Abstract
Background
Relationships between gut microbiomes and airway immunity have been established in murine and human studies of allergy and asthma. Early life Lactobacillus supplementation alters the composition and metabolic productivity of the gut microbiome. However, little is known of how Lactobacillus supplementation impacts the gut microbiota in children with cystic fibrosis (CF) and whether specific microbiota states that arise following gut microbiome manipulation relate to pulmonary outcomes.
Methods
Stool samples were collected from CF patients enrolled in a multi-center, double-blind, randomized placebo-controlled trial of daily Lactobacillus rhamnosus strain GG (LGG) probiotic supplementation over a 12-month period. Fecal 16S rRNA biomarker sequencing was used to profile fecal bacterial microbiota and analyses were performed in QiiME.
Results
Bifidobacteria-dominated fecal microbiota were more likely to arise in LGG-treated children with CF (P = 0.04). Children with Bifidobacteria-dominated gut microbiota had a reduced rate of pulmonary exacerbations (IRR = 0.55; 95% CI 0.25 to 0.82; P = 0.01), improved pulmonary function (+ 20.00% of predicted value FEV1; 95% CI 8.05 to 31.92; P = 0.001), lower intestinal inflammation (Calprotectin; Coef = − 16.53 μg g−1 feces; 95% CI − 26.80 to − 6.26; P = 0.002) and required fewer antibiotics (IRR = 0.43; 95% CI 0.22 to 0.69; P = 0.04) compared to children with Bacteroides-dominated microbiota who were less likely to have received LGG.
Conclusions
The majority of pediatric CF patients in this study possessed a Bacteroides- or Bifidobacteria-dominated gut microbiota. Bifidobacteria-dominated gut microbiota were more likely to be associated with LGG-supplementation and with better clinical outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-022-02078-9.
Keywords: Cystic fibrosis, microbiome, Lactobacillus rhamnosus GG
Background
Mutations in the cystic fibrosis transmembrane conductance regulator, the genetic hallmark of cystic fibrosis (CF), leads to increased mucus secretion across organ systems and mucosal surfaces resulting in altered microbial colonization. CF patients experience chronic pulmonary inflammation punctuated by pulmonary exacerbations primarily due to acute microbial infection, resulting in reduction in pulmonary function with disease progression. Because pulmonary exacerbations represent a major clinical manifestation of the disease, much effort has appropriately been focused on this organ system. However, mucin secretion abnormalities associated with CF are system-wide, including in the gastrointestinal tract [1] and CF patients commonly experience failure to thrive and low BMI, phenotypes associated with gut microbiome dysfunction [2, 3]. In addition, in an attempt to manage acute pulmonary infections, this patient population is frequently administered antimicrobials, resulting in gut microbiome perturbations which have been described both in adult [4, 5] and pediatric [6] patients with CF.
The gut microbiome has emerged as a key player in regulating host immunity [4], protecting against pathogen overgrowth [5], providing energy to the human host [7] and reacting to or modifying specific drugs, including antimicrobials [8]. More recently its role in modulating airway mucosal and hematopoietic immunity has been revealed in murine studies [9, 10]. In human cohorts relationships between early life gut microbiota and subsequent airway disease development have been observed and more recent studies have indicated that at least in early life, Lactobacillus rhamnosus GG supplementation modifies the microbiota composition of high-risk for asthma infants [7]. Thus, the emerging view is that the gut microbiome may represent a modifiable mediator of immune function that influences susceptibility to airway infection.
Recent studies in adults with CF demonstrated dysbiosis in the gut microbiota as well as measurable changes in functionality of the gut microbiota compared to non-CF controls [4, 5]. However, an association between the gut-microbiota and lung function or other clinical outcomes in CF patients has not been shown. Conflicting results from studies indicate that Lactobacillus rhamnosus GG (LGG) supplementation is effective in reducing pulmonary exacerbations in CF patients [8, 11–15]. At the cellular level it’s been shown that LGG supplementation affects the expression of genes related to immune response and inflammation in humans [16]. Within Cystic Fibrosis patients, it’s been reported that probiotic supplementation improves intestinal inflammation and function [11, 12, 17], and reduces the rate of pulmonary exacerbations [13–15], but there has been inconsistent findings for other clinical outcomes such as pulmonary function, hospitalizations, or days of prescribed antibiotics [13, 15, 18]. A recent randomized clinical trial found a lack of evidence that Lactobacillus rhamnosus strain GG (LGG) probiotic supplementation was effective in reducing pulmonary exacerbations and hospital admissions in children with Cystic Fibrosis overall [8]. LGG may be associated with changes in the gut microbiota in some but not all children, for several reasons including the composition of the pre-treatment gut microbiome [19, 20], lack of adherence to treatment, diet, or other environmental factors.
Here, we hypothesized that the composition of the pediatric CF gut microbiota is altered by LGG supplementation in some but not all subjects and that distinct post-supplementation microbiota would be evident and related to clinical features of disease. Using samples collected from a multi-center, double-blinded, randomized placebo-controlled trial (which showed no statistically significant association between treatment and clinical outcomes [8]), we assessed whether, rather than LGG supplementation per se, the specific type of microbiome that arises following daily probiotic supplementation relates to the primary and secondary clinical endpoints in this population.
Methods
Study design
The methods for the randomized clinical trial have been discussed in detail previously [8]. The University Federico II, Naples, Italy, ethical committee approved the study in accordance with the Declaration of Helsinki. Briefly, children aged 2 to 16 years with a confirmed diagnosis of CF were enrolled and randomized to receive daily oral supplementation of either LGG or placebo, and were followed for 12 months. Compliance was evaluated with the parents/caregivers of the enrolled children by counting empty and full capsules, however we do not have access to the compliance data.
Clinical outcomes and stool samples were obtained at the time of randomization as well 12 months after treatment was started. Clinical outcomes assessed included number of pulmonary exacerbations, pulmonary function measured by the patients first forced expiratory volume (FEV1), intestinal inflammation (fecal calprotectin), and hospitalizations within the preceding 6-months (yes/no).
Sample collection and processing
Stool swabs were transported in a nucleic acid preservative (RNALater, Ambion, CA) on dry ice. DNA was extracted from stool samples suing the MoBio fecal extraction kit (MoBIO, CA). The 16S rRNA gene was amplified (using extracted DNA as template) with universal bacterial primers Bact-27F 5′-AGAGTTTGATCCTGGCTCAG-3′ and Bact-1492R 5′-GGTTACCTTGTTACGAC TT-3′ and the high fidelity Takara Taq polymerase (Takara Mirus Bio Inc., WI). Reaction mixtures (50 μl final volume) contained 5 μl 10 × PCR buffer, 5 μl dNTPs (10 mM), 5 μl forward primer and reverse primer (25 pmol), 1 μl l Takara Taq polymerase (5 U μl−1) and 100 ng of template DNA. PCR was performed using an Eppendorf MasterCycler gradient PCR machine (Eppendorf, NY). Amplified products from samples were pooled using using the QIAquick gel extraction kit (Qiagen, CA) and DNA concentration determined by gel electrophoresis using the Invitrogen Low Mass Ladder (Invitrogen, CA). Following quantification and standardization of sample concentrations, each sample will be spiked with known concentrations of control oligonucleotides (ranging from 5.02 × 108 and 7.29 × 1010 molecules) that act as internal standards for normalization.
Statistical analysis
Continuous baseline characteristics were compared between the two treatment groups using Wilcoxon rank-sum and Fishers exact tests for categorical methods in StataIC (ver. 13). Richness (number of OTU’s), evenness (Pielou’s), and Phylogenetic (Faith’s) diversity indices were chosen to measure alpha diversity (measure to estimate diversity within a sample) by arm and visit. Beta Diversity (measure to estimate diversity between samples) was measured using weighted unifrac, unweighted unifrac, Bray Curtis, and Canberra indices. All diversity indices were constructed as distance matrices using QIIME (14). Relationships between bacterial community composition (beta diversity matrices) and clinical outcomes, dominant genus, family, order, and treatment were assessed using adonis found in the R-package, vegan. To assess if dominant genus was associated with the clinical outcomes reported at baseline and 12-month visits, we used a robust mixed effect model (negative binomial for count data, linear for continuous data, and logistic for binary data), clustering by patient, and controlling for age, gender, and sputum organisms. A zero-inflated negative binomial model was chosen for modeling the counts of exacerbations due to over dispersion and excess of zeros, while negative binomial was chosen for days of prescribed antibiotics. And a Fishers Exact test was used to compare dominant taxa found in samples treated with placebo versus probiotics.
Results
Study cohort characteristics
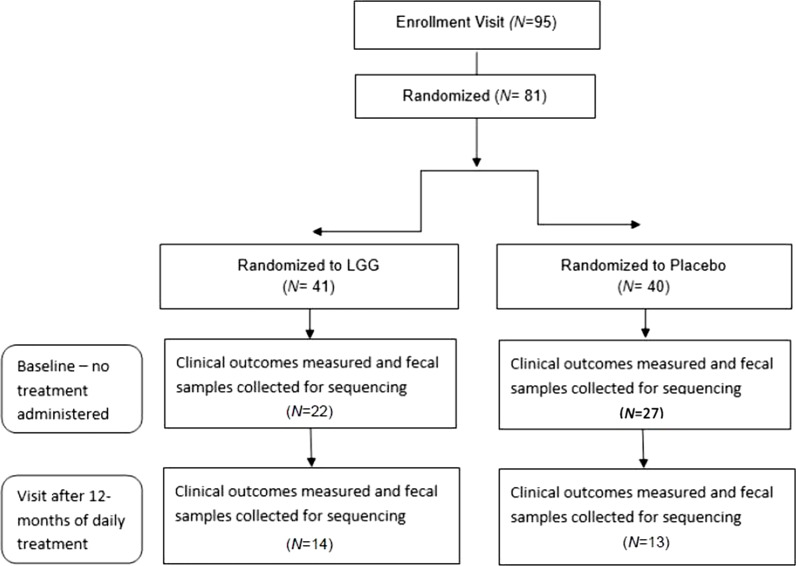
At the baseline (pre-treatment) visit, 50 patients had fecal samples collected and profiled for bacterial microbiota. Of these, 44% were randomized to LGG treatment (N = 22) and 54% to placebo (N = 27; Fig. 1) and 1 was missing treatment assignment data; enrollment sites included Firenze (N = 17, 34%), Milano (N = 19, 38%), and Napoli (N = 14, 28%), Italy. Baseline demographic, clinical and microbiota characteristics are outlined in Table 1; no significant difference was identified at baseline between those who were randomized to the LGG or placebo arms of the trial. Amongst those enrolled, 25 were male, 24 were female, with an overall mean age of 8.4 years and a mean BMI of 17.3 kg/m2. Clinical culture identified Staphylococcus aureus, Pseudomonas aeruginosa and Stenotrophomonas maltophilia as the predominant bacterial pathogens cultured from sputum, however at baseline, no significant difference in the frequency of culture-positivity for these pathogens was observed between treatment groups.
Fig. 1.
Flowchart
Table 1.
Baseline characteristics measured prior to treatment assignment of patients who had fecal samples processed
| Baseline characteristic | LGG (N = 22) | Placebo (N = 28) | Totalc | pa,b |
|---|---|---|---|---|
| Demographics | ||||
| Sex, No. males (%) | 10 (45%) | 16 (57%) | 26 (52%) | 0.57 |
| Age (years), median (IQR) | 9.5 (4.2, 13.0) | 7.1 (4.0, 12.3) | 7.4 (4.1, 12.8) | 0.39 |
| Weight (kg), median (IQR) | 32 (18.8, 40.2) | 24.3 (16.3, 40.7) | 30.7 (17.5, 40.2) | 0.39 |
| Height (cm), median (IQR) | 140.0 (110.0, 153.0) | 123.5 (101.5, 152.0) | 128.0 (105.0, 153.0) | 0.23 |
| BMI Z-scoresb, mean (SD) | 0.06 (1.15) | 0.60 (1.07) | 0.36 (1.13) | 0.10 |
| Clinical features | ||||
| Pulmonary exacerbations, yes/no (%) | 12/10 (55%) | 22/6 (79%) | 34/16 (68%) | 0.13 |
| FEV1 (median, IQR) | 88.8 (67.5, 96.1) | 89.6 (80.0, 108) | 89.6 (80, 100) | 0.42 |
| Calprotectin (median, IQR) | 77.0 (35.0, 240.0) | 71.9(15.0, 138.0) | 75.0 (28.0, 149.7) | 0.37 |
| Days on antibiotics in past 6 mos, median (IQR) | 7 (0, 22) | 15 (2, 25) | 10 (0, 24.5) | 0.18 |
| Hospitalizations in past 6 mos, yes/no (%) | 3/19 (14%) | 3/25 (11%) | 6/44 (12%) | 1 |
| Microbiological | ||||
| Sputum organism | ||||
| P. aeruginosa, yes/no (%) | 5/16 (24%) | 2/24 (8%) | 7/40 (15%) | 0.22 |
| S. aureus, yes/no (%) | 5/15 (25%) | 11/14 (44%) | 16/29 (36%) | 0.22 |
| S. maltophilia, yes/no (%) | 1/20 (5%) | 5/21 (19%) | 6/41 (13%) | 0.20 |
aContinuous and categorical characteristics compared using Wilcoxon rank-sum and Fishers exact tests, respectively
bBMI Z-scores compared using T-test
c50 Baseline samples were processed, but treatment assignment was missing for 1 sample at baseline
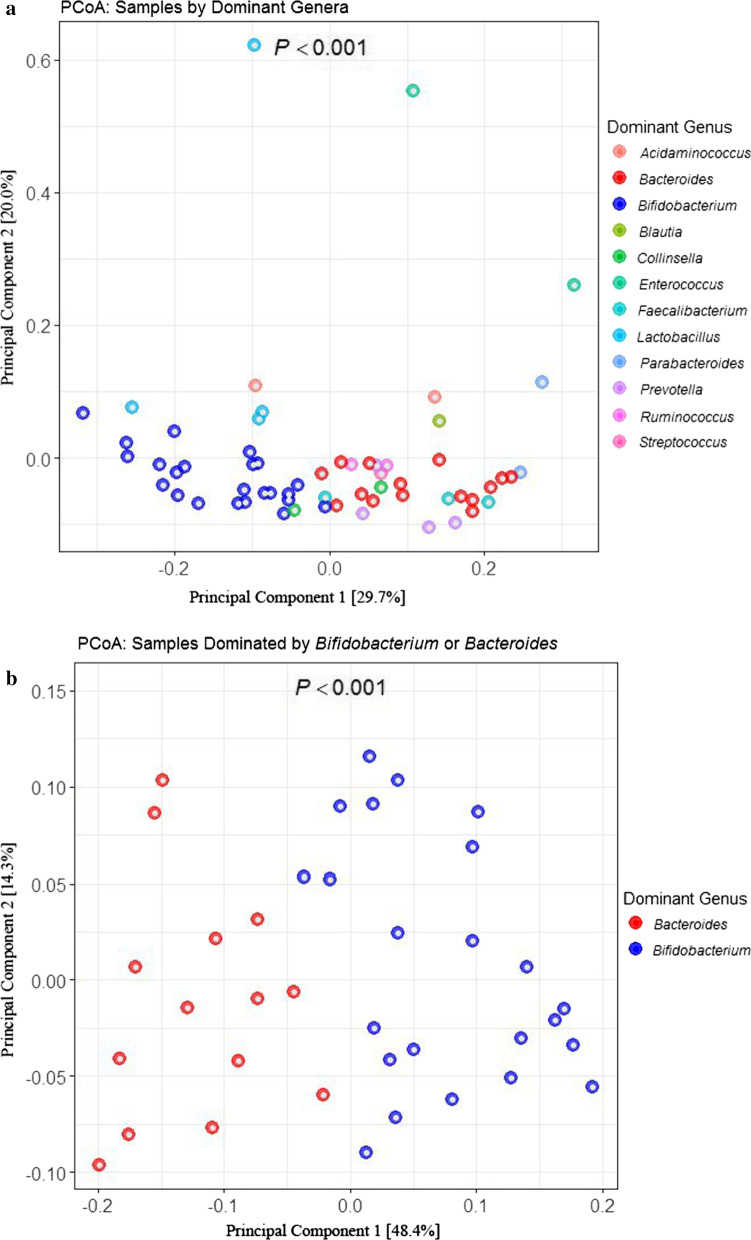
Upon examination of the gut microbiota of baseline samples (n = 49) we found no significant association between microbiota composition and treatment arm or any measured clinical factors, but did note that the dominant bacterial genus present explained a large proportion of variance in community composition (Permanova, R2 = 0.62, P = 0.001; Table 2, Fig. 2). Further examination of this data indicated that the majority of patients were dominated by taxa belonging to the Bifidobacteria (32%, N = 16) or Bacteroides (18%, N = 9), with smaller proportions of subjects exhibiting Prevotella (8%, N = 4), Lactobacillus (6%, N = 3), Enterococcus (4%, N = 2), Acidaminococcus (2%, N = 1), Blautia (2%, N = 1), Collinsella (2%, N = 1), Coprococcus (2%, N = 1), Faecalibacterium (2%, N = 1), Parabacteroides (2%, N = 1), Proteus (2%, N = 1), Ruminococcus (2%, N = 1) and Unidentified Genera (16%, N = 8).
Table 2.
Permutation Analysis of Variance (PERMANOVA) results using Adonis in the R environment to determine factors that significantly (P < 0.05) explained variation in microbiota beta diversity
| N | R2 | P value | |
|---|---|---|---|
| Baseline | |||
| Dominant genus* (category) | 50 | 0.62 | 0.0001 |
| Exacerbations, (N) | 50 | 0.01 | 0.81 |
| Hospitalization (yes/no) | 50 | 0.01 | 0.70 |
| Calprotectin (μg/g of feces) | 46 | 0.01 | 0.91 |
| Days prescribed antibiotic coverage (N) | 50 | 0.01 | 0.73 |
| Treatment (LGG or Placebo) | 49 | 0.03 | 0.64 |
| 12-Month visit | |||
| Dominant genus* (category) | 27 | 0.53 | 0.0001 |
| Exacerbations, (N) | 19 | 0.18 | 0.003 |
| Hospitalization (yes/no) | 19 | 0.13 | 0.02 |
| Calprotectin (μg/g of feces) | 23 | 0.09 | 0.048 |
| Days prescribed antibiotic coverage (N) | 19 | 0.19 | 0.001 |
| Treatment (LGG or Placebo) | 26 | 0.03 | 0.71 |
*Based on a weighted Unifrac distance
Fig. 2.
a PCoA showing weighted UniFrac beta diversity for all baseline and 12-month samples color-coded by dominant genus found in the sample. Principal components 1 and 2 [amount of variance explained by PC] are shown on the X and Y axis respectively. b PCoA showing weighted UniFrac beta diversity for all baseline and 12-month samples that were either dominated by Bacteroides or Bifidobacterium
LGG supplementation of CF patients does not lead to significant differences in fecal alpha diversity
Of the 95 CF patients enrolled in the trial, samples for stool microbiota analyses included 50 at baseline and 27 at 12-months of which 24 represented paired repeated samples from individual patients. Data describing treatment assignment for 1 patient of the 24 paired repeated samples was also missing. Among the 23 patients with complete data 11 were treated for with LGG probiotics for 1 year and 12 were treated with placebo for 1 year. Alpha diversity indices, including richness (number of observed operational taxonomic units; OTUs), Pielou’s evenness (distribution of OTUs within a sample) and Faith’s phylogenetic diversity (which incorporates community richness, evenness and phylogenetic relatedness) were calculated for each sample and compared between the treatment groups cross-sectionally using all samples available and longitudinally, using the sub-set of paired samples (Additional file 1: Table S2). No significant differences in fecal alpha diversity was observed between treatment groups when all samples were considered, or when paired samples were examined by linear mixed effect model testing (Additional file 1: Table S2). However, we noted that, even in this small study, LGG-treated children exhibited a trend towards increased fecal microbiota alpha diversity over time (Additional file 2: Fig. S1).
CF fecal beta diversity is related to dominant bacterial genus and 12-month clinical outcomes
Because the effect of LGG supplementation has recently been shown to impact only a discrete population of bacterial taxa within the early-life gut microbiota [7], we hypothesized that this may also be true in pediatric CF patient populations. To address this hypothesis, we first examined fecal bacterial beta-diversity to determine whether significant compositional differences in gut microbiota existed within the baseline-visit samples or 12-month-visit samples. The largest proportion of variance (R-squared) was attributed to the dominant bacterial genus in each samples at both baseline or 12 month visits (Table 2). This indicates that each dominant bacterial genus co-associates with a distinct group of bacteria, and that these relatively reproducible gut microbiota structures explain a large proportion of variance in gut microbiota profiles of pediatric CF patients in our study (Fig. 2). Next, we examined the relationship between beta diversity and clinical outcomes at each visit. There were no associations between baseline gut microbiota beta diversity and clinical outcomes also measured at baseline. However, at 12 months, by the end of the supplementation period, a number of clinical outcomes measured at the 12-month visit significantly associated with gut bacterial beta-diversity at this timepoint. These included the number of exacerbations (R2 = 0.18, P = 0.003), hospitalization (R2 = 0.13; P = 0.02), fecal calprotectin concentration (R2 = 0.09; P = 0.048), and days of prescribed antibiotic coverage (R2 = 0.19; P = 0.001; Table 2). These data were largely consistent irrespective of whether a weighted UniFrac, Canberra, or Bray–Curtis distance matrix was employed (Additional file 3: Table S1).
Gut microbiota dominated by Bifidobacterium or Bacteroides differentially associate with clinical outcomes
Since the bacterial genus dominating the microbiota in our pediatric CF patient explained the largest proportion of beta-diversity variance and microbiota composition was related to clinical outcomes at the end of the intervention period, we next determined which bacterial genera dominated pediatric CF patient gut microbiota. Amongst all baseline and 12-month fecal samples profiled (N = 77), the majority were dominated by Bifidobacterium (29%; N = 22) or Bacteroides (19%, N = 15; Fig. 2). The rate of pulmonary exacerbations among those patients with fecal microbiota dominated by Bifidobacterium was 0.54 less than those dominated with Bacteroides (IRR = 0.45; 95% CI 0.25 to 0.82; P = 0.01; Table 3) shown in Table 3. Consistent with this observation, patients with gut microbiota dominated by Bifidobacterium had 20.0% increase in FEV1 compared to those dominated by Bacteroides (Coef = 20.00; 95% CI 8.05 to 31.92; P = 0.001; Table 3). Patients with Bifidobacterium-dominated gut microbiota also had significantly lower concentrations of fecal calprotectin (− 16.53 μg g−1 feces; − 26.80 to − 6.26; P = 0.002), and fewer days of prescribed antibiotics (IRR = 0.47; 95% CI 0.22 to 0.97; P = 0.04; Table 3).
Table 3.
Comparison of clinical outcomes in those patients who had gut dominated by bifidobacterium versus bacteroides genera using mixed effect models correcting for age, gender, sputum organisms (except for FEV1) and clustering by patient
| Clinical outcomes | Dominant genus | Bifidobacterium versus Bacteroides (95% CI) | P | |||
|---|---|---|---|---|---|---|
| Bifidobacterium | Bacteroides | |||||
| N | Adjusted estimate ± sd | N | Adjusted estimate ± sd | |||
| Pulmonary exacerbations (N) | 19 | 1.6 ± 1.5 | 13 | 2.9 ± 1.6 | IRR = 0.55 (0.25 to 0.82) | 0.01 |
| FEV1 (percentage of predicted value) | 22 | 103.48 ± 1.39 | 15 | 83.97 ± 1.48 | Coef = 20.00 (8.05 to 31.92) | 0.001 |
| Calprotectin (µg/g of feces) | 19 | 80.05 ± 61.43 | 13 | 107.73 ± 37.97 | Coef = − 16.53 (− 26.80 to − 6.26) | 0.002 |
| Days of prescribed antibiotics in past 6-months (N) | 19 | 25.8 ± 6.8 | 13 | 60.3 ± 5.9 | IRR = 0.43 (0.22 to 0.69) | 0.04 |
Data includes dominant genus and clinical outcomes reported at baseline and 12-month visits
Distribution of dominant genus differs by LGG treatment
In the 23 paired samples sequenced at baseline and 12-months, there was a statistically significant difference between treatment arms when comparing the distribution of dominant genus found in the microbiome after 12 months of treatment (P = 0.005). No difference in the distribution of dominant genus was evident at baseline prior to treatment (P = 1). Patients with undefined genus at either visit (n = 7) were excluded from the analysis. All taxonomic ranks were tested to account for any questions about undefined taxonomic levels within samples. The demonstrated difference between treatment groups held for taxa levels: order (P = 0.02), class (P = 0.016), and phylum (P = 0.03), but not at the family level (P = 0.11). No difference in the distribution of dominant taxa was evident at baseline prior to treatment indicating the randomization balanced treatment between the smaller set of 23 subjects with paired data; family (P = 1), order (P = 1), class (P = 1), and phylum (P = 0.7).
Those treated with LGG transitioned to either Bifidobacterium- (N = 4), Faecalibacerium- (N = 2), Collinsella- (N = 1), or Acidaminococcus-dominated (N = 1) microbiota following 12-months of LGG supplementation (4 samples were undefined at the genus level). In comparison, the majority of placebo-treated patients transitioned to a Bacteroides- (N = 4), Parabaceroides- (N = 1), Streptococcus- (N = 1), Enterococcus- (N = 1), Ruminococcus- (N = 1), Proteus- (N = 1), or Bifidobacterium-dominated (N = 1) gut microbiota within this same 12-month period (Additional file 4: Fig. S2). These data suggest that daily supplementation with LGG promotes development of distinct gut microbiota, more frequently dominated by Bifidobacteria.
Discussion
Our analysis of the gut microbiota of 50 children aged 2–16 years with cystic fibrosis who underwent daily probiotic or placebo supplementation indicate there was not a statistically significant association in alpha or beta diversity across treatment groups. A relatively small number of distinct bacterial genera dominated the gut microbiota of the CF patients and explained the largest proportion of beta-diversity variance. Gut analysis based on these distinct microbiota states indicated that they differed in their exacerbation frequency, pulmonary function, intestinal inflammation and need for antibiotic treatment. LGG supplementation was associated with Bifidobacterium-dominated fecal microbiota, whereas placebo-supplemented patients typically exhibited Bacteroides-dominated microbiota. By the end of the intervention period, microbiota composition was related to clinical outcomes, with Bifidobacteria-dominated gut microbiota associated with decreased exacerbation, intestinal inflammation and antibiotic administration coupled with increased pulmonary function. Although, not statistically significant, LGG treatment increased alpha diversity over a period of 12 months. This is the first RCT that treated patients daily with LGG for an extended time period of 1 year. Sample size was limited at 12 months, which may have limited our ability to detect significant findings.
Beta-diversity variance related to several clinical outcomes as well as the dominant genus detected. Previous studies have shown that distinct bacterial microbiota compositions, each dominated by different bacteria, associate with clinical outcomes [21–23]. Here we strengthen that argument, showing that children with Bifidobacteria-dominated gut microbiota experience fewer exacerbations and hospitalizations, require less antibiotic coverage, and have decreased inflammation in the gut. Moreover, we show that LGG supplementation of pediatric CF patients is associated with increased prevalence of Bifidobacteria-dominated gut microbiota, offering a plausible explanation for why some children administered LGG in this trial exhibit improved clinical and immunological outcomes.
Limitations of this study include its small size and that it contains subgroup analyses which both limit the number of samples as well as generalizability of findings. Additionally, there were significantly less samples available from the 12-month visit, however balance of treatment groups was maintained in this smaller group. Additionally, extrinsic factors that can shape the gut microbiota such as diet were not captured in this study. Nonetheless, these findings suggest that daily supplementation with LGG is associated with Bifidobacteria-dominated fecal microbiota in some, but not all CF children and that Bifidobacteria-dominated microbiota are associated with improved clinical outcomes. These findings require validation in larger clinical trials. They may also offer an explanation for the varied response across CF patient populations to probiotic supplementation and provide early evidence that gut microbiome manipulation is associated with improvements in airway disease status in CF patients.
Supplementary Information
Additional file 1. Table S2. Alpha diversity (Richness, Pielou's Evenness and Faith's phylogenetic diversity) indices of all samples analyzed cross-sectionally between treated and placebo groups.
Additional file 2. Figure S1. Alpha Diversity by treatment group over a 12 month period.
Additional file 3. Table S1. Beta Diversity: Permutation Analysis of Variance (PERMANOVA) results using Adonis in the R environment to determine factors that significantly (P < 0.05) explained variation in microbiota beta diversity.
Additional file 4. Figure S2. a–e Shift table showing composition of gut microbiota of patients (n = 23) having paired samples at baseline (displayed horizontally) and 12-month visit (displayed vertically) are shown at each taxonomic level. Red shading represents patients treated with LGG probiotics (N = 11), blue shading represents patient’s treated with placebo (N = 12). Patients with undefined taxonomy at either visit were excluded from the analysis. In the 23 patients (that had samples sequenced at baseline and 12-months), there was a statistically significant difference between the distribution of dominant taxa by treatment arms found in the microbiome after 12 months of treatment at the genera (P = 0.005), order (P = 0.02), class (P = 0.016), and phylum (P = 0.03) levels, but not at the family level (P = 0.11). All ranks were tested to account for any questions about undefined taxonomic levels within samples. No difference in the distribution of dominant genus was evident at baseline prior to treatment indicating the randomization held for this subset of patients that had fecal samples at 12-months at the genera (P = 1), family (P = 1), order (P = 1), class (P = 1), and phylum (P = 0.7)
Acknowledgements
We would like to acknowledge the generous provision of samples and data from Alfredo Guarino and Eugenia Bruzzese for this study.
Author contributions
SVL conceived and designed the study, interpreted the data and helped draft and substantively revised the manuscript; KR performed statistical analyses, interpreted the data, and drafted and substantively revised the manuscript; CS generated microbiota data for the study; KM performed statistical analyses and interpreted the data; AP generated microbiota data and interpreted for the study. All authors read and approved the manuscript.
Funding
The study was supported by a Cystic Fibrosis Foundation award CA0073548.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The University Federico II, Naples, Italy, ethical committee approved the study in accordance with the Declaration of Helsinki. Additionally, the ethical committees of the Department of Paediatric Medicine, CF Center, “A. Meyer” Children’s Hospital in Florence, Italy and Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena in Milano, Italy approved the study. Enrolment was allowed only after signed written informed consent was obtained from patients and their caregivers/parents. At enrolment, each subject received an informative letter from his/her general practitioner. All enrolled patients were informed about the rationale, the type of intervention and the specific aims of the study by the doctors of the Regional CF Clinical Center. Medical doctors discussed with the patient and parents about the utility and the burden of intervention related to their participation to the study. During the assessment period patients could understand the required task and decide about their participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Lisle RC, Borowitz D. The cystic fibrosis intestine. Cold Spring Harb Perspect Med. 2013;3(9):a009753. doi: 10.1101/cshperspect.a009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The human microbiome and child growth—first 1000 days and beyond. Trends Microbiol. 2019 doi: 10.1016/j.tim.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke DG, et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol. 2017 doi: 10.1186/s12866-017-0968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouhy F, et al. A pilot study demonstrating the altered gut microbiota functionality in stable adults with Cystic Fibrosis. Sci Rep. 2017 doi: 10.1038/s41598-017-06880-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen S, et al. Disrupted progression of the intestinal microbiota with age in children with cystic fibrosis. Sci Rep. 2016 doi: 10.1038/srep24857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durack J, et al. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat Commun. 2018 doi: 10.1038/s41467-018-03157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruzzese E, et al. Lack of efficacy of Lactobacillus GG in reducing pulmonary exacerbations and hospital admissions in children with cystic fibrosis: a randomised placebo controlled trial. J Cyst Fibros. 2018 doi: 10.1016/j.jcf.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca W, et al. Lactobacillus johnsonii supplementation attenuates respiratory viral infection via metabolic reprogramming and immune cell modulation. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadava K, et al. Microbiota promotes chronic pulmonary inflammation by enhancing IL-17A and autoantibodies. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201504-0779OC. [DOI] [PubMed] [Google Scholar]
- 11.del Campo R, et al. Improvement of digestive health and reduction in proteobacterial populations in the gut microbiota of cystic fibrosis patients using a Lactobacillus reuteri probiotic preparation: a double blind prospective study. J Cyst Fibros. 2014 doi: 10.1016/j.jcf.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Infante Pina D, et al. Improvement of intestinal function in cystic fibrosis patients using probiotics. An Pediatr. 2008 doi: 10.1016/S1695-4033(08)75231-7. [DOI] [PubMed] [Google Scholar]
- 13.Weiss B, Bujanover Y, Yahav Y, Vilozni D, Fireman E, Efrati O. Probiotic supplementation affects pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Pediatr Pulmonol. 2010 doi: 10.1002/ppul.21138. [DOI] [PubMed] [Google Scholar]
- 14.Jafari SA, Mehdizadeh-Hakkak A, Kianifar HR, Hebrani P, Ahanchian H, Abbasnejad E. Effects of probiotics on quality of life in children with cystic fibrosis; a randomized controlled trial. Iran J Pediatr. 2013;23:669. [PMC free article] [PubMed] [Google Scholar]
- 15.Di Nardo G, et al. Lactobacillus reuteri ATCC55730 in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2014 doi: 10.1097/MPG.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 16.Di Caro S, et al. Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Dig Liver Dis. 2005 doi: 10.1016/j.dld.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Bruzzese E, et al. Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment Pharmacol Ther. 2004 doi: 10.1111/j.1365-2036.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- 18.Bruzzese E, et al. Effect of Lactobacillus GG supplementation on pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Clin Nutr. 2007 doi: 10.1016/j.clnu.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Zmora N, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–1405.e21. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 20.Suez J, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406–1423.e16. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 21.Cope EK, Goldberg AN, Pletcher SD, Lynch SV. Compositionally and functionally distinct sinus microbiota in chronic rhinosinusitis patients have immunological and clinically divergent consequences. Microbiome. 2017 doi: 10.1186/s40168-017-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015 doi: 10.1016/j.chom.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCauley K, et al. Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J Allergy Clin Immunol. 2019 doi: 10.1016/j.jaci.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S2. Alpha diversity (Richness, Pielou's Evenness and Faith's phylogenetic diversity) indices of all samples analyzed cross-sectionally between treated and placebo groups.
Additional file 2. Figure S1. Alpha Diversity by treatment group over a 12 month period.
Additional file 3. Table S1. Beta Diversity: Permutation Analysis of Variance (PERMANOVA) results using Adonis in the R environment to determine factors that significantly (P < 0.05) explained variation in microbiota beta diversity.
Additional file 4. Figure S2. a–e Shift table showing composition of gut microbiota of patients (n = 23) having paired samples at baseline (displayed horizontally) and 12-month visit (displayed vertically) are shown at each taxonomic level. Red shading represents patients treated with LGG probiotics (N = 11), blue shading represents patient’s treated with placebo (N = 12). Patients with undefined taxonomy at either visit were excluded from the analysis. In the 23 patients (that had samples sequenced at baseline and 12-months), there was a statistically significant difference between the distribution of dominant taxa by treatment arms found in the microbiome after 12 months of treatment at the genera (P = 0.005), order (P = 0.02), class (P = 0.016), and phylum (P = 0.03) levels, but not at the family level (P = 0.11). All ranks were tested to account for any questions about undefined taxonomic levels within samples. No difference in the distribution of dominant genus was evident at baseline prior to treatment indicating the randomization held for this subset of patients that had fecal samples at 12-months at the genera (P = 1), family (P = 1), order (P = 1), class (P = 1), and phylum (P = 0.7)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.