Abstract
The gut microbiota and its metabolites have been shown to play a pivotal role in the regulation of metabolic, endocrine and immune functions. Though the exact mechanism of action remains to be fully elucidated, available knowledge supports the ability of microbiota-fermented short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, to influence epigenetic and metabolic cascades controlling gene expression, chemotaxis, differentiation, proliferation, and apoptosis in several non-immune and immune cell subsets. While used as preferred metabolic substrates and sources of energy by colonic gut epithelial cells, most recent evidence indicates that these metabolites regulate immune functions, and in particular fine-tune T cell effector, regulatory and memory phenotypes, with direct in vivo consequences on the efficacy of chemotherapy, radiotherapy and immunotherapy. Most recent data also support the use of these metabolites over the course of T cell manufacturing, paving the way for refined adoptive T cell therapy engineering. Here, we review the most recent advances in the field, highlighting in vitro and in vivo evidence for the ability of SCFAs to shape T cell phenotypes and functions.
Keywords: T-lymphocytes; lymphocyte activation; immunotherapy, adoptive; review
Introduction
In recent years, the influence of the gut microbiome on remote organs, and mucosal and immune functions has been uncovered and exploited in the management of inflammatory conditions.1 Indeed, a role for the gut microbiome has been recognized in gastrointestinal disorders, and in the development and potential treatment of graft vs host disease, cardiovascular and neurodegenerative diseases, and cancer.2–5 Because of the possibility to influence the microbiome composition and the seemingly beneficial impact of this strategy on several disease states, research efforts have been focused on the identification of critical microbial-derived metabolites, and their therapeutic exploitation. In particular, short chain fatty acids (SCFAs) have recently gained specific attention for their impact on colon health, and for their immune-modulatory activities, which include both anti-inflammatory and tumor-suppressive functions.6 As their relative concentration can be sensitive to diet,7 and to prebiotics (complex carbohydrates that can be fermented by colonic bacteria) and probiotics (live bacteria that promote colonic health), increasingly being used in clinical practice,8 their modulation is raising interest as a therapeutic option.
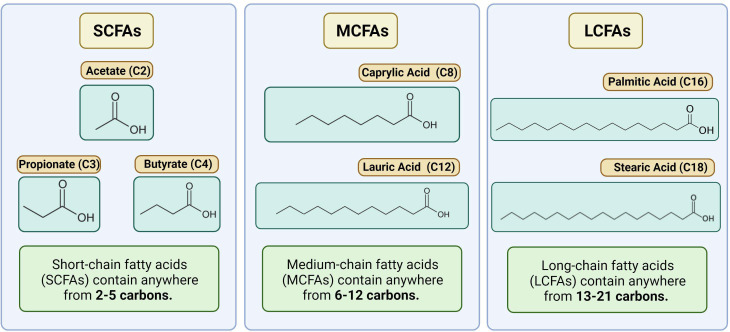
SCFAs are part of the larger fatty acids (FAs) family, classified according to the length in carbon atoms, their degree of saturation (saturated, monounsaturated, and polyunsaturated) and their cisorientation or transorientation of double bonds (for a more extensive description of the general structure of FAs and their impact on lipid metabolism in T cells, please refer to Howie et al)9 SCFAs are saturated monocarboxylic FAs with up to five carbons in a single chain. These include formate (C1), acetate (C2), propionate (C3), butyrate (C4), and valerate (C5).10 They are distinguished from longer FAs, which include medium-chain (6–12 carbons, MCFAs), long-chain FAs (13–21 carbons, LCFAs), and very long-chain FAs (beyond 21) (a schematic representation of the most studied C2-C4, and examples of MCFAs and LCFAs are depicted in figure 1). SCFAs are generated by gut microbes through the fermentation of non-digestible fibers and dietary carbohydrates, thus their concentration might vary according to the microbe composition and the anatomical location (refer to Cong et al for a recent comprehensive review).11 Thanks to shorter hydrophobic chains and the hydrophilic carboxyl group, SCFAs are water soluble and readily absorbed or transported into colon epithelial cells and used as the preferred energy substrates. In the proximal and distal portion of the human colon, the luminal concentrations of C2, C3, and C4 can, respectively, reach a relative concentration of ~130 nmol/Kg and 80 nmol/kg.12 13 In another study, Rombeau et al approximated SCFA concentrations in the content of the human colon to be 75 mM for acetate (C2), 30 mM for propionate (C3), and 20 mM for butyrate (C4).14 While a significant fraction is directly consumed by colonocytes, passive diffusion and active transport grant that micromolar concentrations are found in the portal blood and the liver (~250 µM for C2, 20–200 µM for C3, and 15–65 µM for C4), and also in the peripheral blood (20–150 µM for C2, 1–13 µM for C3, and 1–12 µM for C4),13 15 with various effects on different organ sites.16 The finding that all the major SCFAs are present in portal blood at concentrations several times greater than peripheral venous blood strongly suggests the colon is the major source of these FAs.17 18 Accordingly, dietary changes can alter their relative concentrations, as also infectious agents and health conditions.19 As an example, patients with cirrhosis revealed higher SCFA levels in the liver and in the portal vein than those measured in healthy controls.20
Figure 1.
Schematic representation of SCFA, MCFA and LCFA. The picture depicts the most common C2-C4 SCFAs, and representative members of the MCFA and LCFA family members.
T lymphocytes are sensitive and dependent on extra and intracellular FAs over the course of activation, proliferation, and memory differentiation. Numerous FA receptor/binding proteins regulate their relative representation in the extracellular environment, or their ability to signal via membrane-anchored or nuclear receptors.9 Several FAs act as ligands for nuclear receptors, a group of ligand-binding transcription factors and mediators of various metabolic and signaling pathways.21 In particular, long-chain polyunsaturated fatty acyls are considered the preferred ligands for the peroxisome proliferator-activated receptors,22 which are able to control lipid metabolism and Th1, Th2, Th17, and Treg cell differentiation.23 SCFAs instead are mostly known to act via G-protein-coupled receptor (GPCR) signaling, histone deacetylase (HDAC) inhibition, acetyl-CoA production, and metabolic integration.24 Multiple studies have established that T cell functions are sensitive to SCFA exposure, both in vitro and in vivo. Indeed, SCFAs can either promote a regulatory T cell phenotype or imprint T cells with effector functions, opening their possible exploitation to fine-tune adoptive T cell therapy (ACT) protocols. This review aims to highlight putative benefits and drawbacks of the use of SCFAs as immunomodulators, in both animal models and clinical studies, and to suggest how available knowledge can be translated to the most effective ACT protocols against cancer.
SCFAs: mechanisms and specificities in T cells
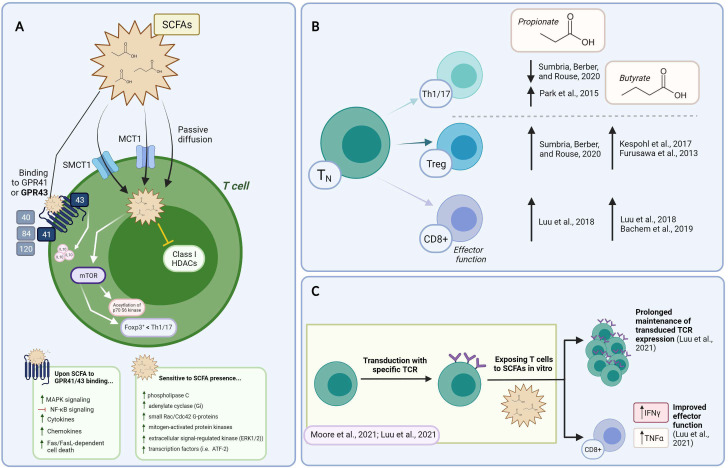
SCFAs reach the cytoplasm either via passive diffusion across the plasma membrane or membrane transporters including MCT1 (monocarboxylate transporter-1/Slc16a1) and SMCT1 (sodium-coupled monocarboxylate transporter-1/Slc5 a8) or (summarized in figure 2A).25 MCT1 is a proton-linked monocarboxylic acid transporter, and a member of the MCTs family. MCT1 is constitutively expressed by colonic epithelial cells and upregulated over the course of T cell activation.26 It is well known for its role in transporting lactate, the most common short chain hydroxy- FA, which can be converted to other SCFAs by a sub-group of lactate-fermenting bacterial species.27 Its inhibition hinders T lymphocyte glycolysis,28 while promoting responses to anti-PD1 therapy.26 MCT1 can bind to the immunoglobulin family member CD147 or extracellular matrix metalloproteinase inducer (Emmprin or Basigin), which is also expressed on activated T cells,29 30 and was found to identify subsets of memory T cells in rheumatoid arthritis patients and of highly suppressive subset T regulatory cells.31 32 SMCT1 is a Na+-coupled electrogenic transporter for SCFAs. It has a preferred affinity for butyrate, followed by propionate, lactate, and acetate. It is predominantly expressed by colonocytes, dendritic cells (DCs), kidney, retinal and brain cells, while absent in T cells.33
Figure 2.
Constructing the mechanism of action and the impact of SCFAs on T cell functions in vitro. (A) Summary of SCFAs mechanism of action in T cells. The ability of SCFA to freely diffuse across the plasma membrane, be transported or bind to GPCR is shown. Their reported mechanism of action mediated by intracellular signaling events and HDAC is also depicted. (B) Supplementing T cell cultures with SCFAs impacts on Th1/17, Treg, and CD8+ T cell functional phenotypes (described within the text). Supporting references are indicated. (C) Exploiting SCFA over the course of TCR/CAR-T manufacturing. Data supports the ability of SCFAs to prolong transgene expression and increase effector functions. CAR, chimeric antigen receptor; HADS, histone deacetylase; SCFAs, short-chain fatty acids;
SCFAs can also bind GPCRs on the cell membrane. Although the precise mechanism of action remains to be defined, SCFAs are thought to exert their functions mainly through GPCR-induced signaling, the inhibition of HDACs, metabolism tuning, and protein acetylation (summarized in figure 2A).34 Five GPRs with different binding affinity for FAs of various lengths have been described: GPR 40, 41, 43, 84, and 120. SCFAs mostly interact with GPR41 and 43, with different affinities for C2, C3 and C4, while GPR84 binds MCFAs, and GPR40 and GPR120 LCFAs (summarized in figure 2A).9 Another SCFA receptor belonging to a sub-class of GPCRs, the olfactory receptor 78 (Olfr78), senses C2 and C3. Most mature T cells have minimal expression of SCFA-sensing GPRs, except some memory CD8+ T cells which express Olfr78, and colonic regulatory T cells (Tregs) described to express GPR43.35 36 In addition, GPR43 has also been found expressed on colonic group 3 innate lymphoid cells (ILC s3) and on γδ T cells.37 Phospholipase C, adenylate cyclase, small Rac/Cdc42 G-proteins, mitogen-activated protein kinases (p38, c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK1/2)) and transcription factors (ie, ATF-2) are among the signaling molecules sensitive to SCFAs (summarized in figure 2A).38 Concentrations above 3 mM were reported to induce Fas upregulation and cause Fas/FasL-dependent cell death (summarized in figure 2A).39
Data support the ability of SCFAs to mediate intracellular signaling events linked to the shaping of T cell fate, via both GPCR-dependent and independent mechanisms. For instance, Sun et al found that acetate (C2, 10 mM), propionate (C3, 0.5 mM), and butyrate (C4, 0.5 mM) favored the upregulation of IL-10 in Th1 cells with regulatory functions via GPR43 and mTOR and STAT3-dependent Blimp-1 expression (summarized in figure 2A).36 These authors also found that oral feeding of mice with butyrate (C4, 200 mM) in the drinking water recapitulated these events and protected mice from DSS-induced colitis.36 Likewise, Trompette et al found that GPR41 was needed for butyrate to improve cytotoxic T lymphocytes (CTL) expansion and effector functions.40 Indeed, provision of butyrate over the course of naïve CD8+ T cell activation in vitro improved the acquisition of cytolytic activities (0.5–1 mM), while oral administration of butyrate over a 2 week period (500 mM) boosted anti-viral T cell immunity.40 Pentanoate and butyrate (0.25–10 mM) were also recently described to augment IFNγ and TNFα secretion by activated CTLs via GPR41 and GPR43-independent mechanisms.35 41 In the case of γδ T cells, Dupraz et al found that propionate (10 mM) inhibited IL-17 and IL-22 production by intestinal murine and human γδ T cells, by a mechanism independent of GPR43 and also of MCT1.42 Thus, data generated so far suggests that SCFA play a role in lymphocyte differentiation, only in part through GPR41 and GPR43.
Other important functions of SCFAs reside in their role as a source of acetyl-CoA and also in their ability to inhibit HDACs. Indeed, SCFAs can be converted to acetyl-CoA, which fuels major metabolic processes, such as the mitochondrial tricarboxylic acid (TCA) cycle, FA synthesis, and protein acetylation. When integrated into the Krebs cycle, acetyl-CoA increases energy (ATP/ADP) production, leading to mTOR activation. This has been found to favor T cell differentiation into effector T cells such as Th1 and Th17 cells at the expense of FoxP3+ T cells (summarized in figure 2A).25 Acetyl groups derived from acetyl-CoA are also used by histone acetyltransferases (HATs) to decorate histone tails and promote gene transcription.43 This has been described in epithelial cells where SCFAs were found to regulate HAT and hypoxia inducible factor (HIF) stability.44 Also, non-histone proteins, including signaling molecules and transcription factors, can undergo acetylation in response to SCFA, ultimately regulating transcriptional processes.45 For instance, in CD4+ T cells activated in Th17 conditions, Park et al reported that acetate (10 mM), propionate (1 mM) and butyrate (0.1–0.5 mM) controlled acetylation of p70 S6 kinase, downstream to mTOR signaling independently of GPR41 and GPR43 (summarized in figure 2A).25 Accordingly, propionate and butyrate were found to be capable of inhibiting class I HDACs.46 47 These remove acetyl groups, leading to the tightening of chromatin and transcriptional repression. In T cells, the FoxP3 and IL-10 gene loci are targets of such regulation.48 Via their activities as HDAC inhibitors, acetate, propionate and butyrate also enhance aryl hydrocarbon receptor (AhR) ligand-induced responses in gene- and cell context-dependent events.20 49 50 Although whether SCFAs control HDAC activity directly or indirectly requires further investigation, it is interesting to note that these metabolites can simultaneously increase availability of acetyl groups and block HDACs, jointly increasing acetylation events and favoring gene expression. Additional studies will improve our understanding of SCFA-controlled events linked to metabolic and epigenetic pathways.
SCFAs shape T cell differentiation in vitro
Antigen recognition via the T cell receptor (TCR) and the engagement of costimulatory and cytokine receptors induces a complex signaling cascade that leads to the activation, proliferation, and differentiation of naïve T cells and the acquisition of effector or regulatory activities. Effector T cells fight pathogens and cause tissue inflammation, while regulatory T cells counterbalance such events. T cell differentiation involves heritable changes to the epigenetic landscape, and also metabolic rewiring involving Akt-mTOR (mammalian target of rapamycin) signaling.51 52 While naïve T cells mostly rely on the TCA cycle and oxidative phosphorylation, activated T cells switch to glycolysis, glutaminolysis and FA synthesis to accommodate an increased demand for energy.53 Nutrients and metabolites provide significant regulatory signals for metabolic adaptation and ensuing T cell differentiation. It is now clear that the gut microbiota as well as SCFAs, in the form of acetate, butyrate, and propionate shape the generation of both effector and regulatory T cells via epigenetic and metabolic events.54
In the case of CD4+ T cells, the effects of SCFAs vary according to the activation conditions and the cytokine milieu. Furusawa et al found that the addition of 0.1 mM butyrate to cultures of CD4+ T cells activated with anti-CD3 and -CD28 antibodies, TGF-β1 and IL-2, promoted an increase in genome-wide histone H3 acetylation and Foxp3 expression, leading to Treg differentiation (summarized in figure 2B).55 More recently, Kespohl et al extended this study and found that while low butyrate concentrations (0.1–0.5 mM) facilitated the differentiation of Foxp3+ Tregs, higher concentrations (1 mM) induced the expression of T-bet and IFNγ via the control of promoters histone acetylation (summarized in figure 2B).56 Park et al also found that titrating amounts of acetate (1–10 mM) and propionate (0.1–1 mM) and butyrate (0-1-0.5mM) supported Th1 or Th17 effector cell differentiation via HDAC inhibition and independently of GPR41or GPR43. These SCFA-conditioned cells, however, also produced IL-10 proving less inflammatory in vivo compared unconditioned ones (summarized in figure 2B).25 In another study, increasing concentrations of propionate (100–400 uM) in 5-day CD3/CD28 cultures of naïve CD4+ T cells inhibited Th1 and Th17 T cells differentiation, while promoting FoxP3+ Tregs (summarized in figure 2B).57 Yang et al also found that SCFAs (acetate, 10 mM; propionate, 0.5 mM; butyrate, 0.5 mM) promoted IL-22 production in CD4+ T cells and ILCs through GPR41 and HDAC inhibition and the expression of the AhR and the HIF1α transcription factors.50 While results might appear contradictory, it should be noted that the level of CD3 stimulation, the cytokine milieu, and the relative concentration of SCFAs adopted in the above-mentioned studies varied, suggesting that SCFAs might balance CD4+ T cell regulatory/effector function according to the milieu. In the case of CD8+ T cells, exposure to 1 mM propionate and butyrate was mostly shown to augment expression of IFNγ and Granzyme B (GzB), and effector function at large (summarized in figure 2B).41 Mechanistically, butyrate increased acetylation of histone H4 at Tbx21 and IFNγ promoters, supporting an HDAC-dependent mechanism. Of note in this study, acetate also promoted IFNγ expression, but via a mechanism less dependent on HDAC inhibition, and rather linked to CTLs metabolism. This observation is in line with a previous report showing that acetate conversion to acetyl-CoA supported GAPDH/glycolytic activity, linked to rapid recall responses of memory CD8+ T cells.58 Similarly, Qiu et al reported that 1 mM acetate could rescue IFNγ expression by CD8+ T cells cultured in glucose-restricted conditions, by controlling histone acetylation and chromatin accessibility in an acetyl-CoA synthetase-dependent manner. The authors found that the same applied to exhausted T cells, through which the ability to express IFNγ was rescued by ex vivo acetate supplementation.59 Bachem et al more recently found that SCFAs also promote long-lasting memory phenotypes.60 In this work, authors found that acetate, propionate, and butyrate (0.5 mM) were able to support long-term survival of antigen-activated T cells, through the upregulation of FoxO1, a transcription factor required for memory T cell differentiation. These authors also reported that the transition to a memory phenotype was due to butyrate uncoupling the TCA from glycolysis, favoring the use of FA and glutaminolysis (summarized in figure 2B).60
Thus, there is evidence supporting the ability of SCFAs to influence T cell differentiation in vitro, via cell-autonomous events involving epigenetic and/or metabolic regulation. Although these effects might be peculiar to supraphysiological concentrations (in the mM range, ie, ~1000 and ~100 fold higher than those recently measured in the peripheral and the hepatic portal blood61) data also support the notion that SCFAs are active in vivo. Indeed, they can promote tolerogenic and anti-inflammatory profiles (by skewing Treg phenotypes), and also effector and memory functions (Th1/Th17, CTL), possibly via the integration of extracellular clues (TCR/CD28 stimuli, cytokines, nutrients). Such contrasting effects might account for some discrepant reports in vivo, and yet represent solid ground for SCFA exploitation in ACT protocols (summarized in figure 2C).
SCFAs have immunomodulatory effects on T cell-dependent responses in vivo
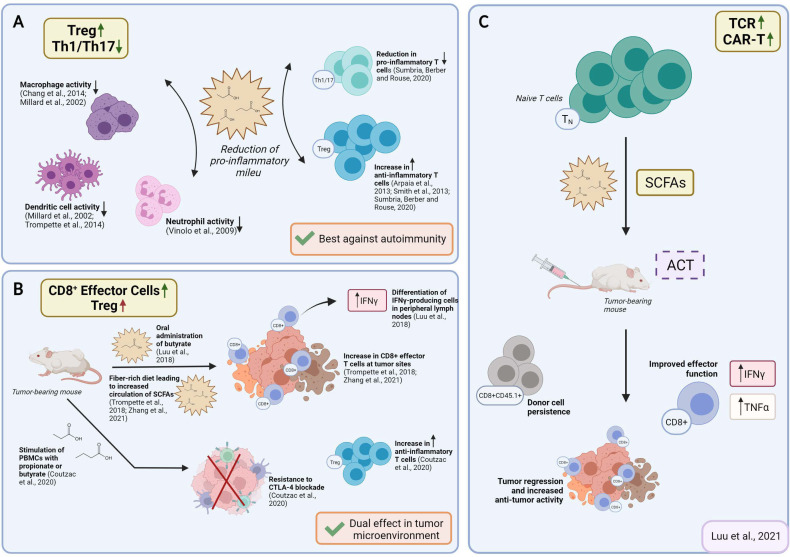
SCFAs in vivo have been reported to induce IL-10–expressing Foxp3+ and Foxp3− regulatory T cells and antagonize inflammation or effector T cells such as Th1 and Th17 cells and mediate inflammatory responses, depending on the physiological state, the immunological and the pathological milieu, and the relative SCFAs abundance. Beneficial anti-inflammatory functions of SCFAs were observed in several disease models, including colitis, and colitis-associated colon cancer, autoimmune manifestations, airway disease, metabolic syndrome, and ischemia-reperfusion injury of the kidney.6 62 In vivo, acetate, propionate, and butyrate feeding, was also shown to dampen immune responses via regulatory T cells,35 48 and also DCs,17 63 macrophages17 64 and neutrophils65 (summarized in figure 3A). Such effects were demonstrated in models as small as embryonic zebrafish, where exposing wound sites to butyrate (30 mM) significantly reduced the recruitment of M1-type proinflammatory macrophages, ultimately revealing anti-inflammatory activity.66 In a mouse model of corneal infection, propionate feeding (500 mM over 3 weeks) was found to have immunomodulatory effects at ocular lesion sites by concomitantly increasing Treg representation and reducing Th1 and Th17 pro-inflammatory T cells (summarized in figure 3A).57 Likewise, oral administration of propionate (150–200 mM) augmented Treg suppressive activity in an experimental autoimmune encephalomyelitis model,67 and Treg expansion in hypertensive cardiovascular damage models.68 Propionate was also reported to lower the number of proinflammatory Th17 cells in these models,68 and to hinder IL-17 production by mouse and human intestinal γδ T cells.42 Additionally, in a preclinical non-obese diabetic (NOD) model for autoimmune diabetes, acetate supplementation (200 mM) reduced the disease incidence, while butyrate or acetate-fortified diets respectively increased Treg number and activity and reduced autoreactive CD8+ effector T cells in lymphoid tissues.61 Nevertheless, supraphysiological doses of orally administered SCFAs (200 mM over 4 weeks) induced Th1 and Th17 effectors and IL-10+ regulatory T cells in ureter and kidney tissues, overall leading to T cell-mediated ureteritis, and kidney hydronephrosis.69 In two independent studies, administration of butyrate or a mix of acetate, butyrate, and propionate revealed protective effects against the development and the growth of colorectal cancer.70 71 Whether protective effects involved changes in T cell functions was not investigated.
Figure 3.
Reported effects of SCFAs on T cell functions in vivo and possible application for ACT strategies. (A) Reported effects of SCFAs in autoimmune manifestations. SCFAs can dampen the pro-inflammatory activities of macrophages, dendritic cells, and neutrophils, and Th1/17 cells, while upregulating anti-inflammatory Treg cells. (B) Reported pro- and anti-inflammatory activities of SCFAs in the context of cancer. Studies in tumor-bearing mice demonstrate that in vivo administration of SCFAs or of fiber rich diets can promote CD8+ T cell effector functions, but also increase the representation of Tregs. This is evidence for SCFAs having counter-regulatory effects according to the disease or the therapeutic setting. (C) Suggested use of SCFA in ACT settings. To take advantage of SCFAs in the context of ACT and overcome possibly pleiotropic effects observed by in vivo administration, preconditioning of T cell product by SCFAs supplementation represents a valuable strategy. ACT, adoptive T cell therapy; SCFAs, short-chain fatty acids.
Results from human trials reported so far appeared to support an anti-inflammatory effect secondary to SCFA administration to patients. SCFA supplementation was indeed tested in patients with multiple sclerosis patients, due to their reduced propionate concentration compared with healthy controls. Daily dietary supplementation with propionate was reported to restore plasma propionate concentration and also immunological parameters in MS patients with MS to those of healthy individuals.72 When tested as an adjunct to disease modifying therapy, propionate promoted an increase in Treg cell numbers and function, and a significant decrease in Th1 and Th17 cells. This was paralleled by a reduced annual relapse rate and delayed disease progression, although participant numbers were low and results should be considered with caution.73 In another trial, a propionate prescription to end-stage renal disease human patients significantly reduced C reactive protein and this correlated with the expansion of Treg cells in circulation.74 In contrast, a randomized, controlled type 1 diabetes clinical trial recently failed to report significant changes to CD4+ and CD8+ T cells, B cells, and natural killer cells in circulation after oral butyrate administration.75 The authors suggested the contrasting data observed in the clinical trial compared with previous preclinical studies could be due to inconsistencies in administration, dosing, and timing, which are all critical factors to consider when establishing a standardized protocol of SCFAs for in vivo administration.
In contrast to the above-mentioned studies, reports related to CD8+ T cell-driven immunity are in favor of SCFAs mostly having pro-inflammatory effects. Indeed, Luu et al found that exposing CD8+ T cells to butyrate in vivo ameliorated CTL function and persistence. While pretreating Ly5.1+ CD8+ T cells with 1 mM butyrate for 3 days imprinted T cells for long-lasting effector in vitro functions, oral administration of butyrate promoted differentiation of IFNγ-producing cells in peripheral lymph nodes (summarized in figure 3B).41 These effects were found independently of T cells expressing GPR41 and GPR43 and relied instead on butyrate-induced HDAC inhibition and metabolic reprogramming. In line with these findings, the administration of SCFA-rich, high fiber diets was associated with augmented CD8+ effector T cell function at sites of inflammation or tumors (summarized in figure 3B).40 76 In the case of infection, the exposure of mice to high fiber diets blunted CXCL1 production and neutrophil recruitment to infected lungs, limiting tissue immunopathology, while promoting metabolic activity and effector function of CD8+ T cells.40 Likewise, in tumor-bearing mice, administration of pectin, a fiber found in vegetable and fruits, improved CD8 effector T cell responses, and sensitivity to anti-PD-1 therapy in a mouse model of colon carcinoma.76 Dose-dependent effects might explain discrepancies, which should be further investigated in models, allowing to dissect the relative contribution of CD4 and CD8 regulatory/effector subsets. The possibility to reach relative subset balance via controlled diets and ad hoc pre/post-biotic supplementation yet appears a pursuable approach.
A role of SCFAs in chemotherapy, radiotherapy and immunotherapy of cancer
Preclinical and clinical reports indicate that chemotherapy or radiotherapy induces major changes in the composition of the gut microbiota. Data also indicate that anticancer therapeutic efficacy of chemotherapyor radiotherapy, and also of immunotherapy benefits of selected gut microbiota composition. This is because commensal microbes, and their SCFA derivatives (as well as antibiotic treatments) directly modulate cancer development and growth, tune intestinal inflammation, and influence efficacy and safety of anticancer therapy.70 This is most evident for, but not limited to, colon cancer. Indeed, SCFAs can boost barrier immunity, limiting persistent bacterial invasion secondary to the damaged gut barrier, the latter of which is responsible for chronic inflammatory responses and colon cancer progression. Concomitantly, SCFAs can also mediate therapy resistance.77
The impact of the gut microbiome on chemotherapy efficacy and toxicity has long been recognized.78 Chemotherapy (and also radiotherapy) often cause intestinal microbial dysbiosis, which leads to intestinal mucositis due to a number of effects: (1) inflammation and oxidative stress, (2) gastrointestinal permeability destruction, (3) mucus layer formation alteration, (4) epithelial repair, and (5) secretion of immune factors.70 Provision of SCFA-producing bacteria contributed to mucosa recovery and intestinal homeostasis. Direct application of SCFAs such as butyrate reported to increase IL-10 and reduce IL-12 and TNF-α in chemotherapy-induced colitis.79 Instead, recent work by He et al demonstrated that butyrate in combination with oxaliplatin promoted antitumor immunity. It favored CTL effector function in MC38 colorectal cancer tumor-bearing mice, ameliorating tumor control.80 Of note, in this setting, butyrate supplementation in vivo also granted better therapeutic activity to anti-PD-1 immune checkpoint blocker (ICB) therapy.80 This result is in line with a recent clinical study proving the efficacy of the association of SCFAs and PD-1 ICB in solid cancers.81
Radiotherapy efficacy and safety has also been reported to be influenced by the microbiome composition and SCFA representation. Radiation-induced intestinal injury involves a decrease in the diversity of intestinal flora and the concentration of SCFAs.82 In a recent report, Guo et al found that selected gut microbial families (Lachnospiraceae and Enterococcaceae) were associated with radioprotection both in patients and mice, and that treatment with propionate (and tryptophan metabolites) attenuated DNA damage and the release of reactive oxygen species in hematopoietic and gastrointestinal tissues, mitigating proinflammatory responses and promoting hematopoiesis and intestinal repair after radiation.83 Zhu et al found that non myeloablative total body irradiation, frequently used to precondition patients and create ‘space’ for adoptive T cell therapy products, impeded the growth and maintenance of SCFA-producing bacteria in the intestine with consequences on intestinal barrier integrity and functionality, ultimately impacting immune cell subset representation.84 A recent study by Yang et al found that the presence of butyrate and/or butyrate-producing bacteria hindered antitumor responses to irradiation.85 When mice were given a gram-positive-targeting antibiotic known to select for butyrate-producing bacteria prior to irradiation treatment, outcomes were improved, in both the MC38 colorectal cancer and B16 melanoma transplantable tumor models. Mechanistically, intratumoral accumulation of butyrate suppressed the activation of STING and production of IFNβ in DCs within the tumor, lowering protective T cell responses.85
In the case of immunotherapy, the microbiome composition has been reported to enhance or dampen antitumor responses, according to the therapeutic setting, and especially in the case of ICBs.86 In an experimental subcutaneous tumor model, a defined commensal consortium made of human bacterial strains elicited strong CD8+ T cell-mediated antitumor immunity, also improving responses to checkpoint blockers.87 In clinical settings, the enrichment of Faecalibacterium and other genera belonging to the phyla Firmicutes was similarly associated with beneficial clinical response to ICBs.88 Also the relative abundance of Akkermansia muciniphila was found a useful clinical predictor of poor responses to ICB in patients with lung and kidney cancers. This was paralleled by the observations that antibiotic-treatments correlated with lower, progression-free survival and overall survival when compared with untreated controls, both in patients and in mice, and that bacteria supplementation to antibiotic-treated mice restored the response to anti-PD-1 therapy.89 In a parallel study, it was found that efficacy of anti–PD-L1 therapy correlated with improved representation of Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium.90 In line with these studies, first-in-human clinical trials recently reported that fecal microbiota transplantation can affect how metastatic melanoma patients respond to anti-PD-1 immunotherapy. Results from these trials highlighted how the gut microbiome regulated changes in distinct proteomic and metabolomic signatures previously shown to be associated with responses to anti-PD-1, increased CD8+ T cell activation, and decreased frequency of interleukin-8-expressing myeloid cells.91
Nevertheless, in a recent study, Coutzac et al observed that, in mouse models of melanoma and in metastatic melanoma patients, high serum levels of butyrate and propionate were associated with resistance to CTLA-4 blockade and a higher proportion of Treg cells. Butyrate supplementation to melanoma tumor-bearing mice in combination with αCTLA-4 restrained DC maturation and the accumulation of both effector and memory tumor-specific CTLs. Likewise, when baseline PBMC samples from metastatic melanoma patients were stimulated with increasing concentration of butyrate ex vivo, there was a correspondent increase in Tregs (summarized in figure 3B).92 Thus, while SCFAs can promote potent CTLs, they can also favor Treg accumulation, and restrain DC maturation.
It should however be considered that while several reports emphasize a role for the microbiome and microbiome-derived SCFAs in vivo in promoting responses to therapy, data also underline the ability of these compounds to exert anti-inflammatory activities, ultimately supporting complex pleiotropic effects and thus, the difficulty in predicting clear outcomes. Indeed, timing of administration, relative abundance, and the physiological or disease context can simultaneously influence the consequence of SCFA exposure. We argue that these are important variables to consider when envisaging the exploitation of SCFAs in the setting of ACT.
SCFAs for the development of an effective ACT protocol
ACT foresees the in vitro expansion/generation of large numbers of tumor-specific T cells and infusion to patients. Tumor-reactive T cells can be taken from tumor samples and expanded into sufficient numbers, or from peripheral blood and genetically-engineered with TCR or chimeric antigen receptor (CAR).93–95 TCR-redirected T cells recognize both extracellular and intracellular antigens presented in the context of major histocompatibility complex molecules (MHC/HLA) molecules, while CAR-T cells bind surface antigens without MHC/HLA restrictions.96 T cell cloning, and TCR sequencing,97 and the isolation of several single-chain antibodies95 has allowed the isolation of a number of TCR and of synthetic CAR molecules with optimized antigen binding and signaling abilities. In addition, the identification of T cell subsets endowed with effector functions or memory potential, together with the exploitation of homeostatic cytokines, such as IL-7 and IL-15, has allowed to optimize manufacturing processes to grant extensive T cell expansion in the absence of terminal differentiation.98–100
It has been recognized that ideal T cell products should have potential for rapid memory recall responses, homing to appropriate anatomical locations, in vivo re-expansion and also long-term survival. Given the notion that SCFAs can fine-tune T cell responsiveness and memory formation, their use over the course of manufacturing has recently been explored. Vodnala et al studied the phenotype of tumor infiltrating lymphocytes (TILS) and found that conditions that determine a diminished uptake and consumption of local nutrients (such as necrosis that leads to a profound alteration of the potassium ion gradient within the tumor microenvironment) leads to a reduction in acetyl coenzyme A (AcCoA).101 This caused a decrease in histone acetylation and silencing of effector genes. They also proved that treating expanding T cells with acetate (5 mM), the immediate precursor of AcCoA, restored nucleo/cytosolic AcCoA and IFNγ production, reversing stemness programs and enabling the acquisition of effector programs. These data underline the notion that nutrient availability and processing controls metabolic features and acts to epigenetically imprint T cell fate.101 Moore et al investigated the impact of combining homeostatic cytokines and SCFAs over T cell manufacturing. They found that T cells expressing a tyrosinase-reactive TCR (TIL 1383I) gradually lost TCR expression over a 12-day long culture. HDAC inhibition reverted this event, suggesting that transgene downregulation observed during T cell cultures was epigenetically regulated. In this study, butyrate supplementation (1 mM) preserved the expression of the TIL 1383I TCR in both lenti-transduced and retroviral-transduced CD4+ and CD8+ T cells (summarized in figure 2C).102 SCFAs or HDAC inhibitors-imprinted long-lasting transgene expression correlated with improved therapeutic activities. Luu et al also investigated the use of SCFAs on antigen-specific CD8+ CTLs and CAR T cells in adoptive T cell therapy settings. Treatment with pentanoate and butyrate improved CTLs and CAR T cells effector functions, which correlated with increased antitumor reactivity and superior therapeutic outcomes in syngeneic murine melanoma and pancreatic cancer models (summarized in figures 2C and 3C).103 SCFA-reprogramming improved CD25, IFNγ and TNF-α expression, via GPR41/GPR43-independent events involving HDAC-inhibition and metabolic modulation. The authors also validated the bacterial source producing the highest amount of pentanoate and butyrate among the top 14 bacterial strains representing the expected microbiome profile of the human intestine: Megasphaera massiliensis of the phyla Actinobacteria. Supernatant derived from M. massiliensis had a significant impact on the production of IFNγ and TNFα of CD8+ effector T cells, demonstrating that the presence of specific microbial strains can have a direct influence over T cell function in vivo. In parallel studies, we similarly found that supplementing IL-7/IL-15 driven cultures of TCR transduced T cells with propionate (1–10 mM) or butyrate (0.2–1 mM) favored the expansion of IFNγ-producing and TNFα-producing cells, without causing their terminal differentiation (unpublished data). This represents an interesting phenotype given that such T cell products might simultaneously be capable of immediate effector function and also of long-term persistence.
Thus, although further studies would be needed to better understand SCFA-instructed phenotypes, including the epigenetic and metabolic events subtending the observed effects, current data support the use of SCFAs during T cell manufacturing. SCFAs imprint genetically engineered T cells with more stable TCR/CAR transgene expression, optimal functionality and memory potential, and in vivo improved therapeutic activity. While it might be tempting to suggest that supplementary SCFA treatments might further support engineered T cells on in vivo transfer, data available to-date might also predict counter-productive effects due to pleiotropic SCFA activities. Of note, searching the NIH clinicaltrial.gov site with ‘SCFA’ or ‘SCFA and immune’ as keywords identified 557 and 72 ongoing trials, respectively. Such a considerable number of trials likely reflects the fact that propionate is classified as a food product in the European Union and the USA, and thus generally considered safe, and as such of interest as a natural immune modulator. It is predictable that new data on the ability of SCFAs to shape the immune landscape within and outside the gut might thus become available soon, and better instructs their therapeutic exploitation in patients.
Conclusions
ACT with TILs or TCR/CAR-engineered T cells represents a valuable therapeutic opportunity to fight cancer. While highly efficacious in the setting of some hematological malignancies, optimization is still needed and required in the setting of solid tumors to improve efficacy while limiting toxicity. Strategies able to ameliorate functional and survival potential of T cell products are continuously being tested to improve efficacy and reduce treatment related toxicity and costs. Several factors can influence T cell engraftment and persistence. The microbiota and microbiota-derived components have been shown to contribute to the success of chemotherapy and radiotherapy and of ICB. SCFAs represent important links between the microbiota and the immune system. They have been shown to impact T cell function, and to epigenetically imprint T cell products with superior transgene expression, cytokine production, and survival. However, as the relative SCFA representation and local concentration at given anatomical districts remains largely unknown and given the SCFA pleiotropic effects on various immune and non-immune cell subsets (T cells, neutrophils, antigen presenting cells and epithelial cells) their direct exploitation in vivo might be currently questioned. Nevertheless, their adoption in vitro even at supraphysiological concentrations as a supplement in the generation of T cell product appears very approachable. Then, once the complex host–microbiota interplay would be more comprehensively understood, in vivo supplementation might be envisaged, and dosing, scheduling, and route of delivery optimized to balance local versus distal, proinflammatory versus anti-inflammatory effects, and ameliorate conventional and more innovative anticancer treatments.
Acknowledgments
The authors wish to thank Dr Arianna Pocaterra and Dr Marco Catucci (IRCCS Ospedale San Raffaele, Milan, Italy) for critical reading of the manuscript. Figures were created with BioRender.com.
Footnotes
Contributors: PR prepared a first draft of the manuscript, figures, and contributed to revision. AM edited the original and revised manuscripts and supervised all contents.
Funding: The authors acknowledge the support of the Associazione Italiana per la Ricerca sul Cancro (AIRC: IG 2018 Id.21763). PR is a recipient of an iCARE-2 fellowship (funded by Fondazione AIRC and the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 800924).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med 2019;216:20–40. 10.1084/jem.20180448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng WY, Wu C-Y, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut 2020;69:1867. 10.1136/gutjnl-2020-321153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitra S, Drautz-Moses DI, Alhede M, et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 2015;3:38. 10.1186/s40168-015-0100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghaisas S, Maher J, Kanthasamy A. Gut microbiome in health and disease: linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther 2016;158:52–62. 10.1016/j.pharmthera.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol 2016;17:505–13. 10.1038/ni.3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseinkhani F, Heinken A, Thiele I, et al. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes 2021;13:1–22. 10.1080/19490976.2021.1882927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan J, McKenzie C, Potamitis M, et al. The role of short-chain fatty acids in health and disease. Adv Immunol 2014;121:91–119. 10.1016/B978-0-12-800100-4.00003-9 [DOI] [PubMed] [Google Scholar]
- 8.Salminen S, Collado MC, Endo A, et al. The International scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 2021;18:649–67. 10.1038/s41575-021-00440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howie D, Ten Bokum A, Necula AS, et al. The role of lipid metabolism in T lymphocyte differentiation and survival. Front Immunol 2017;8:1949. 10.3389/fimmu.2017.01949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol 2020;11:25. 10.3389/fendo.2020.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong J, Zhou P, Zhang R. Intestinal microbiota-derived short chain fatty acids in host health and disease. Nutrients 2022;14:1977. 10.3390/nu14091977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruppin H, Bar-Meir S, Soergel KH, et al. Absorption of short-chain fatty acids by the colon. Gastroenterology 1980;78:1500–7. [PubMed] [Google Scholar]
- 13.Cummings JH, Pomare EW, Branch WJ, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987;28:1221–7. 10.1136/gut.28.10.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rombeau JL, Kripke SA, Settle RG. Short-chain fatty acids. Production, absorption, metabolism, and intestinal effects. In: Kritchevsky D, Bonfield C, Anderson JW, eds. Last dietary fiber. New York: Plenum Press, 1990: 317e37. [Google Scholar]
- 15.Kim M, Qie Y, Park J, et al. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016;20:202–14. 10.1016/j.chom.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frampton J, Murphy KG, Frost G, et al. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metab 2020;2:840–8. 10.1038/s42255-020-0188-7 [DOI] [PubMed] [Google Scholar]
- 17.Millard A L, Mertes PM, Ittelet D. Maturation and function of human monocyte-derived dendritic cells and macrophages: butyrate impairs monocyte-derived APC function. Clin Exp Immunol 2002;130:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloemen JG, Venema K, van de Poll MC, et al. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr 2009;28:657–61. 10.1016/j.clnu.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 19.Ktsoyan ZA, Mkrtchyan MS, Zakharyan MK. Systemic concentrations of short chain fatty acids are elevated in salmonellosis and exacerbation of familial mediterranean fever. Front Microbiol 2016:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juanola O, Ferrusquía-Acosta J, García-Villalba R, et al. Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis. Faseb J 2019;33:11595–605. 10.1096/fj.201901327R [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Chen W-D, Wang Y-D. Nuclear receptors: a bridge linking the gut microbiome and the host. Mol Med 2021;27:144. 10.1186/s10020-021-00407-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echeverría F, Ortiz M, Valenzuela R, et al. Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: relationship to tissue development and aging. Prostaglandins Leukot Essent Fatty Acids 2016;114:28–34. 10.1016/j.plefa.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 23.Choi J-M, Bothwell ALM. The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol Cells 2012;33:217–22. 10.1007/s10059-012-2297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim CH. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol Immunol 2021;18:1161–71. 10.1038/s41423-020-00625-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, Kim M, Kang SG, et al. Short-Chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-s6K pathway. Mucosal Immunol 2015;8:80–93. 10.1038/mi.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renner K, Bruss C, Schnell A, et al. Restricting glycolysis preserves T cell effector functions and augments checkpoint therapy. Cell Rep 2019;29:135–50. 10.1016/j.celrep.2019.08.068 [DOI] [PubMed] [Google Scholar]
- 27.Certo M, Tsai C-H, Pucino V, et al. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol 2021;21:151–61. 10.1038/s41577-020-0406-2 [DOI] [PubMed] [Google Scholar]
- 28.Murray CM, Hutchinson R, Bantick JR, et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chem Biol 2005;1:371–6. 10.1038/nchembio744 [DOI] [PubMed] [Google Scholar]
- 29.Paterson DJ, Jefferies WA, Green JR, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol 1987;24:1281–90. 10.1016/0161-5890(87)90122-2 [DOI] [PubMed] [Google Scholar]
- 30.Kasinrerk W, Fiebiger E, Stefanová I, et al. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol 1992;149:847–54. [PubMed] [Google Scholar]
- 31.Guo N, Ye S, Zhang K, et al. A critical epitope in CD147 facilitates memory CD4+ T-cell hyper-activation in rheumatoid arthritis. Cell Mol Immunol 2019;16:568–79. 10.1038/s41423-018-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solstad T, Bains SJ, Landskron J, et al. Cd147 (Basigin/Emmprin) identifies FoxP3+CD45RO+CTLA4+-activated human regulatory T cells. Blood 2011;118:5141–51. 10.1182/blood-2011-02-339242 [DOI] [PubMed] [Google Scholar]
- 33.Priyamvada S, Saksena S, Alrefai WA. Intestinal anion absorption. In: Physiology of the gastrointestinal tract [online]. Elsevier, 2018: 1317–62. https://linkinghub.elsevier.com/retrieve/pii/B9780128099544000578 [Google Scholar]
- 34.Sun M, Wu W, Liu Z, et al. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol 2017;52:1–8. 10.1007/s00535-016-1242-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569–73. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun M, Wu W, Chen L, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun 2018;9:3555. 10.1038/s41467-018-05901-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chun E, Lavoie S, Fonseca-Pereira D, et al. Metabolite-Sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity 2019;51:871–84. 10.1016/j.immuni.2019.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009;461:1282–6. 10.1038/nature08530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmerman MA, Singh N, Martin PM, et al. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol 2012;302:G1405–15. 10.1152/ajpgi.00543.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trompette A, Gollwitzer ES, Pattaroni C, et al. Dietary fiber confers protection against flu by shaping Ly6c- patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 2018;48:992–1005. 10.1016/j.immuni.2018.04.022 [DOI] [PubMed] [Google Scholar]
- 41.Luu M, Weigand K, Wedi F, et al. Regulation of the effector function of CD8+ T cells by gut microbiota-derived metabolite butyrate. Sci Rep 2018;8:14430. 10.1038/s41598-018-32860-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dupraz L, Magniez A, Rolhion N, et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep 2021;36:109332. 10.1016/j.celrep.2021.109332 [DOI] [PubMed] [Google Scholar]
- 43.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 2007;26:5541–52. 10.1038/sj.onc.1210620 [DOI] [PubMed] [Google Scholar]
- 44.Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015;17:662–71. 10.1016/j.chom.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gui C-Y, Ngo L, Xu WS, et al. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci U S A 2004;101:1241–6. 10.1073/pnas.0307708100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fellows R, Varga-Weisz P. Chromatin dynamics and histone modifications in intestinal microbiota-host crosstalk. Mol Metab 2020;38:100925. 10.1016/j.molmet.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng X-xia, Zhou T, Wang X-A, et al. Histone deacetylases and atherosclerosis. Atherosclerosis 2015;240:355–66. 10.1016/j.atherosclerosis.2014.12.048 [DOI] [PubMed] [Google Scholar]
- 48.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013;504:451–5. 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin U-H, Cheng Y, Park H, et al. Short chain fatty acids enhance aryl hydrocarbon (Ah) responsiveness in mouse colonocytes and Caco-2 human colon cancer cells. Sci Rep 2017;7:10163. 10.1038/s41598-017-10824-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang W, Yu T, Huang X, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun 2020;11:4457. 10.1038/s41467-020-18262-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009;30:832–44. 10.1016/j.immuni.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol 2012;12:325–38. 10.1038/nri3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chapman NM, Boothby MR, Chi H. Metabolic coordination of T cell quiescence and activation. Nat Rev Immunol 2020;20:55–70. 10.1038/s41577-019-0203-y [DOI] [PubMed] [Google Scholar]
- 54.Atarashi K, Honda K. Microbiota in autoimmunity and tolerance. Curr Opin Immunol 2011;23:761–8. 10.1016/j.coi.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 55.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446–50. 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- 56.Kespohl M, Vachharajani N, Luu M, et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4+ T cells. Front Immunol 2017;8:1036. 10.3389/fimmu.2017.01036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sumbria D, Berber E, Rouse BT. Supplementing the diet with sodium propionate suppresses the severity of viral immuno-inflammatory lesions. Sandri-Goldin RM, editor. J Virol 2020;95:e02056-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balmer ML, Ma EH, Bantug GR, et al. Memory CD8(+) T cells require increased concentrations of acetate induced by stress for optimal function. Immunity 2016;44:1312–24. 10.1016/j.immuni.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 59.Qiu J, Villa M, Sanin DE, et al. Acetate promotes T cell effector function during glucose restriction. Cell Rep 2019;27:2063–74. 10.1016/j.celrep.2019.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bachem A, Makhlouf C, Binger KJ, et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 2019;51:285–97. 10.1016/j.immuni.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 61.Mariño E, Richards JL, McLeod KH, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 2017;18:552–62. 10.1038/ni.3713 [DOI] [PubMed] [Google Scholar]
- 62.Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016;165:1332–45. 10.1016/j.cell.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 63.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014;20:159–66. 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- 64.Chang PV, Hao L, Offermanns S, et al. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 2014;111:2247–52. 10.1073/pnas.1322269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vinolo MAR, Hatanaka E, Lambertucci RH, et al. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem Funct 2009;27:48–55. 10.1002/cbf.1533 [DOI] [PubMed] [Google Scholar]
- 66.Cholan PM, Han A, Woodie BR, et al. Conserved anti-inflammatory effects and sensing of butyrate in zebrafish. Gut Microbes 2020;12:1824563. 10.1080/19490976.2020.1824563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haghikia A, Jörg S, Duscha A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015;43:817–29. 10.1016/j.immuni.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 68.Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 2019;139:1407–21. 10.1161/CIRCULATIONAHA.118.036652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park J, Goergen CJ, HogenEsch H, et al. Chronically elevated levels of short-chain fatty acids induce T cell-mediated Ureteritis and hydronephrosis. J Immunol 2016;196:2388–400. 10.4049/jimmunol.1502046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian T, Zhao Y, Yang Y, et al. The protective role of short-chain fatty acids acting as signal molecules in chemotherapy- or radiation-induced intestinal inflammation. Am J Cancer Res 2020;10:3508–31. [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H, Bian Z, Zhang Q, et al. Sodium butyrate inhibits colitis-associated colorectal cancer through preventing the gut microbiota dysbiosis and reducing the expression of NLRP3 and IL-1β. J Funct Foods 2021;87:104862. [Google Scholar]
- 72.Gold R, Montalban X, Haghikia A. Multiple sclerosis and nutrition: back to the future? Ther Adv Neurol Disord 2020;13:175628642093616. 10.1177/1756286420936165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duscha A, Gisevius B, Hirschberg S, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 2020;180:1067–80. 10.1016/j.cell.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 74.Meyer F, Seibert FS, Nienen M, et al. Propionate supplementation promotes the expansion of peripheral regulatory T-cells in patients with end-stage renal disease. J Nephrol 2020;33:817–27. 10.1007/s40620-019-00694-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Groot PF, Nikolic T, Imangaliyev S, et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia 2020;63:597–610. 10.1007/s00125-019-05073-8 [DOI] [PubMed] [Google Scholar]
- 76.Zhang S-L, Mao Y-Q, Zhang Z-Y, et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 2021;11:4155–70. 10.7150/thno.54476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim M, Friesen L, Park J, et al. Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice. Eur J Immunol 2018;48:1235–47. 10.1002/eji.201747122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ciernikova S, Mego M, Chovanec M. Exploring the potential role of the gut microbiome in chemotherapy-induced neurocognitive disorders and cardiovascular toxicity. Cancers 2021;13:782. 10.3390/cancers13040782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008;105:16731–6. 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Y, Fu L, Li Y, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab 2021;33:988–1000. 10.1016/j.cmet.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 81.Nomura M, Nagatomo R, Doi K, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open 2020;3:e202895. 10.1001/jamanetworkopen.2020.2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y, Zhang Y, Wei K, et al. Review: effect of gut microbiota and its metabolite SCFAs on radiation-induced intestinal injury. Front Cell Infect Microbiol 2021;11:577236. 10.3389/fcimb.2021.577236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo H, Chou W-C, Lai Y, et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020;370:eaay9097. 10.1126/science.aay9097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu S, Liang J, Zhu F, et al. The effects of myeloablative or non-myeloablative total body irradiations on intestinal tract in mice. Biosci Rep 2021;41:BSR20202993. 10.1042/BSR20202993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang K, Hou Y, Zhang Y, et al. Suppression of local type I interferon by gut microbiota-derived butyrate impairs antitumor effects of ionizing radiation. J Exp Med 2021;218:e20201915. 10.1084/jem.20201915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zitvogel L, Ma Y, Raoult D, et al. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science 2018;359:1366–70. 10.1126/science.aar6918 [DOI] [PubMed] [Google Scholar]
- 87.Tanoue T, Morita S, Plichta DR, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019;565:600–5. 10.1038/s41586-019-0878-z [DOI] [PubMed] [Google Scholar]
- 88.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359:97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91–7. 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 90.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359:104–8. 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021;371:595–602. 10.1126/science.abf3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coutzac C, Jouniaux J-M, Paci A, et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun 2020;11:2168. 10.1038/s41467-020-16079-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62–8. 10.1126/science.aaa4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Met Özcan, Jensen KM, Chamberlain CA, et al. Principles of adoptive T cell therapy in cancer. Semin Immunopathol 2019;41:49–58. 10.1007/s00281-018-0703-z [DOI] [PubMed] [Google Scholar]
- 95.Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer 2021;21:145–61. 10.1038/s41568-020-00323-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 2021;11:69. 10.1038/s41408-021-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manfredi F, Cianciotti BC, Potenza A, et al. TCR redirected T cells for cancer treatment: achievements, hurdles, and goals. Front Immunol 2020;11:1689. 10.3389/fimmu.2020.01689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knochelmann HM, Smith AS, Dwyer CJ, et al. CAR T cells in solid tumors: blueprints for building effective therapies. Front Immunol 2018;9:1740. 10.3389/fimmu.2018.01740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cieri N, Camisa B, Cocchiarella F, et al. Il-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013;121:573–84. 10.1182/blood-2012-05-431718 [DOI] [PubMed] [Google Scholar]
- 100.Herda S, Heimann A, Obermayer B, et al. Long‐term in vitro expansion ensures increased yield of central memory T cells as perspective for manufacturing challenges. Int J Cancer 2021;148:3097–110. 10.1002/ijc.33523 [DOI] [PubMed] [Google Scholar]
- 101.Vodnala SK, Eil R, Kishton RJ, et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019;363:eaau0135. 10.1126/science.aau0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moore TV, Scurti GM, DeJong M, et al. HDAC inhibition prevents transgene expression downregulation and loss-of-function in T-cell-receptor-transduced T cells. Mol Ther Oncolytics 2021;20:352–63. 10.1016/j.omto.2021.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luu M, Riester Z, Baldrich A, et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat Commun 2021;12:4077. 10.1038/s41467-021-24331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]