Abstract
Background
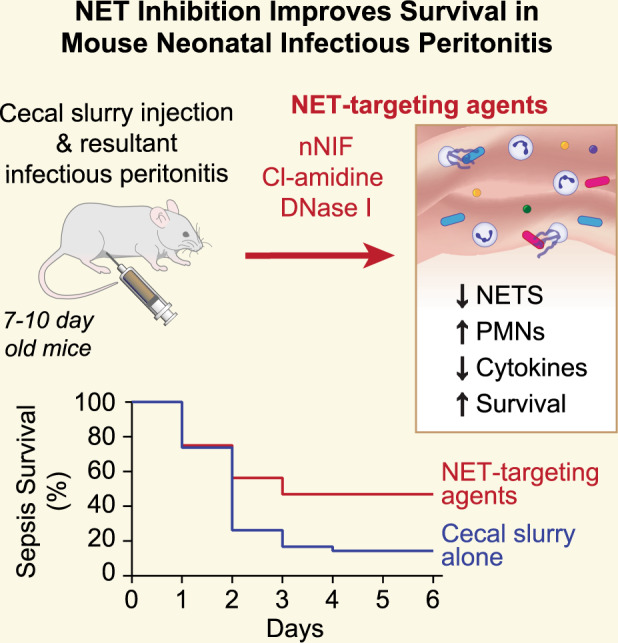
Treatment of neonatal peritonitis and sepsis is challenging. Following infection, neutrophils elaborate neutrophil extracellular traps (NETs)—extracellular lattices of decondensed chromatin decorated with antimicrobial proteins. NETs, however, can augment pathogenic inflammation causing collateral damage. We hypothesized that NET inhibition would improve survival in experimental neonatal infectious peritonitis.
Methods
We induced peritonitis in 7 to 10-day-old mice by intraperitoneal injection with cecal slurry. We targeted NETs by treating mice with neonatal NET-Inhibitory Factor (nNIF), an endogenous NET-inhibitor; Cl-amidine, a PAD4 inhibitor; DNase I, a NET degrading enzyme, or meropenem (an antibiotic). We determined peritoneal NET and cytokine levels and circulating platelet–neutrophil aggregates. Survival from peritonitis was followed for 6 days.
Results
nNIF, Cl-amidine, and DNase I decreased peritoneal NET formation and inflammatory cytokine levels at 24 h compared to controls. nNIF, Cl-amidine, and DNase I decreased circulating platelet–neutrophil aggregates, and NET-targeting treatments significantly increased survival from infectious peritonitis compared to controls. Finally, nNIF administration significantly improved survival in mice treated with sub-optimal doses of meropenem even when treatment was delayed until 2 h after peritonitis induction.
Conclusions
NET inhibition improves survival in experimental neonatal infectious peritonitis, suggesting that NETs participate pathogenically in neonatal peritonitis and sepsis.
Impact
Neutrophil extracellular trap formation participates pathogenically in experimental neonatal infectious peritonitis.
NET-targeting strategies improve outcomes in a translational model of neonatal infectious peritonitis.
NET inhibition represents a potential target for drug development in neonatal sepsis and infectious peritonitis.
Introduction
Severe neonatal infections, including sepsis, represent a significant cause of neonatal mortality and long-term morbidity.1–3 Neonatal sepsis occurs at an estimated rate of 1 to 2 cases per 1000 live births in the U.S.2,4 Worldwide, there are an estimated 1 to 4 million annual neonatal sepsis cases reported, and the mortality rate ranges from 5 to 10% in developed countries and is even higher in underdeveloped countries.2,5 Neonatal sepsis outcomes and prognosis depend particularly on early and efficient treatment.6–8 Indeed, up to an estimated 80% of neonatal deaths due to infection could be prevented through measures such as early diagnosis and timely, appropriate clinical management. Moreover, while necessary, empirical antibiotics often provide sub-optimal pathogen coverage and may result in widespread, unnecessary exposure to adverse drug effects and risk for increased antibiotic resistance.1,7,9 In this setting, adjunctive anti-inflammatory treatment may mitigate neonatal sepsis, but to date, no adjunctive intervention has demonstrated clinical benefit in clinical trials.10 Therefore, a critical need exists to better understand the mechanistic drivers of neonatal sepsis pathophysiology and find novel therapeutic targets that can be used adjunctively with standard antimicrobial therapy to improve outcomes in neonatal sepsis.
Neutrophils play a key role in the innate immune response against invading pathogens.11 Neutrophils rapidly respond to infections and limit pathogen dissemination through phagocytosis, intracellular microbial killing, the release of cytokines and chemokines, and the formation of neutrophil extracellular traps (NETs).12 NETs are lattices of extracellular decondensed chromatin coated with cytotoxic histones and antimicrobial proteins originating from the neutrophil granules such as neutrophil elastase, myeloperoxidase, and calprotectin.13,14 However, excessive NET formation induces endothelial cell toxicity leading to vascular leakage and blood coagulation inducing systemic thrombosis.14 Therefore, in cases of overwhelming infection, NETs may have a negative impact.
The role of NETs in adult sepsis is well-characterized.15 However, neonates manifest a different host immune response compared to adults.16–19 In this study, we used a well-characterized translational model of cecal slurry induced neonatal peritonitis to investigate the impact of NETs on neonatal sepsis and the therapeutic potential of targeting NETs to improve outcomes. We report for the first time that NET-targeting strategies improve survival in a translational model of neonatal infectious peritonitis, and that neonatal NET-Inhibitory Factor (nNIF) improves survival as an adjunctive treatment in this model following sub-optimal antimicrobial treatment.
Materials and methods
Ethics statement
All murine experiments were approved by the University of Utah Institutional Animal Care and Use Committee (no. 21-09012) in the Center for Comparative Animal Studies at the University of Utah, which is approved by the American Association of Laboratory Animal Care.
Animal husbandry
We purchased 16 female and 4 male outbred Swiss Webster mice for breeding (Charles River Laboratories). Outbred Swiss Webster mice were chosen strategically given their superior nurturing capacity and vitality in models of experimental infection. We housed our mice on a 12-h light/12-h dark cycle with a constant temperature in the University of Utah Center for Comparative Animal Studies in specific pathogen-free microisolator cages (5 mice/cage) with access to standard rodent food and water ad libitum. After experimental treatments and cecal slurry injection, the mice continued to be fostered by the birth dam.
Cecal slurry model of neonatal infectious peritonitis
The cecal slurry stock was prepared from mature mice as described in the literature with minor modifications.20–22 Briefly, five adult Swiss-Webster mice were euthanized, and the content of the cecum was harvested and pooled. Cecal contents were mixed with sterile water at a concentration of 200 mg/mL and pressed through a 70 µm filter. Subsequently, the slurry was added to an equal volume of a 30% glycerol/PBS solution and mixed by stirring, after which it was frozen at −80 °C until use. Peritonitis was induced by injecting seven to 10-day-old Swiss-Webster mice with cecal slurry or vehicle control intraperitoneally at a dose of 1.3 mg/g mouse weight. This age was chosen because murine neutrophils routinely become NET-competent at postnatal day 7.18,22,23 The mice were injected intraperitoneally with nNIF (10 mg/kg), inactive nNIF-SCR control peptide (10 mg/kg), DNase I (Dornase alfa, 25 mg/kg, University of Utah Pharmacy), 40 mg/kg Cl-amidine (Cayman Chemical), or meropenem (25 mg/kg, University of Utah Pharmacy) 1 h before and 4 h after cecal slurry injection. Treatments were randomized in every litter. For continued care, mouse pups were returned to the dams and observed for either mortality over 6 days or euthanized after 24 h for various experimental analysis. We further examined the combination therapy of nNIF and meropenem. Varying dosages of meropenem with or without nNIF (10 mg/kg) were injected 1 h pre- and 4 h post-cecal slurry injection intraperitoneally. In additional experiments in the model of sub-optimal antibiotic treatment, mice were treated with nNIF 2 and 6 h after cecal slurry injection. Neonatal mice were returned to dams and followed for survival for 6 days.
Peritoneal lavage
Twenty-four hours after cecal slurry injection, seven to 10-day-old mice were euthanized by IACUC approved procedures. The abdomen was carefully opened to reveal the peritoneal cavity. Each side of the cavity was flushed with 200 μL of PBS (total volume 400 μL). Samples from both sides were combined and placed into a fresh tube pending further analysis, including myeloperoxidase (MPO)-DNA ELISA, cytokine analysis, NET visualization, cell differential, and aerobic bacterial load determination.
Live-cell imaging of NETs
We evaluated 7 to 10-day-old mice for peritoneal NET formation via live-cell imaging with confocal microscopy as previously described.22 Sham and cecal slurry injected mice ± experimental conditions were euthanized 24 h after cecal slurry injection and peritoneal fluid samples were collected as described above. On a glass coverslip coated with poly-l-lysine, 100 μL of peritoneal fluid was incubated for 1 h at 37 °C in 5% CO2/95% air. DNA fluorescent stains for cell-permeable (SYTO Green, Molecular Probes) and cell-impermeable (SYTOX Orange, Molecular Probes) were used to assess peritoneal NET formation. Images of NETs were acquired using a FV3000 1 × 81 confocal microscope and FluoView software (Olympus) with 20x and 60x objectives. Z-stacked images were obtained over a range of 10 μm with a 1 μm step size for each randomly selected high-power field and processed using Olympus FluoView (Olympus), Adobe Photoshop (Adobe), and ImageJ (NIH) software.
MPO–DNA complex ELISA
An in-house ELISA was used to quantify MPO–DNA complexes in peritoneal fluid from sham and cecal slurry treated seven to 10-day-old mice, 24 h after injection, as previously described.24 Briefly, after overnight coating with anti-MPO capture antibody (2 µg/mL; 0400–0002, Bio-Rad) at 4 °C, a 96-well plate was blocked with 2.5% bovine serum albumin in PBS for 2 h at room temperature. The plate was subsequently washed, before incubating for 90 min at room temperature with 10% peritoneal fluid in blocking buffer. The plate was washed five times, and then incubated for 90 min at room temperature with anti-DNA detection antibody (1:20; Cell Death detection ELISA, 11544675001, Sigma). After five washes, the plate was developed with ABTS substrate (Sigma).
Peritoneal fluid leukocyte differential
Peritoneal lavage samples were acquired from sham and experimental mice 24 h after cecal slurry injection ± experimental treatments. Peritoneal lavage samples were incubated with CD45 APC-cy7 (Biolegend), CD11b PE-cy7 (Ebioscience), Ly6G BV510 (Biolegend) and CD16/CD32 (Fc-block, EBioscience) for 30 min at room temperature in PBS. Thirty minutes later, cells were washed, fixed, and analyzed on a Beckman Coulter Cytoflex located in the Utah Flow Cytometry Core.
Platelet–neutrophil aggregates
Whole-blood samples from seven to 10-day-old mice were obtained by cardiac puncture into EDTA coated microtubes (Sarstedt). To measure platelet–neutrophil aggregates (PNAs), we diluted whole blood 1:10 with M-199 serum-free media (12–117F; Lonza) containing 100 U/mL heparin.25,26 Neutrophils were labeled with Ly6G BV510 (Biolegend) and platelets were labeled with APC anti-mouse CD41 (BD Biosciences) for 15 min at 37 °C. For platelet activation studies, staining was performed in the presence of 50 ng/mL of convulxin (Santa Cruz). Samples were fixed with FACS lysis buffer, centrifuged at 500 x g for 10 min and resuspended in PBS before analysis.
Colony forming unit determination following peritoneal lavage after cecal slurry injection
Peritoneal lavage fluid was collected 24 h after cecal slurry injection ± experimental treatments and diluted 1:10,000 with PBS. A 25 μL sample of peritoneal lavage fluid was spread across an LB agar plate and incubated overnight at 37 °C in 5% CO2/95% air. Colony forming unit number was determined via serial dilution and manual counting.
Cytokine analysis
Various cytokine levels were analyzed in the peritoneal lavage samples of seven to 10-day-old neonatal mice 24 h after cecal slurry injection intraperitoneally. Using separate Duo-set ELISA assays, we determined peritoneal fluid levels for TNF-α, CXCL1 (murine homolog to human IL- 8), and IL-6 (all R&D Systems).
Neonatal mouse neutrophil isolation
Seven to 10-day-old mice were euthanized as per IACUC approved protocol. The spleens of several mice were removed, placed in PBS, and cut into small pieces using micro-scissors. Proceeding from 18-gauge to 22-gauge to 27-gauge needles, tissues were flushed through each needle size three times with a 1 mL syringe. Cell suspension was passed through a 70 μm pre-filter (Miltenyi Biotec) and washed with PBS. Cell suspensions were then centrifuged at 300 x g for 10 min. Tissue homogenate samples were subsequently resuspended in Miltenyi running buffer at 5 × 107 cells/mL. Finally, neutrophils were isolated using the Miltenyi Biotec Mouse Neutrophil Isolation Kit (130–097–658) on the Miltenyi Biotec AutoMACS immune cell sorter and resuspended at 2 × 106 cells/mL in 37 °C M-199 serum-free media.
Reactive-oxygen species generation
Neutrophils isolated from the spleens of seven to 10-day mice were resuspended in 37 °C M-199 serum-free media at 2 × 106 cells per mL and preincubated with either nNIF (1 nM) or nNIF-SCR peptide control (1 nM) for 1 h at 37 °C in 5% CO2/95% air and stimulated with 20 nM phorbol 12-myristate 13-acetate (PMA) for 30 min at 37 °C in 5%CO2/95% air. Following stimulation, cells were incubated for 10 min at 37 °C with a working solution containing dihydrorhodamine (DHR)/catalase (DHR 7.25 mM, Molecular Probes; Catalase 1000 Units/mL, Sigma in HBSS). Levels of DHR fluorescence were detected and quantified using flow cytometry (Beckman Coulter; CytExpert Software).22
Phagocytosis assay
Seven to 10-day-old mouse neutrophils in serum-free M-199 media were preincubated with nNIF (1 nM) and nNIF-SCR (1 nM) for 1 h at 37 °C and then incubated with fluorescently labeled E. coli bioparticles-488 (Molecular Probes) for 2 h at 37 °C (Multiplicity of Infection (MOI): 3:1). Neutrophils were then washed three times with PBS to remove extra bioparticles in the cell preparation and spun onto glass coverslips. Neutrophils were then fixed and permeabilized with 4% paraformaldehyde (10 min) and 0.1% Triton-X-100 PBS solution (10 min), respectively. After washing with PBS, cells were stained with DAPI and WGA633 for 1 h at room temperature. Images of randomly selected visual fields were captured using an Olympus FV3000 confocal microscope, and the fluorescent intensity of E. coli bioparticles was quantified per cell.
Statistics
We used GraphPad Prism software (version 9.3) for all statistical analyses. Data on each figure is presented as a bar graph with the mean ± standard deviation. In all datasets, we compared nNIF treatment to treatment with its inactive scrambled peptide control, nNIF-SCR. Additionally, in all datasets, we compared other treatment groups to the vehicle control groups. All data used in each statistical test met the assumptions of the specific test. Prior to statistical analysis, a D’Agostino and Pearson normality test was used to check data distribution. We used a one-way ANOVA with Tukey’s post-hoc testing when multiple comparisons were made for normally distributed data. For multiple comparisons on non-normally distributed data, we used the Kruskal-Wallis statistical tool. An unpaired student’s t-test or Mann–Whitney test was used when comparing two groups. Survival curves were assessed for statistical significance using the Mantel-Cox Log-rank test. We considered P < 0.05 statistically significant.
Results
nNIF does not alter stimulated mouse neutrophil reactive-oxygen species generation, phagocytosis, or platelet-neutrophil aggregation
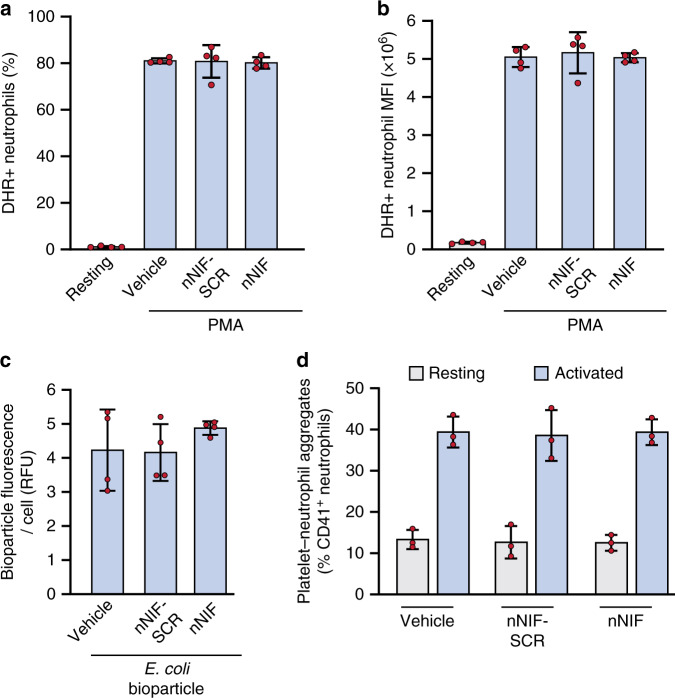
We have previously shown that nNIF inhibits NET formation in seven to 10-day-old mice.22 Here, we examined the effects of nNIF on key neutrophil activities besides NET formation in seven to 10-day-old mice, as we have done before in mature mouse neutrophils.23 We found that nNIF or nNIF-SCR pretreatment of spleen-isolated neutrophils did not alter neutrophil reactive-oxygen species generation in response to PMA (Fig. 1a, b) or phagocytosis of fluorescently labeled E. coli bioparticles (Fig. 1c) when compared to stimulated neonatal mouse neutrophils assayed in parallel. Similarly, platelet–neutrophil aggregate formation in whole blood was unaltered in mice injected with nNIF compared to nNIF-SCR injected mice (Fig. 1d).
Fig. 1. nNIF does not alter stimulated mouse neutrophil reactive-oxygen species generation, phagocytosis, or platelet-neutrophil aggregation.
a–c Neutrophils were isolated from spleens of seven to 10-day-old outbred Swiss Webster mouse pups and incubated with vehicle, 1 nM nNIF, or 1 nM nNIF-SCR. N = 4 separate experiments; Mean ± SD. a, b ROS generation upon PMA stimulation was assessed by flow cytometry and is represented as (a) % cells that were DHR positive or as (b) the mean fluorescent intensity of the DHR staining on the neutrophils. c Neutrophils were incubated with fluorescently labeled E. coli bioparticles-488 for 2 h after which phagocytosis was measured by fluorescence per cell of washed neutrophils. d Seven to 10-day-old outbred Swiss Webster mouse pups were injected with vehicle, nNIF (10 mg/kg) or nNIF-SCR (10 mg/kg). One hour later, blood was drawn and stimulated with convulxin (50 ng/mL) or left unstimulated; after 15 min platelet–neutrophil complexes were measured by flow cytometry. N = 3 mice per group; Mean ± SD.
NET inhibition/degradation decreases peritoneal NET formation in experimental neonatal infectious peritonitis
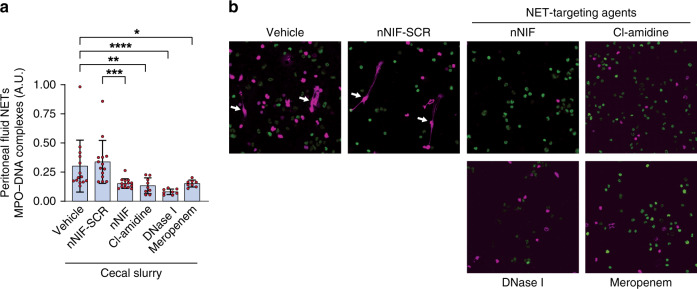
We pretreated 7 to 10-day-old mice intraperitoneally with vehicle, nNIF-SCR, nNIF, Cl-amidine (pharmacologic PAD4 inhibitor), DNase I (NET degrading agent) or meropenem (negative control for infection) 1 h prior to cecal slurry injection, with a second dose of each agent given 4 h after injection of the cecal slurry. At 24 h after cecal slurry injection, we detected significantly decreased NET levels both quantitatively and qualitatively in peritoneal fluid samples collected from mice treated with nNIF, Cl-amidine, DNase I, and meropenem in comparison to their respective controls (Fig. 2a, b).
Fig. 2. NET inhibition/degradation decreases peritoneal NET formation in experimental neonatal infectious peritonitis.
Seven to 10-day old, outbred Swiss-Webster mice were treated with vehicle, nNIF-SCR (10 mg/kg), nNIF (10 mg/kg), Cl-amidine (40 mg/kg), DNase I (25 mg/kg), or meropenem (25 mg/kg) 1 h prior and 4 h after cecal slurry injection (1.3 mg/gram mouse weight) to induce infectious peritonitis. Twenty-four hours after cecal slurry injection, peritoneal lavage fluid was collected and analyzed quantitatively and qualitatively for the presence of NETs by a MPO–DNA complex ELISA and b live-cell imaging and confocal microscopy. Statistical analysis was performed by comparing treatment groups with their respective controls, i.e., nNIF was compared with nNIF-SCR; the other treatment groups were compared to the vehicle control. N = 8–14 mice per group; Mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Representative images of peritoneal lavage samples from 8 individual mice per group.
NET inhibition/degradation does not alter peritoneal leukocyte accumulation, but NET inhibition does decrease bacterial killing in experimental neonatal infectious peritonitis
We next assessed the peritoneal fluid total leukocyte and neutrophil counts 24 h after cecal slurry challenge. As expected, we found a significant increase in peritoneal leukocytes after cecal slurry challenge (Fig. 3a), which were predominantly neutrophils (Fig. 3b). No significant difference was found in peritoneal total leukocyte counts for any of the treatment groups compared to their respective controls (Fig. 3a). However, consistent with NET inhibition, we detected increased neutrophil counts in peritoneal fluid from nNIF and Cl-amidine treated mice compared to their respective controls (Fig. 3b). In contrast, the NET degrading compound, DNase I, did not demonstrate an increase in neutrophil numbers in the peritoneal fluid compared to vehicle control treated mice (Fig. 3b). In contrast to the leukocyte and neutrophil accumulation in the peritoneal fluid, we detected significantly increased levels of aerobic bacterial colony forming units (CFU) in the nNIF-treated mice compared to nNIF-SCR treated mice (Fig. 3c). However, this was not seen in the Cl-amidine or DNase I treatment groups when compared to vehicle control. As expected, sham surgery and cecal slurry mice treated with meropenem had no detectable CFU in their peritoneal fluid (Fig. 3c).
Fig. 3. NET inhibition/degradation does not alter peritoneal leukocyte accumulation, but NET inhibition does decrease bacterial killing in experimental neonatal infectious peritonitis.
Seven to 10-day old, outbred Swiss-Webster mice were treated with vehicle, nNIF-SCR (10 mg/kg), nNIF (10 mg/kg), Cl-amidine (40 mg/kg), DNase I (25 mg/kg), or meropenem (25 mg/kg) 1 h prior and 4 h after cecal slurry injection (1.3 mg/gram mouse weight) to induce infectious peritonitis. a, b Twenty-four hours after cecal slurry injection, peritoneal lavage fluid was collected and total leukocyte (a) as well as neutrophil (b) recruitment was analyzed by flow cytometry. c Twenty-four hours after cecal slurry injection, peritoneal lavage fluid was collected, diluted 1:10,000 with PBS, grown on an LB agar plate after which colony forming unit (CFU) number was determined via serial dilution and manual counting. Statistical analysis was performed by comparing treatment groups with their respective controls, i.e., nNIF was compared with nNIF-SCR;the other treatment groups were compared to the vehicle control. N = 4–15 mice per group; Mean ± SD. *P < 0.05; **P < 0.01.
NET inhibition/degradation decreases pro-inflammatory cytokine levels in experimental neonatal infectious peritonitis
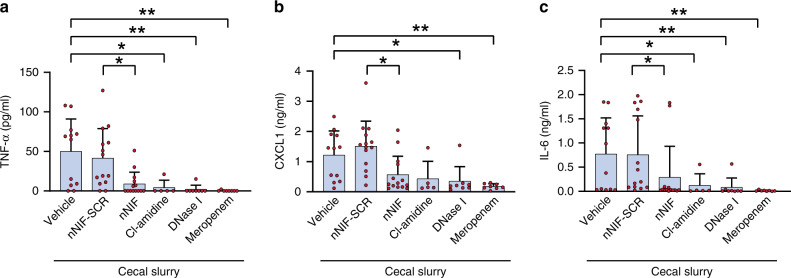
As NETs can potentiate inflammation, we next assessed cytokine levels in the peritoneal fluid of cecal slurry injected mice. We detected significantly decreased peritoneal fluid levels of TNF-α, CXCL1, and IL-6 in nNIF, Cl-amidine, DNase 1, and meropenem treated mice compared to their respective controls (Fig. 4).
Fig. 4. NET inhibition/degradation decreases pro-inflammatory cytokine levels in experimental neonatal infectious peritonitis.
Seven to 10-day old, outbred Swiss-Webster mice were treated with vehicle, nNIF-SCR (10 mg/kg), nNIF (10 mg/kg), Cl-amidine (40 mg/kg), DNase I (25 mg/kg), or meropenem (25 mg/kg) 1 h prior and 4 h after cecal slurry injection (1.3 mg/gram mouse weight) to induce infectious peritonitis. Twenty-four hours after cecal slurry injection, peritoneal lavage fluid was collected and the concentrations of TNF-alpha (a), CXCL1 (b), and IL-6 (c) were determined by ELISA. Statistical analysis was performed by comparing treatment groups with their respective controls, i.e., nNIF was compared with nNIF-SCR; the other treatment groups were compared to the vehicle control. N = 8–14 mice per group; Mean ± SD. *P < 0.05; **P < 0.01.
NET inhibition/degradation reduces systemic platelet–neutrophil interactions in experimental neonatal infectious peritonitis
Systemic platelet activation has been reported in adult sepsis,27 and platelet–neutrophil interactions drive NET formation which, in turn, can activate platelets.28,29 We hypothesized that in our model of neonatal peritonitis excessive NET formation would lead to increased systemic platelet activation. To examine the effect of NET-targeting strategies on systemic platelet activation, we measured platelet–neutrophil aggregates in the circulation of cecal slurry injected mice. As hypothesized, cecal slurry injection induced a strong increase in circulating platelet–neutrophil aggregates compared to sham mice (Fig. 5). Importantly, we found a significant decrease in whole-blood platelet–neutrophil aggregates in the nNIF, Cl-amidine, DNase I, and meropenem treated mice compared to their respective controls (Fig. 5).
Fig. 5. NET inhibition/degradation reduces systemic platelet–neutrophil interactions in experimental neonatal infectious peritonitis.

Seven to 10-day old, outbred Swiss-Webster mice were treated with vehicle, nNIF-SCR (10 mg/kg), nNIF (10 mg/kg), Cl-amidine (40 mg/kg), DNase I (25 mg/kg), or meropenem (25 mg/kg) 1 h prior and 4 h after cecal slurry injection (1.3 mg/gram mouse weight) to induce infectious peritonitis. Twenty-four hours after cecal slurry injection, blood was drawn, and platelet–neutrophil aggregate formation was determined by flow cytometry. Statistical analysis was performed by comparing treatment groups with their respective controls, i.e., nNIF was compared with nNIF-SCR; the other treatment groups were compared to the vehicle control. N = 3–7 mice per group; Mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
NET inhibition/degradation improves survival in experimental neonatal infectious peritonitis, and adjunctive treatment with nNIF rescues survival in this model following sub-optimal antibiotic therapy
To assess the potential role of NET formation in exacerbating the systemic inflammatory response and sequelae of infectious peritonitis in neonates, we assessed survival over a 6-day period in our model of neonatal infectious peritonitis. We detected a 14% survival rate in vehicle treated cecal slurry treated mice and a 9% survival in nNIF-SCR treated mice, suggesting a high severity for this model (Fig. 6a). As expected, all mice in the sham treated group (data not shown) and nearly all mice in the meropenem treated group (93%; P < 0.0001) survived (Fig. 6a). nNIF (47%; P = 0.03) and DNase I (43%; P = 0.009) treated groups showed significantly increased survival at 6 days compared to the nNIF-SCR or vehicle group, respectively (Fig. 6a). The Cl-amidine group trended towards increased survival (38%; P = 0.07) as compared to the vehicle group (Fig. 6a).
Fig. 6. NET inhibition/degradation improves survival in experimental neonatal infectious peritonitis, and adjunctive treatment with nNIF rescues survival in this model following sub-optimal antibiotic therapy.
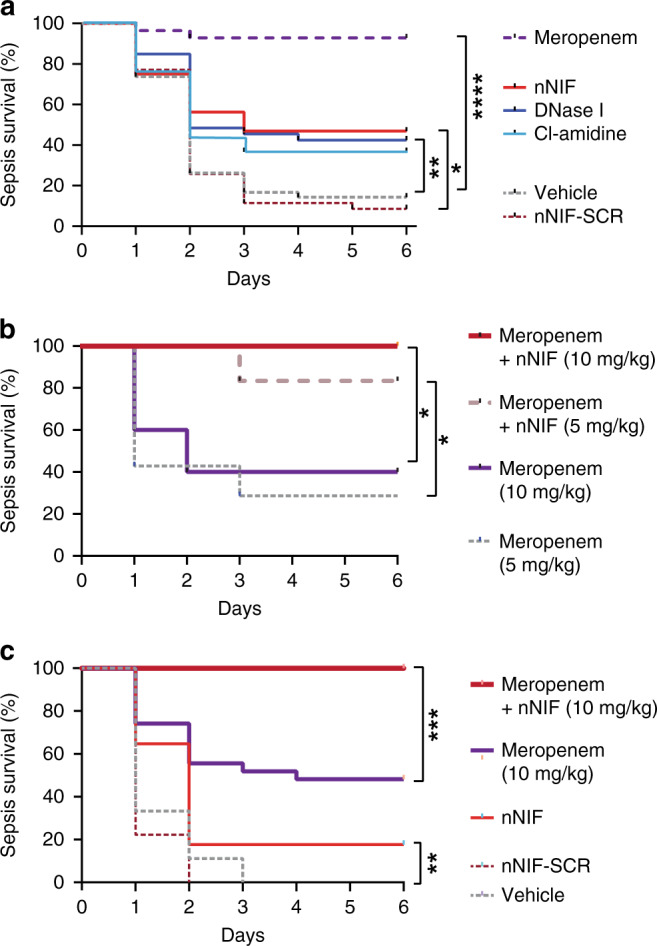
a Seven to 10-day old, outbred Swiss-Webster mice were treated with vehicle, nNIF-SCR (10 mg/kg), nNIF (10 mg/kg), Cl-amidine (40 mg/kg), DNase I (25 mg/kg), or meropenem (25 mg/kg) 1 h prior and 4 h after cecal slurry injection (1.3 mg/gram mouse weight) to induce infectious peritonitis. Sham animals did not receive cecal slurry. Survival was monitored for 6 days. N = 28–42 mice per group. b Seven to 10-day old, outbred Swiss-Webster mice were treated with meropenem (5 mg/kg or 10 mg/kg) ± nNIF (10 mg/kg) 1 h prior and 4 h post injection of cecal slurry (1.3 mg/gram mouse weight) to induce infectious peritonitis. Survival was monitored for 6 days. c Seven to 10-day old, outbred Swiss-Webster mice were treated with vehicle, nNIF-SCR (10 mg/kg), nNIF (10 mg/kg), or meropenem (10 mg/kg) ± nNIF (10 mg/kg) 2 h and 6 h after cecal slurry injection (1.3 mg/gram mouse weight) to induce infectious peritonitis. Survival was monitored for 6 days. Statistical analysis was performed by comparing treatment groups with their respective controls, i.e., nNIF was compared with nNIF-SCR; the other treatment groups were compared to the vehicle control. N = 5–7 mice per group. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We next examined nNIF in a translationally relevant model of adjunctive treatment with sub-optimal antibiotic dosing. To this aim, we investigated different meropenem concentrations (5 and 10 mg/kg) administered 1 h before and 4 h after cecal slurry injection and evaluated their impact on survival. Subsequently, we evaluated combination treatment of nNIF with meropenem. A dose of 5 mg/kg meropenem led to a survival of 29% and a dose of 10 mg/kg of meropenem improved survival to 40% (Fig. 6b). Importantly, treatment with nNIF (10 mg/kg) in combination with the sub-optimal meropenem doses of 5 or 10 mg/kg significantly improved survival to 83% (P = 0.04) and 100% (P = 0.02), respectively (Fig. 6b).
Based on these positive results, we next delayed treatment until 2 h after cecal slurry challenge to increase the translatability of our findings. When treatment was delayed, nNIF treatment still significantly increased survival compared to nNIF-SCR (P = 0.006), but survival was only 18% 5 days after cecal slurry challenge. Similar to pretreatment, 10 mg/kg of meropenem resulted in increased survival (48%) after cecal slurry challenge. However, adjunctive nNIF treatment in combination with sub-optimal dosing of meropenem, completely averted mortality in this model (P = 0.0005; Fig. 6c).
Discussion
We report a key role for NETs in exacerbating experimental peritonitis in seven to 10-day-old mice. Furthermore, treatment of mice with the NET-targeting agents Cl-amidine, DNase I, or nNIF, an endogenous inhibitor of NET formation, significantly improved survival in this translational model. Of additional translational importance, we also demonstrate that nNIF rescued survival when given adjunctively with a sub-optimal dose of meropenem, suggesting that NET inhibition may work synergistically with antibiotic treatment in preventing mortality.
Neutrophils perform essential functions in the acute inflammatory response to infection. Upon infection, pathogen recognition receptors on dendritic cells and macrophages sense infection and respond by releasing pro-inflammatory cytokines and chemokines.11,30 These innate immune effector molecules combat infection directly through cytotoxic effects as well as by recruiting neutrophils.31 Neutrophils aid in host defense against pathogens through degranulation, microbe phagocytosis, and intracellular and NET-mediated microbial killing.13,31 As the inflammatory reaction progresses, pro-inflammatory mediators yield to anti-inflammatory cytokines and resolving macrophages, limiting tissue damage and systemic inflammation.32 While neutrophils are essential to fight off infections, excessive NET formation results in widespread vascular injury, micro-thrombosis, and multi-organ failure in many inflammatory diseases.14,15,29 Systemic NET levels have been associated with increased disease severity and mortality in lupus33,34, adult and pediatric sepsis35–38, and COVID-19.24,39,40 Furthermore, preclinical studies have shown a great potential for anti-NET strategies in limiting inflammation-associated collateral damage in sepsis models.23,38,41–43 Our study confirms these earlier reports, in that we found that NET inhibition or NET degradation significantly improved outcomes in a mouse model of neonatal peritonitis. Targeting NETs reduced the levels of inflammatory cytokines in the peritoneal cavity and reduced circulating platelet–neutrophil aggregates, a sensitive marker for systemic platelet activation. These results imply that NETs contribute to the cytokine storm and platelet activation in neonatal peritonitis. Platelets are key mediators of NET formation;44,45 however, platelets also bind NETs and get activated in the process, contributing to thrombus formation and tissue ischemia.28,46–48
Unique to our study is the use of a recently identified, endogenous inhibitor of NET formation, nNIF.18,22,23 nNIF is part of a family of neutrophil inhibitory peptides (NIPs), cleavage fragments from alpha-1-antitrypsin, detected in umbilical cord blood and blood from neonates.22,23 NIPs are conserved across species, result from placental expression of the serine protease HTRA1, and disappear within 3 to 7 days after birth in humans and mice.22 They are not detected in plasma from healthy adults. Therefore, NIPs may represent part of the innate system maintaining immune tolerance before and after birth. NET inhibition by NIPs in the peripartum period most likely protects the neonate from organ dysfunction and mortality as it identifies and responds to pathogenic and nonpathogenic colonizing microbes.22 Critically, NIPs do not alter key additional neutrophil activities, including chemotaxis, ROS generation, degranulation, and intracellular killing.22,23 Here, more neutrophils migrated to the peritoneal cavity after cecal slurry injection of nNIF-treated mice as compared to the nNIF-SCR treated control group, an observation best attributed to the reduced amount of suicidal NET formation by neutrophils.23
In our study, nNIF treatment resulted in similar neonatal sepsis outcomes compared to proven anti-NET strategies. Cl-amidine (hydrochloride) is an irreversible PAD4 inhibitor, blocking histone citrullination, a key early step in the formation of NETs.49 The mechanism by which Cl-amidine improves outcomes is probably similar to nNIF, as we have previously found reduced histone citrullination in stimulated neutrophils pretreated with nNIF.23 However, Cl-amidine is a pan-PAD inhibitor, inhibiting all PADs (PAD1–4) and is likely to have several off-target effects, limiting its clinical use. DNase I (Dornase alpha) is an FDA-approved treatment for cystic fibrosis, often used in preclinical studies to limit pathogenicity of NETs by degrading the DNA backbone of NETs.50 The clinical utility of DNase I treatment in the setting of sepsis is more complicated than PAD-4 inhibition, mainly due to the critical timing of administration essential to achieve optimal efficacy and safety.38,51–53 Some of the reported outcomes of DNase I treatment in sepsis likely result from DNase I liberating pathogens from the already formed NETs, potentiating systemic dissemination and overall pathogenicity.54,55 Depending on the timing post infection, DNase I treatment can, however, limit excessive NET formation and inflammatory injury.38,56,57 Similarly in our study, DNase I treatment was effective in improving survival in our translational model of neonatal peritonitis.
Despite the damaging collateral effects of NETs in many diseases, NETs efficiently trap and kill pathogens in vitro and in vivo.13,54,58,59 Therefore, limiting NET formation must be evaluated carefully in the context of infectious disease. In our study, blocking NETs significantly improved survival after infectious peritonitis arguing an overall pathogenic effect of NETs in this model. However, for each treatment group, we observed substantial mortality despite NET inhibition. Possible explanations for this observation include the severity of the model (survival of 14% in the vehicle control group) and/or the relative increase in peritoneal bacterial load in nNIF and Cl-amidine treated mice 24 h after injection of cecal slurry. Complete inhibition of NET formation potentially limits efficient clearance of pathogens resulting in an overwhelming microbial burden.53,55 Our observation, however, that increased peritoneal bacterial load in nNIF-treated mice did not increase overall mortality, once again highlights the complex balancing act between infection control and immune system activation.
In the clinical situation, once infection is suspected as the etiology of symptoms, antibiotic treatment will be initiated immediately. However, while necessary, empirical antibiotics often provide sub-optimal pathogen coverage and may result in widespread, unnecessary exposure to adverse drug effects and risk for increased antibiotic resistance.1,7,9 Whether NET inhibition could be employed as an adjunctive treatment to antimicrobial agents in experimental neonatal infectious peritonitis remained unknown. We, therefore, investigated whether a combination therapy of antibiotic treatment and NET inhibition using nNIF would be synergistic and improve outcomes in this translational model. Accordingly, we combined sub-optimal meropenem treatment with nNIF and observed almost complete protection from mortality in our model of neonatal infectious peritonitis. Importantly, this synergistic effect was also effective when treatment was delayed until after peritonitis onset. These results suggest the potential importance for adjunctive, NET-targeting therapies for infectious inflammatory syndromes such as sepsis and peritonitis in the neonate.
In conclusion, we report that early NET formation exacerbates outcomes in a translational model of neonatal infectious peritonitis and that combining adjunctive, NET-targeting agents with sub-optimal antibiotics improves mortality. These results will need validation in other models of neonatal sepsis; however, they show great promise for NET inhibition as a therapeutic target.
Author contributions
C.C.Y. and F.D. conceived the project and designed the experiments. F.D., J.L.R., I.P., J.L.C., C.V.d.A., and M.J.C. performed experiments and analyzed data. F.D., M.J.C., R.A.C., and C.C.Y. wrote and edited the manuscript for important intellectual content.
Funding
This work was supported in part by the US NIH (R01HD093826 to C.C.Y.—NICHD; K01AG059892 to R.A.C.—NIA), PEEL Therapeutics, Inc. (Sponsored Research Agreement to C.C.Y.), the American Heart Association (2021Post830138 to F.D. and 2022Post906231 to I.P.), and by the University of Utah Department of Pediatrics, Division of Neonatology.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
C.C.Y. authors a US patent (patent 232,023 B2) held by the University of Utah for the use of NET-inhibitory peptides for the “treatment of and prophylaxis against inflammatory disorders,” for which PEEL Therapeutics, Inc. holds the exclusive license. The other authors declare that no additional conflict of interest exists.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reinhart K, et al. Recognizing sepsis as a global health priority—a WHO resolution. N. Engl. J. Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 2.Fleischmann-Struzek C, et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir. Med. 2018;6:223–230. doi: 10.1016/S2213-2600(18)30063-8. [DOI] [PubMed] [Google Scholar]
- 3.Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390:1770–1780. doi: 10.1016/S0140-6736(17)31002-4. [DOI] [PubMed] [Google Scholar]
- 4.Weston EJ, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatr. Infect. Dis. J. 2011;30:937–941. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seale AC, et al. Estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, south Asia, and Latin America for 2012: a systematic review and meta-analysis. Lancet Infect. Dis. 2014;14:731–741. doi: 10.1016/S1473-3099(14)70804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kermorvant-Duchemin E, Laborie S, Rabilloud M, Lapillonne A, Claris O. Outcome and prognostic factors in neonates with septic shock. Pediatr. Crit. Care Med. 2008;9:186–191. doi: 10.1097/PCC.0b013e31816689a8. [DOI] [PubMed] [Google Scholar]
- 7.Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin. Perinatol. 2010;37:439–479. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortese F, et al. Early and late infections in newborns: where do we stand? A review. Pediatr. Neonatol. 2016;57:265–273. doi: 10.1016/j.pedneo.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am. J. Perinatol. 2013;30:131–141. doi: 10.1055/s-0032-1333413. [DOI] [PubMed] [Google Scholar]
- 10.Schüller SS, et al. Immunomodulation to prevent or treat neonatal sepsis: past, present, and future. Front. Pediatr. 2018;6:199. doi: 10.3389/fped.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133:2178–2185. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 14.Sorvillo, N., Cherpokova, D., Martinod, K. & Wagner, D. D. Extracellular DNA NET-works with dire consequences for health. Circ. Res.125, 470–488 (2019). [DOI] [PMC free article] [PubMed]
- 15.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 16.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–1041. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Zhivaki D, Lo-Man R. Unique aspects of the perinatal immune system. Nat. Rev. Immunol. 2017;17:495–507. doi: 10.1038/nri.2017.54. [DOI] [PubMed] [Google Scholar]
- 18.Yost, C. C. et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood113, 6419–6427 (2009). [DOI] [PMC free article] [PubMed]
- 19.Zonneveld R, et al. Soluble adhesion molecules as markers for sepsis and the potential pathophysiological discrepancy in neonates, children and adults. Crit. Care. 2014;18:204. doi: 10.1186/cc13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wynn JL, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28:675–683. doi: 10.1097/shk.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 21.Starr ME, et al. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS ONE. 2014;9:e115705. doi: 10.1371/journal.pone.0115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell RA, et al. Placental HTRA1 cleaves α1-antitrypsin to generate a NET-inhibitory peptide. Blood. 2021;138:977–988. doi: 10.1182/blood.2020009021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yost, C. C. et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J. Clin. Invest.126, 3783–3798 (2016). [DOI] [PMC free article] [PubMed]
- 24.Middleton, E. A. et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood136, 1169–1179 (2020). [DOI] [PMC free article] [PubMed]
- 25.Denorme, F. et al. Platelet necrosis mediates ischemic stroke outcome in mice. Blood135, 429–440 (2020). [DOI] [PMC free article] [PubMed]
- 26.Denorme, F., Portier, I., Kosaka, Y. & Campbell, R. A. Hyperglycemia exacerbates ischemic stroke outcome independent of platelet glucose uptake. J. Thromb. Haemost.19, 536–546 (2021). [DOI] [PMC free article] [PubMed]
- 27.Guo L, Rondina MT. The era of thromboinflammation: platelets are dynamic sensors and effector cells during infectious diseases. Front. Immunol. 2019;10:2204. doi: 10.3389/fimmu.2019.02204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denorme F, Rustad JL, Campbell RA. Brothers in arms: platelets and neutrophils in ischemic stroke. Curr. Opin. Hematol. 2021 doi: 10.1097/MOH.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denorme F, et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Invest. 2022 doi: 10.1172/JCI154225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinchieri G, Sher A. Cooperation of toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y, Kim J, Ernits H, Remick D. The septic neutrophil-friend or foe. Shock. 2021;55:147–155. doi: 10.1097/SHK.0000000000001620. [DOI] [PubMed] [Google Scholar]
- 32.Ye Q, Du L-Z, Shao W-X, Shang S-Q. Utility of cytokines to predict neonatal sepsis. Pediatr. Res. 2017;81:616–621. doi: 10.1038/pr.2016.267. [DOI] [PubMed] [Google Scholar]
- 33.Hakkim A, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czaikoski PG, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S, et al. Neutrophil extracellular traps promote hypercoagulability in patients with sepsis. Shock. 2017;47:132–139. doi: 10.1097/SHK.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 37.Hoppenbrouwers T, et al. Neutrophil extracellular traps in children with meningococcal sepsis. Pediatr. Crit. Care Med. 2018;19:e286–e291. doi: 10.1097/PCC.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 38.Colón DF, et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit. Care. 2019;23:113. doi: 10.1186/s13054-019-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuo, Y. et al. Neutrophil extracellular traps in COVID-19. JCI Insight5, e138999 (2020). [DOI] [PMC free article] [PubMed]
- 40.Nicolai L, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biron BM, et al. Cl-amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. J. Innate Immun. 2017;9:22–32. doi: 10.1159/000448808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biron BM, et al. PAD4 deficiency leads to decreased organ dysfunction and improved survival in a dual insult model of hemorrhagic shock and sepsis. J. Immunol. 2018;200:1817–1828. doi: 10.4049/jimmunol.1700639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawez A, et al. MiR-155 regulates neutrophil extracellular trap formation and lung injury in abdominal sepsis. J. Leukoc. Biol. 2021 doi: 10.1002/JLB.3A1220-789RR. [DOI] [PubMed] [Google Scholar]
- 44.Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 45.Sreeramkumar, V. et al. Neutrophils scan for activated platelets to initiate inflammation. Science (1979)346, 1234–1238 (2014). [DOI] [PMC free article] [PubMed]
- 46.Jorch, S. K. & Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med.23, 279–287 (2017). [DOI] [PubMed]
- 47.Massberg, S. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med.16, 887–896 (2010). [DOI] [PubMed]
- 48.Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021 doi: 10.1038/s41569-021-00552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knight JS, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J. Clin. Invest. 2013;123:2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuchs TA, et al. Extracellular DNA traps promote thrombosis. Proc. Natl Acad. Sci. USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lauková L, et al. Exogenous deoxyribonuclease has a protective effect in a mouse model of sepsis. Biomed. Pharmacother. 2017;93:8–16. doi: 10.1016/j.biopha.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Mai SHC, et al. Delayed but not early treatment with dnase reduces organ damage and improves outcome in a murine model of sepsis. Shock. 2015;44:166–172. doi: 10.1097/SHK.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 53.Meng W, et al. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit. Care. 2012;16:R137. doi: 10.1186/cc11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yipp BG, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menegazzi R, Decleva E, Dri P. Killing by neutrophil extracellular traps: fact or folklore? Blood. 2012;119:1214–1216. doi: 10.1182/blood-2011-07-364604. [DOI] [PubMed] [Google Scholar]
- 56.McDonald B, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carestia A, Davis RP, Davis L, Jenne CN. Inhibition of immunothrombosis does not affect pathogen capture and does not promote bacterial dissemination in a mouse model of sepsis. Platelets. 2020;31:925–931. doi: 10.1080/09537104.2019.1704711. [DOI] [PubMed] [Google Scholar]
- 58.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Li P, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.