean (SD) age was 82.4 (7.7) years. LVEF improvement, defined as an increase of 10% points or more at 30 days post-TAVR, occurred in 216 patients (32.8%) (eFigure 1 in the Supplement), and 86 patients (13.1%) had LVEF of 50% or more at 30 days post-TAVR. Among patients with LVEF improvement, mean (SD) ΔLVEF was 16.4% (5.7%).
Patients with LVEF improvement were less likely to be male individuals, had higher STS score, lower prevalence of comorbidities such as CAD, prior MI, prior coronary artery bypass grafting, peripheral artery disease, cancer, and atrial fibrillation, lower baseline LVEF and SVi, higher AV mean gradient and AV peak velocity, smaller AVA, and larger LVEDD and LV end-systolic diameter, compared with those without LVEF improvement (Table 1). There were no significant differences in rates of more than mild paravalvular leak, permanent pacemaker implantation, or new atrial fibrillation within 30 days post-TAVR in patients with vs without LVEF improvement.
Table 1. Baseline Characteristics of Patients Undergoing TAVR With vs Without Early LVEF Improvement.
| Characteristic | No./total No. (%) | P value | |
|---|---|---|---|
| No LVEF improvement (n = 443) | LVEF improvement (n = 216) | ||
| Age, mean (SD), y | 82.1 (7.9) | 83.2 (7.4) | .09 |
| Sex | |||
| Male | 339/443 (76.5) | 129/216 (59.7) | <.001 |
| Female | 104/443 (23.5) | 87/216 (40.3) | |
| Race and ethnicitya | |||
| Asian | 2/443 (0.4) | 2/216 (0.9) | .90 |
| Black | 14/443 (3.2) | 9/216 (4.2) | |
| Hispanic | 8/443 (1.8) | 4/216 (1.8) | |
| White | 399/443 (90.1) | 192/216 (88.9) | |
| Other race | 20/443 (4.5) | 9/216 (4.2) | |
| BMI, mean (SD) | 27.4 (5.6) | 27.7 (6.6) | .59 |
| STS score, mean (SD) | 8.1 (4.4) | 8.9 (4.4) | .02 |
| NYHA class III/IV | 382/443 (86.2) | 197/216 (91.2) | .08 |
| Hypertension | 409/443 (92.3) | 189/216 (87.5) | .06 |
| Diabetes | 174/443 (39.3) | 71/216 (32.9) | .12 |
| Coronary artery disease | 367/443 (82.8) | 162/216 (75.0) | .02 |
| Prior MI | 154/443 (34.8) | 46/216 (21.3) | <.001 |
| Prior PCI | 163/442 (36.9) | 73/216 (33.8) | .49 |
| Prior CABG | 190/443 (42.9) | 61/216 (28.2) | <.001 |
| Prior BAV | 60/443 (13.5) | 28/216 (13.0) | .90 |
| Atrial fibrillation | 185/416 (44.5) | 57/175 (32.6) | .008 |
| Cerebrovascular disease | 37/443 (8.4) | 20/216 (9.3) | .77 |
| Peripheral artery disease | 137/443 (30.9) | 50/216 (23.1) | .04 |
| Chronic kidney diseaseb | 63/442 (14.3) | 21/216 (9.7) | .11 |
| COPD | 145/443 (32.7) | 73/216 (33.8) | .79 |
| Oxygen-dependent lung disease | 13/443 (2.9) | 10/216 (4.6) | .27 |
| Cancer | 146/443 (33.0) | 49/216 (22.7) | .007 |
| Katz Activities of Daily Living Index, mean (SD) | 5.5 (1.0) | 5.2 (1.3) | .002 |
| Hemoglobin, mean (SD), g/dL | 12.5 (6.5) | 11.9 (1.6) | .09 |
| Albumin <3.5 g/dL | 88/418 (21.1) | 46/184 (25.0) | .29 |
| Baseline echocardiographic data, mean (SD) | |||
| LVEF, % | 38.8 (8.4) | 35.6 (9.1) | <.001 |
| LV mass, g | 266.7 (81.8) | 260.5 (74.2) | .34 |
| LV mass index, g/m2 | 139.3 (39.5) | 140.9 (40.0) | .63 |
| LVEDD, cm | 5.2 (0.8) | 5.0 (0.7) | <.001 |
| LVESD, cm | 4.2 (0.9) | 4.0 (0.8) | .02 |
| AV peak velocity, cm/s | 406.1 (60.6) | 418.9 (71.6) | .03 |
| AV mean gradient, mm Hg | 39.8 (12.5) | 43.0 (15.1) | .006 |
| AV area, cm2 | 0.7 (0.2) | 0.6 (0.2) | .001 |
| LVOT Doppler stroke volume, mL | 62.0 (15.8) | 58.6 (17.5) | .02 |
| LVOT Doppler Stroke Volume Index, mL/m2 | 32.5 (8.1) | 31.5 (8.6) | .16 |
| ≥Moderate AR | 41/431 (9.5) | 23/210 (11.0) | .58 |
| ≥Moderate MR | 120/424 (28.3) | 54/209 (25.8) | .57 |
| Procedural characteristics | |||
| Valve size, mm | <.001 | ||
| 20 | 7/443 (1.6) | 1/216 (0.5) | |
| 23 | 81/443 (18.3) | 81/216 (37.5) | |
| 26 | 229/443 (51.7) | 97/216 (44.9) | |
| 29 | 126/443 (28.4) | 37/216 (17.1) | |
| Valve type | <.001 | ||
| SAPIEN | 84/443 (19.0) | 62/216 (28.7) | |
| SAPIEN XT | 205/443 (46.3) | 68/216 (31.5) | |
| SAPIEN 3 | 154/443 (34.8) | 86/216 (39.8) | |
| Predilation | 435/441 (98.6) | 214/216 (99.1) | .99 |
| Postdilation | 88/441 (20.0) | 54/213 (25.4) | .13 |
| Concomitant PCI | 3/343 (0.9) | 3/166 (1.8) | .40 |
| Need for second THV | 7/443 (1.6) | 1/216 (0.5) | .28 |
| Discharge medications | |||
| β-Blocker | 317/443 (71.6) | 156/216 (72.2) | .93 |
| ACE inhibitor/ARB | 234/443 (52.8) | 115/216 (53.2) | .93 |
| Procedural complications within 30 d | |||
| PPM | 36/443 (8.1) | 11/216 (5.1) | .20 |
| New atrial fibrillation | 21/443 (4.7) | 9/216 (4.2) | .84 |
| >Mild PVL | 39/434 (9.0) | 19/213 (8.9) | >.99 |
| 30-d Post-TAVR echocardiographic data | |||
| AV gradient, mean (SD), mm Hg | 9.6 (3.95) | 10.1 (3.40) | .10 |
| Severe prosthesis-patient mismatchc | 63/429 (14.7) | 22/208 (10.6) | .17 |
| ΔLVEF (30-d change from baseline), mean (SD), % | 1.1 (5.4) | 16.4 (5.7) | <.001 |
Abbreviations: ACE, angiotensin-converting enzyme; AR, aortic regurgitation; ARB, angiotensin II receptor blocker; AV, aortic valve; BAV, balloon aortic valvuloplasty; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVOT, left ventricular outflow tract; MI, myocardial infarction; MR, mitral regurgitation; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PPM, permanent pacemaker; PVL, paravalvular leak; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement; THV, transcatheter heart valve.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; creatinine to micromoles per liter, multiply by 88.4; hemoglobin to grams per liter, multiply by 10.
Race and ethnicity data were self-reported.
Serum creatinine >2 mg/dL.
Defined as indexed effective orifice area <0.65 cm2/m2.
Baseline characteristics stratified by sex and LVEF improvement are shown in eTables 1 to 4 in the Supplement. Baseline characteristics of male and female individuals with vs without early LVEF improvement following TAVR were consistent with those of the overall cohort (eTables 1 and 2 in the Supplement). Compared with male individuals, female individuals had higher STS score, lower prevalence of CAD, prior MI, prior percutaneous coronary intervention, prior coronary artery bypass grafting, and chronic obstructive pulmonary disease, lower Katz index, lower hemoglobin, higher baseline LVEF, higher AV mean gradient and AV peak velocity, smaller AVA, smaller LVEDD and LV end-systolic diameter, and higher prevalence of moderate or severe mitral regurgitation (eTables 3 and 4 in the Supplement). Female individuals were more likely to undergo TAVR with a smaller valve size and had higher 30-day AV mean gradient but had similar rates of severe prosthesis-patient mismatch compared with male individuals. These differences in baseline, procedural, and postprocedural characteristics between male vs female individuals were consistent among patients with and without early LVEF improvement following TAVR (eTables 3 and 4 in the Supplement).
Multivariable Predictors of Early LVEF Improvement After TAVR
In multivariable analysis, prior MI, diabetes, cancer, higher baseline LVEF, larger LVEDD, and larger AVA were associated with lower likelihood of early LVEF improvement, whereas higher BMI and higher SVi were associated with increased odds of early LVEF improvement after TAVR (Table 2). When LVEF improvement was modeled as a continuous variable, prior MI, prior coronary artery bypass grafting, hypertension, cancer, higher baseline LVEF, larger LVEDD, and lower post-TAVR AV mean gradient were associated with smaller increases in LVEF (eTable 5 in the Supplement).
Table 2. Multivariable Predictors of Early LVEF Improvement (Increase of ≥10% Points) After TAVR.
| Characteristic | Full model | Parsimonious model | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.00 (0.97-1.03) | .98 | NA | NA |
| Male | 0.98 (0.53-1.83) | .96 | NA | NA |
| BMI | 1.06 (1.02-1.10) | .005 | 1.06 (1.02-1.10) | <.001 |
| STS score | 0.99 (0.93-1.05) | .67 | NA | NA |
| NYHA class III/IV | 1.59 (0.83-3.04) | .16 | NA | NA |
| Hypertension | 0.73 (0.37-1.42) | .35 | NA | NA |
| Diabetes | 0.55 (0.34-0.86) | .01 | 0.61 (0.40-0.92) | .02 |
| Coronary artery disease | 1.20 (0.69-2.07) | .52 | NA | NA |
| Prior MI | 0.64 (0.40-1.04) | .07 | 0.65 (0.42-0.98) | .04 |
| Prior PCI | 1.49 (0.93-2.36) | .09 | NA | NA |
| Prior CABG | 0.76 (0.46-1.24) | .27 | NA | NA |
| Prior BAV | 0.57 (0.31-1.05) | .07 | NA | NA |
| Cerebrovascular disease | 1.08 (0.54-2.18) | .83 | NA | NA |
| Peripheral artery disease | 0.78 (0.49-1.24) | .30 | NA | NA |
| Chronic kidney diseasea | 0.80 (0.43-1.49) | .48 | NA | NA |
| COPD | 1.22 (0.77-1.94) | .41 | NA | NA |
| Oxygen-dependent lung disease | 2.03 (0.69-5.98) | .20 | NA | NA |
| Cancer | 0.50 (0.32-0.80) | .003 | 0.56 (0.37-0.86) | .008 |
| Hemoglobin, g/dL | 0.97 (0.86-1.09) | .63 | NA | NA |
| Baseline LVEF, % | 0.93 (0.90-0.96) | <.001 | 0.93 (0.90-0.95) | <.001 |
| LV mass index, g/m2 | 1.00 (1.00-1.01) | .72 | NA | NA |
| LVEDD, cm | 0.61 (0.43-0.87) | .006 | 0.59 (0.44-0.78) | <.001 |
| AV area, cm2 | 0.16 (0.03-0.78) | .02 | 0.19 (0.05-0.73) | .02 |
| LVOT Doppler stroke volume index | 1.02 (0.99-1.06) | .20 | 1.03 (1.00-1.06) | .04 |
| ≥Moderate AR | 0.96 (0.49-1.86) | .90 | NA | NA |
| ≥Moderate MR | 0.80 (0.50-1.28) | .34 | NA | NA |
| Discharge medication | ||||
| β-Blocker | 0.96 (0.60-1.54) | .87 | NA | NA |
| ACE inhibitor/ARB | 1.05 (0.70-1.58) | .81 | NA | NA |
| Procedural complications (≤7 d) | 1.18 (0.69-2.00) | .55 | NA | NA |
| Post-TAVR AV mean gradient | 1.05 (0.98-1.11) | .15 | NA | NA |
| Severe prosthesis-patient mismatch | 0.52 (0.27-1.01) | .054 | 0.59 (0.32-1.07) | .08 |
| Valve type (SAPIEN 3 vs SAPIEN/SAPIEN XT) | 0.66 (0.41-1.08) | .10 | NA | NA |
| Valve size | 0.94 (0.83-1.07) | .37 | 0.91 (0.83-1.01) | .07 |
Abbreviations: ACE, angiotensin-converting enzyme; AR, aortic regurgitation; ARB, angiotensin II receptor blocker; AV, aortic valve; BAV, balloon aortic valvuloplasty; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; MI, myocardial infarction; MR, mitral regurgitation; NA, not applicable; NYHA, New York Heart Association; OR, odds ratio; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement.
SI conversion factors: To convert creatinine to micromoles per liter, multiply by 88.4; hemoglobin to grams per liter, multiply by 10.
Serum creatinine >2 mg/dL.
Association Between LVEF Improvement and 5-Year Outcomes
The Kaplan-Meier rate of the primary end point of 5-year all-cause death was 55.6% in the overall study cohort. In univariate analysis, patients with vs without early LVEF improvement had lower rates of 5-year all-cause death (102 [50.0%; 95% CI, 43.3-57.1] vs 246 [58.4%; 95% CI, 53.6-63.2]; P = .04) and cardiac death (52 [29.5%; 95% CI, 23.2-37.1] vs 135 [38.1%; 95% CI, 33.1-43.6]; P = .05) but similar rates of noncardiac death (38 [22.4%; 95% CI, 16.7-29.7] vs 292 [27.6%; 95% CI, 22.9-33.0]; P = .21) and rehospitalization (85 [44.2%; 95% CI, 37.3-51.8] vs 184 [47.3%; 95% CI, 42.2-52.8]; P = .52) (Figure). There were no significant differences in the rates of the composite of all-cause death or rehospitalization (133 [63.5%; 95% CI, 57.0-70.1] vs 300 [70.3%; 95% CI, 65.8-74.7]; P = .18), cardiac death or rehospitalization (114 [56.7%; 95% CI, 49.8-63.8] vs 216 [59.7%; 95% CI, 54.7-64.8]; P = .62), NYHA functional class III/IV (6 of 76 [7.9%; 95% CI, 3.0-16.4] vs 16 of 119 [13.4%; 95% CI, 7.9-20.9]; P = .26), and mean (SD) Kansas City Cardiomyopathy Questionnaire Overall Summary Score (75.6 [19.6] vs 73.9 [21.8]; P = .58) at 5 years between the 2 groups (eFigures 2-4 in the Supplement).
Figure. 5-Year Outcomes in Patients With vs Without Early Left Ventricular Ejection Fraction (LVEF) Improvement After Transcatheter Aortic Valve Replacement.
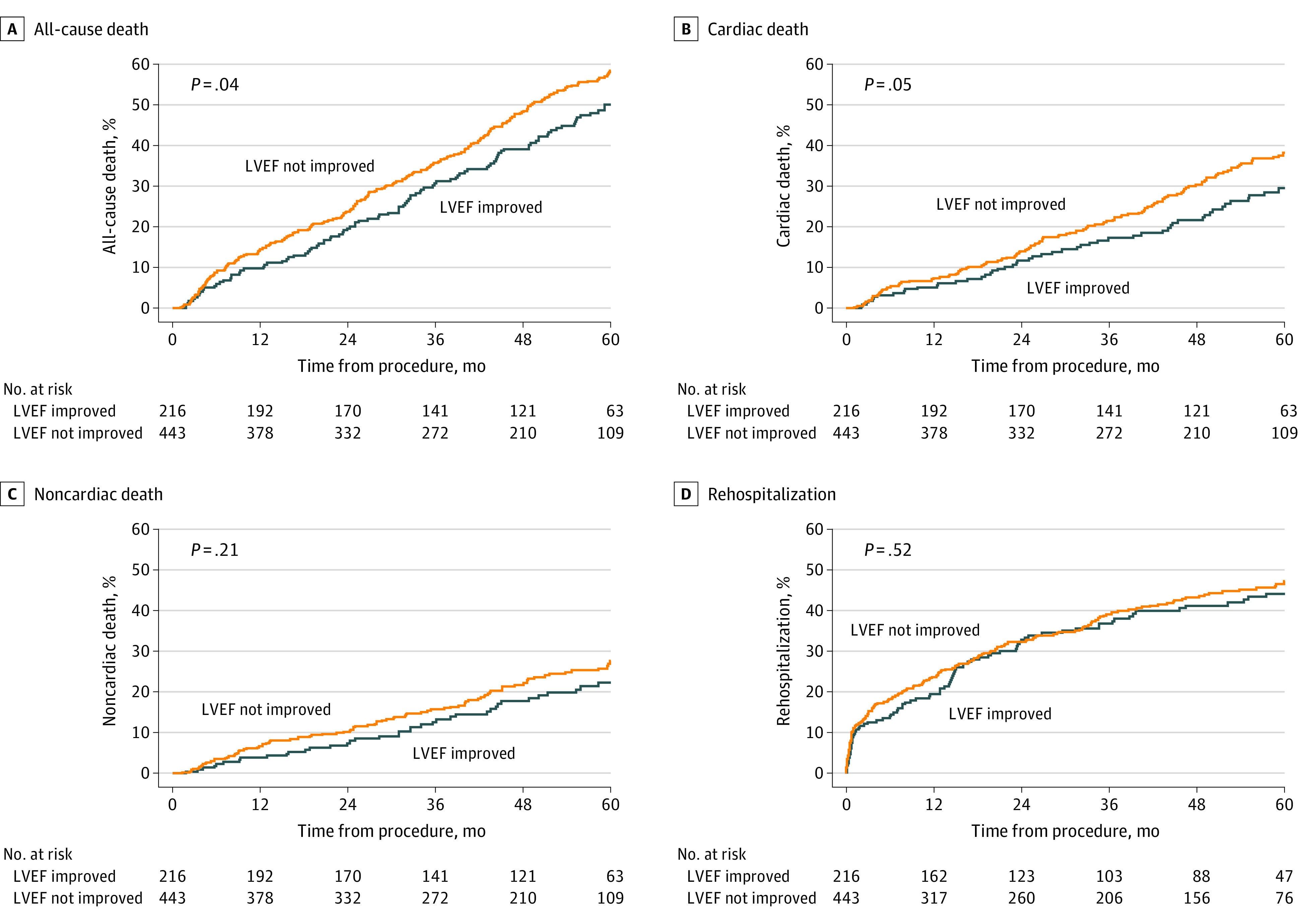
Time to event curves of 5-year all-cause death (A), cardiac death (B), noncardiac death (C), and rehospitalization (D) in patients with vs without LVEF improvement (defined as an increase of ≥10% points over baseline LVEF at 30 days) after transcatheter aortic valve replacement.
In multivariable analyses, early improvement in LVEF (modeled as a continuous variable) was associated with lower 5-year all-cause death (adjusted hazard ratio per 5% increase in LVEF, 0.94 [95% CI, 0.88-1.00]; P = .04) and cardiac death (adjusted hazard ratio per 5% increase in LVEF, 0.90 [95% CI, 0.82-0.98]; P = .02) after TAVR (Table 3). Restricted cubic spline analysis demonstrated a visual inflection point at ΔLVEF of 10% beyond which there was a steep decline in all-cause mortality with increasing degree of LVEF improvement (eFigure 5 in the Supplement). When modeled as a dichotomous variable, early LVEF improvement of 10% points or more was associated with lower 5-year all-cause death and cardiac death; however, this difference did not reach statistical significance (all-cause death vs cardiac death: adjusted hazard ratio, 0.79 [95% CI, 0.62-1.02]; P = .06 vs 0.75 [95% CI, 0.53-1.05]; P = .09).
Table 3. Adjusted Association Between Early LVEF Improvement and 5-Year Clinical Outcomes After Transcatheter Aortic Valve Replacementa.
| Outcome | LVEF improvement (per 5% increase) | Increase in LVEF ≥10% points | ||
|---|---|---|---|---|
| aHR (95% CI) | P value | aHR (95% CI) | P value | |
| All-cause death | 0.94 (0.88-1.00) | .04 | 0.79 (0.62-1.02) | .06 |
| Cardiac death | 0.90 (0.82-0.98) | .02 | 0.75 (0.53-1.05) | .09 |
| Noncardiac death | 0.97 (0.87-1.07) | .51 | 0.82 (0.54-1.22) | .32 |
| Rehospitalization | 0.98 (0.92-1.06) | .64 | 0.93 (0.70-1.22) | .59 |
| Death or rehospitalization | 0.97 (0.92-1.03) | .31 | 0.88 (0.70-1.09) | .24 |
| Cardiac death or rehospitalization | 0.99 (0.93-1.05) | .66 | 0.97 (0.76-1.24) | .82 |
Abbreviations: aHR, adjusted hazard ratio; LVEF, left ventricular ejection fraction.
Multivariable models adjusted for the following covariates: sex, body mass index, Society of Thoracic Surgeons score, diabetes, prior myocardial infarction, cancer, and baseline LVEF.
In secondary analyses, LVEF improvement at 1 year was associated with significantly lower 5-year all-cause death, cardiac death, all-cause death or rehospitalization, and cardiac death or rehospitalization (eTable 6 and eFigure 6 in the Supplement).
The association between early LVEF improvement and outcomes was consistent regardless of the presence or absence of CAD or prior MI (Table 4). Subgroup analyses demonstrated a significant interaction by sex such that early LVEF improvement was associated with lower 5-year all-cause death, cardiac death, all-cause death or rehospitalization, and cardiac death or rehospitalization in female individuals but not in male individuals (Table 4; eTable 7 and eFigure 7 in the Supplement).
Table 4. Association Between Early LVEF Improvement and 5-Year All-Cause Death After Transcatheter Aortic Valve Replacement in Prespecified Subgroups.
| Subgroup | LVEF improvement (per 5% increase) | Increase in LVEF ≥10% points | ||||
|---|---|---|---|---|---|---|
| aHR (95% CI) | P value | aHR (95% CI) | P value | |||
| Effect | Interaction | Effect | Interaction | |||
| Age, y | ||||||
| <80 | 0.89 (0.79-1.00) | .06 | .26 | 0.6 (0.38-1.03) | .06 | .30 |
| ≥80 | 0.96 (0.90-1.03) | .27 | 0.8 (0.65-1.09) | .19 | ||
| Sex | ||||||
| Male | 1.01 (0.94-1.08) | .88 | .01 | 1.0 (0.80-1.37) | .73 | .004 |
| Female | 0.84 (0.75-0.95) | .005 | 0.5 (0.31-0.75) | .001 | ||
| Coronary artery disease | ||||||
| No | 0.98 (0.86-1.11) | .71 | .60 | 1.05 (0.65-1.69) | .83 | .17 |
| Yes | 0.94 (0.88-1.00) | .06 | 0.72 (0.55-0.94) | .02 | ||
| Prior myocardial infarction | ||||||
| No | 0.96 (0.89-1.03) | .23 | .68 | 0.83 (0.64-1.09) | .18 | .57 |
| Yes | 0.93 (0.83-1.04) | .22 | 0.71 (0.44-1.14) | .16 | ||
| STS score | ||||||
| <4 | 1.06 (0.74-1.50) | .76 | .20 | 1.1 (0.31-3.60) | .93 | .17 |
| 4-8 | 0.95 (0.86-1.05) | .29 | 0.9 (0.59-1.27) | .47 | ||
| >8 | 0.89 (0.82-0.96) | .002 | 0.6 (0.42-0.76) | <.001 | ||
| Baseline LVEF, % | ||||||
| ≤35 | 0.96 (0.88-1.05) | .37 | .56 | 0.9 (0.61-1.22) | .39 | .44 |
| >35 | 0.92 (0.85-1.00) | .06 | 0.7 (0.52-0.98) | .04 | ||
| Baseline AVA, cm2 | ||||||
| <0.6 | 0.97 (0.89-1.05) | .43 | .49 | 0.7 (0.47-0.96) | .03 | .26 |
| ≥0.6 | 0.93 (0.85-1.01) | .09 | 0.9 (0.65-1.22) | .47 | ||
| Baseline AV mean gradient, mm Hg | ||||||
| <50 | 0.94 (0.82-1.09) | .41 | .85 | 0.8 (0.64-1.07) | .15 | .82 |
| ≥50 | 0.96 (0.90-1.03) | .22 | 0.8 (0.45-1.30) | .32 | ||
| Baseline AR | ||||||
| None/mild | 1.05 (0.87-1.25) | .63 | .28 | 0.8 (0.60-0.98) | .01 | .17 |
| ≥Moderate | 0.94 (0.88-1.01) | .07 | 1.3 (0.64-2.62) | .47 | ||
| Baseline MR | ||||||
| None/mild | 0.92 (0.82-1.03) | .14 | .40 | 0.8 (0.63-1.10) | .20 | .53 |
| ≥Moderate | 0.97 (0.90-1.05) | .46 | 0.7 (0.45-1.11) | .13 | ||
Abbreviations: aHR, adjusted hazard ratio; AR, aortic regurgitation; AV, aortic valve; AVA, aortic valve area; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; STS, Society of Thoracic Surgeons.
Discussion
In this study of high- or intermediate-risk patients with symptomatic severe AS and baseline LVEF less than 50% undergoing TAVR, we report the following findings: (1) 1 in 3 patients experienced early improvement in LVEF of 10% points or more; (2) prior MI, diabetes, cancer, higher baseline LVEF, larger LVEDD, and larger AVA were associated with lower likelihood of early LVEF improvement, whereas higher BMI and higher SVi were associated with increased odds of early LVEF improvement after TAVR; (3) LVEF improvement at 30 days or 1 year after TAVR was associated with lower 5-year all-cause and cardiac death; and (4) there was significant interaction by sex such that early LVEF improvement was associated with improved 5-year outcomes in female individuals but not in male individuals.
Approximately 20% to 30% of patients with severe AS have LVEF less than 50%.7,12 LV systolic dysfunction in patients with AS may be caused by afterload mismatch when LV pressure overload results in decrease in stroke volume and ejection fraction, decreased contractility due to myocyte loss and myocardial fibrosis as a result of LV hypertrophy or superimposed myocardial ischemia/infarction, or an underlying myocardial process that leads to decline in LVEF even before AS becomes severe.12,13,14 Prior studies have shown that in extreme-/high-risk patients with symptomatic severe AS and LVEF less than 50%, approximately 50% to 60% experience early LVEF improvement (increase of ≥10% points within 30 days) after TAVR.2,3,4 However, in the current study early improvement in LVEF was seen in only 33% of patients, likely due to the relatively higher baseline LVEF, especially in the intermediate-risk patient cohort.
Prior MI, diabetes, and cancer—comorbidities that are known to adversely influence LV systolic function either directly or indirectly (eg, cardiotoxic chemotherapy in patients with cancer) independent of severe AS—were associated with lower likelihood of LVEF improvement following TAVR. Higher baseline LVEF, larger LVEDD (a marker of LV dilation), and larger AVA (suggestive of less severe AS, and likely non-AS–related cardiomyopathy) were also associated with lower likelihood of LVEF improvement. In contrast, higher BMI and higher SVi were associated with higher odds of LVEF improvement after TAVR. Patients with severe AS and reduced SVi (or low flow) tend to have lower LVEF and greater myocardial fibrosis, which could explain the positive association between SVi and LVEF recovery following TAVR.15,16 The mechanism underlying the association of higher BMI with LVEF improvement is unclear but may play in role in the obesity paradox (lower 1-year mortality in patients with overweight and obesity compared with patients with normal weight) seen after TAVR.17 The independent predictors of early LVEF improvement identified in this study may help counsel patients about the expected benefits of TAVR in terms of LVEF recovery and to individualize treatment decisions. For instance, patients with severe AS with prior MI, diabetes, or cancer, and baseline LV dysfunction may require further optimization of guideline-directed medical therapy for heart failure with reduced ejection fraction even after TAVR.18,19 Future studies are needed to determine if earlier intervention (eg, TAVR for moderate AS) with or without additional imaging (eg, cardiac magnetic resonance to identify different patterns of myocardial fibrosis/late gadolinium enhancement) is warranted in this patient population to improve the likelihood of LVEF recovery and clinical outcomes post-TAVR.20,21,22
Data on the association between baseline LV dysfunction and outcomes in patients with severe AS undergoing TAVR have been inconsistent.2,7,8 However, in patients with severe AS and LV systolic dysfunction, failure to improve LVEF after TAVR is associated with increased 1-year mortality. Similarly, incomplete LV recovery is independently associated with increased mortality and heart failure after surgical aortic valve replacement in patients with LV systolic dysfunction.23 Our current study extends these observations to 5-year clinical outcomes by demonstrating that LVEF improvement at 30 days or 1 year after TAVR was associated with reduced all-cause and cardiac death at 5 years. Each improvement of 5% points in LVEF at 30 days was associated with a 6% and 10% lower risk of all-cause and cardiac death at 5 years, respectively. Restricted cubic spline analysis demonstrated a visual inflection point at ΔLVEF of 10% beyond which there was a steep decline in all-cause mortality with increasing degree of early LVEF improvement. We found no statistically significant differences in rehospitalization, NYHA functional class, or Kansas City Cardiomyopathy Questionnaire Overall Summary Score in patients with vs without early LVEF improvement. Thus, the pathophysiologic mechanism mediating reduced mortality in patients with LVEF recovery likely extends beyond improvement in heart failure symptoms (eg, improvement in kidney function, reduction in sudden cardiac death) and warrants further investigation.24
We found that early LVEF improvement after TAVR was associated with lower all-cause and cardiac death in female individuals but not in male individuals. Several studies have demonstrated sex differences in LV remodeling in response to AS with more concentric LV hypertrophy, lower LV mass index, smaller LV cavity dimensions, smaller end-systolic and diastolic volumes, and less myocardial fibrosis in female vs male individuals.25,26,27,28 Maladpative LV remodeling is associated with impaired survival in female individuals but not male individuals after surgical aortic valve replacement.29 However, data on sex differences in reversal of LV remodeling following aortic valve replacement are conflicting. Petrov et al30 reported more frequent reversal of LV hypertrophy in female vs male individuals after surgical aortic valve replacement for severe AS. Similarly, Chen et al31 found that female individuals with AS had favorable reverse LV remodeling with greater and earlier LV mass regression post-TAVR compared with male individuals. In contrast, other studies in patients undergoing TAVR have shown similar regression of hypertrophy as measured by LV mass index in female and male individuals.32,33 Why LVEF improvement is associated with lower mortality in female individuals but not in male individuals remains unclear and warrants further scrutiny. Because LVEF underestimates the degree of LV systolic dysfunction in presence of concentric hypertrophy, which is more prevalent and more pronounced in female vs male individuals, it is possible that post-TAVR, the regression of the concentric hypertrophy may, at least in part, mask the magnitude of improvement in LV systolic function and LVEF such that an improvement of 10% points in LVEF in female individuals may actually represent a more important true improvement in LV systolic function than the same improvement of 10% points in male individuals. Another potential explanation is that female individuals enrolled in the PARTNER trials and registries had less comorbidities (eg, lower prevalence of CAD) and hence fewer competing risks compared with male individuals, and were therefore more likely to translate improvement in LVEF post-TAVR into mortality benefit. Nonetheless, our findings suggest that the favorable survival in female vs male individuals after TAVR demonstrated in previous studies may at least partly be mediated via improvement in LVEF.34
Limitations
Our study has certain limitations that must be acknowledged. First, patients with very severe LV dysfunction (LVEF <20%) and those with low-gradient AS (AV mean gradient <40 mm Hg) were excluded from the PARTNER trials. Therefore, our findings may not be applicable to those 2 groups of patients. Second, the exact etiology of LV dysfunction was not known. Patients with LV dysfunction secondary to nonvalvular heart disease (eg, CAD, amyloidosis) may not experience LVEF recovery even after treatment of AS with TAVR. Third, our study only included patients at high or intermediate risk for surgery, so these observations may not be generalizable to low-risk patients. Lastly, these findings may not be generalizable to patients with severe AS and LV dysfunction treated with TAVR bioprostheses other than SAPIEN, SAPIEN XT, or SAPIEN 3 (Edwards).
Conclusions
In high- or intermediate-risk patients with symptomatic severe AS and LVEF less than 50% undergoing transfemoral TAVR, 1 in 3 experienced early improvement in LVEF. Prior MI, diabetes, cancer, higher baseline LVEF, larger LVEDD, and larger AVA were associated with lower likelihood of early LVEF improvement, whereas higher BMI and higher SVi were associated with increased likelihood of early LVEF improvement after TAVR. Early LVEF improvement after TAVR was associated with lower 5-year all-cause death and cardiac death. Further studies are needed to understand the pathophysiologic mechanism(s) mediating reduced mortality in patients with early LVEF recovery after TAVR.
eFigure 1. Early LVEF Improvement After TAVR in Patients With Severe AS and Baseline LVEF <50%
eFigure 2. Composite of All-Cause Death or Rehospitalization and Cardiac Death or Rehospitalization at 5 Years in Patients With vs. Without Early LVEF Improvement After TAVR
eFigure 3. NYHA Functional Class in Patients With vs. Without Early LVEF Improvement After TAVR
eFigure 4. KCCQ-OS in Patients With vs. Without Early LVEF Improvement After TAVR
eFigure 5. Association Between ΔLVEF and 5-Year All-Cause Mortality After TAVR
eFigure 6. 5-Year Clinical Outcomes in Patients With vs. Without LVEF Improvement at 1 Year After TAVR
eFigure 7. 5-Year Outcomes in Men and Women With vs. Without Early LVEF Improvement After TAVR
eTable 1. Baseline Characteristics of Men With vs. Without Early LVEF Improvement Following TAVR
eTable 2. Baseline Characteristics of Women With vs. Without Early LVEF Improvement Following TAVR
eTable 3. Baseline Characteristics of Men vs. Women Without Early LVEF Improvement Following TAVR
eTable 4. Baseline Characteristics of Men vs. Women With Early LVEF Improvement Following TAVR
eTable 5. Predictors of Degree of LVEF Improvement (ΔLVEF) After TAVR
eTable 6. Adjusted Association Between LVEF Improvement at 1 Year and 5-Year Clinical Outcomes After TAVR
eTable 7. Association Between Early LVEF Improvement and 5-Year Outcomes After TAVR by Sex
References
- 1.Otto CM, Nishimura RA, Bonow RO, et al. ; Writing Committee Members . 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25-e197. doi: 10.1016/j.jacc.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 2.Elmariah S, Palacios IF, McAndrew T, et al. ; PARTNER Investigators . Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: results from the Placement of Aortic Transcatheter Valves (PARTNER) trial (cohort A). Circ Cardiovasc Interv. 2013;6(6):604-614. doi: 10.1161/CIRCINTERVENTIONS.113.000650 [DOI] [PubMed] [Google Scholar]
- 3.Dauerman HL, Reardon MJ, Popma JJ, et al. Early recovery of left ventricular systolic function after CoreValve transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2016;9(6):e003425. doi: 10.1161/CIRCINTERVENTIONS.115.003425 [DOI] [PubMed] [Google Scholar]
- 4.Passeri JJ, Elmariah S, Xu K, et al. ; PARTNER Investigators . Transcatheter aortic valve replacement and standard therapy in inoperable patients with aortic stenosis and low EF. Heart. 2015;101(6):463-471. doi: 10.1136/heartjnl-2014-306737 [DOI] [PubMed] [Google Scholar]
- 5.Poulin F, Carasso S, Horlick EM, et al. Recovery of left ventricular mechanics after transcatheter aortic valve implantation: effects of baseline ventricular function and postprocedural aortic regurgitation. J Am Soc Echocardiogr. 2014;27(11):1133-1142. doi: 10.1016/j.echo.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Deste W, Gulino S, Zappulla P, et al. Early recovery of left ventricular systolic function after transcatheter aortic valve implantation. J Cardiovasc Echogr. 2018;28(3):166-170. doi: 10.4103/jcecho.jcecho_13_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron SJ, Arnold SV, Herrmann HC, et al. Impact of ejection fraction and aortic valve gradient on outcomes of transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67(20):2349-2358. doi: 10.1016/j.jacc.2016.03.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furer A, Chen S, Redfors B, et al. Effect of baseline left ventricular ejection fraction on 2-year outcomes after transcatheter aortic valve replacement: analysis of the PARTNER 2 trials. Circ Heart Fail. 2019;12(8):e005809. doi: 10.1161/CIRCHEARTFAILURE.118.005809 [DOI] [PubMed] [Google Scholar]
- 9.Smith CR, Leon MB, Mack MJ, et al. ; PARTNER Trial Investigators . Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 10.Leon MB, Smith CR, Mack MJ, et al. ; PARTNER 2 Investigators . Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609-1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 11.Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387(10034):2218-2225. doi: 10.1016/S0140-6736(16)30073-3 [DOI] [PubMed] [Google Scholar]
- 12.Ito S, Miranda WR, Nkomo VT, et al. Reduced left ventricular ejection fraction in patients with aortic stenosis. J Am Coll Cardiol. 2018;71(12):1313-1321. doi: 10.1016/j.jacc.2018.01.045 [DOI] [PubMed] [Google Scholar]
- 13.Ross J Jr. The concept of afterload mismatch and its implications in the clinical assessment of cardiac contractility. Jpn Circ J. 1976;40(8):865-875. doi: 10.1253/jcj.40.865 [DOI] [PubMed] [Google Scholar]
- 14.Huber D, Grimm J, Koch R, Krayenbuehl HP. Determinants of ejection performance in aortic stenosis. Circulation. 1981;64(1):126-134. doi: 10.1161/01.CIR.64.1.126 [DOI] [PubMed] [Google Scholar]
- 15.Herrmann S, Störk S, Niemann M, et al. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58(4):402-412. doi: 10.1016/j.jacc.2011.02.059 [DOI] [PubMed] [Google Scholar]
- 16.Rosa VEE, Ribeiro HB, Sampaio RO, et al. Myocardial fibrosis in classical low-flow, low-gradient aortic stenosis. Circ Cardiovasc Imaging. 2019;12(5):e008353. doi: 10.1161/CIRCIMAGING.118.008353 [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Lavie CJ, Elmariah S, et al. Relationship of body mass index with outcomes after transcatheter aortic valve replacement: results from the National Cardiovascular Data-STS/ACC TVT Registry. Mayo Clin Proc. 2020;95(1):57-68. doi: 10.1016/j.mayocp.2019.09.027 [DOI] [PubMed] [Google Scholar]
- 18.Goel SS, Kleiman NS, Zoghbi WA, Reardon MJ, Kapadia SR. Renin-angiotensin system blockade in aortic stenosis: implications before and after aortic valve replacement. J Am Heart Assoc. 2020;9(18):e016911. doi: 10.1161/JAHA.120.016911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito T, Yoshijima N, Hase H, et al. Impact of beta blockers on patients undergoing transcatheter aortic valve replacement: the OCEAN-TAVI registry. Open Heart. 2020;7(2):e001269. doi: 10.1136/openhrt-2020-001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitzer E, Van Mieghem NM, Pibarot P, et al. Rationale and design of the Transcatheter Aortic Valve Replacement to UNload the Left ventricle in patients with ADvanced heart failure (TAVR UNLOAD) trial. Am Heart J. 2016;182:80-88. doi: 10.1016/j.ahj.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 21.Jean G, Van Mieghem NM, Gegenava T, et al. Moderate aortic stenosis in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77(22):2796-2803. doi: 10.1016/j.jacc.2021.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bing R, Cavalcante JL, Everett RJ, Clavel MA, Newby DE, Dweck MR. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC Cardiovasc Imaging. 2019;12(2):283-296. doi: 10.1016/j.jcmg.2018.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Une D, Mesana L, Chan V, et al. Clinical impact of changes in left ventricular function after aortic valve replacement: analysis from 3112 patients. Circulation. 2015;132(8):741-747. doi: 10.1161/CIRCULATIONAHA.115.015371 [DOI] [PubMed] [Google Scholar]
- 24.Cubeddu RJ, Asher CR, Lowry AM, et al. ; PARTNER Trial Investigators . Impact of transcatheter aortic valve replacement on severity of chronic kidney disease. J Am Coll Cardiol. 2020;76(12):1410-1421. doi: 10.1016/j.jacc.2020.07.048 [DOI] [PubMed] [Google Scholar]
- 25.Carroll JD, Carroll EP, Feldman T, et al. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86(4):1099-1107. doi: 10.1161/01.CIR.86.4.1099 [DOI] [PubMed] [Google Scholar]
- 26.Aurigemma GP, Gaasch WH. Gender differences in older patients with pressure-overload hypertrophy of the left ventricle. Cardiology. 1995;86(4):310-317. doi: 10.1159/000176895 [DOI] [PubMed] [Google Scholar]
- 27.Douglas PS, Otto CM, Mickel MC, Labovitz A, Reid CL, Davis KB; NHLBI Balloon Valvuloplasty Registry . Gender differences in left ventricle geometry and function in patients undergoing balloon dilatation of the aortic valve for isolated aortic stenosis. Br Heart J. 1995;73(6):548-554. doi: 10.1136/hrt.73.6.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tastet L, Kwiecinski J, Pibarot P, et al. Sex-related differences in the extent of myocardial fibrosis in patients with aortic valve stenosis. JACC Cardiovasc Imaging. 2020;13(3):699-711. doi: 10.1016/j.jcmg.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 29.Petrov G, Dworatzek E, Schulze TM, et al. Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc Imaging. 2014;7(11):1073-1080. doi: 10.1016/j.jcmg.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 30.Petrov G, Regitz-Zagrosek V, Lehmkuhl E, et al. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation. 2010;122(11)(suppl):S23-S28. doi: 10.1161/CIRCULATIONAHA.109.927764 [DOI] [PubMed] [Google Scholar]
- 31.Chen SC, Leu HB, Chang HH, et al. Women had favourable reverse left ventricle remodelling after TAVR. Eur J Clin Invest. 2020;50(1):e13183. doi: 10.1111/eci.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stangl V, Baldenhofer G, Knebel F, et al. Impact of gender on three-month outcome and left ventricular remodeling after transfemoral transcatheter aortic valve implantation. Am J Cardiol. 2012;110(6):884-890. doi: 10.1016/j.amjcard.2012.04.063 [DOI] [PubMed] [Google Scholar]
- 33.Chau KH, Douglas PS, Pibarot P, et al. Regression of left ventricular mass after transcatheter aortic valve replacement: the PARTNER Trials and Registries. J Am Coll Cardiol. 2020;75(19):2446-2458. doi: 10.1016/j.jacc.2020.03.042 [DOI] [PubMed] [Google Scholar]
- 34.Zhao ZG, Liao YB, Peng Y, et al. Sex-related differences in outcomes after transcatheter aortic valve implantation: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6(5):543-551. doi: 10.1161/CIRCINTERVENTIONS.113.000529 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Early LVEF Improvement After TAVR in Patients With Severe AS and Baseline LVEF <50%
eFigure 2. Composite of All-Cause Death or Rehospitalization and Cardiac Death or Rehospitalization at 5 Years in Patients With vs. Without Early LVEF Improvement After TAVR
eFigure 3. NYHA Functional Class in Patients With vs. Without Early LVEF Improvement After TAVR
eFigure 4. KCCQ-OS in Patients With vs. Without Early LVEF Improvement After TAVR
eFigure 5. Association Between ΔLVEF and 5-Year All-Cause Mortality After TAVR
eFigure 6. 5-Year Clinical Outcomes in Patients With vs. Without LVEF Improvement at 1 Year After TAVR
eFigure 7. 5-Year Outcomes in Men and Women With vs. Without Early LVEF Improvement After TAVR
eTable 1. Baseline Characteristics of Men With vs. Without Early LVEF Improvement Following TAVR
eTable 2. Baseline Characteristics of Women With vs. Without Early LVEF Improvement Following TAVR
eTable 3. Baseline Characteristics of Men vs. Women Without Early LVEF Improvement Following TAVR
eTable 4. Baseline Characteristics of Men vs. Women With Early LVEF Improvement Following TAVR
eTable 5. Predictors of Degree of LVEF Improvement (ΔLVEF) After TAVR
eTable 6. Adjusted Association Between LVEF Improvement at 1 Year and 5-Year Clinical Outcomes After TAVR
eTable 7. Association Between Early LVEF Improvement and 5-Year Outcomes After TAVR by Sex


