Abstract
Retrospective studies showed a relationship between vitamin D status and COVID-19 severity and mortality, with an inverse relation between SARS-CoV-2 positivity and circulating calcifediol levels. The objective of this pilot study was to investigate the effect of vitamin D supplementation on the length of hospital stay and clinical improvement in patients with vitamin D deficiency hospitalized with COVID-19. The study was randomized, double blind and placebo controlled. A total of 50 subjects were enrolled and received, in addition to the best available COVID therapy, either vitamin D (25,000 IU per day over 4 consecutive days, followed by 25,000 IU per week up to 6 weeks) or placebo. The length of hospital stay decreased significantly in the vitamin D group compared to the placebo group (4 days vs. 8 days; p = 0.003). At Day 7, a significantly lower percentage of patients were still hospitalized in the vitamin D group compared to the placebo group (19% vs. 54%; p = 0.0161), and none of the patients treated with vitamin D were hospitalized after 21 days compared to 14% of the patients treated with placebo. Vitamin D significantly reduced the duration of supplemental oxygen among the patients who needed it (4 days vs. 7 days in the placebo group; p = 0.012) and significantly improved the clinical recovery of the patients, as assessed by the WHO scale (p = 0.0048). In conclusion, this study demonstrated that the clinical outcome of COVID-19 patients requiring hospitalization was improved by administration of vitamin D.
Keywords: vitamin D, cholecalciferol, calcifediol, COVID-19, SARS-CoV-2, hospitalization
1. Introduction
The general metabolism and actions of vitamin D in regulating serum calcium concentrations and, in a feedback loop, parathyroid hormone, are well known [1]. There is ample evidence that having enough vitamin D can help prevent many diseases, such as heart disease, bone disease and cancer. Recent data also showed that vitamin D can reduce the risk of respiratory tract infections, and particularly, the risk of viral infections [2,3,4,5,6,7,8,9]. Vitamin D can interfere with viral replication but also has immunomodulatory and anti-inflammatory effects [10,11,12]. These effects can be of importance during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Indeed, SARS-CoV-2 uses immune evasion mechanisms as a common pathogenic mechanism of acute respiratory disease syndrome and systemic inflammatory response syndrome development [13]. Importantly, vitamin D is also involved in renin–angiotensin system regulation. The SARS-Cov-2 virus enters into cells via the angiotensin converting enzyme 2 (ACE 2) receptor, leading to cytokine storms and fatal respiratory distress syndrome [14]. An independent correlation between low serum concentrations of calcifediol (the main vitamin D metabolite) and susceptibility to acute respiratory infection was shown in observational trials [15]. Other studies suggested a protective effect of vitamin D against viral and bacterial respiratory pathogens [3,15,16,17,18]. This positive input of vitamin D is also observed in hospitalized and critically ill patients [19,20]. Indeed, several studies have established a link between a lack of vitamin D and clinical outcomes, such as increased hospital length of stay, readmission rates, sepsis and mortality [19,20,21,22,23,24,25,26,27,28].
Recently, retrospective studies showed a relationship between vitamin D status and coronavirus disease (COVID-19) severity and mortality. The studies observed an inverse relationship between SARS-CoV-2 positivity and calcifediol levels. This correlation persists across latitudes, races, ethnicities, sexes and age ranges [29]. Studies have shown that deficiency in vitamin D was linked to an increased COVID-19 risk and inversely associated with mortality and the need for invasive mechanical ventilation [30,31]. This is supported by the positive results of a double-blind, randomized control trial performed on mechanically ventilated adult intensive care unit (ICU) patients and vitamin D supplementation [32]. Another study recently performed in Spain with patients hospitalized with COVID-19 infection demonstrated that a high dose of calcifediol significantly decreased the need for ICU treatment [33].
Currently available data regarding the beneficial impact of vitamin D in COVID-19 in prospective, interventional and well-designed clinical trials are lacking. There is therefore no consensus regarding vitamin D supplementation for prevention or treatment of COVID-19. Grant et al. suggested that raising serum calcifediol concentrations to 40–60 ng/mL could decrease the risk of COVID-19 infection and death [34]. High prevalence of vitamin D deficiency in patients with comorbidities makes investigations of its role as a secondary therapeutic agent in COVID-19 conceivable. It is therefore necessary to assess serum calcifediol levels in patients with COVID-19 to identify the need for promptly increasing and maintaining the levels of calcifediol in the optimal range. In order to fill this gap, it was of utmost interest to conduct a prospective, interventional study to determine the beneficial effects of vitamin D supplementation as an adjuvant therapy for patients with suboptimal vitamin D status and hospitalized for COVID-19.
The study objective was to assess whether the proposed dosing regimen of a daily dose of 25,000 international units (IU) vitamin D administered over 4 consecutive days, followed by a weekly dose of 25,000 IU, was adequate to rapidly increase the concentrations of calcifediol in patients hospitalized with COVID-19 and to explore its impact on hospital length and other clinical outcomes of the disease.
2. Materials and Methods
2.1. Methodology
This was an interventional, randomized, parallel, two-treatment, two-arm, double-blind and placebo-controlled pilot study, carried out in one clinical site in Belgium. The study was performed according to the ethical principles of the Declaration of Helsinki, the Good Clinical Practice and the National Drug Law. All patients provided written informed consent, and the study was approved by the Ethics Committee of the University Hospital of Liège, Belgium (local reference: 2020/177, approved on 26 May 2020 and amended on 20 November 2020). The study protocol was registered on ClinicalTrials.gov (Identifier: NCT04636086).
2.2. Study Population
All the patients were recruited from the University Hospital of Liège (Liège, Belgium) from August 2020 to August 2021. Caucasian subjects, male and female, aged 18 years or older, with vitamin D deficiency (defined as serum calcifediol concentration ≤ 20 ng/mL) and hospitalized for confirmed SARS-CoV-2 infection at screening were recruited. To be included in the study, the patients were expected to survive for at least 96 h after study entry. The main exclusion criteria were patients presenting acute impairment of renal function or nephrolithiasis. Patients with hypercalcemia and/or hypercalciuria and/or pseudohypoparathyroidism were also excluded at screening. Concomitant medications susceptible to interfere with the study results were not allowed, and subjects who had used any type of vitamin D supplement at screening visit were excluded.
2.3. Study Intervention
The patients were randomized in the two different treatment groups (vitamin D or placebo) in a 1:1 ratio. Patients participated in the study for a maximum of 9 weeks, including an up to 6-week treatment period and a maximum of 3-week follow-up period. The study duration was defined by the length of patient’s hospitalization. The patients stayed at the hospital during the overall treatment period. The last day of treatment period was the last day of hospitalization or day 36, whichever came first. The intervention group received the best available treatment plus oral vitamin D (ampoule of 1 mL containing 25,000 IU of cholecalciferol (vitamin D3), D-CURE®, Laboratoires SMB SA). A daily dose of 25,000 IU of vitamin D over 4 consecutive days was given to rapidly restore the calcifediol levels. Then, 25,000 IU per week up to six weeks was given to maintain this level. This dose was proposed on the basis of previous studies performed with oral vitamin D [35,36,37]. The control group received the best available treatment for COVID plus placebo (ampoule of 1 mL of excipient). The study medications were identical in consistency, smell and taste. The placebo and active study treatments were given either orally or via enteral feeding tube. The study was double blinded. The patients, investigators and any other persons involved in the data handling were blinded to the trial medications. To maintain the blind, the calcifediol levels were not provided to the clinical staff after randomization until database lock. The patients in ICU with enteral nutrition received 600 IU vitamin D per day in addition to the study treatments, assuring a standard supplementation of vitamin D to patients with possibly more severe deficiency in vitamin D. The study design is summarized in Table 1.
Table 1.
Study design.
| Day 1, 2, 3, 4, 8, 15, 22, 29 and 36 | |
| Group 1: Placebo group | 1 ampoule of placebo + standard of care treatment |
| Group 2: Vitamin D group | 1 ampoule of vitamin D 25,000 IU + standard of care treatment |
2.4. Study Endpoints
Outcomes of effectiveness included calcifediol serum level, ordinal scale for clinical improvement as recommended by the World Health Organization (WHO), hospitalization length, intensive care unit admission, time until absence of fever, need for supplemental oxygen, non-invasive ventilation, high-flow oxygen devices, invasive mechanical ventilation or additional organ support and death [38].
2.5. Laboratory Tests
Clinical specimens required for SARS-CoV-2 diagnostic procedures were collected on admission by nasopharyngeal exudate sampling following WHO guidelines and recommendations [39]. Blood samples were collected to determine the serum concentration of calcifediol. The Fujirebio 25-OH Vitamin D assay on Lumipulse G1200 analyzer (Fujirebio, Tokyo, Japan) was used to screen the calcifediol concentrations of the patients at the inclusion of the study. This assay indeed showed excellent concordance with the LC–MS/MS method used in our laboratory and has the advantage of providing results in a fast turnaround time, compatible with the needs of a screening [40,41,42]. All the other samples were measured in a single batch with our LC–MS/MS method, which is certified by the Vitamin D Standardization and Certification Program (VDSCP) to be traceable to the Centers for Disease Control reference method. The details of our methods have been published previously [43]. All analyses were performed at the ISO 15,198 clinical chemistry laboratory of the University of Liège (Liège, Belgium).
2.6. Statistical Analyses
The number of participants was determined on the basis of feasibility, based on resources, capacity of clinical staff and available patients. Given that the minimal clinically important difference between the groups for length of stay among patients with COVID-19 is unknown, no formal calculation of sample size was performed, and it was decided that 25 subjects per treatment group would be included. All statistical computations were performed using the SAS/STAT software version 9.4. (SAS Institute Inc., Cary, NC, USA). Continuous variables were presented as mean ± standard deviation (median and interquartile range for non-normalized data), and categorical variables were presented as frequencies and percentages. The endpoints were compared between treatment groups and were analyzed as follows: categorical variables were analyzed using the chi-square test or Fisher’s exact test for categorical variables and independent-sample t-test for continuous variables. Furthermore, in case the Student’s t-test was not applicable for parameters not normally distributed, a non-parametric Wilcoxon (Mann–Whitney U-test) was applied to compare the two independent groups (placebo vs. Vitamin D) for these parameters. A 2-sided p value of less than 0.05 was considered significant. A post hoc adjusted analysis for the outcome length of hospital stay was performed by estimating a multiple regression equation relating the outcome of interest to independent variables representing the treatment assignment—the co-founding variables. These cofounders were age, gender, height, weight, BMI, arterial hypertension, diabetes, hepatic failure, renal failure, cardiac pathology, chronic lung disease and vaccinal status. The number of cofounders being high with regard to the sample size, two different models were established.
3. Results
3.1. Baseline Characteristics
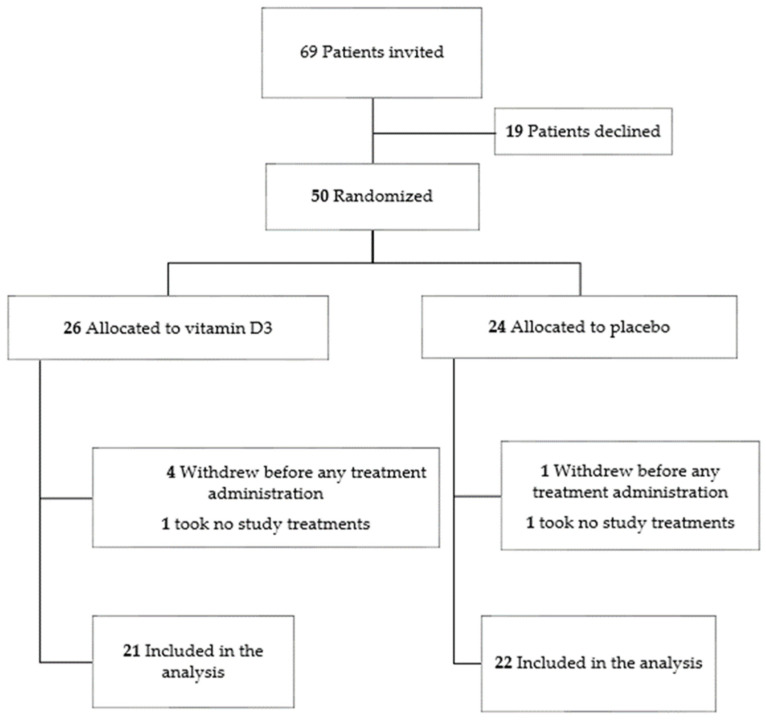
A total of 50 subjects signed their informed consent and were randomized to either the vitamin D group (n = 26) or the placebo group (n = 24). A total of 43 of them completed the study: 21 in the vitamin D group and 22 in the placebo group (Figure 1). Out of the 26 patients randomized in the vitamin D group, 4 of them withdrew from the study or were discharged from hospital before the planned treatment administration, and 1 of them refused to take any study treatments. Out of the 24 patients randomized in the placebo group, 1 of them was discharged from the hospital before the planned study treatment administration, and 1 refused to take any study treatments. Baseline demographic and clinical characteristics were similar in both groups (p > 0.05) (Table 2). At baseline, comorbidities, including cardiac and lung diseases, renal and hepatic failure, diabetes, arterial hypertension and body mass index (BMI), did not differ between groups. The vaccinal status was comparable across both groups, the majority of the subjects not being vaccinated.
Figure 1.
Flow chart of patients included in the study.
Table 2.
Baseline demographic characteristics.
| Placebo Group n = 22 |
Vitamin D Group n = 21 |
p Value | ||
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 68.73 ± 10.97 | 63.24 ± 14.46 | 0.167 | |
| min–max | 41.00–88.00 | 36.00–98.00 | ||
| Gender | ||||
| Male | n (%) | 10 (45%) | 13 (62%) | 0.364 |
| Female | n (%) | 12 (54%) | 8 (38%) | |
| Weight (kg) | ||||
| Mean ± SD | 79.46 ± 17.54 | 75.85 ± 12.03 | 0.728 | |
| min–max | 47.70–106.00 | 63.00–108.00 | ||
| BMI (kg/m2) | ||||
| Mean ± SD | 28.92 ± 6.92 | 26.52 ± 3.24 | 0.133 | |
| min–max | 19.11–44.89 | 21.30–32.97 | ||
| Calcifediol concentration at screening (ng/mL) | n | 22 | 21 | |
| Mean ± SD | 16.87 ± 9.48 | 17.87 ± 10.15 | 0.741 | |
| min–max | 4.80–43.60 | 5.00–44.60 | ||
| Vaccinal Status | ||||
| Not vaccinated | n (%) | 20 (91%) | 17 (81%) | 0.169 |
| 1 Dose | n (%) | 2 (9.1%) | 1 (5%) | |
| 2 Doses | n (%) | 0 (0.0%) | 3 (14%) | |
| Cardiac Pathology | ||||
| Yes | n (%) | 9 (42%) | 7 (33%) | |
| Hepatic Failure | ||||
| Yes | n (%) | 0 (0.0%) | 2 (9.5%) | |
| Renal Failure | ||||
| Yes | n (%) | 4 (18%) | 4 (19%) | |
| Diabetes | ||||
| Yes | n (%) | 8 (36%) | 8 (38%) | |
| Arterial Hypertension | ||||
| Yes | n (%) | 13 (59%) | 11 (52%) | |
| Chronic lung disease | ||||
| Yes | n (%) | 9 (42%) | 5 (24%) |
BMI: body mass index; SD: standard deviation.
3.2. Safety Assessment
The proposed dosing regimen was well tolerated, and no specific adverse events in relation to vitamin D supplementation were identified during the study.
3.3. Measurements of Calcifediol
Baseline mean serum calcifediol concentrations were comparable across the two groups (p = 0.7415) and below 20 ng/mL (Table 2). After supplementation, in the vitamin D group, the calcifediol concentrations rapidly increased, reaching mean value of 29.9 ± 14.81 ng/mL, whereas no changes vs. baseline were observed in the placebo group.
3.4. Clinical Outcomes
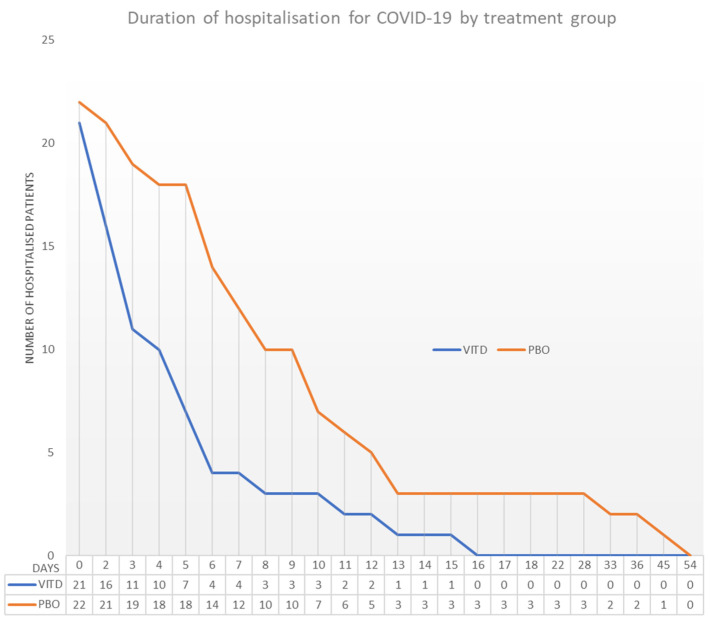
The median length of hospital stay significantly decreased in the vitamin D group compared to the placebo group (4 days for the vitamin D group vs. 8 days for the placebo group; p = 0.003) (Table 3). These results were confirmed by the adjusted post hoc analysis (Unadjusted treatment effect size for hospital length of stay −7.22 [−13.16; −1.29], p = 0.0183; Adjusted treatment effect size −7.35 [−14.10; −0.59], p = 0.034 and −8.76 [−15.88; −1.64], p = 0.018). At Day 7, a significantly lower proportion of patients were still hospitalized in the vitamin D group than in the placebo group (19% vs. 54%; p = 0.0161), and none of the patients treated with vitamin D were hospitalized after 21 days compared to 14% of the patients treated with placebo (Figure 2). Multiple regression models were performed to analyze the relationship between the primary endpoint “hospital length of stay” and the identified cofounders. The analysis confirmed there was no effect of age, BMI, height, weight, gender, cardiac pathology, arterial hypertension, diabetes, hepatic failure and vaccinal status on the primary endpoint (p > 0.05). The relationship was independent, regardless of the treatment administered (Interaction treatment*cofounders p > 0.05). Five patients were admitted to the intensive care unit in the placebo group vs. two in the vitamin D group (23% vs. 9.5%; p = 0.4121). The average intensive care unit length of stay was shorter in the vitamin D group compared to the placebo group (4.0 days ± 4.2 vs. 12.4 days ± 14.3; p = 0.4724). A positive trend of supplementation with vitamin D was also noticed in a smaller proportion of patients who needed supplemental conventional oxygen, non-invasive ventilation, high-flow nasal oxygen and invasive mechanical ventilation (86.4% in the placebo group vs. 62% in the vitamin D group; p = 0.0545). Among all the patients who needed supplemental conventional oxygen, the administration of vitamin D significantly decreased the duration of treatment (4 days vs. 7 days; p = 0.012). The duration of recovery from fever was also shorter in the vitamin D group but without statistical significance (7.7 days ± 4.7 vs. 14.1 days ± 13.1; p = 0.0593). No significant differences were observed regarding mortality. Three patients died due to COVID-19 in the placebo group and one in the vitamin D group (Table 3).
Table 3.
Clinical outcomes.
| Placebo Group n = 22 |
Vitamin D Group n = 21 |
p Value | ||
|---|---|---|---|---|
| Hospital length of stay (Days) | ||||
| Median | 8.0 | 4.0 | 0.003 | |
| Q1–Q3 | 6.0–12.0 | 3.0–6.0 | ||
| Proportion of patients hospitalized | ||||
| At Day 7 | n (%) | 12 (54) | 4 (19.) | 0.016 |
| At Day 14 | n (%) | 3 (14) | 1 (4.8)) | 0.262 |
| At Day 21 | n (%) | 3 (14) | 0 (0.0) | 0.125 |
| At Day 28 | n (%) | 3 (14) | 0 (0.0) | 0.125 |
| At Day 36 | n (%) | 2 (9.1%) | 0 (0.0) | 0.256 |
| Admission in intensive care unit | n (%) | 5 (23) | 2 (9.5) | 0.412 |
| Intensive Care Unit length of stay (Days) | Mean ± SD | 12.4 ± 14.3 | 4.0 ± 4.2 | 0.472 |
| min–max | 3.0–36.0 | 1.0–7.0 | ||
| Proportion of patients requiring supplemental oxygen, non-invasive ventilation or high-flow oxygen devices, invasive mechanical ventilation | n (%) | 19 (86) | 13 (62) | 0.054 |
| Duration of supplemental conventional oxygen (Days) | ||||
| Median | 7.0 | 4.0 | 0.012 | |
| Q1–Q3 | 5.0–11.0 | 0.0–6.0 | ||
| Duration of non-invasive ventilation or high-flow nasal oxygen, invasive mechanical ventilation or additional organ support (Days) | Mean ± SD | 1.3 ± 4.2 | 0.3 ± 1.3 | 0.306 |
| min–max | 0.0–16.0 | 0.0–16.0 | ||
| Time until absence of fever for more than 48 h without antipyretics (Days) | Mean ± SD | 14.1 ± 13.1 | 7.7 ± 4.7 | 0.059 |
| min–max | 0.0–52.0 | 2.0–18.0 | ||
| Mortality All causes | n (%) | 3 (14) | 4 (19) | 0.286 |
| Mortality related to COVID-19 | n (%) | 3 (12) | 1 (4.8) | 0.129 |
Figure 2.
Number of hospitalized patients by treatment group.
3.5. WHO Scale
At randomization, the severity of the disease assessed by the ordinal WHO scale for clinical improvement was comparable across both groups, the majority of the patients (95%) being categorized in the moderate clinical assessment. After vitamin D supplementation, a more rapid and greater improvement was observed compared to the placebo group (Table 4). At Day 7, 71% of the patients supplemented with vitamin D switched from the moderate to the mild category of the scale compared to 18% in the placebo group (p = 0.0048). At day 36, 90% of the patients from the vitamin D group were assessed as “no more infected” compared to 77% in the placebo group.
Table 4.
WHO ordinal scale.
| Placebo Group n = 22 |
Vitamin D Group n = 21 |
p Value | ||
|---|---|---|---|---|
| Ordinal Scale for clinical improvement by severity * at baseline (Day 1) | 0.512 | |||
| No infection | n (%) | 0 (0.0) | 0 (0.0) | |
| Mild | n (%) | 0 (0.0) | 0 (0.0) | |
| Moderate | n (%) | 21 (95) | 20 (95) | |
| Severe | n (%) | 1 (4.5) | 1 (4.8) | |
| Death | n (%) | 0 (0.0) | 0 (0.0) | |
| Ordinal Scale for clinical improvement by severity * at Day 7 | 0.005 | |||
| No infection | n (%) | 0 (0.0) | 0 (0.0) | |
| Mild | n (%) | 4 (18) | 15 (71) | |
| Moderate | n (%) | 14 (63) | 4 (19) | |
| Severe | n (%) | 3 (13) | 1 (4.8) | |
| Death | n (%) | 1 (4.5) | 1 (4.8) | |
| Ordinal Scale for clinical improvement by severity * at Day 15 | 0.549 | |||
| No infection | n (%) | 0 (0.0) | 0 (0.0) | |
| Mild | n (%) | 15 (68) | 18 (85) | |
| Moderate | n (%) | 4 (18) | 1 (4.8) | |
| Severe | n (%) | 1 (4.5) | 1 (4.8) | |
| Death | n (%) | 1 (4.5) | 1 (4.8) | |
| Ordinal Scale for clinical improvement by severity * at Day 22 | 0.543 | |||
| No infection | n (%) | 0 (0.0) | 0 (0.0) | |
| Mild | n (%) | 17 (77) | 19 (90) | |
| Moderate | n (%) | 1 (4.5) | 0 (0.0) | |
| Severe | n (%) | 2 (9.1) | 0 (0.0) | |
| Death | n (%) | 2 (9.1) | 2 (9.5) | |
| Ordinal Scale for clinical improvement by severity * at Day 29 | 0.543 | |||
| No infection | n (%) | 0 (0.0) | 0 (0.0) | |
| Mild | n (%) | 17 (77) | 19 (90) | |
| Moderate | n (%) | 1 (4.5) | 0 (0.0) | |
| Severe | n (%) | 2 (9.1) | 0 (0.0) | |
| Death | n (%) | 2 (9.1) | 2 (9.5) | |
| Ordinal Scale for clinical improvement by severity * at Day 36 | 0.318 | |||
| No infection | n (%) | 0 (0.0) | 0 (0.0) | |
| Mild | n (%) | 17 (77.) | 19 (90) | |
| Moderate | n (%) | 2 (9.1) | 0 (0.0) | |
| Severe | n (%) | 0 (0.0) | 0 (0.0) | |
| Death | n (%) | 3 (14) | 2 (9.5) |
* No infection = 0; Mild = Score 1, 2; Moderate = Score 3, 4, Severe = Score 5, 6, 7 and Death = Score 8.
4. Discussion
In this randomized, double-blind, placebo-controlled clinical trial, a proposed dosing regimen of 25,000 IU vitamin D per day over four consecutive days followed by 25,000 IU per week did significantly reduce the hospitalization length, the need for supplemental oxygen, and it improved the WHO score among patients with COVID-19. In our trial, all efforts were made to adequately control the possible cofounding factors. All patients should have a calcifediol level ≤ 20 ng/mL at study entry to have comparable baseline mean values. The study treatment was taken under the supervision of the clinical staff, which led to 100% compliance in both treatment groups. All patients were recruited from one site. Therefore, the standard of care treatment and the management of patients were the same for all patients. It is interesting to highlight that both groups of patients had comparable percentage of unfavorable risk factors at baseline. Indeed, there was no significant difference in subjects regarding age, sex, BMI, diabetes, arterial hypertension and cardiac, hepatic, renal or lung disorders. Moreover, the multiple regression analysis confirmed these cofounding factors could be ruled out as possible bias. It should also be noted that the vaccinal status was comparable for both groups, the majority of the patients being not yet vaccinated. Importantly, all the patients received the same best available treatment at the hospital, which included the use of corticosteroids, paracetamol, heparin and remdesevir.
It was observed that higher doses of vitamin D than usual were given in case of deficiency in patients at risk of developing respiratory infections [34]. Our study assessed a dosing regimen of 25,000 IU vitamin D per day for four consecutive days, followed by 25,000 IU per week until discharge. The study results confirmed that this regimen was adequate to rapidly raise the calcifediol level above 20 ng/mL and improve the clinical outcome of patients requiring hospitalization for COVID-19. Whether that would also apply to patients with an earlier stage of the disease is unknown.
Our study complements the recent findings of Castillo et al., 2020 study performed in Spain with 76 patients hospitalized with COVID-19 infection, which showed that the administration of high dose of calcifediol (21,280 IU on the day of admission, 10,640 IU on day 3 and 7, and then weekly until discharge) significantly reduced the need for ICU treatment of patients requiring hospitalization due to proven COVID-19 (p < 0.001) [33].
This is also supported by the positive results of a double-blind, randomized control trial performed on mechanically ventilated adult ICU patients and vitamin D supplementation in two hospitals in Atlanta [32]. Subjects were administered either placebo, 50,000 IU vitamin D or 100,000 IU vitamin D daily for 5 consecutive days enterally. The high-dose vitamin D safely increased plasma calcifediol concentrations to the sufficient range and was associated with decreased hospital length of stay (25 ± 14 and 18 ± 11 days compared to 36 ± 19 days in placebo group; p = 0.03).
The lack of benefit observed in the Brazilian study of Murai et al., 2021 could have been affected by the single higher dose of vitamin D administered once as a bolus dose (200,000 IU) and the heterogeneity of the sample underlined by the authors due to several coexisting diseases and diverse medication regimens [44]. The same conclusions were drawn in the recent COVID-VIT-D trial where a single oral bolus of 100,000 IU vitamin D did not improve the outcomes of the disease compared with patients who did not receive it, even if the cohort analysis showed that high serum calcifediol level at hospital admission was associated with better outcomes [45]. As observed by the authors, the different sun exposure of the patients being recruited from different countries with different latitudes could have influenced the calcifediol levels and the study results. In our study, all patients were recruited over one year and could have been potentially impacted by the seasonal changes [46,47]. However, they were recruited from the same hospital and consecutively assigned to the active or the placebo group considering a 1:1 ratio, decreasing the risk of having unbalanced randomization in terms of any potential seasonal changes. Moreover, all patients stayed at the hospital during the whole study period, which avoided any bias related to sun exposure. Therefore, the potential impact of the seasonal changes on the human immune response can be ruled out.
Our study adds to the current literature of vitamin D supplementation as an adjuvant therapy for patients with COVID-19 requiring hospitalization, particularly by focusing on patients with vitamin D deficiency (≤20 ng/mL).
The strengths of the study include the randomized, placebo-controlled and double-blind experimental design, the comparable groups at baseline and the assessment of calcifediol serum level with clinical outcomes at baseline and during the study. The study shows some limitations, which are the low number of subjects and the minimal clinically important difference in hospital length among patients with COVID-19, which remains to be determined. Our study showed that vitamin D deficiency is an easily modifiable risk factor for COVID-19 that should be actively corrected. Vitamin D supplementation is a simple, safe and inexpensive measure, which is effective in correcting hypovitaminosis D. Even a small improvement in COVID-19 clinical outcome would easily justify this intervention.
5. Conclusions
In this study, including COVID-19 patients requiring hospitalizations, administration of cholecalciferol significantly reduced the hospital length of stay, reduced the duration of supplemental oxygen and improved the clinical status assessed by the WHO scale. Further studies with a larger number of patients would be needed to confirm our observations.
Acknowledgments
The vitamin D (D Cure® 25,000 ampoules) and placebo were kindly provided by Laboratoires SMB SA who supported services related to study management and statistical analyses.
Author Contributions
E.C., J.G., A.-F.R., S.D.N. and M.T. contributed to the study conception and methodology. A.-F.R., J.G., D.C., A.-N.F. and F.G. supervised recruitment. A.-F.D., E.G., M.D., M.H. and A.S. supervised patient screening, assessment, data management and procurement of samples. E.C. analyzed the samples. D.M. supervised randomization and treatment preparation. S.D.N. and M.T. performed statistical analysis and wrote the manuscript with M.C., R.L., E.C., J.G. and A.-F.R. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University Hospital of Liège, Belgium (local reference: 2020/177, approved on 26 May 2020 and amended on 20 November 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
Sophie De Niet, Mickaël Trémège and Monte Coffiner are employees of Laboratoires SMB SA.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011;50:194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coussens A.K. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem. Photobiol. Sci. 2017;16:314–338. doi: 10.1039/c6pp00355a. [DOI] [PubMed] [Google Scholar]
- 5.Lang P.O., Aspinall R. Vitamin D Status and the Host Resistance to Infections: What It Is Currently (Not) Understood. Clin. Ther. 2017;39:930–945. doi: 10.1016/j.clinthera.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Gruber-Bzura B.M. Vitamin D and Influenza-Prevention or Therapy? Int. J. Mol. Sci. 2018;19:2419. doi: 10.3390/ijms19082419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rondanelli M., Miccono A., Lamburghini S., Avanzato I., Riva A., Allegrini P., Faliva M.A., Peroni G., Nichetti M., Perna S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds-Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid.-Based Complement. Altern. Med. ECAM. 2018;2018:5813095. doi: 10.1155/2018/5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gombart A.F., Pierre A., Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jolliffe D.A., Camargo Jr C.A., Sluyter J.D., Aglipay M., Aloia J.F., Ganmaa D., Bergman P., Bischoff-Ferrari H.A., Borzutzky A., Damsgaard C.T., et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9:276–292. doi: 10.1016/S2213-8587(21)00051-6. [DOI] [PubMed] [Google Scholar]
- 10.Hewison M. Vitamin D and immune function: An overview. Proc. Nutr. Soc. 2012;71:50–61. doi: 10.1017/S0029665111001650. [DOI] [PubMed] [Google Scholar]
- 11.Wei R., Christakos S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients. 2015;7:8251–8260. doi: 10.3390/nu7105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabbri A., Infante M., Ricordi C. Editorial—Vitamin D status: A key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4048–4052. doi: 10.26355/eurrev_202004_20876. [DOI] [PubMed] [Google Scholar]
- 13.Teymoori-Rad M., Marashi S.M. Vitamin D and COVID-19: From potential therapeutic effects to unanswered questions. Rev. Med. Virol. 2021;31:e2159. doi: 10.1002/rmv.2159. [DOI] [PubMed] [Google Scholar]
- 14.Dijkman R., Jebbink M.F., Deijs M., Milewska A., Pyrc K., Buelow E., van der Bijl A., van der Hoek L. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J. Gen. Virol. 2012;93:1924–1929. doi: 10.1099/vir.0.043919-0. [DOI] [PubMed] [Google Scholar]
- 15.Jolliffe D.A., Griffiths C.J., Martineau A.R. Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies. J. Steroid Biochem. Mol. Biol. 2013;136:321–329. doi: 10.1016/j.jsbmb.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Hansdottir S., Monick M.M., Hinde S.L., Lovan N., Look D.C., Hunninghake G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J. Immunol. Baltim. Md. 1950. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olliver M., Spelmink L., Hiew J., Meyer-Hoffert U., Henriques-Normark B., Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J. Infect Dis. 2013;208:1474–1481. doi: 10.1093/infdis/jit355. [DOI] [PubMed] [Google Scholar]
- 18.Golpour A., Bereswill S., Heimesaat M.M. Antimicrobial and Immune-Modulatory Effects of Vitamin D Provide Promising Antibiotics-Independent Approaches to Tackle Bacterial Infections—Lessons Learnt from a Literature Survey. Eur. J. Microbiol. Immunol. 2019;9:80–87. doi: 10.1556/1886.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moromizato T., Litonjua A.A., Braun A.B., Gibbons F.K., Giovannucci E., Christopher K.B. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit. Care Med. 2014;42:97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- 20.Thickett D.R., Moromizato T., Litonjua A.A., Amrein K., Quraishi S.A., Lee-Sarwar K.A., Mogensen K.M., Purtle S.W., Gibbons F.K., Camargo C.A., et al. Association between prehospital vitamin D status and incident acute respiratory failure in critically ill patients: A retrospective cohort study. BMJ Open Respir. Res. 2015;2:e000074. doi: 10.1136/bmjresp-2014-000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins D.M., Wischmeyer P.E., Queensland K.M., Sillau S.H., Sufit A.J., Heyland D.K. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. JPEN J. Parenter. Enter. Nutr. 2012;36:713–720. doi: 10.1177/0148607112444449. [DOI] [PubMed] [Google Scholar]
- 22.Nair P., Lee P., Reynolds C., Nguyen N.D., Myburgh J., Eisman J.A., Center J.R. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013;39:267–274. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 23.Braun A.B., Gibbons F.K., Litonjua A.A., Giovannucci E., Christopher K.B. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit. Care Med. 2012;40:63–72. doi: 10.1097/CCM.0b013e31822d74f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quraishi S.A., Litonjua A.A., Moromizato T., Gibbons F.K., Camargo C.A., Jr., Giovannucci E., Christopher K.B. Association between prehospital vitamin D status and hospital-acquired Clostridium difficile infections. JPEN J. Parenter. Enter. Nutr. 2015;39:47–55. doi: 10.1177/0148607113511991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnett N., Zhao Z., Koyama T., Janz D.R., Wang C.Y., May A.K., Bernard G.R., Ware L.B. Vitamin D deficiency and risk of acute lung injury in severe sepsis and severe trauma: A case-control study. Ann. Intensive Care. 2014;4:5. doi: 10.1186/2110-5820-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amrein K., Schnedl C., Holl A., Riedl R., Christopher K.B., Pachler C., Purkart T.U., Waltensdorfer A., Münch A., Warnkross H., et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 27.McNally J.D., Menon K., Chakraborty P., Fisher L., Williams K.A., Al-Dirbashi O.Y., Doherty D.R. The association of vitamin D status with pediatric critical illness. Pediatrics. 2012;130:429–436. doi: 10.1542/peds.2011-3059. [DOI] [PubMed] [Google Scholar]
- 28.Kempker J.A., West K.G., Kempker R.R., Siwamogsatham O., Alvarez J.A., Tangpricha V., Ziegler T.R., Martin G.S. Vitamin D status and the risk for hospital-acquired infections in critically ill adults: A prospective cohort study. PLoS ONE. 2015;10:e0122136. doi: 10.1371/journal.pone.0122136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman H.W., Niles J.K., Kroll M.H., Bi C., Holick M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelidi A.M., Belanger M.J., Lorinsky M.K., Karamanis D., Chamorro-Pareja N., Ognibene J., Palaiodimos L., Mantzoros C.S. Vitamin D Status Is Associated With In-Hospital Mortality and Mechanical Ventilation: A Cohort of COVID-19 Hospitalized Patients. Mayo Clin. Proc. 2021;96:875–886. doi: 10.1016/j.mayocp.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw. Open. 2020;3:e2019722. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han J.E., Jones J.L., Tangpricha V., Brown M.A., Hao L., Hebbar G., Lee M.J., Liu S., Brown L.A.S., Ziegler T.R., et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J. Clin. Transl. Endocrinol. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castillo M.E., Costa L.M.E., Barrios J.M.V., Díaz J.F.A., Miranda J.L., Bouillon R., Gomez J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavalier E., Faché W., Souberbielle J.C. A Randomised, Double-Blinded, Placebo-Controlled, Parallel Study of Vitamin D3 Supplementation with Different Schemes Based on Multiples of 25,000 IU Doses. Int. J. Endocrinol. 2013;2013:327265. doi: 10.1155/2013/327265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleck M.L., Souberbielle J.C., Jandrain B., Da Silva S., De Niet S., Vanderbist F., Scheen A., Cavalier E. A Randomized, Double-Blind, Parallel Study to Evaluate the Dose-Response of Three Different Vitamin D Treatment Schemes on the 25-Hydroxyvitamin D Serum Concentration in Patients with Vitamin D Deficiency. Nutrients. 2015;7:5413–5422. doi: 10.3390/nu7075227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Niet S., Coffiner M., Da Silva S., Jandrain B., Souberbielle J.C., Cavalier E. A Randomized Study to Compare a Monthly to a Daily Administration of Vitamin D3 Supplementation. Nutrients. 2018;10:659. doi: 10.3390/nu10060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diagnostic Testing for SARS-CoV-2. [(accessed on 30 May 2022)]. Available online: https://www.who.int/publications-detail-redirect/diagnostic-testing-for-sars-cov-2.
- 40.Wise S.A., Camara J.E., Sempos C.T., Lukas P., Le Goff C., Peeters S., Burdette C.Q., Nalin F., Hahm G., Durazo-Arvizu R.A., et al. Vitamin D Standardization Program (VDSP) intralaboratory study for the assessment of 25-hydroxyvitamin D assay variability and bias. J. Steroid Biochem. Mol. Biol. 2021;212:105917. doi: 10.1016/j.jsbmb.2021.105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavalier E., Fraser C.G., Bhattoa H.P., Heijboer A.C., Makris K., Ulmer C.Z., Vesper H.W., Vasikaran S., Lukas P., Delanaye P., et al. Analytical Performance Specifications for 25-Hydroxyvitamin D Examinations. Nutrients. 2021;13:431. doi: 10.3390/nu13020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavalier E., Lukas P., Bekaert A.C., Peeters S., Le Goff C., Yayo E., Delanaye P., Souberbielle J.C. Analytical and clinical evaluation of the new Fujirebio Lumipulse®G non-competitive assay for 25(OH)-vitamin D and three immunoassays for 25(OH)D in healthy subjects, osteoporotic patients, third trimester pregnant women, healthy African subjects, hemodialyzed and intensive care patients. Clin. Chem. Lab. Med. 2016;54:1347–1355. doi: 10.1515/cclm-2015-0923. [DOI] [PubMed] [Google Scholar]
- 43.Cavalier E., Lukas P., Crine Y., Peeters S., Carlisi A., Le Goff C., Gadisseur R., Delanaye P., Souberbielle J.C. Evaluation of automated immunoassays for 25(OH)-vitamin D determination in different critical populations before and after standardization of the assays. Clin. Chim. Acta Int. J. Clin. Chem. 2014;431:60–65. doi: 10.1016/j.cca.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 44.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S., Silva C.B., Franco A.S., Macedo M.B., Dalmolin H.H., et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannata-Andía J.B., Díaz-Sottolano A., Fernández P., Palomo-Antequera C., Herrero-Puente P., Mouzo R., Carrillo-López N., Panizo S., Ibañez G.H., Cusumano C.A., et al. A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: The COVID-VIT-D-a randomised multicentre international clinical trial. BMC Med. 2022;20:83. doi: 10.1186/s12916-022-02290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milani G.P., Simonetti G.D., Edefonti V., Lava S.A., Agostoni C., Curti M., Stettbacher A., Bianchetti M.G., Muggli F. Seasonal variability of the vitamin D effect on physical fitness in adolescents. Sci. Rep. 2021;11:182. doi: 10.1038/s41598-020-80511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dopico X.C., Evangelou M., Ferreira R.C., Guo H., Pekalski M.L., Smyth D.J., Cooper N., Burren O.S., Fulford A.J., Hennig B.J., et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 2015;6:7000. doi: 10.1038/ncomms8000. [DOI] [PMC free article] [PubMed] [Google Scholar]