Abstract
Background
In patients with Parkinson’s disease (PD), real-time quaking-induced conversion (RT-QuIC) detection of pathological α-synuclein (α-syn) in olfactory mucosa (OM) is not as accurate as in other α-synucleinopathies. It is unknown whether these variable results might be related to a different distribution of pathological α-syn in OM. Thus, we investigated whether nasal swab (NS) performed in areas with a different coverage by olfactory neuroepithelium, such as agger nasi (AN) and middle turbinate (MT), might affect the detection of pathological α-syn.
Methods
NS was performed in 66 patients with PD and 29 non-PD between September 2018 and April 2021. In 43 patients, cerebrospinal fluid (CSF) was also obtained and all samples were analyzed by RT-QuIC for α-syn.
Results
In the first round, 72 OM samples were collected by NS, from AN (NSAN) or from MT (NSMT), and 35 resulted positive for α-syn RT-QuIC, including 27/32 (84%) from AN, 5/11 (45%) from MT, and 3/29 (10%) belonging to the non-PD patients. Furthermore, 23 additional PD patients underwent NS at both AN and MT, and RT-QuIC revealed α-syn positive in 18/23 (78%) NSAN samples and in 10/23 (44%) NSMT samples. Immunocytochemistry of NS preparations showed a higher representation of olfactory neural cells in NSAN compared to NSMT. We also observed α-syn and phospho-α-syn deposits in NS from PD patients but not in controls. Finally, RT-QuIC was positive in 22/24 CSF samples from PD patients (92%) and in 1/19 non-PD.
Conclusion
In PD patients, RT-QuIC sensitivity is significantly increased (from 45% to 84%) when NS is performed at AN, indicating that α-syn aggregates are preferentially detected in olfactory areas with higher concentration of olfactory neurons. Although RT-QuIC analysis of CSF showed a higher diagnostic accuracy compared to NS, due to the non-invasiveness, NS might be considered as an ancillary procedure for PD diagnosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40035-022-00311-3.
Keywords: Parkinson disease, Alpha-synuclein, Real-time quaking-induced conversion assay, Olfactory mucosa, Cerebrospinal fluid
Introduction
Parkinson’s disease (PD) is the most common form of parkinsonism, clinically characterized by motor symptoms (e.g., bradykinesia, tremor, rigidity, and postural instability) and non-motor symptoms (e.g., cognitive decline, depression, overactive bladder, and constipation) [1, 2]. Several years before the clinical onset, PD patients might complain of hyposmia and rapid eye movement sleep behavior disorder (RBD) [1, 2]. Around 80% of patients with PD eventually develop dementia later in the disease course [3]. Neuroimaging investigations such as magnetic resonance imaging (MRI) and striatal dopamine-transporter imaging are supportive of clinical diagnosis.
The definitive diagnosis of PD is only achieved at autopsy. The detection of intracellular deposits of α-synuclein (α-syn) aggregates forming Lewy bodies is the neuropathological hallmark of PD [4]. Therefore, the detection of α-syn aggregates in biofluids or peripheral tissues of patients with PD would be essential in clinical practice for early diagnosis and prognostic assessment. In this context, a novel assay called real-time quaking induced conversion (RT-QuIC) has shown capability to amplify trace amounts of α-syn aggregates in cerebrospinal fluid (CSF) from patients with PD, with variable diagnostic sensitivity (~ 80%–90%) and specificity (~ 90%–100%) [5–9]. Using RT-QuIC, α-syn aggregates propagate with a prion-like replication mechanism by inducing conversion of recombinant α-syn to the misfolded form. Then, the converted α-syn initiates amyloid fibril formation which, in turn, enhances the fluorescence of Thioflavin T (ThT).
Parallel studies have been conducted to identify easily accessible peripheral tissues such as olfactory mucosa (OM) and skin of patients with PD, multiple system atrophy (MSA) and dementia with Lewy bodies (DLB) with reliable diagnostic robustness [10–14]. As opposed to CSF, in which α-syn aggregates are dissolved homogeneously, in peripheral tissues a regional distribution of α-syn aggregates has been assessed. In this context, seminal post-mortem studies on PD have identified distinct areas of peripheral tissues (i.e., skin, submandibular glands or gastroenteric mucosa) with the highest concentration of phospho-α-syn. These findings were subsequently conveyed in patients for obtaining successful tissue sampling [15, 16]. In skin, a map of α-syn aggregate distribution shows a higher distribution in the cervical region, thigh, and leg [17]. Skin biopsies from patients with PD display phospho-α-syn deposits in dermal nerves and RT-QuIC testing showed sensitivity and specificity of 77%–96% and 95.1%–100%, respectively [13, 14, 17, 18]. Submandibular glands also show variable phospho-α-syn deposits positive to RT-QuIC, but these studies are on autoptic samples and clinical studies are still pending [19].
The OM has been targeted for RT-QuIC analysis of α-syn in patients with PD, MSA and DLB, with a relevant diagnostic accuracy in MSA (82%) and DLB (86.4%), but not in PD patients (range from 44% to 48%) [10–12].
Given the previous evidence of the low sensitivity of RT-QuIC test in OM of PD patients, we explored the performance of RT-QuIC in samples collected from two different regions of OM, the AN, a small ridge located in the top of the nasal vault, and the MT (Fig. 1a). These areas were chosen for their different coverage by olfactory neuroepithelium, known to be more represented at the level of AN [20, 21]. In addition, in smears of NS we aimed to characterize the cell population and to assess the level of α-syn and phospho-α-syn expression. Finally, in a restricted number of patients the diagnostic accuracy of RT-QuIC was also tested in the CSF as compared with the OM.
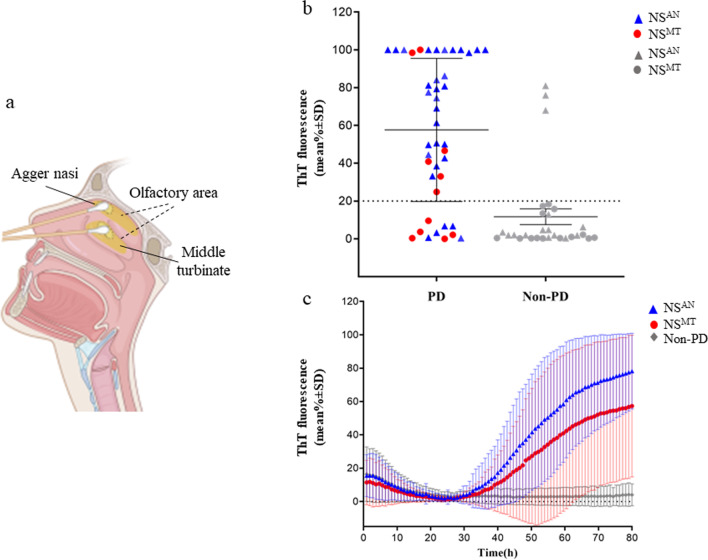
Fig. 1.
RT-QuIC detection of α-syn seeding activity in the nasal swabs (NSs) of patients with PD and non-PD. a Illustration of olfactory mucosa (OM) swab sampling from agger nasi (AN) (NSAN) and middle turbinate (MT) (NSMT) (created with BioRender.com). b Average thioflavin T (ThT) fluorescence from four replicate readings obtained in NS for each subject with PD (n = 43) and non-PD (n = 29) at 80 h. NSAN are shown as blue triangles (n = 46) while red dots denote NSMT (n = 26). c Traces represent the relative average percentage of ThT fluorescence readings from four replicate reactions (normalized as described in the Methods section) from PD and non-PD samples. The means (thick lines) with standard deviations (thin lines) are shown as a function of RT-QuIC reaction time
Methods
Ethics statement
The study was conducted according to the revised Declaration of Helsinki and Good Clinical Practice guidelines. Informed consent was given by study participants or the next-of-kin. OM or CSF sample collection was performed under protocols approved by the Ethical Committee of each unit, University of Trieste (UNITS) (CEUR-2020-Sper-013, February 11th, 2020), University of Udine (UNIUD) (UD: 26499) and University of Verona (UNIVR) (Prot. n. 28917 June 15th, 2012). Written informed consent was obtained from patients or legal representatives.
Patients and clinical assessment
Patients were recruited at three neurology units including UNITS, UNIUD and UNIVR. Sixty-six patients received a clinical diagnosis of probable PD based on the international diagnostic criteria [2, 3]. Additional 29 patients with other clinical diagnoses, based on established diagnostic criteria, such as Alzheimer’s disease (AD) (n = 13), progressive supranuclear palsy (PSP) (n = 6), Creutzfeldt-Jakob disease (CJD) (n = 7) and essential tremor (n = 3), were recruited as non-PD patients. Based on the clinical history and neurological evaluation performed by neurology specialists in neurodegenerative disorders, appropriate diagnostic investigations were performed, including MRI, FDG PET, 123I-Ioflupane SPECT and CSF measurement of biomarkers of dementia. Next, for each patient a final diagnosis was made by applying the latest recommended criteria for PD, AD, PSP, CJD and essential tremor (Table 1) [22–25]. RT-QuIC analysis of OM and CSF samples was carried out at UNIVR by clinical diagnosis investigators who were blinded to the patients’ diagnosis. All patients included in this study underwent NS and 43 of them further underwent CSF collection. For immunocytochemical study, NS samples from 12 healthy volunteers (6 males and 6 females, mean age 56 ± 5 years) were also included.
Table 1.
Demographics, clinical characteristics and RT-QuIC results of PD patients who underwent NS and CSF sampling
| Patients underwent NS at the (n = 46) | Patients underwent NS at the MT (n = 26) | P-value (effect size) | ||||
|---|---|---|---|---|---|---|
| PD (n = 32) | Non-PD (n = 14) | PD (n = 11) | Non-PD (n = 15) | AN vs MT in PD | AN vs MT in non-PD | |
| Age at diagnosis, years | 67 (60–70) | 73 (70–76) | 65 (53–70) | 68 (64–75) | 0.3 (R2 = .02) | 0.2 (R2 = 0.07) |
| Sex Female/Male | 12/20 | 4/10 | 3/8 | 5/10 | 0.7 (φc = 0.09) | 0.9 (φc = 0.05) |
| Age at onset, years | 66 (59–69) | 70 (61–74) | 64 (51–69) | 68 (63–71) | 0.4 (R2 = 0.02) | 0.4 (R2 = 0.02) |
| Disease duration (from diagnosis), years | 2 (1–3) | ND | 2 (1–2) | ND | 0.9 (R2 = 0.00) | – |
| Interval between clinical diagnosis and NS, months | 1 (1–8) | 12 (12–24) | 12 (2–12) | 12 (6–24) | 0.009 (R2 = 0.17)* | 0.9 (R2 = 0.00) |
| Interval between lumbar puncture and NS, months | 1 (1–3) (n = 23) | 12 (1–18) (n = 8) | 2 (2–2) (n = 1) | 2 (2–12) (n = 11) | 0.6 (R2 = 0.04) | 0.7 (R2 = 0.01) |
| MDS-UPDRS III score | 18 (11–33) | 5 (2–17) | 20 (12–31) | ND | 0.9 (R2 = 0.02) | – |
| MoCA score | 27 (24–29) | 21 (19–24) | 25 (24–30) | ND | 0.5 (R2 = 0.01) | – |
| Hyposmia n (%) | 17/32 (53) | ND | 5/11 (46) | ND | 0.7 (φc = 0.07) | – |
| RT-QuIC-positive OM (%) | 27 (84)* | 2 (14) | 5 (45)* | 1 (7) | 0.018 (φc = 0.39)* | 0.6 (φc = 0.12) |
| RT-QuIC-positive CSF (%) | 21/23 (91) | 1/8 (12) | 1/1 | 0/11 | 0.76 | 0.4 |
Data are shown as median (IQR) or n (%)
AN, agger nasi; MT, middle turbinate; ND, not determined
*Significant differences in group comparisons; –, not tested due to extreme or low counts
OM sample collection
NS was performed by otolaryngologists in each unit, independently. A step-by-step tutorial video of NS procedure is available at https://www.youtube.com/watch?v=wYb9W3u6uMY. At UNITS and UNIUD, NS procedure was performed at the level of AN and the patients were assigned in the NSAN group. At UNIVR, OM samples were collected through the MT and this group of patients was named NSMT (Fig. 1a). Two to four OM samples were collected from both sides in each subject, depending on individual’s tolerability. The procedure was well tolerated by all subjects without complications and we did not observe individual reluctance to NS, which is a well-known test for COVID-19. Following the NS procedure, swabs were placed in polypropylene tubes containing saline or fixative (Diacyte, Diapath, Martinengo, Italy) solutions, sealed and sent to UNIVR. Upon arrival, tubes were vortexed and centrifuged at 1700 rpm for 15 min. The OM pellets in saline were frozen and kept at −80 °C for RT-QuIC analyses. The fixed cells were counted with Countess™ II FL Automated Cell Counters (Invitrogen) and diluted if necessary. Cell suspension was cyto-centrifuged onto slides using a cytospin (CYTOSPIN IV, AHSI, Italy) and slides were kept frozen until immunostaining [26].
Immunocytochemical analysis
NS samples collected from AN and MT of 18 patients with PD were processed for immunocytochemistry. Slides were incubated overnight at 4 °C with primary anti-β-tubulin III (1:400; T2200 Sigma), anti-α-syn (1:400; BioLegend) and anti-phospho-α-syn (1:1000; Invitrogen, Waltham, MA) antibodies. On the next day, the slides were washed and incubated for 1 h at room temperature with Alexa Fluor-conjugated secondary antibodies (1:1000, Life Technologies, Waltham, MA). After washing, the slides were incubated with DAPI (1:2000) for 5 min and mounted with DABCO (Sigma-Aldrich, Milan, Italy). Images were acquired using the Axiolab fluorescence microscope (Zeiss, Oberkochen, Germany).
Expression and purification of recombinant human α-synuclein
Recombinant α-syn was expressed and purified from the periplasmic fraction as reported [27]. Briefly, wild-type human α-syn cDNA was cloned in the pET-28a plasmid (Novagen, Milan, Italy) and transformed into Escherichia coli BL21(DE3). Cell cultures (1 L) were grown at 37 °C to an OD600 of 0.3–0.4 and the expression was induced with 0.1 mM isopropyl b-D-1-thiogalactopyranoside for 5 h. The cells were collected by centrifugation, resuspended in 100 ml of osmotic shock buffer (30 mM Tris-HCl pH 7.2, 40% sucrose, and 2 mM EDTA) and incubated for 10 min at room temperature. The pellet was centrifuged at 12,000 rpm, resuspended in 90 ml of cold water added with 37.5 µl of saturated MgCl2 solution and centrifuged again after 5-min incubation on ice. The supernatant containing periplasm proteins was boiled for 15 min and cleared by centrifugation. The soluble fraction, enriched in α-syn, was subjected to ammonium sulfate precipitation followed by extensive dialysis against 20 mM Tris–HCl, pH 8.0. Alpha-syn was further purified by anion exchange chromatography: the protein was loaded on a Q-Sepharose column (GE Healthcare, Milan, Italy) equilibrated with the same buffer and eluted with a 0–500 mM linear gradient of NaCl. The purity of α-syn was checked by SDS-PAGE. The protein was then dialyzed against 10 mM sodium phosphate buffer pH 7.4 and stored at -80 °C until use.
Alpha-synuclein RT-QuIC in OM swabs
OM samples were thawed and a disposable inoculating loop (Fisherbrand, Milan, Italy) was dipped into the pellet to transfer ∼2 μl of the pellet into a tube containing 120 μl phosphate-buffered saline (PBS). The latter tube was sonicated at 120 W (Digital ultrasonic bath Mod.DU-32, Argo Lab, Carpi, Italy) for at least 1 min until the pellet was dispersed. For each test, 2 μl of the diluted OM sample was plated in 98 µl of reaction buffer composed of 100 mmol/l phosphate buffer (pH 8.2), 10 μmol/l ThT, and 0.05 mg/ml human recombinant full-length (1–140 aa) α-syn and 37 ± 3 mg of 0.5 mm glass beads (Sigma). The plate was sealed with a plate sealer film (Nalgene Nunc International, Rochester, NY) and then incubated at 30 °C in a BMG FLUOstar® Omega plate reader with cycles of 1-min shaking (200 rpm double orbital) and 14-min rest. ThT fluorescence measurements (450 ± 10 nm excitation and 480 ± 10 nm emission; bottom read) were taken every 45 min. Four replicate reactions were tested for each sample.
RT-QuIC analysis in NS and CSF samples
Alpha-syn RT-QuIC test has been previously optimized in OM and CSF samples from patients with PD, DLB and RBD, as described [11, 12, 27]. Four replicate reactions were tested for each sample. A sample was considered positive when at least two of four replicate wells crossed the calculated threshold (20% maximum ThT fluorescence). In detail, for each set of replicate reactions, the mean baseline relative fluorescence units (rfu) value was calculated over a 10-h period spanning the lowest part of a plot of the mean rfu of all replicates versus reaction time. This value was then subtracted from the mean rfu values at each time point to give the baseline‐adjusted mean rfu values. The latters were then normalized as percentages of the baseline‐adjusted maximal fluorescence rfu values as follows: baseline-adjusted rfu values/baseline-adjusted maximum rfu value × 100% [11, 12]. These normalized values were plotted versus reaction time. ThT fluorescence positivity threshold was calculated as the average fluorescence for all samples between 15 and 17 h of incubation plus three standard deviations (SD). Cut-off time was assessed at 80 h for both NS and CSF samples, based on the results from definite cases, in order to obtain the best specificity and sensitivity. Absence of RT-QuIC seeding reaction was also determined in OM samples from PD patients without substrate and substrate without OM samples (Additional file 1: Fig. S1).
Statistical analysis
RT-QuIC relative fluorescence responses were analysed and plotted using the software Graphpad Prism 8.3. We compared the mean relative ThT fluorescence and the lag phase responses in NS and CSF samples by either two-tailed unpaired t-test or by following ascertainment of normal distribution of data by Shapiro–Wilk normality test. Numerical variables were assessed using histograms, Q-Q plots and Kolmogorov–Smirnov’s test for normality. First, we tested the proportions of positive OM and CSF samples, demographic, clinical and instrumental data from the two centres in the original cohort for homogeneity using Fisher’s exact test and Kruskal–Wallis H test for numerical variables. Cramer’s V (φc) and Eta-squared () were used to estimate the effect size. We then tested the same variables from the supplementary cohort and the original cohort for homogeneity using Fisher’s exact test for binomial variables and Mann–Whitney U-test for numerical variables. Cramer’s V (φc) and R2 were used to estimate the effect size. Finally, agreement between the anterior and posterior sampling methods in the supplementary cohort was tested for different results by McNemar test, using Cohen’s kappa (k) to estimate the effect size. P values < 0.05 were considered statistically significant.
Results
NS sampling and RT-QuIC assay in patients with PD and other diagnoses
At the first round, 72 patients with PD and other neurodegenerative disorders underwent NS (Table 1). In total, 72 OM samples were obtained, including 46 AN samples from 32 PD patients and 14 non-PD, and 26 MT samples 11 PD patients and 15 non-PD.
Thirty-five samples were positive for α-syn in RT-QuIC. In particular, 32 samples were from patients having a clinical diagnosis of probable PD while 3 were from non-PD patients, including one patient with a clinical diagnosis of AD, one with PSP and one with CJD (Fig. 1b). As such, α-syn RT-QuIC was positive in OM of 32/43 (74%) patients with PD and 3/29 (10%) patients with non-PD. The proportion of positive samples from PD patients was significantly higher in NSAN (27/32, 84%) compared to NSMT (5/11, 45%) (P = 0.018, φc = 0.39) (Table 1). Interestingly, among the 32 PD patients positive for RT-QuIC α-syn, 17 complained of hyposmia (53%), but 4 out of 5 patients with AN sampling who were OM negative did not complain of olfactory dysfunction.
In addition, the average α-syn seeding reactivity of NSAN samples was higher than that of NSMT samples (the maximum ThT fluorescence at the endpoint of 80 h was 80% for NSAN compared to 57% for NSMT; P < 0.05), and the average reaction time required to exceed the designated positivity threshold was 41 h for NSAN and 47 h for NSMT (see also Materials and Methods) (Fig. 1c).
Evaluation of RT-QuIC sensitivity in OM samples obtained at the level of AN and through the MT
To confirm that the RT-QuIC sensitivity would be higher when OM samples were obtained at the AN, 23 additional patients with PD were recruited. The demographic and clinical characteristics of the PD patients for OM swab sampling did not differ from the first group of patients (Table 2) and each patient underwent OM swab sampling either at the AN or through the MT.
Table 2.
Comparative analysis of demographic and clinical characteristics between PD patients tested at the first and the second rounds
| PD patients of the first round (n = 43) | PD patients of the second round (n = 23) | P value | |
|---|---|---|---|
| Age at diagnosis, years | 66 (59–70) | 70 (61–75) | 0.14 |
| Sex female/male | 15/28 | 7/16 | 0.79 |
| Age at onset, years | 65 (57–69) | 68 (60–71) | 0.24 |
| Interval between clinical diagnosis and NS, months | 2 (1–12) | 2 (1–12) | 0.99 |
| MDS-UPDRS III score | 20 (12–32) | 18 (9–33) | 0.95 |
| MoCA score | 26 (24–29) | 24 (24–28) | 0.11 |
| RT-QuIC-positive NSAN (%) | 27/32 (84) | 18/23 (78) | – |
| RT-QuIC-positive NSMT (%) | 5/11 (45) | 10/23 (43) | – |
Data are shown as median (IQR) or n (%)
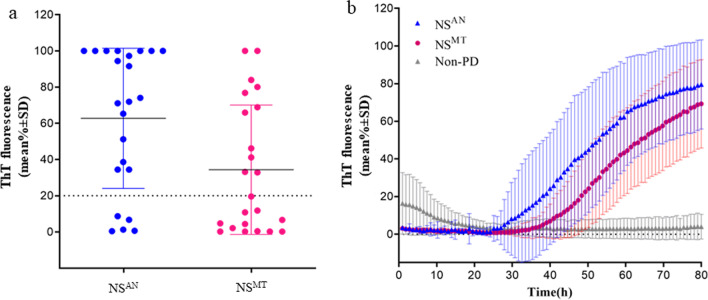
Different proportions of RT-QuIC-positive samples were observed between NSAN and NSMT. In particular, NSAN resulted RT-QuIC positive in 18 out of 23 (78%), while NSMT in 10 out of 23 (44%) (Fig. 2a) (κ = 0.19, 95% CI 0.00 to 0.49, P = 0.021). In particular, four patients were identified to be negative for α-syn by both NSAN and NSMT RT-QuIC; nine patients were identified to be positive for α-syn by NSAN RT-QuIC but negative by NSMT RT-QuIC; and one patient was negative in the NSAN RT-QuIC but positive in the NSMT RT-QuIC. In addition, the average seeding reactivity of the positive samples was statistically higher in NSAN than in NSMT, similar as that in the first round of testing (79% and 69% maximum ThT fluorescence at 80 h) (P < 0.05) (Fig. 2b).
Fig. 2.
RT-QuIC detection of α-synuclein seeding activity in the nasal swabs (NS) of PD patients at agger nasi (AN) and middle turbinate (MT). a The final average relative ThT fluorescence from four replicate readings obtained from NS of each individual case (n = 23) at the level of AN and through the MT at 80 h. Bars show the average ± SD for all the cases in each group. The dashed line shows the fluorescence threshold for a positive result. b Traces represent the relative average percentage of ThT fluorescence readings of positive samples from AN (blue trace) and the MT (magenta trace), and non-PD (grey trace) as negative controls. The means (thick lines) with SDs (thin lines) of those averages are shown as a function of RT-QuIC reaction time
These results suggest similar RT-QuIC sensitivity between the first and the second rounds, in both NSAN (84% in the first vs 78% in the second round) and NSMT (45% vs 43%). Moreover, the results of average α-syn seeding activity were also similar between the first and the second rounds in both NSAN and NSMT, confirming the higher seeding reactivity of α-syn aggregates in NSAN.
Immunocytochemical analysis of NSAN and NSMT
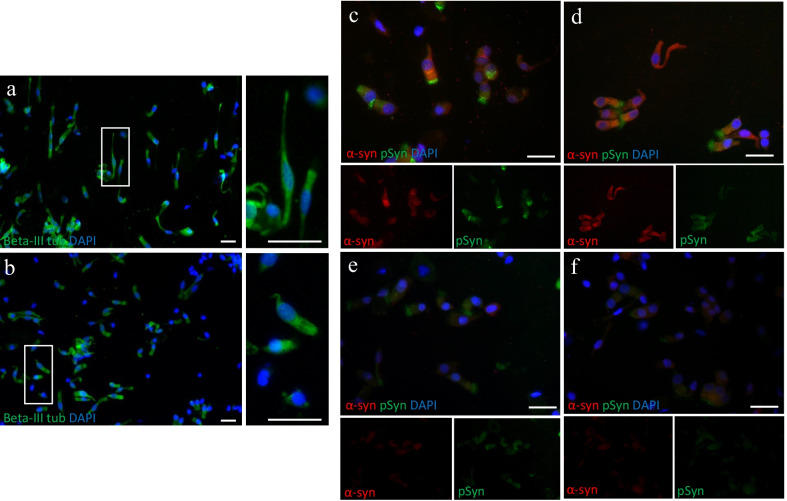
We next aimed to demonstrate that RT-QuIC results obtained from NSAN and NSMT correlated with a different representation of olfactory neuroepithelium. OM samples from 18 PD patients were immunostained with anti-β-tubulin III antibody, a phenotypic marker of olfactory neuron and its precursors, and a higher number of β-III tubulin-positive cells was observed in NSAN compared to NSMT (Fig. 3a vs b). Furthermore, the OM preparations were immunostained for α-syn and phospho-α-syn. In NS from 18 PD patients, a diffuse α-syn positivity was observed in olfactory neurons and supporting cells. Sustentacular-like ciliated cells showed phospho-α-syn deposits in the cytoplasm, more concentrated in the apical portion (Fig. 3c, d). Conversely, OM preparations from 12 non-PD patients or 12 normal controls showed only a faint positivity to both α-syn and phospho-α-syn (Fig. 3e, f).
Fig. 3.
Immunocytochemical analyses of nasal swabs (NS) of controls and PD patients. a, b OM cell preparations from NS of AN (a) and MT (b) of a PD patient and immunostained with anti-β-tubulin III antibody (green) (scale bars 25 µm, magnification 20×). Thin and elongated β-III tubulin-positive cells with a neuronal-shape morphology are dominant in AN (inset, scale bar 25 µm) compared to MT (inset, scale bar 25 µm). c, d Immunostaining with anti-α-syn (red) and anti-phospho-α-syn (green) antibodies of NS from the PD patient showed α-syn and phospho-α-syn deposits in neuronal-shaped and ciliated cells. In contrast, NS from a non-PD patient (e) and normal control (f) showed a weak positivity (scale bar 25 µm, magnification 40×). Nuclei were stained with DAPI (blue)
Diagnostic accuracy of CSF RT-QuIC
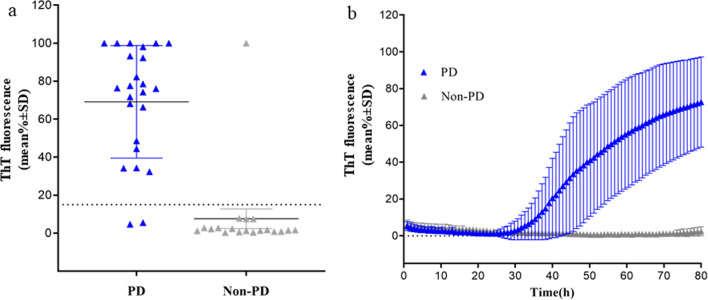
Twenty-four patients with PD enrolled at the first round also underwent lumbar puncture and 22 CSF samples were positive for α-syn RT-QuIC (92%) (Fig. 4a, b). Nineteen CSF samples from patients with a clinical diagnosis of non-PD (5 with AD, 3 with PSP and 11 with CJD) were included as controls, of which one CSF sample from a patient with AD was positive for α-syn RT-QuIC (Fig. 4a, b).
Fig. 4.
RT-QuIC detection of α-syn seeding activity in CSF samples of patients with PD and non-PD. a The average of relative ThT fluorescence from four replicate readings obtained from CSF of each individual cases with PD (n = 24) and non-PD (n = 19) at 80 h. Bars show the average ± SD for each group. The dashed line shows the fluorescence threshold for a positive result. b Traces represent the average percentage of ThT fluorescence readings from positive 22/24 CSF samples from patients with PD and 1/19 non-PD . The means (thick lines) with SD (thin lines) of those averages are shown as a function of RT-QuIC reaction time
RT-QuIC analysis of CSF provided a slightly higher diagnostic accuracy compared to OM (94% vs 82%). In 43 patients with both CSF and OM samples (31 collected from AN and 12 from MT), the RT-QuIC results did not differ (P = 0.9) between the two samples (k = 0.72, 95%CI 0.70–0.74). It should be noted that of the two CSF-negative samples, one was positive in paired OM while the other was negative in OM.
Discussion
NS was originally targeted as an innovative procedure for intravital diagnosis of human prion disorders [28, 29]. More recently, α-syn RT-QuIC has been explored in NS of patients with DLB, PD and iRBD, providing variable diagnostic accuracies [11, 12]. However, a yet unexplored issue is the performance of the RT-QuIC assay in samples obtained from different OM areas, as done for other peripheral tissues during optimization of the sampling procedure. In patients with PD, OM samples had been always collected at the level of MT and RT-QuIC sensitivity was 46% in one study which included 63 patients, or 56% in another on 18 PD patients [10, 11].
Here, we obtained an overall agreement of 74% between RT-QuIC positivity and clinical diagnosis (32/43), higher than that obtained in previous studies. However, when analyzed separately, the RT-QuIC assay in NSAN and NSMT had sensitivity of 84% and 45%, respectively. The findings were reproduced in a second round when 23 additional PD patients underwent the RT-QuIC assay in both NSAN and NSMT, providing a sensitivity of 78% and 43%, respectively. In conclusion, this is the first study showing that NS performed in two distinct areas of OM provided a significant difference in RT-QuIC sensitivity (P = 0.021) and that the higher RT-QuIC positivity was correlated to the higher distribution of β-III tubulin-positive olfactory cells in NSAN [20, 21].
Notably, in patients with Parkinson’s disease dementia (PDD) (data not shown), DLB or prodromal DLB, the RT-QuIC sensitivity was ~ 80% regardless of the NS procedure, and no significant difference in the sensitivity between OM samples collected from AN and MT was observed [12]. Thus, it is tempting to hypothesize that in the presence of cognitive impairment, α-syn aggregates are involved in a larger area of OM.
Previous post-mortem studies have shown Lewy body (LB) pathology in the olfactory bulb but less frequently in the olfactory neuroepithelium of PD cases. In addition, α-syn aggregates or phospho-α-syn deposits were not detected in OM biopsy from subjects with PD. The lack of LB pathology was explained by the rapid turnover of olfactory cells, estimated to be few months, not sufficient for harbouring LB pathology [30, 31]. Here, we showed that α-syn and phospho-α-syn deposits, detected in the OM of PD patients but not in non-PD patients or controls, have seeding activity, indicating that even if LB pathology is not detected, α-syn is pathologically folded. It remains to be clarified whether in patients with PD, α-syn is highly expressed or has impaired degradation, and which metabolic dysfunctions lead to abnormal α-syn phosphorylation. It is well known that these aberrant changes of α-syn represent predisposing conditions for generating pathologic α-syn aggregates [32]. However, the phospho-α-syn deposition detected in the OM of PD patients should be further analyzed in a larger number of individuals.
Likely, the detection of α-syn aggregates in AN, a distinct area of OM, indicates that AN is a preferential site for α-syn aggregate formation. This occurrence might have a possible mechanical explanation. Normally, odorant molecules slow their flow in the superior area of the nasal cavity to allow their capture by olfactory receptors. This favors the exposure of AN olfactory receptor cells to environmental toxicants, which exerts cell stress damage contributing to α-syn misfolding [33]. A previous study showed that the olfactory bulb of patients with PD is affected by LB pathology in a gradient manner of progression, with neuronal loss at the level of glomeruli first, and subsequently in the anterior olfactory nucleus [34]. In mammals, the olfactory neurons are compartmentalized by expression of receptors in defined areas of the neuro-epithelium which projects to distinct regions of the olfactory bulb, preserving the spatial topography of the nasal epithelium in the glomerular sheet of the olfactory bulb [35–37]. Although this specific organization has not been clearly shown in humans, the preferential distribution of α-syn aggregates in the superior/anterior area of OM might find a correlation to LB pathology in the olfactory bulb of PD.
Diagnostic accuracy of RT-QuIC in CSF and in the combination of CSF and OM
Here, we showed that the sensitivity of RT-QuIC in CSF was 94%, higher than that in the NS. The concordance of CSF RT-QuIC results with clinical diagnosis is comparable to that reported in previous studies, which showed a sensitivity ranging from 84% to 96% and specificity from 82% to 100% [5, 6, 38–42]. Notably, in this study, one of the two PD patients with negative results of CSF RT-QuIC, showed a positive result in OM (sampled from the AN). These findings support the assumption that a double tissue testing should be the ideal diagnostic approach. We previously showed in human prion disorders that the sensitivity of RT-QuIC in CSF and OM increased from 95% when the two samples were tested separately to 100% when both samples were tested [28, 29]. In patients with DLB, α-syn RT-QuIC assay in CSF and OM showed a concordance with clinical diagnosis of 94% and 86%, respectively, which increased to 100% when the results were combined. More recently, α-syn RT-QuIC in skin biopsies has also been considered supportive for CSF analysis [14]. However, compared to α-syn RT-QuIC testing in the skin, a greater diagnostic accuracy has been obtained by immunofluorescence detection of phospho-syn deposits in skin nerves of PD patients. These findings provide a further rationale for the use of NS in the diagnostic assessment of PD and related disorders [43].
This study is not without limitations. This is a clinical study and the results obtained need to be further confirmed in definite PD. Further, 20% of the PD patients were RT-QuIC negative in NS and 73% of these PD patients did not complain hyposmia. It might be not excluded that these PD patients represent a distinct phenotypic subgroup with relative sparing of OM or may be affected by other forms of Parkinsonisms [7]. These patients, as well as non-PD patients with NS positive for α-syn RT-QuIC, will be followed-up.
In conclusion, following NS optimization, the sensitivity of RT-QuIC in OM was increased to ~80%, comparable to that of skin biopsies. Specific protocols of sampling have been reported for skin biopsy, providing a sensitivity of 82.4% or 76.9% based on the number of skin samples obtained from each patient [17]. Further, NS allows collection of optimal OM samples with a single procedure, and can be done for multiple times, providing the optimal approach for follow-up studies.
Conclusion
In this study, we optimized the protocol of NS in patients with PD and we showed that the sensitivity of α-syn RT-QuIC was significantly increased when OM was collected at the level of AN as compared to MT (~ 80% vs ~ 45%). However, the above sampling protocol did not provide significant differences in sensitivity of the test, when performed in patients with PDD or DLB. Taken together, these results suggest that, in PD, deposition of aberrant α-syn preferentially occurs in AN, which eventually spreads to the entire OM. In line with other studies, RT-QuIC analysis of CSF showed a sensitivity of 94%. Combining the CSF results with the results of NS can increase the diagnostic accuracy to nearly 100%.
Supplementary Information
Additional file 1: Fig. S1 Olfactory mucosa (OM) sample without substrate (recombinant α-syn and reaction buffer) (red trace) and substrate without OM (black trace) were tested by RT-QuIC.
Acknowledgements
We thank all participants and their families for olfactory mucosa donation. We deeply thank Dr. Santina Castriciano and Ilaria Triva (COPAN group) for providing nasal swabs.
Abbreviations
- AD
Alzheimer disease
- α-syn
α-Synuclein
- CBS
Corticobasal syndrome
- DLB
Dementia with Lewy bodies
- MSA
Multiple system atrophy
- OM
Olfactory mucosa
- p-α-syn
Phosphorylated α-synuclein
- PSP
Progressive supranuclear palsy
- REM
Rapid eye movement
- RT-QuIC
Realtime quaking-induced conversion
- ThT
Thioflavin T
Author contributions
PM and GZ conceived the idea, designed the study, and supervised the project. MB, MC, and DP contributed to the conception and design of the study. MC, FJ, CB, PP, VT, GB, AB, MV, ST, LB, GG, SM, PM and GZ contributed to the clinical diagnosis and patient recruitment. LS and RB performed nasal swab sampling. SC and EAK prepared the recombinant synuclein substrate. MB and DP performed the RT-QuIC assays. MF analyzed the RT-QuIC data. EF processed samples, performed immunocytochemistry, image acquisition and analyses. MT and GZ performed and reviewed the statistical analysis. GZ wrote the first draft. GZ and EF edited the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Fondazione Cariverona: “Development and validation of a novel molecular assay for α-synuclein in patients with Parkinson’s disease and other α-synucleinopathies” # 2018.0708 to GZ and Brain Research Verona Foundation to GZ. Dr. Elena Fontana is attending a doctoral fellowship supported by COPAN group.
Availability of data and materials
All the data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and conset to participate
This was a cross-sectional study performed at three clinical academic centres (University of Trieste, Udine and Verona, Italy). The study was approved by the local ethics committees of University of Trieste (CEUR-2020-Sper-013, February 11th, 2020), University of Udine (UD: 26499) and University of Verona (Prot. n. 28917 June 15th, 2012). All participants provided written informed consent according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
All authors report no competing interests.
Footnotes
Matilde Bongianni, Mauro Catalan and Daniela Perra have been contributed equally to this work.
References
- 1.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3:1–21. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 2.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 3.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 5.Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol. 2016;3(10):812–818. doi: 10.1002/acn3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahnawaz M, Tokuda T, Waraga M, Mendez N, Ishii R, Trenkwalder C, et al. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 2017;74(2):163–172. doi: 10.1001/jamaneurol.2016.4547. [DOI] [PubMed] [Google Scholar]
- 7.van Rumund A, Green AJE, Fairfoul G, Esselink RAJ, Bloem BR, Verbeek MM. α-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann Neurol. 2019;85(5):777–781. doi: 10.1002/ana.25447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun. 2018;6:1–10. doi: 10.1186/s40478-018-0508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi M, Candelise N, Baiardi S, Capellari S, Giannini G, Orrù CD, et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020;140(1):49–62. doi: 10.1007/s00401-020-02160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Luca CMG, Elia AE, Portaleone SM, Cazzaniga FA, Rossi M, Bistaffa E, et al. Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy. Transl Neurodegener. 2019;8(1):1–14. doi: 10.1186/s40035-018-0141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefani A, Iranzo A, Holzknecht E, Perra D, Bongianni M, Gaig C, et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain. 2021;144(4):1118–1126. doi: 10.1093/brain/awab005. [DOI] [PubMed] [Google Scholar]
- 12.Perra D, Bongianni M, Novi G, Janes F, Bessi V, Capaldi S, et al. Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun. 2021;3(2):1–11. doi: 10.1093/braincomms/fcab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Becker K, Donadio V, Siedlak S, Yuan J, Rezaee M, et al. Skin α-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol. 2021;78(1):30–40. doi: 10.1001/jamaneurol.2020.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mammana A, Baiardi S, Quadalti C, Rossi M, Donadio V, Capellari S, et al. RT-QuIC detection of pathological α-synuclein in skin punches of patients with Lewy body disease. Mov Disord. 2021;36(9):2173–2177. doi: 10.1002/mds.28651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beach TG, Adler CH, Sue LI, Vedders L, Lue LF, White CL, et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, et al. Multiple organ involvement by alpha-synuclein pathology in lewy body disorders. Mov Disord. 2014;29(8):1010–1018. doi: 10.1002/mds.25776. [DOI] [PubMed] [Google Scholar]
- 17.Donadio V, Incensi A, Leta V, Giannoccaro MP, Scaglione C, Martinelli P, et al. Skin nerve α-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology. 2014;82(15):1362–1369. doi: 10.1212/WNL.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 18.Manne S, Kondru N, Jin H, Serrano GE, Anantharam V, Kanthasamy A, et al. Blinded RT-QuIC analysis of α-synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov Disord. 2020;35(12):2230–2239. doi: 10.1002/mds.28242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manne S, Kondru N, Jin H, Anantharam V, Huang X, Kanthasamy A, et al. α-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov Disord. 2020;35(2):268–278. doi: 10.1002/mds.27907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leopold DA, Hummel T, Schwob JE, Hong SC, Knecht M, et al. Anterior distribution of human olfactory epithelium. Laryngoscope. 2000;110:417–421. doi: 10.1097/00005537-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Escada PA, Lima C, Da Silva JM. The human olfactory mucosa. Eur Arch Otorhinolaryngol. 2009;266(11):1675–1680. doi: 10.1007/s00405-009-1073-x. [DOI] [PubMed] [Google Scholar]
- 22.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osaki Y, Ben-Shlomo Y, Lees AJ, Daniel SE, Colosimo C, Wenning GK, et al. Accuracy of clinical diagnosis of progressive supranuclear palsy. Mov Disord. 2004;19(2):181–189. doi: 10.1002/mds.10680. [DOI] [PubMed] [Google Scholar]
- 24.Höglinger G, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors: from the task force on tremor of the international Parkinson and movement disorder society. Mov Disord. 2018;33:75–87. doi: 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brozzetti L, Sacchetto L, Cecchini MP, Avesani A, Perra D, Bongianni M, et al. Neurodegeneration-associated proteins in human olfactory neurons collected by nasal brushing. Front Neurosci. 2020;14:145. doi: 10.3389/fnins.2020.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bongianni M, Ladogana A, Capaldi S, Klotz S, Baiardi S, Cagnin A, et al. α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann Clin Transl Neurol. 2019;6(10):2120–2126. doi: 10.1002/acn3.50897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orrú CD, Bongianni M, Tonoli G, Ferrari S, Hughson AG, Groveman BR, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371(6):519–529. doi: 10.1056/NEJMoa1315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bongianni M, Orrù C, Groveman BR, Sacchetto L, Fiorini M, Tonoli G, et al. Diagnosis of human prion disease using real-time quaking-induced conversion testing of olfactory mucosa and cerebrospinal fluid samples. JAMA Neurol. 2017;74(2):155–162. doi: 10.1001/jamaneurol.2016.4614. [DOI] [PubMed] [Google Scholar]
- 30.Witt M, Bormann K, Gudziol V, Pehlke K, Barth K, Minovi A, et al. Biopsies of olfactory epithelium in patients with Parkinson’s disease. Mov Disord. 2009;24(6):906–914. doi: 10.1002/mds.22464. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Shioya A, Sano T, Sumikura H, Murata M, Murayama S. Lewy body pathology involves the olfactory cells in Parkinson’s disease and related disorders. Mov Disord. 2016;31(1):135–138. doi: 10.1002/mds.26463. [DOI] [PubMed] [Google Scholar]
- 32.Awa S, Suzuki G, Masuda-Suzukake M, Nonaka T, Saito M, Hasegawa M. Phosphorylation of endogenous α-synuclein induced by extracellular seeds initiates at the pre-synaptic region and spreads to the cell body. Sci Rep. 2022;12(1):1–13. doi: 10.1038/s41598-022-04780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23(2):1–13. doi: 10.1038/nm.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson TJ, Murray HC, Turner C, Faull RLM, Dieriks BV, Curtis MA. α-synuclein inclusions are abundant in non-neuronal cells in the anterior olfactory nucleus of the Parkinson’s disease olfactory bulb. Sci Rep. 2020;10(1):1–10. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286(5440):711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- 36.Levai O, Breer H, Strotmann J. Subzonal organization of olfactory sensory neurons projecting to distinct glomeruli within the mouse olfactory bulb. J Comp Neurol. 2003;458(3):209–220. doi: 10.1002/cne.10559. [DOI] [PubMed] [Google Scholar]
- 37.Maresh A, Rodriguez Gil D, Whitman MC, Greer CA. Principles of glomerular organization in the human olfactory bulb–implications for odor processing. PLoS ONE. 2008;3(7):e2640. doi: 10.1371/journal.pone.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang UJ, Boehme AK, Fairfoul G, Shahnawaz M, Ma TC, Hutten SJ, et al. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov Disord. 2019;34(4):536–544. doi: 10.1002/mds.27646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manne S, Kondru N, Hepker M, Jin H, Anantharam V, Lewis M, et al. Ultrasensitive detection of aggregated α-synuclein in glial cells, human cerebrospinal fluid, and brain tissue using the RT-QuIC assay: new high-throughput neuroimmune biomarker assay for Parkinsonian disorders. J Neuroimmune Pharmacol. 2019;14(3):423–435. doi: 10.1007/s11481-019-09835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russo MJ, Orru CD, Concha-Marambio L, Giaisi S, Groveman BR, Farris CM, et al. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol Commun. 2021;9(1):179. doi: 10.1186/s40478-021-01282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Concha-Marambio L, Shahnawaz M, Soto C. Detection of misfolded α-synuclein aggregates in cerebrospinal fluid by the protein misfolding cyclic amplification platform. Methods Mol Biol. 2019;1948:35–44. doi: 10.1007/978-1-4939-9124-2_4. [DOI] [PubMed] [Google Scholar]
- 42.Poggiolini I, Gupta V, Lawton M, Lee S, El-Turabi A, Querejeta-Coma A, et al. Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain. 2022;145(2):584–595. doi: 10.1093/brain/awab431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donadio V, Wang Z, Incensi A, Rizzo G, Fileccia E, et al. In vivo diagnosis of synucleinopathies: a comparative study of skin biopsy and RT-QuIC. Neurology. 2022;96(20):e2513–e2524. doi: 10.1212/WNL.0000000000011935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1 Olfactory mucosa (OM) sample without substrate (recombinant α-syn and reaction buffer) (red trace) and substrate without OM (black trace) were tested by RT-QuIC.
Data Availability Statement
All the data generated or analysed during this study are included in this published article.