Abstract
Synthetic phytochelatins (ECs) are a new class of metal-binding peptides with a repetitive metal-binding motif, (Glu-Cys)nGly, which were shown to bind heavy metals more effectively than metallothioneins. However, the limited uptake across the cell membrane is often the rate-limiting factor for the intracellular bioaccumulation of heavy metals by genetically engineered organisms expressing these metal-binding peptides. In this paper, two potential solutions were investigated to overcome this uptake limitation either by coexpressing an Hg2+ transport system with (Glu-Cys)20Gly (EC20) or by directly expressing EC20 on the cell surface. Both approaches were equally effective in increasing the bioaccumulation of Hg2+. Since the available transport systems are presently limited to only a few heavy metals, our results suggest that bioaccumulation by bacterial sorbents with surface-expressed metal-binding peptides may be useful as a universal strategy for the cleanup of heavy metal contamination.
Mercury is one of the most toxic heavy metals in the environment. The principal sources of contamination in wastewater are chloralkali plants, battery facilities, mercury switches, and medical wastes (12). In an aqueous environment, Hg2+ in sediment is subject to methylation, forming more toxic methylmercury (4). Bioaccumulation of methylmercury through the food chains is a potential risk to consumers of contaminated fish or shellfish (7). One of the most severe cases of mercury poisoning occurred in Minamata Bay, Japan, in which hundreds of people died and thousands were affected by consuming contaminated fish.
Common treatments to remove Hg2+ from contaminated sources are based on adsorption with ion-exchange resins (14). These technologies, however, are inadequate to reduce Hg2+ concentrations to acceptable regulatory standards. Another emerging technology that is receiving more attention is the use of biosorbents. The first commercial biosorbents developed (MRA and Algasorb) were based on sequestration of toxic metals by cell-surface moieties (8). These biosorbents, however, generally lack the required affinity and specificity.
The availability of genetic engineering technology provides the possibility of specially tailoring microbial biosorbents with the required selectivity and affinity for Hg2+. One emerging strategy that is receiving more attention is the use of metal-binding peptides. Naturally occurring metal-binding peptides, such as metallothioneins (MTs) and phytochelatins (17), are the main metal-sequestering molecules used by cells to immobilize metal ions, offering selective, high-affinity binding sites. However, the de novo design of metal-binding peptides is an attractive alternative to MTs, as they offer the potential of enhanced affinity and selectivity for heavy metals. Recently, a new class of metal-binding peptides known as synthetic phytochelatins (ECs) with the repetitive metal-binding motif (Glu-Cys)nGly were shown to have improved Cd2+ binding capability over that of MTs (1).
Overexpression of metal-binding proteins such as MTs in bacterial cells resulted in enhanced Hg2+ accumulation and thus offers a promising strategy for the development of microbe-based biosorbents (13, 15) for the removal and recovery of Hg2+ from contaminated water or soil. However, Hg2+ removal by intracellular accumulation has been problematic because of the limited metal uptake (2). This uptake limitation could be potentially overcome either by coexpressing an Hg2+ transport system (3) or by anchoring the metal-binding proteins directly on the cell surface (1). In this paper, we describe the characterization of recombinant Escherichia coli strains with EC20 either anchored on the cell surface or coexpressed intracellularly with the mercury transport proteins MerP and MerT (2, 10). The ability of these strains to accumulate Hg2+ was investigated.
Expression of ECs.
Synthetic genes coding for EC20 were synthesized as described previously (1). To express EC20 intracellularly, plasmid pM20 (1) was digested with BamHI and HindIII and the DNA fragment coding for EC20 was inserted into pMAL-c2x (New England BioLabs), resulting in pMC20. This construct allows the cytoplasmic expression of EC20 as a fusion to the maltose-binding protein (MBP).
To facilitate the transport of Hg2+ across the cell membrane, the Hg2+ transport proteins MerP and MerT were coexpressed with MBP-EC20. Plasmid pCLTP (2), containing the merT and merP genes, was cotransformed with pMC20. Transformed cells were selected on Luria-Bertani (LB) plates containing ampicillin and spectinomycin. For comparison, E. coli strain JM109, carrying only pMC20, was also used. An alternate strategy to bypass Hg2+ uptake is to directly anchor EC20 on the cell surface. We have successfully demonstrated this possibility using the Lpp-OmpA fusion system (1). Plasmid pLO20, expressing the Lpp-OmpA-EC20 fusion, was used in this study. Bacterial strains and vectors used are listed in Table 1.
TABLE 1.
Plasmids and strains
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strain | ||
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 thi Δ(lac-proAB) F′ (traD36, proAB, lacIq ZΔM15) | 18 |
| Plasmids | ||
| pUC18 | Cloning vector | 18 |
| pLO20 | A pUC18 derivative containing the lpp-ompA-ec20 fusion | 1 |
| pMAL-c2x | MBP gene fusion vector | New England BioLabs |
| pMC20 | A pMAL-c2x derivative containing the malE-ec20 fusion | This work |
| pCLTP | A pCL1921 derivative containing the merT and merP genes | 2 |
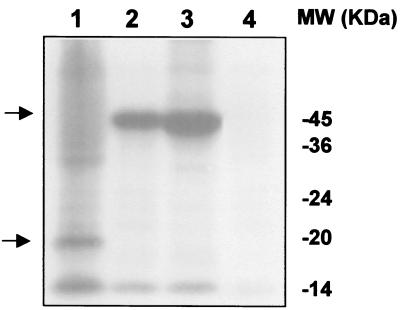
To confirm the production of Lpp-OmpA-EC20 and MBP-EC20, cultures were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and radiolabeled cysteine (35S, 1,075 Ci/mmol; ICN) was added at the time of induction. After 15 h, total cell lysates were separated by a sodium dodecyl sulfate (SDS)–12.5% polyacrylamide gel (6). The gel was then dried and exposed to an X-ray film. The high cysteine content of EC20 enables detection of these proteins by autoradiography. Synthesis of full-length Lpp-OmpA-EC20 (21 kDa) and MBP-EC20 (47.5 kDa) fusions was detected at the expected molecular weight (Fig. 1). The intensity of the protein bands was quantified using a Bio-Rad Gel Doc 2000 Gel Documentation System and Quantity One software. Intracellular expression of MBP-EC20 was approximately 12 times higher than expression on the cell surface, and coexpression of the MerT-MerP transporters reduced MBP-EC20 production by about twofold.
FIG. 1.
Expression of EC fusion proteins. [35S]cysteine was added to the cultures at an OD600 of 0.3. The cultures were further grown for 15 h. Total cell proteins were separated on SDS–12.5% polyacrylamide gel electrophoresis. The gel was dried and autoradiographed. Expression from induced cultures harboring pLO20 (lane 1), pMC20/pCLTP (lane 2), pMC20 (lane 3), and pMAL-c2x (lane 4), respectively, is shown. The desired fusion proteins are marked with arrows. Molecular mass is shown in kilodaltons on right.
Hg2+ binding to EC20.
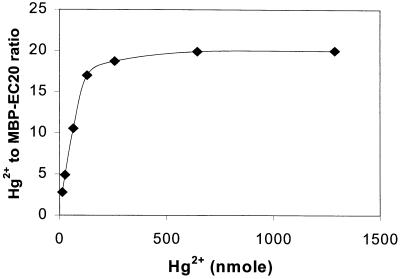
To investigate the Hg2+ binding stoichiometry of EC20, MBP-EC20 fusion proteins were purified from cultures of JM109/pM20 using an amylose resin affinity column (New England BioLabs). The purity of the protein was confirmed through SDS–12.5% polyacrylamide gel electrophoresis. Five nanomoles of the purified fusion protein was resuspended in 50 mM Tris-Cl buffer (pH 7.4) supplemented with 5 mM dithiothreitol and incubated with 1 to 1,200 nmol of Hg2+ for 2 h. Hg(II)-glutathione complexes were used instead of HgCl2 in order to prevent precipitation as reported previously (9). The protein-Hg2+ complex was recovered using a Microcon centrifugal filter membrane (Millipore), and the amount of bound Hg2+ was measured by cold-vapor atomic absorption spectroscopy (Coleman Model 5B Mercury Analyzer System). The Hg2+-to-MBP-EC20 stoichiometry was determined by plotting the initial Hg2+ concentration against the molar ratio of bound Hg2+ to MBP-EC20 (Fig. 2). A saturating ratio of 20 Hg2+ per MBP-EC20 was obtained, a value much higher than the typical ratio of 7 reported for MTs (11). A similar binding experiment was conducted with purified MBP, with no significant binding of Hg2+ observed.
FIG. 2.
The Hg2+-to-MBP-EC20 stoichiometry expressed as the plot of initial Hg2+ concentration against the complexed Hg2+-to-peptide ratio. Five nanomoles of purified MBP-EC20 was incubated with 1 to 1,200 nmol of Hg2+ in 5 mM dithiothreitol for 1 h. The portion of bound and unbound Hg2+was determined by a mercury analyzer.
Bioaccumulation of Hg2+
To investigate the effect of uptake on bioaccumulation of Hg2+, the binding capabilities of various E. coli strains were compared. Overnight cultures grown in LB medium at 37°C were harvested, washed with distilled water twice, and resuspended to a final optical density at 600 nm (OD600) of 1.0 in LB medium containing 5 μM Hg2+. The Hg2+ contents were determined after 1 h. As shown in Fig. 3, E. coli strain JM109/pUC18 accumulated a very low level of Hg2+. The intracellular accumulation of Hg2+ increased by sixfold for cells overexpressing MBP-EC20 (JM109/pMC20). By elimination of Hg2+ uptake, cells with EC20 anchored on the cell surface (JM109/pLO20) accumulated about threefold more Hg2+ than did cells with EC20 expressed in the cytoplasm. This threefold improvement is in good agreement with our earlier observation of Cd2+ accumulation using cells with surface-expressed EC20 (1). In the presence of the Hg2+ transporters (JM109/pCLTP/pMC20), intracellular accumulation of Hg2+ also increased significantly. The level of Hg2+ accumulation was similar to that for cells expressing EC20 on the surface. In both cases, 100% of the added Hg2+ was removed after 1 h. These results indicate that uptake is indeed the rate-limiting step for the intracellular accumulation of Hg2+.
FIG. 3.
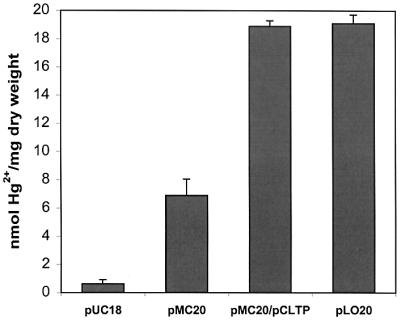
Bioaccumulation of Hg2+ by resuspended cultures harboring various plasmids from LB medium containing 5 μM Hg2+. Data were obtained from three independent experiments.
Localization of the accumulated Hg2+ was determined by separating cells into cytoplasmic and membrane fractions as described before (1). Consistent with the localization of EC20, 80% of the accumulated Hg2+ was associated with the cytoplasmic fraction for both JM109/pMC20 and JM109/pCLTP/pMC20 cells, while over 90% of the accumulated Hg2+ was found in the membrane fraction of JM109/pLO20 cells. These results demonstrate that bioaccumulation proceeds in the virtual absence of Hg2+ uptake for cells with EC20 displayed on the surface. Such an approach should be beneficial not only to the overall capacity but also to the kinetics of the bioaccumulation.
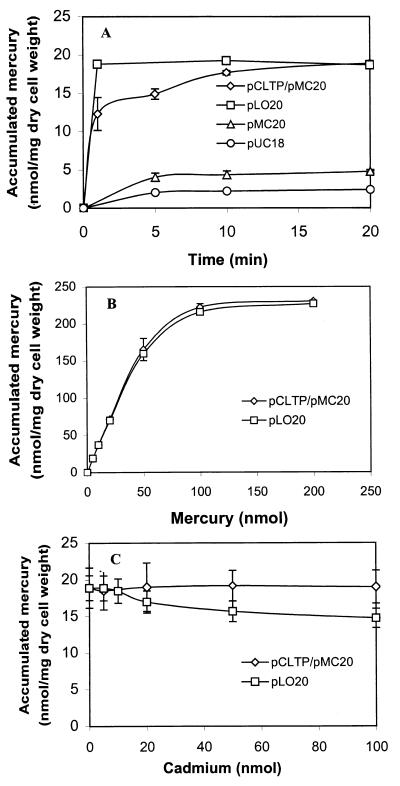
To determine the benefits on the rate of Hg2+ bioaccumulation, a time course assay was carried out. Overnight cultures were harvested, washed with distilled water twice, and resuspended to a final OD600 of 1.0 in LB medium containing 5 μM Hg2+. As shown in Fig. 4A, cells expressing only MBP-EC20 (JM109/pMC20) accumulated Hg2+ at a very low rate, with less than 20% removed after 20 min. Coexpression of the Hg2+ transporters and MBP-EC20 (JM109/pCLTP/pMC20) improved the bioaccumulation rate significantly, with 95% of the added Hg2+ removed within 20 min. These results again confirmed that Hg2+ uptake is the rate-limiting step in the bioaccumulation of Hg2+. However, the rate of bioaccumulation was further improved for JM109/pLO20 cells with EC20 expressed on the surface; over 95% of the added Hg2+ was removed within 1 min. It appears that the introduction of EC20 on the cell surface is even more effective in eliminating the uptake limitation, resulting in virtually instantaneous removal of Hg2+.
FIG. 4.
(A) Time course of mercury uptake by resting cultures harboring various plasmids. Resting cultures were resuspended in LB medium containing 5 μM Hg2+ and were incubated for 20 min. Cells were harvested at various times, and the supernatant was removed by centrifugation for 30 s. (B) Hg2+ bioaccumulation capacity of JM109 cells (0.265 mg [dry weight]) harboring either pLO20 or pCLTP/pMC20. Bioaccumulation of Hg2+ was measured at various concentrations after 1 h of incubation. (C) Effect of cadmium on bioaccumulation of Hg2+. JM109 cells harboring either pLO20 or pCLTP/pMC20 were incubated with 5 nmol of Hg2+ and various concentrations of Cd2+. The amount of Hg2+ accumulated by cells after 1 h was determined.
Evaluation of bioaccumulation.
The maximum bioaccumulation capacity of two best cell lines overexpressing EC20 (JM109/pLO20 and JM109/pMC20/pCLTP) was determined over a range of Hg2+ concentrations (Fig. 4B). At the lower levels (<20 nmol), 100% of the added Hg2+ was removed within 1 h. Although the level of EC20 expressed on the surface is approximately fivefold lower than the expression of MBP-EC20 from JM109/pCLTP/pMC20, the highest level of accumulation was around 230 nmol/mg (dry weight) for both cell lines. It has been reported previously that the bioaccumulation of Cd2+ by cells expressing MT on the surface exceeds by at least 1 order of magnitude the theoretical amount contributed by the surface-exposed MT moiety (16). It appears that the surface-exposed MT helps to increase the local metal concentration around the cells and facilitates interactions of the metal ions with other cell wall components. A similar situation may have occurred here for cells with surface-exposed EC20. It should be noted that the maximum bioaccumulation observed in this study is twofold higher than that reported for cells overexpressing MT and the mercury transporters (3) and falls within the higher range reported for other microorganisms (15 to 290 μmol/g of cells [dry weight]). This increase in capacity may also reflect the improved Hg2+ binding stoichiometry offered by EC20 over that of MT.
The selectivity of the different cell lines for Hg2+ bioaccumulation was investigated by performing the Hg2+ accumulation experiments in the presence of various amounts of cadmium (Fig. 4C). Cd2+ was selected because it is commonly found in sites contaminated with Hg2+ and is also one of the most toxic heavy metals. Because of the specificity of the Hg2+ transporters (3), no effect on Hg2+ bioaccumulation was observed with JM109/pCLTP/pMC20 cells even in the presence of a 20-fold excess of Cd2+. JM109/pLO20 cells with EC20 displayed on the surface were slightly less selective for Hg2+; the amount of accumulated Hg2+ declined gradually with an increasing excess of Cd2+. However, even in the presence of a 20-fold excess of Cd2+, JM109/pLO20 cells retained about 80% of their Hg2+ bioaccumulation activity. Since the binding affinity of Hg2+ to MTs and phytochelatins is reported to be much stronger than that of Cd2+ (11, 17), this slight decrease in selectivity may be due to the nonspecific binding of Cd2+ to the other cell wall components, which contributes greatly to the overall bioaccumulation of Hg2+.
The effects of ionic strength and metal chelators on Hg2+ bioaccumulation were investigated. The addition of up to 200 mM NaCl and 1 mM EDTA did not change the Hg2+ bioaccumulation levels for either JM109/pCLTP/pMC20 or JM109/pLO20 cells. The resistance of both systems to the presence of EDTA and NaCl makes them ideal for the removal of Hg2+ in contaminated wastewaters.
Conclusions.
Two different strategies were used to enhance the uptake and bioaccumulation of Hg2+ by cells overexpressing EC20. Our results indicate that the expression of EC20 on the cell surface is as efficient as the coexpression of Hg2+ transporters in alleviating the uptake limitation, resulting in rapid, selective, and high-level bioaccumulation of Hg2+. Since specific transporters have been identified only for a few heavy metals such as mercury and nickel (5, 10), surface expression of metal-binding peptides may be useful as a common strategy to bypass the uptake of any heavy metal of interest, a highly desirable property not associated with the metal transporter systems.
Acknowledgments
This work was supported by grants from the UC Biotechnology Research and Education Program and the U.S. Environmental Protection Agency (R827227).
We thank David Wilson for providing the plasmid pCLTP. We thank the reviewers for their helpful suggestions.
REFERENCES
- 1.Bae W, Chen W, Mulchandani A, Mehra R. Enhanced bioaccumulation of heavy metals by bacterial cells displaying synthetic phytochelatins. Biotechnol Bioeng. 2000;70:518–523. doi: 10.1002/1097-0290(20001205)70:5<518::aid-bit6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Wilson D B. Construction and characterization of Escherichia coli genetically engineered for bioremediation of Hg2+-contaminated environments. Appl Environ Microbiol. 1997;63:2442–2445. doi: 10.1128/aem.63.6.2442-2445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Wilson D B. Genetic engineering of bacteria and their potential for Hg2+ bioremediation. Biodegradation. 1997;8:97–103. doi: 10.1023/a:1008233704719. [DOI] [PubMed] [Google Scholar]
- 4.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 5.Krishnaswamy R, Wilson D B. Construction and characterization of an Escherichia coli strain genetically engineered for Ni(II) bioaccumulation. Appl Environ Microbiol. 2000;66:5383–5386. doi: 10.1128/aem.66.12.5383-5386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 7.Mason R P, Reinfelder J R, Morel F M M. Uptake, toxicity, and trophic transfer of mercury in a coastal diatorn. Environ Sci Tech. 1996;30:1835–1845. [Google Scholar]
- 8.Mattison P L. Metal biosorbents. In: Mattison P L, editor. Bioremediation of metals. Santa Rosa, Calif: Cognis; 1992. [Google Scholar]
- 9.Mehra R J, Miclat J, Kodati V R, Abdullah R, Hunter T C, Mulchandani P. Optical spectroscopic and reverse-phase HPLC analyses of Hg(II) binding to phytochelatins. Biochem J. 1996;314:73–82. doi: 10.1042/bj3140073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morby A D, Hobman J L, Brown N L. The role of cysteine residues in the transport of mercuric ions by the Tn501 MerT and MerP mercury-resistance proteins. Mol Microbiol. 1995;17:25–35. doi: 10.1111/j.1365-2958.1995.mmi_17010025.x. [DOI] [PubMed] [Google Scholar]
- 11.Nielson K B, Atkin C L, Winge D R. Distinct metal-binding configurations in metallothionein. J Biol Chem. 1985;260:5342–5350. [PubMed] [Google Scholar]
- 12.Nriagu J O, Pacyna J M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1989;333:34–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- 13.Pazirandeh M, Chrisey L A, Mauro J M, Campbell J R, Gaber B P. Expression of the Neurospora crassa metallothionein gene in Escherichia coli and its effect on heavy-metal uptake. Appl Microbiol Biotechnol. 1995;43:1112–1117. doi: 10.1007/BF00166934. [DOI] [PubMed] [Google Scholar]
- 14.Ritter J A, Bibler J P. Removal of mercury from waste water: large scale performance of an ion exchange process. Water Sci Technol. 1992;25:165–172. [Google Scholar]
- 15.Romeyer F M, Jacobs F A, Brousseau R. Expression of a Neuospora crassa metallothionein and its variants in Escherichia coli. Appl Environ Microbiol. 1990;56:2748–2754. doi: 10.1128/aem.56.9.2748-2754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sousa C, Kotrba P, Ruml T, Cebolla A, de Lorenzo V. Metalloadsorption by Escherichia coli cells displaying yeast and mammalian metallothioneins anchored to the outer membrane protein LamB. J Bacteriol. 1998;180:2280–2284. doi: 10.1128/jb.180.9.2280-2284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winklemann G, Winge D R. Metal ions in fungi. New York, N.Y: Marcel Dekker, Inc; 1994. [Google Scholar]
- 18.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–109. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]