Abstract
Background
Patients with multiple sclerosis (MS) on some disease modifying therapies (DMTs), particularly anti-CD20 and sphingosine-1-phosphate (S1P) modulators, are at increased risk of severe Coronavirus Disease 19 (COVID-19) and death. COVID-19 vaccinations are effective in preventing infection and severe disease, but humoral response to vaccination and outcomes of COVID-19 infection after vaccination in MS patients on DMTs remain less understood.
Methods
In this retrospective single-center study, patients enrolled in the CLIMB (Comprehensive Longitudinal Investigation of Multiple Sclerosis at Brigham and Women's Hospital) study and biorepository who had been vaccinated against COVID-19 and had SARS-CoV-2 spike antibody (anti-SARS-CoV-2 S Roche-Elecsys) testing were identified and compared to healthy controls. Demographic data, serum immune profiles including lymphocyte count, B-cell count, and immunoglobulins, and clinical outcome of COVID-19 infection were collected.
Results
254 patients (73.2% female, mean (SD) age 52.9 (11.2) years) were identified. When controlling for age, time since vaccination, and vaccine type, patients on fingolimod, ocrelizumab, rituximab, mycophenolate mofetil, natalizumab and teriflunomide had significantly lower levels of spike antibodies compared to healthy controls (n = 34). Longer duration of treatment was associated with lower spike antibody levels in patients on anti-CD20 therapy (p = 0.016) and S1P modulators (p = 0.016) compared to healthy controls. In patients on anti-CD20 therapy, higher spike antibody levels were associated with higher CD20 cell count (p<0.001), and longer time since last anti-CD20 therapy infusion (p<0.001). 92.8% (13/14) vaccine responders (spike antibody titer >100 ug/dL) on anti-CD20 therapy demonstrated B-cell reconstitution (mean CD20 3.6%). Only 1 out of 86 patients with CD20 of 0% had a measurable spike antibody response to vaccination. During follow-up (mean 270 days), five patients were diagnosed with COVID-19 after vaccination (incidence 1.9%), all of whom had spike antibody < 20 ug/dL. No patients required ICU care or died.
Conclusions
Patients on some DMTs demonstrate reduced humoral immunity after Sars-CoV-2 vaccination. Longer duration of anti-CD20 therapy and reduced CD20 cell count is associated with blunted humoral response to vaccination. CD20 reconstitution >0.1% appears necessary, but not always sufficient, for humoral response to vaccination. Breakthrough COVID-19 infection in our cohort of MS patients on DMT was higher than in population studies. We propose that adjustment of B-cell therapy administration to allow for B-cell reconstitution prior to vaccination should be considered.
Keywords: Multiple sclerosis, Disease modifying therapy, COVID-19, Vaccination, Anti-CD20
1. Introduction
Multiple sclerosis (MS) is a central nervous system autoimmune disease that is estimated to affect over 2.8 million people worldwide. (Walton et al., 2020) There are currently a wide range of immune suppressing treatment options for patients with MS with varying strength and efficacy profiles. (Zhang et al., 2021) Understanding the effect of these disease modifying therapies (DMTs) on coronavirus disease 19 (COVID-19) severity and response to the COVID-19 vaccine is essential to care of patients with MS during the COVID-19 pandemic. Patients on high-efficacy anti-CD20 therapies, such as rituximab and ocrelizumab, are at increased risk of severe COVID-19 and death after infection with SARS-CoV-2 virus. (Jones et al., 2021) While COVID-19 vaccinations are effective in preventing COVID-19 infection and severe disease (Baden et al., 2021, Polack et al., 2020) and have been shown to be safe in patients with multiple sclerosis (MS), (Lotan et al., 2021) the ability for MS patients on DMTs to mount an effective immune response after vaccination remains unclear.
Prior work has demonstrated reduced spike antibody response to COVID-19 vaccines in patients on anti-CD20 and S1P modulators. (Sormani et al., 2021, Mrak et al., 2021, Brill et al., 2021, Disanto et al., 2021, Sabatino et al., 2021, Tortorella et al., 2021, Cohen et al., 2022, Apostolidis et al., 2021) Research targeted at understanding how to increase immune response to vaccination in these patients is of great importance. Previous studies have shown that even minimal B-cell reconstitution may correlate with an increased humoral response to COVID-19 vaccination. (Disanto et al., 2021, Apostolidis et al., 2021, Rico et al., 2021, van Kempen et al., 2021) In one study, while 1 of 36 patients with undetectable B cells seroconverted after vaccination, 45% of patients with between 0 and 1% of CD19+ B cells seroconverted. (Mrak et al., 2021) Studies have also found a significant correlation between time since last anti-CD20 infusion as well as length of anti-CD20 therapy and SARS-CoV-2 antibody titer. (Disanto et al., 2021, Sabatino et al., 2021, Tortorella et al., 2021, Apostolidis et al., 2021)
Recent work has shown a significant correlation between breakthrough COVID-19 infection and SARS-CoV-2 spike antibody level (Sormani et al., 2022), indicating spike antibody level (i.e. humoral vaccination response) may represent some level of protection from clinical COVID-19. However, the quantitative spike antibody response post-vaccination necessary to prevent COVID-19 infection is not fully understood. A recent editorial identified areas for future research including additional data on humoral response after a wider array of DMTs, as well as identification of the necessary levels of humoral and cellular immunity needed to provide sufficient protection against COVID infection. (Graves and Killestein, 2021) Case series have reported mild cases of COVID-19 in small numbers of vaccinated patients on DMTs, (Januel et al., 2021) but have not commented on the necessary immunity to prevent such infections.
We performed a retrospective, single-center cohort study comparing vaccinated MS patients on DMTs to healthy controls. In this work we sought to expand the literature characterizing immune responses after COVID-19 vaccination in a large cohort of MS patients treated with a wide range of DMTs. Additionally, we sought to identify the relationship between humoral response to vaccination (SARS-CoV-2 spike antibody level), DMT type and treatment duration, immune system markers (IgG, absolute lymphocyte count, CD20 count), and clinical outcomes of infection with COVID-19.
2. Methods
2.1. Study design and patients
This was a retrospective, single-center cohort study. Patients enrolled in the CLIMB (Comprehensive Longitudinal Investigation of Multiple Sclerosis at Brigham and Women's Hospital) study and CLIMB biorepository of the Brigham MS Center with a diagnosis of multiple sclerosis, who had received the mRNA-1273 (Moderna mRNA), BNT162b2 (Pfizer mRNA) or Ad26.COV2.S (Johnson and Johnson) COVID-19 vaccine and had SARS-CoV-2 spike antibody serologic testing were identified. Fully vaccinated was defined as two doses of mRNA vaccination, or one dose of Ad26.COV2.S vaccination. Sex, age, body mass index (BMI), disease duration, last recorded expanded disability status scale (EDSS), DMT type, vaccine type, date(s) of vaccination, date of spike antibody testing, and spike antibody level were extracted from the CLIMB dataset. Patient charts were individually reviewed for accuracy, and additional data were collected. Laboratory values of Immunoglobulin G (IgG, mg/dL) level, absolute lymphocyte count (ALC, K/ul), and CD20 cell count (measured as % of total lymphocytes) were recorded. For IgG and ALC, the value available from the date closest to the date of first COVID-19 vaccination was used. For CD20 cell count, the closest value to date of first COVID-19 vaccination available after the patient's last anti-CD20 infusion was used. Per protocol at our infusion center, most CD20 cell counts are drawn immediately prior to administration of anti-CD20 infusion, so values prior to last infusion were not used given they would not accurately reflect B-cell counts. Clinical data including dates of last DMT treatment and development of COVID-19 infection (defined as positive nasopharyngeal SARS-CoV-2 PCR) were collected. Healthy patients enrolled in a COVID-vaccine study between June 2021-December 2021 were included in this report and spike antibody levels collected.
2.2. Standard Protocol approvals, registrations, and patient consents
The CLIMB study and CLIMB biorepository are approved by the Massachusetts General Brigham Human Research Committee (IRB #1999P010435, #2017P001169, # 2002P001045). Healthy controls were enrolled from an IRB approved vaccine study (IRB # 2021P001156). Patients provided written informed consent for these studies.
2.3. Statistics
Summary statistics were calculated for demographic characteristics using the mean and standard deviation for continuous variables and proportions for categorical variables. For the spike antibody levels, the median and range for each treatment group was calculated. The difference between treatment groups was assessed using pairwise Wilcoxon rank sum tests to account for subject below (<0.40) and above (>2500) the limit of detection. To adjust for age, time since vaccination and type of vaccine, the treatment groups were compared using a proportional odds logistic regression model. Finally, the association between spike antibody level and several patient characteristics were estimated using Spearman's correlation coefficient. All analyses were completed in the statistical software package R version 3.6.3 (www.r-project.org).
2.4. Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
3. Results
3.1. Demographics
254 patients were identified, 186 (73.2%) were female with a mean (SD) age of 52.9 (11.2) years and BMI of 27.1 (6.3). The mean (SD; range) time between the final vaccination and SARS-CoV-2 spike antibody sample was 83.0 (46.7; 5, 224) days. 133 (52.4%) patients received the Pfizer vaccine, 110 (43.3%) received the Moderna vaccine, 11 (4.3%) Johnson and Johnson. 186 (73.2%) of patients had relapse-remitting MS, 11 (4.3%) had primary progressive MS, 51 (20.1%) had secondary progressive MS, 3 (1.2%) had progressive relapsing MS, and 3 (1.2%) had a clinically isolated syndrome. Mean (SD) EDSS at time of data collection was 2.9 (2.1). Demographic characteristics of included patients are shown in Table 1 .
Table 1.
Demographics.
| N | Age | Female/Male | BMI | EDSS | |
|---|---|---|---|---|---|
| All MS patients | 254 | 52.9 (11.2) | 186 / 68 | 27.1 (6.3) | 2.9 (2.0) |
| Dimethyl Fumarate | 14 | 56.8 (11.7) | 12 / 2 | 23.7 (4.0) | 1.5 (1.2) |
| Fingolimod | 42 | 51.7 (10.9) | 29 / 13 | 26.5 (6.6) | 1.9 (1.4) |
| Glatiramer acetate | 4 | 60.0 (16.2) | 2 / 2 | 27.6 (4.2) | 2.9 (3.0) |
| Interferon beta | 7 | 51.0 (12.0) | 6 / 1 | 24.1 (4.3) | 1.7 (0.5) |
| Mycophenolate mofetil | 4 | 59.3 (3.9) | 4 / 0 | 25.2 (4.5) | 5.4 (1.6) |
| Natalizumab | 8 | 46.2 (13.4) | 6 / 2 | 27.4 (7.2) | 1.4 (1.0) |
| Ocrelizumab | 116 | 51.7 (11.2) | 80 / 36 | 28.1 (6.0) | 3.1 (2.1) |
| Ofatumumab | 2 | 59.8 (2.7) | 2 / 0 | 26.3 (3.2) | 5.0 (2.1) |
| Rituximab | 37 | 53.1 (11.0) | 30 / 7 | 26.6 (7.8) | 3.7 (2.1) |
| Teriflunomide | 11 | 55.6 (6.9) | 8 / 3 | 29.7 (7.5) | 3.7 (2.2) |
| Untreated | 9 | 64.3 (7.3) | 7 / 2 | 23.8 (4.2) | 4.4 (2.6) |
| Healthy controls | 34 | 38.4 (13.5) | 15 / 19 | NA | NA |
Data shown is mean (SD).
BMI = Body mass index in kg/m2.
3.2. Humoral response to vaccination and disease modifying therapy
3.2.1. Quantification of spike antibody levels by DMT
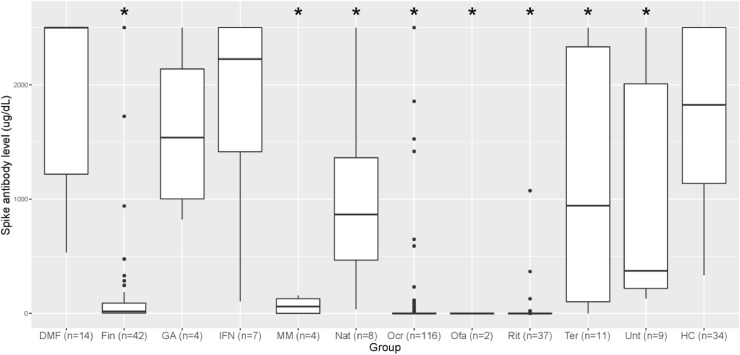
The median (range) spike antibody level for each treatment group and the healthy controls are provided in Table 2 and Fig. 1 . For between DMT group comparison, we removed patients who had spike antibody levels checked after only a single dose of mRNA vaccines (n = 8). We excluded DMT groups with only 1 patient (n = 4). Older age has been associated with decreased antibody response after COVID-19 vaccination, (Collier et al., 2021) as has longer time since vaccination, (Levin et al., 2021) so multiple group comparisons were performed between DMTs and healthy controls controlling for age, time since vaccination, and vaccine type. When controlling for these factors, patients on fingolimod, ocrelizumab, rituximab, mycophenolate mofetil, natalizumab, and teriflunomide had significantly lower levels of spike antibodies compared to healthy controls (Fig. 1).
Table 2.
SARS-CoV-2 Spike Antibody Level after controlling for age, time since vaccination and vaccine type.
| Treatment group | Median spike antibody (range) | Wilcoxon rank sum test comparison vs. healthy controls (p-value) | Proportional odds model comparison vs. healthy controls (p-value) |
|---|---|---|---|
| Dimethyl fumarate (n = 14) | >2500 (535.6, >2500) | 0.35 | 0.26 |
| Fingolimod (n = 42) | 16.8 (<0.40, >2500) | 4.0*10−12 | 2.0*10−13 |
| Glatiramer acetate (n = 4) | 1539.5 (823, >2500) | 0.66 | 0.76 |
| Interferon beta (n = 7) | 2225 (105.9, >2500) | 0.78 | 0.56 |
| Mycophenolate mofetil (n = 4) | 59.8 (<0.40, 157) | 0.0011 | 0.0006 |
| Natalizumab (n = 8) | 865.9 (36.8, >2500) | 0.022 | 0.019 |
| Ocrelizumab (n = 116) | <0.40 (<0.40, >2500) | < 2*10−16 | < 2*10−16 |
| Ofatumumab (n = 2) | <0.40 (<0.40, <0.40) | 0.018 | ** |
| Rituximab (n = 37) | <0.40 (<0.40, 1074) | 1.4*10−13 | < 2*10−16 |
| Teriflunomide (n = 11) | 942.4 (<0.40, >2500) | 0.037 | 0.048 |
| Untreated (n = 9) | 372.8 (132.6, >2500) | 0.018 | 0.095 |
| Healthy controls (n = 34) | 1825 (337.4, >2500) | reference | reference |
Legend: Median spike antibody level ( ug/dL) separated by DMT group. Spike antibody level at our institution is reported as a single value from <0.4 to >2500 ug/dL.Wilcoxon rank sum comparison between each treatment group and healthy controls was performed and p-values are shown. Proportional odds model adjusting for age, vaccine type, and time since vaccination comparing each DMT group to healthy controls was performed and p-values are shown. **missing since all the measurements for this treatment were at the lowest value
Fig. 1.
Sars-CoV-2 Spike Antibody level by Disease Modifying Therapy Type. Legend: Box plots demonstrate mean (stdev) spike antibody level ( ug/dL) for patients on each DMT. *Indicates statistically significant (p<0.05) difference compared to healthy controls. Spike antibody level at our institution is reported as a single value from <0.4 to >2500 ug/dL. DMF= Dimethyl fumarate; Fin = Fingolimod; GA= Glatiramer acetate; IFN = Interferon beta; MM= Mycophenolate mofetil; Nat= Natalizumab; Ocr= Ocrelizumab; Ofa= Ofatumumab; Rit=Rituximab; Ter=Teriflunomide; Unt= Untreated; HC: Healthy controls. Between group comparison statistics are shown in Table 2.
3.2.2. Duration of anti-CD20 and S1P modulator treatment and spike antibody levels
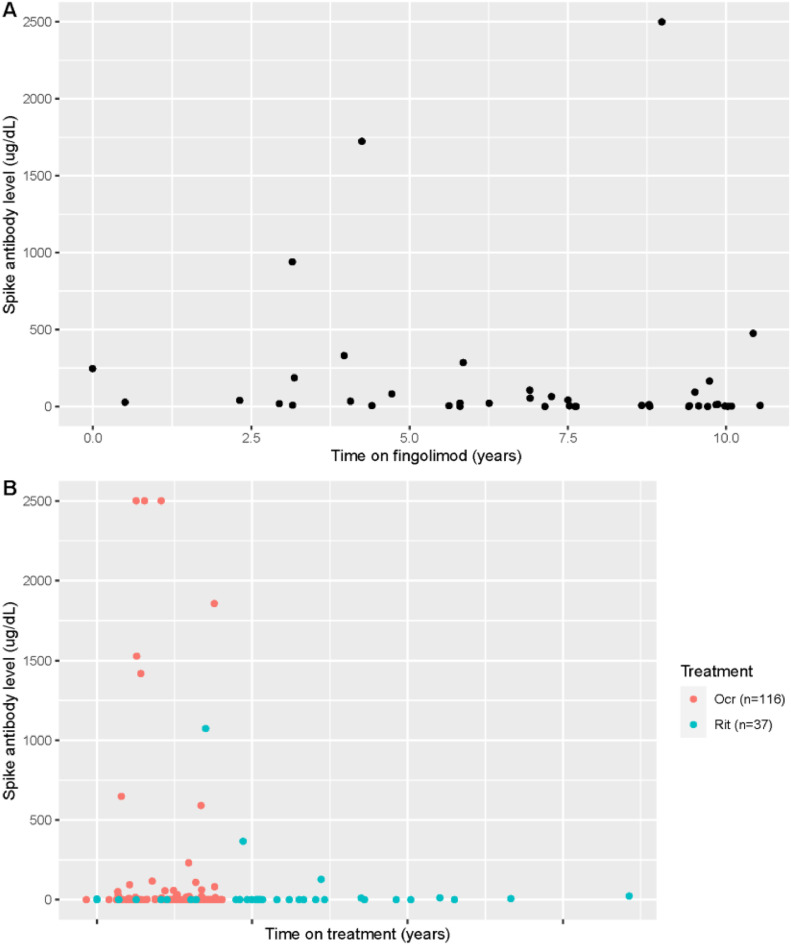
Given the large number of patients on anti-CD20 and S1P modulator therapy in our cohort, and prior evidence that these DMTs reduce spike antibody response after vaccination, we evaluated the relationship of duration of DMT treatment and spike antibody levels for these two patient groups. In patients on S1P modulator therapy (fingolimod, n = 42), we found the estimated Spearman's correlation coefficient to be -0.37 (p = 0.016), indicating that as time on treatment with fingolimod increases, the spike antibody level decreases (Fig. 2 A). Similarly, we found a relationship between longer duration of treatment with anti-CD20 therapy and lower SARS-CoV-2 spike antibody levels (estimated Spearman's correlation coefficient between time on treatment and spike antibody level -0.19 (p = 0.016) (Fig. 2B).
Fig. 2.
Relationship of Spike Antibody titer and Time on DMT Treatment. Legend: A. Association between time on treatment and spike antibody levels ( ug/dL) in subjects treated with fingolimod (estimated Spearman's correlation coefficient -0.37 (p = 0.016). B: Association between time on treatment and spike antibody levels ( ug/dL) in subjects treated with ocrelizumab or rituximab (estimated Spearman's correlation coefficient -0.19 (p = 0.016).
3.3. Relationship of immune system markers and spike antibody levels
3.3.1. Immunoglobulin G levels
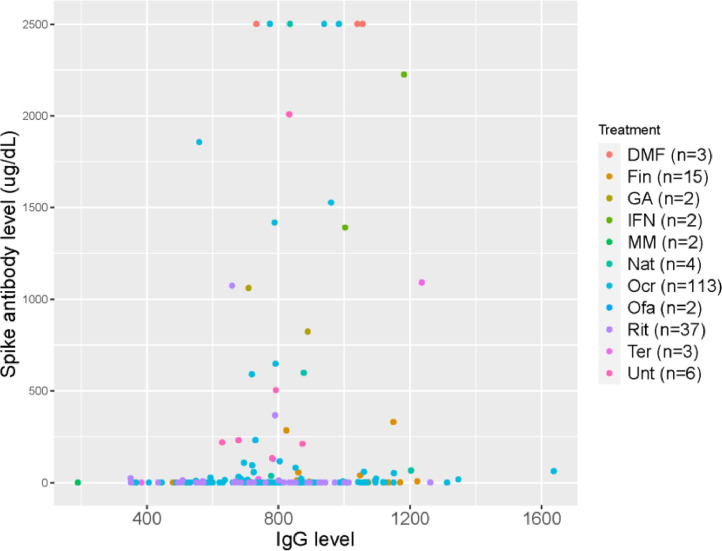
We examined the relationship of Immunoglobulin G (IgG) level and spike antibody across all patients. IgG level was recorded from the lab value in the chart available closest to date of vaccination. Average (SD) time between date of IgG level and date of vaccination was 197 (363) days. We found a significant relationship between higher levels of IgG and higher spike antibody level (estimated Spearman's correlation coefficient 0.23 (p = 0.0017) (Fig. 3 ). All patients with hypogammaglobulinemia (IgG <600 mg/dL) were on anti-CD20 except for four patients: two on mycophenolate mofeteil, one on teriflunomide, and one on fingolimod. Hypogammaglobulinemia has been reported in patients on fingolimod (Zoehner et al., 2019) and mycophenolate. (Kaplan and Bonagura, 2019) The patient on teriflunomide had been on ocrelizumab within 1 year of this study and was switched due to hypogammaglobulinemia, thus their persistent hypogammaglobulinemia was likely secondary to ocrelizumab and not teriflunomide.
Fig. 3.
Antibody response by IgG level in all patients. Legend: Relationship of IgG level ( ug/dL) and spike antibody level ( ug/dL) in all patients. Estimated Spearman's correlation coefficient was 0.23 (p = 0.0017). DMF= Dimethyl fumarate; Fin = Fingolimod; GA= Glatiramer acetate; IFN = Interferon beta; MM= Mycophenolate mofetil; Nat= Natalizumab; Ocr= Ocrelizumab; Ofa= Ofatumumab; Rit=Rituximab; Ter=Teriflunomide; Unt= Untreated.
3.3.2. Absolute lymphocyte count
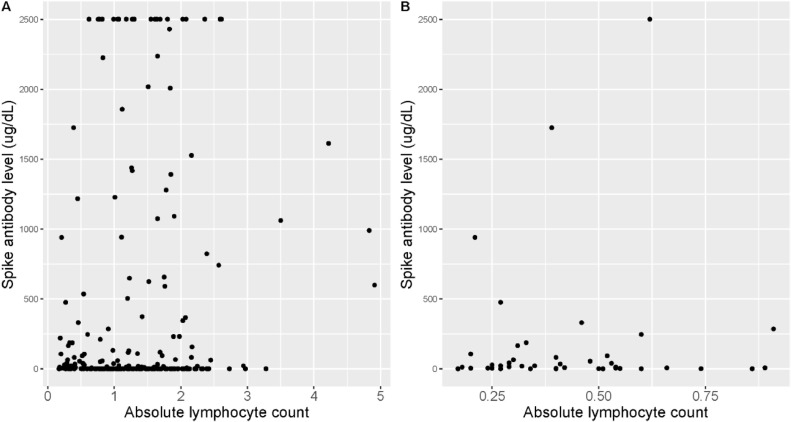
We next sought to further classify the relationship of spike antibody level and lymphocyte count, particularly for patients on S1P modulators which are known to cause lymphopenia. Absolute lymphocyte count value was chosen from the closest available date to date of vaccination. Average time between date of vaccination and serum sample of absolute lymphocyte count was 101.7 days. The estimated Spearman's correlation coefficient between absolute lymphocyte count and spike level was -0.002 (p = 0.97) (Fig. 4 A). The estimated Spearman's correlation coefficient between absolute lymphocyte count and spike level among patients treated with fingolimod was -0.04 (p = 0.80) (Fig. 4B). These data do not indicate a significant relationship between lymphocyte count and humoral vaccine response in this cohort.
Fig. 4.
Relationship of spike antibody level and absolute lymphocyte count. Legend: Absolute lymphocyte count vs spike antibody level. A: All patients (n = 254) estimated Spearman's correlation coefficient 0.002 (p = 0.97). B: Patients on S1P modulator therapy (fingolimod, n = 42) estimated Spearman's correlation coefficient -0.04 (p = 0.80) (Fig. 3B).
3.3.3. CD20 lymphocyte counts
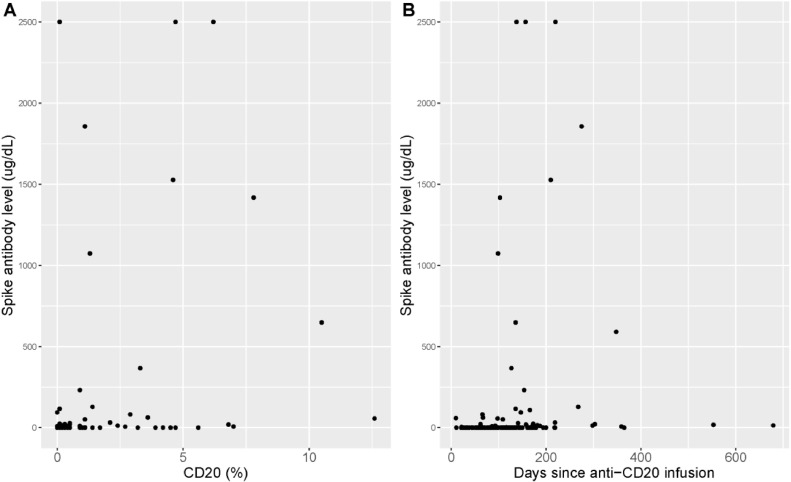
We next evaluated the relationship of spike antibody level and CD20 cell count for patients on anti-CD20 infusion therapy (ocrelizumab, rituximab). Date of last infusion prior to vaccination was recorded to calculate time between infusion and vaccination. The first CD20 cell count available after vaccination was chosen as a proxy for CD20 count at the time of vaccination. The average time between vaccination and available CD20 count was 118.6 days. Mean (stdev) CD20 cell count for all patients on anti-CD20 therapy was 0.9 (2.1). We found a significant relationship between reduced CD20 cell count and lower spike antibody level (estimated Spearman's correlation coefficient 0.59 (p<0.001) (Fig. 5 A). We also found a significant relationship between longer time since anti-CD20 infusion and vaccination date, and higher spike antibody levels (estimated Spearman's correlation coefficient 0.38 (p<0.001) (Fig. 5B).
Fig. 5.
SARS-CoV-2 spike antibody in patients on anti-CD20 therapy. Legend: A: Association between spike antibody level and CD20 cell count (estimated Spearman's correlation coefficient 0.59 (p<0.001). B: Association between spike antibody level and days since last B-cell infusion (estimated Spearman's correlation coefficient 0.38 (p<0.001).
3.4. Clinical outcomes
3.4.1. Characterizing anti-CD20 therapy vaccine responders vs non-responders
Given that most patients on anti-CD20 therapy do not demonstrate a humoral response to COVID-19 vaccination, an in-depth chart review was performed to further characterize vaccine “responders,” which we defined as patients on anti-CD20 therapy who had a spike antibody titer >100 ug/dL. One patient did not have an available CD20 count after vaccination. Six out of 152 patients on anti-CD20 therapy (3.9%) had high titer spike antibody levels (>1000 ug/dL). Five of these six patients demonstrated B-cell reconstitution (CD20 >0.1%) at the first available CD20 count after vaccination (at an average of 6.4 months after last infusion). These five patients had an average CD20 cell count of 3.6% (SD 2.2; range 1.1-6.2). One patient (48-year-old female) initially had low spike antibody titer after two Moderna vaccinations (5.71 ug/dL) but demonstrated an increase to spike antibody of >2500 ug/dL after her third vaccination despite CD20 count of 0%. An additional 8 patients on anti-CD20 therapy had low positive spike antibody levels >100 ug/dL. Seven of these patients demonstrated B-cell reconstitution at the first available CD20 count after vaccination, at an average of 5.8 months after last infusion. These seven patients had an average CD20 count of 5.31% (SD 4.35; range 0.1-10.5). Taken together, the 14 patients with spike antibody levels >100 ug/dL were 78% female, 53% RRMS, had had a mean (SD) age of 48.7 (12) years, a mean (SD) EDSS of 3.17 (2.0), and a mean treatment duration of 39.9 months (range 14-96 months). They had a mean of 181.2 days between last ocrelizumab infusion and vaccination (range 99-348 days). None of these patients contracted COVID-19 during follow-up.
In contrast, we identified 49 patients on anti-CD20 therapy who had evidence of B-cell reconstitution (CD20 > 0.1%), but a negative spike antibody as defined as a titer of < 20 ug/dL, or vaccine “non-responders.” This patient group was 78% female, 73% RRMS, had a mean (SD) age of 52.8 (12.8) years, a mean (SD) EDSS of 3.2 (2.2), and a mean treatment duration of 45.1 months (range 12-165 months). They had an average of 139.6 days between last treatment and vaccination (range 22-679 days). They had an average CD20 count of 1.33% (SD 1.83)
When comparing vaccine responders vs non-responders on anti-CD20 therapy using two-tailed T tests, there was no significant difference in age (p = 0.358), duration of anti-CD20 therapy (p = 0.556), or time between last B-cell infusion and vaccination (p = 0.249). However, there was a significantly higher average B-cell count in the vaccine responder group (mean CD20 1.3% vs 3.9%, p = 0.00038).
Finally, we evaluated all patients who had a CD20 count of 0% (n = 86) to see if any had a positive humoral response to vaccination. Only one patient had a spike antibody level >20 ug/dL, with spike antibody measured at 94.4 ug/dL two weeks after second vaccination. This patient was a 33yo male who had been treated with ocrelizumab for one year, with his last infusion four months prior to first vaccination.
3.4.2. COVID-19 infection after vaccination
Mean (SD) length of follow-up after vaccination for our cohort was 270.8 (36.3) days. Five patients were diagnosed with COVID-19 after vaccination and are characterized in Table 3 . Two patients were on teriflunomide, two on ocrelizumab, and one on rituximab with a mean (range) duration of DMT of 31.2 (2-59) months and mean (range) EDSS of 3.5 (0-6.5). All patients had a spike antibody level <20 ug/dL after vaccination, with 4 out of 5 patients having level below the threshold of detection. The mean (SD) time from vaccination to infection was 5.75 (1.04) months. Two patients required supplemental oxygen (World Health Organization (WHO) COVID severity of 4), two had limitation of activities but not hospitalized (WHO grade 2), and one patient was symptomatic but ambulatory (WHO grade 1). None were intubated. Two patients with EDSS measurements after COVID-19 infection were stable. Two patients were treated with monoclonal antibody treatment.
Table 3.
Clinical Characteristics of Patients Who Contracted COVID-19 After Vaccination.
| Patient | Age | Sex | EDSS Pre-COVID | DMT | Duration of DMT (months) | Vaccine type | CD20 count | Spike Ab level ( ug/dL) | Time from vaccine to infection (months) | WHO COVID Severity | EDSS post-COVID |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | F | 6.5 | teriflunomide | 2 | Pfizer | n/a | 19.3 | 5.5 | 4 | 6.5 |
| 2 | 55 | M | 2.5 | teriflunomide | 50 | J&J | n/a | <0.4 | 6 | 1 | n/a |
| 3 | 47 | F | 2.5 | ocrelizumab | 37 | Moderna | 0 | <0.4 | 4.5 | 2 | 2.5 |
| 4 | 31 | F | 0 | ocrelizumab | 8 | Pfizer | 0.3 | <0.4 | 7 | 2 | n/a |
| 5 | 59 | F | 6 | rituximab | 59 | Pfizer | 0 | <0.4 | 3* | 4 | n/a |
Patient received 3rd vaccination
4. Discussion
Understanding the effect of disease modifying therapies on the ability to mount a humoral response to SARS-CoV-2 vaccination is imperative for clinicians caring for patients with MS during the ongoing COVID-19 pandemic. In this study, we add to prior work (Disanto et al., 2021, Sabatino et al., 2021, Apostolidis et al., 2021, Sormani et al., 2021) supporting observations that the humoral response to COVID-19 vaccination is reduced in patients on B-cell therapies and S1P modulators compared to untreated controls. However, we also show reductions in spike antibody levels in patients on mycophenolate, natalizumab, and teriflunomide, though to a lesser degree. We also add that this observation persists even when controlling for age and time since vaccination, supporting evidence that treatment with these therapies is an independent cause of reduced spike antibody levels after vaccination. We show that treatment with other DMTs including dimethyl fumarate, interferons, and glatiramer acetate do not significantly reduce antibody response compared to untreated controls. While we observed a trend towards reduced average antibody response in our group of untreated control patients with MS which has not been seen in some prior work, (Achiron et al., 2021) of our nine patients, seven had been on DMT in the past (5 on ocrelizumab, 1 on rituximab, 1 on glatiramer acetate). The mean time since last therapy was 1.2 years, making effect of prior treatment a possible explanation for this reduction.
When examining duration of DMT treatment, we confirm prior work showing longer duration of treatment with S1P modulators and anti-CD20 therapy is associated with reduced SARS-CoV-2 spike antibody levels. (Sabatino et al., 2021) Additionally, we demonstrate that several humoral and cellular immune measures correlate significantly with antibody response to vaccination. While hypogammaglobulinemia has been shown to impair responses to certain vaccinations, (Szczawinska-Poplonyk et al., 2015) there have been fewer reports regarding IgG levels and COVID-19 vaccine response. Here we show that lower levels of IgG were associated with lower levels of spike antibody in our cohort, suggesting this may be an important risk factor for poorer humoral response to vaccination. S1P modulators potentially cause circulating lymphopenia, and data regarding the relationship between absolute lymphocyte count and spike antibody levels in patients on S1P modulators have previously been mixed, with some studies reporting significant correlation between lymphopenia and reduced humoral response (Sormani et al., 2021) while others do not. (Sabatino et al., 2021) In our cohort we did not see a significant relationship between absolute lymphocyte count and spike antibody level across all patients or within the subgroup of patients on S1P modulators, indicating that lymphocyte count may not directly influence humoral response to vaccination. Early work indicates lymphocyte count may correlate with T-cell mediated vaccination responses (Tortorella et al., 2021), and additional investigation on this topic is underway.
Study of patients on anti-CD20 therapy is of particular interest given there is widespread use of these medications and there is evidence that their use leads to reduced humoral response to vaccination, (Sormani et al., 2021) which we also demonstrate in our cohort. We show a significant correlation between reduced spike antibody and lower CD20 count as well as higher spike antibody level and longer time since last anti-CD20 infusion. While these findings are consistent with prior work (Sabatino et al., 2021, Sormani et al., 2021), the relationship between anti-CD20 therapy dosing interval, B-cell reconstitution, antibody response, and real-world risk of COVID infection has remained unclear. We sought to further elucidate these relationships through in depth chart review of our single-center cohort. We demonstrate that in vaccine responders (defined as spike antibody titer >100 ug/dL), all patients except 1 had B-cell reconstitution >0.1%. Conversely, only 1 patient with a B-cell count of 0% had a positive spike antibody level, and at a low titer. However, we also show that not all patients with B-cell reconstitution mounted an antibody response, with almost 50 patients in our cohort demonstrating a spike antibody level below the threshold of detection despite a CD20 count of at least 0.1%. Interpretation of this data is limited given this is a retrospective cohort and CD20 counts were not drawn at the exact same time when vaccination occurred. However, our data indicate that B-cell reconstitution >0.1% is necessary but not sufficient to mount a humoral vaccine response. Recent data indicate that COVID-19 breakthrough infection strongly correlates with SARS-CoV-2 antibody level. (Sormani et al., 2022) Thus, we suggest that individualized dosing and consideration of extended interval dosing with monitoring of B-cell counts may be safe (Rolfes et al., 2021) and of benefit to some patients to allow for B-cell reconstitution and humoral response to vaccination in vaccine non-responders.
One patient on anti-CD20 therapy in our cohort had a minimal antibody response after two doses of mRNA vaccination but had an increase to spike antibody of >2500 ug/dL after third vaccination despite CD19/20 count of 0. While extrapolation from a single case is limited, it is possible that third vaccination may boost humoral immune response for patients on anti-CD20 therapy even if they had minimal response to initial vaccination. Additional data is needed to confirm this finding.
Finally, we report longitudinal clinical outcomes in our patients. We found a rate of COVID infection after vaccination of 1.9% (5/254). This occurred in patients only with antibody response <20 ug/dL, which could suggest that an antibody titer of >20 ug/dL is needed to prevent infection. However, further large-scale studies will be needed to determine if there is a necessary minimum antibody titer to prevent COVID-19 infection, which may vary from person to person based on individual history and comorbidities. The rate of post-vaccination COVID-19 infection in our cohort is higher than other reports in the general population, which have reported infection after vaccination (as measured by positive nasopharyngeal PCR) at .2%, (Levin et al., 2021) and is likely explained by lack of humoral response in a large number of patients in this cohort.
Strengths of this study include the in-depth analysis of a large cohort of multiple sclerosis patients and healthy controls from a single center. We report on humoral response after vaccination from a larger range of disease modifying therapies than most prior studies, and control for factors including age and time since vaccination. We also have an extended follow-up period with an average of 9 months after vaccination and are one of the first studies to report on outcomes of COVID-19 infection after vaccination in MS patients on DMTs.
Our study has several weaknesses. The COVID data collection in the MS patients is retrospective which limits the available data. Specifically, the dates of CD20 cell counts were not drawn on the same date as vaccination, so first available B-cell counts following vaccination dates were used as a proxy. While we do not know exact B-cell counts at time of vaccination, we can infer they were equal or less than those drawn after. While our sample size is large for a single-center study, our sample size is limited compared to larger population-based studies. We did not include T cell responses in this study, which may play an important role in COVID-19 infection severity and vaccine response (Apostolidis et al., 2021) and will be the subject of future reports.
5. Conclusions
In summary, we demonstrate reduced humoral immunity to SARS-CoV-2 after vaccination in patients on a variety of DMTs, especially S1P modulators and B-cell therapies, even when controlling for age. We also demonstrate a correlation of decreased humoral response to vaccination and low IgG, reduced B-cell count, and longer duration of B-cell and S1P therapy. We suggest that extended interval dosing of B-cell therapies to allow for B-cell reconstitution >0.1% helps to promote humoral response to vaccination and should be considered.
6. Disclosures
K.H reports no disclosures. B.H has received research support from Analysis Group, Celgene (Bristol-Myers Squibb), Verily Life Sciences, Merck-Serono, Novartis, and Genzyme. S.C reports no disclosures. M.H has served as a consultant for biogen, Roche Genentech, Novartis, Genzyme. She has received research support from Biogen, Genentech, and Genzyme. R.B reports no disclosures. S.B. has received consulting fees from Teladoc Health and Alexion Pharmaceuticals; publishing honorarium from UpToDate and American Academy of Neurology; research support from Alexion Pharmaceuticals. G.B. has received an MS PostDoctoral Fellowship award from the Multiple Sclerosis Society of Canada. K.G has received consulting compensation from Glaxo Smith Kline that is not relevant to this project. T.K has received consulting and advisory board fees from Biogen, Genzyme-Sanofi, Roche-Genentech/Roche, Novartis, and Bristol Myers Squibb. C. S. has received consulting fees from Genzyme, Novartis, and Biogen.T.S has received consulting compensation from Novartis Pharmaceuticals and research support from Novartis Pharmaceuticals and Genzyme-Sanofi. L.S reports no disclosures. J.Z reports no disclosures. M.P.T reports no disclosures. S.S reports no disclosures. A.P reports no disclosures. B.G reports no disclosures. H.L.W reports no disclosures. T.C. has received compensation for consulting from Banner Life Sciences, Biogen, Bristol Myers Squibb, Novartis Pharmaceuticals, Roche Genentech, and Sanofi Genzyme. She has received research support from the National Institutes of Health, National MS Society, US Department of Defense, Sumaira Foundation, Brainstorm Cell Therapeutics, Bristol Myers Squibb, EMD Serono, I-Mab Biopharma, Mallinckrodt ARD, Novartis Pharmaceuticals, Octave Bioscience, Roche Genentech, Sanofi Genzyme, and Tiziana Life Sciences.
Funding
The authors report no funding supporting this work.
Author contributions
K.H contributed to data collection, data analysis, drafting and revising the manuscript. B.H contributed to data analysis, drafting, and revising the manuscript. S.C., M.H., R.B., S.B., G.B., K.G., T.K, C.S., T.S., L.S., J.Z., contributed to data collection and manuscript revision. M.P.T, S.S., A.P., B.G. contributed to data collection and study design. H.L.W and T.C. contributed to data collection, data analysis, and manuscript revision.
References
- Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: up to 6 months cross-sectional study. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577746. 12 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. 02 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill L, Rechtman A, Zveik O, et al. Humoral and T-Cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.3599. Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Bermel RA, Grossman CI, et al. Immunoglobulin G immune response to SARS-CoV-2 vaccination in people living with multiple sclerosis within multiple sclerosis partners advancing technology and health solutions. Mult. Scler. 2022 doi: 10.1177/13524585211061343. Jan 0713524585211061343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. doi: 10.1038/s41586-021-03739-1. 08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanto G, Sacco R, Bernasconi E, et al. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.3609. Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JS, Killestein J. Reading the "T" leaves of COVID-19 vaccine responses in multiple sclerosis. Neurology. 2021 doi: 10.1212/WNL.0000000000013166. Dec 08. [DOI] [PubMed] [Google Scholar]
- Januel E, De Seze J, Vermersch P, et al. Post-vaccine COVID-19 in patients with multiple sclerosis or neuromyelitis optica. Mult. Scler. 2021 doi: 10.1177/13524585211049737. Dec 2113524585211049737. [DOI] [PubMed] [Google Scholar]
- Jones JM, Faruqi AJ, Sullivan JK, Calabrese C, Calabrese LH. COVID-19 outcomes in patients undergoing b cell depletion therapy and those with humoral immunodeficiency states: a scoping review. Pathog. Immun. 2021;6(1):76–103. doi: 10.20411/pai.v6i1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B, Bonagura VR. Secondary hypogammaglobulinemia: an increasingly recognized complication of treatment with immunomodulators and after solid organ transplantation. Immunol. Allergy Clin. North Am. 2019;39(1):31–47. doi: 10.1016/j.iac.2018.08.005. 02. [DOI] [PubMed] [Google Scholar]
- Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2114583. Oct 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. 12 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I, Wilf-Yarkoni A, Friedman Y, Stiebel-Kalish H, Steiner I, Hellmann MA. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): early experience from a tertiary MS center in Israel. Eur. J. Neurol. 2021 doi: 10.1111/ene.15028. Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann. Rheum. Dis. 2021;80(10):1345–1350. doi: 10.1136/annrheumdis-2021-220781. 10. [DOI] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. 12 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico A, Ninove L, Maarouf A, et al. Determining the best window for BNT162b2 mRNA vaccination for SARS-CoV-2 in patients with multiple sclerosis receiving anti-CD20 therapy. Mult. Scler. J. Exp. Transl. Clin. 2021;7(4) doi: 10.1177/20552173211062142. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes L, Pawlitzki M, Pfeuffer S, et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID-19. Neurol. Neuroimmunol. Neuroinflamm. 2021;8(5) doi: 10.1212/NXI.0000000000001035. 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino JJ, Mittl K, Rowles W, et al. Impact of multiple sclerosis disease-modifying therapies on SARS-CoV-2 vaccine-induced antibody and T cell immunity. medRxiv. 2021 doi: 10.1101/2021.09.10.21262933. Sep 20. [DOI] [Google Scholar]
- Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMed. 2021;72 doi: 10.1016/j.ebiom.2021.103581. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani MP, Schiavetti I, Inglese M, et al. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMed. 2022;80 doi: 10.1016/j.ebiom.2022.104042. May 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczawinska-Poplonyk A, Breborowicz A, Samara H, Ossowska L, Dworacki G. Impaired antigen-specific immune response to vaccines in children with antibody production defects. Clin. Vaccine Immunol. 2015;22(8):875–882. doi: 10.1128/CVI.00148-15. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-Cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with ms using different disease-modifying therapies. Neurology. 2021 doi: 10.1212/WNL.0000000000013108. Nov 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kempen ZLE, Wieske L, Stalman EW, et al. Longitudinal humoral response after SARS-CoV-2 vaccination in ocrelizumab treated MS patients: to wait and repopulate? Mult. Scler. Relat. Disord. 2021;57 doi: 10.1016/j.msard.2021.103416. Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult. Scler. 2020;26(14):1816–1821. doi: 10.1177/1352458520970841. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Salter A, Jin S, et al. Disease-modifying therapy prescription patterns in people with multiple sclerosis by age. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoehner G, Miclea A, Salmen A, et al. Reduced serum immunoglobulin G concentrations in multiple sclerosis: prevalence and association with disease-modifying therapy and disease course. Ther. Adv. Neurol. Disord. 2019;12 doi: 10.1177/1756286419878340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.