Abstract
Background
SARS-CoV-2 is a highly infectious respiratory virus associated with coronavirus disease (COVID-19). Discoveries in the field revealed that inflammatory conditions exert a negative impact on bone metabolism; however, only limited studies reported the consequences of SARS-CoV-2 infection on skeletal homeostasis. Inflammatory immune cells (T helper—Th17 cells and macrophages) and their signature cytokines such as interleukin (IL)-6, IL-17, and tumor necrosis factor-alpha (TNF-α) are the major contributors to the cytokine storm observed in COVID-19 disease. Our group along with others has proven that an enhanced population of both inflammatory innate (Dendritic cells—DCs, macrophages, etc.) and adaptive (Th1, Th17, etc.) immune cells, along with their signature cytokines (IL-17, TNF-α, IFN-γ, IL-6, etc.), are associated with various inflammatory bone loss conditions. Moreover, several pieces of evidence suggest that SARS-CoV-2 infects various organs of the body via angiotensin-converting enzyme 2 (ACE2) receptors including bone cells (osteoblasts—OBs and osteoclasts—OCs). This evidence thus clearly highlights both the direct and indirect impact of SARS-CoV-2 on the physiological bone remodeling process. Moreover, data from the previous SARS-CoV outbreak in 2002–2004 revealed the long-term negative impact (decreased bone mineral density—BMDs) of these infections on bone health.
Methodology
We used the keywords “immunopathogenesis of SARS-CoV-2,” “SARS-CoV-2 and bone cells,” “factors influencing bone health and COVID-19,” “GUT microbiota,” and “COVID-19 and Bone health” to integrate the topics for making this review article by searching the following electronic databases: PubMed, Google Scholar, and Scopus.
Conclusion
Current evidence and reports indicate the direct relation between SARS-CoV-2 infection and bone health and thus warrant future research in this field. It would be imperative to assess the post-COVID-19 fracture risk of SARS-CoV-2-infected individuals by simultaneously monitoring them for bone metabolism/biochemical markers. Importantly, several emerging research suggest that dysbiosis of the gut microbiota—GM (established role in inflammatory bone loss conditions) is further involved in the severity of COVID-19 disease. In the present review, we thus also highlight the importance of dietary interventions including probiotics (modulating dysbiotic GM) as an adjunct therapeutic alternative in the treatment and management of long-term consequences of COVID-19 on bone health.
Keywords: SARS-CoV-2, COVID-19, Bone health, Inflammation, Probiotics, Gut microbiota
Introduction
In March 2020, the COVID-19 disease was declared a pandemic by World Health Organization (WHO). To date, more than 570 million confirmed cases of COVID-19 along with 6.38 million deaths have been reported globally (https://coronavirus.jhu.edu/map.html). It is gradually becoming clear that patients recovered from COVID-19 are reporting various long-term ill-health consequences, but little is known about its long-term sequelae on bone health. The relevance of the skeletal system is supported by the fact that it is involved in calcium homeostasis, hematopoiesis, mobility, etc. The role of the skeletal system as a bona fide endocrine organ (osteocalcin—Ocn and fibroblast growth factor—FGF) in controlling the physiological energy metabolism and mineral ion homeostasis is well established [1]. Ocn is a bone matrix protein that in its gamma (γ)-carboxylated form regulates the size and shape of hydroxyapatites. However, the undercarboxylated form of Ocn regulates glucose metabolism, testosterone biosynthesis (by Leydig cells), etc. A study reported that daily injection of Ocn improved glucose tolerance and insulin sensitivity and prevented the development of type 2 diabetes in mice [2]. Consistent with this, a study reported that deficiency of Ocn hormone impaired glucose homeostasis in Ocn null mice [3]. Of note, recently a study showed that lower levels of Ocn can be employed as a good prognostic marker for monitoring stress-induced hyperglycemia in COVID-19 infected patients [4]. In addition, exosomes-ExoFlo™ generated from bone marrow mesenchymal stem cells (MSCs-osteoblast progenitors) increased the survival rate in COVID-19 disease patients, by increasing oxygenation, reducing the inflammatory environment, and considerably lowering the neutrophil count [5]. Together these studies emphasize the importance of bone in maintaining the physiological functioning of the body under both normal and disease conditions.
The intriguing interaction between the skeletal system and the host immune system reveals the fascinating feature supporting the adaptability of the skeletal system and its cells. An interdisciplinary field called “Osteoimmunology” was founded in 2000 by the Takayanagi et al. group as a result of the relationship between the immune system and bone [6]. In the past 2 decades, critical work in this enthralling field has resulted in the identification of the role played by the RANKL-OPG (osteoprotegerin) axis in the preservation of skeletal homeostasis, the earlier being a key regulator of osteoclastogenesis. Numerous immune cells, including activated T cells and B cells, which are crucial in the pathophysiology of numerous bone-related illnesses, including osteoporosis, express RANKL in addition to bone-forming OBs cells. Experimental evidence supports the idea that pro-inflammatory cytokines such as IL-1β, IL-6, IL-17, and TNF-α promote bone resorption by activating the RANK-RANKL axis, which causes the appearance of a variety of bone-related illnesses [7, 8] (Fig. 1). Immune system activation in response to SARS-CoV-2 infection also leads to the aggressive production and accumulation of these inflammatory cytokines (IL-1β, IL-6, IL-17, and TNF-α) resulting in an event termed as “Cytokine Storm” [9]. Enhancement in the level of inflammatory cytokines causes the influx of several immune cells such as macrophages, T cells, and neutrophils to the site of infection, resulting in devastating effects on the vascular barrier, capillaries, alveoli, cell-to-cell interactions ultimately leading to damage in multiple organs (including bone) in SARS-CoV-2-infected patients [9]. A study reported in 2005 demonstrated that during the SARS-CoV outbreak patients who were treated with corticosteroids showed lower bone mineral density (BMD) in comparison to normal controls in long-term studies [10]. In addition, WHO approved the use of corticosteroids to suppress the inflammatory response in critical COVID-19-infected patients thus alarming an enhanced fracture risk in SARS-CoV-2-infected patients. Accordingly, a better understanding of SARS-CoV-2 pathogenesis in inducing bone loss would result in improved therapeutic and preventative measures in a current pandemic. In the present review, we thus highlight the role of various factors underpinning the risk of developing skeletal disorders in various viral diseases including COVID-19 (Fig. 2). In addition, we further discuss the role of GM and probiotics in managing the long-term consequences of COVID-19 infection on bone health.
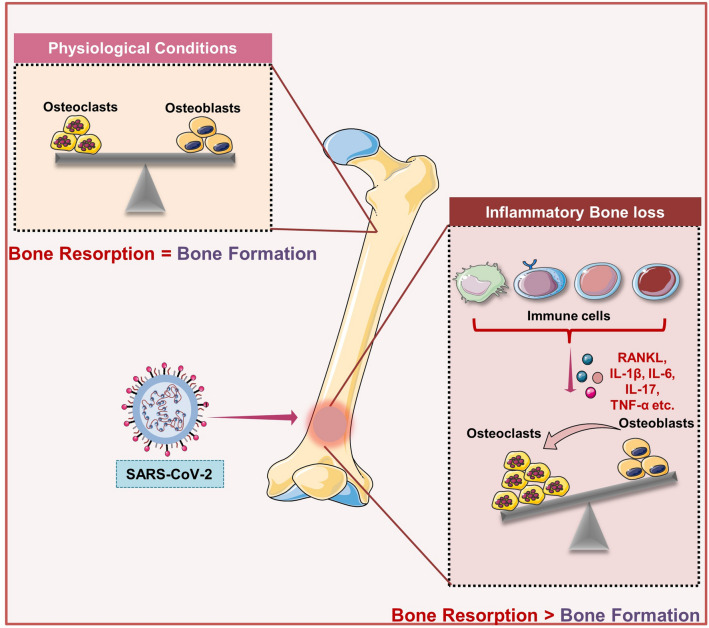
Fig. 1.
SARS-CoV-2 infection induce bone loss: In physiological conditions, the homeostatic balance between osteoblasts and osteoclasts maintains the bone health, whereas upon SARS-CoV-2 infection enhanced production of inflammatory cytokines shifts balance towards the bone-resorbing osteoclasts that further augments bone loss
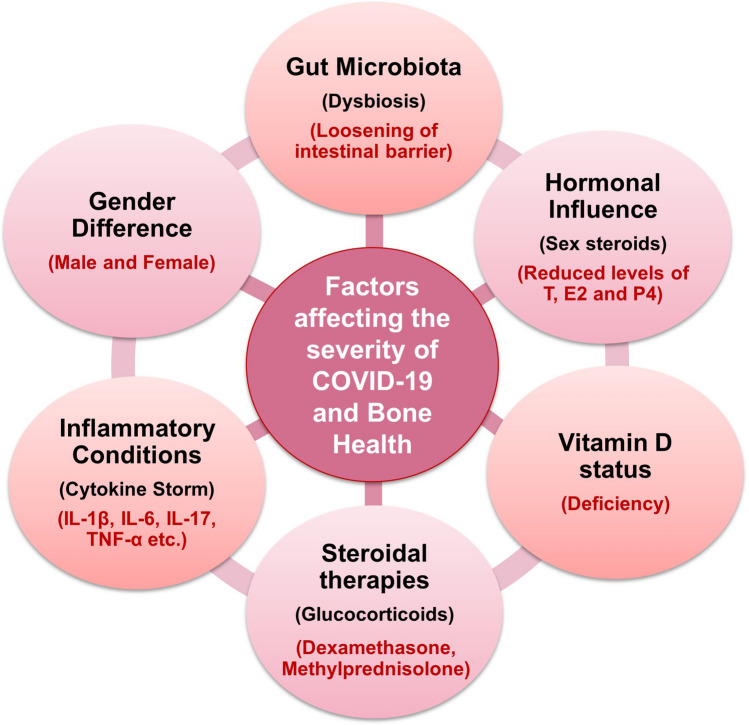
Fig. 2.
Factors influencing COVID-19 disease severity and bone loss: various factors such as dysbiosis of gut microbiota (GM), cytokine storm, administration of steroidal therapies, deficiency of vitamin D, gender and, hormonal factors influence COVID-19 and bone health
Inflammatory bone loss in viral diseases
The role of virus-induced bone loss has long been neglected, but investigations in recent decades revealed the involvement of virus infections in the augmentation of bone loss. Available reports suggest that human immunodeficiency virus (HIV)-infected patients showed a higher prevalence of bone-related diseases, and the use of corticosteroids further augments this in HIV-infected patients [11]. In the case of HIV, it has been observed that disruption of the immune-skeleton interface enhanced bone loss and thus intensified the risk of fracture in infected patients. Patients with HIV experience bone loss due to a variety of causes, but recently, the significance of B cells in HIV-induced bone loss has also come to light. It has been observed that dysregulated expression of RANKL and OPG on B cells elicits bone loss in HIV-infected patients [12]. In addition, a study demonstrated that highly active anti-retroviral therapies (HAART) control viral replication but the regimens such as tenofovir disoproxil fumarate (TDF) may also accelerate bone loss by inducing osteolytic activity of OCs along with dampening the OBs bone-forming potential [13]. In contrast to this, a study demonstrated that HIV-infected patients showed a higher prevalence of reduced BMD, irrespective of their anti-retroviral therapies status [11]. Of note, it has been observed that HIV exhibits the potential of inducing bone loss by directly increasing the adhesion and bone degradation machinery of OCs. In particular, the viral protein “nef” induces bone loss by promoting the assembly of OCs on the bone-resorbing sealing zone by activating Src (a key regulator of podosome formation in OCs) [14]. Furthermore, a cross-sectional study reported that the use of nucleoside/nucleotide analogs such as tenofovir reduced the BMD in hepatitis B virus (HBV) infected patients thereby simultaneously enhancing the risk of developing osteoporosis-related fractures in the long run [15, 16]. Importantly, bone resorptive biochemical marker such as deoxypyridinoline in the urine of HBV-infected patients further supports its bone resorptive potential. Alphaviruses have also been reported to interfere with the bone remodeling process by inducing the production of inflammatory cytokine levels such as IL-6 that further by dysregulating the RANKL-OPG axis augments bone loss [17]. Various bone anomalies were also observed in SARS-CoV-recovered patients which are partly explained by the rampant use of various steroidal therapies. In 2009, a study reported that human monocytes (precursor of OCs) express ACE2 (a receptor of SARS-CoV) that aid the entry of SARS-CoV directly into OCs, and by expressing 3a/X1protein, SARS-CoV increase the nuclear factor kappa B (NFκB) activity and promote RANKL-induced osteoclastogenesis [18]. Long-term follow-up studies in SARS-CoV-infected patients demonstrated the distinct possible mechanisms such as prolonged use of steroidal therapies, and inflammatory conditions may further induce bone loss in recovered patients. According to a recent cohort study, 36% of COVID-19-infected patients had thoracic vertebral fractures (VFs) that could affect their ability to breathe in the future. This is suggestive of the evaluation of the morphometric VFs parameters in all the COVID-19 suspected and infected patients [19]. Together, these studies suggest an enhanced risk of fractures in COVID-19 recovered patients.
SARS-CoV-2 infection and its implications on bone health
The initial step involved in the pathogenesis of COVID-19 is the invasion of the SARS-CoV-2 virus into target host cells. Spike protein (S) of SARS-CoV-2 aids the entry of the virus into the host cell via interacting with ACE2 receptors expressed on cellular targets such as alveolar cells of lungs, myocardial cells, testis, and bone cells (Fig. 3). The physiological functioning of ACE2 is to regulate the inflammatory condition by hydrolyzing the angiotensin II (inflammatory) of the renin-angiotensin system (RAS) to angiotensin 1–7 (anti-inflammatory). This leads to an enhancement in controlling the inflammatory conditions. However, it has been observed that upon infecting the target cells, SARS-CoV-2 downregulates the expression of ACE2 and enhances the levels of angiotensin II [20]. Increased angiotensin II further induces inflammatory conditions via upregulating the expression of E and P selectin, IL-8, chemokine ligands (CCL)-2 and 5. In addition, angiotensin II stimulates innate immunity by activating the toll-like receptor (TLR)-4 [21] and thus, facilitates the massive production of inflammatory responses in respective organs including the skeletal system. Shimizu et al. demonstrated that angiotensin II exhibits osteoclastogenic potential via enhancing the expression of RANKL on OBs, which further enhances differentiation of OCs thereby accelerating osteoporosis development in the rat model [22]. Of note, a study demonstrated enhancement in OCs number along with a robust reduction in several bone parameters in the COVID-19 mice model [23]. From the TRAP staining data, it has been observed that infection of SARS-CoV-2 significantly enhanced OCs number by 64%, OCs surface by 27%, and 38% enhancement in OCs per bone surface in COVID-19 disease mice model after 2 weeks of post-infection [23]. Thus, it is vital to delineate and understand the long-term sequelae of SARS-CoV-2 infection on bone loss in COVID-19-recovered patients. Moreover, it is correspondingly important to understand the mechanism of COVID-19-induced bone loss. In further sections, we discuss the possible mechanisms that may enhance bone deterioration in COVID-19-recovered patients under distinct categories.
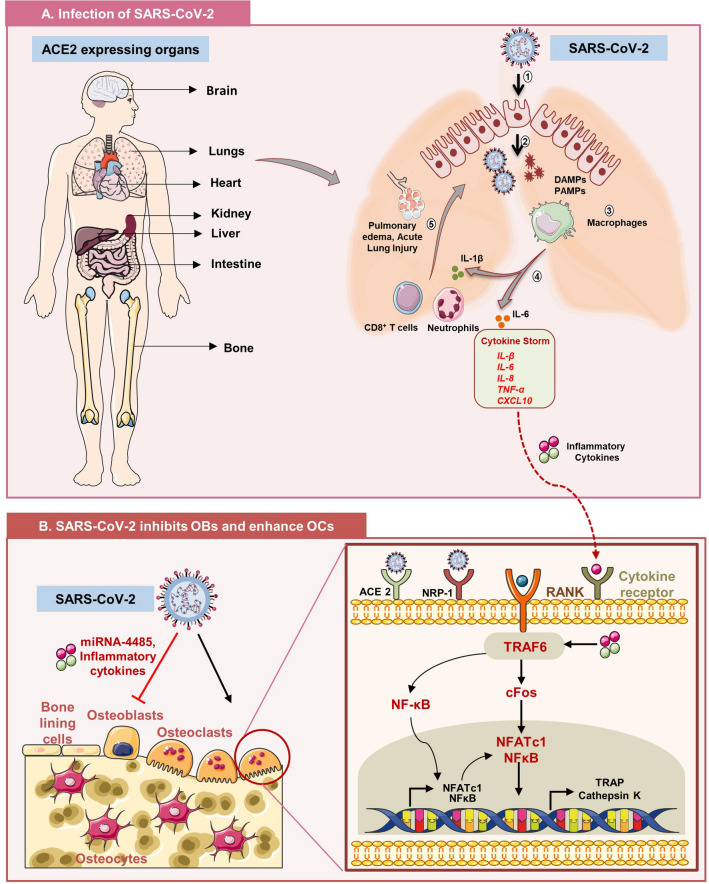
Fig. 3.
SARS-CoV-2 affects bone health either directly or via cytokine storm: A Various organs of the body including skeleton system express angiotensin convertase enzyme 2 (ACE2) receptor that aids the entry of SARS-CoV-2 inside host cells. (1): Internalization of COVID-19 into target cells, (2): after pyroptosis waning cells generate death-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), (3): virus-derived DAMPs and PAMPs engulfed by macrophages in turn produce pro-inflammatory cytokines, (4) interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, chemokine (CC) ligand-2, CCL-3 and CXCL10, (5): recruitment of neutrophils and CD8+ T cells to the site of infection that further causes tissue injury in lung parenchyma, (6): “Cytokine Storm Syndrome”, i.e., flooding of cytokines into circulation that leads to multiorgan failure including skeleton system. B Inside the skeleton system, SARS-CoV-2 binds to ACE2 receptor on osteoclasts and induces its differentiation. Moreover, inflammatory cytokines further augment bone loss by binding to cytokine receptors. In addition, by inducing production of micro-RNA-4485, SARS-CoV-2 inhibits osteogenic ability of osteoblasts.
SARS-CoV-2 and bone cells
Several studies demonstrated that SARS-CoV-2 exhibits the potential to infect the skeletal system by directly infecting the bone cells (OCs and OBs) and affecting the bone remodeling process. A study reported that along with owning the inflammatory potential, ACE2/Ang (1–7) axis is found to be linked with osteoporosis marked by the expression of ACE2 receptors on OBs and OCs [24] (Fig. 3). Moreover, it suggests that SARS-CoV-2 invades bone cells and may alter their ability to modulate bone health by targeting the ACE2 receptor. Furthermore, in situ immunofluorescence analysis and single-cell RNA sequencing data revealed that entry of SARS-CoV-2 in bone marrow macrophages (BMMs) is dependent on the expression of neuropilin-1 (NRP-1). The expression of NRP-1 on BMMs makes these cells target for SARS-CoV-2 [25]. However, the expression of NRP-1 is observed to be reduced upon differentiation of BMMs into mature OCs, thus indicating the reduced tendency of SARS-CoV-2 to infect mature OCs. A recent study described a novel method by which SARS-CoV-2 reduces the osteogenic potential of OBs by increasing the expression of microRNA-4485 via targeting TLR4 (found to perturb the fracture healing) in COVID-19 disease patients [26] (Fig. 3). Together these studies are indicative of the strong disruption of bone health in post-COVID-19 patients. Along with directly targeting the bone cells, SARS-CoV-2 can also influence bone health in multiple ways such as by either augmenting inflammatory conditions or even by causing the dysbiosis of GM. Thus, in further sections, we enlist these factors in detail.
Cytokine storm
In response to SARS-CoV-2, immediate activation of innate immune cells, viz. macrophages, neutrophils, etc. occurs that further limits the infection by causing the downstream activation of the long-lasting adaptive immune system to produce neutralizing antibodies and T cell response against the virus. Nevertheless, when inflammation does not resolve after serving its purpose then it can cause dysregulated hyperinflammation, i.e., “cytokine storm” and results in inhibition of the adaptive immune system thereby further escalating tissue damage and organ failure. Cytokine storm is manifested by unrestrained production of several inflammatory cytokines such as IL-6, IL-7, IL-2, IL-17, TNF-α, monocyte chemoattractant protein (MCP)-1, and macrophage inhibitory protein (MIP)-1α (Fig. 3) (Table 1). These cytokines are considerably higher in intensive care unit (ICU) patients infected with SARS-CoV-2 in comparison to non-ICU patients having the same viral load [27, 28]. Thus, these studies suggest that viral load is not the pertinent factor in determining the severity in COVID-19 patients. But the elevated levels of inflammatory cytokines by promoting hyperinflammation, acute respiratory distress syndrome (ARDS), and multiorgan failure define the severity of COVID-19 disease. In addition to inducing inflammatory conditions, disease severity is also marked by the reduced expression of anti-viral cytokine IFNγ by CD4+ T cells [29]. Both IL-6 and TNF-α cytokines via inducing apoptosis in T and B lymphocytes causes lymphopenia and increased IL-2 levels thereby causing activation induced cell death [28]. In addition, the expression of ACE2 on lymphocytes also aids the apoptosis of lymphocytes. In support of this, a study demonstrated (golden Syrian hamster model) that cytokine storm causes the loss of bone in the trabecular region of long bones and lumbar-vertebrae of SARS-CoV-2-infected model [30]. It has been observed that bone loss aggravates from the acute phase to the post-recovery phase. A mechanistic view of the enhanced bone loss deciphered the ability of inflammatory cytokines such as IL-1β, IL-6, and TNF-α in inducing pathological bone loss in the hamster model [30]. Since the immune system is strongly associated with the management of healthy bone and any dysregulation in the levels of inflammatory cytokines leads to unfavorable effects on bone health. To further emphasize the importance of the immune system in inflammatory bone loss conditions such as osteoporosis, Srivastava et al. have recently coined the term “Immunoporosis”, i.e., immunology of osteoporosis [31]. A recent study proved that higher levels of inflammatory cytokines such as IL-1β, IL-6, IL-17, and TNF-α in postmenopausal osteoporotic women enhance bone deterioration (low BMD) in comparison to the control group [32]. These inflammatory cytokines by causing an imbalance in the metabolism of the skeletal system favor bone resorption by promoting the expression RANKL on OBs. Altogether, these studies suggest that therapeutic strategies proven to be effective for treating inflammatory conditions in COVID-19 disease may also enhance the risk of developing secondary osteoporosis and destruction of joints in recovered patients.
Table 1.
Cytokine storm and its effect on bone health
| S. no | Inflammatory cytokines | Source | Levels in COVID-19 disease patients | Effect on bone health | Reference |
|---|---|---|---|---|---|
| 1 | IL-1β | Monocytes, macrophages, neutrophils, CD4+ T cells | High | Stimulates osteoclastogenesis and inhibits bone formation by directly activating a RANK mediated signaling pathway | [66] [67] |
| 2 | IL-6 | Monocytes, macrophages, neutrophils, CD4+ T cells | High | Promotes bone resorption by enhancing the interaction of OCs and OBs/osteocytes via the RANK-RANKL axis | [68, 69] |
| 3 | IL-8 | Monocytes, macrophages, fibroblasts, endothelial cells | High | Stimulates osteoclastogenesis and promotes osteolysis | [27, 70] |
| 4 | IL-17 | Th17 cells | High | Stimulates osteoclastogenesis by augmenting the levels of M-CSF and RANKL and by promoting autophagy in bone marrow cells | [71, 72] |
| 5 | IL-18 | Monocytes, macrophages, neutrophils, dendritic cells | High | Enhance OCs differentiation via upregulating RANKL on T cells | [73, 74] |
| 6 | TNF-α | Monocytes, macrophages, Th17 cells | High | Promotes OCs differentiation via upregulating RANKL on OBs via NFκB signaling pathway | [75, 76] |
Steroidal therapies and bone health
Corticosteroid drugs are synthetic steroid hormones that are produced by the adrenal cortex, and it is employed to treat a wide range of inflammatory diseases. Corticosteroids include both glucocorticoids and mineralocorticoids. Primarily glucocorticoids such as dexamethasone are considered a double edge sword for treating critical COVID-19 patients (Table 2). Dexamethasone mimics the nature of natural compounds that the body produces to quell the inflammation and is used to treat various inflammatory disease conditions including chronic obstructive lung diseases (COLD), asthma, allergies, and skin conditions. Dexamethasone is being given in dosage ranging from 0.5 to 10 mg daily which in turn depends on the nature and severity of the disease. In the recovery clinical trial, it was observed that the use of dexamethasone also resulted in lowered mortality rate in COVID-19-infected patients who were receiving oxygen support or mechanical ventilation (The recovery collaborative group; https://www.nejm.org/doi/full/10.1056/nejmoa2021436). However, extended administration of glucocorticoids was found to be detrimental. Strikingly, epidemiological data suggest that glucocorticoid therapy enhanced the risk of fracture occurrence and was found to be the reason for iatrogenic osteoporosis commonly known as glucocorticoid-induced osteoporosis (GIOP) [33]. Glucocorticoids by altering the bone metabolism shift the balance of bone remodeling towards OCs thus enhancing bone loss. Recently, a cross-sectional study reported a confirmed case of osteonecrosis in a cohort of Wuhan COVID-19 patients treated with glucocorticoids [34, 35]. Moreover, dexamethasone via inhibiting the osteogenesis of stem cells reduced the ability of cells to repair the bone and thus lead to the onset of osteonecrosis. According to a longitudinal research, 58 percent of SARS-CoV-infected patients who received corticosteroid treatment went on to develop avascular osteonecrosis [36]. Debating the use of corticosteroids, led to disagreements about whether the short-term advantages may outweigh the severe side effects that are seen over the long term. The leading theory behind the observed consequences is that corticosteroid treatment leads to the accumulation of fat droplets in blood and swelling of fat cells inside BM that may further lead to clogging of small blood vessels thereby starving bone cells for oxygen and nutrients. Moreover, a study demonstrated that the treatment of rabbits with methylprednisolone activates the RAS system in bone and induces osteonecrosis by enhancing the expression of ACE receptors on OCs [36]. These advancements in research are thus indicating a considerable increase in skeletal disorders in COVID-19-recovered patients, highlighting the need for a long-term follow-up studies.
Table 2.
Corticosteroidal therapies given to COVID-19-infected patients and their impact on bone health
| S. no | Steroidal therapies | Durability/dosage | Mechanism of action in COVID-19 | Effect on bone health | Reference |
|---|---|---|---|---|---|
| 1 | Dexamethasone (C22H29FO5) | Long-acting half-life (36–72 h) once daily | It markedly reduces the levels of IL-1β, IL-6, CRP, and ferritin and thus inhibits the inflammatory conditions in COVID-19 diseases patients | It causes osteonecrosis and osteoporosis as it is resistant to 11β-hydroxysteroid dehydrogenase-mediated inactivation | [77, 78] |
| 2 | Methylprednisolone (C22H30O5) | Intermediate-acting half-life (12–36 h) twice daily | It inhibits activation of T cells, reduces the expression of inflammatory genes along with inducing the expression of anti-inflammatory genes via blocking the NFκB transcription factor. It also suppresses the expression of cyclooxygenase (COX-2) and thus inhibits inflammatory cascade by acting at multiple dimensions | Enhances bone loss by promoting the deterioration and micro-damage in bone by inducing apoptosis of osteoblasts | [79–81] |
| 3 | Hydrocortisone (C21H30O5) | Intermediate-acting half-life (8–12 h) 2–4 doses daily | It is employed to manage septic shock in COVID-19 patients; it reduces levels of inflammatory cytokines such as IL-1β, IL-6, CRP, TNF-α, MIP, MCP, and nitric oxide in COVID-19 patients | Reduce bone mineral density (BMD) | [82–84] |
Gender and hormonal influences
Across geographics, different findings reported the association between gender and the risk of developing worse outcomes due to COVID-19. Though the prevalence of COVID-19 infection is the same in both males and females, clinical data indicate a higher number of fatalities in males in comparison to females in most countries [37]. However, in some countries such as India, Nepal, Vietnam, and Slovenia, fatality rate due to COVID-19 is higher in women in comparison to men [38]. Further to investigate whether the observed disparities are linked with the alterations in the hormonal levels. It has been observed that sex steroids regulate the expression of ACE2, thus controlling the entry of SARS-CoV-2 inside the host cells. A cohort study demonstrated that testosterone concentration is inversely related to the concentrations of inflammatory cytokines such as IL-6, interferon-gamma inducible protein 10 (IP10), interleukin 1 receptor antagonist, and C-reactive protein (CRP) in COVID-19-infected males [39]. Moreover, gene enrichment analysis showed upregulation in hormone signaling pathways in both classical (CD14+CD16−) and non-classical (CD14−CD16+) monocytes, indicating that low testosterone levels activate the inflammatory signaling cascade and are associated with the disease severity [39]. This study clearly indicates that lower levels of testosterone hormone predispose men towards COVID-19 infection. Recently, a study reported that hospitalized women are less likely to die due to COVID-19 infection, but once severe symptoms develop the risk of mortality is similar in both females and males [40]. These shreds of evidence suggest that gonadal hormones in females mainly estrogen (E2) and progesterone (P4) give a likely explanation for the sexual dimorphism in the COVID-19 infection and disease severity. It has been observed that E2 and P4 favor the anti-inflammatory state in females via reducing the innate immunity along with enhancing the immune tolerance and antibody production in females. A study reported in mice showed that estradiol treatment reduces the expression of ACE2 in kidney and airway epithelial cells via activating the estrogen receptor alpha (ERα) thus inhibiting viral entry, whereas ovariectomy (estrogen-deficient condition) in mice enhances the expression of ACE2 in distinct tissues and thus may enhance virus entry [41] in elderly women. However, the protective effect of estrogen is diminished in postmenopausal women resulting in a higher incidence of COVID-19 in menopausal subjects (estrogen-deficient condition) [42]. Together, these studies represent the effects of sex hormones estrogen and testosterone in regulating the expression of receptors involved in SARS-CoV-2 virus entry, modulating the immune system, etc.
Vitamin D status
An active form of vitamin D (1,25-dihydroxy vitamin D) is responsible for the regulation of calcium and phosphate metabolism. Several studies reported that lower levels of vitamin D enhance the development of multiple immune-related diseases such as rheumatoid arthritis (RA), multiple sclerosis (MS), type 1 diabetes (T1D), and COVID-19. It has been observed that older patients with vitamin D deficiency (< 30 nmol/L) showed higher D-dimer levels and were found to be associated with worse outcomes of COVID-19 infection [43]. Consistent with this, a study recently demonstrated that COVID-19-positive patients with vitamin D levels > 30 ng/ml showed lower levels of D-dimer, CRP, and shorter hospital stays in contrast to COVID-19 patients with severe vitamin D deficiency (< 10 ng/ml) [44]. Thus, these studies suggest that vitamin D levels may be employed as a prognosis indicator. A clinical trial (NCT04344041): COVIT-TRIAL is a randomized clinical trial where a high-risk elderly population was supplemented with vitamin D and the outcome of the trial described that a high dose of vitamin D is found to be an effective and immediately approachable treatment for COVID-19 [45]. Furthermore, a vitamin D deficiency’s secondary effect is a disturbance in the process of bone mineralization, which results in bone loss, muscle weakness, and the onset of osteoporosis, which may eventually progress to osteomalacia [46]. Notably, the majority of patients admitted to ICU and dying concomitantly suffered from hypovitaminosis D. In agreement with this, a study showed that decreased serum vitamin D levels were linked to an increase in mortality in critically ill patients infected with SARS-CoV-2 [47]. Vitamin D is also a key modulator of the renin–angiotensin system which is exploited by the SARS-CoV-2 to enter the host cells [48]. In addition, vitamin D controls the immune system via dampening the pro-inflammatory immune response along with enhancing the anti-inflammatory immune response, anti-microbial peptides (AMPs), along with activating the macrophages thereby exhibiting the potential to destroy the SARS-CoV-2. According to a study, a feedback loop between vitamin D deficiency and the COVID-19-linked coagulopathy enhances platelet activation, which heightens thrombosis [49]. Notably, vitamin D insufficiency and low platelet count have been connected to illnesses that cause pathological bone loss, such as osteoporosis, and have also been proven to play a critical role in the metabolism of minerals and the preservation of bone health. Thus, to prevent the pathological bone loss caused by the SARS-CoV-2 infection, it is crucial to monitor both platelet count and vitamin D levels.
COVID-19, gut microbiome, and bone health
Enormous variations among the COVID-19-infected patients are largely unknown and are described as “Immunological Dark Matter (IDM)”. Emerging evidence demonstrated that GM is likely to be a pertinent contributing factor to IDM. Though COVID-19 is a respiratory disease, several emerging pieces of evidence suggest that GM is also involved in the severity of the disease. GM harbors hundreds of bacterial genera that reside either in the luminal stream or adhere to the gut mucosa. It has been observed that SARS-CoV-2 infection alters the bacteriome and virome which could reflect the contribution of altered GM in the disease severity and recovery [50]. Since the major method of SARS-CoV-2 infection may involve respiratory droplets and fomite but the fecal–oral pathway may also be used to disseminate SARS-CoV-2 [51]. SARS-CoV-2 has likewise selected the GUT as its target because this organ also expresses ACE2 and TMPRSS2. In addition, SARS-CoV-2 viral RNA was also detected in the patient’s feces samples [51]. In consistent, the endoscopic evaluation revealed that individuals also suffered from colon injury when infected with SARS-CoV-2. Moreover, the fundamental etiology of post-acute COVID-19 syndrome (PACS) has been determined to be dysbiosis of the Gut microbiota. A 6-month follow-up study in COVID-19-recovered patients revealed the significant difference in the GM among the patients developing PACS and without PACS [52]. Moreover, patients with PACS showed reduced bacterial diversity and richness in comparison to healthy individuals. Together, these results point forth how GM dysbiosis contributes to the development of PACS and to the aggravation of COVID-19 illness.
A study demonstrated that Bacteroides spp. is observed to be inversely interrelated with the SARS-CoV-2 fecal load in hospitalized patients [53]. Bacteroides spp. has also been linked with the altered expression and functionality of the ACE2 receptor in the gut. Remarkably, Bacteroides were observed to modulate the lung heparan sulfate which is a glycosaminoglycan. In addition, the endoscopic evaluation revealed that the individuals had colon injury that in turn alters the adhesion of virion to the host cells [54]. In addition, a recent study showed a direct correlation with increased levels of inflammatory cytokines and markers such as CRP, aspartate aminotransferase (AAT), lactate dehydrogenase (LDH), and gamma-glutamyl transferase [53]. These studies highlight the involvement of GM in enhanced inflammation and disease severity in COVID-19 disease patients. Thus, strategies that can alter the GM should be employed to manage the long-term effects of COVID-19 in infected patients. This evidence supports the fact that altered GM and the associated leaky gut may also contribute to the multiorgan complications observed in long run. Growing evidence suggests that dysbiosis of the GM was also found to be the pivotal factor in augmenting bone loss which in turn promotes the development of several bone-related diseases such as RA and osteoporosis. A series of reports revealed that GM influences bone strength by altering the quality of bone tissue. The mechanism through which GM modulates bone health is via promoting the generation of regulatory metabolites such as indole derivatives, trimethylamine N-oxide (TMAO: amine oxide), short-chain fatty acids (SCFAs), and gasotransmitter such as hydrogen sulfide (H2S). It was observed that H2S-donating compound GYY4137 enhances bone health by activating the Wnt10b production thereby increasing bone formation with reducing the trabecular bone loss in the ovariectomy (ovx) induced postmenopausal osteoporotic mice model [55]. SCFAs such as acetate (C2), propionate (C3), and butyrate (C4) induce metabolic reprogramming in bone-resorbing cells viz. OCs resulting in enhanced glycolysis, thereby reducing the osteoclast’s specific genes such as NFATc1 and TRAF6, potent regulators of skeletal homeostasis [56].
The main producers of butyrate in the human gut are bacteria belonging to the phylum Firmicutes, mainly Faecalibacterium prausnitzii [57]. A study demonstrated that treatment with fermented products of Lactobacillus plantarum and Lactobacillus fermentum significantly improved the bone mass in the ovx mice model by enhancing the abundance of genus Faecalibacterium prausnitzii and Lactobacillus [58]. Notably, shotgun metagenome sequencing data of fecal samples collected from the 15 SARS-CoV-2-infected patients showed baseline abundance of Clostridium hathewayi, Clostridium ramosum, and Coprobacillus, and reduced levels of Faecalibacterium prausnitzii was observed to be associated with disease severity. These reports thereby strongly suggest that modulation of GM could also be employed to reduce the disease severity [53]. A recent study reported that gut commensals exhibiting immunomodulatory potentials such as Eubacterium rectale, Faecalibacterium prausnitzii, and bifidobacteria were observed to be reduced in COVID-19-infected patients in comparison to non-COVID-19 individuals irrespective of the condition whether they received medication or not. Moreover, GM analysis of 212 patients via 16S rRNA sequencing showed lower bacterial richness and diversity in the GM composition with a lower abundance of Streptococcus, Bifidobacterium, and Collinsella in SARS-CoV-2-infected patients in connection with the reduction in the distinct network of anti-inflammatory genera. Dysbiosis of GM persists for a long even after the clearance of the virus in patients who recovered from COVID-19 disease. In addition, emerging evidence suggests that dysbiosis of GM is one of the major causative factors for the onset and progression of osteoporosis [59]. Thus, targeting dysbiosis in SARS-CoV-2-infected patients can prevent the risk of developing osteoporosis-related fractures (Fig. 4).
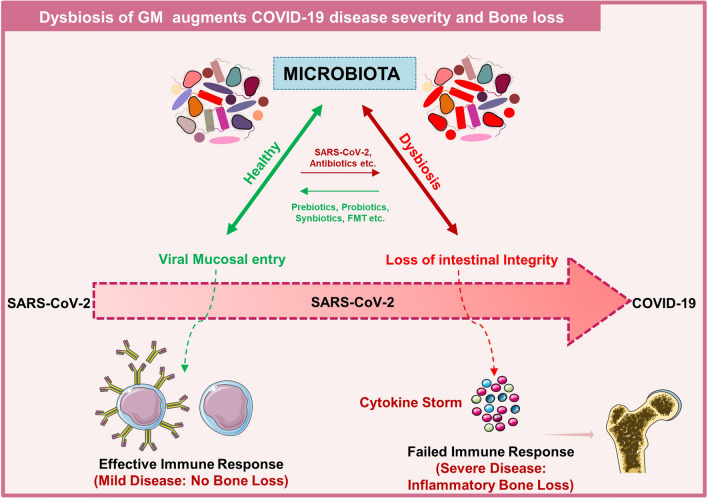
Fig. 4.
Dysbiosis of Gut microbiota (GM) enhances COVID-19 disease severity and bone loss: healthy GM maintains the intestinal barrier that further generates the effective immune response resulting in clearance of virus and no bone loss. On the other hand, dysbiosis of GM results in loss of intestinal barrier integrity which further enhances cytokine storm and thus bone loss
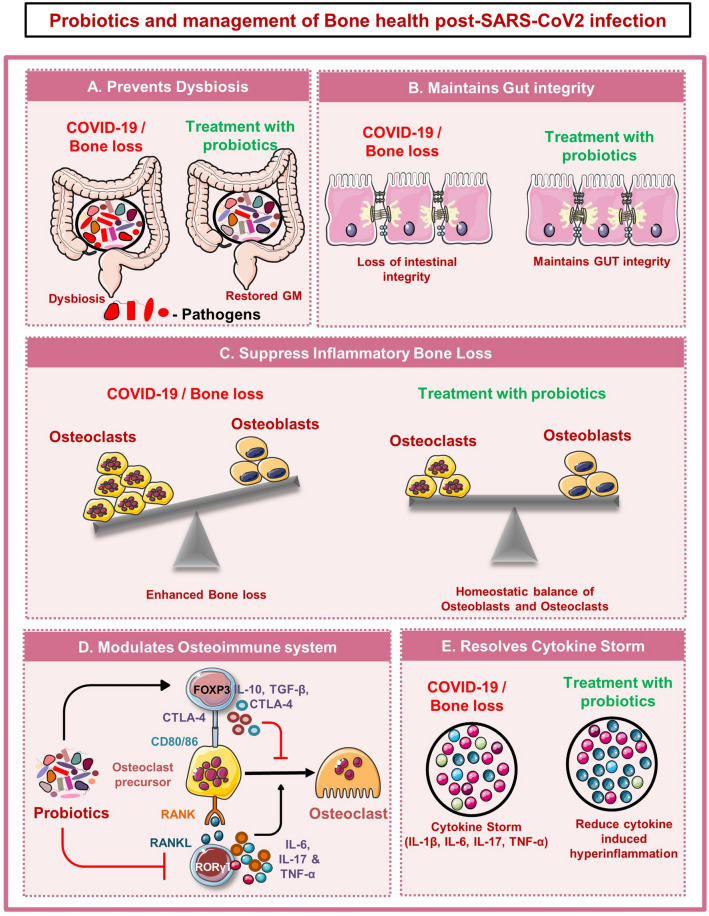
Probiotics and management of bone health post-SARS-CoV-2 infection
Presently, there is no ideal promising therapy for the treatment and management of COVID-19 disease. All the above-mentioned studies prove that SARS-CoV-2 infection imparts its negative impact by altering the gut microbial species. Emerging evidence suggests that intestinal microbial richness does not restore to normal levels even after months of recovery from COVID-19 disease. Probiotics are specified as viable microorganisms that impart numerous health benefits when administered in satisfactory amounts. Importantly, various studies have already established the role of probiotics in ameliorating various inflammatory conditions. The administration of probiotics as an adjunctive therapy will thus restore the healthy gut microbial species in post-SARS-CoV-2-infected individuals. Here, we discuss the possible mechanisms that may aid the beneficial effects of probiotics (Fig. 5).
Fig. 5.
Probiotics and management of bone health post-SARS-CoV-2 infection: A Probiotics prevent COVID-19-induced dysbiosis by restoring gut microbiota composition. B Probiotics maintains Gut integrity and thus inhibit inflammation induced bone loss. C Probiotics can directly promote osteoblasts differentiation and suppress osteoclastogenesis. D Probiotics modulate bone health via modulating immune system. E Probiotics resolve inflammation by reducing inflammatory cytokines
Probiotics by adhering to the gut epithelial cells competitively inhibit the colonization of pathogenic organisms in the intestinal region. Upon adhesion, probiotics secrete several metabolites, and modulating the intestinal permeability strengthens the epithelial barrier and thus attenuates the inflammation. Probiotics mediate enhancement in intestinal barrier function by releasing mucin in the intestinal cavity to form the mucus layer [60]. The mucus layer forms the first line of defense that ensures the integrity of intestinal barriers and thus prevents the invasion of pathogenic organisms to reach gut epithelial cells. In addition, secreting anti-microbial factors such as antibacterial peptides, SCFAs, defensins, and bacteriocins, prevent the growth of pathogenic organisms [61]. In addition, it maintains the intestinal epithelial gut integrity via enhancing the expression of tight junction proteins [60]. Thus, by employing probiotics as adjunctive therapy microbial composition of the gut may be restored similar to that of healthy individuals and thus would lessen the risk of developing secondary bone-related diseases.
Several studies demonstrated that probiotics exhibit immunomodulatory potential that enhances the host immunity by regulating the production of inflammatory and anti-inflammatory cytokines. Under pathological conditions, probiotic administration provokes the differentiation of Th1, Th2, Treg, Th17, Bregs, etc. via modulating the intestinal microbial species. In general, Lactobacilli and Bifidobacteria make up the majority of the gut microbial niche that by producing several metabolites such as SCFAs upregulates the anti-inflammatory immune response. A study demonstrated that Lactobacilli and Bifidobacteria reduced the inflammatory immune response against allergens by promoting the secretion of IL-10 and transforming growth factor (TGF)-β, etc. Recently, a double-blind prospective trial revealed that Lactobacillus plantarum significantly enhanced the cytokine index in correspondence to an early immune response to COVID-19 infection, thereby mimicking the cytokine environment produced during SARS-CoV infection [62]. This study suggests that daily intake of Lactobacillus plantarum may be employed as an anti-COVID-19 therapy as a possible way to prevent the occurrence of COVID-19 during the pandemic. Moreover, recently our group showed that probiotics such as Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum and Bacillus clausii mediate their beneficial effects on the host by modulating the dysregulated balance of Tregs and Th17 immune cells [8, 63–65]. Together these studies suggest that probiotics exhibit strong immunomodulatory potential and thus restoring the GM composition via probiotics treatment would balance both the inflammatory and anti-inflammatory immune cells thereby imparting its beneficial effects.
Conclusion and future outlook
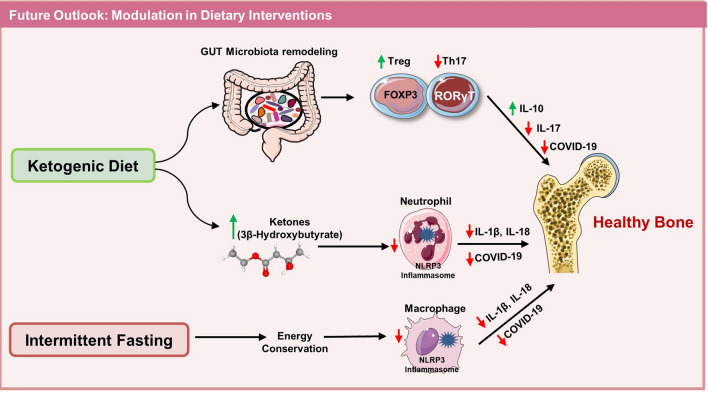
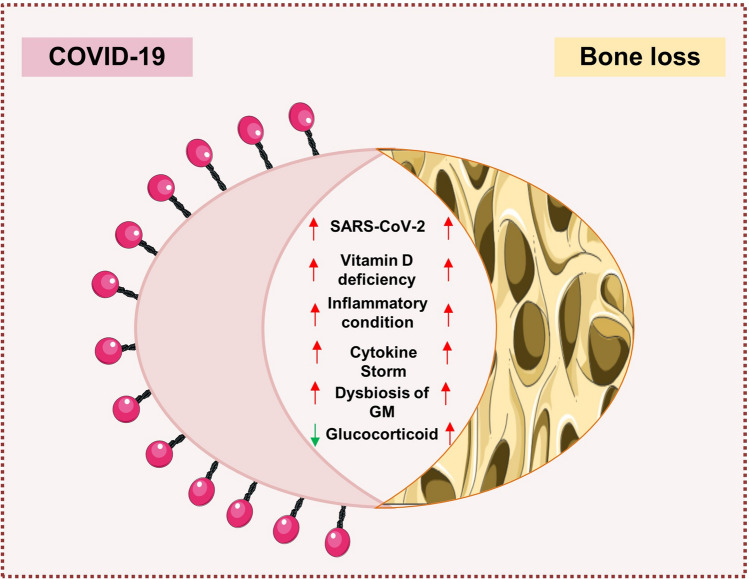
The present review summarizes the correlation and implications of COVID-19 disease on bone health. Numerous possibilities suggest that SARS-CoV-2 affects bone health either directly or indirectly. Cytokine storm and dysbiosis of the GM are the two predominant contributing factors in influencing the severity of COVID-19 disease. Total 33 clinical trials have highlighted the potential of probiotics (undergoing and completed) in reducing the severity of COVID-19 by modulating the GM and reducing cytokine storm (PROVID-LD-NCT05080244; PROVID-19-NCT04621071; NCT04390477; NCT04458519; NCT04734886; NCT05043376; NCT04734886). One clinical trial showed probiotics with promising results even in COVID-19 patients suffering from co-morbidities (NCT04507867) https://clinicaltrials.gov/. These studies clearly highlight the potential of probiotics in reducing the severity of COVID-19 disease and its negative impact on bone health thereby attesting to its therapeutic employment in future. Several emerging evidence suggests that intake of a low fiber diet along with a high carbohydrate/fat diet leads to the dysbiosis of GM leading to modulated immune response and thus compromised bone health. Thus, modulation of dietary interventions in the form of ketogenic diet and intermittent fasting might be a valuable strategy in reducing the long-term implications of COVID-19 disease on bone health. In summary, robust studies are warranted in the field along with long-term follow-up of recovered COVID-19-infected patients for establishing the mechanism of COVID-19-induced bone pathologies (Figs. 6 and 7).
Fig. 6.
Modulation of dietary interventions reduces the COVID-19 disease severity and maintains bone health: intake of a ketogenic diet by remodeling the GM enhances the Tregs and reduces Th17 cells that further reduce COVID-19 disease severity and maintains bone health by producing IL-10 and reducing IL-17 cytokine. On the other hand, production of ketone bodies such as 3β-Hydroxybutyrate reduces the NLR family pyrin domain containing 3 (NLRP3) inflammasomes mediated production of IL-1β and IL-18 cytokines and thus maintains bone health. A perusal of intermittent fasting conserves energy and reduces macrophage (NLRP3 inflammasomes) and maintains bone health
Fig. 7.
Correlation between Covid-19 and Bone Health: List of factors affecting the severity of COVID-19 disease and Bone loss
Acknowledgements
This work was financially supported by the Intramural project (A-COVID-98) from the All India Institute of Medical Sciences (AIIMS), New Delhi-India sanctioned to RKS. LS, CS, BV, and RKS acknowledge the Department of Biotechnology, AIIMS, New Delhi—India for providing infrastructural facilities. LS thanks UGC for the research fellowship. Figures are created with the help of Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license (https://smart.servier.com).
Abbreviations
- OCs
Osteoclasts
- OBs
Osteoblasts
- RANK
Receptor activator of nuclear factor kappa B
- RANKL
Receptor activator of nuclear factor kappa B ligand
- Th
Helper T cells
- Treg
Regulatory T cells
- IL
Interleukins
- TGF-β
Tumor growth factor-β
- Ocn
Osteocalcin
- MCP
Monocyte chemoattractant protein
- MIP
Macrophage inhibitory protein
Author contributions
RKS contributed to the conceptualization and writing of the manuscript. LS: participated in the writing and editing of the review. RKS suggested and LS: created the illustrations. CS, BG, RG, and BV: provided valuable inputs in preparing the manuscript. All the authors made a significant contribution in drafting, revising, and critically reviewing the article and gave final approval of the version to be published.
Funding
All India Institute of Medical Sciences, A-COVID-98.
Declarations
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guntur AR, Rosen CJ. Bone Endocrine Organ. 2012;18:758–762. doi: 10.4158/EP12141.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–575. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth SL, Centi A, Smith SR, Gundberg C. The role of osteocalcin in human glucose metabolism: marker or mediator? Nat Rev Endocrinol. 2013;9:43–55. doi: 10.1038/nrendo.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrieta F, Martinez-Vaello V, Bengoa N, Rosillo M, de Pablo A, Voguel C, et al. Stress hyperglycemia and osteocalcin in COVID-19 critically III patients on artificial nutrition. Nutrients. 2021;13:3010. doi: 10.3390/nu13093010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell Develop. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-Cell-Med Regul Osteoclastogenesis Sig Cross-Talk Between RANKL IFN-γ. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 7.Sapra L, Azam Z, Rani L, Saini C, Bhardwaj A, Shokeen N, et al. “Immunoporosis”: Immunology of Osteoporosis. Proceed Natl Acad Sci India Sec B: Biol Sci. 2021;91:511. doi: 10.1007/s40011-021-01238-x. [DOI] [Google Scholar]
- 8.Sapra L, Dar HY, Bhardwaj A, Pandey A, Kumari S, Azam Z, et al. Lactobacillus rhamnosus attenuates bone loss and maintains bone health by skewing Treg-Th17 cell balance in Ovx mice. Sci Rep. 2021;11:1807. doi: 10.1038/s41598-020-80536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: the current evidence and treatment strategies. Front immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau EMC, Chan FWK, Hui DSC, Wu AKL, Leung PC. Reduced bone mineral density in male Severe Acute Respiratory Syndrome (SARS) patients in Hong Kong. Bone. 2005;37:420–424. doi: 10.1016/j.bone.2005.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glesby MJ. Bone Disord Human Immunodefic Virus Infect. 2003;37:S91–95. doi: 10.1086/375884. [DOI] [PubMed] [Google Scholar]
- 12.Titanji K. Beyond antibodies: B cells and the OPG/RANK-RANKL pathway in health, non-HIV disease and HIV-induced bone loss. Front Immunol. 2017;8:1851. doi: 10.3389/fimmu.2017.01851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delpino MV, Quarleri J. Influence of HIV infection and antiretroviral therapy on bone homeostasis. Front Endocrinol. 2020;11:502. doi: 10.3389/fendo.2020.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raynaud-Messina B, Bracq L, Dupont M, Souriant S, Usmani SM, Proag A, et al. Bone degradation machinery of osteoclasts: An HIV-1 target that contributes to bone loss. Proceed Nat Acad Sci. 2018;115:E2556–E2565. doi: 10.1073/pnas.1713370115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y-Y, Fang W-H, Wang C-C, Kao T-W, Chang Y-W, Yang H-F, et al. Crosssectional assessment of bone mass density in adults with hepatitis B virus and hepatitis C virus. Infection. 2019;9:5069. doi: 10.1038/s41598-019-41674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dessordi R, Watanabe LM, Guimarães MP, Romão EA, de LourdesCandoloMartinelli A, de CarvalhoSantana R, et al. Bone Loss Hepatitis B Virus-Infect Patient Assoc Gt Osteoclastic Act Indep Retrovir Use. 2021;11:10162. [Google Scholar]
- 17.Chen W, Foo S-S, Rulli NE, Taylor A, Sheng K-C, Herrero LJ, et al. Arthr Alphaviral Infec Perturb Osteoblast Funct Trigger Pathol Bone Loss. 2014;111:6040–6045. doi: 10.1073/pnas.1318859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obitsu S, Ahmed N, Nishitsuji H, Hasegawa A, Nakahama K, Morita I, et al. Potential enhancement of osteoclastogenesis by severe acute respiratory syndrome coronavirus 3a/X1 protein. Archiv Virol. 2009;154:1457–1464. doi: 10.1007/s00705-009-0472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.di Filippo L, Formenti AM, Doga M, Pedone E, Rovere-Querini P, Giustina A. Radiological thoracic vertebral fractures are highly prevalent in covid-19 and predict disease outcomes. J Clin Endocrinol Meta. 2021;106:e602–e614. doi: 10.1210/clinem/dgaa738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuba K, Imai Y, Penninger J. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6:271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biancardi VC, Bomfim GF, Reis WL, Al-Gassimi S, Nunes KP. 2017 The interplay between Angiotensin II, TLR4 and hypertension 120: 88–96. [DOI] [PubMed]
- 22.Shimizu H, Nakagami H, Osako MK, Hanayama R, Kunugiza Y, Kizawa T, et al. Angiotensin II accel osteoporos activ osteoclast. 2008;22:2465–2475. doi: 10.1096/fj.07-098954. [DOI] [PubMed] [Google Scholar]
- 23.Awosanya OD, Dalloul CE, Blosser RJ, Dadwal UC, Carozza M, Boschen K, et al. Osteoclast-mediated bone loss observed in a COVID-19 mouse model. Bone. 2022;154:116227. doi: 10.1016/j.bone.2021.116227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Queiroz-Junior CM, Santos ACPM, Galvão I, Souto GR, Mesquita RA, Sá MA, et al. The angiotensin converting enzyme 2/angiotensin-(1–7)/Mas Receptor axis as a key player in alveolar bone remodelling. Bone. 2019;128:115041. doi: 10.1016/j.bone.2019.115041. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Mei H, Sun J, Li H, Huang Y, Tang Y, et al. Neuropilin-1 Mediates SARS-CoV-2 Infection in Bone Marrow-derived Macrophages 2021 [DOI] [PMC free article] [PubMed]
- 26.Mi B, Xiong Y, Zhang C, Zhou W, Chen L, Cao F, et al. SARS-CoV-2-induced overexpression of miR-4485 suppresses osteogenic differentiation and impairs fracture healing. Intern J Biol Sci. 2021;17:1277–1288. doi: 10.7150/ijbs.56657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhardwaj A, Sapra L, Saini C, Azam Z, Mishra PK, Verma B, et al. COVID-19: immunology, immunopathogenesis and potential therapies. Intern Rev Immunol. 2021;41:1–36. doi: 10.1080/08830185.2021.1883600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manjili RH, Zarei M, Habibi M, Manjili MH. COVID-19 as an Acute Inflammatory Disease. J Immunol. 2020;205:12–19. doi: 10.4049/jimmunol.2000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen SF, Ho Y-C. SARS-CoV-2: a storm is raging. J Clin Investig. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao W, Lau HE, Xie H, Poon VK-M, Chan CC-S, Chu H, et al. SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters. Nature communications 2021: 2021.10.08.463665. [DOI] [PMC free article] [PubMed]
- 31.Srivastava RK, Dar HY, Mishra PK. Immunoporosis: immunology of osteoporosis—Role of T cells. Front Immunol. 2018 doi: 10.3389/fimmu.2018.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilesanmi-Oyelere BL, Schollum L, Kuhn-Sherlock B, McConnell M, Mros S, Coad J, et al. Inflamm Mark Bone Health Postmenopausal Women Cross-Sec Overv. 2019;16:15. doi: 10.1186/s12979-019-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Compston J. Glucocorticoid-Induced Osteoporos Update. 2018;61:7–16. doi: 10.1007/s12020-018-1588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G. Glucocorticoid, Covid-19, bone and nerve repair. J Ortho Trans. 2021;31:A1–2. doi: 10.1016/j.jot.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Huang Z, Tan B, Chen G, Li X, Xiong K, et al. General recommendation for assessment and management on the risk of glucocorticoid-induced osteonecrosis in patients with COVID-19. J Ortho Trans. 2021;31:1–9. doi: 10.1016/j.jot.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Wang K, Song Q, Liu R, Ji W, Ji L, et al. Role Local Bone Renin-Angiot Sys Steroid-Induc Osteonecros Rabbit. 2014;9:1128–1134. [Google Scholar]
- 37.Jin J-M, Bai P, He W, Wu F, Liu X-F, Han D-M, et al. Gender differences in patients With COVID-19: focus on severity and mortality. Fron Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehingia N, Raj A. Sex differences in COVID-19 case fatality: do we know enough? Lancet Global Health. 2021;9:e14–15. doi: 10.1016/S2214-109X(20)30464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Net Open. 2021;4:e2111398. doi: 10.1001/jamanetworkopen.2021.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raimondi F, Novelli L, Ghirardi A, Russo FM, Pellegrini D, Biza R, et al. Covid-19 and gender: lower rate but same mortality of severe disease in women—an observational study. BMC Pulm Med. 2021;21:96. doi: 10.1186/s12890-021-01455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Shoemaker R, Thatcher SE, Batifoulier-Yiannikouris F, English VL, Cassis LA. Administration of 17β-estradiol to ovariectomized obese female mice reverses obesity-hypertension through an ACE2-dependent mechanism. Am J Phy-Endocrinol Meta. 2015;308:E1066–E1075. doi: 10.1152/ajpendo.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costeira R, Lee KA, Murray B, Christiansen C, Castillo-Fernandez J, Ni Lochlainn M, et al. Estrogen and COVID-19 symptoms: Associations in women from the COVID Symptom Study. PLoS ONE. 2021;16:e0257051. doi: 10.1371/journal.pone.0257051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baktash V, Hosack T, Patel N, Shah S, Kandiah P, Van den Abbeele K, et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Post Med J. 2021;97:442–447. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demir M, Demir F, Aygun H. Vitamin D deficiency is associated with COVID-19 positivity and severity of the disease. J Med Virol. 2021;93:2992–2999. doi: 10.1002/jmv.26832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annweiler C, Beaudenon M, Gautier J, Simon R, Dubée V, Gonsard J, et al. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials. 2020;21:1031. doi: 10.1186/s13063-020-04928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lips P, van Schoor NM. Effect Vitamin D Bone Osteoporos. 2011;25:585–591. doi: 10.1016/j.beem.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Parra-Ortega I, Alcara-Ramírez DG, Ronzon-Ronzon AA, Elías-García F, Mata-Chapol JA, Cervantes-Cote AD, et al. 25-Hydroxyvitamin D level is associated with mortality in patients with critical COVID-19: a prospective observational study in Mexico City. Nutri Res Prac. 2021;15:S32. doi: 10.4162/nrp.2021.15.S1.S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar R, Rathi H, Haq A, Wimalawansa SJ, Sharma A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 2021;292:198235. doi: 10.1016/j.virusres.2020.198235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salamanna F, Maglio M, Sartori M, Landini MP, Fini M. Vitamin D and platelets: a menacing duo in COVID-19 and potential relation to bone remodeling. Intern J Mole Sci. 2021;22:10010. doi: 10.3390/ijms221810010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao J, Wang C, Zhang Y, Lei G, Xu K, Zhao N, et al. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut microbes. 2021;13:1887722. doi: 10.1080/19490976.2021.1887722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang B, Zhang L, Wang Y, Dai T, Qin Z, Zhou F, et al. Alterations in microbiota of patients with COVID-19: potential mechanisms and therapeutic interventions. Sig Trans Target Therapy. 2022;7:143. doi: 10.1038/s41392-022-00986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC-Y, Ng SSS, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71:544–552. doi: 10.1136/gutjnl-2021-325989. [DOI] [PubMed] [Google Scholar]
- 53.Yeoh YK, Zuo T, Lui GC-Y, Zhang F, Liu Q, Li AYL, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martino C, Kellman BP, Sandoval DR, Clausen TM, Marotz CA, Song SJ, et al. Bacterial modification of the host glycosaminoglycan heparan sulfate modulates SARS-CoV-2 infectivity. BioRxiv 2020: 2020.08.17.238444.
- 55.Grassi F, Tyagi AM, Calvert JW, Gambari L, Walker LD, Yu M, et al. Hydrog Sul Nov Reg Bone Form Implicat Bone Loss Induc Estrog Def. 2016;31:949–963. [Google Scholar]
- 56.Lucas S, Omata Y, Hofmann J, Böttcher M, Iljazovic A, Sarter K, et al. Short-Chain Fatty Acid Reg Sys Bone Mass Prot Pathol Bone Loss. 2018;9:55. doi: 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in immunology 2019; 10. [DOI] [PMC free article] [PubMed]
- 58.Lee CS, Kim J-Y, Kim BK, Lee IO, Park NH, Kim SH. Lacto Ferment Milk Prod Atten Bone Loss Exper Rat Model Ovariectomy-Ind Post-Menopaus Prim Osteoporos. 2021;130:2041–2062. [Google Scholar]
- 59.Bhardwaj A, Sapra L, Tiwari A, Mishra PK, Sharma S, Srivastava RK. “Osteomicrobiology”: The Nexus Between Bone and Bugs. Frontiers in Microbiology 2022; 12. [DOI] [PMC free article] [PubMed]
- 60.Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, et al. The Potential of gut commensals in reinforcing intestinal barrier function and alleviating. Inflammation. 2018;10:988. doi: 10.3390/nu10080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu LC-H. Host-microbial interactions and regulation of intestinal epithelial barrier function: from physiology to pathology. World J Gastrointest Pathophy. 2012;3:27. doi: 10.4291/wjgp.v3.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kageyama Y, Nishizaki Y, Aida K, Yayama K, Ebisui T, Akiyama T, et al. Lactobacillus plantarum induces innate cytokine responses that potentially provide a protective benefit against COVID-19: A single-arm, double-blind, prospective trial combined with an in vitro cytokine response assay. Exper Therapeutic Med. 2021;23:20. doi: 10.3892/etm.2021.10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dar HY, Shukla P, Mishra PK, Anupam R, Mondal RK, Tomar GB, et al. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. 2018;8:46–56. doi: 10.1016/j.bonr.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dar HY, Pal S, Shukla P, Mishra PK, Tomar GB, Chattopadhyay N, et al. Bacillus clausii inhibits bone loss by skewing Treg-Th17 cell equilibrium in postmenopausal osteoporotic mice model. Nutrition. 2018;54:118–128. doi: 10.1016/j.nut.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Sapra L, Shokeen N, Porwal K, Saini C, Bhardwaj A, Mathew M, et al. Bifidobacterium longum ameliorates ovariectomy-induced bone loss via enhancing anti-osteoclastogenic and immunomodulatory potential of regulatory B Cells (Bregs) Fron Immunol. 2022;13:875788. doi: 10.3389/fimmu.2022.875788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee Y-M, Fujikado N, Manaka H, Yasuda H, Iwakura Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Intern Immunol. 2010;22:805–816. doi: 10.1093/intimm/dxq431. [DOI] [PubMed] [Google Scholar]
- 67.van de Veerdonk FL, Netea MG. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit Care. 2020;24:445. doi: 10.1186/s13054-020-03166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Q, Zhou X, Huang D, JI Y, Kang F. IL-6 Enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cell Phy Biochem. 2017;41:1360–1369. doi: 10.1159/000465455. [DOI] [PubMed] [Google Scholar]
- 70.Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/S8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 71.Hasan MZ, Islam S, Matsumoto K, Kawai T. SARS-CoV-2 infection initiates interleukin-17-enriched transcriptional response in different cells from multiple organs. Sci Rep. 2021;11:16814. doi: 10.1038/s41598-021-96110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song L, Tan J, Wang Z, Ding P, Tang Q, Xia M, et al. Interleukin-17A facilitates osteoclast differentiation and bone resorption via activation of autophagy in mouse bone marrow macrophages. Mol Med Rep. 2019;19(6):4743–4752. doi: 10.3892/mmr.2019.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen P-K, Lan J-L, Huang P-H, Hsu J-L, Chang C-K, Tien N, et al. Interleukin-18 is a potential biomarker to discriminate active adult-onset still’s disease from COVID-19. Fron Immunol. 2021;12:2997. doi: 10.3389/fimmu.2021.719544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dai S-M. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 and tumour necrosis factor. Ann Rheum Dis. 2004;63:1379–1386. doi: 10.1136/ard.2003.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo G, Li F, Li X, Wang Z-G, Zhang B. TNF-α RANKL Promote Osteoclastogenes Upreg Rank Via Nf-Κb Path. 2018;17:6605–6611. doi: 10.3892/mmr.2018.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmed MH, Hassan A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Comp Clin Med. 2020;2639:1–10. doi: 10.1007/s42399-020-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weinstein RS. Glucocorticoid-Induc Osteoporos Osteonecros. 2012;41:595–611. [Google Scholar]
- 79.Sloka JS, Stefanelli M. Mech Act Methylprednisolone Treat Mult Scleros. 2005;11:425–432. doi: 10.1191/1352458505ms1190oa. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Dai R, Xiao J, Chen D. Liao E [Effect of methylprednisolone on bone mass, microarchitecture and microdamage in cortical bone of ulna in rats] Med Sci. 2015;40:25–30. doi: 10.11817/j.issn.1672-7347.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 81.Salton F, Confalonieri P, Meduri GU, Santus P, Harari S, Scala R, et al. Prolonged Low-Dose Methylprednisolone in Patients With Severe COVID-19 Pneumonia 7. US: Oxford University Press; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y, Cui X, Shiloach J, Wang J, Suffredini DA, Xu W, et al. Hydrocortisone decreases lethality and inflammatory cytokine and nitric oxide production in rats challenged with B. anthracis cell wall peptidoglycan. Inten Care Med Exp. 2020;8:67. doi: 10.1186/s40635-020-00358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dequin P-F, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. 2020 Effect of Hydrocortisone on 21 Day Mortality or Respiratory Support Among Critically III Patients With COVID-19.JAMA 324: 1298. [DOI] [PMC free article] [PubMed]
- 84.Schulz J, Frey KR, Cooper MS, Zopf K, Ventz M, Diederich S, et al. Red Daily Hydro Dose Improv Bone Health Prim Adrenal Insuff. 2016;174:531–538. doi: 10.1530/EJE-15-1096. [DOI] [PubMed] [Google Scholar]