Abstract
The imbalance between reactive oxygen species (ROS) production and antioxidant defense systems leads to macromolecule and tissue damage as a result of cellular oxidative stress. This phenomenon is considered a key factor in fatigue and muscle damage following chronic or high-intensity physical exercise. In the present study, the antioxidant effect of Moringa oleifera leaf extract (MOLE) was evaluated in C2C12 myotubes exposed to an elevated hydrogen peroxide (H2O2) insult. The capacity of the extract to influence the myotube redox status was evaluated through an analysis of the total antioxidant capacity (TAC), glutathione homeostasis (GSH and GSSG), total free thiols (TFT), and thioredoxin (Trx) activity, as well as the enzyme activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) and transferase (GST). Moreover, the ability of MOLE to mitigate the stress-induced peroxidation of lipids and oxidative damage (TBARS and protein carbonyls) was also evaluated. Our data demonstrate that MOLE pre-treatment mitigates the highly stressful effects of H2O2 in myotubes (1 mM) by restoring the redox status (TFT, Trx, and GSH/GSSG ratio) and increasing the antioxidant enzymatic system (CAT, SOD, GPx, GST), thereby significantly reducing the TBARs and PrCAR levels. Our study provides evidence that MOLE supplementation has antioxidant potential, allowing myotubes better able to cope with an oxidative insult and, therefore, could represent a useful nutritional strategy for the preservation of muscle well-being.
Keywords: Moringa oleifera leaf extract (MOLE), C2C12 skeletal muscle cells, redox status, enzymatic antioxidant system, oxidative stress
1. Introduction
Skeletal muscles represent 30–40% of the total body mass and have a key role in the well-being of the entire organism. They are involved in movement, heat production, breathing, and have a primary role in maintaining glycemic levels and an efficient body energy balance. Skeletal muscles are very variable in ATP request, varying from a resting, low-energy consuming condition to a very high-energy request following intense muscle contraction [1]. To fulfill energy demand, more oxygen is released from hemoglobin, and in mitochondria, oxidative phosphorylation increases their working capacity, producing the necessary ATP [2]. At the same time, mitochondria are also the main reactive oxygen species (ROS) generators through the leak of electrons in complexes I and III of the electron transport chain [3].
Among ROS, hydrogen peroxide (H2O2) is a Janus-faced molecule. It exerts an opposite role on skeletal muscle function depending on its concentration [4,5,6,7]. Levels from low to moderate act as signals for cell adaptation and are necessary for muscle growth and repair [4,5,6,7]. On the contrary, high levels of H2O2 with the consequent formation of oxidized macromolecules may contribute to the loss of myoblast function, increase cell death, and worsen muscle-repair mechanisms [8,9,10]. Muscle contractions during physical exercise, especially intense or unaccustomed, are usually accompanied by the high production of ROS that ultimately leads to oxidative stress, which potentially results in myofiber damage evidenced by increased biomarkers of oxidation in both skeletal muscle and blood [11,12]. Moreover, oxidative stress is one of the key factors for the development of fatigue, a phenomenon that many sports practitioners experience and that leads to a deterioration of exercise performance. In addition, oxidative stress has been reported as being involved in such diverse phenomena as aging, diabetes mellitus, cancers, and Alzheimer’s disease [13,14].
In physiological conditions, ROS are maintained at a low level by the action of several types of antioxidants. Among them, an important role is played by natural dietary antioxidants (e.g., vitamins, polyphenols, flavonoids), endogenous antioxidant enzymes (e.g., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione transferase (GST)), and endogenous antioxidant molecules (mainly by the thiol system, by glutathione (GSH) and thioredoxin (Trx)) [15,16,17,18,19,20].
The disruption of the homeostasis of cellular antioxidant systems is the most central feature of oxidative stress occurrence. In the effort to prevent and/or contain such harmful imbalances, researchers have investigated nutritional strategies to improve physical capabilities, such as a reduction in fatigue and increased exercise endurance [21,22,23,24].
During recent decades, the natural products of Moringa oleifera Lam. (Family Moringaceae; Order Brassicaceae) have been extensively investigated in various biological systems [25,26,27,28,29].
It has been shown that Moringa oleifera has antioxidant features that are due to the many bioactive compounds present in different parts of the plant. Specifically, tannins, saponins, flavonoids, and terpenoids are very well represented, especially in the leaves. These molecules demonstrate beneficial features acting as antioxidants, and/or antimicrobial, and/or anti-carcinogenic agents [30,31]. Moreover, these molecules have proven themselves to be effective in treating several chronic pre-pathological conditions. In fact, hypercholesterolemia, insulin resistance, and inflammation, whose onset is based on the increase in reactive oxygen species, have been shown to be reduced by the effect of flavonoids and other glycosides [32,33,34,35]. Moreover, phenolic acids (e.g., chlorogenic acid and ferulic acid) are also present at moderate concentrations in extracts of Moringa oleifera leaves. They are known to act as primary antioxidants, for example, by inactivating lipid free radicals or by acting in the prevention of the decomposition of hydroperoxides into free radicals [36,37,38].
Recently we demonstrated that Moringa oleifera leaf extract (MOLE) improved oxidative capacity in C2C12 myotubes by the activation of the SIRT1-PPARα pathway leading the cells to an increased reliance on lipid metabolism [36]. Moreover, MOLE has a beneficial effect on the antioxidant system of skeletal muscle cells through the induction of the Nrf2-HO-1 pathway [29].
Based on these findings, we have hypothesized that MOLE, having the ability to switch on the pathways that upregulate the antioxidant defense system, could efficiently protect skeletal muscle cells subjected to a pro-oxidant environment.
In the present study, the goal is to provide scientific evidence regarding the effect of Moringa oleifera leaf extract on restoring redox balance after a strong oxidative insult that mimicks the distress condition that happens in high, intense muscle contractions. To this end, differentiated C2C12 skeletal muscle cells were treated with MOLE for 24 h and then exposed to 1 mM H2O2 for 1 h and analyzed for: (a) cell viability and the total antioxidant capacity (TAC), an assay that measures lipo- and hydro-philic antioxidants; (b) GSH homeostasis, the level of intracellular free thiols and the activity of thioredoxin, as markers of redox status; (c) antioxidant enzymatic defense network: superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione transferase (GST) activities; and (d) protein carbonyls (PrCar) and lipid peroxidation (TBARS) as markers of oxidative damage.
2. Materials and Methods
All chemical reagents, unless otherwise specified, were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA).
2.1. MOLE: Methanolic Extract of Moringa oleifera Leaves
One gram of Moringa oleifera leaf powder (PureBodhi Nutraceuticals Ltd., London, UK) was dissolved in 10 mL of methanol (100%) and then sonicated (Vibra-Cell CV 18 SONICS VX 11, Sonics & Materials, Newtown, CT, USA) twice for 10 min at +4 °C. The obtained extract was then centrifuged (2000× g for 10 min at +4 °C) and then collected and stored at −20 °C (stock solution).
2.2. MOLE Qualitative Profiling
Qualitative profiling of the tested MOLE extract was obtained by ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC/Q-TOF-MS, SCIEX X500B, AB SCIEX GmbH, Landwehrstraße 54, Darmstadt, Germany) technique with a high-resolution SCIEX X500B QTOF electrospray ion source operated in negative ion mode as previously described [39]. The digital fingerprint of the sample was characterized with SWATH® analysis.
The data obtained were processed using SCIEXOS Software 2.1 (AB SCIEXGmbH, Landwehrstraße 54, Darmstadt, Germany), and the SCIEX Natural Products 2.1 Library (AB SCIEX GmbH, Landwehrstraße 54, Darmstadt, Germany) was used to search for database compound spectra.
2.3. Cell Cultures
C2C12 myoblasts (ATCC, Manassas, VA, USA) were cultured, as previously described [29]. Preconfluent cells (85% confluency) were induced to differentiate by lowering the FBS to 2% in a culture medium. Cell differentiation was monitored by microscopy and assessed by myogenin and MHC expression by Western blot analysis [40].
C2C12 myotubes were treated with H2O2 dose-dependently (0.1–1 mM) for 1 h to verify cytotoxicity. The methyl-thiazolyl-diphenyl-tetrazolium bromide (MTT) assay was performed to test cell viability [41]. Then, 1 mM H2O2 was chosen for further experiments. Then, the cells were treated with working solutions with 1/1000 or 1/100 MOLE solutions or vehicle-only (methanol) in a culture media for 24 h. In these working solutions, the methanol concentration (0.1%, v/v) does not have any specific effect on myotubes.
Subsequently, hydrogen peroxide (1 mM) was added to samples pre-treated with vehicle or MOLE for a further hour, MTT assays were performed, and the samples were prepared for biochemical analysis.
The cells were trypsinized, collected, and centrifuged at 1200× g for 10 min at room temperature. Gentle lysis was then performed, and the lysate obtained was then used for biochemical analysis or tested for protein content using the Bradford method (Sigma-Aldrich, St. Louis, MO, USA).
2.4. TAC: Trolox® Equivalents Antioxidant Capacity
The total antioxidant capacity (TAC) was performed spectrophotometrically by the Trolox® equivalents antioxidant capacity assay, as previously described [42]. This assay evaluates the ability of cell lysates to prevent ABTS+ radical formation in ABTS-metMyo-PBS buffer after the addition of H2O2 (450 μM) compared to the vitamin E analogue Trolox® standards.
The variation in absorbance detected at 734 nm was compared to those obtained using Trolox® standards (0.125–2.0 mM) and expressed as micromoles/mg of protein tested.
To check the efficiency of the extraction method, 10 μl of different MOLE stock solution dilutions (0.015, 0.075, 0.15, and 1.5 mg/mL of dried powder corresponding to 1/1000–1/500–1/100, and 1/10 working solution, respectively) were tested and the antioxidant capacity obtained were comparable with those already reported [29,39] (data not shown).
2.5. GSH, GSSG and GSH/GSSG Evaluation
Myotubes reduced (GSH), oxidized (GSSG) glutathione contents, and the GSH/GSSG ratio were quantified by a DTNB–glutathione reductase recycling assay, as previously described [43].
GSSG was selectively measured in samples in which reduced glutathione was masked using a 2-vinylpyridine (2%) pretreatment. The variation in TNB formation absorbance was followed at 412 nm and compared to those obtained by using glutathione standards, and the results were normalized for protein content.
2.6. Total Free Thiol Levels and Thioredoxin Activity Analysis
The cellular total free thiol concentration was quantified by the Ellman assay, as previously described [44], by following the TNB anion formation upon the reaction of thiols with DTNB. The mean intrinsic absorbance was subtracted from the mean absorbance of TNB release. The molar concentration of the thiols was calculated from the molar absorbance of the TNB anion and expressed as micromole –SH/g cell lysate amounts [44].
Thioredoxin activity was measured using the insulin disulfide reduction assay according to Holmgren and Björnstedt [45]. The total cellular protein lysate was incubated with reaction buffer (50 mM HEPES pH 7.6, 1 mM EDTA, 1 mg/mL BSA, 2 mM DTT) at 37 °C for 15 min and then incubated with thioredoxin reductase (American Diagnostica Inc., Greenwich, CT, USA) in a reaction buffer (20 mM HEPES pH 7.6, 1 mM EDTA, 200 μM NADPH and 0.3 mM insulin) at 37 °C for 20 min. After that, the stop mix buffer (6 M guanidine HCl, 1 mM DTNB in 0.2 M Tris-HCl pH 8.0) was added, and the absorption at 412 nm was measured. The Trx activity level was compared with standards and expressed as micrograms of Trx per milligram of the total cellular proteins tested.
2.7. Lipid and Protein Oxidation
Thiobarbituric acid reactive substances. The TBARS levels were assayed by spectrophotometric analysis [46]. The methodology measures malondialdehyde (MDA) and other aldehydes produced by lipid peroxidation induced by hydroxyl free radicals. Sample absorbance was determined spectrophotometrically at 535 nm and compared to a standard MDA (1,1,3,3-tetramethoxypropane) solution. The levels of TBARS were expressed in terms of nmol/mg protein [46].
Protein carbonyls. The protein carbonyl levels (PrCAR) were determined by measuring the reactivity of carbonyl derivatives with 2,4-dinitrophenylhydrazine (DNPH) according to Colamartino et al. [47]. The protein carbonyl content was calculated from the absorbance measurement of the conjugated DNTB at 380 nm. The millimolar extinction coefficient for DNPH is ε380 = 22.00. The PrCAR content was expressed in terms of nmol/mg protein [47].
2.8. Antioxidant Enzymatic Activities
Intracellular superoxide dismutase, catalase, and glutathione peroxidase activities were assayed spectrophotometrically using commercial assay kits (Cayman Chemical Company, Ann Arbor, MI, USA) following the manufacturer’s instructions and the results were expressed as the units/milligrams of protein tested as previously described [44,46,48].
Intracellular glutathione transferase activity was assayed with spectrophotometric methods as previously described [49]. Cell extracts (100 μL) were incubated with 1 mM glutathione and 1 mM of 1-chloro-2,4-dinitrobenzene in 1 mL of 0.1 M potassium-phosphate buffer, pH 6.5. S-glutathionyl-2,4-dinitrobenzene formation was monitored at 340 nm (ε340 nm = 9600 M/cm). For each spectrophotometric determination, the spontaneous reaction of glutathione with 1-chloro-2,4-dinitrobenzene was subtracted. The GST activity was expressed in enzymatic units (U) at 37 °C and normalized for protein content.
2.9. Statistical Analysis
The distribution of the data was evaluated using the Kolmogorov–Smirnov test. All of the data are expressed as the means ± S.D. of three independent experiments, each performed in triplicate. One-way ANOVA for repeated measures and Bonferroni post-hoc analyses were performed in order to determine significant variation among groups for each variable tested. A value of p < 0.05 was accepted as statistically significant. The SPSS program for Windows (Version 17.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The analysis performed between untreated controls and control vehicles (CTRLm) showed no statistical differences for all variables tested (data not shown).
3. Results
3.1. MOLE Metabolomic Fingerprint
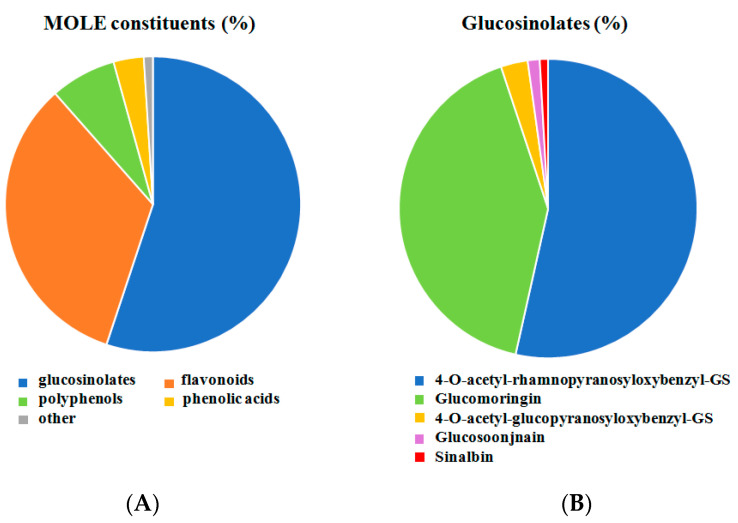
UHPLC-MS analysis highlights the presence of four main secondary metabolite groups in MOLE extract: glucosinolates, flavonoids, polyphenols, and phenolic acids. A digital record of the sample was acquired by SWATH® analysis with MS/MS spectral information for all of the detectable precursor ions in the defined mass range. SCIEX Natural The Products Library 2.1, a database for potential compound identification (library match score > 75% and mass error +/−2 ppm), was used for compound identification based on the product’s ion spectral information. About 27 secondary metabolites among flavonoids, polyphenols, and phenols were identified with a high library score (78–100%) [39].
Figure 1A shows the relative percentage of metabolite categories. Glucosinolates (55.1%), flavonoids (33.4%), polyphenols (7.2%), and phenolic acids (3.3%) were the most highly represented in the metabolomic fingerprint.
Figure 1.
Relative amounts of constituents of MOLE. Metabolomic analysis was performed on MOLE working solution. Relative percent of different categories of compound was shown in panel (A). SWATH MS/MS spectrum of MOLE extract was performed for glucosinolates analysis. Relative percent were shown in panel (B).
The most intense peaks were represented by GLs. The lack of reference standards for some GLs and as a consequence of their MS/MS spectral information, a loss of number in the number of identified GLs may occur. Then, a different approach was undertaken for their identification. These molecules are thioglucoside compounds containing a sulfated aldoxime moiety. Moreover, a variable side chain derived from amino acids is present. Glucosinolates have a particular chemical structure producing diagnostic typical fragment ions in the MS/MS spectra. In particular, the GLs sulfated glucose moiety (fragment 259.01 m/z) and a sulfate group (fragment 96.96 m/z) could be identified in spectra and assigned [50,51].
Glucosoonjnain (tR: 3.8; 1.3%), glucomoringin (tR: 4.2; 41.4%), sinalbin (tR: 4.3; 0.9%), 4-O-acetyl-rhamnopyranosyloxybenzylGS (tR: 6.0, 6.4, 7.7; 53.5%), and 4-O-acetyl-glucopyranosyloxybenzylGS (tR: 5.3, 7.0; 2.9%). were identified from ion chromatograms XIC, MS, and SWATH MS/MS spectrum for identified glucosinolates and expressed as a percentage (Figure 1B).
3.2. Cell Viability
Cell viability was assessed by the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay [52].
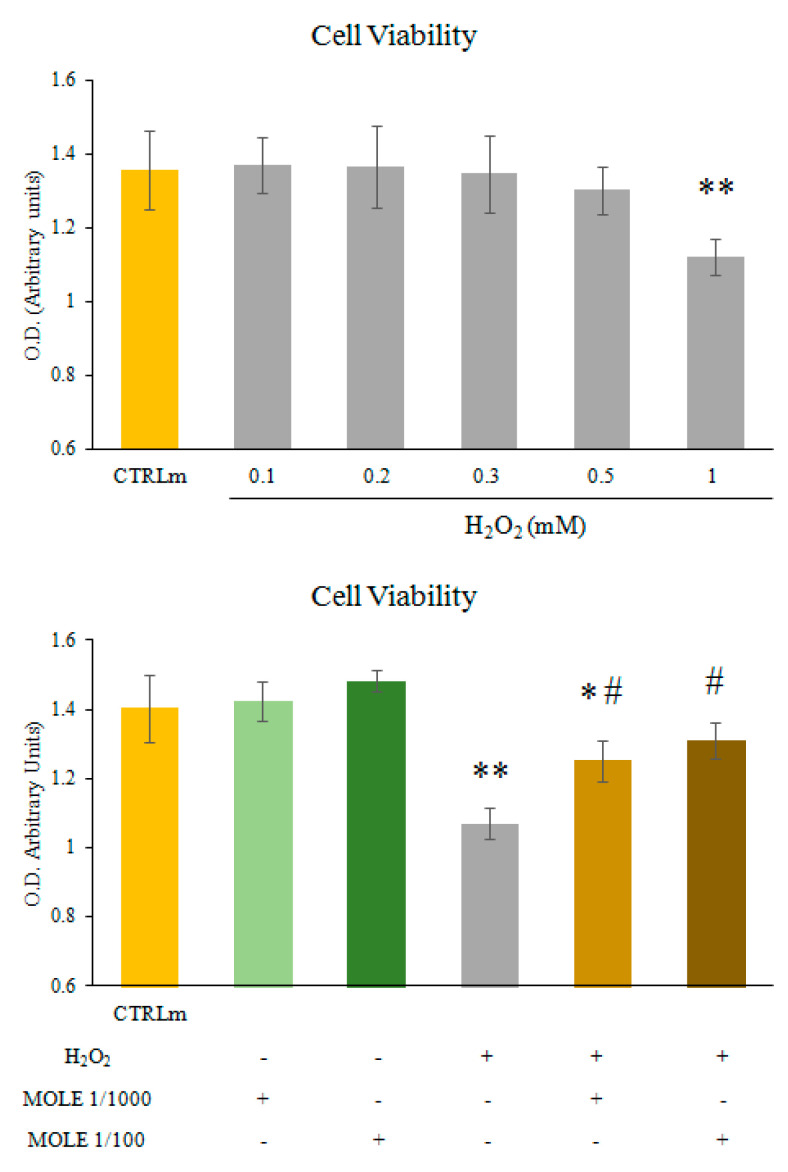
The cells were treated with a range of H2O2 (0.1–1 mM, dose-dependence) for 1 h.
We found that H2O2 1 mM induced a statistically significant reduction in myotube viability (Figure 2, upper panel). Therefore, 1 mM of H2O2 was selected for all of the subsequent experiments. To test the protective effect of MOLE, C2C12 myotubes were treated with MOLE stock solution dilutions (1/1000 and 1/100 working solution) or a vehicle (methanol) in culture media for 24 h.
Figure 2.
MTT assay. C2C12 myotubes were treated with different H2O2 concentrations (0.1–1 mM) for 1 h (dose-dependence, upper panel). Then the effects of MOLE pre-treatments were assayed. C2C12 myotubes were treated with MOLE stock solution dilutions (1/1000 and 1/100 working solution) or vehicle (methanol) in culture media for 24 h. Then, hydrogen peroxide (1 mM) was added to samples pre-treated with vehicle or MOLE for a further hour (lower panel). Cell viability was assessed by the MTT assay. Data presented are the mean ± S.D. of three experiments, each performed in triplicate. * p < 0.05; ** p < 0.01 vs. CTRLm; # p < 0.05 vs. H2O2.
After incubation, hydrogen peroxide (1 mM) was added to the samples pre-treated with vehicle or MOLE for a further hour, and an MTT assay was performed (Figure 2, lower panel). No statistically significant differences were found between the single MOLE treatments. We found that myotubes pre-treated with MOLE and then exposed to H2O2 showed markedly greater cell viabilities, of 17% and 22%, compared to single H2O2 for 1/1000 and 1/100 dilutions of MOLE, respectively (Figure 2, lower panel).
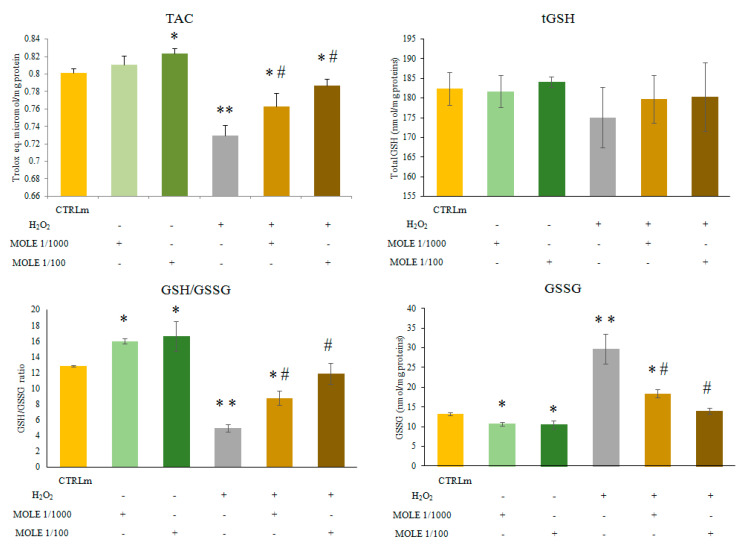
3.3. Evaluation of Glutathione Homeostasis and Total Antioxidant Capacity
The evaluation of glutathione homeostasis revealed significant differences in the GSH/GSSG ratio, a well-known marker of redox status, between the different treatments. The MOLE treatment markedly decreased the GSSG levels (19% and 20% for MOLE 1/1000 and 1/100, respectively, p < 0.01) compared to CTRLm (Figure 3).
Figure 3.
Total antioxidant capacity (TAC) and glutathione homeostasis analysis. Measurement of total antioxidant capacity (TAC), total glutathione (tGSH), oxidized glutathione (GSSG), and reduced to oxidized glutathione ratio (GSH/GSSG) was performed in C2C12 myotubes treated with MOLE stock solution dilutions (1/1000 and 1/100 working solution) or vehicle (methanol) in culture media for 24 h and in samples treated for a further hour with hydrogen peroxide (1 mM) with pre-treatment with vehicle or MOLE. Data presented are the mean ± S.D. of three experiments. * p < 0.05; ** p < 0.01 vs. CTRLm; # p < 0.05 vs. H2O2.
The H2O2 (1 mM, 1 h) treatment increased the GSSG levels (225% p < 0.01) compared to CTRLm (Figure 3). The MOLE pre-treatment decreased the H2O2-induced rise in GSSG levels in a statistically significant manner (38% and 53% for MOLE 1/1000 and 1/100, respectively, p < 0.01) compared to H2O2 (Figure 3).
No statistically significant differences were found after treatments in the total glutathione (tGSH) levels. Only a slight, non-significant decrease was found after the H2O2 treatments.
No statistically significant differences were found after treatments in the total glutathione (tGSH) levels. Only a slight and non-significant decrease was found after H2O2 treatments.
Because the total glutathione levels were not affected by the treatments, the evaluation of the ratio between the reduced and oxidized glutathione (GSH/GSSG) showed a statistically significant increase after the MOLE treatment (25% and 29% for MOLE 1/1000 and 1/100, respectively, p < 0.01) compared to CTRLm (Figure 3).
The 1-h H2O2 1 mM treatment induced a decrease in the GSH/GSSG ratio (61% p < 0.01) compared to CTRLm (Figure 3). The pre-treatment with MOLE increased the H2O2-induced fall in GSH/GSSG ratio in a statistically significant manner (177% and 239% for MOLE 1/1000 and 1/100, respectively, p < 0.01) compared to H2O2 (Figure 3).
The analysis of myotube total antioxidant capacity showed a statistically significant increase after MOLE 1/100 treatment (p < 0.05) and a reduction after H2O2 treatment (p < 0.01). The pre-treatment with MOLE was able to partially revert in a statistically significant manner the H2O2-induced decrease in total antioxidant capacity (p < 0.05 for MOLE 1/1000 and p < 0.01 for MOLE 1/100; Figure 3).
3.4. Total Free Thiols and Thioredoxin Activity Analysis
Free thiol residues play a fundamental role in the homeostasis of the cellular redox state and in the detoxification of reactive oxygen species [53]. We investigated whether MOLE was able to keep these groups in the reduced-activity state. The MOLE treatment did indeed induce an increase in the myotube total free thiol levels (7% and 10% for MOLE 1/1000 and 1/100, respectively, p < 0.05) compared to CTRLm (Figure 3). Similarly, 1-h H2O2 1 mM treatment induced a decrease in TFT levels (p < 0.05) compared to CTRLm (Table 1). Finally, a pre-treatment with MOLE was able to revert the H2O2-induced decrease in TFT levels in a statistically significant manner (7% and 13% for MOLE 1/1000 and 1/100, respectively, p < 0.05) compared to H2O2 (Table 1).
Table 1.
Total free thiols and Thioredoxin activity analysis.
| CTRLm | MOLE 1/1000 | MOLE 1/100 | H2O2 | MOLE 1/1000 
H2O2 |
MOLE 1/100  H2O2 |
|
|---|---|---|---|---|---|---|
| TFT a) | 3.69 ± 0.02 | 3.94 ± 0.08 * | 4.07 ± 0.05 * | 3.56 ± 0.03 * | 3.82 ± 0.11 # | 4.03 ± 0.25 # |
| Total Trx b) | 20.46 ± 0.75 | 22.46 ± 0.28 * | 24.31 ± 0.06 * | 17.80 ± 0.77 * | 19.44 ± 1.41 | 21.91 ± 0.80 # |
| Active Trx b) | 3.41 ± 0.17 | 4.76 ± 0.71 * | 5.59 ± 0.16 * | 1.43 ± 0.24 * | 2.62 ± 0.03 * # | 3.37 ± 0.20 # |
| Active Trx (%) | 16.68 ± 0.21 | 21.15 ± 2.29 | 22.98 ± 0.61 * | 8.04 ± 0.99 * | 13.53 ± 0.83 * # | 15.42 ± 1.47 # |
TFT, total free thiols; Trx, thioredoxin; Active Trx, free active form of Trx. C2C12 myotubes were treated with MOLE stock solution dilutions (1/1000 and 1/100 working solution) or vehicle (methanol) in culture media for 24 h. Then, hydrogen peroxide (1 mM) was added to samples pre-treated with vehicle or MOLE for a further hour. After treatments, cells were lysed, and then lysates were used for biochemical analysis. Data presented are the means ± S.D. of three experiments performed in triplicate. a) nmol -SH/g proteins; b) ng Trx/mg proteins. * p < 0.05 vs. CTRLm; # p < 0.05 vs. H2O2.
The same effects were found in the analysis of the total levels of thioredoxin and its reactive active form. MOLE was able to increase the total Trx (10% and 19% for MOLE 1/1000 and 1/100, respectively, p < 0.05) and active Trx levels (27% and 38% for MOLE 1/1000 and 1/100, respectively, p < 0.05), and in pre-treated myotubes, MOLE was able to revert the H2O2-induced decrease in total Trx and active Trx levels in a statistically significant manner (9% and 23% for MOLE 1/1000 and 1/100, respectively, for total Trx, p < 0.05; and 69% and 92% for MOLE 1/1000 and 1/100, respectively, for active Trx, p < 0.05) compared to H2O2 (Table 1).
3.5. Evaluation of Antioxidant Enzyme Activities
Compared to CTRLm, MOLE was able to increase the activity of all of the antioxidant enzymes in C2C12 myotubes.
MOLE administration induced a treatment effect (p < 0.05) and a dose-dependent increase in SOD (8% and 24% for MOLE 1/1000 and 1/100, respectively) and GST (11% and 17% for MOLE 1/1000 and 1/100, respectively) (p < 0.05, Table 2). A treatment effect was found for CAT (13% and 17% increase for MOLE 1/1000 and 1/100, respectively) and GPx (27% and 31% increase for MOLE 1/1000 and 1/100, respectively; p < 0.05, Table 2). However, no dose effect was found for these enzymatic activities (Table 2).
Table 2.
Enzymatic activity analysis.
| CTRLm | MOLE 1/1000 | MOLE 1/100 | H2O2 | MOLE 1/1000 
H2O2 |
MOLE 1/100 
H2O2 |
|
|---|---|---|---|---|---|---|
| SOD a) | 0.35 ± 0.01 | 0.38 ± 0.01 * | 0.44 ± 0.05 * | 0.29 ± 0.02 ** | 0.35 ± 0.03 # | 0.36 ± 0.02 ## |
| CAT a) | 3.46 ± 0.16 | 3.92 ± 0.23 * | 4.04 ± 0.09 * | 2.97 ± 0.06 ** | 3.61 ± 0.13 ## | 3.81 ± 0.27 ## |
| GPx a) | 368.49 ± 27.48 | 468.20 ± 50.01 * | 483.92 ± 31.65 * | 312.27 ± 17.17 ** | 407.63 ± 16.34 ## | 432.13 ± 22.66 * ## |
| GST a) | 144.07 ± 6.54 | 160.26 ± 6.49 * | 168.92 ± 3.75 * | 123.31 ± 2.61 ** | 150.85 ± 5.74 ## | ± 10.94 ## |
SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GST, glutathione transferase. C2C12 myotubes were treated with MOLE stock solution dilutions (1/1000 and 1/100 working solution) or vehicle (methanol) in culture media for 24 h. Then, hydrogen peroxide (1 mM) was added to samples pre-treated with vehicle or MOLE for a further hour. After treatments, the cells were lysed and then cell lysates were used for biochemical analysis. Data presented are the means ± S.D. of three experiments performed in triplicate. a) U/mg proteins. * p < 0.05 and ** p < 0.01 vs. CTRLm; # p < 0.05 and ## p < 0.01 vs. H2O2.
The H2O2 (1 mM, 1 h) treatment induced a decrease in all enzymatic activity levels compared to CTRLm (SOD: 17%, CAT: 14%, GPx: 15%, GST: 15%; p < 0.01, Table 2).
MOLE pre-treatment was able to revert the decrease in the activities H2O2-induced for all enzymes in a statistically significant manner when compared to H2O2 (21% and 24% increase for MOLE 1/1000 and 1/100, respectively, for SOD; 22% and 28% increase for MOLE 1/1000 and 1/100, respectively, for CAT; 30% and 38% increase for MOLE 1/1000 and 1/100, respectively, for GPx; 23% and 29% increase for MOLE 1/1000 and 1/100, respectively, for GST; p < 0.05; Table 2).
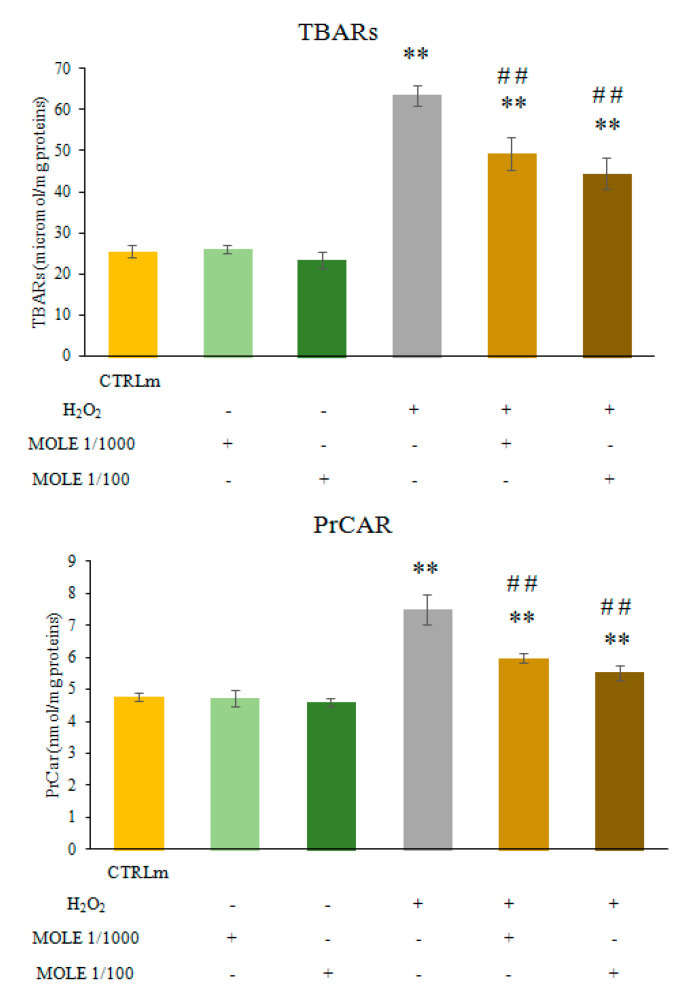
3.6. Evaluation of Oxidative Damage Markers
As shown in Figure 4, the MOLE treatment did not induce any significant change in PrCAR and TBARs (malondialdehyde (MDA) and other aldehydes) levels if compared to untreated cells. H2O2 (1 mM, 1 h) exposure induced a statistical increase in protein carbonyls and TBARs (172% and 249%, respectively; p < 0.01) compared to CTRLm (Figure 4).
Figure 4.
Thiobarbituric acid reactive substances (TBARs) and protein carbonyls (PrCar) analysis. Measurement of TBARs and PrCar analysis was performed in C2C12 myotubes treated with MOLE stock solution dilutions (1/1000 and 1/100 working solution) or vehicle (methanol) in culture media for 24 h and in samples treated for a further hour with hydrogen peroxide (1 mM) with pre-treatment with vehicle or MOLE. Data presented are the mean ± S.D. of three experiments. ** p < 0.01 vs. CTRLm; ## p < 0.01 vs. H2O2.
MOLE pre-treatment was able to lower the H2O2-induced increase in oxidative damage markers in a statistically significant manner (p < 0.01) compared to H2O2 (27% for both MOLE 1/1000 and 1/100 for PrCAR, p < 0.01; and 23% and 30% for MOLE 1/1000 and 1/100, respectively, for TBARs, p < 0.01; Figure 4).
4. Discussion
In the present study, we found that MOLE efficiently counteracts the oxidative damage induced by a high dose of hydrogen peroxide in C2C12 myotubes. MOLE pretreatment blunts the oxidative insult by stimulating antioxidant enzyme activities and restoring redox status, thereby limiting the damage to lipids and proteins.
Skeletal muscle is a tissue that is often exposed to pro-oxidizing conditions due to its high oxygen consumption rates. [54,55]. In fact, every increase in metabolic activity leads to increased levels of ROS. In physiological amounts, they regulate numerous cellular signaling pathways and modulate the expression of many redox-sensitive genes. ROS production is usually matched by endogenous antioxidant defenses, but if ROS are in excess, oxidative stress occurs, predisposing organisms to pathological conditions [56,57,58].
Our previous data show that, in C2C12 myotubes, treatment with MOLE caused the activation of the oxidative metabolism through the SIRT1-PPARα pathway along with the activation of the nuclear factor erythroid 2-related factor (Nrf2) and its target gene heme oxygenase-1 (HO-1), both regulators of cellular resistance to oxidants. MOLE treatment counterbalanced the stimulated oxidative metabolism with an improved glutathione redox homeostasis and increased antioxidant enzymatic activities [29,39].
It is well established that vigorous, unaccustomed physical exercise increases muscle energy requests enormously, leading to very high anion superoxide (O2°-) production by mitochondria and NADPH oxidase activities. The successive dismutation of O2°- leads to uncontrolled, high intracellular levels of H2O2, a condition that sportsmen take pains to avoid because it results in contractile dysfunction and fatigue. In this situation, cell damage occurs due to the impairment of the redox state and the functionality of various proteins, including antioxidant enzymes, up to the events that lead to cell death [5,6,7,59,60].
Examining the effect of H2O2 in vitro at the cellular level, we have shown that the exposure of C2C12 myotubes to H2O2 1 mM decreased cell viability, antioxidant capacity, GSH/GSSG ratio (a primary marker of redox status), and the levels of total free thiols and thioredoxin. The disruption of the homeostasis of myotube thiol systems is the most central feature of the oxidative stress occurrence induced by H2O2 exposure. Moreover, the levels of superoxide dismutase, catalase, glutathione peroxidase, and glutathione transferase activities were also found to be decreased by H2O2 exposure. The adverse effects of H2O2 were mirrored by an increase in oxidative damage markers such as PrCAR and TBARs.
To preserve muscle function and protect myotubes from excessive exposure to ROS, the use of antioxidants is a common strategy, and our results show that Moringa offers a useful resource for such strategies. The appropriate use of antioxidants has proved to be beneficial in balancing the ratio between oxidants and antioxidants in most physio-pathological conditions [21,61,62].
Moringa oleifera leaf extracts are rich in glucosinolates, polyphenols, flavonoids, and phenolic acids that are known to act as antioxidants, either inactivating lipid free radicals or preventing the decomposition of hydroperoxides into free radicals [36,37,38].
Following these findings, here we examined whether the antioxidant molecules in Moringa oleifera leaf extracts are present in sufficient concentrations to counteract the damage provoked by the oxidizing-/oxidative environment in myotubes exposed to 1 mM H2O2. We have previously observed that MOLE showed, per se, in a cell-free system a dose-dependent total antioxidant capacity indicating that its ability depends on the amount of antioxidant molecules present in the mixture [39].
Further, since our preliminary data highlighted the incapacity of MOLE to exert a significant protective effect on C2C12 myotubes when added at the same time point of H2O2 treatment (data not shown), we adopted a pre-supplementation strategy by which MOLE was added in culture medium 24 h before the acute H2O2 treatment.
Our data demonstrate that the presence of MOLE restores the redox status as evidenced by blunting the negative effects of H2O2 on the intracellular GSH/GSSG ratios, the level of free thiols, and the activity of thioredoxin, and furthermore positively modulates antioxidant enzyme activities. These features lead to a decrease in H2O2-induced oxidative damage with the result of improving myotubes viability.
The pre-treatment of myotubes with MOLE increased free thiols, thioredoxin activity, and intracellular TAC. The TAC assay allows for the determination of the levels of the total non-enzymatic antioxidant capacity of biological samples in terms of lipid-soluble antioxidants such as tocopherols, carotenes, vitamin A, ubiquinols, and water-soluble antioxidants such as glutathione, as well as ascorbate and proteins with redox-sensitive thiols. In particular, the levels of intracellular free thiols are a fundamental component for maintaining the redox state and the total antioxidant capacity [53,63,64]. In this assay, the contribution of the antioxidant enzymatic system contribution [65,66] is not valued, and this should be taken into consideration.
The analysis of the enzymatic antioxidant system confirmed a dose-dependent increase in the enzymatic activities of SOD, CAT, GPx, and GST in the myotubes after MOLE administration. We have previously demonstrated that MOLE induces the SIRT1-Nrf2 system [29]. SIRT1 acts as a modulator of Nrf2 [67]. Nrf2 is an important transcription factor that potentiates the cellular defense system through its ability to bind and regulate the antioxidant-responsive elements (AREs) [68,69,70]. It is well-known that Nrf2 activation prevents oxidative damage, and its signaling plays a key role in the oxidative-stress-mediated beneficial effects of exercise [71]. When a redox status imbalance occurs, Nrf2 dissociates from its sequestration complex and then translocates to the nucleus. In this way, it can interact with the AREs of antioxidant genes, leading to the transcriptional activation of its target genes such as superoxide dismutase, glutathione peroxidase, glutathione S-transferase, and heme oxygenase-1 [72,73,74].
Here we found that MOLE pretreatment is able to maintain the activity levels of all antioxidant enzymes in myotubes exposed to oxidative insult. Our data show that MOLE positively modulates SOD, an enzyme that catalyzes the dismutation of superoxide radicals to generate hydrogen peroxide, which was restored to the level found in the controls. Interestingly, a stronger effect of MOLE was found in the myotubes exposed to H2O2, with the induction of CAT and GPx, enzymes involved in the neutralization of H2O2 to H2O in peroxisomes, and the cytosol, respectively, with respect to the myotubes challenged with oxidative insult alone. Additionally, the activity of GST, involved in the detoxification of molecules through the formation of S-conjugates with GSH, was found to be significantly increased compared to the myotubes treated with H2O2 and higher than the control values.
Our results can be explained by the fact that MOLE is an inducer of cellular antioxidant systems, in addition to acting as a direct scavenger of ROS. Given that MOLE exerts its protective antioxidant effect against H2O2-induced oxidative stress only in pretreated cells, we speculate that the activation of the Nrf2 pathway is particularly important in enabling cells to respond when subjected to oxidative stress promptly. In fact, our data on muscle cells are in agreement with recent reports on the effects of the different components of Brassicaceae, and Moringa oleifera in particular, on other cellular models exposed to oxidative stress [75,76,77,78,79,80,81].
Finally, these data allow us to assert that Moringa oleifera leaf extract represents a useful nutritional supplement for preventing harmful oxidative stress conditions in skeletal muscles. In doing so, the compounds in MOLE may exert antioxidant activity either directly by scavenging ROS or indirectly by inducing an antioxidant response, thereby ameliorating the cellular redox status.
5. Conclusions
The present study shows that MOLE pretreatment has a beneficial effect on the antioxidant system of skeletal muscle cells, increasing enzymatic capacity and redox status in the stressful condition of an oxidizing environment.
Our data encourage further studies to better elucidate the action of MOLE on the redox state of different physiological and pathological states in humans in order to generalize the role of MOLE as a supporter of skeletal muscle health.
Acknowledgments
Each author of this study further declares no relationships with the companies or manufacturers who will benefit from the results of the present study.
Abbreviations
Moringa oleifera leaf extract (MOLE), glucosinolates (GLs), reduced (GSH) and oxidized (GSSG) glutathione, reduced to oxidized glutathione ratio (GSH/GSSG), total antioxidant capacity (TAC), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione transferase (GST), total free thiols (TFT), thioredoxin (Trx), thiobarbituric acid reactive substances (TBARS), protein carbonyls (PrCar), reactive oxygen species (ROS).
Author Contributions
Conceptualization, G.D. and R.C.; methodology, G.D., M.M., R.C.; investigation, G.D., M.M., R.C.; writing—Original draft preparation, G.D., R.C., M.E.O., M.M., I.D.; writing—Review and editing, G.D., R.C., M.E.O., D.C., K.H., I.D.; supervision, G.D., S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors of this article declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sato S., Basse A.L., Schönke M., Chen S., Samad M., Altıntaş A., Laker R.C., Dalbram E., Barrès R., Baldi P., et al. Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab. 2019;30:92–110.e4. doi: 10.1016/j.cmet.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Murphy R.M., Watt M.J., Febbraio M.A. Metabolic communication during exercise. Nat. Metab. 2020;2:805–816. doi: 10.1038/s42255-020-0258-x. [DOI] [PubMed] [Google Scholar]
- 3.Zhao R.Z., Jiang S., Zhang L., Yu Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int. J. Mol. Med. 2019;44:3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers S.K., Lennon S.L. Analysis of cellular responses to free radicals: Focus on exercise and skeletal muscle. Proc. Nutr. Soc. 1999;58:1025–1033. doi: 10.1017/S0029665199001342. [DOI] [PubMed] [Google Scholar]
- 5.Jackson M.J. Reactive oxygen species and redox-regulation of skeletal muscle adaptations to exercise. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:2285–2291. doi: 10.1098/rstb.2005.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson M.J. Free radicals generated by contracting muscle: By-products of metabolism or key regulators of muscle function? Free Radic. Biol. Med. 2008;44:132–141. doi: 10.1016/j.freeradbiomed.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Jackson M.J. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid. Redox Signal. 2011;15:2477–2486. doi: 10.1089/ars.2011.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson M.J., McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. Pt 9J. Physiol. 2011;589:2139–2145. doi: 10.1113/jphysiol.2011.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasilaki A., Mansouri A., Van Remmen H., van der Meulen J.H., Larkin L., Richardson A.G., McArdle A., Faulkner J.A., Jackson M.J. Free radical generation by skeletal muscle of adult and old mice: Effect of contractile activity. Aging Cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 10.Fulle S., Protasi F., Di Tano G., Pietrangelo T., Beltramin A., Boncompagni S., Vecchiet L., Fanò G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klaunig J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018;24:4771–4778. doi: 10.2174/1381612825666190215121712. [DOI] [PubMed] [Google Scholar]
- 15.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Cabrera M.C., Viña J., Ji L.L. Interplay of oxidants and antioxidants during exercise: Implications for muscle health. Phys. Sportsmed. 2009;37:116–123. doi: 10.3810/psm.2009.12.1749. [DOI] [PubMed] [Google Scholar]
- 17.Veal E., Jackson T., Latimer H. Role/s of ‘Antioxidant’ Enzymes in Ageing. Subcell. Biochem. 2018;90:425–450. doi: 10.1007/978-981-13-2835-0_14. [DOI] [PubMed] [Google Scholar]
- 18.Fougere B., van Kan G.A., Vellas B., Cesari M. Redox Systems, Antioxidants and Sarcopenia. Curr. Protein Pept. Sci. 2018;19:643–648. doi: 10.2174/1389203718666170317120040. [DOI] [PubMed] [Google Scholar]
- 19.Alkadi H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets. 2020;20:16–26. doi: 10.2174/1871526518666180628124323. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Cabrera M.C., Carretero A., Millan-Domingo F., Garcia-Dominguez E., Correas A.G., Olaso-Gonzalez G., Viña J. Redox-related biomarkers in physical exercise. Redox Biol. 2021;42:101956. doi: 10.1016/j.redox.2021.101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonioni A., Fantini C., Dimauro I., Caporossi D. Redox homeostasis in sport: Do athletes really need antioxidant support? Res. Sports Med. 2019;27:147–165. doi: 10.1080/15438627.2018.1563899. [DOI] [PubMed] [Google Scholar]
- 22.Pingitore A., Lima G.P., Mastorci F., Quinones A., Iervasi G., Vassalle C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition. 2015;31:916–922. doi: 10.1016/j.nut.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Edenfield K.M. Sports Supplements: Pearls and Pitfalls. Prim. Care. 2020;47:37–48. doi: 10.1016/j.pop.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Kerasioti E., Stagos D., Priftis A., Aivazidis S., Tsatsakis A.M., Hayes A.W., Kouretas D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014;155:271–278. doi: 10.1016/j.foodchem.2014.01.066. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira P.M.P., Farias D.F., Oliveira J.T.D.A., Carvalho A.D.F.U. Moringa oleifera: Bioactive compounds and nutritional potential. Rev. Nutr. 2008;21:431–437. doi: 10.1590/S1415-52732008000400007. [DOI] [Google Scholar]
- 26.Mishra G., Singh P., Verma R., Kumar R.S., Srivastava S., Khosla R.L. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: An overview. Der. Pharm. Lett. 2011;3:141–164. [Google Scholar]
- 27.Tejas G.H., Umang J.H., Payal B.N., Tusharbinu D.R., Pravin T.R. A panoramic view on pharmacognostic, pharmacological, nutritional, therapeutic and prophylactic values of Moringa olifera Lam. Int. Res. J. Pharm. 2012;3:1–7. [Google Scholar]
- 28.Lopez-Teros V., Ford J.L., Green M.H., Tang G., Grusak M.A., Quihui-Cota L., Muzhingi T., Paz-Cassini M., Astiazaran-Garcia H. Use of a “Super-child” Approach to Assess the Vitamin A Equivalence of Moringa oleifera Leaves, Develop a Compartmental Model for Vitamin A Kinetics, and Estimate Vitamin A Total Body Stores in Young Mexican Children. J. Nutr. 2017;147:2356–2363. doi: 10.3945/jn.117.256974. [DOI] [PubMed] [Google Scholar]
- 29.Duranti G., Maldini M., Crognale D., Horner K., Dimauro I., Sabatini S., Ceci R. Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells. Molecules. 2021;26:5041. doi: 10.3390/molecules26165041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayoola G.A., Coker H.A.B., Adesegun S.A., Adepoju-Bello A.A., Obaweya K., Ezennia E.C. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop. J. Pharm. Res. 2008;7:1019–1024. [Google Scholar]
- 31.Davinelli S., Bertoglio J.C., Zarrelli A., Pina R., Scapagnini G. A randomized clinical trial evaluating the efficacy of an anthocyanin-maqui berry extract (Delphinol®) on oxidative stress biomarkers. J. Am. Coll Nutr. 2015;34((Suppl. S1)):28–33. doi: 10.1080/07315724.2015.1080108. [DOI] [PubMed] [Google Scholar]
- 32.Posmontie B. The medicinal qualities of Moringa oleifera. Holist. Nurs. Pract. 2011;25:80–87. doi: 10.1097/HNP.0b013e31820dbb27. [DOI] [PubMed] [Google Scholar]
- 33.Stohs S.J., Hartman M.J. Review of the safety and efficacy of Moringa Oleifera. Phytother Res. 2015;29:796–804. doi: 10.1002/ptr.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahar S., Faisal F.M., Iqbal J., Rahman M.M., Yusuf M.A. Antiobesity activity of Moringa Oleifera leaves against high fat diet-induced obesity in rats. Int. J. Basic Clin. Pharmacol. 2016;5:1263–1268. doi: 10.18203/2319-2003.ijbcp20162427. [DOI] [Google Scholar]
- 35.Bais S., Singh G.S., Sharma R. Anti obesity and hypolipidemic activity of Moringa Oleifera leaves against high fat diet induced obesity in rats. Adv. Biol. 2014;2014:162914. doi: 10.1155/2014/162914. [DOI] [Google Scholar]
- 36.Murillo A.G., Fernandez M.L. The relevance of dietary polyphenols in cardiovascular protection. Curr. Pharmacol. Rev. Curr. Pharm. Des. 2017;23:2444–2452. doi: 10.2174/1381612823666170329144307. [DOI] [PubMed] [Google Scholar]
- 37.Pokorny J. Introduction. In: Pokorny J., Yanishlieva N., Gordon N.H., editors. Antioxidant in Foods: Practical Applications. Woodhead Publishing Limited; Cambridge, UK: 2001. pp. 1–3. [Google Scholar]
- 38.Zheng W., Wang S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 39.Duranti G., Maldini M., Crognale D., Sabatini S., Corana F., Horner K., Ceci R. Moringa oleifera leaf extract influences oxidative metabolism in C2C12 myotubes through SIRT1-PPAR𝛼 pathway. Phytomedicine PLUS. 2021;1:100014. doi: 10.1016/j.phyplu.2020.100014. [DOI] [Google Scholar]
- 40.Ceci R., Duranti G., Rossi A., Savini I., Sabatini S. Skeletal muscle differentiation: Role of dehydroepiandrosterone sulfate. Horm. Metab. Res. 2011;43:702–707. doi: 10.1055/s-0031-1285867. [DOI] [PubMed] [Google Scholar]
- 41.Antinozzi C., Duranti G., Ceci R., Lista M., Sabatini S., Caporossi D., Di Luigi L., Sgrò P., Dimauro I. Hydrogen peroxide stimulates dihydrotestosterone release in C2C12 myotubes: A new perspective for exercise-related muscle steroidogenesis? Int. J. Mol. Sci. 2022;23:6566. doi: 10.3390/ijms23126566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duranti G., Ceci R., Sgrò P., Sabatini S., Di Luigi L. Influence of the PDE5 inhibitor tadalafil on redox status and antioxidant defense system in C2C12 skeletal muscle cells. Cell Stress Chaperones. 2017;22:389–396. doi: 10.1007/s12192-017-0778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colamartino M., Santoro M., Duranti G., Sabatini S., Ceci R., Testa A., Padua L., Cozzi R. Evaluation of levodopa and carbidopa antioxidant activity in normal human lymphocytes in vitro: Implication for oxidative stress in Parkinson’s disease. Neurotox Res. 2015;27:106–117. doi: 10.1007/s12640-014-9495-7. [DOI] [PubMed] [Google Scholar]
- 44.Ceci R., Duranti G., Di Filippo E.S., Bondi D., Verratti V., Doria C., Caporossi D., Sabatini S., Dimauro I., Pietrangelo T. Endurance training improves plasma superoxide dismutase activity in healthy elderly. Mech. Ageing Dev. 2019;185:111190. doi: 10.1016/j.mad.2019.111190. [DOI] [PubMed] [Google Scholar]
- 45.Holmgren A., Björnstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-687952023-6. [DOI] [PubMed] [Google Scholar]
- 46.Di Luigi L., Duranti G., Antonioni A., Sgrò P., Ceci R., Crescioli C., Sabatini S., Lenzi A., Caporossi D., Del Galdo F., et al. The phosphodiesterase type 5 inhibitor sildenafil improves dna stability and redox homeostasis in systemic sclerosis fibroblasts exposed to reactive oxygen species. Antioxidants. 2020;9:E786. doi: 10.3390/antiox9090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colamartino M., Duranti G., Ceci R., Sabatini S., Testa A., Cozzi R. A multi-biomarker analysis of the antioxidant efficacy of parkinson’s disease therapy. Toxicol. In Vitro. 2017;47:1–7. doi: 10.1016/j.tiv.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Magi F., Dimauro I., Margheritini F., Duranti G., Mercatelli N., Fantini C., Ripani F.R., Sabatini S., Caporossi D. Telomere length is independently associated with age, oxidative biomarkers, and sport training in skeletal muscle of healthy adult males. Free Radic. Res. 2018;52:639–647. doi: 10.1080/10715762.2018.1459043. [DOI] [PubMed] [Google Scholar]
- 49.Ceci R., Duranti G., Leonetti A., Pietropaoli S., Spinozzi F., Marcocci L., Amendola R., Cecconi F., Sabatini S., Mariottini P., et al. Adaptive responses of heart and skeletal muscle to spermine oxidase overexpression: Evaluation of a new transgenic mouse model. Free Radic. Biol. Med. 2017;103:216–225. doi: 10.1016/j.freeradbiomed.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 50.Bennett R.N., Mellon F.A., Foidl N., Pratt J.H., Dupont M.S., Perkins L., Kroon P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tis- sues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003;51:3546–3553. doi: 10.1021/jf0211480. [DOI] [PubMed] [Google Scholar]
- 51.Fahey J.W., Olson M.E., Stephenson K.K., Wade K.L., Chodur G.M., Odee D., Nouman W., Massiah M., Alt J., Egner P.A., et al. The diversity of chemoprotective glucosinolates in moringaceae (Moringa spp.) Sci. Rep. 2018;8:7994. doi: 10.1038/s41598-018-26058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stockert J.C., Horobin R.W., Colombo L.L., Blázquez-Castro A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018;120:159–167. doi: 10.1016/j.acthis.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Ulrich K., Jakob U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019;140:14–27. doi: 10.1016/j.freeradbiomed.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinzel F.R., Luo Y., Dodoni G., Boengler K., Petrat F., Di Lisa F., de Groot H., Schulz R., Heusch G. Formation of reactive oxygen species at increased contraction frequency in rat cardiomyocytes. Cardiovasc. Res. 2006;71:374–382. doi: 10.1016/j.cardiores.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Kozakowska M., Pietraszek-Gremplewicz K., Jozkowicz A., Dulak J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015;36:377–393. doi: 10.1007/s10974-015-9438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 57.Niess A.M., Simon P. Response and adaptation of skeletal muscle to exercise—The role of reactive oxygen species. Front. Biosci. 2007;12:4826–4838. doi: 10.2741/2431. [DOI] [PubMed] [Google Scholar]
- 58.Meng S.J., Yu L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010;11:1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 61.Zhao X., Wang J., Deng Y., Liao L., Zhou M., Peng C., Li Y. Quercetin as a protective agent for liver diseases: A comprehensive descriptive review of the molecular mechanism. Phytother. Res. 2021;35:4727–4747. doi: 10.1002/ptr.7104. [DOI] [PubMed] [Google Scholar]
- 62.Aaseth J., Alexander J., Alehagen U. Coenzyme Q10 supplementation—In ageing and disease. Mech. Ageing Dev. 2021;197:111521. doi: 10.1016/j.mad.2021.111521. [DOI] [PubMed] [Google Scholar]
- 63.Deneke S.M. Thiol-based antioxidants. Curr. Top. Cell Regul. 2000;36:151–180. doi: 10.1016/s0070-213780007-8. [DOI] [PubMed] [Google Scholar]
- 64.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sies H. Total Antioxidant Capacity: Appraisal of a Concept. J. Nutr. 2007;137:1493–1495. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- 66.Fraga C.G., Oteiza P.I., Galleano M. In vitro measurements and interpretation of total antioxidant capacity. Biochim. Biophys. Acta. 2014;1840:931–934. doi: 10.1016/j.bbagen.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 67.Kawai Y., Garduño L., Theodore M., Yang J., Arinze I.J. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J. Biol. Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh C.K., Chhabra G., Ndiaye M.A., Garcia-Peterson L.M., Mack N.J., Ahmad N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018;28:643–661. doi: 10.1089/ars.2017.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J., Ren D., Fedorova J., He Z., Li J. SIRT1/SIRT3 Modulates Redox Homeostasis during Ischemia/Reperfusion in the Aging Heart. Antioxidants. 2020;9:858. doi: 10.3390/antiox9090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hyun D.H. Insights into the New Cancer Therapy through Redox Homeostasis and Metabolic Shifts. Cancers. 2020;12:1822. doi: 10.3390/cancers12071822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vargas-Mendoza N., Morales-González Á., Madrigal-Santillán E.O., Madrigal-Bujaidar E., Álvarez-González I., García-Melo L.F., Anguiano-Robledo L., Fregoso-Aguilar T., Morales-Gonzalez J.A. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants. 2019;8:196. doi: 10.3390/antiox8060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 73.Samimi F., Baazm M., Eftekhar E., Rajabi S., Goodarzi M.T., Jalali Mashayekhi F. Possible antioxidant mechanism of coenzyme Q10 in diabetes: Impact on Sirt1/Nrf2 signaling pathways. Res. Pharm. Sci. 2019;14:524–533. doi: 10.4103/1735-5362.272561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang G., Xie X., Yuan L., Qiu J., Duan W., Xu B., Chen X. Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. Biofactors. 2020;46:441–453. doi: 10.1002/biof.1599. [DOI] [PubMed] [Google Scholar]
- 75.Guerrero-Beltrán C.E., Calderón-Oliver M., Pedraza-Chaverri J., Chirino Y.I. Protective effect of sulforaphane against oxidative stress: Recent advances. Exp. Toxicol. Pathol. 2012;64:503–508. doi: 10.1016/j.etp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Förster N., Mewis I., Glatt H., Haack M., Brigelius-Flohé R., Schreiner M., Ulrichs C. Characteristic single glucosinolates from Moringa oleifera: Induction of detoxifying enzymes and lack of genotoxic activity in various model systems. Food Funct. 2016;7:4660–4674. doi: 10.1039/C6FO01231K. [DOI] [PubMed] [Google Scholar]
- 77.Jana S., Patel D., Patel S., Upadhyay K., Thadani J., Mandal R., Das S., Devkar R. Anthocyanin rich extract of Brassica oleracea L. alleviates experimentally induced myocardial infarction. PLoS ONE. 2017;12:e0182137. doi: 10.1371/journal.pone.0182137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J., Ma G., Wang Y., Zhang Y. Moringa oleifera leaf flavonoids protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative stress in vitro. Reprod. Domest. Anim. 2020;55:711–719. doi: 10.1111/rda.13670. [DOI] [PubMed] [Google Scholar]
- 79.Soliman M.M., Aldhahrani A., Alkhedaide A., Nassan M.A., Althobaiti F., Mohamed W.A. The ameliorative impacts of Moringa oleifera leaf extract against oxidative stress and methotrexate-induced hepato-renal dysfunction. Biomed. Pharmacother. 2020;128:110259. doi: 10.1016/j.biopha.2020.110259. [DOI] [PubMed] [Google Scholar]
- 80.Sailaja B.S., Aita R., Maledatu S., Ribnicky D., Verzi M.P., Raskin I. Moringa isothiocyanate-1 regulates Nrf2 and NF-κB pathway in response to LPS-driven sepsis and inflammation. PLoS ONE. 2021;16:e0248691. doi: 10.1371/journal.pone.0248691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim C.G., Chang S.N., Park S.M., Hwang B.S., Kang S.A., Kim K.S., Park J.G. Moringa oleifera mitigates ethanol-induced oxidative stress, fatty degeneration and hepatic steatosis by promoting Nrf2 in mice. Phytomedicine. 2022;100:154037. doi: 10.1016/j.phymed.2022.154037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.