Abstract
We have determined that concentrations of copper considered to be toxic can induce a fraction of a population of Escherichia coli to enter the viable but nonculturable (VBNC) condition. Copper-induced VBNC cells could be resuscitated for up to 2 weeks after entering the VBNC state.
Excess levels of copper are toxic to aerobic bacteria (14). It has been suggested that toxicity occurs due to membrane-bound copper catalyzing the formation of hydroperoxide free radicals (16). Toxicity levels are determined by examining bacterial growth in the presence of various concentrations of an agent on solid or liquid growth medium (5, 9, 13). A lack of growth is considered to indicate that the agent has killed cells. However, a lack of growth could also result from the cells entering the viable but nonculturable (VBNC) condition.
The dormant-like VBNC condition occurs in response to a variety of environmental stresses and is considered to be a long-term survival mechanism employed primarily by gram-negative bacteria (7). VBNC cells do not undergo visible growth under conditions that would normally support growth. Because VBNC cells do not readily grow, growth-independent viability assays are used to document the VBNC condition (11). That these assays are by necessity indirect indicators of viability is what makes the VBNC condition controversial (2, 12). Regaining the ability to grow (i.e., resuscitation) is the most definitive proof of cells having been VBNC; however, resuscitation does not always occur by reversing the initial VBNC-inducing conditions. There is a lack of molecular and genetic information about this condition due in part to the existence of no known chemical inducer of the VBNC state. In Agrobacterium tumefaciens and Rhizobium meliloti, copper was reported to be the first such chemical VBNC inducer (1). Resuscitation of these VBNC cells was not examined, and no comparison of the VBNC-inducing concentrations of copper with those concentrations considered to be toxic was made.
To determine if toxic concentrations of copper (9, 14) could induce the VBNC condition, mid- to late-exponential-stage cells of Escherichia coli strain ED8739 (9) were grown with aeration at 37°C in Luria-Bertani (LB) liquid medium and then added to LB plates containing various concentrations of copper sulfate. Copper toxicity has previously been examined in this wild-type strain using these conditions (9). After 2 days of incubation at 37°C, cells were harvested from the plate as follows. Five milliliters of 0.9% NaCl was repipetted over the plate surface; the released cells were then collected onto a 0.22-μm-pore-size polyvinylidene difluoride filter (Millipore Corp., Bedford, Mass.), washed with 0.9% NaCl, and then removed from the filter by shaking in a culture tube in 1 ml of 0.9% NaCl. Cells were assayed for viability using the LIVE/DEAD BacLight bacterial viability kit (Molecular Probes, Inc., Eugene, Oreg.) as described in the manufacturer's instructions. This viability assay, which differentiates cells with an intact (i.e., viable) cell membrane from those with a compromised (i.e., dead) cell membrane based on the differential ability of two fluorescent dyes to permeate them, has previously been used to study the VBNC condition (4, 8, 15). Cells collected onto a 0.2-μm-pore-size black polycarbonate filter (Poretics Corp., Livermore, Calif.) were scored using an Olympus BX-60 epifluorescence microscope and a fluorescein isothiocyanate filter. At least 100 cells over at least four fields of vision were scored per tested sample. A difference between the numbers of viable and culturable cells was used to quantitate the number of VBNC cells. The average results of two trials, given in Table 1, indicate that growth was observed on agar plates of LB medium and of LB medium containing 4 mM CuSO4 and that no growth was observed on LB medium containing either 6 mM or 25 mM CuSO4. A previous study reported similar results and concluded that 6 mM CuSO4 was toxic to E. coli (9). Significant concentrations of viable cells were recovered from the plates lacking observable growth (Table 1), indicating that VBNC cells were present.
TABLE 1.
Copper induction on LB agar of the VBNC state in E. coli ED8739
| Copper concn (mM) | Visible growth | % of cells viable (mean ± SD) |
|---|---|---|
| 0 | + | 67.5 ± 21 |
| 4 | + | 55.7 ± 11 |
| 6 | − | 17.9 ± 4.8 |
| 25 | − | 20.4 ± 2.0 |
To determine if this response to copper was strain specific, the same experiment was conducted with E. coli strain ES80 (a spontaneous rifampin-resistant derivative of the uropathogenic E. coli strain 536 [10]) with similar results (data not shown). Because the efficiency of recovery of bacteria in different physiological states from agar plates is unknown, the percentage of cells in each plate that were VBNC could not be determined. Therefore, cells in liquid cultures were examined.
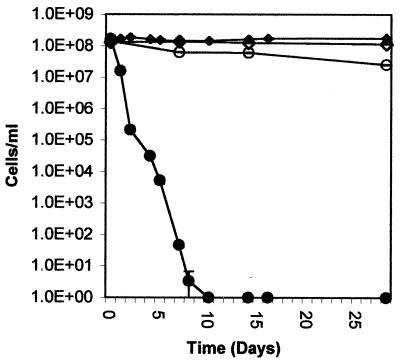
Liquid cultures of strain ES80 were established as follows. Cells grown in LB medium were harvested by centrifugation when at an optical density at 600 nm of 0.8 to 1.0, washed three times in 0.9% NaCl, added to a final concentration of 1 × 108 to 3 × 108 cells per ml in 20 ml of 0.9% NaCl with or without 500 μM CuSO4 in a 125-ml Erlenmeyer flask, and incubated at 25°C. This concentration of copper sulfate was chosen because it would be considered toxic in liquid medium; previous experiments had shown that this concentration as well as lower concentrations of copper sulfate (100 μM) resulted in the removal of all culturable cells from a liquid culture within a few days (unpublished results). At various intervals over a 4-week period, samples were removed and subjected to culturability and viability assays. Viability was determined as described above. Culturability was determined as follows. Cells collected onto a 0.2-μm-pore-size cellulose nitrate filter (Whatman, Inc., Clifton, N.J.) were washed and suspended in 0.9% NaCl, diluted as necessary in saline, and plated in triplicate onto LB plates. Colonies were counted after the plates were incubated at 37°C for at least 3 days. Occasionally, plates were recounted after 10 days; when this was done, no difference in colony counts was observed. The results of one representative experiment are given in Fig. 1. For all three trials, no culturable cells were observed by day 10. When viability was measured, all cultures contained at least 107 viable cells per ml throughout the experiment. Therefore, although CuSO4 killed over 90% of the cells, those cells that escaped killing were in the VBNC state. Similar results were obtained with a starting cell concentration ranging from 107 to 109 cells per ml (data not shown).
FIG. 1.
Copper induction in liquid cultures of the VBNC state in E. coli. Cells grown and harvested as described in the text were placed in 0.9% NaCl liquid cultures with (○) or without (◊) 500 μM CuSO4. At various times, culturability (closed symbols) and viability (open symbols) were quantitated as described in the text. Samples lacking detectable culturable cells are plotted on the log scale as 1 cell per ml. The results presented here are from one of three trials. Bars represent standard errors.
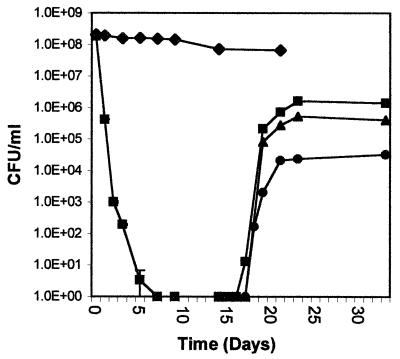
To confirm that the lack of observed growth was due to cells being VBNC and not dead, resuscitation of copper-induced VBNC ES80 cells was examined. At various times after VBNC induction, samples were removed and cells were collected onto a cellulose nitrate filter, washed with 3 to 5 volumes of 500 μM EDTA, suspended in the original volume of 0.9% NaCl, and incubated at 25°C. Samples of cells were diluted 25- and 1,000-fold in 0.9% NaCl. At various times, samples of cells were removed, diluted if necessary in 0.9% NaCl, and plated onto LB medium to monitor culturability; any resulting colonies were transferred to LB medium containing rifampin (100 μg per ml). It was found that, if this treatment was performed within 2 weeks of loss of culturability, the washed cells regained culturability to a level proportional to their dilution and to a level expected if resuscitation, and not growth, was occurring (Fig. 2). That all colonies were rifampin resistant indicated that the colonies did not arise from contamination. Cultures that contained no detectable culturable cells for 4 weeks did not undergo resuscitation under these conditions.
FIG. 2.
Resuscitation of copper-induced VBNC E. coli. Cells grown and harvested as described in the text were placed in 0.9% NaCl liquid cultures with (■) or without (⧫) 500 μM CuSO4. At various times, culturability was quantitated as described in the text. At day 14, VBNC cells were harvested, washed, and suspended in copper-free 0.9% NaCl either undiluted (■), after 25-fold dilution (▴), or after 1,000-fold dilution (●), as described in the text. Culturability was then monitored. Samples lacking detectable culturable cells are plotted on the log scale as 1 CFU per ml. The results presented here are from one of three trials. Bars represent standard errors.
The reappearance of culturable cells could be due to VBNC cells being resuscitated or to the growth of a few undetected culturable cells (12). That no nutrients were known to be present in the liquid cultures argues against the latter possibility. If growth of a few culturable cells was occurring due to the presence of some unknown nutrients, the concentrations of culturable cells present in the EDTA-washed cultures would be approximately the same regardless of the dilution and would not have been proportional to the fold dilution. Furthermore, that the cells were washed makes it unlikely that lysed dead cells could have provided nutrients, especially in an amount sufficient to support growth to the observed levels.
An alternate explanation for the reappearance of culturable cells is that the viable cells present prior to washing with EDTA were nonculturable, not because they were VBNC but because they were in a hydrogen peroxide-sensitive state. The observation of a hydrogen peroxide-sensitive culturable cell population (3) was used to argue against an earlier report of resuscitation of nonculturable Vibrio vulnificus cells (18). Hydrogen peroxide-sensitive cells grow on rich medium only when plates are supplemented with sodium pyruvate or catalase (3). Hence, V. vulnificus cells that did not give rise to colonies on unsupplemented rich medium may not have all been VBNC, and the subsequent appearance of culturable cells following warming (18) may not have been the result of resuscitation but may have resulted instead from the growth of residual hydrogen peroxide-sensitive culturable cells. We wanted to determine if copper was inducing a similar hydrogen peroxide-sensitive culturable cell population. The culturability of copper-treated cells was assayed as described above; in addition, samples were plated on 25-ml agar plates supplemented with 80 mg of sodium pyruvate (3). In both of two trials, there was no difference in the decrease in the number of culturable cells on plates containing or lacking sodium pyruvate (data not shown). Therefore, the appearance of culturable cells shown in Fig. 2 is due to resuscitation and not the growth of a hydrogen peroxide-sensitive culturable cell population.
These results indicate that copper can induce E. coli to enter the VBNC condition. The concentrations of copper used in this study include those considered toxic (9, 13) and are higher than those used to examine copper-induced cell injury (6, 17). That high concentrations of copper do not kill all cells suggests that current growth-based microbiological methods for assaying toxicity result in an undercount of the number of viable cells through incorrect scoring of VBNC cells as dead. Additional non-growth-based assays should be used to obtain accurate toxicity data. Although cells could be resuscitated only from VBNC cultures less than 1 month old, it is possible that other conditions could induce resuscitation in older cultures. Current studies are under way to determine if similar results can be achieved with other heavy metals.
Acknowledgments
We are grateful to Joerg Hacker (University of Wurzburg) and Henry Wu (Uniformed Services University, Bethesda, Md.; deceased) for bacterial strains and to Jordan Gottlieb for assistance with collecting preliminary data.
This work was supported by the University of North Carolina at Charlotte.
REFERENCES
- 1.Alexander E, Pham D, Steck T R. The viable-but-nonculturable condition is induced by copper in Agrobacterium tumefaciens and Rhizobium leguminosarum. Appl Environ Microbiol. 1999;65:3754–3756. doi: 10.1128/aem.65.8.3754-3756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barer M R, Harwood C R. Bacterial viability and culturability. Adv Microb Physiol. 1999;41:93–97. doi: 10.1016/s0065-2911(08)60166-6. [DOI] [PubMed] [Google Scholar]
- 3.Bogosian G, Aardema N D, Bourneuf E V, Morris P J L, O'Neil J P. Recovery of hydrogen peroxide-sensitive culturable cells of Vibrio vulnificus gives the appearance of resuscitation from a viable but nonculturable state. J Bacteriol. 2000;182:5070–5075. doi: 10.1128/jb.182.18.5070-5075.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole S P, Cirillo-Daniela D, Kagnoff M F, Guiney D G, Eckmann L. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infect Immun. 1997;65:843–846. doi: 10.1128/iai.65.2.843-846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz-Baez M C, Roldan F. Evaluation of the agar plate method for rapid toxicity assessment with some heavy metals and environmental samples. Environ Toxicol Water Qual. 1996;11:259–263. [Google Scholar]
- 6.Domek M J, Robbins J E, Anderson M E, McFeters G A. Metabolism of Escherichia coli injured by copper. Can J Microbiol. 1987;33:57–62. doi: 10.1139/m87-010. [DOI] [PubMed] [Google Scholar]
- 7.Gauthier M J. Environmental parameters associated with the viable but nonculturable state. In: Colwell R R, Grimes D J, editors. Nonculturable microorganisms in the environment. Washington, D.C.: American Society for Microbiology; 2000. pp. 87–112. [Google Scholar]
- 8.Ghezzi J I, Steck T R. Induction of the viable but non-culturable condition in Xanthomonas campestris pv. campestris in liquid microcosms and sterile soil. FEMS Microbiol Ecol. 1999;30:203–208. doi: 10.1111/j.1574-6941.1999.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S D, Lee B T O, Camakaris J, Wu H C. Identification of cutC and cutF (nlpE) genes involved in copper tolerance in Escherichia coli. J Bacteriol. 1995;177:4207–4215. doi: 10.1128/jb.177.15.4207-4215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hacker J, Ott M, Blum G, Marre R, Heeseman J, Tschape H, Goebel W. Genetics of Escherichia coli uropathogenicity: analysis of the O6:K15:H31 isolate 536. Zentbl Bakteriol. 1992;276:165–175. doi: 10.1016/s0934-8840(11)80003-4. [DOI] [PubMed] [Google Scholar]
- 11.Huq, A., I. N. G. Rivera, and R. R. Colwell. Epidemiological significance of viable but nonculturable microorganisms, p. 301–323. In R. R. Colwell and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. American Society for Microbiology, Washington, D.C.
- 12.Kell D, Kaprelyants A, Weichart D, Harwood C, Barer M. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Leeuwenhoek. 1998;73:169–187. doi: 10.1023/a:1000664013047. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Kwasniewska K. An improved agar plate method for rapid assessment of chemical inhibition to microbial populations. Bull Environ Contam Toxicol. 1981;27:289–294. doi: 10.1007/BF01611022. [DOI] [PubMed] [Google Scholar]
- 14.Nies D H. Microbial heavy-metal resistance. Appl Microbiol Biotechnol. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 15.Rigsbee W, Simpson L M, Oliver J D. Detection of the viable but nonculturable state in Escherichia coli O157:H7. J Food Saf. 1997;16:255–262. [Google Scholar]
- 16.Rodriguez-Montelongo L, de la Cruz-Rodriguez L C, Farias R N, Massa E M. Membrane-associated redox cycling of copper mediates hydroperoxide toxicity in Escherichia coli. Biochim Biophys Acta. 1993;1144:77–84. doi: 10.1016/0005-2728(93)90033-c. [DOI] [PubMed] [Google Scholar]
- 17.Singh A, Yeager R, McFeters G A. Assessment of in vivo revival, growth, and pathogenicity of Escherichia coli strains after copper- and chlorine-induced injury. Appl Environ Microbiol. 1986;52:832–837. doi: 10.1128/aem.52.4.832-837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitesides M D, Oliver J D. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl Environ Microbiol. 1997;63:1002–1005. doi: 10.1128/aem.63.3.1002-1005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]