Abstract
Heparin is a subject of ever-growing interest for laboratory researchers and pharmaceutical industry. One of the driving factors is its critical life-saving drug status, which during the COVID-19 pandemic has assumed a central role in disease treatment and/or prevention. Apart, heparin is one amongst few drugs enjoying a “demand constant” status. In 2020, heparin market size was valued to US$6.5 bn., and given the ongoing stability in the COVID-19 health crisis, it is expected to reach US$11.43 bn. by 2027 with yearly growth rate momentum (CAGR) of 3.9% during the forecast period (Pepi et al., Mol Cell Proteomics 20:100,025, 2021). As patent is a limited monopoly, every year, many patents on low molecular weight heparin (LMWH; a chemically or enzymatically degraded product of unfractionated heparin) are losing market exclusivity worldwide, inviting the generic/biosimilar drug manufacturers to capture market share with cheaper drug products. By tracking patent expiration, drugs in patent litigation, regulatory setbacks for innovator companies (such as those seeking data exclusivity or patent term extension), or other unexpected events affecting market demand and competition, generics can make investment decisions in manufacturing off-patent LMWH drug products of commercial significance. However, given the US Food and Drug Administration (FDA), European Medicine Agency (EMA), Drug Regulatory Authority of Pakistan (DRAP), and other regulatory authorities scientifically rigorous standards for generic/biosimilar LMWH drug products marketing approval, the market is secured and momentous for drug makers that could demonstrate through scientific and clinical dataset that the generic/biosimilar LMWH drug product is of the same quality and purity as the innovator drug product. This study presents an overview of the patent landscape of commercially available LMWHs and advanced analytical techniques for their structural and biochemical characterization for quality control and quality assurance during manufacturing and post-marketing. The study also covers FDA, EMA, Health Canada, and DRAP’s current approaches to evaluating the generic/biosimilar LMWH drug products for quality, safety including immunogenicity, and efficacy.
Keywords: Biosimilars, Enoxaparin, Generics, Heparin, Low molecular weight heparin, Patent, Regulatory authority
Introduction
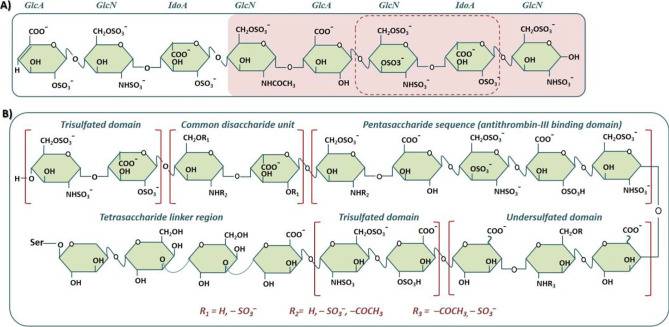
Heparin (also known as “standard heparin”) is a highly sulfated glycosaminoglycan (GAG) of animal origin having weight-average molecular weight (Mw) of ~ 12,000 Da corresponding to ~ 40 saccharide units [1, 2]. Since 1939, it has been used as a pharmaceutical intervention in a number of diseases including thrombosis, which is the formation of thrombus (blood clot) that sometimes may lead to pulmonary embolism, a potentially life-threatening condition [3]. Heparin structure is characterized by (i) repeating, 1 → 4-glycosidically linked disaccharide building blocks composed of one uronic acid (either glucuronic acid (GlcA) or iduronic acid (IdoA) and one glucosamine moiety (GlcN) that is either N-sulfated or N-acetylated; (ii) various substitution patterns of sulfation in the form of O-sulfation at the C2 position of IdoA, and C3 and C6 position of GlcN, or in the form of N-sulfation at the C2 position of GlcN [4]; (iii) a unique pentasaccharide sequence — GlcNS/Ac6S-GlcA-GlcNS3S6S-IdoA2S-GlcNS6S — distributed within the heparin polymer, having high affinity for binding to antithrombin III (ATIII); and (iv) a partially characterized octadecasaccharide sequence that together with pentasaccharide sequence activate ATIII to inhibit coagulation factor Xa and factor II (prothrombin) [5] (Fig. 1A).
Fig. 1.
A Heparin with a representative polysaccharide containing four disaccharide building blocks composed of one uronic acid (UA) and one glucosamine (GlcN) moiety. The disaccharide sequence — GlcNS3S6S-IdoA2S — in the dashed frame (FG) constitutes the highly sulfated region and major repeating structural unit within heparin while the block-shaded pink is the pentasaccharide sequence (or antithrombin II–binding domain). One of the two UA residues (iduronic acid, IdoA) present in the pentasaccharide sequence is consistently sulfated at the C-2 position, whereas the hydroxyl groups (OH) at both C-2 and C-3 of the other uronic moiety (glucuronic acid, GlcA) are unsubstituted. B A representative heparin comprising (left to right continuing to lower panel): a trisulfated domain, a common disaccharide unit, a pentasaccharide sequence (antithrombin III–binding domain), a trisulfated domain, and a tetrasaccharide linker region, GlcA-Gal-Gal-Xyl-Ser (source: Wang and Chi [5] Recent advances in mass spectrometry analysis of low molecular weight heparins. J. Chin. Chem. Lett., 2018, 29(1): 11–18; Ekre et al. Use of chemically modified heparin derivatives in sickle cell disease. US9480702 (2016))
Heparin also has a linkage tetrasaccharide sequence — GlcA-Gal-Gal-Xyl-Ser — at its reducing end that covalently link heparin to serine core of proteoglycan during synthesis and elongation [5] (Fig. 1B). The disaccharide sequence — IdoA2S-GlcNS6S — is the major repeating structural unit and constitutes the highly sulfated region of heparin [5].
Heparin exerts its anti-coagulation activity (anti-IIa activity) through high-affinity binding to and activation of ATIII, which is a protease inhibitor including thrombin (factor IIa) and factor Xa [6]. Commercial preparations of heparin are available from marketing companies including the following: Abbott, Organon, Riker, Invenex, Baxter, Calibiochem, Sigma Tau, Changzhou Qianhong CQ Biopharma, Nanjing King Friend, and Upjohn. A major adverse effect associated with heparin is the heparin-induced thrombocytopenia (HIT). Several studies have concluded heterogeneity in the GAG chains, their length, molecular weight (MW) distribution, and degree of sulfation as key factors for heparin varying binding tendency with other components in the blood plasma and consequent neutralization [1, 7]. Parallel studies regard non-template-driven biosynthesis, the principal cause of complexity, and polydispersity in the GAG chains [1, 8]. Consequent upon Andersson et al.’s [9] findings that LMWHs of different MWs influence the coagulation process differently, several heparin fragments with more selective and predictable pharmacological action had been developed [2] and are the subject of proprietary rights in the USA, Europe, and other countries where substantive patent laws are practiced and enforced.
The very first patent on LMWH dalteparin by Lindahl et al. [10] was expired in 2000, opening-up the market for low costs generic/biosimilar competition, and in parallel fuelling the quest for further innovations and patent protection. As more innovative LMWH products went generic, analytical characterization, control of quality, and regulations including consistency in heparin-derived products supply chains become more and more challenging. Several top-down and bottom-up studies on extensive characterization of LMWH complex structural features for quality control and quality assurance and understanding their diversifying role in medicine and pharmacology [11], decrypting their non-template driven biosynthesis to fix heterogeneity and polydispersity in their quality attributes [8], establishing correlation between their structure and biological activity [12], and discovering new clinical applications [13] are available in the published literature. However, amongst these, we find very few reports that specifically highlight the patent stats of commercial LMWH drug products for the purposes of defining the boundaries where the generic drug manufacturers have freedom to operate, discuss advances in the analytical techniques that generic/biosimilar drug manufacturers can utilize for the purposes of establishing that the generic/biosimilar LMWHs contain the same active ingredient as the innovator drug product, and conduct residual uncertainty risk assessment studies to demonstrate the absence of any clinically meaningful differences between the generic and the innovator drug products for the purposes of regulatory approval. This review provides information on this little studied area of technical advancements and commercial value, which the scientific researchers, medical practitioners, and generic/biosimilar drug makers shall find of equal interest.
LMWHs and Production Methods
European Pharmacopoeia (EP) 6.0 (01/2008:0828,2041–2043) defines LMWHs as “salts of sulfated glycosaminoglycan (GAGs) having a weight-average molecular weight (Mw) less than 8000 Da and for which at least 60% of the total mass has a molecular mass less than 8000 Da.” On an average, LMWH has Mw of ~ 4000 to ~ 8000 DA corresponding to ~ 6 to ~ 12 disaccharide units, which is nearly one third the size of the unfractionated heparin polysaccharide chains [14]. This reduction in molecular size renders the LMWHs more selective and predictable in therapeutic response, high in bioavailability and anticoagulation effect, and practical for subcutaneous infusion [15]. The LMWHs essentially retain the backbone structure of heparin, and only the termini of newly created oligosaccharide chains, modified through chemical or enzymatic depolymerization, display different chemical structures at the reducing and non-reducing ends [5]. These distinctive terminal structures are representative of the characteristics of each type of LMWHs. Nonetheless, a preferential cleavage either towards the highly sulfated region or the undersulfated region of heparin can substantially affect the disaccharide sequence distribution of resulting LMWHs and consequently the binding affinity of the pentasaccharide sequence for AT [16]. As a whole, these structural and compositional differences affect the pharmacological activity of the LMWH drug product; hence, deeper investigation of LMWH structure and function is critical for pharmaceutical control of quality and consistency in reproduction. So far, approved and commercially available LMWHs in various markets include the following: dalteparin (Fragmin®), enoxaparin (Lovenox®), nadroparin (Fraxodi®), tinzaparin (Innohep®), parnaparin (Fluxum®), bemiparin (Hibor®, Ivor®, Ivorat®, Ivormax®, Badyket®, Zibor®), sevuparin, ardeparin (Normiflo®), reviparin (Clivarin®), and certoparin (Sandoparin®, Embolex®). Table 1 provides production methods and structural characteristics including MWs, signature structures, and degree of sulfation of commercially available LMWHs.
Table 1.
Summary of commercial LMWH and ULMWH production methods and structural characteristics including molecular weight range, chain end groups, anti-coagulant activity (anti-Xa, anti-IIa), and degree of sulfation
| LMWH/ULMWH (INN) | Brand name | Manufacturer/marketing company | Mode of depolymerization/method of preparation | MW (Da) | MW (Da) | NRE | RE | Anti-Xa:anti-IIa ratio | Anti-Xa activity (IU/mg) | Anti-IIa activity (IU/mg) | Degree of sulfation/saccharide unit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dalteparin sodium |
Fragmin Boxol FR 860 Tedelparin |
Pfizer/Kabi/Pharmacia-Upjohn (US) | Deaminative cleavage with nitrous acid (HONO) | 5600–6400 | 6000–5000 | 2-O-sulfo-α-L-idopyranosuronic acid | 6-O-sulfo-2,5-anhydro-D-mannitol | 1.9–3.2:1 | 110–210 | 35–100 | 2.0–2.5 |
| Enoxaparin sodium |
Lovenox Clexane |
Sanofi-Aventis /Rhone-Poulenc/Aspen Pharma/Eurofarma Lab | Alkaline ß-eliminative cleavage of benzyl ester of heparin | 3500–5500 | 4500 | 2-O-sulfo-4-enepyranosuronic acid (or 2-sulfated 4,5-unsaturated uronic acid) | 2-N-sulfated-D-glucosamine; characterized by 1,6-anhydro ring structure/2-N,6-O-disulfo-D-glucosamine | 3.3–5.3:1 | 100–210 | 20–35 | ~ 2.0 |
| Tinzaparin sodium |
Innohep Logiparin |
LEO Pharma/Novo Nordisk/Braun/DuPont/Pharmion | ß-eliminative cleavage by heparinase/ | 5500 to 7500 | 6500 | 2-O-sulfo-4-enepyranosuronic acid (or 2-sulfated 4,5-unsaturated uronic acid) | 2-N,6-O-disulfo-D-glucosamine | 1.5–2.5:1 | 70–120 | 45–50 | 2.66 |
| Nadroparin calcium |
CY-216 Fraxiparin Seleparina |
Sanofi-Winthrop/Choay/Aspen/Italfarmaco | Deaminative cleavage with nitrous acid (HONO) | 4200 to 5500 | 4300 | 2-O-sulfo-α-L-idopyranosuronic acid | 6-O-sulfo-2,5-anhydro-D-mannitol | 2.5–4.0:1 | 95–130 | 27–37 | 2.0–2.5 |
| Bemiparin sodium |
Badyket Ivor Zibor Hibor Beparine |
Laboratorios Farmaceuticos Rovi S.A. /Sigma Tau/UCB /Biological Evans | Alkaline treatment with quaternary ammonium (NH4) salt of heparin | 3000 to 4200 | 3600 | 2-O-sulfo-4-enepyranosuronic acid | 2-N,6-O-disulfo-D-glucosamine | 8.0:1 | 80–100 | 10–12.5 | About 2 (WHO) |
| Sevuparin (DF02) | N/A | Modus Therapeutics AB/Dilafor AB | Selective oxidation of non-sulfated uronic acid residues in heparin by periodate | 6500 to 9500 | 5000 | 2-N,6-O-disulfo-D-glucosamine |
Glucosamine bound to a “remnant” residue (remnant = D-threonic acid)*
|
1.5:1 | < 10 | < 10 | 2.4 |
| Parnaparin sodium |
Alpha LMWH 86–02 Fluxum Minidalton OP-21–23 |
Alfa Wassermann SpA | Oxidative depolymerization with cupric ions (Cu2+) and hydrogen peroxide (H2O2) | 4500 to 5000 | 5000 | 2-O-sulfo-α-L-idopyranosuronic acid | 2-N,6-O-disulfo-D-glucosamine | 1.5–3.0:1 | 75–110 | 25–30 | 2.15 |
| Reviparin sodium |
Clivarin LU 473,111 |
Knoll AG/Abbott GmbH | Deaminative cleavage with nitrous acid (HONO) | 3400 to 4650 | 3900 | 2-O-sulfo-α-L-idopyranosuronic acid | 6-O-sulfo-2,5-anhydro-D-mannitol | 4.2:1 | 124 | 29 | 2.0–2.6 |
|
Ardeparin sodium (withdrawn from US market in 2000) (www.drugs.com) |
Normiflo RD 11,885 WY-90493-RD |
Wyeth-Ayerst/Hepar Industries/Pfizer | Oxidative depolymerization with hydrogen peroxide (H2O2) | 2000 to 15,000 | 5300–6500 | 2-O-sulfo-α-L-idopyranosuronic acid or saturated uronic acid | 2-N-acetyl-6-O-sulfo-D-glucosamine | 1.8:1 | 95–145 | 45–75 | 2.0–2.7 |
| Certoparin sodium |
Alphaparin Sandoparin Mono-Embolex NM, Troparin |
Novartis/Sandoz/Aspen in EU | Deaminative cleavage with isoamyl nitrite | 4200–6200 | 5200 | 2-O-sulfo-α-L-idopyranosuronic acid | 6-O-sulfo-2,5-anhydro-D-mannitol | 1.5–2.5:1 | 80–120 | 30–35 | 2.0–2.5 |
| Fondaparinux sodium | Arixtra | Aspen | Chemically synthesized by O-sulfation-hydrogenation-N-sulfation of pentasaccharide | 1500–3000 | 1728 | 2-N,6-O-disulfo-D-glucosamine | Methyl-2-N,6-O-disulfo-D-glucosamine (or 6-O-sulfo-2-(sulfoamino)-α-D-glucopyranoside) | Specific FXa inhibitor* | 930 | 0 | 1.6 |
|
AVE5026* (A derivative of enoxaparin) |
Semuloparin | Sanofi-Aventis | Chemoselective depolymerization by BEMP following ß-elimination | 2000 to 3000 | 2400 | 2-O-sulfo-4-enepyranosuronic acid (or 4,5 unsaturated uronic acid or 4-enopyranosyl uronate) | - | > 30:1 | 150–200 | 0.2 or < 5 | 2.2 |
|
RO-14 (A derivative of bemiparin) |
N/A | Laboratorios Farmaceuticos Rovi S.A | Chemoselective depolymerization of heparin in non-aqueous medium following ß-elimination | 1800 to 3000 | 2200 | 2-O-sulfo-α-L-idopyranosuronic acid (or 4-enopyranosyl uronate) | 2-N,6-O-disulfo-D-glucosamine | 9.7:1 | 80–140 | ≤ 10 | 2.0 |
Sources: Min Qiu, Shengjie Huang, Chuanhong Luo, Zhenfeng Wu, Binzhu Liang, Haozhou Huang, Zhimin Ci, Dingkun Zhang, Li Han, Junzhi Lin, Pharmacological and clinical application of heparin progress: An essential drug for modern medicine, Biomedicine & Pharmacotherapy 139 (2021) 111,561; Yan Y, Ji Y, Su N, Mei X, Wang Y, Du S, Zhu W, Zhang C, Lu Y, Xing XH. Non-anticoagulant effects of low molecular weight heparins in inflammatory disorders: A review. Carbohydr Polym. 2017 Mar 15;160:71–81. https://doi.org/10.1016/j.carbpol.2016.12.037. Epub 2016 Dec 21. PMID: 28,115,102; Hao, C., Sun, M., Wang, H., Zhang, L., & Wang, W. (2019). Low molecular weight heparins and their clinical applications. Progress in Molecular Biology and Translational Science.https://doi.org/10.1016/bs.pmbts.2019.02.003; Akhtar F, Wan X, Wu G, Kesse S, Wang S, He S. Low-Molecular-Weight Heparins: Reduced Size Particulate Systems for Improved Therapeutic Outcomes. Molecules. 2018 Jul 18;23(7):1757. https://doi.org/10.3390/molecules23071757. PMID: 30,021,958; PMCID: PMC6100363; Lühn S, Grimm JC, Alban S. Simple and rapid quality control of sulfated glycans by a fluorescence sensor assay–exemplarily developed for the sulfated polysaccharides from red algae Delesseria sanguinea. Mar Drugs. 2014 Apr 10;12(4):2205–27. https://doi.org/10.3390/md12042205. PMID: 24,727,392; PMCID: PMC4012468; Bisio A, Urso E, Guerrini M, de Wit P, Torri G, Naggi A. Structural Characterization of the Low-Molecular-Weight Heparin Dalteparin by Combining Different Analytical Strategies. Molecules. 2017 Jun 24;22(7):1051. https://doi.org/10.3390/molecules22071051. PMID: 28,672,818; PMCID: PMC6152074; *Gerotziafas GT, Petropoulou AD, Verdy E, Samama MM, Elalamy I. Effect of the anti-factor Xa and anti-factor IIa activities of low-molecular-weight heparins upon the phases of thrombin generation. J Thromb Haemost. 2007 May;5(5):955-62. 10.1111/j.1538-7836.2007.02477.x
BEMP 2-tert-butylimino-2-diethylamino-1,3-dimethyl-perhydro-1,2,3-diaza-phosphorine
*Source: WO2009007224A1; NIH — National Center for Advancing Translational Sciences, Inxight: Drugs;, https://drugs.ncats.io/substance/V72OT3K19I, accessed: June 30, 2022
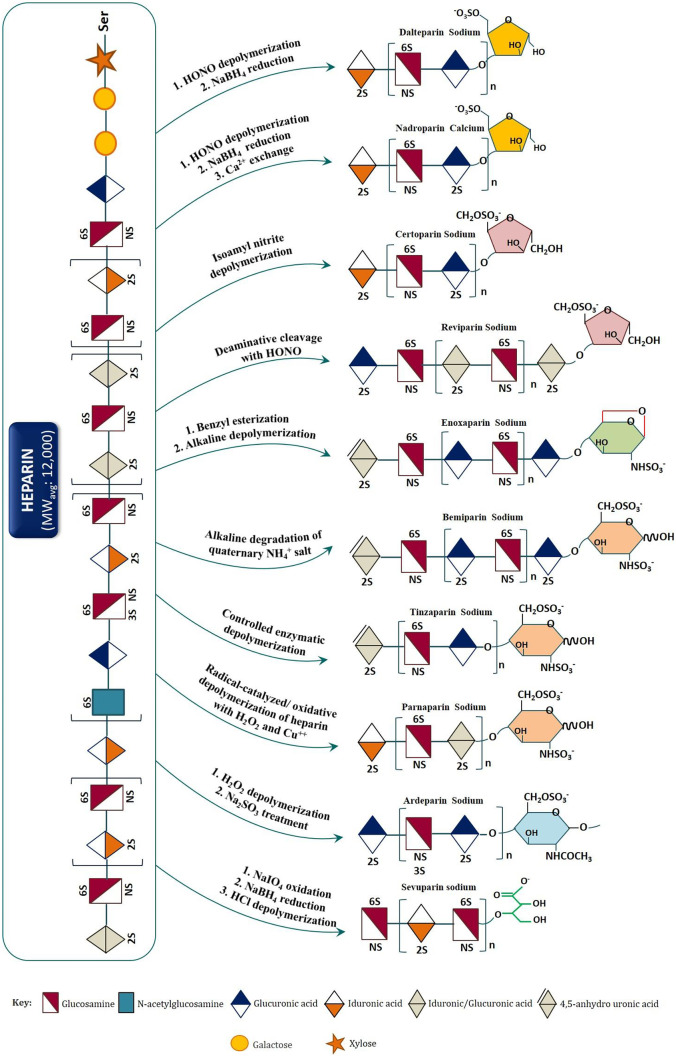
Enoxaparin sodium is the most commonly used LMWH derivative. It is derived from standard heparin through a controlled chemical ß-elimination reaction comprising (i) cleaving the polysaccharide backbone and (ii) hydrolyzing the residual esters under alkaline conditions [17]. The most part of oligosaccharide chains in enoxaparin have unsaturated 4-enopyranosuronate residues at their non-reducing ends, and only a minority, accounting 15–25% of the total oligosaccharide chains, has unnatural 1,6-anhydro amino sugar residues at the reducing ends [18]. These unnatural structures at termini constitute the fingerprints or signature structure of enoxaparin [19]. In other instances of LMWH derivatives, such as dalteparin, nadroparin, and reviparin, depolymerization of heparin polysaccharides is carried out through controlled deaminative cleavage using nitrous acid (HNO2), followed by sodium borohydride (NaBH4) reduction. This cleavage results in oligosaccharide fragments with reducing ends that are modified to 6-O-sulfo-2,5-anhydro-D-mannitol [20]. In case of tinzaparin sodium, the depolymerization is carried out enzymatically, in particular, with a highly purified heparinase of bacterial origin. Heparinase breaks the heparin saccharide chains between the anomeric carbon (carbonyl carbon) of an N-sulfate-glucosamine and the following uronic acid motif, creating an unsaturated uronic acid structure at its NRE. Ardeparin sodium represents a LMWH that is obtained by oxidative depolymerization of heparin with hydrogen peroxide while parnaparin sodium is obtained by oxidative depolymerization of heparin with Cu+ [2] and hydrogen peroxide. Both have saturated uronic acid residues at the NRE of the chain. Still, selective oxidation of non-sulfated uronic acid residues in heparin by periodate (NaIO4), alkaline treatment with quaternary ammonium (NH4) salt of heparin, deaminative cleavage of heparin polysaccharide using isoamyl nitrite, and chemo-selective depolymerization of heparin in non-aqueous medium (or using 2-tert-butylimino-2-diethylamino-1,3-dimethyl-perhydro-1,2,3-diaza-phosphorine (BEMP)) have resulted in different LMWH and ultra-LMWH products, with distinct groups at non-reducing and reducing ends of the oligosaccharide/polysaccharide chains. Summarily, different cleavage methods (enzymatic or chemical) create LMWH products containing different active ingredients with specific MWs, disaccharide building block compositions, and distribution of their sequences in the oligosaccharide chains, as well as distinct structural signatures and hence are not substitutable (see Fig. 2).
Fig. 2.
Scheme of depolymerization (enzymatic or chemical) used to prepare commercially available LMWHs from standard heparin. Signature structures (reducing end groups) are at the extremes right end. Identical groups are shown with same colors. (Sources: Wang Z. and Chi L. Recent advances in mass spectrometry analysis of low molecular weight heparins (2018). J. Chin. Chem. Lett., 29(1): 11–18; Yan Y, Ji Y, Su N, Mei X, Wang Y, Du S, Zhu W, Zhang C, Lu Y, Xing XH (2017). Non-anticoagulant effects of low molecular weight heparins in inflammatory disorders: A review. Carbohydr Polym., 160:71–81. 10.1016/j.carbpol.2016.12.037; Fu L. et al. (2015). Bioengineered heparins and heparan sulfates, Adv. Drug Deliv. Rev., http://dx.doi.org/10.1016/j.addr.2015.11.002; Sánchez-Ferrer, C. F. (2010). Bemiparin. Drugs, 70, 19–23. 10.2165/1158581-s0-000000000-00000; Patents: US9475888; US10023659; WO2009007224A1; US9012229B2 (Assignee: Hangzhou Jiuyuan Gene Engineering Co., Ltd. and Shanghai Institute of Organic Chemistry, CAS); Web: Japanese Accepted Names (JAN) Name and Structure Database (National Institute of Health Sciences, https://jpdb.nihs.go.jp/jan/DetailList_en.aspx?submit=all_alp+Search&keyword=Reviparin+Sodium, accessed: April 20, 2021) Japanese Accepted Names for Pharmaceuticals (JAN Database) jpdb.nihs.go.jp)
Commercial LMWH Patent Ecosystem
Globally, there are more than 5000 patents covering production and/or purification methods, new therapeutic applications, dosing regimens, new formulations/mixtures, and advanced analytical approaches for structural characterization of LMWHs including the most widely used enoxaparin/enoxaparin sodium [21], but our focus here is to discuss some of the key patents (see Table 2) on the commercially available LMWHs and ultra-low molecular weight heparins (ULMWHs) so that manufacturers of generic/biosimilar LMWHs may know the boundaries of competitor’s IP portfolio and their freedom-to-operate in the relevant IP ecosystem.
Table 2.
Summary of commercial LMWH and ULMWH patent position including the principal claim defining the scope of patent (whether product, process, product-by-process patent) and the date of expiry
| LMWH | Patent | Current Owner/ Assignee | Filed on | Claim Type/Claim 1 | Patent Expiry |
|---|---|---|---|---|---|
| Dalteparin | EP0014184 | Pfizer Health/ Kabi AB |
07-01-1980 (08-01-1979 SE) |
“Compound/ Heparin fragments, characterized by 14-18 sugar units, the disaccharide unit L-iduronosyl-2-O-sulphate-N-sulpho-D-glucosamine-6-O-sulphate” | 18-10-2006 |
| US4303651 | Pfizer Health / Kabi AB |
04-01-1980 (08-01-1979 SE) |
“Compound/Heparin fragments having 14-18 sugar units, wherein the main component is the disaccharide unit L-iduronosyl-2-O-sulphate-N-sulpho-D-glucosamine-6-O-sulphate” | 04-01-2005 | |
| EP0048231 | Phadia AB/ Kabi AB |
10-09-1981 (15-09-1980 SE) |
“Compound/ Oligosaccharide comprising 4-8 monosaccharide units” | 29-06-2005 | |
| Enoxaparin | US4990502 | Sanofi-Aventis |
15-04-1988 (16-04-1987 FR) |
“Product / A composition of heparins of low molecular weight and their pharmaceutically acceptable salts” | 15-04-2008 |
| US4841041 |
Sanofi/ Akzo Nobel |
12-07-1988 (20-07-1987 EP) |
Product/ Pentasaccharide of the formula: | 12-07-2008 | |
| EP0287477 | Sanofi-Aventis |
15-04-1988 (16-04-1987 FR) |
“Product / Composition of heparins of which 90% have a molecular weight between 3,600 and 11,000 Da” | 15-04-2008 | |
| US5707973 | Aventis/Rhone Poulenc |
09-12-1994 (23-04-1991 FR) |
“Product/mixture of sulfated oligosaccharides having the structure of constituent oligosaccharides of heparin, that has a mean molecular mass of 6±0.6 kD” | 13-01-2015 | |
| US5389618 (Re-issued 38743) | Aventis/ Rhone Poulenc |
16-07-1993 (26-06-1990 FR) |
“Compound/heterogeneous intimate admixture of sulfated heparinic polysaccharides” | 14-02-2012 | |
| US5849721 | Aventis Pharma SA |
06-06-1995 (07-02-1992 FR) |
“Product / A mixture of sulphated oligosaccharides obtained from native or depolymerized heparin comprising at least 70% of oligosaccharides having a molecular weight between 5,400 and 7,800 daltons” | 15-12-2015 | |
| US6534481 | Sanfo-Sythelabo/Akzo Nobel |
08-07-1997 (19-07-1996 FR) |
“Product/Polysaccharide comprising a region for binding to antithrombin III consisting of a sequence of five monosaccharides bearing in total two carboxylic acid functions” | 08-07-2017 | |
| US6617316 | Aventis Pharma SA |
20-10-2000 (22-10-1999 FR) |
64118920891500“Compound/A purified oligosaccharide of formula”: | 14-02-2022 | |
| US6969705 | Aventis Pharma SA |
23-07-2001 (21-07-2000 FR) |
“Product/ A composition comprising at least one salt chosen from alkali and alkaline-earth metal salts of at least one sulphated polysaccharide of heparin” | 24-10-2021 | |
| US6608042 | Aventis Pharma SA |
26-03-2001 (28-03-2000 FR) |
70231030099000“Product/pharmaceutical composition comprising one or more oligosaccharides of formula”: | 26-03-2021 | |
| US7956046 | Aventis Pharma SA |
27-07-2004 (24-07-2003 FR) |
“Compound/An oligosaccharide mixture, wherein: the oligosaccharide mixture comprises an anti-Xa activity of from 200 IU/mg to 450 IU/mg” | 22-03-2026 | |
| US7812007 | Aventis Pharma SA |
01-04-2005 (21-07-200 FR) |
“Method/Method of treating the proliferation of smooth muscle cells in a patient” | 23-07-2021 | |
| US7687274 | Aventis Pharma SA |
14-01-2008 (22-02-2004 FR) |
“Method/Method of assaying a sample chosen from heparin, low-molecular-weight heparin, ultra low molecular weight heparin, and oligosaccharides” | 25-03-2024 | |
| US8492352 | Sanofi-Aventis |
24-08-2009 (26-08-2008) |
“Product/ Synthetic polysaccharide of formula: Methyl (2-[N-(6-aminohexanoyl)]-2-deoxy-3,4-di-O-methyl-6-O-sulfonato-α-D-glucopyranosyl)-(1→4)-(2,3-di-O-methyl-β-D-glucopyranosyluronic acid)-(1→4)-(2,3,6-tri-O-sulfonato-α-D-glucopyranosyl)-(1→4)-(2,3-di-O-methyl-α-L-idopyranosyluronic acid)-(1→4)-2,3,6-tri-O-sulfonato-α-D-glucopyranoside sodium salt” | 01-09-2029 | |
| US8071570 | Aventis Pharma SA |
09-05-2011 (10-10-2002 FR) |
“Product/ A mixture of sulfated oligosaccharides” | 08-10-2023 | |
|
Tinzaparin B-eliminative cleavage by heparinase/enzymatic depolymerization |
EP0244235 | Novo Nordisk AS |
29-04-1987 (30-04-1986 DK) |
“Process/ process for the production of low molecular weight heparin (LMW-heparin) by enzymatic depolymerization of heparin” | 29-04-2007 |
| US5106734 | LEO/ Pharmion/Novo Nordisk AS |
29-04-1987 (30-04-1986 DK) |
“Process/ A continuous process for the production of a low molecular weight heparin product of a predetermined molecular weight by enzymatic depolymerization of heparin” | 21-04-2009 | |
|
Nadroparin Fraxiparin Nitrous acid depolymerization |
DE2944792 | Choay SA/ GlaxoSmithkline/Aspen |
11-06-1979 (11-06-1978 FR) |
“Compound/Mucopolysaccharide fraction obtainable from a material based on heparin” | 11-07-1999 |
| US4401662 | Choay SA |
06-10-1980 (05-10-1979 GB) |
“Compound/Oligosaccharide fraction (1) comprises not more than 8 saccharide units, (2) of which one is an N-sulfate-3-O-sulfate-D-glucosamine unit ….” | 30-08-2000 | |
| US4486420 | Choay SA |
14-09-1981 (06-11-1978 FR) |
“Compound/Heparinic mucopolysaccharide fractions” | 04-12-2001 | |
| EP0037319 | DROPIC/Choay |
20-03-1981 (20-03-1981 FR) |
“Process/ A process for the preparation of mucopolysaccharides having higher antithrombic activity and a lower ratio of YW/USP” | 20-03-2001 | |
| US4500519 | Choay SA |
20-11-1981 (06-11-1978 FR) |
“Process/ A process for making mucopolysaccharide heparinic fractions which have the L-iduronosyl-2-O-sulfate-(1-alpha-4)-N-sulfo-D-glucosamine-6-O-sulfate disaccharide structural units of heparin with the O-sulfated iduronic component of heparin” | 19-02-2002 | |
| US4607025 | Choay SA |
20-12-1982 (28-04-1981 FR) |
167894016700500“Compound/A disaccharide of the formula wherein M is hydrogen, --SO3 Na or acetyl; Z is --SO3 Na or acetyl and R1 is hydrogen, methyl or sodium.” | 19-08-2003 | |
| US4474770 | Choay SA |
22-08-1983 (05-10-1979 GB) |
“Compound/Oligosaccharide fraction (1) comprises not more than 8 saccharide units, (2) of which one is an N-sulfate-D-glucosamine unit” | 02-10-2001 | |
| US4686288 | DROPIC/Choay |
13-12-1984 (20-03-1980 FR) |
“Process/ A process for the preparation of mucopolysaccharides having higher antithrombic activity and a lower ratio of YW/USP and lower YW titer than the starting mucopolysaccharides” | 11-08-2004 | |
| US4692435A | Sanofi-Aventis/ Choay SA |
24-03-1985 (06-11-1978 FR) |
“Process/ process for obtaining heparinic mucopolysaccharides” | 24-12-2004 | |
| US4801583 | Choay SA |
15-05-1985 (15-01-1982 FR) |
10293351016000“A pure, synthetic tetrasaccharide having the formula:” | 31-01-2006 | |
| US4804652 | Choay SA |
19-02-1985 (06-11-1978 FR) |
“Process/A process for making mucopolysacharide heparinic fractions” | 14-02-2006 | |
| US4788307 | Choay SA and Sanofi-Aventis |
29-01-1987 (30-04-1986 FR) |
“Compound/An oligosaccharide fraction of the heparin chain which has antithrombotic activity in vivo (as measured by the (Yin-Wessler test) lower than that of heparin” | 29-11-2005 | |
| US5599801 | Choay SA and Sanofi-Aventis |
06-05-1994 (07-05-1993 FR) |
“Compound/ heparin fraction resulting from nitrous depolymerization of heparin of natural origin having a content of total N-nitroso compounds not exceeding 500 ppb” | 06-05-2014 | |
| EP2314632 | Aventis Pharma SA |
22-03-2005 (24-03-2004EP) |
14136392148100“Compound/Substantially pure compound having the formula:” | 22-03-2025 | |
| Ardeparin | US4281108 | Hepar Industries |
02-06-1980 (28-01-1980 US) |
“Process/ Process for obtaining low molecular weight heparins endowed with elevated pharmacological properties” | 28-01-2000 |
| US4757057 | Pfizer Health AB |
07-01-1986 (09-08-1977 IT) |
“Process/Method of increasing the antithrombotic activity of mammalian blood relative to the anticoagulant activity” | 12-07-2010 | |
| US9068957 | Momenta Pharmaceuticals |
21-02-2012 (21-02-2011 US) |
“Method/Method of evaluating heparin preparations….” | 15-08-2032 | |
| Sevuparin | WO2009007224A1 | Sigma-Tau Industrie Farmaceutiche Riunite S.p.A |
10-07-2007 (10-07-2007 EP) |
11690355270500“Compound/ A heparin derivative having the formula”: | N/A |
| US9475888 | Dilafor AB |
19-12-2012 (19-12-2011 SE) |
“Compound/ chemically modified glycosaminoglycan, which glycosaminoglycan is selected from the group consisting of heparin and heparan sulfate” | 19-12-2032 | |
| US9480701 |
Modus Therapeutics AB/ Dilaforette AB |
19-12-2012 (19-12-2011 SE) |
“Compound/ Chemically modified heparin having an antifactor IIa activity and an antifactor Xa activity” | 19-12-2032 | |
| US9480702 |
Modus Therapeutics AB/ Dilaforette AB |
19-12-2012 (19-12-2011 SE) |
“Method/A method of treating sickle cell disease” | 19-12-2032 | |
| US10023659 | Dilafor AB |
19-06-2014 (19-06-2013 GB) |
“Process/Process for the preparation of a heparin derivative, the process comprising the consecutive steps of: (i) oxidising an acidic aqueous solution of unfractionated heparin by addition of an oxidising agent” |
10-09-2034 | |
| Parnaparin | US4791195 | Opocrin SpA |
21-10-1986 (08-03-1983 IT) |
“Compound/ heparin fraction which is a mixture of oligosaccharides containing an average of 13-17 monosaccharides” | 13-12-2005 |
| US4973580 | Opocrin SpA |
10-05-1989 (17-05-1985 IT) |
“Compound/ The oligosaccharide fraction from dermatan sulfate which has molecular weight 4800 daltons” | 27-11-2007 | |
| US5010063 | Alfa Wassermann SpA |
26-05-1989 (10-06-1988 IT) |
“Compound/ Heparin derivative which exhibits signals in the 13C-NMR spectrum at about 53 and about 54 ppm” | 26-05-2009 | |
| EP0347588 | Alfa Wassermann SpA |
19-05-1989 (10-06-1988 IT) |
“Compound/Heparin derivative characterized by signals in the 13C-NMR spectrum at 53 and 54 ppm” | 19-05-2009 | |
| US5104860 | Alfa Wassermann SpA |
09-01-1990 (30-01-1989 IT) |
“Compound/Heparin derivative having a 13 C-NMR spectrum in the zone between 102 and 92 p.p.m. with the presence of a characteristic signal at about 101.3 p.p.m.” | 09-01-2010 | |
| EP0380943 | Alfa Wassermann SpA |
12-01-1990 (30-01-1989 IT) |
“Process/ process for the preparation of new heparinic derivatives” | 12-01-2010 | |
| EP0497162 | Alfa Wassermann SpA |
17-01-1992 (30-01-1991 IT) |
“Process/ Process for preparing Pharmaceutical compositions containing orally absorbable glycosaminoglycans” | 17-01-2012 | |
| US5430132 | Alfa Wassermann SpA |
13-04-1993 (17-04-1992 IT) |
“Compound/Glycosaminoglycan of molecular weight 3,000-50,000 Daltons” | 13-04-2013 | |
| US5430133 | Alfa Wassermann SpA |
13-04-1993 (17-04-1992 IT) |
“Compound/Glycosaminoglycan of molecular weight 3,000-50,000” | 13-04-2013 | |
| US5410039 | Alfa Wassermann SpA |
07-03-1994 (29-03-1993 IT) |
“Process/Process for the synthesis of a product glycosaminoglycan” | 07-03-2014 | |
| Reviparin | EP1284717 | Abbott GmbH |
29-05-2001 (30-05-2000 DE) |
“Product/Formulation based on at least one heparin, glycosaminoglycan or heparinoids” | 27-12-2006 |
| Bemiparin | EP0293539 | Laboratorios Farmaceuticos Rovi S.A. |
22-07-1987 (05-01-1987 ES) |
“Process/Process of depolymerising heparine having an average molecular weight between 10.000 and 20.000 dalton to produce a depolymerized product” | 22-07-2007 |
| US981955 | Lopez Lorenzo L |
26-02-1990 (28-06-1988 US) |
“Process/Method of depolymerizing heparin comprising contacting a fully salified quaternary ammonium salt of heparin with a quaternary ammonium hydroxide in a non-aqueous polar solvent” | 26-02-2010 | |
| EP2308497 | Laboratorios Farmaceuticos Rovi S.A. |
30-06-2009 (07-01-2008 ES) |
“Product/Pharmaceutical composition comprising low or very low molecular weight heparins for use in the treatment of chronic ulcers, characterized in that the plasma half-life of the heparin is between 2.3 and 6.9 hours and the average daily dose of the heparin is between 5,400 and 10,000 IU/day” | 30-06-2029 | |
| EP2391352 | Laboratorios Farmaceuticos Rovi S.A. |
29-01-2010 (30-01-2009 EP) |
“A pharmaceutical form comprising a glycosaminoglycan selected from the group consisting of: bemiparin, fondaparinux and enoxaparin and its pharmaceutically acceptable salts” | 29-01-2030 | |
| US8802156 | Laboratorios Farmaceuticos Rovi S.A. |
29-07-2011 (14-11-2007 EP) |
“Product/Pharmaceutical form comprising a glycosaminoglycan with anions and a compound presenting cations of pH independent quaternary ammonium groups” | 02-04-2029 | |
| Certoparin | US4351938 | Riker Laboratories |
19-05-1980 (19-05-1980 US) |
“Process/Process which comprises reacting a heparin salt with from about 5 to 100 milliliters of an aqueous nitrous acid solution per gram of the heparin salt” | 19-05-2000 |
| Fondaparinux | US7468358 | ParinGenix/Cantex |
27-10-2004 (16-06-2004 US) |
“Method/Method for treating heparin-induced thrombocytopenia syndrome in a patient comprising administering sulfated polysaccharide comprises 2-O desulfated heparin or 2-O, 3-O desulfated heparin” | 07-09-2025 |
| US8288515 | Reliable Biopharmaceutical LLC. |
30-07-2010 (31-07-2009 US) |
“Compound/A compound of Formula I:” | 11-02-2031 | |
| US8420790 | Reliable Biopharmaceutical LLC. |
29-10-2010 (30-10-2009 US) |
“Process/Process for the preparation of a protected heparinic pentasaccharide precursor to Fondaparinux sodium having the structure” | 18-04-2031 | |
| US9089484 | Novo Nordisk AS |
28-03-2011 (26-03-2010US) |
“Method/A method for obtaining a reproducible bioavailability of fondaparinux” | 13-07-2032 | |
| EP2809678 | Reliable Biopharmaceutical LLC |
02-02-2012 (02-02-2011 PCT) |
“Process/Process for the preparation of a protected heparinic pentasaccharide precursor to Fondaparinux sodium” | 02-02-2032 | |
| US9255119 | Reliable Biopharmaceutical LLC |
18-04-2014 (31-07-2009 US) |
“Process/A process for making a compound of Formula I:” | 30-07-2030 | |
| CN109734757 | Huaibei Normal University |
11-03-2019 (11-03-2018 CN) |
“Process/Preparation method of a related substance B of fondaparinux sodium injection” | 11-03-2039 | |
| CN105175460 | Chongging University |
08-09-2015 (08-09-2014 CN) |
“Preparation method for monosaccharide fragment intermediate of fondaparinux sodium as anticoagulant drug” | 08-09-2035 | |
| Semuloparin AVE5026 |
EP1307491 (~WO 02008295) |
Aventis Pharma SA |
18-07-2001 (21-07-2000 FR) |
“Product/Mixtures of sulphated polysaccharides possessing the general structure of the constituent polysaccharides of heparin” | 18-07-2021 |
|
EP1556414 (~WO2004033503) |
Aventis Pharma SA |
08-10-2003 (10-10-2002 FR) |
“Product/Sulfated oligosaccharides having the general structure of the constituent polysaccharides of heparin” | 08-10-2023 | |
| EP1651677 | Aventis Pharma SA |
22-07-2004 (24-07-2003 EP) |
“Product/Oligosaccharide mixtures having the general structure of the constituent polysaccharides of heparin” | 22-07-2024 | |
| US8003623 | Aventis Pharma SA |
02-08-2007 (10-10-2002 FR) |
“Product/Mixture of sulfated oligosaccharides” | 19-08-2024 | |
| TW201038279 (~WO2010106519) | Sanofi-Aventis |
18-03-2010 (19-03-2009 EP) |
“Selection/A dose of 10 mg of AVE5026 for use in therapy in patients with severe renal impairment” | 18-03-2030 (If granted) | |
| WO2012072799A1 | Aventis Pharma SA |
02-12-2011 (02-12-2010 EP) |
“Assay/A method for the in vitro measurement of the biological activity of an Ultra Low Molecular Weight Heparin (ULMWH) sample, wherein said method is carried out relative to a standard comprising an ULMWH (semuloparin).” | N/A | |
| US9346894 | Sanofi-Aventis |
11-04-2012 (11-04-2011(FR) |
“A sulfonated polysaccharide having a polysaccharide of heparin which has a molecular weight of less than 8000 Daltons” | 23-09-2032 | |
|
RO-14 (a derivative of Bemiparin) |
EP1070503 | Laboratorios Farmaceuticos Rovi S.A. |
13-10-1999 (23-07-1999 ES) |
“Product/Composition of heparin of very low molecular weight, with the general formula:” | 13-10-2019 |
| US6384021 | Laboratorios Farmaceuticos Rovi S.A. |
03-11-1999 (23-07-1999 ES) |
“Product/Composition of heparin of very low molecular weight, with the general formula:” | 03-11-2019 | |
| EP2881404 | Laboratorios Farmaceuticos Rovi S.A. |
02-08-2013 (02-08-2012 ES) |
“Process/ Process for obtaining very low molecular weight heparin (VLMWH)” | 02-08-2033 |
The first European patent EP0014184A2 (~ US Patent No. 4303651A1; Lindahl et al.) describing LMWH — dalteparin sodium — was granted to Kabi AB in 1989 and is currently being marketed by Pfizer Health AB under the brand name Fragmin®. Going into the very details of this significant innovation, in 1980, Lindahl et al. [10] prepared a new heparin fragment through controlled nitrous acid depolymerization of heparin sodium in aqueous medium. The group suggested several other ways to preparing the new heparin fragment such as periodate oxidation, partial depolymerization with heparinase, partial depolymerization by esterification of carboxylic groups and subsequent alkaline R-elimination, and partial depolymerization by partial N-desulfation and subsequent deamination with nitrous acid. With very weak inhibitory effect on thrombin (factor IIa), the new heparin fragments had a very strong inhibitory effect on activated coagulation factor Xa that assumed a central position in the middle of the coagulation cascade. Structural analysis of the Lindahl’s LMWH revealed the same disaccharide unit (i.e., L-iduronosyl-2-O-sulfate-(1)-N-sulfo-D-glucosamine-6-O-sulfate) as the dominating component as in the starting material but with enhanced amount of unsulfated iduronic acid from 6 to 16% relative to the starting material. The US patent on dalteparin sodium was expired in 2005, but the non-patent data exclusivity still authorizes Pfizer to maintain their exclusive hold on the market until May 16, 2022. So far, no generic/biosimilar version of dalteparin is available in the USA through authorized channels [22].
The first US patent 5,389,618 (re-issued under No. 38,473) covering the most widely used LMWH — enoxaparin sodium — was expired on February 14, 2012. The patent was one of the two patents, listed in the Orange Book as the FDA’s approved drug product. The second US Patent 4, 692,435, was expired on December 24, 2004. The 5-year non-patent data exclusivity for enoxaparin as a compound had long expired in 1998. Enoxaparin sodium is manufactured by Sanofi-Aventis under the brand name Lovenox® (enoxaparin sodium injection in the USA) and Clexane® or Klexane® (in other countries). Sanofi-Aventis obtained enoxaparin sodium through ß-eliminative degradation of heparin benzyl ester under alkaline conditions (pH > 10), which they derived from porcine intestinal mucosa. For generic drug product manufacturers, to establish bioequivalence has been a potentially difficult phenomenon [23]. They need high-throughput technology to ensure reliable analytical characterization, pre-clinical evaluation, and immunogenicity assessment of generic/biosimilar LMWHs. Sandoz and Momenta had taken lead to seek approval from US-FDA to sell first generic version of Lovenox® post-patent expiry [24]. Earlier (2003), during the validity term of enoxaparin sodium patent, several companies such as Amphastar Pharmaceuticals and Teva Pharmaceutical Industries made joint efforts to seek US-FDA approval to sell generic versions of Lovenox in the US market. Sanofi filed patent infringement lawsuits against the said generic concerns before the US District Court, California [25]. The Court ruled against Sanofi and invalidated the patent on Lovenox on the ground of “inequitable conduct.” Sanofi appealed to the US Court of Appeals for the Federal Circuit that affirmed the District Court decision [26]. More so, the Supreme Court denied the petition for certiorari to Sanofi [27].
In a parallel action before FDA, on February 19, 2003, Sanofi filed a Citizen Petition requiring the authorities to withhold any abbreviated new drug application (ANDA) for generic version of Lovenox until (i) enoxaparin structure is fully characterized; (ii) the manufacturing process used to make generic enoxaparin is demonstrated to be equivalent to the process used by Sanofi for branded enoxaparin, or the ANDA is supported by safety and effectiveness data gathered through clinical trials; and (iii) the generic product contains 1,6-anhydro ring structure at the reducing ends of between 15 and 25% of its polysaccharide chains. Excepting the last request for presence of enoxaparin signature structure in the generic enoxaparin, FDA denied the petition on all other respects. Responding to Sanofi’s request for “manufacturing equivalence” of generic enoxaparin, FDA remarked that “to manufacture enoxaparin an ANDA applicant will- i) depolymerize heparin by chemical (alkaline) ß-elimination; and ii) adjust the process conditions such that they result in the same active ingredient as Lovenox enoxaparin.” FDA extended that to manufacture enoxaparin, the process conditions may be the same as used for the originator enoxaparin but these not necessarily need to [28].
While the US courts and FDA rulings had long cleared the road towards development of generic/biosimilar versions of off-patent Lovenox® at low costs and for increasing access to patients, nonetheless Sanofi’s concerns about therapeutic non-equivalence of the generic/biosimilar enoxaparin drug product due to chemical diversity in the disaccharide building blocks and corresponding distribution of their sequences in the polysaccharide chains with the innovator drug product shall still be remaining there, invigorating the need for developing methods for fine structural characterization and compositional analysis of LMWHs.
Sevuparin sodium (also known as DF02) is another distinctive heparin derivative invented by Ekre et al. and protected by Modus Therapeutics under the US Patent 9480701B2. Ekre et al.’s heparin fragment has a low anticoagulant activity and effectiveness for treating heparin-associated disorders such as malaria. The research group also developed a method for use of DF02 in the treatment of vaso-occlusive crisis in sickle cell disease. As per Ekre’s patent description, DF02 is a chemically modified heparin composed of polysaccharide chains having (i) at least 90% of the sulfate groups of source heparin; (b) a reduction in chemically intact saccharide sequences and a reduction in unsulfated iduronic acid and/or glucuronic acid units, when compared to source heparin; and (c) a predominant disaccharide represented by the structure: -IdoA2S-GlcNS6S-OR′- (wherein R′ = threonate (C4H7O5) residue). The fragment is derived from heparin sodium and involved selective oxidation of non-sulfated iduronic acid residues in heparin by sodium periodate (NaIO4), followed by reduction with sodium borohydrate (NaBH4) and treatment with HCl for polymer cleavage at the oxidized site. (Fig. 3 shows schematic reaction scheme of sevuparin preparation.) Structural analysis of DF02 using proton nuclear magnetic resonance (1H-NMR) confirmed the absence of unidentified residues or structures that are unexpected in the [1] H-NMR spectrum, hence the product’s greater stability. Both the patents stand granted in the name of Modus Therapeutics AB and shall expire on December 19, 2032. Right now, Modus Therapeutics is considering clinical development of sevuparin for the potential treatment of sepsis/septic shock and other inflammatory complications. This substantiates appreciation by leading pharmaceutical companies of the significance of understanding the precise structure of individual components in LMWHs for stringent quality control and improving clinical applications of LMWHs. The first clinical trial for new indications is expected to take start by the end of 2021 [29].
Fig. 3.
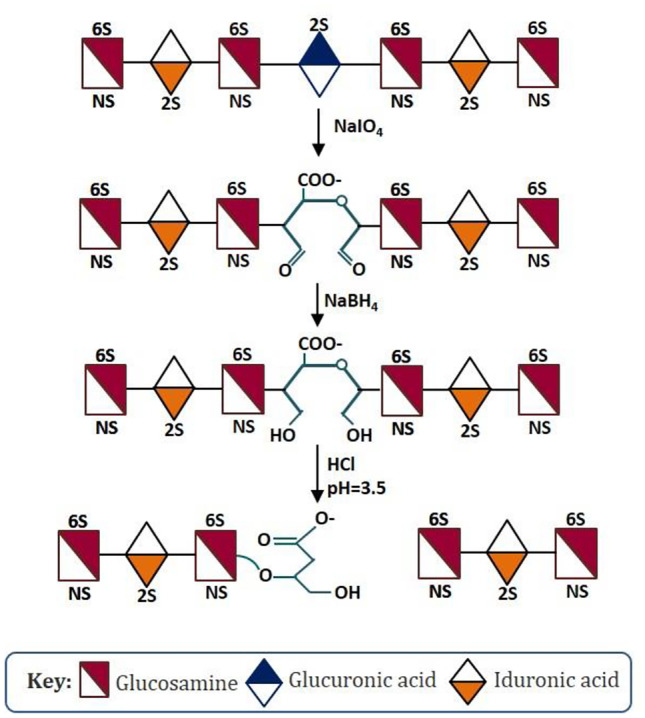
Schematic reaction scheme of sevuparin sodium preparation
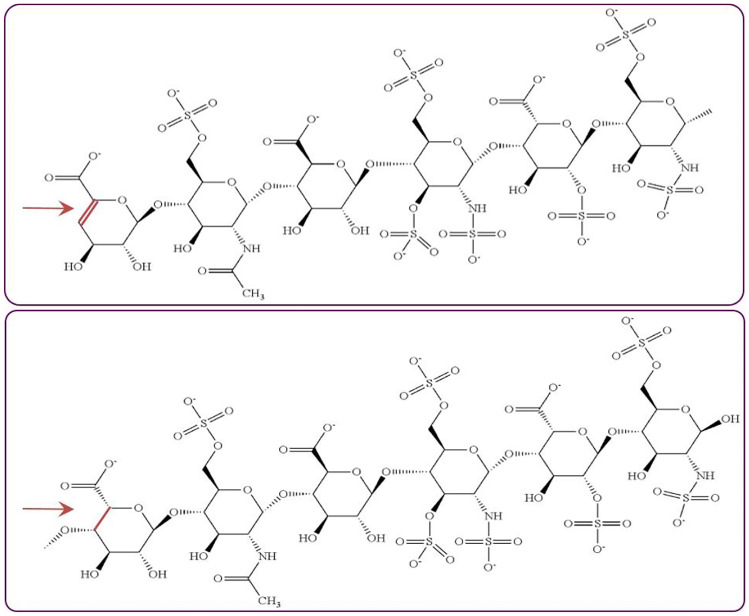
Notwithstanding LMWHs increased bioavailability, more selective activity with respect to activated factor Xa and factor IIa, and predictability in pharmacological action over unfractionated heparin, researchers believe they are still having high anti-IIa activity. To reduce hemorrhage risks, ULMWHs with high anti-Xa activity and zero or very low anti-IIa activity have been developed. US Patent 6,384,021 owned by Laboratorios Farmaceuticos Rovi S.A. discloses ULMWH (RO-14, bemiparin derivative) with anti-Xa activity value 120 lU/mg and anti-Xa/anti-IIa ratio between 15 and 50. Using a different preparation scheme, international patent application WO2002008295A1 by Sanofi-Aventis discloses another ULMWH (AVE5026, semuloparin sodium) that exhibits an anti-Xa activity value 100–150 lU/mg, an anti-IIa activity of 0–10 lU/mg, and anti-Xa/anti-IIa ratio > 10. Through controlling the % age of water (less than 0.6%) during depolymerizing a quaternary ammonium salt of a benzyl ester of heparin in the presence of a base selected from phosphazenes, Sanofi-Aventis developed and disclosed in US Patent 8,003,623 another improved ULMWH (semuloparin sodium) with a mean MW between 1500 and 3000 Da, anti-Xa activity between 161 and 192 lU/mg, an anti-IIa activity < 10 lU/mg, and an anti-Xa/anti-IIa ratio > 30 [30]. Sanofi’s new sulfated oligosaccharides are further characterized by the following: 2–26 saccharide units, 4,5-unsaturated uronic acid 2-O-sulfate at one of their ends and a hexasaccharide having high binding affinity for ATIII and an anti-Xa activity > 740 lU/mg (US8003623) [31]. The hexasaccharide represents about 15–25% of the mixture of oligosaccharides. Sanofi also developed and disclosed in WO2012072799A1 new method (amidolytic assays on chromogenic substrate) for in vitro measurement of the biological activity of semuloparin sodium. Another US patent 9,346,894 (~ WO2012140580A1) by Sanofi SA discloses a heparin derivative (a “double site” sulfonated polysaccharides) comprising two ATIII-binding hexasaccharide sequences (Fig. 4). Earlier, Jordan et al. [32] reported on the presence of two ATIII-binding sites in high molecular weight heparin fractions of 18,000 to 22,000. In parallel, analysis of LMWHs having MW ~ 6000–8000 Da had established the presence of single site for interaction with Viskov et al.'s very hypothesis that LMWH can interact with at least two AT proteins and consequently to be having at least two possibilities of inhibiting factor Xa has unveiled a new generation of hemisynthetic LMWHs (~ 2000 to 3000 Da) with a new antithrombotic profile [30].
Fig. 4.
Polysaccharides comprising two antithrombin III–binding hexasaccharide sequences. When hexasaccharide is located at the NRE of the polysaccharide, the bond between carbon atoms 4 and 5 of the first saccharide is a double bond; else, the bond between carbon 4 and 5 of the first saccharide is a single bond
(Source: Mourier P. and Viskov C. Polysaccharides comprising two antithrombin III-binding sites, preparation thereof and use thereof as antithrombotic medicaments. US9346894B2 (2016))
Additionally, current approaches to develop selective factor Xa inhibitors for oral administration such as synthetic pentasaccharides (fondaparinux) in the form of water-in-oil microemulsion (Novo Nordisk), rivaroxaban (Bayer HealthCare AG and Scios, Inc.), apixaban (Bristol-Myers Squibb), and 813,893 (GlaxoSmithKline) are promising and provide a reproducible and predictable bioavailability of the LMWHs (see US9346894B2).
Continuous development of new heparin fragments with distinct characteristics and clinical applications and, in parallel, gradual expiry of old patents and data exclusivity are clear indicators of an ever competitive and a never-ending market for heparin and heparin-derived products worldwide. Controlling quality pre- and post marketing is the core issue that need to be addressed squarely especially in countries where local pharmaceutical industry is lacking modern laboratory facilities at one hand, while compromising good manufacturing practices at the other. In the next section, the article lays out how reorientation in analytical approaches can better characterize LMWH structure and compositions and discover new applications of known heparin and heparin-derived products.
Advanced Analytical Approaches for LMWH Characterization and Control of Quality in Patent Ecosystem
Since the first generic LMWH enoxaparin entry into the US market in 2010, quality assurance and reproducibility with claimed safety and efficacy have become even greater challenges for FDA and other regulatory authorities worldwide. The very first inquiry for assessing generic/biosimilar drug product similarity with the innovator drug product is what scientific data are required to sanction a conclusion that the generic/biosimilar drug product has the same active ingredient as the innovator drug product for which quality and safety stand established by the innovator company. While clinical studies can support a conclusion of generic/biosimilar drug product safety and effectiveness, these studies are inert towards distinguishing the two drug products at the molecular levels [3]. Recognizing the scientific and regulatory complexities in the way to approval for generic LMWH drug products, and 2007–2008 heparin contamination crises, FDA presented a framework that requires characterization of several key aspects of LMWH drug products with advanced analytical techniques in the active ingredient sameness assessment quest. The framework is followed by many developing countries that are members of WTO’s agreement on TRIPs mutatis mutandis while many least developed countries have adopted the FDA framework as verbatim transcription.
Conventional approaches for structural characterization of LMWHs (such as proton and carbon nuclear magnetic resonance (NMR) spectroscopy, capillary electrophoresis (CE) and mass spectrometry (MS)) have unveiled many of their critical quality attributes; nonetheless, the chemical diversity of GAG polysaccharides resulting from a variety of differential pattern of O- and N-sulfation and uronic acid C-5 epimerization creates a need for development of new approaches for understanding the still latent structural and functional attributes of LMWHs as a measure to control quality and improving their clinical applications [33]. To support the inference that modes of depolymerization used to produce generic and innovator LMWH drug products produce the same degree and cleavage pattern of parent heparin, FDA has made determination of MW distribution and relative abundance of oligosaccharides of different MWs, analyzing the overall chemical composition in the generic LMWH drug product sourced from two different manufacturers and contrasting them with the innovator LMWH drug product a mandatory requirement. This adds to scoring the need for still more analytically sensitive, specific, and technically impeccable approaches for profiling LMWH complex and heterogeneous mixtures with great variations in sulfation and sequence composition [28]. In this quest, advances in (i) mass spectrometry (such as LC–MS, CE-MS, CE-LIF (laser-induced fluorescence) and tandem mass spectrometry (also known as MS/MS or MS2)) that involve pairing of two or more mass analyzers for increasing their capacity to analyze; (ii) online separations including HPLC (such as size-exclusion chromatography (SEC), strong anion exchange (SAX), reverse-phase ion pairing (RPIP), and hydrophilic interaction chromatography (HILIC)), ion mobility spectrometry (IMS), high-field asymmetric waveform ion mobility spectrometry (FAIMS), and capillary zone electrophoresis (CZE); and (iii) automated analysis software have greatly revolutionized the top-down and bottom-up approaches for precise structural characterization of LMWHs [8]. Table 3 provides a listing of modern analytical tools for LMWH fine structural and compositional analyses that also need listing in the US and EU pharmacopoeias for assuring quality and purity of the LMWH drugs approved for marketing.
Table 3.
Patent position and pros and cons of analytical tools that generics can utilize for profiling generic/biosimilar LMWH drug products
| Technique | Advantages | Challenges | Patent | Inventor(s) | Current assignee | Patent expiry |
|---|---|---|---|---|---|---|
| RP-LC–MS | Capability to perform intact chain mapping analysis; offer rich information about chain distribution and composition of LMWHs; can identify trace amounts of unusual structures or impurities, can characterize by-products, and degradation products | Insufficient separation resolution, detection sensitivity or specificity; restricted ability to identify different disaccharide building blocks of LMWHs (12 to 17) | US7329353 | Thomas Dillon et al. | Amgen Inc | 06–03-2026 |
| CE-MS | Capability to perform intact chain mapping analysis; offer rich information about chain distribution and composition of LMWHs; can identify trace amounts of unusual structures or impurities rapid, economic, ultra-sensitive | Insufficient separation resolution, detection sensitivity or specificity; restricted ability to identify different disaccharide building blocks of LMWHs (12 to 17) | WO2015121366 | Johann FAR et al. | Analis S.A | N/A |
| LIT-MS/MS/MS (or MS3) | Better in-trap fragmentation, high ion trap sensitivity, and high selectivity | Difficult high selection resolution | US9852895 | Daniel James Kenny | Micromass UK Ltd | 20–01-2030 |
| MSI-CE-MS | High-throughput screening of ions within a single capillary; enhanced data processing with quality assurance | Low mechanical robustness, poor reproducibility | US9490110 | Phillip Britz-McKibbin | McMaster University | 14–05-2034 |
| CE-ESI–MS | Simple, inexpensive, good in resolution, easy capillary replacement, highly compatible with MS | Insufficient capacity, low selectivity, not suitable for high MW analysis | EP2250490B1 | Elizabeth Jane Maxwell, Xuefei Zhong, Hong Zhang, David Da Yong Chen | University of British Columbia | 06–03-2029 |
| ESI FT-ICR MS | Ultra high spectral resolution and mass accuracy; facilitate application of top-down MS strategies | High cost, requires large space, long signal acquisition time, not suitable for clinical laboratory applications | US8530834 | Alan G. Marshall et al. | Florida State University Research Foundation, Inc | 05–02-2030 |
| DE-MALDI-TOF MS | High spectral resolution and mass accuracy, obviating the need for MS tuning during single sample signal acquisition | Presents different results under similar sample preparation using similar conditions | US9536726B2 | James VanGordon, Bradford Clay | Biomerieux, Inc | 27–08-2035 |
| SEC-UPLC/Q-TOF HRMS | Simple; robust; capacity to reflect process-based structural changes; potential to reveal contribution of each individual components to the overall bioactivity of LMWHs; capacity to identify oligosaccharide with sizes upto dp30.* [37] | Cost, insufficient storage capacity | US202002000719A1 | Navya Sama et al. | Alembic Pharmaceuticals Ltd |
Pending (05–09-2038, if granted) |
| IP-HILIC LC–ESI–MS | Simple, effective for analysis of –vely charged saccharides, suitable for coupling with MS, low IP concentration increases resolution and strength of MS, highly sensitive, low contamination rate, useful for quality control of LMWHs | Sensitivity fluctuates during runs, low separation efficiency for polar compounds | WO2016051170A1 | Lingzhi Gong | Isis Innovation Limited | N/A |
| HILIC-UPLC-CAD/MS | Capacity to separate highly sulfated and very polar heparin oligosaccharides; universal adaptability to any MS instrument; capacity to identify oligosaccharides with sizes up to dp28; robust, reproducible and easy-to-use, offers impressive increase in sensitivity when compared with RP-LC–MS | High reliance on aprotic solvent acetonitrile, less flexible | US9441053B2 | ChungYao Wang, Imin Huang, Chia Yen Wu, YungTe Chiang Helen Chao | ScinoPharm Taiwan Ltd | 20–02-2035 |
| SEC-MS/RPIP-HPLC–MS | Fast, capacity to disclose size distributions and sequences; better in oligosaccharide resolution, provide interesting information about oligosaccharide structure, chain length, and chemical modifications | Expensive, low tolerance to contaminants | WO2013139478A1 | Giangiacomo Torri, Antonella Bisio | Instituto di Ricerche Chimiche e Biochimiche “G. Ronzoni” | N/A |
| SAX-HPLC or RP-HPLC ESI–MS | Capacity to profile disaccharide building blocks; offers better resolution; signal enhancement, effective in costs | Signal suppression, low tolerance to contaminants | US9139876 | Zachary Shriver, Naveen Bhatnagar, Nur Sibel Gunay, Jennifer Ozug, Elaine Y. Sun | Momenta Pharmaceuticals, Inc | 23–08-2031 |
| Orbitrap MS/MS | High resolution/ionization efficiency; high mass accuracy; miniature design, offers identification and characterization of all known disaccharide building blocks | Lower sensitivity, false negatives, low-abundance peaks | US7728290B2 | Alexander Makarov | Thermo Finnigan LLC | 22–10-2024 |
| HPLC FTICR + LTQ-FTMS | Posttranslational modification analysis of complex molecules | Low-abundance peaks, limited mass accuracy and resolution | WO2006026569A2 | Shiaw-Lin Wu et al. | Northeastern University | N/A |
| LC–MS/MS | Capable of sequencing short oligosaccharide mixture with saccharides as large as dodecasaccharides; highly sensitive, specific and rapid in detection | Higher operational cost; more limited sample throughput; less favorable concentration sensitivity | US8945933 | Anthony Le, Tina Cowan | The Board of Trustees of the Leland Stanford Junior University | 20–10-2032 |
| FT-ICR MS | Incomparable high mass resolution, mass measurement accuracy; small, simple | Relatively slow acquisition rate | US5886346 | Alexander Alekseevich Makarov | Thermo Finnigan LLC | 29–03-2016 |
| IMS-MS | Simple, selective, sensitive, inexpensive, | Low-resolution, limited dynamic response range, low tolerance to contamination | US9607820B2 | Robert Harold Bateman et al | Micromass UK Ltd | 21–05-2030 |
| ECD-CID MS/MS | Simple, low costs, selectively cleaves c-z site on the amino acid backbone; suitable for analyzing post-translational modifications | Low in fragmentation efficiency; requires high signal-to-noise ratio for precursor ions | US8080786B2 | Takashi Baba, Hiroyuki Satake, Izumi Waki | Hitachi High Tech Corp | 10–07-2026 |
| CID MS/MS | Creates MS spectra with high accuracy and efficiency; produce useful fragmentation | Reduces multiplicity of bond cleavages; Ineffective for large molecule samples; Easily detached functional groups cause loss of fragment-derived information on location and provide limited structural information | US8269166 | Daisuke Okumura | Shimadzu Corp | 05–02-2029 |
| IRMPD MS/MS | Fast, free from blind spots, no degradation of high vacuum; allows product ion formation on axis | Fails to conduct complete structural analysis; easily loses side chains involves in post translational modifications of biomolecules; allows secondary fragmentation | US6717137B2 | Steven A. Hofstadler, Jared J. Drader | Ibis Biosciences Inc | 11–06-2022 |
| ETD MS/MS | Cleaves main amino acid residues sequence chain while preserving the post-translationally modified site; allows more complete sequence information; works well with partially ionized precursor ion; can be practiced on a variety of other analytical tools | Not feasible for large-scale analysis, high in cost; technically complex | US8692187 | Donald F. Hunt et al. | University of Virginia Patent Foundation | 19–04-2025 |
| ECD MS/MS | Efficient, suitable for analyzing complex mixtures and large biomolecules, produce more structurally important cleavages than CID and IRMPD; produce simple/predictable fragmentation pattern | Can be employed in Penning cell ion cyclotron resonance mass spectrometers | US6958472 | Roman Zubarev | Syddansk Universitet | 22–03-2022 |
| EID MS/MS | Suitable for dissociating high-mass, even-electron ions produced by thermo spray and other soft ionization tools; yields extensive and reproducible fragmentation characteristics; can analyze qualitatively and quantitatively greater quantity of samples; can be easily implemented on a variety of analytical tools | Need electron sourcing and singly charged precursor ions | US4731533 | Marvin L. Vestal | Applied Biosystems LLC | 15–10-2006 |
| IT-MS | Selectively trap multivalent ions having variety of charge to mass ratio | Can cause outgassing, Need for large radio frequency (RF) potentials can aggravate RF breakdown | US6847037 | Yoshikatsu Umemura | Shimadzu Corp | 19–05-2023 |
| 2D-Tandem MS | Much faster, offers large increase in peak capacity, can characterize intact chains or originally stable non- fragmenting ions | High cost | US6770871 | Houle Wang, Kerry D. Nugnet |
Bruker Corp Bruker Scientific LLC |
01–06-2022 |
| Chip cube Nano-LC–ESI–MS/MS ion-trap system | Easy, reliable, efficient, highly sensitive, can investigate a wide range of biomolecules | Reproducibility, sensitivity dependency on shape of the capillary tip and cone distance | US20160305919A1 | Gregory Staples | Agilent Technologies Inc | Abandoned |
RP-LC–MS reverse phase-liquid chromatography-mass spectrometry, CE capillary electrophoresis, LIT linear ion trap, FT-MS Fourier transform mass spectrometry, FTICR Fourier transform ion cyclotron resonance, MSI multi-segment injection, DE delayed extraction, MALDI matrix-assisted laser desorption ionization, ESI electrospray ionization, IT ion trap, CID collision-induced dissociation, IRMPD infrared multiphoton dissociation, HPLC high-performance liquid chromatography, HILIC hydrophilic interaction chromatography, Q-TOF quadruple time-of-flight, RPIP reversed phase ion pairing chromatography, SEC size exclusion chromatography, UPSEC ultra-performance size exclusion chromatography, SAX strong anion exchange chromatography, CTA-SAX cetyltrimethylammonium-coated SAX, UPLC ultra-performance liquid chromatography, HRMS high-resolution-MS, UVPD ultra-violet photodissociation, EDD electron detachment dissociation, EID electron-induced dissociation, NETD negative electron transfer dissociation
Sources: (i) For patents: www.patents.google.com, www.epo.org, www.wipo.org, www.uspto.com, www.drugfuture.com; (ii) For advantages and disadvantages of techniques: Lauren E. Pepi, Patience Sanderson, Morgan Stickney, I. Jonathan Amster, Developments in Mass Spectrometry for Glycosaminoglycan Analysis: A Review, Molecular & Cellular Proteomics, Volume 20, 2021, 100,025, ISSN 1535–9476, https://doi.org/10.1074/mcp.R120.002267; (iii) Zhangjie Wang, Lianli Chi. Recent advances in mass spectrometry analysis of low molecular weight heparins [J]. Chin. Chem. Lett., 2018, 29(1): 11–18
As an outcome of these innovative efforts, today, researchers and pharmaceutical industry have in their toolkits several independent and hyphenated approaches for LMWHs’ deeper structural and compositional analyses including but not limited to the following: (i) 2D-NMR for identifying LMWH types and calculating their monosaccharide composition (1D 1H-NMR identification test is part of US pharmacopoeia and EU pharmacopoeia monographs for heparin or LMWH sodium) [34]; (ii) 2D 1H-13C- heteronuclear single quantum coherence (HSQC) spectroscopy for characterizing normal structural variations in the intact LMWH molecular composition [34] (Using the approach, commercially available LMWHs (enoxaparin, dalteparin and tinzaparin) were tested for results. The technique identified the major as well as the minor components present at the termini of the oligosaccharide chains.) [35]; (iii) 2D-chromatography combining SEC and RPIP approaches, offering better resolution of LMWH components of different sizes and of same sizes but with different charges and polarities, and providing information on both size distribution and sequences of LMWHs for control of quality (Using electrospray quadruple time-of-flight (Q-TOF) mass-spectrometer more than 80 oligosaccharides in nadroparin and more than 120 in enoxaparin were identified.) [36]; (iv) HILIC-LC–MS for characterizing major chains of LMWH octadecasaccharide in size [37]; (v) ultra-performance size exclusion chromatography (UPSEC) coupled to electrospray quadruple time-of-flight (Q-TOF) mass-spectrometer (UPSEC-Q-TOF–MS) [38] for profiling LMWH oligosaccharide chains with sizes upto dp30 (The technique identified more than 70 components in enoxaparin including oligosaccharides with unnatural structures 1,6-anhydro rings and saturated uronic acid at the non-reducing ends.) [39]; (vi) HILIC LC–MS supported by Agilent’s chip-based nanospray amide HILIC LC–MS system to analyze LMWHs up to dp18 [40]; (vii) a universal diol-based HILIC Fourier transform (FT)-ESI–MS platform for direct characterization of intact LMWHs (coupled with bioinformatics software package (GlycReSoft 1.0) [41], a quantitative comparison of up to 200–500 components in two commercial LMWH products, innovator enoxaparin (Lovenox) and generic enoxaparin, was made possible.) [42]; (viii) LC–MS for profiling LMWH longer chains (More than 80 compositions were identified using the method.) [35]; (ix) RPIP-ESI–MS for profiling intact LMWHs (Using the method, Chi et al. identified more than 200 intact components in enoxaparin sodium.) [43]; (x) a HILIC-MS for separating highly sulfated and polar heparin oligosaccharides using ambient mobile phases with low ammonium salt concentration [44]; (xi) CE-MS for intact chain mapping analysis with speed and cost-effectiveness [45]; (xii) RPIP-ESI–MS method performed on an ion trap-time-of-flight hybrid mass spectrometer IT-TOF for oligosaccharide fragments mapping [46]; (xiii) HILIC, multiple reaction monitoring (MRM)-MS methods for identifying and quantifying disaccharide building blocks [47] (HILIC-MRM-MS has been used successfully for dalteparin and nadroparin disaccharide building blocks analysis.) [48]; (xiv) LC-MRM-MS for determining composition of disaccharide building blocks; (xv) ion mobility spectrometry (IMS) — a powerful technique for GAGs separation based on ions mobility, when coupled to MS/MS resolves GAG negative ions having the same mass-to-charge ratio [49] and analyze GAG chains qualitatively and quantitatively [33]; (xvi) electron-based ion activation techniques (such as collision-induced dissociation (CID), infrared multi-photon dissociation (IRMPD), electron-induced dissociation (EID), electron detachment dissociation (EDD), ultra-violet photodissociation (UVPD), and negative electron transfer dissociation (NETD) for extensive crossing cleavages of polysaccharide chains), allowing identification of epimers, distinction between IdoA and GlcA, number of sulfo-modifications, determination of position of sulfo-group substitutions [50], and sequence information of highly sulfated GAGs [51]; (xvii) gated trapped IMS paired with NETD MS/MS for characterization and quantification of highly sulfated GAG isomers without decomposition of sulfo-group [52]; (xviii) online LC and CID MS/MS for sequencing chemically derivatized mixtures of oligosaccharides [53]; (xix) CZE coupled with NETD MS/MS for structural analysis of GAG in human urinary samples [54]; (xx) HILIC-LC–MS for analyzing GAG oligosaccharides up to dp30 [55] (Applying the technique to a mixture of dalteparin and nadroparin, depolymerized through nitrous acid, Sun et al. separated and identified 36 building blocks) [56]; (xx) HILIC LC-NETD MS/MS for separating and sequencing chemically synthesized tetra- and hexasaccharide isomers without permethylation [57]; and (xxi) traveling wave ion mobility spectrometry (TWIMS)-MS for separating and characterizing mixtures of chondroitin sulfate oligomers [58].
Evaluating the strengths and efficiencies of analytical advances made in the past decade, the CE-based method for separating and determining the ratio of 1,6-anhydro structure in enoxaparin as developed by Kang and Zhan and disclosed in US 9012229B2 is a substantial advancement in the fine structural analysis of enoxaparin sodium [59] and for controlling the drug product quality during manufacturing. The method could separate and measure, qualitatively and quantitatively, all the building blocks of enoxaparin including disaccharides, trisaccharides, tetrasaccharides, and specifically oligosaccharides having 1,6-anhydro ring structure. The US Pharmacopoeia (USP) has used ratio of 1,6-anhydro as a standard in drug product quality control of enoxaparin. In an exhaustively digested product of enoxaparin sodium with a mixture of heparin lyases I, II, and III in a ratio of 1:1:1, Kang and Zhan detected the following components: (i) four oligosaccharides with 1,6-anhydro ring structure; (ii) a trisaccharide of structure I; (iii) 8 disaccharides represented by the symbols — ∆IA, ΔIS, ∆IIA, ΔIIS, ∆IIIA, ΔIIIS, ∆IVA, and ΔIVS; (iv) two non-naturally occurring disaccharide ΔIISgal and ΔIVSgal comprising a galacturonic acid produced by 2-O-desulfation of -IdoA(2S)-GlacNS(6S) and -IdoA(2S)-GlacNS; (v) two 3-O-sulfo containing tetrasaccharides (ΔIIA-IISglu and ΔIIa-IVSglu) [60] may or may not be having affinity to ATIII.
The structural identification of above components received endorsement from the USP monograph for enoxaparin sodium [61]. For calculating the molar %age of oligosaccharide chains with 1,6-anhydro ring structure in enoxaparin sodium, Kang and Zhan used the following equations/formulae:
Wherein Wx is the weight-average molecular weight of enoxaparin sodium.
The Wx of enoxaparin sodium was 4500 Da (US5389618A) ranging from 3800 to 5000 Da wherein (i) about 20% oligosaccharides have MW less than 2000 Da, (ii) more than 68% oligosaccharides have MW between 2000 and 8000 Da, and (iii) no more than 18% oligosaccharides have MW higher than 8000 Da (US9012229B2). The characteristic oligosaccharide chains in enoxaparin contain a pentasaccharide sequence, originally displayed in the parent heparin polysaccharide chains, that accounts for 15–25% in enoxaparin sodium.
Adding to the LMWH fine structural characterization efforts as one of several measures to control quality of pharmaceutical drug product during first manufacturing as a whole and subsequently in batch-to-batch variation, in 2017, Arnold et al. reported on the development of a new technique that they claimed to be more reliable, sustainable, and efficient for determination of Mw and MW distribution of LMWHs [18]. The research team coupled chemo-enzymatically synthesized oligosaccharides with a predictive in silico model, developed from a library of chemosynthetically synthesized heparin oligosaccharides for enoxaparin MW determination. The group believed that the chemo-enzymatic process allows production of pure oligosaccharides that is reproducible and can be used as standard for a MW analysis of enoxaparin. To have their approach translated into practice, Arnold group had accomplished the following: (i) synthesized a panel of oligosaccharides covering the enoxaparin MW range between 2226 and 5176 Da; (ii) developed a predictive in silico model for MW analysis; (iii) developed a set of guidelines for testing system suitability; and (iv) analyzed commercially available enoxaparin from different manufacturers [18]. While Arnold group acknowledged that using refractive index (RI) detection method in lieu of HPLC as stated in the USP monograph is a limitation of the approach, nonetheless development of homogenous oligosaccharide for MW analysis of LMWHs is promising and must be projected as a measure to ensure quality and consistency of the product [18]. In the same year, Bisio et al. [14] worked on a similar approach of combining different analytical strategies (such as LC–MS and NMR) for deep structural characterization of LMWHs particularly dalteparin. The group found the approach effective for comparative studies of dalteparin samples or assessment of batch-to-batch variability [14].
For addressing the pharmaceutical quality control issue, Karawdeniya et al. [62] reported the use of silicon nitride nanopores for easy differentiation of clinical heparin sample and contaminated over-sulfated chondroitin sulfate (OSCS). In another study for LMWH control of quality, Im et al. [63] used solid-state nanopores sensor with a support vector machine (SVM) learning algorithm for GAGs single molecule identification and quantification. The technique is capable of identifying impurity in a heparin sample with high accuracy (> 90%) at the level of 0.8% (w/w). Still, another study implicating production and characterization of LMWHs for better therapeutic results and providing an alternative anticoagulant therapy is made by Oliveira et al. [64] Working on the proposed strategy, the group first produced and characterized LMWH nanoparticles through the solvent-evaporated double emulsion method, making use of polylactic-co-glycolic acid (PLGA) and polyvinyl alcohol (PVA), followed by quantification of encapsulation efficiency (EE), and evaluation of stability. Homogenous and stable nanoparticles with low polydispersity index (IPD) of 0.067 ± 0.05 and EE value of 66.5% suggested effectiveness of the nano-encapsulation method and its use as an alternative anticoagulation therapy. [64]
The research group — Stickney et al. [65] — has made parallel attempts for structural characterization of LMWHs in 2019, making use of LC–MS, UHPLC-MS, CE-MS, and MS/MS techniques. Recently, Miller et al. [66] used a combinatorial approach coupling NETD and CE and applied it to LMWH (enoxaparin) deeper characterization. The strategy allowed enoxaparin separation within 30 min, identified 37 unique molecular compositions, and assigned 9 structures using MS/MS [66]. In another latest study, Saad and Leary [67] developed a “shotgun ion mobility mass spectrometry sequencing” (SIMMS2) for heparin saccharide intact chain mapping. Contrasting data for intact and fragment ions against standards HS oligosaccharide structure has allowed determination of heparin unnatural saccharides including 3-O-sulfo groups containing variants [67].
Bioinformatics Tools for LMWH Oligosaccharide Mass Spectra Annotation to Succeed Control over Drug Product Quality
Apart from advances in analytical approaches for detailed structural characterization and compositional analysis of LMWHs, either as part of regulatory compliance for marketing approval or pharmacovigilance after the drug products have been licensed for use, efficient software/bioinformatics tools that allow accurate analysis of datasets or interpretation of spectra generated by various analytical techniques (such as LC/MS, LC–MS/MS) are equally desirable to achieve the drug product quality control and consistent reproduction goal. In the past one decade, several bioinformatics tools have been developed for translating and/or interpreting mass spectra into meaningful information. Starting from early developed software package HOST for heparin/HS oligosaccharide sequencing [68], today, researchers and pharmaceutical industry have variety of choices for undertaking identification of GAG oligosaccharides different structures and elemental composition analysis. Amongst these, bioinformatics tools include the following: (i) GlycoWorkbench [69] for analyzing carbohydrate mass spectra through matching an artificially generated library of possible glycan structures, fragments, and compositions using all known and modified disaccharide building blocks against the dataset of peaks derived from the MS sequencing (the system aims to fully facilitate the everyday manual interpretation of MS data); (ii) Manatee for rapid extracting, assigning, and contrasting glycan compositions from LC–MS datasets [70]; (iii) GlycReSoft [71] for processing bottom-up and top-down data auto-processed by open source software Decon2LS/DeconTools to generate the identity and quantitative information for LMWHs; (iv) GlyTouCan by Kinoshita et al. to unify databases for extensive glycan analysis [72]; (v) GlycCompSoft for automated comparison of LMWHs using top-down LC/MS data [73]; (vi) GlycoDeNovo — an efficient algorithm for accurate de novo glycan topology reconstruction from tandem mass spectra [74]; (vii) GAGfinder — the first software package for MS/MS spectrum peak ending and elemental composition analysis of GAG [75] (GAGfinder is a targeted approach to spectrum analysis and annotating peak isotopes composition that exploits precursor product information for generating hypothetical library of fragments for experimental result matching); and (viii) GAG-ID — a multivariate mixture model to estimate the automate assignment of LC–MS/MS-fragmented derivatized heparins [76].
In addition to getting benefits from the above analytical advances for commercial LMWH quality and consistent reproduction, many pharmaceutical companies are outsourcing characterization of unfractionated heparin/LMWH samples to the commercial companies. Such companies may provide drug manufacturers a broad range of structural and physicochemical data sufficient to sustain rigorous assessment of LMWH similarity with the originator drug product by the regulatory authorities [77].
Analytical advances for structural characterization of uniquely modified and more complicated LMWHs than the precursor heparin are increasingly helping the new and generic LMWH drug product manufacturers in the successful preparation of their application dossier for marketing authorization approval process at one end while the regulatory authorities (FDA-EMA-DRAP-Health Canada etc.) to revise and/or upgrade heparin sodium monographs in light of these advances to ensure release of quality and safe generic/biosimilar LMWH drug products in the local and global markets.
Current Pharmacopeial Monographs for LMWH Marketing Approval
While new advances in technology influence compendial standards for drug products characterization and quality assurances as a matter of routine, adverse drug reactions or immunogenic responses may also require development of new tests and specifications not only to address the concerns but also to improve the quality and purity of the drug product in question. Following 2007–2008 heparin contamination crisis with over-sulfated chondroitin sulfate (OSCS), the pharmacopoeias for LMWHs had undergone extensive revisions worldwide. In the USA, FDA urged the need for introduction of new analytical approaches for detecting the presence of OSCS impurities in the heparin preparations and tools for determination of MW distribution and weight-average Mw in the heparin monograph of the USP as a measure to control quality and purity [78]. Succeeding 2009 revision in enoxaparin sodium monograph, new assays including 1D 1H NMR, CE, and SAX-HPLC have been added in the USP for detection of impurities in the heparin sodium monograph [79]. While different regulatory authorities have set the level of impurity tolerance at varying degree, the USP for heparin sodium and heparin calcium has set specification for galactosamine-containing glycosaminoglycans component not more than 1% [19].
For MW determination, Mulloy et al. [80] reported on the development of a broad standard calibrant for USP heparin sodium MW calibrant reference standard (RS). The group also developed a simplified SEC method with light scattering detection for determination of MW distribution of heparin sodium for incorporation into the USP to help ensuring safety and quality of heparin sodium [80]. Following experts’ discussions and lapse of public comment period, the below criteria was accepted for incorporation into the USP heparin sodium monograph [81]:
-
i)
M24,000 not more than 20%
-
ii)
Mw between 15,000 and 19,000
-
iii)
The ratio of M8000–16000 to M16,000–24,000 not less than 1.0
This development has made direct comparison between MW values for standard heparin determined by different laboratories possible [80].
Another effort for MW determination using homogenous calibrants as a measure to control quality testing has been reported by Arnold et al. [18] in 2017. The group used support vector machine technique for modeling as a reliable, consistent, and improved substitute for USP recommended nonlinear regression analysis to account for enoxaparin’s heterogeneity. The previously used standards (i.e., mixtures of oligosaccharide derived from the heparin source material) because of heparin source heterogeneity have imprecise structure, hence difficult reproduction.
In countries like Australia, Canada, and Pakistan, the respective regulatory authorities (Therapeutic Goods Administration (TGA), Health Canada, and DRAP) have adopted mutatis mutandis principal revisions in USP for enoxaparin sodium while European Medicines Agency (EMA) has defined their own reference standards for assessment of enoxaparin’s quality, purity, and efficacy. Generally, EMA follows EP Reference Standards H0185000 for heparin low molecular mass for assay BRP (biological reference preparations) and Y0001282 for heparin physico-chemical analysis but, specifically, from quality perspective, EMA requires performance of extensive comparability studies encompassing the following: (i) comparison of heparin source material and mode of depolymerization; (ii) physicochemical characteristics; (iii) structural comparison for assessing oligosaccharide sequence similarity, oligosaccharide fragment similarity, disaccharide building block similarity, and high affinity/no-affinity component similarity; (iv) in vitro biological assays (clotting tests such as activated partial thromboplastin time [aPTT] and HEPTEST) and biochemical activity (inhibition of coagulation factors Xa (anti-FXa) and factor IIa (anti-FIIa) [82]. Apart, EMA also requires the following: assessment of degree of sulfation and sodium content of the oligosaccharide chains, MW determination using HP-SEC with Triple Detector Arrays, and assessment of monosaccharides forming an integral part of the disaccharide building blocks in enoxaparin chain including assessment of residues at both the reducing and non-reducing ends. From clinical perspective, EMA can accept a single PK/PD study or otherwise drop a dedicated efficacy trial provided a comparison of the physicochemical characteristics, biological activity/potency, and PD fingerprint profiles of a candidate biosimilar and the reference product leads to a convincing inference of “similar efficacy,” based on the use of highly sensitive and specific methods [83].
In Australia, TGA mostly follows the Ph. Eur. monographs for LMWHs (0828) [84] (EUP-LMWH-2014) and enoxaparin sodium (1097) [85], but additional considerations are also given to the following: mass-average relative molecular mass percentage, benzyl alcohol, nitrogen, loss on drying, and anti-factor Xa and anti-factor IIa assays [86].
In Pakistan, the DRAP follows the US Pharmacopoeia and British Pharmacopoeia standards for testing the quality and comparability of LMWH biosimilars with the reference listed drug. In particular, DRAP requires the following: The weight average molecular weight of LMWHs must be less than 8000 Da; more than 60% of the total must have MW less than 8000 Da; and anti-Fxa/anti-IIa ratio must be greater than 1.5 [87]. The DRAP (Registration Board) has declared that drug products registered in any of the regulatory authorities, namely US FDA, EMA, PMDA Japan, TGA Australia, and Health Canada shall receive registration grant. In addition, the molecules/formulations (in same dosage form) and clinical trials approved by the regulatory authorities of UK, Germany, France, Switzerland, Netherlands, Austria, Denmark, Sweden, and Norway will also be taken as reference/guidelines for consideration of Registration Board, as authorities of these countries have robust drug regulatory mechanisms and long standing strong litigation systems. However, the DRAP (Registration Board) reminded the stakeholders that should the domestic circumstances be justifying, they shall also consider their own safety and efficacy parameters of the drug; and decision shall be made on case-by-case basis.
Regulatory Pathways for Generic/Biosimilar Marketing Approval: FDA Exempts ANDA Applicants for Generic LMWHs to Submit Clinical Studies While EMA, WHO, Health Canada, TGA, and DRAP Sanction the Requirement
In USA, ANDA pathway specified under Sect. 505(j) of Federal Food, Drug, and Cosmetic Act of 1938 (FDCA) is exclusive for generics (small molecule drugs) approval while Biosimilar Biologics License Applications (BBLA) pathway specified under 351(k) of Public Service Health Act (PHSA) is reserved for biosimilars. FDA considers unfractionated heparin and LMWHs a drug (not a biologic), hence requires applicants to follow NDA pathway as specified under Sect. 505(b)(1) and 505(b)(2) of FDCA for new LMWHs and ANDA pathway (505(j)) for generic LMWH approval [88]. Placing reliance on the FDA’s previous findings about the clinical safety and effectiveness of originator drug product, an ANDA applicant is not required to submit clinical studies for re-establishing safety and efficacy of the generic drug product. In contrast, EMA, WHO, Health Canada, and DRAP consider LMWHs as biologically active products and hence urge the need for conducting clinical trials to reliably predict pharmacological effect of the biosimilar LMWHs.
Generally, a generic drug product approved following ANDA pathway is considered to be therapeutically equivalent to the reference drug product in terms of safety and clinical efficacy and hence can be substituted at the pharmacy-level without additional formalities (such as physician recommendation, dose adjustment, safety monitoring) [3]. For generic LMWH drug products, however, since a different source material and mode of depolymerization (chemical or enzymatic) introduces unique changes in the structure and consequently pharmacological activity of the resulting LMWHs, FDA considers each NDA LMWH to be a different drug product and hence not substitutable at the pharmacy level [89]. US FDA approved the first generic version of enoxaparin in 2010 after satisfying itself that the generic enoxaparin contains a 1,6-anhydro ring structure at the reducing ends of between 15 and 25% of its oligosaccharide chain. This new elemental approach for generic enoxaparin approval was the consequence of Aventis’s citizen petition No. FDA-2003-P-0273 that required FDA to withhold approval of any generic version of enoxaparin unless the generic manufacturer satisfied certain conditions including the presence of signature structure (1,6 anhydro) at the reducing ends of the polysaccharide chains. Recognizing the scientific and regulatory complexities in the way to approval for ANDAs for enoxaparin, FDA developed a five-tier approach each of which access the active ingredient ‘similarity’ goal from a different perspective. Summarily, the five-tier approach includes assessment of equivalence/biosimilarity between the generic enoxaparin and the reference drug product (Lovenox®) in respect of the below aspects: (i) physicochemical attributes; (ii) heparin source material and mode of depolymerization; (iii) nature and arrangements of components that constitute enoxaparin (such as disaccharides building blocks, fragment mapping, and sequence of oligosaccharides species); (iv) laboratory measurements of anticoagulant activity (biological and biochemical assays for determining anti-factor Xa and anti-factor IIa activity); and (v) drug effects on humans (in vivo pharmacodynamic profile) [89].
The equivalence evaluation against the five-tier criteria is based on a comparative qualitative and quantitative analysis of generic enoxaparin for the multiple batches of the originator enoxaparin. Such comparability exercise takes into account the expected batch-to-batch variability, sampling, and analytical test variability expected from every biological product. Equivalent molecular diversity tilts the balance of probabilities towards equivalence in the clinical efficacy and safety profiles of the generic and the originator enoxaparin. Depending upon the future advances in the analytical techniques used for characterization of LMWHs, FDA may require a different or improved criteria, or tests for active ingredient similarity demonstration and quality assurance [28].
In European Union, EMA has introduced a centralized procedure under Article 3(2)(b) of the Regulation (EC) No. 726/2004 for generic enoxaparin sodium marketing approval. This procedure meets EMA’s Committee for Medicinal Products for Human use (CHMP) agreement. Apart from complete quality data, EMA/CHMP requires enoxaparin biosimilar manufacturers to support application dossier for a similar biological medicinal product with non-clinical and clinical data [82]. However, in cases where similar efficacy can be strongly inferred from a comparison of the physicochemical attributes, biological activity/potency, and PD fingerprint profiles, EMA may withdraw clinical efficacy studies. According to EMA, enoxaparin biosimilarity assessment exercise is not a “one-stop service.” A technically sound combination of conventional and modern techniques and optimization of Ph. Eur. based parameters and tests are desirable for full characterization of LMWHs as part of biosimilarity assessment exercise. EMA based their marketing authorization decisions on “totality-of-evidence” approach and clinical data derived from comparative PK/PD trial establishing biosimilarity at the level of quality, clinical safety, and efficacy.
In Australia, all marketing authorization applications are considered and published by TGA. For biosimilar version of enoxaparin, TGA follows the US FDA multiple point criteria for approval [28]. For comparability exercise, TGA follows the EMA guidelines on biosimilar drug products [90]. In parallel, TGA gives due consideration to the conclusions drawn by the reference member states (such as New Zealand, Mexico, and EU) on biosimilar enoxaparin approval. Following EMA’s Guideline on non-clinical and clinical development of similar biological medicinal products containing low-molecular-weight-heparins using complex mixtures, TGA may allow clinical efficacy studies where the generic and reference drug products sponsors have established convincing similarity in the analytical characteristics (structural and functional attributes) and PD fingerprint profiles [91].
Parallel to the position in Australia and Health Canada, DRAP (Pakistan) in its 281st meeting held on April 11–13, 2018, adopted the same 5-tier approach as developed by US FDA when they approved enoxaparin ANDA pathway in 2010 [3]. In particular, following FDA guideline, DRAP requires applicants seeking approval of biosimilar version of enoxaparin (Lovenox) to provide the multiple point data/information along with application Form-5A under Rule 26(1) of the Drugs (Licensing, Registering and Advertising) Rules, 1976, for registration of an imported enoxaparin drug. Prior to reaching their conclusion, DRAP had taken into account WHO Technical Report Series No. 999 (2016), wherein the Health Canada pointed out the oversight in the regulatory approval pathway for LMWHs and clarified that these should be regulated as biologicals and not as small-molecule drugs [92]. Subject to (i) establishing convincing similarity/equivalence to the innovator drug product as per the USP monograph at the levels of physicochemical properties; active pharmaceutical ingredient sameness; levels of impurities, weight-average Mw, and distribution of sequences of disaccharide building block units in the oligosaccharide chains; and in vitro biological and biochemical assay results; and in vivo PD profile and (ii) assurance by the manufacturer that a confirmatory clinical efficacy and safety trial would not provide any additional information about the drug product safety and efficacy than previously established by the innovator company, DRAP (Registration Board) may waive a pre-approval phase III efficacy/safety trial to avoid duplicating efforts and resources.
Conclusion
While development of new strategies for structural characterization of highly complex LMWHs and discovering their new clinical applications is a continuing challenge, so far, advances in analytical techniques (such as “in silico” modeling, digitalization, orthogonal separations and online testing) are encouraging for LMWH control of quality and purity during manufacturing and post-marketing. A continuum of patented or non-patented high-throughput separation techniques coupled to high-resolution mass spectrometry (MS, MS/MS) and bioinformatics platforms for spectral interpretation have made GAG chains analysis fast, more accurate, dependable, and meaningful for scientists and laboratory researchers. Where these scientific advancements and increased technical know-how are resulting in development of new therapeutic drug targets, understanding the process-dependent variations in structural characteristics of LMWHs, and discovery of new applications of old active ingredients, in appropriate cases (where a sponsor of generic drug product provides a reasonable scientific justification for the same), these may let regulatory authorities to relax or obviate the need for expensive and extensive in vivo animal testing and clinical trials [92]. Foregoing clinical trials subject to rigorous scientific evidence in support of active ingredient sameness quest may increase availability of low-cost generic/biosimilar LMWHs to the public at one hand, save substantial cost for the health care system at the other, a patent-friendly ecosystem where pharmaceutical and biotechnology companies may have increasing opportunities to make substantial investments in innovation and development. In parallel, advances in uses of old structural analysis tools such as CE and NMR are providing new tracks for innovative thinking and translating them into practice.
Author Contribution
Both authors (Z.I. and S.S.) contributed equally in the preparation of this article.
Data Availability
All data analyzed/literature reviewed is included in the article.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Highlights
• Underlines LMWHs’ significance as a life-saving drug and increase in market demand during COVID-19 crisis.
• Accentuates impact of loss of exclusivity over LMWH drug products of commercial significance and increase in opportunities for market entry with low-cost generic drug products.
• Elucidates scientifically rigorous standards for generic drug makers to get marketing approval of generic LMWH drug products to prove bioequivalence (same quality, clinical efficacy, safety including immunogenicity as the innovator drug product).
• Presents overview of patent landscape of commercially significant LMWHs and analytical techniques developed for quality characterization (structural and biochemical).
• Describes current approaches on analytical characterization and challenges for evaluating and maintaining control over quality and consistency in reproduction of generic LMWH drug products.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zarina Iqbal, Email: pakpat@pakpat.com.pk.
Saima Sadaf, Email: saima.sbb@pu.edu.pk.
References
- 1.Pepi LE, Sanderson P, Stickney M, et al. Developments in mass spectrometry for glycosaminoglycan analysis: a review. Mol Cell Proteomics. 2021;20:100025. doi: 10.1074/mcp.R120.002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Zhang F, Zaia J, Linhardt RJ. Top-down approach for the direct characterization of low molecular weight heparins using LC-FT-MS. Anal Chem. 2012;84(20):8822–8829. doi: 10.1021/ac302232c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Raw A, Yu L, et al. Scientific considerations in the review and approval of generic enoxaparin in the United States. Nat Biotechnol. 2013;31(3):220–226. doi: 10.1038/nbt.2528. [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, Zhu Y, Song X, et al. 3-O-sulfation of heparan sulfate enhances tau interaction and cellular uptake. Angew Chem Int Ed Engl. 2020;59(5):1818–1827. doi: 10.1002/anie.201913029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Chi L. Recent advances in mass spectrometry analysis of low molecular weight heparins. Chin Chem Lett. 2018;29(1):11–18. doi: 10.1016/j.cclet.2017.08.050. [DOI] [Google Scholar]
- 6.Köwitsch A, Zhou G, Groth T. Medical application of glycosaminoglycans: a review. J Tissue Eng Regen Med. 2018;12(1):e23–41. doi: 10.1002/term.2398. [DOI] [PubMed] [Google Scholar]
- 7.Guerrini M, Elli S, Gaudesi D, et al. Effects on molecular conformation and anticoagulant activities of 1,6-anhydrosugars at the reducing terminal of antithrombin-binding octasaccharides isolated from low-molecular-weight-heparin enoxaparin. J Med Chem. 2010;53,8030−8040. [DOI] [PubMed]
- 8.Szekeres GP, Pagel K, Heiner Z. Analytical challenges of glycosaminoglycans at biological interfaces. Anal Bioanal Chem. 2022;414(1):85–93. doi: 10.1007/s00216-021-03705-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson LO, Barrowcliffe TW, Holmer E, et al. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin III and by gel filtration. Thromb Res. 1976;9(6):575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl U, Bäckström G, Höök M, et al. Structure of the antithrombin-binding site in heparin. Proc Natl Acad Sci USA. 1979;76(7):3198–3202. doi: 10.1073/pnas.76.7.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G, Steppich J, Wang Z, et al. Bottom-up low molecular weight heparin analysis using liquid chromatography-Fourier transform mass spectrometry for extensive characterization. Anal Chem. 2014;86(13):6626–6632. doi: 10.1021/ac501301v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugahara K, Kitagawa H. Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr Opin Struct Biol. 2000;10:518–527. doi: 10.1016/S0959-440X(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 13.Margolis RU, Margolis RK. Heparin: structure, cellular functions and clinical application. Academic Press, New York, NY. 1979.
- 14.Bisio A, Urso E, Guerrini M, et al. Structural characterization of the low-molecular-weight heparin dalteparin by combining different analytical strategies. Molecules. 2017;22(7):1051. doi: 10.3390/molecules22071051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsh J, Bauer KA, Donati MB, et al. Parenteral anticoagulants: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6):141S–159S. [DOI] [PubMed]
- 16.Bisio A, Vecchietti D, Citterio L, et al. Structural features of low-molecular-weight heparins affecting their affinity to antithrombin. Thromb Haemost. 2009;102(5):865–873. doi: 10.1160/TH09-02-0081. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder M, Hogwood J, Gray E, et al. Protamine neutralization of low molecular weight heparins and their oligosaccharide components. Anal Bioanal Chem. 2011;399(2):763–771. doi: 10.1007/s00216-010-4220-8. [DOI] [PubMed] [Google Scholar]
- 18.Arnold KM, et al. Modernization of enoxaparin molecular weight determination using homogeneous standards. Pharmaceuticals. 2017;10:66. doi: 10.3390/ph10030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Pharmacopeia–National Formulary (USP–NF). Test for 1,6-anhydro derivative for enoxaparin sodium. Rockville, MD: U.S. Pharmacopeial Convention. 2009.
- 20.Council of Europe, European Pharmacopoeia, Fifth ed., Directorate for the Quality of Medicines & HealthCare, France, 2013.
- 21.EPO searching for patents (search keywords: low molecular weight heparins). https://worldwide.espacenet.com/patent/search/family/053118510/publication/CN104592421A?q=enoxaparin%20. Accessed last: May 31, 2021.
- 22.Generic fragmin availability. Medically reviewed by Drugs.com. https://www.drugs.com/availability/generic-fragmin.html. Accessed: 11 May 2021.
- 23.Sanofi-Aventis loses US Lovenox patent case. The PharmaLetter dated 19–02–2007. www.phramaletter.com. Accessed: 05 June 2021.
- 24.Sandoz leads the way with first generic version of ‘gold standard’ anti-thrombotic Lovenox. https://www.fiercepharma.com/pharma/sandoz-leads-way-first-generic-version-of-gold-standard-anti-thrombotic-lovenox. Accessed: 29 May 2021.
- 25.See Aventis Pharma SA. v. Amphastar Pharmaceuticals, Inc., 475 F. Supp. 2nd 970 C.D. Cal. Feb. 8, 2007.
- 26.See Aventis Pharma SA. v. Amphastar Pharmaceuticals, Inc., 525 F.3d. 1334 Fed. Cir. May 14, 2008.
- 27.Aventis Pharma SA. v. Amphastar Pharmaceuticals Inc., et al. 129 S.Ct. 2053 (April 27, 2009).
- 28.US Food and Drug Administration. Response to citizen petition, docket no. FDA-2003-P-0273. http://www.regulations.gov. 2010. Accessed: 20 June 2021.
- 29.Modus Therapeutics announces new strategy for the clinical development of sevuparin as a potential treatment for sepsis/septic shock. March 10, 2021. https://finance.yahoo.com/news/modus-therapeutics-announces-strategy-clinical-070000121.html. Accessed: 18 June 2021.
- 30.Viskov C, Just M, Laux V, et al. Description of the chemical and pharmacological characteristics of a new hemisynthetic ultra-low-molecular-weight heparin, AVE5026. J Thromb Haemostasis. 2009;7:1143–1151. doi: 10.1111/j.1538-7836.2009.03447.x. [DOI] [PubMed] [Google Scholar]
- 31.Biberovic V, et al. Mixtures of sulfated oligosaccharides. US8003623. 2011.
- 32.Jordan R, et al. Heparin with two binding sites for antithrombin or platelet factor 4. J Bio Chem. 1982;257(1):400–406. doi: 10.1016/S0021-9258(19)68378-X. [DOI] [PubMed] [Google Scholar]
- 33.Song Y, Zhang F, Linhardt RJ. Analysis of the glycosaminoglycan chains of proteoglycans. J Histochem Cytochem. 2021;69(2):121–135. doi: 10.1369/0022155420937154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keire DA, Buhse LF, Al-Hakim A. Characterization of currently marketed heparin products: composition analysis by 2D-NMR. Anal Methods. 2013;5(12):2984–2994. doi: 10.1039/c3ay40226f. [DOI] [Google Scholar]
- 35.Guerrini M, Guglieri S, Naggi A, et al. Low molecular weight heparins: structural differentiation by bidimensional nuclear magnetic resonance spectroscopy. Semin Thromb Hemost. 2007;33(5):478–487. doi: 10.1055/s-2007-982078. [DOI] [PubMed] [Google Scholar]
- 36.Ouyang Y, Zeng Y, Rong Y, et al. Profiling analysis of low molecular weight heparins by multiple heart-cutting two dimensional chromatography with quadruple time-of-flight mass spectrometry. Anal Chem. 2015;87:8957–8963. doi: 10.1021/acs.analchem.5b02218. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, Buhse LF, Al-Hakim A, et al. Characterization of currently marketed heparin products: analysis of heparin digests by RPIP-UHPLC-QTOF-MS. J Pharm Biomed Anal. 2012;67–68:42–50. doi: 10.1016/j.jpba.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Chen X, Zhu Z, et al. Structural analysis of low molecular weight heparin by ultraperformance size exclusion chromatography/time of flight mass spectrometry and capillary zone electrophoresis. Anal Chem. 2013;85:1819–1827. doi: 10.1021/ac303185w. [DOI] [PubMed] [Google Scholar]
- 39.Zaia J, Khatri K, Klein J, et al. Complete molecular weight profiling of low-molecular weight heparins using size exclusion chromatography-ion suppressor-high-resolution mass spectrometry. Anal Chem. 2016;88:10654–10660. doi: 10.1021/acs.analchem.6b03081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lingzhi GONG. Patent WO2016051170A1.
- 41.Maxwell E, Tan Y, Tan Y, Hu H, et al. GlycReSoft: a software package for automated recognition of glycans from LC/MS data. PLoS ONE. 2012;7:e45474. doi: 10.1371/journal.pone.0045474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Zhang F, Zaia J, Linhardt RJ. Top-down approach for the direct characterization of low molecular weight heparins using LC-FT-MS. Anal Chem. 2012;84:8822–8829. doi: 10.1021/ac302232c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, Chi L, Jin L, et al. Mapping of low molecular weight heparins using reversed phase ion pair liquid chromatography-mass spectrometry. Carbohydr Polym. 2014;99:339–344. doi: 10.1016/j.carbpol.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Ange KS, Lin L, et al. Top-down and bottom-up analysis of commercial enoxaparins. J Chromatogr A. 2017;1480:32–40. doi: 10.1016/j.chroma.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Lin L, Liu X, et al. Capillary electrophoresis–mass spectrometry for the analysis of heparin oligosaccharides and low molecular weight heparin. Anal Chem. 2016;88:1937–1943. doi: 10.1021/acs.analchem.5b04405. [DOI] [PubMed] [Google Scholar]
- 46.Lin L, Liu X, Zhang F, et al. Analysis of heparin oligosaccharides by capillary electrophoresis–negative-ion electrospray ionization mass spectrometry. Anal Bioanal Chem. 2017;409:411–420. doi: 10.1007/s00216-016-9662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X, Sheng A, Liu X, et al. Comprehensive identification and quantitation of basic building blocks for low-molecular weight heparin. Anal Chem. 2016;88:7738–7744. doi: 10.1021/acs.analchem.6b01709. [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Guo Z, Yu M, et al. Hydrophilic interaction chromatography-multiple reaction monitoring mass spectrometry method for basic building block analysis of low molecular weight heparins prepared through nitrous acid depolymerization. J Chromatogr A. 2017;1479:121–128. doi: 10.1016/j.chroma.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 49.Kailemia MJ, Park M, Kaplan DA, et al. Highfield asymmetricwaveform ion mobility spectrometry and electron detachment dissociation of isobaric mixtures of glycosaminoglycans. J Am Soc Mass Spectrom. 2013;25(2):258–268. doi: 10.1007/s13361-013-0771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agyekum I, Zong C, Boons GJ, et al. Single stage tandem mass spectrometry assignment of the C5 uronic acid stereochemistry in heparan sulfate tetrasaccharides using electron detachment dissociation. J Am Soc Mass Spectrom. 2017;28(9):1741–50. [DOI] [PMC free article] [PubMed]
- 51.Guo Q, Reinhold VN. Advancing MSn spatial resolution and documentation for glycosaminoglycans by sulfate-isotope exchange. Anal Bioanal Chem. 2019;411:5033–5045. doi: 10.1007/s00216-019-01899-8. [DOI] [PubMed] [Google Scholar]
- 52.Wei J, Wu J, Tang Y, et al. Characterization and quantification of highly sulfated glycosaminoglycan isomers by gatedtrapped ion mobility spectrometry negative electron transfer dissociation MS/MS. Anal Chem. 2019;91(4):2994–3001. doi: 10.1021/acs.analchem.8b05283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang Q, Chopra P, Boons G-J, Sharp JS. Improved de novo sequencing of heparin/heparan sulfate oligosaccharides by pro-pionylation of sites of sulfation. Carbohydr Res. 2018;465:16–21. doi: 10.1016/j.carres.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han X, Sanderson P, Nesheiwat S, et al. Structural analysis of urinary glycosaminoglycans from healthy human subjects. Glycobiology. 2020;30:143–151. doi: 10.1093/glycob/cwz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antia IU, Mathew K, Yagnik DR, et al. Analysis of procainamide-derivatised heparan sulphate disaccharides in biological samples using hydrophilic interaction liquid chromatography mass spectrometry. Anal Bioanal Chem. 2018;410:131–143. doi: 10.1007/s00216-017-0703-1. [DOI] [PubMed] [Google Scholar]
- 56.Wu J, Wei J, Chopra P, et al. Sequencing heparan sulfate using HILIC LC-NETD-MS/MS. Anal Chem. 201;91:11738–11746. [DOI] [PMC free article] [PubMed]
- 57.Poyer S, Lopin-Bon C, Jacquinet JC, et al. Isomer separation and effect of the degree of polymerization on the gas-phase structure of chondroitin sulfate oligosaccharides analyzed by ion mobility and tandem mass spectrometry. Rapid Commun Mass Spectrom. 2017;31:2003–2010. doi: 10.1002/rcm.7987. [DOI] [PubMed] [Google Scholar]
- 58.Zhan X. Fine structural analysis of enoxaparin sodium based on capillary electrophoresis. Master’s Thesis, Donhua University, January 13, 2011. pp 1–57.
- 59.Kang J, Zhan X. Capillary electrophoresis method for fine structural analysis of enoxaparn sodium. US 9012229B2. 2015.
- 60.Enoxaparin Sodium United States Pharmacopeia; USP Rockville. MD, USA. 2016;39:3695–3697. [Google Scholar]
- 61.Karawdeniya BI, Bandara YND, Nichols JW, et al. Surveying silicon nitride nanopores for glycomics and heparin quality assurance. Nat Commun. 2018;9(1):1–8. doi: 10.1038/s41467-018-05751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Im J, Lindsay S, Wang X, Zhang P. Single molecule identification and quantification of glycosaminoglycans using solid-state nanopores. ACS Nano. 2019;13(6):6308–6318. doi: 10.1021/acsnano.9b00618. [DOI] [PubMed] [Google Scholar]
- 63.Oliveira RCRD, Araujo TGD. Production and characterization of low molecular weight heparin obtained by modified double emulsion method with solvent evaporation. Pharm Pharmacol Int J. 2019;7(6):271–273. doi: 10.15406/ppij.2019.07.00263. [DOI] [Google Scholar]
- 64.Sadowski R, Gadzała-Kopciuch R, Buszewski B. Recent developments in the separation of low molecular weight heparin anticoagulants. Curr Medicinal Chem. 2019;26:166. doi: 10.2174/0929867324666171005114150. [DOI] [PubMed] [Google Scholar]
- 65.Stickney M, Sanderson P, Leach FE, et al. Online capillary zone electrophoresis negative electron transfer dissociation tandem mass spectrometry of glycosaminoglycan mixtures. Int J Mass Spectrom. 2019;445:116209. doi: 10.1016/j.ijms.2019.116209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller RL, Guimond SE, Schwörer R, et al. Shotgun ion mobility mass spectrometry sequencing of heparan sulfate saccharides. Nat Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-15284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saad OM, Leary JA. Heparin sequencing using enzymatic digestion and ESI-MSn with HOST: a heparin/HS oligosaccharide sequencing tool. Anal Chem. 2005;77(18):5902–5911. doi: 10.1021/ac050793d. [DOI] [PubMed] [Google Scholar]
- 68.Ceroni A, Maass K, Geyer H, et al. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res. 2008;7:1650–1659. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- 69.Dreyfuss JM, Jacobs C, Gindin Y, et al. Targeted analysis of glycomics liquid chromatography/ mass spectrometry data. Anal Bioanal Chem. 2011;399:727–735. doi: 10.1007/s00216-010-4235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maxwell E, Tan Y, Tan Y, et al. GlycReSoft: a software package for automated recognition of glycans from LC/MS data. PLoS ONE. 2012;7:e45474. doi: 10.1371/journal.pone.0045474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aoki-Kinoshita K, Agravat S, Aoki NP, et al. GlyTouCan1.0–the international glycan structure repository. Nucleic Acids Res. 2015;44:D1237–D1242. [DOI] [PMC free article] [PubMed]
- 72.Wang X, Liu X, Li L, et al. GlycCompSoft: software for automated comparison of low molecular weight heparins using top-down lc/ms data. PLoS ONE. 2016;11(12):e0167727. doi: 10.1371/journal.pone.0167727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong P, Sun H, Sha L, et al. GlycoDeNovo–an efficient algorithm for accurate de novo glycan topology reconstruction from tandem mass spectra. J Am Soc Mass Spectrom. 2017;28(11):2288–2301. doi: 10.1007/s13361-017-1760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hogan JD, Klein JA, Wu J, et al. Software for peak ending and elemental composition assignment for glycosaminoglycan tandem mass spectra. Mol Cell Proteomics. 2018;17(7):1448–1456. doi: 10.1074/mcp.RA118.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiu Y, Schliekelman P, Orlando R, Sharp JS. A multivariate mixture model to estimate the accuracy of glycosaminoglycan identifications made by tandem mass spectrometry (MS/MS) and database search. Mol Cell Proteomics. 2017;16(2):255–264. doi: 10.1074/mcp.M116.062588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.www.biopharmaspec.com. [https://biopharmaspec.com/biosimilar-testing/low-molecular-weight-heparin-lmwh-products/. Last Accessed: June 26, 2022]
- 77.Szajek AY, Chess E, Johansen K, et al. The US regulatory and pharmacopeia response to the global heparin contamination crisis. Nat Biotechnol. 2016;34:625. doi: 10.1038/nbt.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ye H, Toby TK, Sommers CD, et al. Characterization of currently marketed heparin products: key tests for LMWH quality assurance. J Pharm Biomed Anal. 2013;85:99–107. doi: 10.1016/j.jpba.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 79.Mulloy B, et al. USP compendial methods for analysis of heparin: chromatographic determination of molecular weight distributions for heparin sodium. Anal Bioanal Chem. 2014;406:4815–4823. doi: 10.1007/s00216-014-7940-3. [DOI] [PubMed] [Google Scholar]
- 80.United States Pharmacopeial Convention. USP 37 official monographs: heparin sodium. In: United States pharmacopeia and national formulary (USP 37-NF 32). United States Pharmacopeial Convention, Rockville. 2014.
- 81.EMA/536977/2016 - Committee for Medicinal Products for Human Use (CHMP). Assessment report for Inhixa.
- 82.Guideline on biosimilar low molecular weight heparins (EMEA/CHMP/BMWP/118264/2007 Rev. 1).
- 83.European Pharmacopoeia 8.0 volume II, Heparins, low-molecular-mass monograph 0828, Council of Europe. 2014.
- 84.European Pharmacopoeia 8.0 volume II, Enoxaparin sodium monograph 1097, Council of Europe. 2014.
- 85.Australian Public assessment report for enoxaparin sodium. 2017.
- 86.Gray E, Mulloy B. Biosimilar low molecular weight heparin products. J Thromb Haemost. 2009;7:1218–1221. doi: 10.1111/j.1538-7836.2009.03461.x. [DOI] [PubMed] [Google Scholar]
- 87.Ofosu FA. The United States Food and Drugs Administration approves a generic enoxaparin. Clin Appl Thromb Hemost. 2011;17(1):5–8. doi: 10.1177/1076029610389028. [DOI] [PubMed] [Google Scholar]
- 88.US Food and Drug Administration. Generic enoxaparin questions and answers (updated July 23, 2010). .http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm220037.htm. (Accessed 15 Dec 2020).
- 89.EMA/CHMP/437/04: guideline on similar biological medicinal products.
- 90.EMEA/CHMP/BMWP/118264/2007 Rev. 1: guideline on non-clinical and clinical development of similar biological medicinal products containing low molecular-weight-heparins using complex mixtures.
- 91.Policy Statement: clarifying the appropriate regulatory pathway for subsequent entry low molecular weight heparins. Ottawa: Health Canada; Draft date 12 August 2013 (http://www.hc-sc.gc.ca/dhp-mps/brgtherap/applic-demande/guides/lmwh-pol-hfmm-eng.php. Accessed: 15 June 2021)
- 92.Agbogbo FK, et al. Current perspectives on biosimilars. J Industrial Microbiol Biotechnol. 2019;46(9–10):1297–1311. doi: 10.1007/s10295-019-02216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed/literature reviewed is included in the article.