Abstract
Background & Aims:
Lynch syndrome is associated with pathogenic variants in 4 mismatch repair (MMR) genes that increase lifetime risk of colorectal cancer (CRC). Guidelines recommend intensive CRC surveillance with colonoscopy every 1–2 years starting at 25 years for all carriers of Lynch syndrome-associated variants, regardless of gene product. We constructed a simulation model to analyze the effects of different ages of colonoscopy initiation and surveillance intervals for each MMR gene (MLH1, MSH2, MSH6, and PMS2) on CRC incidence and mortality, quality-adjusted life-years (QALYs), and cost.
Methods:
Using published literature, we developed a Markov simulation model of Lynch syndrome progression for patients with each MMR variant. The model simulated clinical trials of Lynch syndrome carriers, varying age of colonoscopy initiation (5-year increments from 25–40 years) and surveillance intervals (1–5 years). We assessed the optimal strategy for each gene, defined as the strategy with the highest QALYs and incremental cost-effectiveness ratio below a $100,000 willingness-to-pay threshold (WTP).
Results:
Optimal surveillance for patients with pathogenic variants in the MLH1 and MSH2 genes was colonoscopy starting at 25 years of age, with 1–2 year surveillance intervals. Initiating colonoscopy at age 35 and 40 years, with 3-year intervals, was cost effective for patients with pathogenic variants in MSH6 or PMS2, respectively.
Conclusions:
We developed a simulation model to select optimal surveillance starting ages and intervals for patients with Lynch syndrome based on MMR variant. The model supports recommendations for intensive surveillance of patients with Lynch syndrome-associated variants in MLH1 or MSH2. However, for patients with Lynch syndrome-associated variants of MSH6 or PMS2, later initiation of surveillance at 35 and 40 years, respectively, and at 3-year intervals, can be considered.
Keywords: cost-effectiveness, colorectal cancer, surveillance, genetic cancer syndromes
Background
Lynch syndrome is a common inherited cancer syndrome that affects about 1 in 300 individuals.1 It is most often associated with colorectal and endometrial cancer and caused by pathogenic variants in the mismatch repair (MMR) genes MLH, MSH2, MSH6, PMS2, and deletions in the EPCAM (epithelial cellular adhesion molecule) gene, which causes epigenetic silencing of MSH2. The prevalence of Lynch syndrome is similar to Hereditary Breast and Ovarian Cancer Syndrome associated with pathogenic variants in the BRCA1 and BRCA2 genes, although awareness and identification of individuals with Lynch syndrome is less common.2 In the US, it has been estimated that more than one million people have Lynch syndrome, many of whom are unaffected by cancer.
Colorectal cancer (CRC) is preventable and surveillance that involves early and frequent colonoscopy is recommended for Lynch syndrome carriers. Early prospective studies of Lynch syndrome families found that colonoscopy every three years reduced CRC incidence and mortality by 62% and 65% respectively.3 However, studies also reported interval CRC development despite intensive surveillance;4–6 to reduce these cancers, shortening the colonoscopy interval to 1–2 years was adopted in multiple international guidelines.7–10 The current recommendation for all Lynch syndrome carriers is intensive CRC surveillance with colonoscopy every 1–2 years starting at age 25–30 years.9,10
However, the lifetime risk of CRC varies by MMR gene. Carriers of MLH1 and MSH2 pathogenic variants have a 58–82% lifetime risk of CRC with mean age of diagnosis between 44–61 years.11–17 CRC risk is markedly lower for MSH6 and PMS2 mutation carriers at 10–22%, with a later mean age of 55–66 years.14,16–21 CRC surveillance starting at age 25 years is based on the premise that Lynch syndrome-associated colorectal neoplasia develops at younger ages and progresses more rapidly than sporadic CRCs. However, these conclusions were based on the phenotypic expression of the more penetrant MMR gene variants, MLH1 and MSH2, where these carriers were ascertained based on a young CRC diagnosis or strong family history of CRC. With the implementation of universal tumor testing to screen all newly diagnosed CRC cases for Lynch syndrome, irrespective of CRC age or family history, MSH6 and PMS2 mutation carriers are more often identified, at older ages and with less or no family CRC history than MLH1 and MSH2 mutation carriers.
Results from the Prospective Lynch Syndrome Database on 6,350 carriers emphasizes the difference in CRC risk between the four MMR genes and the need for gene-specific recommendations; it has been suggested that carriers of MSH6 and PMS2 pathogenic variants not be counselled similarly to those with MLH1 and MSH2 variants.17 This has significant implications since pathogenic variants in PMS2 are the most common in Lynch syndrome, affecting 1 in 716 individuals, followed by MSH6 (1 in 758), MSH2 (1 in 2,841), and MLH1 (1 in 1,946).1 As the majority of Lynch syndrome carriers carry the lowest risk of CRC, gene-specific recommendations could prevent colonoscopy overuse and harms associated with intensive surveillance.
Using a decision analytic model, our goal was to determine the optimal CRC surveillance strategies for Lynch syndrome carriers by specific MMR gene. We analyzed the impact of varying age at time of colonoscopy initiation and surveillance intervals for each of the four MMR genes on CRC incidence and mortality, quality-adjusted life-years (QALYs), and cost.
Methods
Model Overview and Target Population
We developed a state-transition (Markov) cohort-level model that recapitulated the clinical course of CRC development in patients with Lynch syndrome CRC development using Python 3.7 (Figure 1). The model was used to simulate trials by following hypothetical cohorts of Lynch syndrome carriers with each of the four MMR genes (MLH1, MSH2, MSH6, PMS2) undergoing surveillance protocols at various intervals over a lifetime. The model cohort was comprised of healthy 25-year-old individuals at initiation and cycled annually until age 75 (end of screening) or death, including CRC diagnosis. To maintain model parsimony and transparency, after a CRC diagnosis, patients remained in the stage-specific state until death from cancer or all cause. Our model did not include remission, as the primary focus was CRC prevention. However, to account for differences in costs and quality of life in initial treatment compared to continuing care, we applied separate utility values and costs to the initial year of diagnosis and subsequent years. Given favorable outcomes for stage I CRC,22–23 we used age-adjusted rates of death from colectomy complications as the cancer-specific death rate for these patients.
Figure 1.
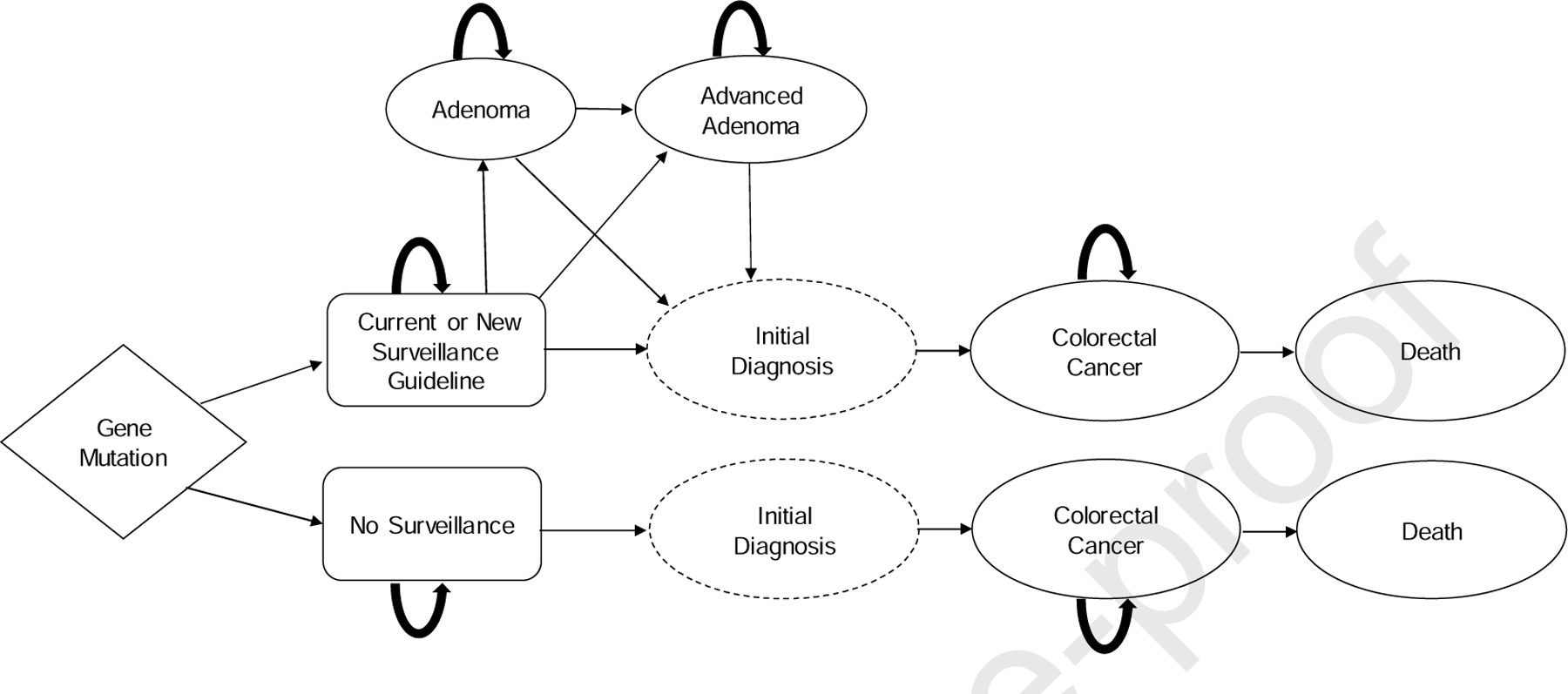
Model Schematic.
Surveillance strategies were tested separately for each of the four MMR gene cohorts. Due to the complexity of strategies, we aggregated lifetime risk and life expectancy data for women and men. We did not account for gynecological cancer risks given our study’s focus on CRC prevention. Institutional review board approval was not necessary.
Study Perspective and Outcomes
We assessed the incremental cost-effectiveness of various surveillance protocols, stratified by gene, from the perspective of the U.S. healthcare system. The primary endpoint was the optimal strategy for each gene, defined by the highest QALYs with an incremental cost-effectiveness ratio (ICER) below a willingness-to-pay threshold (WTP) of $100,000 (2019 USD). Secondary endpoints included unadjusted life-years gained, cancer incidence, and cancer mortality. Costs and QALYs were discounted at 3% per year,24 and a half-cycle correction was applied to QALYs and unadjusted life-years.25
Surveillance Protocols
The model simulated hypothetical clinical trials of Lynch syndrome patients, varying the age of colonoscopy initiation (5-year increments from 25–40 years) and surveillance intervals (1–5 years); 20 (4 x 5) unique strategies were tested for each gene. The most aggressive strategy was based on current guidelines for annual colonoscopy starting at age 25.10 The least aggressive strategy was based on the current management for individuals with a family history of CRC, namely colonoscopy every 5 years beginning at age 40.26
Health State Transition Probabilities, Model Calibration
Our model was calibrated and validated to external clinical data by ensuring that CRC incidence reproduced Kaplan-Meier curves of lifetime cumulative risk estimates for each MMR gene (Supplemental Table S1), utilizing clinical observational data from cohorts undergoing endoscopic surveillance. The MCLIR methodology was used for validation (Supplemental Figure 6a–6c, Supplemental Figure 7a–7c). The effect of varying surveillance intervals on risk of interval cancers was derived from studies of patients undergoing different surveillance protocols.3,27 These data informed variable risk ratios that attenuated baseline cancer risk. Kaplan-Meier curves of adenoma incidence and advanced adenoma incidence per gene were also used to derive and calibrate progression rates to adenoma development; 5,28,29,30 this was done using a simulated annealing optimization algorithm for all four genotypes.
Cancer dwell time, the time a cancer is present before clinical diagnosis, was modeled implicitly through model calibration to CRC incidence and adenoma incidence data by genotype. Adenoma dwell time is represented in the model as the time it takes for an adenoma to become a symptomatically detected CRC. Cancer stage at diagnosis was based on estimates of cancer stage distributions under varying surveillance intervals (Supplemental Table S2).27,31 Stage-specific cancer death rates were obtained from a study of CRC survival in patients with MSI-high tumors (Table 1)23 as these tumors are a hallmark of Lynch syndrome related CRC, and outcomes for those with MSI-high tumors do not significantly differ by Lynch syndrome status.22,23 Risks of colonoscopy complications and colectomy deaths were obtained from the literature (Table 1).32,33 All-cause mortality was derived from the average of male and female 2016 U.S. life tables.34
Table 1.
Model inputs: Health state transition probabilities, costs, and health state utility valuesa
| Model Inputs | Base Case Value | Range for Sensitivity Analysis | References |
|---|---|---|---|
| HEALTH STATE TRANSITION PROBABILITIES | |||
| All-cause mortality | 2016 U.S. Lifetables | N/A | 32 |
| Death from colectomy | 33 | ||
| < 60 years old | 0.03% | 0.0285%-0.0315% | |
| 60 – 70 years old | 0.08% | 0.076%-0.084% | |
| 70 – 80 years old | 0.06% | 0.057%-0.063% | |
| Colonoscopy Complications | 30 | ||
| Without biopsy | 0.008% | 0.0072%-0.0088% | |
| With biopsy | 0.07% | 0.0063%-0.0077% | |
| Death | 0.0061% | 0.0055%-0.0067% | |
| Cancer death (rates) | 23 | ||
| Stage I | Death from colectomy | ||
| Stage II | 0.019 | 0.018–0.020 | |
| Stage III | 0.069 | 0.066–0.072 | |
| Stage IV | 0.921 | 0.875–0.967 | |
| Cumulative lifetime risk of developing adenomas | 0.776 | 0.737–0.815 | 5,28,29,30 |
| Risk ratio for developing interval CRC | 5,27 | ||
| Colonoscopy every year | 0.215 | 0.204–0.226 | |
| Colonoscopy every 2 years | 0.274 | 0.260–0.288 | |
| Colonoscopy every 3 years | 0.304 | 0.289–0.319 | |
| Colonoscopy every 4 years | 0.600 | 0.570–0.630 | |
| Colonoscopy every 5 years | 0.829 | 0.788–0.870 | |
| COSTS | |||
| Colonoscopy without biopsy/polypectomy | $ 979.14 | $783.31.00-$1,174.97 | 33,34 |
| Colonoscopy with biopsy/polypectomy | $ 1,203.00 | $962.40-$1443.60 | 33,34 |
| Colonoscopy complication | $ 3,981.18 | $3,184.94-$4,777.42 | 33,34 |
| Cost of colectomy | $ 30,673.00 | $24,538.40-$36,807.60 | 33,34 |
| CRC Care | |||
| Stage I | |||
| Initial year | $ 45,409.31 | $36,327.45-$54,491.17 | 33,34 |
| Continued care | $ 3,347.95 | $2,678.36-$4,017.54 | 33,34 |
| End-of-life care | $70,042,44 | $44,666.80-$84,050.93 | 33,34 |
| Stage II | |||
| Initial year | $ 60,945.99 | $48,756.79-$73,135.19 | 33,34 |
| Continued care | $ 3,150.37 | $2,520.30-$3,780.44 | 33,34 |
| End-of-life care | $69,763.63 | $44,488.50-$83,716.93 | 33,34 |
| Stage III | |||
| Initial year | $ 73,974.72 | $59,179.78-$88,769.66 | 33,34 |
| Continued care | $ 4,413.81 | $3,531.05-$5,296.57 | 33,34 |
| End-of-life care | $73,589.67 | $46,928.72-$88,307.61 | 33,34 |
| Stage IV | |||
| Initial year | $ 96,295.01 | $77,036.01-$115,554.01 | 33,34 |
| Continued care | $ 13,367.67 | $10,694.14-$16,041.20 | 33,34 |
| End-of-life care | $96,999.90 | $61,857.64-$116,399.88 | 33,34 |
| Health State Utilities | |||
| Healthy | 0.84 – 1.00 | N/A | 36 |
| Colonoscopy (disutility) | 0.0055 | 0.0052–0.0057 | 33,34 |
| Colonoscopy complication (disutility) | 0.0384 | 0.0369–0.0399 | 33,34 |
| CRC Care | |||
| Stage I | |||
| Initial year | 0.88 | 0.84–0.92 | 33,34 |
| Continued care | 0.95 | 0.912–0.99 | 33,34 |
| End-of-life care | 0.3 | 0.288–0.312 | 33,34 |
| Stage II | |||
| Initial year | 0.82 | 0.79–0.85 | 33,34 |
| Continued care | 0.95 | 0.91–0.99 | 33,34 |
| End-of-life care | 0.3 | 0.288–0.312 | 33,34 |
| Stage III | |||
| Initial year | 0.76 | 0.73–0.79 | 33,34 |
| Continued care | 0.76 | 0.73–0.79 | 33,34 |
| End-of-life care | 0.3 | 0.288–0.312 | 33,34 |
| Stage IV | |||
| Initial year | 0.3 | 0.288–0.312 | 33,34 |
| Continued care | 0.3 | 0.288–0.312 | 33,34 |
| End-of-life care | 0.3 | 0.288–0.312 | 33,34 |
Input parameters are per year unless otherwise specified. CRC: colorectal cancer
Aggregated colonoscopy complications based on frequency and cost of complication
Costs and Health State Utility Values
Costs associated with surveillance, cancer care, and colonoscopy complications were based on Medicare reimbursement rates from past cost-effectiveness analyses of Lynch syndrome carriers or family history of CRC (Table 1).35–37 Health state utility values, used to calculate QALYs, were also from past work including Lynch syndrome and familial CRC.35–37 Age-specific weights were applied to utility values to account for the decreased utility associated with ageing.38 Cost and effectiveness parameters are presented in Table 1. Although we did not model annual colonoscopy following cancer treatment separately, we assumed that the associated costs and quality of life decrements were captured by the overall cost and utilities associated with cancer care. Indirect costs related to lost work by the patient or the chaperone for the colonoscopies were not included in this analysis. We have added the code used for this model to Github to increase transparency: https://github.com/CUMC-HIRE/lynch_syndrome.
Sensitivity Analyses
After initial analyses using base-case input parameters, we tested the impact of model parameter uncertainty on results. One-way (deterministic) sensitivity analyses were conducted by varying one parameter at a time across its plausible range of values (Table 1). Probabilistic sensitivity analyses were conducted by varying all input parameters simultaneously based on sampling according to the distributions in Table 1 across 10,000 Monte Carlo samples.
Results
Lifetime cancer incidence in the base-case without intervention was 57.46% for MLH1, 47.65% for MSH2, 18.66% for MSH6, and 9.20 % for PMS2 (Supplemental Figures). Corresponding cancer mortality rates were 15.54% for MLH1, 10.21% for MSH2, 3.06% for MSH6, and 1.66% for PMS2. These model outputs are concordant with published estimates13–16,18–20 and provide assurance that our results are clinically realistic. All secondary and primary outcomes associated with no intervention are presented by gene in Supplemental Table S4.
Base-case analyses
MLH1:
For MLH1 gene mutation carriers, the optimal strategy was the same as current guidelines for annual surveillance beginning at age 25. This strategy yielded the highest QALYs (29.51) with total cost of $40,783.80, and an ICER of $44,790.96, which is below the $100,000 WTP threshold (Table 2). This approach resulted in the lowest CRC incidence (17.04%) and highest unadjusted life-expectancy (46.48 life-years gained from initiation at age 25; Figures 2–3). Additional results in Table 2 include starting colonoscopy at age 25 with surveillance every 2 years (ICER: refence strategy), and others were strongly dominated (i.e., higher cost with fewer QALYs). Results for initiation at age 30, 35, and 40 with surveillance at 2, 3, 4, and 5 years are in Supplemental Table S5a.
Table 2.
Base-case results by specific MMR gene.
| Strategya | QALYs | Life-Years | CRC Incidence | Cost | ICER |
|---|---|---|---|---|---|
| MLH1 | |||||
| Q5Y, Start age 40 | 27.867 | 44.557 | 52.80% | $102,074.89 | Dominated |
| Q3Y, Start age 35 | 28.558 | 45.241 | 30.75% | $73,022.45 | Dominated |
| Q3Y, Start age 25 | 29.347 | 46.138 | 23.19% | $38,982.50 | Dominated |
| Q2Y, Start age 25 | 29.433 | 46.291 | 21.16% | $37,514.06 | -- |
| Q1Y, Start age 25 | 29.506 | 46.477 | 17.04% | $40,783.80 | $44,790.96 |
| MSH2 | |||||
| Q5Y, Start age 40 | 29.032 | 45.569 | 42.37% | $51,362.57 | Dominated |
| Q3Y, Start age 35 | 29.530 | 46.338 | 19.89% | $30,477.17 | Dominated |
| Q3Y, Start age 25 | 29.668 | 46.568 | 18.21% | $25,323.40 | -- |
| Q2Y, Start age 25 | 29.696 | 46.643 | 16.57% | $26,231.66 | $29,298.71 |
| Q1Y, Start age 25 | 29.699 | 46.734 | 13.27% | $32,261.21 | $2,009,850.42 |
| MSH6 | |||||
| Q5Y, Start age 40 | 29.785 | 46.684 | 15.95% | $18,164.77 | Dominated |
| Q3Y, Start age 40 | 29.885 | 46.823 | 6.83% | $14,272.85 | Dominated |
| Q3Y, Start age 35 | 29.892 | 46.837 | 6.56% | $14,072.41 | -- |
| Q3Y, Start age 25 | 29.895 | 46.842 | 6.13% | $14,813.35 | $246,980.73 |
| Q1Y, Start age 25 | 29.835 | 46.916 | 4.38% | $25,999.77 | Dominated |
| PMS2 | |||||
| Q5Y, Start age 40 | 29.887 | 46.817 | 7.80% | $13,349.79 | Dominated |
| Q4Y, Start age 40 | 29.912 | 46.858 | 5.88% | $12,328.57 | Dominated |
| Q3Y, Start age 40 | 29.941 | 46.890 | 3.25% | $11,491.14 | -- |
| Q3Y, Start age 35 | 29.940 | 46.908 | 2.89% | $11,672.08 | Dominated |
| Q1Y, Start age 25 | 29.853 | 46.938 | 2.05% | $25,094.03 | Dominated |
Strategy refers to colonoscopy interval (years) and starting age. Bold-facing indicates optimal strategies for each gene. QALYs: quality-adjusted life-years, ICER: incremental cost-effectiveness ratio, CRC: colorectal cancer
Figure 2.
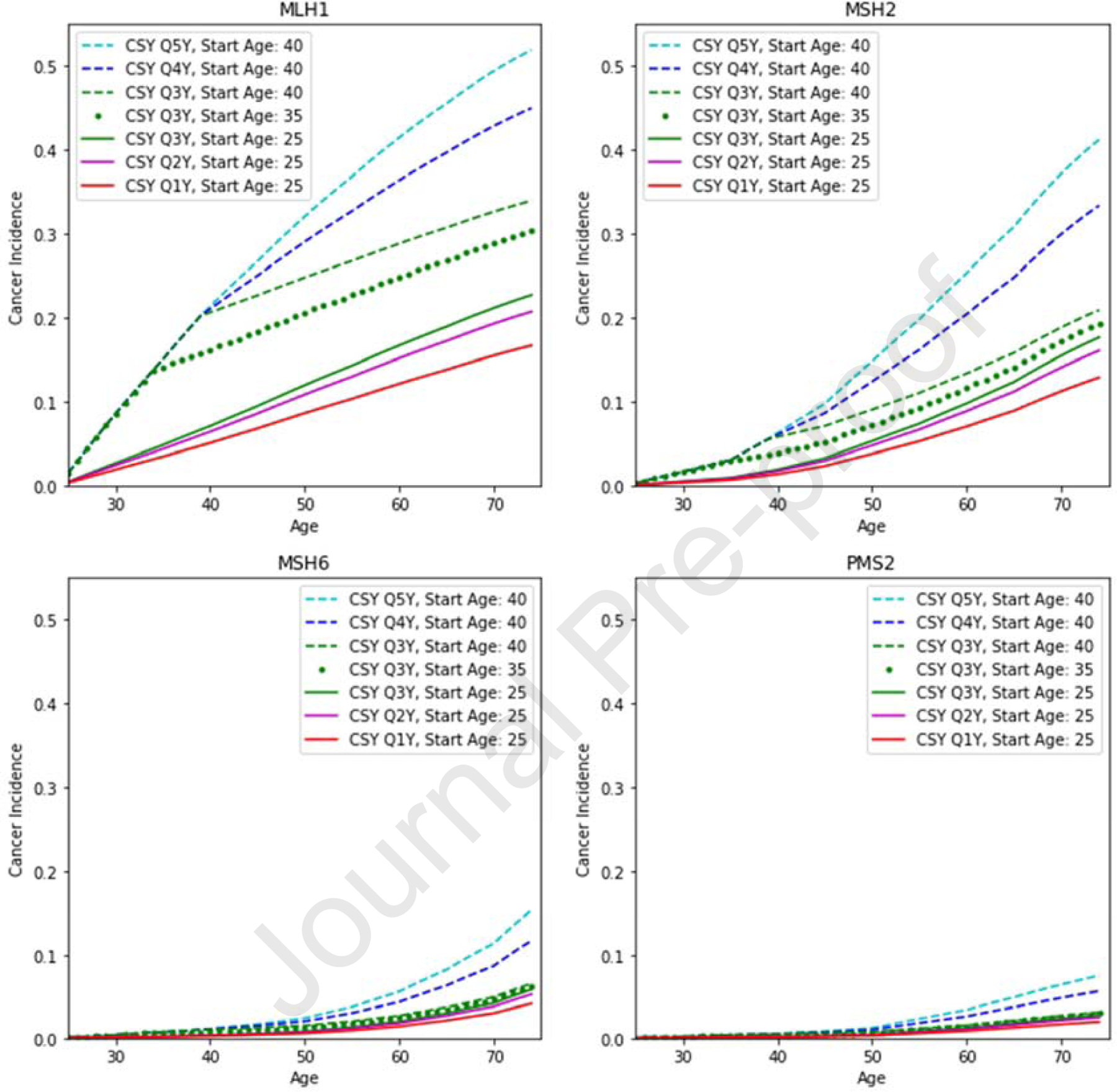
Colon cancer incidence for strategies on the efficiency frontier by age for (A) MLH1, (B) MSH2, (C) MSH6, and (D) PMS2 carriers. CSY, colonoscopy.
Figure 3.
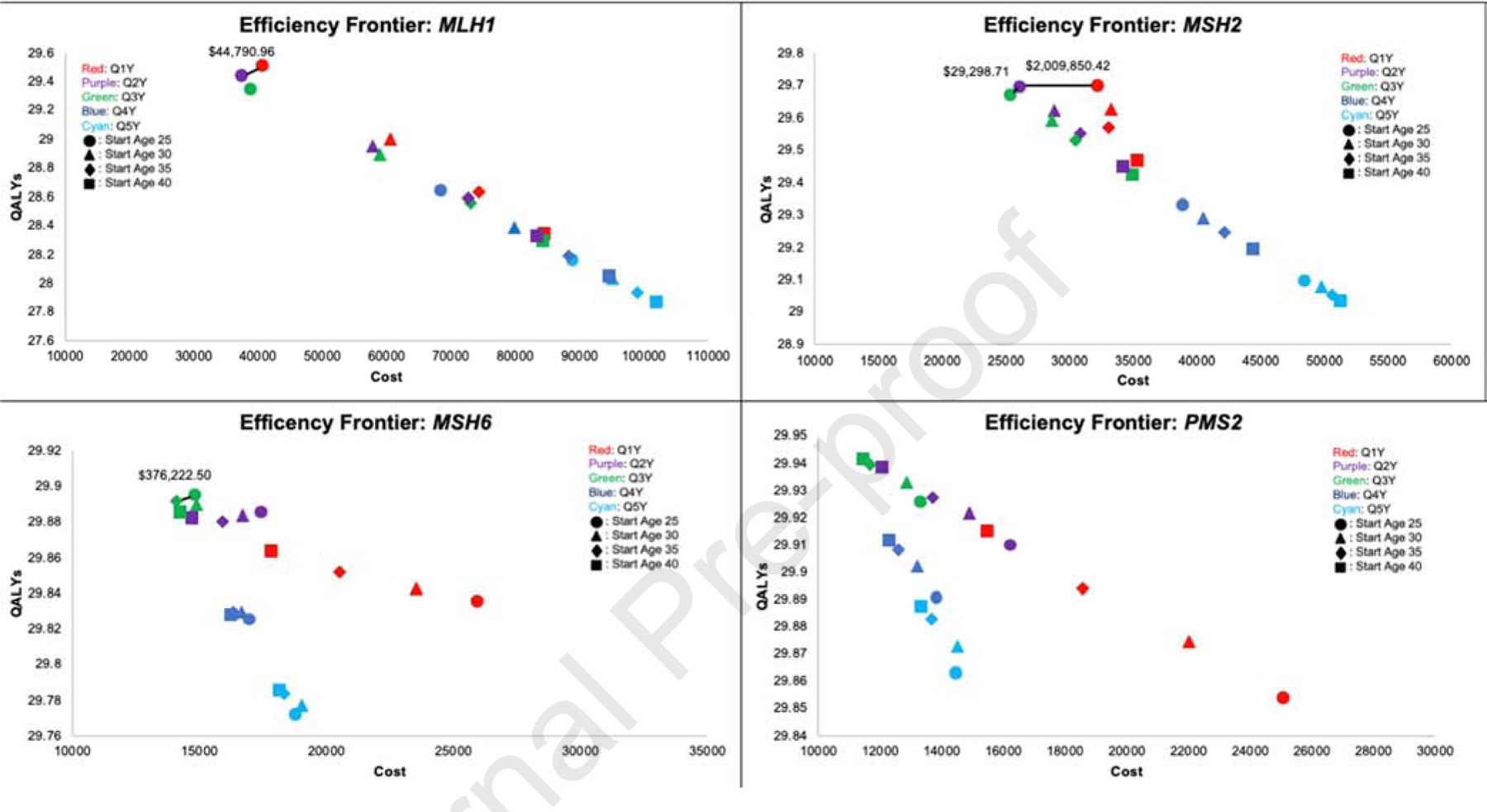
Efficiency frontiers for (A) MLH1, (B) MSH2, (C) MSH6, and (D) PMS2 carriers. QALYs, quality-adjusted life-years.
MSH2:
For MSH2 gene mutation carriers, the optimal strategy was biennial surveillance from age 25, yielding 29.69 QALYs, ICER of $29,298.71, and total cost of $32,261.21 (Table 2, Figures 2–3). This resulted in a lifetime CRC incidence of 16.57% and 46.64 unadjusted life-years gained from age 25. Colonoscopy every three years from age 25 was also cost-effective, as it was the reference strategy, but decreased QALYs relative to the optimal strategy. Annual surveillance at age 25, the current recommendation, resulted in the highest QALYs (29.70), unadjusted life expectancy (46.73 years gained), and lowest cancer incidence (13.27%); however, it was not cost-effective with an ICER of $2,009,850.42 and cost of $32,261.21. Other strategies resulted in decreased QALYs and unadjusted life expectancy and higher cancer incidence than those on the efficiency frontier (Supplementary Table S5b).
MSH6:
For MSH6 gene mutation carriers, the optimal strategy was surveillance every 3 years beginning at age 35. This approach resulted in 29.892 QALYs, 46.84 life-years gained, and 6.56% lifetime cancer incidence, with a cost of $14,072.41 (Table 2). Surveillance every 3 years from age 25 resulted in higher QALYs and life-years gained than colonoscopy beginning at 35 but was cost-prohibitive with an ICER of $246,980.73 (Figures 2–3, Table 2). The guideline recommendation for annual colonoscopy from age 25 was strongly dominated, resulting in fewer QALYs (29.835) and higher cost ($25,999.77) relative to the optimal strategy.
PMS2:
For PMS2 gene mutation carriers, surveillance every 3 years from age 40 was the cost-effective strategy, yielding 29.941 QALYs and an ICER of $2,216.81. This resulted in overall CRC incidence of 3.25%, 46.89 unadjusted life-years gained, and a cost of $11,491.14. Surveillance every 4 years from age 40 had comparable QALYs, life-years gained, and costs, but higher cancer incidence of 5.88%. Notably, current management was not supported, with fewer QALYs (29.853) and higher cost ($25,094.03) than the optimal strategy (Table 2, Figures 2–3).
One-Way Sensitivity Analysis
One-way sensitivity analysis found that the model’s results were most sensitive to risk of interval CRC (Supplemental Tables 6a–b). Decreasing the risk of interval cancers between biennial colonoscopies caused biennial surveillance starting at age 25 to be the optimal strategy for MLH1 (Supplemental Table 6a). The optimal strategy for MSH2 was sensitive to risk of interval cancers; increasing the cancer prevention benefits of surveillance every 3 years relative to surveillance every 2 years (i.e., by increasing the risk of interval cancers in the Q2Y strategy or by decreasing the risk of interval cancers in the Q3Y strategy) led surveillance every 3 years to be the optimal strategy for MSH2 (Supplemental Table 6b). For MSH6 and PMS2, the ICERs for the base-case optimal strategy did not exceed the $100,000 WTP threshold for all the plausible ranges of input parameters tested. The model was also sensitive to the cost of colonoscopy, disutility with stage I and stage II CRC diagnosis and lifetime CRC risk, but changes in these parameters did not change the cost-effectiveness outcomes of the model.
Probabilistic Sensitivity Analysis
Results from probabilistic sensitivity analysis indicated that the base-case results were robust to uncertainty in model input parameters (Supplemental Figure S5). For MLH1, the base-case optimal strategy of annual colonoscopy from age 25 remained optimal in 89% of simulations, compared to 11% for biennial colonoscopy beginning at 25 years. For MSH2, biennial colonoscopy from age 25 was optimal in 86.3% of trials. Results for MSH6 showed 3-year colonoscopy was the optimal colonoscopy interval, with the base-case optimal strategy of every 3-year colonoscopy from age 35 being optimal in 65.3% of simulations and 16.3% for colonoscopy every 3 years from age 40, and 18.4% for colonoscopy every 3 years from age 25. For PMS2, colonoscopy every 3 years was also the optimal colonoscopy interval, with colonoscopy every 3-years beginning at age 40 optimal in 78.2% of simulations and every 3-year colonoscopy from age 30 optimal in 21.6%.
Discussion
Our analysis supports gene-specific CRC surveillance intervals for individuals with Lynch syndrome. Our results concur with current recommendations for intensive surveillance in MLH1 and MSH2 gene mutation carriers with initial colonoscopy at age 25 years and at every 1–2 year intervals.7–10 However, given the lower lifetime estimates of CRC among carriers with MSH6 and PMS2 pathogenic variants,15–21 less aggressive surveillance can be considered with colonoscopy initiation at age 35 for MSH6 and 40 years for PMS2 carriers, and every 3 years thereafter.
To the best of our knowledge, this is the first simulation model-based analysis to assess the gene-specific impact of various surveillance strategies on CRC-related outcomes for Lynch syndrome carriers. Our modeling approach allows for simulation of hypothetical clinical trials in a context where such trials would be infeasible to conduct in gene-specific Lynch syndrome cohorts. We leverage existing data on CRC risk estimates stratified by MMR gene variants in order to test and evaluate a large number of potential management strategies.
Our results, if implemented, could greatly impact the care of MSH6 and PMS2 mutation carriers. Recent studies report infrequent neoplasia before the fourth decade in these individuals, where colonoscopy before this would be of little to no benefit. However, with adherence to current guidelines (colonoscopy every 1–2 years from age 25–75), MSH6 and PMS2 mutation carriers undergo anywhere from 25–50 colonoscopies during their lifetime. This approach is not only associated with risk of complications, but requires missed work, an aggressive bowel cleansing, temporary disability following sedation (which requires a third party to accommodate patients), and medical expenses with potential loss of income. Our results support a delayed initiation of colonoscopy to age 35 and 40 years for carriers of MSH6 and PMS2 pathogenic variants, respectively, with surveillance every three years, thereby decreasing the lifetime number of colonoscopies to approximately 12. Furthermore, the increased risk of complications caused by the aggressive surveillance recommended in current guidelines is associated with lower QALYs than the optimal strategy for both MSH6 and PMS2 gene mutation carriers, thereby rendering current guidelines not cost-effective for these carriers. These results would limit overuse of colonoscopy for select Lynch syndrome carriers and could potentially redirect resources to a larger pool of individuals who would benefit from CRC screening.
Our findings also impact a significant proportion of Lynch syndrome carriers, as MSH6 and PMS2 pathogenic variants are common. Among 34,980 individuals who had germline testing of multiple cancer susceptibility genes, 579 newly identified Lynch syndrome carriers had pathogenic variants in MSH6 (29.3%), PMS2 (24.2%), MSH2 (23.7%), MLH1 (21.6%) and EPCAM (1.2%).39 This differs from previous data where MSH2 and MLH1 variants were estimated to comprise nearly 90% of all Lynch syndrome cases. With increased multigene testing among individuals without cancer, Lynch syndrome carriers with less penetrant phenotypes will continue to unexpectedly be identified. In addition, the recent success of immunotherapy in patients with advanced solid tumors that display MSI, supports such testing irrespective of cancer type. Among 15,045 cancer patients with over 50 tumors screened for MSI, Lynch syndrome was identified across a broader tumor spectrum than previously appreciated, with a high frequency of MSH6 and PMS2 pathogenic variants.40
Our study should be interpreted in the context of potential limitations. As with all modeling analyses, model inputs and assumptions rely on existing data, which are often simplified and extended to populations with limited or unavailable data. The empiric data on gene-specific CRC screening outcomes in Lynch syndrome are limited. The Prospective Lynch Syndrome Database offers data on only 407 PMS2 gene mutation carriers compared to 2,607 MLH1, 2,496 MSH2, and 841 MSH6 mutation carriers, with less robust follow-up for PMS2 carriers.17 There are also limited data to create a comprehensive natural history model that reproduces CRC progression rates in the absence of colonoscopic surveillance because the available clinical data comes almost exclusively from cohorts enrolled in surveillance programs; hence our approach to only model cohorts that were under surveillance. In addition, much data on the disutility of colonoscopy surveillance is extrapolated from familial or sporadic CRC screening studies. Also, colonoscopy sensitivity and specificity are based on a single round of screening and whether performance varies at repeat screenings is unknown. In the absence of data to suggest otherwise, conditional independence of repeat screenings was assumed, so there were no systematic false-negative results for adenomas and cancers. Similar to decision analyses that evaluate colonoscopy screening, we assumed perfect adherence to surveillance for Lynch syndrome carriers, resulting in the maximum achievable benefit for each approach. Adherence was not incorporated because the model may favor a strategy with shorter intervals to accommodate for suboptimal adherence and conversely, over-screening in those who are adherent, leading to unnecessary risks and burden.
A potential shortcoming is that these results are based on simulation of Lynch syndrome cohorts and not intended for individual level decision-making, which would incorporate personal and family cancer risk and patient preferences. While evaluation of personalized scenarios was beyond the scope of this analysis, strategies modified for family history may be warranted as age to initiate colonoscopy may decrease based on the youngest family members’ CRC diagnosis. In addition, strategies may be individualized based on the presence of precursor neoplasia on colonoscopy.
Lastly, we assume that the natural history of Lynch syndrome is similar for each MMR gene variant and follows the adenoma-to-carcinoma sequence. Whether a biological difference in carcinoma development exists between the different Lynch syndrome genes, or an alternate pathway to cancer development occurs without the precursor adenomatous polyp, has been unproven.
In summary, we provide timely, cost-effective approaches for CRC surveillance for Lynch syndrome carriers based on the specific MMR gene and the variable associated risks of CRC. Our results provide a more tailored and precise approach to CRC screening for MSH6 and PMS2 mutation carriers than current guidelines. In turn, implementation of delayed initiation of colonoscopy to age 35 and 40 with three-year surveillance intervals for MSH6 and PMS2 mutation carriers, respectively, will affect the majority of Lynch syndrome carriers who carry the lowest risk of CRC development, de-implementing over-testing and overtreatment and their potential harms by decreasing the total number of colonoscopies by more than half. Such “saved” resources could be potentially better utilized by the LS carriers at highest risk, as well as other patient populations who would garner greater benefit from CRC screening.
Supplementary Material
What you need to know:
Background and Context:
Lynch syndrome is associated with variants in 4 mismatch repair (MMR) genes that increase lifetime risk of colorectal cancer (CRC). Guidelines recommend intensive CRC surveillance with colonoscopy every 1–2 years starting at 25 years for all carriers of Lynch syndrome-associated variants, regardless of gene product
New Findings:
The authors developed a model to select surveillance starting ages and intervals for patients with Lynch syndrome based on MMR variant. The model supports recommendations for intensive surveillance of patients with Lynch syndrome-associated variants in MLH1 or MSH2. However, for patients with Lynch syndrome-associated variants of MSH6 or PMS2, later initiation of surveillance at 35 or 40 years, respectively, and at 3-year intervals, can be considered.
Limitations:
This was a modeling study; prospective studies are needed to support these findings.
Impact:
Patients with Lynch syndrome-associated variants in MLH1 or MSH2 should receive intensive surveillance, as recommended by guidelines. However, for patients with Lynch syndrome-associated variants of MSH6 or PMS2, later initiation of surveillance (age 35 for MSH6 or 40 years for PMS2), at 3-year intervals, can be considered.
Lay Summary:
This study analyzed data on risk of CRC in patients with Lynch syndrome and found that risk varies with genetic alteration associated with the disease, which should be used to determine starting age for colonoscopy surveillance and interval.
Funding:
No external funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
No relevant financial, professional, or personal conflicts to disclose.
References
- 1.Win AK, Jenkins MA, Dowty JG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev 2017;26(3):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maxwell KN, Domchek SM, Nathanson KL, Robson ME. Population frequency of germline BRCA1/2 mutations. J Clin Oncol 2016; 34(34):4183. [DOI] [PubMed] [Google Scholar]
- 3.Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000; 118(5):829–834. [DOI] [PubMed] [Google Scholar]
- 4.Vasen HF, Abdirahman M, Brohet R, et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology 2010; 138(7):2300–6. [DOI] [PubMed] [Google Scholar]
- 5.Mecklin J-P, Aarnio M, Läärä E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology 2007; 133(4):1093–1098. [DOI] [PubMed] [Google Scholar]
- 6.Ladabaum U, Ford JM, Martel M, Barkun AN. American Gastroenterological Association Technical Review on the Diagnosis and Management of Lynch Syndrome. Gastroenterology 2015; 149(3):783–813. [DOI] [PubMed] [Google Scholar]
- 7.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2014; 147(2):502–526. [DOI] [PubMed] [Google Scholar]
- 8.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am J Gastroenterol 2015; 110(2):223–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vangala DB, Cauchin E, Balmaña J, et al. Screening and surveillance in hereditary gastrointestinal cancers: Recommendations from the European Society of Digestive Oncology (ESDO) expert discussion at the 20th European Society of Medical Oncology (ESMO)/World Congress of Gastrointestinal Cancer, Barcelona, June 2018. Eur J Cancer 2018; 104:91–103. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network (NCCN) Guidelines: Genetic/Familial High-Risk Assessment, Colorectal. Version 3.2019; https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Accessed January 2020.
- 11.Hampel H, Stephens JA, Pukkala E, et al. Cancer Risk in Hereditary Nonpolyposis Colorectal Cancer Syndrome: Later Age of Onset. Gastroenterology 2005;129(2):415–421. [DOI] [PubMed] [Google Scholar]
- 12.Quehenberger F, Vasen HFA, Van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet 2005; 42(6):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of Risk of Colorectal and Endometrial Cancer Among Patients With Lynch Syndrome. Gastroenterology 2009;137(5):1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonadona V, Bonaïti B, Olschwang S, et al. Cancer Risks Associated With Germline Mutations in MLH1, MSH2, and MSH6 Genes in Lynch Syndrome. JAMA 2011; 305(22):2304–2310. [DOI] [PubMed] [Google Scholar]
- 15.Møller P, Seppälä T, Bernstein I, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 2017;66(3):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 2018;67(7):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominguez-Valentin M, Sampson J, Seppälä T, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med 2020; 22(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baglietto L, Lindor NM, Dowty JG, et al. Risks of Lynch Syndrome Cancers for MSH6 Mutation Carriers. J Natl Cancer Inst 2010;102(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology 2008;135(2):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ten Broeke SW, Brohet RM, Tops CM, et al. Lynch Syndrome Caused by Germline PMS2 Mutations: Delineating the Cancer Risk. J Clin Oncol 2015; 33(4):319–325. [DOI] [PubMed] [Google Scholar]
- 21.ten Broeke SW, van der Klift HM, Tops CMJ, et al. Cancer Risks for PMS2-Associated Lynch Syndrome. J Clin Oncol 2018;36(29):2961–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haraldsdottir S, Hampel H, Wu C, et al. Patients with colorectal cancer associated with Lynch syndrome and MLH1 promoter hypermethylation have similar prognoses. Genet Med 2016;18(9):863–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malesci A, Laghi L, Bianchi P, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res 2007;13(13):3831–3839. [DOI] [PubMed] [Google Scholar]
- 24.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 25.Naimark DM, Bott M, Krahn M. The half-cycle correction explained: two alternative pedagogical approaches. Med Decis Making 2008;28(5):706–712. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Provenzale D, Llor X, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 2.2019. J Natl Compr Canc Netw 2019;17(9):1032–1041. [DOI] [PubMed] [Google Scholar]
- 27.Engel C, Vasen HF, Seppälä T, et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy Among 3 Countries With Different Lynch Syndrome Surveillance Policies. Gastroenterology 2018;155(5):1400–1409. [DOI] [PubMed] [Google Scholar]
- 28.Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of Colonoscopy Adenoma Findings With Long-term Colorectal Cancer Incidence. JAMA 2018;319(19):2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engel C, Ahadova A, Seppälä T, Aretz S. Associations of Pathogenic Variants in MLH1, MSH2, and MSH6 with Risk of Colorectal Adenomas and Tumors and With Somatic Mutations in Patients with Lynch Syndrome. Gastroenterology 2020;158(5):1326–1333. [DOI] [PubMed] [Google Scholar]
- 30.Goverde A, Eikenboom E, Viskil E, Bruno M. Yield of Lynch Syndrome Surveillance for Patients With Pathogenic Variants in DNA Mismatch Repair Genes. Clinical Gastroenterology and Hepatology 2020;18(5):1112–1120. [DOI] [PubMed] [Google Scholar]
- 31.Seppälä TT, Ahadova A, Dominguez-Valentin M, et al. Lack of association between screening interval and cancer stage in Lynch syndrome may be accounted for by over-diagnosis; a prospective Lynch syndrome database report. Hered Cancer Clin Pract 2019;17. doi: 10.1186/s13053-019-0106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med 2006;145(12):880–886. [DOI] [PubMed] [Google Scholar]
- 33.Visser BC, Keegan H, Martin M, Wren SM. Death After Colectomy: It’s Later Than We Think. Arch Surg 2009;144(11):1021–1027. [DOI] [PubMed] [Google Scholar]
- 34.Arias E, Xu J, Kochanek KD. United States life tables, 2016. National Vital Statistics Reports 2019;68(4). https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_04-508.pdf. [PubMed] [Google Scholar]
- 35.Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med 2010;12:93–104. [DOI] [PubMed] [Google Scholar]
- 36.van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing Colonoscopy Screening for Elderly Individuals by Screening History, Cancer Risk, and Comorbidity Status Could Increase Cost Effectiveness. Gastroenterology 2015; 149(6):1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramsey SD, Burke W, Clarke L. An economic viewpoint on alternative strategies for identifying persons with hereditary nonpolyposis colorectal cancer. Genet Med 2003;5 (5):353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fryback DG, Dunham NC, Palta M, et al. U.S. Norms for Six Generic Health-Related Quality-of-Life Indexes from the National Health Measurement Study. Med Care 2007;45(12): 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espenschied CR, LaDuca H, Li S, et al. Multigene Panel Testing Provides a New Perspective on Lynch Syndrome. J Clin Oncol 2017; 35(22):2568–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latham A, Srinivasan P, Kemel Y et al. Microsatellite Instability is Associated with the Presence of Lynch Syndrome Pan-Cancer. J Clin Oncol 2019;37 (4):286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


