Abstract
Objectives
COVID-19 has been associated with long-term consequences to patient wellness and quality of life. Data on post-COVID-19 conditions are scarce in developing countries. This study aimed to investigate long COVID in a cohort of hospitalized patients in Brazil.
Methods
Surviving patients discharged from the hospital between July 1, 2020 and March 31, 2021 were assessed between 2 and 12 months after acute onset of COVID-19. The outcomes were the prevalence of persistent symptoms, risk factors associated with long COVID, and quality of life as assessed by the EuroQol 5D-3L questionnaire.
Results
Of 439 participants, most (84%) reported at least one long COVID symptom, at a median of 138 days (interquartile range [IQR] 90-201) after disease onset. Fatigue (63.1%), dyspnea (53.7%), arthralgia (56.1%), and depression/anxiety (55.1%) were the most prevalent symptoms. In multivariate analysis, dysgeusia (odds ratio [OR] 2.0, 95% confidence interval [CI] 1.18-3.44, P <0.001) and intensive care unit (ICU) admission (OR 2.6, 95% CI 1.19-6.56, P = 0.03) were independently associated with long COVID. Fifty percent of patients reported a worsened clinical condition and quality of life.
Conclusion
Long-term outcomes of SARS-CoV-2 infection in a low- to middle-income country were relevant. Fatigue was the most common persistent symptom. ICU admission was an independent factor associated with long COVID. Dysgeusia could be a potential predictor of long COVID.
Keywords: COVID-19, Long COVID, Dysgeusia, Risk factors, Quality of life, Brazil
Introduction
A significant number of patients recovering from COVID-19 report new, recurring, or persistent symptoms beyond 4 or more weeks after infection. These patients are referred to as experiencing “long COVID”, an umbrella term for the wide range of post-COVID-19 conditions (Centers for Disease Control and Prevention, 2021; Jennings et al., 2021).
Beyond sequelae from the illness or hospital stay, scientific evidence is evolving on more specific post-acute sequelae of SARS-CoV-2 infection in multiple organ systems (Gupta et al., 2020; Nalbandian et al., 2021). Many of these physical and mental health consequences may have a significant negative impact on the quality of life. Furthermore, patients who had mild or asymptomatic COVID-19 infections also could be affected.
More than 75% of the studies on the post-COVID-19 syndrome, however, were carried out in Europe, USA, and China, where the first reports occurred (Akbarialiabad et al., 2021; Nasserie et al., 2021). Notably, the scarcity of evidence on long COVID from low- and middle-income countries is challenging and demands urgent responses in a pandemic setting.
We conducted a study of surviving individuals with confirmed COVID-19 who were discharged from a reference hospital for infectious diseases in Minas Gerais, Brazil. Our aims in this study were to describe the prevalence and type of consequences of COVID-19 after acute recovery, evaluate the quality of life, and identify potential risk factors associated with long COVID.
Methods
Study design and participants
We conducted an observational, cross-sectional study of individuals discharged from a public hospital for infectious diseases, Eduardo de Menezes Hospital (HEM)/ Fundação Hospitalar do Estado de Minas Gerais (FHEMIG), in Belo Horizonte, Brazil.
All patients >18 years of age consecutively admitted to HEM from July 1, 2020 to March 31, 2021 with COVID-19 (diagnosed by reverse transcriptase-polymerase chain reaction [RT-PCR] positive test results for SARS-CoV-2 in a nasopharyngeal swab) and discharged alive by April 30, 2021 were eligible for the study. Patients transferred to other hospitals or (who escaped or evaded) from HEM were excluded from the analysis. No patient had been previously vaccinated against COVID-19. All eligible individuals were contacted between February 1, 2021 and July 30, 2021 at least 4 weeks after symptom onset (defined as time zero), and outcomes were measured at a single follow-up time.
The main outcomes included the prevalence of long COVID (defined as the persistence of at least one physical and/or mental health symptom 4 or more weeks after disease onset), the range of symptoms of long COVID, and health-related quality of life.
The study protocol was approved by the Ethics Committee of the HEM (Protocol CAAE: 41774720.6.0000.512).
Procedures
Telephone and virtual assessment
Patients were systematically contacted and invited to participate by a trained recruitment team of doctors and nurses from HEM through a telephone call or text message. At least two text messages, at an interval of 24-72 h, were attempted per patient. If participation was accepted, a link was sent, and a standardized questionnaire focused on post-COVID-19 persistent symptoms and quality of life was completed online. The telephone call was the primary assessment for all patients >61 years old. Two or three call attempts, at intervals of 24-72 h and at various times throughout the day to allow for increased chances of success, were made to most of the patients. Some virtual assessments were also changed to telephone calls, according to participant preference. The telephone interview was conducted using the same standardized questionnaire. Patients were directed to self‐management resources, given medical advice, and directed to rehabilitation services and other medical specialists if possible. All study participants or their legal representatives provided an online informed consent to participate in this study.
Patients with proxy respondents were excluded because details about current symptoms and medical conditions were frequently unknown or could be misunderstood. Other exclusion criteria for participation were death post-discharge, unreachability after the third contact attempt, and refusal to participate. Also excluded were patients with neurologic disabilities (such as deafness, aphasia, dementia, or other cognitive impairment), patients with uncontrolled psychiatric disorders, and patients receiving palliative care, as these conditions could complicate the accomplishment of the remote questionnaire or telephone interview.
Health-related quality of life (HRQoL) was assessed using the EuroQol Group Association five-domain, three-level questionnaire (EQ-5D-3L), a validated European questionnaire (EuroQol, 2022) that consists of two sections: the descriptive system and the visual analog scale. Individuals were asked to rate their health states from 1 to 3 (1 = no problems, 2 = some problems, and 3 = extreme problems) in five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and on a visual analog scale (EQ-VAS) from 0 (worst possible health) to 100 (best subjective health experience) at the time of the interview.
Mortality data after hospital discharge were obtained from a government registry database. Every unreachable but otherwise-eligible patient was searched in the “MG Cidadão”, an official application from Minas Gerais state, to have their death and date of death eventually confirmed. Unfortunately, the cause of death was not officially available, although sometimes it was provided by families during the telephone or virtual contacts.
Baseline assessment
Baseline demographics, comorbidity data, clinical data (including the evolution and complications during the hospitalization), blood test results, and images were collected retrospectively from the electronic medical records using a research form. We defined the acute phase as the time between symptom onset and hospital discharge. Demographic and clinical data at admission included age; sex; days since symptom onset; type and number of acute symptoms (fever, cough, sputum, shortness of breath, sore throat, headache, fatigue, myalgia, arthralgia, abdominal pain, vomiting, diarrhea, ageusia/dysgeusia, anosmia, cutaneous rash, red eyes, and vertigo); and type and number of comorbidities (arterial hypertension, heart failure, diabetes mellitus, coronary artery disease, stroke, obesity, chronic obstructive pulmonary disease, asthma, chronic kidney disease, cancer, HIV, immunosuppressive therapy, current alcohol use, and smoking). Obesity was considered if mentioned in electronic records. Smoking status and alcohol consumption were described as “current” or “former”. Information about body mass index and amounts of alcohol consumption and tobacco smoking were not available. Data related to hospitalization and complications of COVID-19 included type of oxygen therapy; admission to intensive care unit (ICU) and length of stay; Sequential Organ Failure Assessment score; need for artificial ventilatory support; acute respiratory distress syndrome; shock; use of vasoactive amines; confirmed peripheral venous thrombosis; confirmed pulmonary thromboembolism; stroke; acute kidney insufficiency; need for hemodialysis; diagnosis and type of others infectious diseases and use of antibiotics; clinical condition at hospital discharge; and length of hospital stay. Laboratory test results at admission included hemoglobin; hematocrit; leukocyte, neutrophil, lymphocyte, and platelet counts; prothrombin time (measured by the international normalized ratio); urea; creatinine; aspartate and alanine aminotransferases; total, direct, and indirect bilirubin; alkaline phosphatase; gamma-glutamyl transferase; lactate; C-reactive protein; lactic acid; amylase; lipase; lactate dehydrogenase; and D-dimer. Chest computed tomography (CT) findings were collected when available, and the extent of lung involvement was categorized.
Outcomes
The primary outcomes were the prevalence of long COVID (defined as the persistence of at least one physical and/or mental health symptom 4 or more weeks after disease onset); the range of long COVID symptoms (fatigue, dyspnea, cough, chest pain, headache, palpitations and/or tachycardia, arthralgia, myalgia, anosmia, dysgeusia, abdominal pain, diarrhea, and rash); and the quality of life (pain or discomfort, anxiety or depression, mobility, personal care, and usual activities).
The secondary endpoint was the identification of potential risk factors for long COVID related to baseline clinical, laboratory, and imaging features of acute COVID-19.
Statistical analysis
Descriptive analysis of variables studied was performed, and the frequency of long COVID symptoms was determined. Demographic characteristics were expressed as absolute values and their respective percentages for categorical variables. In contrast, numerical variables were summarized with medians and interquartile ranges (IQRs). The association of long COVID with categorical variables was assessed using the chi-square or Fisher's exact test. In contrast, the Mann-Whitney U test evaluated median differences between groups for numerical variables. All variables with a P-value <0.20 were entered into an initial logistic regression model.
The final multivariate logistic regression included only significant coefficients (P <0.05) that were converted into odds ratios (ORs) and their 95% confidence intervals (CIs). A multivariable-adjusted linear regression model was used to estimate the β coefficients and 95% CIs for the association between long COVID symptoms and continuous outcomes.
All tests were two-sided, and a P-value <0.05 was considered statistically significant. Participants whose variables of interest were available in the final analysis were included; missing data were not imputed.
All statistical analysis was performed using R statistical software, version 4.0.4.
Results
Descriptive
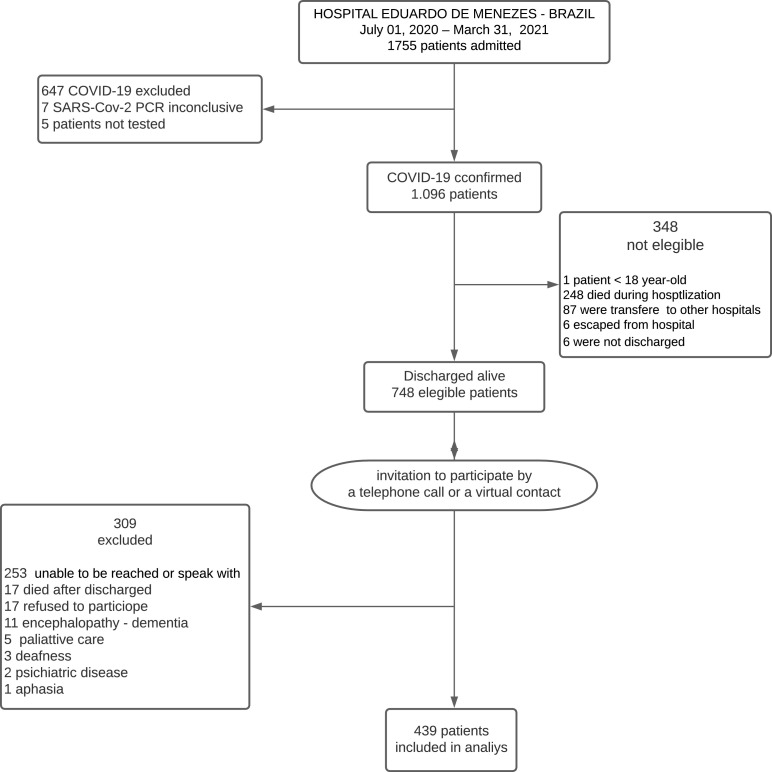
From July 2020 to March 2021, a total of 1755 patients were admitted to HEM/FHEMIG with the severe respiratory syndrome. Of these, 647 (37%) had negative RT-PCR test results for SARS-CoV-2, seven (0.4%) had inconclusive test results, and five (0.3%) patients were not tested. A total of 1096 patients (62%) were confirmed to have COVID-19, but 348 of these (32%) were not eligible (for reasons shown in the study flowchart). A total of 748 of 1096 patients (68%) were eligible and invited to participate by telephone call or text message. A total of 439/748 (59%) agreed to answer the questionnaire and were included in the study analysis (Figure 1 ).
Figure 1.
Patient flowchart. PCR, polymerase chain reaction.
Among the patients studied, the median age was 58 years, and the distribution between genders was nearly even (50.3% were men). Most patients (75%) had some comorbidity, of which the most frequent was arterial hypertension (44%), followed by obesity (33%), and then diabetes mellitus (26%). Dyspnea (99%), cough (78%), and fatigue (76%) were the most frequent symptoms at hospital admission. The median time from symptom onset to hospitalization was 8 (IQR 6-10) days, and the median time between the onset of symptoms and application of the study questionnaire was 138 (IQR 90-201) days. The median length of hospital stay was 8 (IQR 4-12) days. Among the 254 patients (58%) who underwent chest CT, alterations were observed in the majority; pulmonary involvement of 25-50% was most common. Almost all patients received corticosteroid therapy with dexamethasone. ICU admission was required in 77 patients (18%); 37 (18%) needed mechanical ventilation. Other events occurring during hospitalization are described in Table 1, Table 2, Table 3 .
Table 1.
Characteristics, signs, and symptoms at hospital admission and univariate analysis of variables associated with long COVID in Belo Horizonte, Brazil
| Characteristics | OverallN = 439 | No long COVIDN = 70 | Long COVIDN = 369 | Univariate analysisP-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 58 (47-67) | 62 (49-71) | 57 (46-66) | 0.04 |
| Male sex, N (%) | 221 (50.3) | 41(59.0) | 180 (49.0) | 0.13 |
| Signs and symptoms at hospital admission, N (%) | ||||
| Fever | 252 (57.0) | 33 (47.0) | 219 (59.0) | 0.06 |
| Cough | 344 (78.0) | 61 (87.0) | 283 (77.0) | 0.05 |
| Dyspnea | 434 (99.0) | 70 (100) | 364 (99.0) | 0.99 |
| Sore throat | 64 (15.0) | 10 (14) | 54 (15.0) | 0.93 |
| Headache | 105 (24.0) | 20 (29.0) | 85 (23.0) | 0.33 |
| Fatigue | 331 (76.0) | 65 (93.0) | 266 (72.0) | <0.01 |
| Hyporexia | 183 (42.0) | 36 (51.0) | 147 (40.0) | 0.07 |
| Coryza | 182 (41.0) | 29 (41.0) | 153 (41.0) | 0.99 |
| Myalgia | 137 (31.0) | 23 (33.0) | 114 (31.0) | 0.75 |
| Arthralgia | 18 (4.1) | 4 (5.7.0) | 14 (3.8) | 0.51 |
| Anosmia | 160 (36.0) | 23 (33.0) | 137 (37.0) | 0.49 |
| Dysgeusia | 234 (53.0) | 29 (41.0) | 205 (56.0) | 0.03 |
| Abdominal pain | 66 (15.0) | 16 (23.0) | 50 (14.0) | 0.05 |
| Vomiting | 30 (6.8) | 6 (8.6) | 24 (6.5) | 0.60 |
| Diarrhea | 72 (16.0) | 16 (23.0) | 56 (15.0) | 0.11 |
| Skin lesion | 10 (2.3) | 2 (2.9) | 8 (2.2) | 0.66 |
| Conjunctival hyperemia | 19 (4.3) | 2 (2.9) | 17 (4.6) | 0.75 |
| Vertigo | 25 (5.7) | 8 (11.0) | 17 (4.6) | 0.04 |
| Comorbid conditions, N (%) | ||||
| Any comorbid condition | 329 (75.0) | 51 (73.0) | 278 (75.0) | 0.66 |
| Arterial hypertension | 195 (44.0) | 36 (51.0) | 159 (43.0) | 0.19 |
| Diabetes mellitus | 113 (26.0) | 18 (26.0) | 95 (26.0) | 0.99 |
| Obesity | 145 (33.0) | 26 (37.0) | 119 (32.0) | 0.43 |
| COPD | 21 (4.8) | 5 (7.1) | 16 (4.3) | 0.35 |
| Asthma | 11 (2.5) | 0 (0) | 11 (3.0) | 0.23 |
| Smoking | 83 (19.0) | 14 (20.0) | 69 (19.0) | 0.79 |
| Alcoholism | 39 (8.9) | 6 (8.6) | 33 (8.9) | 0.92 |
| CAD | 23 (5.2) | 7 (10.0) | 16 (4.3) | 0.07 |
| HF | 12 (2.7) | 3 (4.3) | 9 (2.4) | 0.42 |
| Chronic renal disease | 11 (2.5) | 2 (2.9) | 9 (2.4) | 0.69 |
| HIV infection | 3 (0.7) | 2 (2.9) | 1 (0.3) | 0.07 |
| Time from symptom onset to hospitalization (days), median (IQR) | 8 (6-10) | 7.0 (6.0-9.0) | 8.0 (6.0-10.0) | 0.04 |
| Time from symptom onset to study questionnaire (days), median (IQR) | 138 (90-201) | 172 (126-272) | 130 (87-192) | <0.01 |
| Time from symptom onset to study questionnaire, stratified by quarters, N (%) | <0.01 | |||
| <90 days | 108 (25.0) | 9 (13.0) | 99 (27.0) | |
| 90-180 days | 198 (45.0) | 29 (41.0) | 169 (46.0) | |
| >180 | 133 (30.0) | 32 (46.0) | 101 (27.0) | |
| Duration of hospital stay (days), median (IQR) | 8 (4-12) | 6 (4-10) | 8 (5-12) | 0.03 |
CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HF, heart failure; IQR, interquartile range.
Table 2.
Laboratory tests at hospital admission, chest computed tomography characteristics, and univariate analysis of variables associated with long COVID in Belo Horizonte, Brazil
| Characteristics | OverallN = 439 | No long COVIDN = 70 | Long COVIDN = 369 | Univariate analysisP-value |
|---|---|---|---|---|
| Hemoglobin (g/dl), median (IQR) | 13.20 (12.50-13.70) | 13.20 (12.60-13.60) | 13.20 (12.50-13.80) | 0.67 |
| Leucocyte count (cells/mm3), median (IQR) | 7500 (5760-9000) | 7465 (5502-8964) | 7500 (5800-9000) | 0.69 |
| Neutrophil count (cells/mm3), median (IQR) | 5860 (4205-7350) | 5980 (4012-7100) | 5842 (4256-7420) | 0.50 |
| Lymphocyte count (cells/mm3), median (IQR) | 1092 (890-1300) | 1030 (890-1315) | 1100 (890- 1300) | 0.49 |
| Platelet count (cells/mm3), median (IQR) | 214,000 (174,000-272,500) | 214,000 (174,000-272,500) | 214,000 (174,000-270,000) | 0.64 |
| Prothrombin time (INR), median (IQR) | 1.00 (1.00-1.10) | 1.00 (1.00-1.09) | 1.00 (1.00-1.10) | 0.09 |
| Urea (mg/dl), median (IQR) | 36 (29-48) | 40 (30-47) | 36 (29-49) | 0.40 |
| Creatinine (mg/dl), median (IQR) | 0.9 (0.7-1.0) | 0.90 (0.70-1.00) | 0.90 (0.70-1.00) | 0.44 |
| AST (U/l), median (IQR) | 58 (45-84) | 58 (46-80) | 58 (45-84) | 0.92 |
| ALT (U/l), median (IQR) | 52 (38-74) | 52 (39-71) | 52 (38-75) | 0.76 |
| GGT (U/l), median (IQR) | 92 (68-142) | 88 (65-107) | 95 (68-146) | 0.13 |
| Alkaline phosphatase (U/l), median (IQR) | 87 (70-116) | 89 (69-108) | 86 (70-118) | 0.66 |
| Total bilirubin (mg/dl), median (IQR) | 0.60 (0.50-0.90) | 0.60 (0.50-0.90) | 0.60 (0.50-0.90) | 0.34 |
| Direct bilirubin (mg/dl), median (IQR) | 0.40 (0.30-0.60) | 0.50 (0.40-0.60) | 0.40 (0.30-0.60) | 0.10 |
| C-reactive protein (mg/l), median (IQR) | 80 (52-120) | 75 (52-100) | 82 (52-120) | 0.37 |
| D-dimer (µ/ml), median (IQR) | 1.02 (0.65-1.45) | 0.96 (0.63-1.62) | 1.02 (0.65-1.45) | 0.21 |
| LDH (U/l), median (IQR) | 362 (296-426) | 326 (296-412) | 365 (298-433) | 0.06 |
| Lactate (mmol/l), median (IQR) | 1.30 (1.00-1.60) | 1.30 (1.00-1.60) | 1.30 (1.00-1.60) | 0.72 |
| Amylase (U/l), median (IQR) | 62 (46-96) | 52 (42-77) | 63 (48-99) | 0.01 |
| Lipase (U/l), median (IQR) | 96 (68-164) | 89 (66-122) | 98 (70-174) | 0.05 |
| Arterial oxygen pressure (mm Hg), median (IQR) | 78 (68-96) | 73 (67-93) | 80 (68-96) | 0.18 |
| Percentage of pulmonary involviment on CCT | 254 (58.0) | 33 (49.0) | 220 (60.0) | |
| <25% | 87 (34.0) | 10 (30.0) | 77 (35.0) | 0.62 |
| 25-50% | 123 (48.0) | 18 (55.0) | 105 (47.0) | 0.44 |
| 50-75% | 38 (15.0) | 5 (15.0) | 33 (15.0) | 0.97 |
| >75% | 5 (2.0) | 0 (0) | 5 (2.3.0) | 0.99 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CCT, chest computed tomography; CI, confidence interval; GGT, gamma-glutamyl transferase; IQR, interquartile range, INR, international normalized ratio; LDH, lactate dehydrogenase.
Table 3.
Events, interventions, and univariate analysis of variables associated with long COVID in Belo Horizonte, Brazil.
| Characteristics | OverallN = 439 | No long COVIDN = 70 | Long COVIDN = 369 | Univariate analysisP-value |
|---|---|---|---|---|
| Events and interventions, N (%) | ||||
| ICU admission | 77 (18.0) | 7 (10.0) | 70 (19.0) | 0.07 |
| Ventilatory support | ||||
| Nasal catheter | 219 (50.0) | 41 (59.0) | 178 (48.0) | 0.99 |
| Face mask | 145 (33.0) | 22 (31.0) | 123 (33.0) | 0.92 |
| Noninvasive ventilation | 37 (8.4) | 3 (4.3) | 34 (9.2) | 0.04 |
| Mechanical ventilation | 37 (8.4) | 4 (5.7) | 33 (8.9) | 0.34 |
| Hemodialysis | 9 (2.1) | 2 (2.9) | 7 (1.9) | 0.64 |
| Shock/use of vasoactive amines | 12 (2.7) | 2 (2.9) | 10 (2.7) | 0.99 |
| Pulmonary thromboembolism | 30 (6.8) | 7 (10.0) | 23 (6.2) | 0.30 |
| Deep vein thrombosis | 2 (0.5) | 0 (0) | 2 (0.5) | 0.99 |
| Presumed bacterial infection | 204 (46.0) | 35 (50.0) | 169 (46.0) | 0.52 |
| Community infection | 177 (40.0) | 26 (37.0) | 151 (41.0) | 0.55 |
| Nosocomial infection | 41 (9.3) | 5 (7.1) | 36 (9.8) | 0.49 |
| Stroke | 1 (0.2) | 0 (0) | 1 (0.3) | 0.99 |
| Acute myocardial infarction | 4 (0.9) | 1 (1.4) | 3 (0.8) | 0.50 |
| Dexamethasone use | 435 (99.0) | 70 (100) | 365 (99.0) | 0.99 |
| Azithromycin use | 205 (47.0) | 32 (46.0) | 173 (47) | 0.86 |
ICU, intensive care unit.
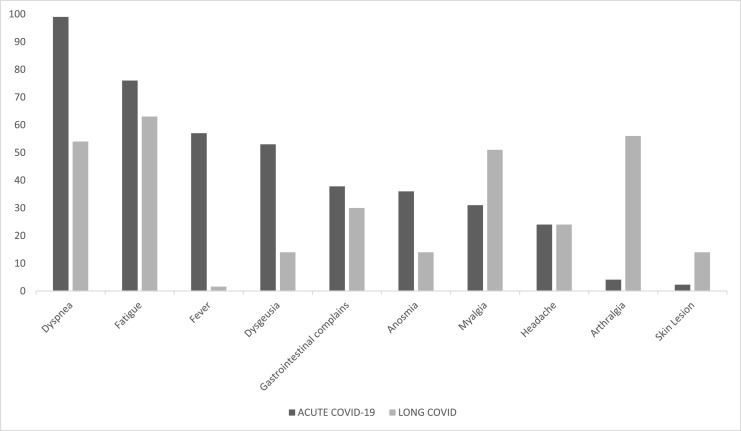
Of the 439 individuals analyzed, 369 (84%) reported at least one long COVID symptom. Fatigue, which was present in 233 patients (63.1%), was the most frequent symptom, followed by arthralgia, depression and anxiety, dyspnea and myalgia (Table 4 ). The distribution of some symptoms in the acute phase of COVID-19 and in long COVID are shown in Figure 2 .
Table 4.
Long COVID symptoms in 369 studied patients in Belo Horizonte, Brazil
| Symptoms Patients, N (%) | |
|---|---|
| Fatigue | 233 (63.1 |
| Arthralgia | 207 (56.1) |
| Depression and anxiety (N = 361)a | 199 (55.1) |
| Dyspnea | 198 (53.7) |
| Myalgia | 189 (51.2) |
| Chest pain | 129 (35.0) |
| Gastrointestinal symptomsb | 110 (29.8) |
| Headache | 90 (24.4) |
| Anosmia | 52 (14.1) |
| Dysgeusia | 50 (13.6) |
| Both anosmia and dysgeusia | 25 (6.8 |
| Skin lesion | 51 (13.8) |
| Palpitations | 15 (4.1) |
| Fever | 6 (1.6) |
| Number of symptoms | |
| 1-5 | 296 (80.2) |
| 6-10 | 73 (19.8) |
Total number of patients who answered the question.
Abdominal pain and/or emesis and/or diarrhea.
Figure 2.
Frequency of long COVID symptoms compared with hospital admission in 369 studied patients in Belo Horizonte, Brazil.
Factors associated with long COVID
Patients who developed long COVID were younger (57 [IQR 46-66] vs 62 [IQR 49-71] years old, P = 0.04), had longer times from symptom onset to hospitalization (8 [IQR 6.0-10.0] vs 7 [IQR 6.0-9.0] days, P = 0.04), and had longer hospital stays (8 [IQR 5-12] vs 6 [IQR 4-10] days, P = 0.03). In multivariate analysis, dysgeusia (OR 2.0, 95% CI 1.18-3.44, P <0.001) and ICU admission (OR 2.6, 95% CI 1.19-6.56, P = 0.03) were independently associated with the presence of long COVID (Table 5 ).
Table 5.
Multivariate analysis of variables associated with long COVID in Belo Horizonte, Brazil
| Variable | OR | CI (95%) | P-value |
|---|---|---|---|
| Dysgeusia | 2.0 | 1.18-3.44 | 0.01 |
| ICU admission | 2.6 | 1.19-6.56 | 0.03 |
| Time from symptom onset to study questionnaire >180 days | 0.24 | 0.10-0.51 | <0.001 |
CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
Long Covid-19 impacts on quality of life
A total of 179/357 patients (50.1%) with long COVID reported a health condition that was worse than before the acute illness. The EQ-5D-3L dimensions for which most long COVID participants reported some worsening (level 2) were mobility (32%, 118/364), usual activities (38%, 140/368), anxiety/depression (46.2%, 167/361), and pain/discomfort (53.3%, 193/362) (Table 6 ). Only patients in the long COVID group presented an extreme level (level 3) of complications (particularly depression and anxiety). EQ-VAS was significatively lower in patients with long COVID than in patients free of persistent symptoms.
Table 6.
EuroQol Group Association five-domain, three-level questionnaire (EQ-5D-3L) and visual analog scale (EQ-VAS) in 439 studied patients in Belo Horizonte, Brazil
| Overall N (%) | No long COVID N (%) | Long COVID N (%) | Univariate analysis P-value | |
|---|---|---|---|---|
| Health today, N=427a | 0.000 | |||
| Worse than before COVID-19 | 179 (42.0) | 0 | 179 (50.1) | |
| Same as before COVID-19 | 194 (45.5) | 56 (80.0) | 138 (38.7) | |
| Better than before COVID-19 | 54 (12.4) | 14 (20.0) | 40 (11.2) | |
| EuroQol-5D-3L | ||||
| Mobility,N=434a | 0.000 | |||
| I have no mobility issues | 308 (70.9) | 64 (91.4) | 244 (67.0) | |
| I have some problems walking | 124 (28.6) | 6 (8.6) | 118 (32.4) | |
| I am limited to staying in bed | 2 (0.5) | 0 (0) | 2 (0.5) | |
| Self-care,N=437a | 0.419 | |||
| I have no problems with my personal care | 408 (93.4) | 68 (97.1) | 340 (92.6) | |
| I have some problems washing or dressing | 27 (6.2) | 2 (2.9) | 25 (6.8) | |
| I am unable to wash or dress myself | 2 (0.5) | 0 (0) | 2 (0.5) | |
| Usual activities,N=438a | 0.000 | |||
| I have no problems performing my usual activities | 294 (67.1) | 69 (98.6) | 225 (61.1) | |
| I have some problems performing my usual activities | 141 (32.2) | 1 (1.4) | 140 (38.0) | |
| I am unable to perform my usual activities | 3 (0.7) | 0 (0) | 3 (0.8) | |
| Pain/discomfort,N=432a | 0.000 | |||
| I have no pain or discomfort | 231 (53.5) | 67 (95.7) | 164 (45.3) | |
| I have moderate pain or discomfort | 196 (45.4) | 3 (4.3) | 193 (53.3) | |
| I have extreme pain or discomfort | 5 (1.2) | 0 (0) | 5 (1,4) | |
| Depression and anxiety,N=428a | 0.000 | |||
| I am not anxious or depressed | 215 (50.2) | 53 (79.1) | 162 (44.9) | |
| I am moderately anxious or depressed | 181 (42.0) | 14 (20.9) | 167 (46.2) | |
| I am extremely anxious or depressed | 32 (7.5) | 0 (0) | 32 (8.9) | |
| EQ-VAS (0-100), median (IQR) | 80 (70-100) | 100 (90-100) | 80 (70-90) | 0.000 |
IQR, interquartile range.
Total number of patients who answered the question.
Discussion
We found high rates of long COVID (84%) and poor long-term outcomes, which were present several months (median 138 [IQR 90-201] days) after hospitalization for COVID-19. Despite the significant growth in knowledge regarding long-term sequelae of the disease worldwide, data in low- and middle-income countries are lacking (Akbarialiabad et al., 2021). This study may contribute to understanding this new condition, particularly in Brazil, where 33,076,779 COVID-19 cases were confirmed (World Health Organization, 2022), and a high frequency of long-term complications can be expected.
Our prevalence of long COVID was consistent with that in many publications based on postdischarge patients (Arnold et al., 2021; Ayoubkhani and Munro, 2022; Carfì et al., 2020; Carvalho-Schneider et al., 2021; Chevinsky et al., 2021; Jacobs et al., 2020; Jacobson et al., 2021; Mandal et al., 2021). The highproportion was observed even later; 180 days after disease onset, 101/133 patients (76%) presented with long COVID. This finding could be related to the study design, considering that hospitalized and more severely ill patients with COVID-19 are expected to report persistent symptoms after discharge. Huang et al. (2021) found that 76% of patients reported at least one symptom at a median follow-up time of 186 days. Taboada et al. (2021) demonstrated an even higher incidence of long COVID (83.5%) 6 months after discharge from ICU. An alternative hypothesis, however, could relate to the particular scenario of COVID-19 in a middle-income country. Limited access to primary healthcare and rehabilitation programs after discharge may represent additional challenges resulting from longstanding socioeconomic disparities. These difficulties in follow-up care could increase the burden of long-term persistent symptoms. Lastly, a bias of information was also possible because of similarities between SARS-CoV-2 health effects and manifestations of other clinical conditions and because of overlap with other postviral syndromes and with chronic post-intensive care symptoms.
Interestingly, our study suggested a relation between long COVID frequency and time of assessment. Despite not studying a prospective cohort, we found a significantly higher rate of long COVID (46%,169/369) between 90 and 180 days after the acute disease, compared with a 27% rate (101/369) after 180 days (OR 0.24, 95% CI 0.10-0.51, P <0.001). The decrease over time was confirmed by Fumagalli et al. (2022) and Huang et al. (2021) in longitudinal studies, suggesting that most patients had a good physical and functional recovery. In fact, the National Institute for Health and Care Excellence emphasized two different phases in the long COVID period. Ongoing symptomatic COVID-19 and post-COVID-19 syndrome related to periods from 4 to 12 weeks and >12 weeks after infection onset, respectively, with significant heterogeneity in frequency of post-COVID symptoms and quality of life (Iqbal et al., 2021; Jennings et al., 2021).
The most-reported long COVID symptoms in our study were fatigue (63.1%), arthralgia (56.1%), anxiety/depression (55.1%), and dyspnea (53.7%), among others. Notably, 82% of patients had between one and five persistent symptoms, emphasizing the potential impact on physical wellness and the psychological and social consequences. Many systematic reviews reported similar rates and patterns of long COVID symptoms (Akbarialiabad et al., 2021); Iqbal et al., 2021; Jennings et al., 2021; Malik et al., 2022; Nasserie et al., 2021; Salamanna et al., 2021).
Our results demonstrated that admission to ICU was significantly associated with the risk of long COVID (OR 2.60, 95% CI 1.19-6.56, P = 0.026), highlighting the need to establish a comprehensive management plan for this group of patients. Before the pandemic, survivors of critical illnesses have previously reported long-term impairments in physical, cognitive, and/or psychological function (often known as post-intensive care syndrome). D'Cruz et al. (2021), in a prospective study of hospitalized patients with severe and critical COVID-19 pneumonia, found a high burden of persistent symptoms including fatigue (68%), sleep disturbance (57%), mental health disorders, and functional disability 2 months after ICU discharge. Taboada et al. (2021) also demonstrated that only 16% of patients who were critically ill with COVID-19 were completely free of symptoms within 6 months of follow-up. In our cohort, no association was found between interventions and events related to critical care, such as mechanical ventilation or hemodialysis, and long COVID.
An interesting aspect of our study was the association between dysgeusia and long COVID. It is well-known that anosmia, ageusia, and headache are the most frequently reported neurological symptoms in the acute phase of COVID-19 (Mahmoud et al., 2021), but no previous publication linking dysgeusia to long COVID was found (Fumagalli et al., 2022; Goërtz et al., 2020; Huang et al., 2021; Sudre et al., 2021; Taboada et al., 2021). In fact, Sudre et al. (2021) had identified anosmia as a predictor of long COVID in patients >70 years old in a multicenter study. A potential pitfall related to dysgeusia could be related to the change in olfaction itself, as the taste sensation depends on the retronasal stimulation pathway. In our cohort, the proportions of anosmia (14.1%, 52 patients) and dysgeusia (13.6%, 50 patients) are quite similar, but only 25 patients (6.8%) reported the association of dysgeusia and anosmia as symptoms of long COVID. Our findings do not suggest a direct impact on taste caused by the loss of smell, but a potential limitation in our data was the subjective character of the self-collected questionnaire. The use of objective methods to evaluate taste and smell disorders in another study design could derive more reliable conclusions regarding the association of dysgeusia and long COVID.
There is an increase in publications focused on risk factors for long COVID. Many other risk factors have been demonstrated, such as female gender (Fumagalli et al., 2022; Sudre et al., 2021), advanced age (Sudre et al., 2021; Taboada et al., 2021), obesity (Sudre et al., 2021), need for hospitalization (Carvalho-Schneider et al., 2021; Sudre et al., 2021), and comorbidities (Goërtz et al., 2020; Sudre et al., 2021). We found no association between these factors and long COVID, in contrast with other publications. However, this aspect may reflect differences in the study samples and subpopulations. In addition, no laboratory test was related to the occurrence of post-COVID conditions.
The EQ‐5D‐3L questionnaire confirmed a high impact of long COVID in all domains in our cohort, particularly in anxiety/depression and in women (OR 1.93, 95% CI 1.16-2.29, P = 0.002; data not shown). Our findings were in agreement with recent studies worldwide (Malik et al., 2022; Taboada et al., 2021), but the consequences of long COVID on quality of life in a low- or middle-income setting have not yet been fully characterized. In a scenario of poverty, the existence of other neglected infectious diseases, and limited access to primary care, multidisciplinary post-COVID care, and rehabilitation centers, a higher impact could be expected. Bonifácio et al. (2022), in a prospective study in Brazil using another instrument (the World Health Organization Quality of Life questionnaire), reported a decrease in the awareness of the good quality of life after COVID-19, but more robust longitudinal surveillance data on post-COVID conditions are lacking.
There are some limitations in our study. Firstly, this is a cross-sectional, single-center study, which may limit the generalizability of the results. Secondly, a high rate of unreachable patients was observed, probably because of logistical problems with telephone and virtual contact. Furthermore, some clinical information was not accurate because of retrospective data collection and a lack of consistent definitions (such as for obesity and elitism) in medical records. In addition, only laboratory data at admission were considered, which limits the understanding of dynamic variation in analytical data during the course of hospitalization. Moreover, the questionnaire for post-COVID-19 was a closed list of symptoms that, although comprehensive, was not necessarily exhaustive. Also, we could not exclude COVID-19 reinfection after discharge. Finally, distinguishing health effects related exclusively to long COVID symptoms from those related to other conditions or comorbidities could be challenging and introduce bias.
Conclusion
In conclusion, our study highlights that long COVID is a very prevalent condition in a middle-income country. Long COVID was most prevalent in the first 180 days after the acute phase of COVID-19, and fatigue was the most commonly reported symptom. ICU admission was a factor independently associated with long COVID. Dysgeusia could be a potential predictor of long COVID. Future multicenter studies will be crucial in understanding post-COVID-19 conditions and their risk factors.
Author contributions
Jacqueline Ferreira de Oliveira: Study design, data collection, data analysis, writing. Renata Eliane de Ávila: Study design, data collection, data analysis, writing. Neimy Ramos de Oliveira: Data collection. Natália da Cunha Severino Sampaio: Data collection. Maiara Botelho: Data collection. Fabíola Araújo Gonçalves: Data collection. Cirilo José Ferreira Neto: Data collection. Ana Carolina de Almeida Milagres: Data collection. Tatiane Cristina Caldeira Gomes: Data collection. Tássia Lopardi Pereira: Data collection. Renan Pedra de Souza: Data analysis. Israel Molina: Data collection, data analysis, writing (revision).
Acknowledgments
Declarations of competing interest
The authors have no competing interests to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This study was conducted under approvals from the Ethics Committee of the Eduardo de Menezes Hospital (Protocol CAAE: 41774720.6.0000.512). All participants or their legal representatives provided digital informed consent to be included in this study
References
- Akbarialiabad H, Taghrir MH, Abdollahi A, Ghahramani N, Kumar M, Paydar S, et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49:1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DT, Hamilton FW, Milne A, Morley AJ, Viner J, Attwood M, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoubkhani D, Munro M. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/3march2022, 2022 (accessed xx Month yyyy).
- Bonifácio LP, Csizmar VNF, Barbosa-Júnior F, Pereira APS, Koenigkam-Santos M, Wada DT, et al. Long-term symptoms among COVID-19 survivors in prospective cohort study. Brazil. Emerg Infect Dis. 2022;28:730–733. doi: 10.3201/eid2803.212020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A, Bernabei R, Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Interim guidance on evaluating and caring for patients with post-COVID conditions. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-index.html, 2021 (accessed 6 April 2022).
- Chevinsky JR, Tao G, Lavery AM, Kukielka EA, Click ES, Malec D, et al. Late conditions diagnosed 1-4 months following an initial coronavirus disease 2019 (COVID-19) encounter: a matched-cohort study using inpatient and outpatient administrative data-United States, 1 March-30 June 2020. Clin Infect Dis. 2021;73:S5–16. doi: 10.1093/cid/ciab338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz RF, Waller MD, Perrin F, Periselneris J, Norton S, Smith LJ, et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021;7:00655–02020. doi: 10.1183/23120541.00655-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuroQol. EQ-5D. https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/, 2022 (accessed 9 April 2022).
- Fumagalli C, Zocchi C, Tassetti L, Silverii MV, Amato C, Livi L, et al. Factors associated with persistence of symptoms 1 year after COVID-19: a longitudinal, prospective phone-based interview follow-up cohort study. Eur J Intern Med. 2022;97:36–41. doi: 10.1016/j.ejim.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:00542–02020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalmedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, Nyirenda T, Friedman T, Gupta A, Rasouli L, Zetkulic M, Balani B, Ogedegbe C, Bawa H, Berrol L, Qureshi N, Aschner JL. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KB, Rao M, Bonilla H, Subramanian A, Hack I, Madrigal M, et al. Patients with uncomplicated coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 2021;73:e826–e829. doi: 10.1093/cid/ciab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings G, Monaghan A, Xue F, Mockler D, Romero-Ortuño R. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs post-COVID-19 syndrome. J Clin Med. 2021;10:5913. doi: 10.3390/jcm10245913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud MM, Abuohashish HM, Khairy DA, Bugshan AS, Khan AM, Moothedath MM. Pathogenesis of dysgeusia in COVID-19 patients: a scoping review. Eur Rev Med Pharmacol Sci. 2021;25:1114–1134. doi: 10.26355/eurrev_202101_24683. [DOI] [PubMed] [Google Scholar]
- Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-a systematic review and meta-analysis. J Med Virol. 2022;94:253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al. Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. a systematic review of the current data. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboada M, Moreno E, Cariñena A, Rey T, Pita-Romero R, Leal S, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth. 2021;126:e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int, 2022 (accessed 17 July 2022).