Abstract
This study examined the effect of caffeine supplementation (CAFF) in a Wingate test (WT), and the behaviour of blood lactate concentrations (BLa) and neuromuscular fatigue (NMF), measured as reduced countermovement jump (CMJ) performance, in response to the WT. In a double-blind crossover study, 16 participants attended the laboratory twice, separated by a 72-hour window. In the sessions, participants first ingested 6 mg·kg-1 of either CAFF or placebo (PLAC), and then performed a WT. BLa was measured before (L-pre), and 0.5 min (L-post-0.5) and 3.5 min (L-post-3.5) after conducting the WT. The CMJ test was conducted before (CMJ pre), after (CMJ post), and 3 min after completing (CMJ post-3) the WT. The results indicated that CAFF enhanced peak power (Wpeak: + 3.22%; p = 0.040), time taken to reach Wpeak (T_Wpeak: -18.76%; p = 0.001) and mean power (Wmean: + 2.7%; p = 0.020). A higher BLa was recorded for CAFF at L-post-0.5 (+ 13.29%; p = 0.009) and L-post-3.5 (+ 10.51%; p = 0.044) compared to PLAC. CAFF improved peak power (PP; + 3.44%; p = 0.003) and mean power (MP; + 4.78%; p = 0.006) at CMJ pre, compared to PLAC, whereas PP and MP were significantly diminished at CMJ post and CMJ post-3 compared to pre (p < 0.001 for all comparisons) under both the CAFF and PLAC conditions. PP and MP were increased at post-3 compared to post (p < 0.001 for all comparisons) for both conditions. In conclusion, CAFF increased WT performance and BLa without affecting NMF measured by CMJ. Thus, CAFF may allow athletes to train with higher workloads and enhance the supercompensation effects after an adequate recovery period.
Keywords: Countermovement jump, Ergogenic aids, Glycolytic metabolism, Lactate, Sport nutrition, Wingate
INTRODUCTION
Caffeine (CAFF) is one of the most widely used sport supplements. An example is that some 97% of professional soccer clubs in the UK provide their players with caffeine to improve performance [1]. In the 1970s, the first studies that analysed the effect of CAFF on sports performance reported an improvement in time to exhaustion (TTE), attributing this ergogenic effect to increased lipolysis and sparing of muscle glycogen. In this regard, in vitro studies have observed that CAFF leads to high calcium (Ca2 +) bioavailability in the myoplasm through an effect on the velocity of the rate of release from the sarcoplasmic reticulum [2]. However, CAFF’s ergogenic effect is currently mainly attributed to its antagonistic function to adenosine receptors, as CAFF has a high binding capacity to these receptors. Thus, through the blockade of receptors A1, A2a, and A2b [3], CAFF prevents the inhibitory effects of adenosine on neuro-excitability, increasing the synthesis of excitatory neurotransmitters in the brain (particularly dopamine), and promoting analgesic effects that could help to reduce the rate of perceived exertion (RPE) [4], along with improved cognitive function [5]. Furthermore, although there are reports that CAFF supplementation improves performance in endurance sports with a predominance of aerobic metabolism [6], interest in the possible ergogenic effects of CAFF on anaerobic exercise efforts has increased.
When executing high-intensity anaerobic exercises, concentrations of blood lactate (BLa) and hydrogen ions (H +) rise, lowering the pH of the muscle cell [7] and reducing its capacity to generate adenosine triphosphate (ATP) for muscle contraction [8]. One of the most valid and reliable tools used to assess anaerobic performance is the 30-s Wingate test (WT). This test assesses the capacity of the muscle to generate power through anaerobic energy systems [9]. The accumulation of H + during WT inhibits phosphocreatine (PCr) resynthesis and phosphofructokinase activity responsible for the main stages of glycolytic metabolism leading to muscle fatigue [10]. In this way, recent studies have also highlighted that CAFF can improve mean and peak power registered during the WT [11].
The decline in the ability to generate strength and/or power output with repetitive stimuli, measured through an objective performance marker, can be defined as fatigue [12]. The countermovement jump (CMJ) test is commonly used in high-performance sport athletes in order to monitor neuromuscular fatigue (NMF) [13], recovery status [14], and training-induced adaptations [15]. The CMJ test can be used to repeatedly assess jump ability over a short period of time in an individual, promoting scarce physiological strain, which makes it a very useful tool to measure muscular fatigue [16]. Power and force produced during CMJ, along with jump height – which is directly associated with jump total time – are indicators of lower limb muscular power output [17]. Diminished post-exercise CMJ performance is considered appropriate to monitor neuromuscular status [18]. In addition, the incorporation of metabolic variables, such as BLa concentration, helps to improve our understanding of the post-exercise fatigue recovery process [14].
For athletes practising anaerobic sport modalities, it needs to be stablished whether CAFF can be considered ergogenic during competition and training as any possible ergogenic effect will likely affect post-exercise NMF. Accordingly, the aims of the present study were: (a) to examine the ergogenic effect of CAFF on WT performance and (b) to determine its impacts on post-exercise BLA, and fatigue measured thorough loss of CMJ ability. The hypothesis of our study was that CAFF would increase performance in a WT and BLa concentration without affecting post-exercise NMF.
MATERIALS AND METHODS
Design of the study
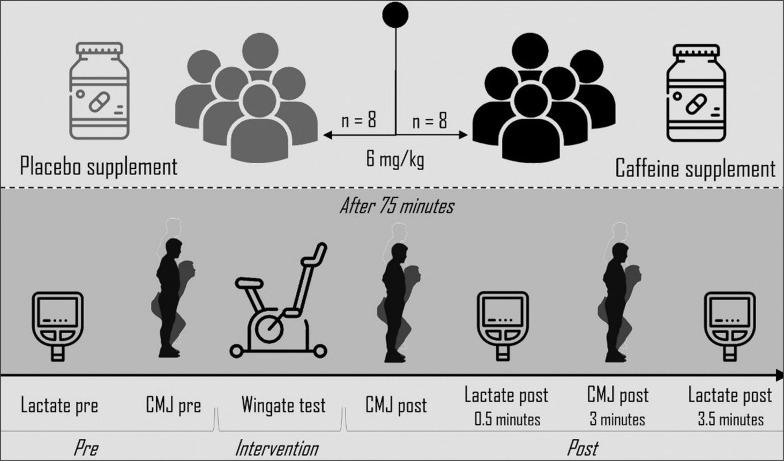
In a double-blind crossover study, participants attended twice in a 72-hour window, within the same time frame (± 0.5 hours) to avoid bias due to the circadian rhythm interaction associated with caffeine intake [19]. In each session, 50% of the participants were randomly assigned to CAFF or placebo (PLAC) supplementation (www.randomizer.org). Each experimental session consisted of a WT, and measurement of BLa and CMJ performance to assess metabolic and neuromuscular recovery, respectively. Thus, BLa concentrations was measured before (L-pre), 0.5 (L-post), and 3.5 (L-post-3.5) minutes after completing the WT. Jump ability was assessed before and after the WT (CMJ post) and after 3 min after the WT test (CMJ post-3) (see Figure 1).
FIG. 1.
Summary of the experimental session. CMJ: countermovement jump.
Participants
Sixteen male degree sports science students participated in the study (age: 22.69 ± 2.12 years; height: 1.78 ± 0.06 m; weight: 78.09 ± 10.27 kg; body mass index: 24.72 ± 2.74 kg·m-2). In order to participate in the study, researchers checked in an informative session that all the volunteers fulfilled the following inclusion criteria: i) continuous resistance training with a minimum of 3 sessions per week during at least 18 months before the study; ii) resistance training performance: their one-repetition maximum strength (1RM) normalized per kg of body mass was 1 for bench press and >1.5 for the back squat exercise; iii) no sports supplements taken in the 3 months leading up to the study outset; iv) not smoking; v) not considered to be an elite athlete; vi) not being diagnosed with a cardiovascular, respiratory, or metabolic disease; vii) no orthopaedic problem that could affect cycling technique; viii) previous experience with performing the WT. All subjects were informed of the procedure and the nutritional guidelines before giving their written informed consent. The study protocol fulfilled ethical international standards as approved by the Ethics Committee of the University Alfonso X El Sabio (code number 1.010.704).
Supplementations and dietary control
Participants arrived at the laboratory 75 min before starting WT and were instructed to take the corresponding CAFF (6 mg·kg-1) or PLAC (6 mg·kg-1 of sucrose) supplement. Capsules used were no. 1 opaque red (Guinama S.L.U, 0044634, La Pobla de Valbona, Spain) with a capacity of 0.50 mL/capsule where a researcher adjusted the individualized content of CAFF or PLAC using a semi-automatic manual filling machine Capsunorm 2000 (Miranda de Ebro, Spain). Supplementation timing was based on the time needed to achieve peak plasma caffeine levels (60 minutes) [20] and values reported on a disaggregation quality assay (13.4 min) [21]. To ensure similar nutritional intake (60% carbohydrates, 30% lipids, and 10% proteins) from 72 hours before the study onset, until its end, participants were provided with a set of guidelines. Furthermore, since the half-life for caffeine elimination has been reported in the range of 2.5–10 hours [22], caffeine intake was restricted 24 h before each experimental session in order to avoid potential interaction with the study results. Hence, participants were given a list of foodstuffs rich in caffeine (coffee, tea, mate, energizing drinks, cola drinks, chocolate drinks, and chocolate) that should be avoided.
Wingate test
The WT was executed on a Monark cycle ergometer (Ergomedic 828E, Vansbro, Sweden) and it was preceded by a standardized warm-up. The WT procedure has been described elsewhere [14]. During the test, power values (W) were recorded every second. For data analysis, peak power (Wpeak), time (s) taken to reach Wpeak (T_Wpeak), mean power (Wmean), and minimum power (Wmin), considered as the minimum W registered in the last 10 s of the test, were extracted. Mean power was also calculated for 6 five-second splits of 5 seconds during the WT (W_split0–5, W_split5–10, W_split10–15, W_split15–20, W_split20–25, and W_split25–30).
Blood lactate
Before the warm-up (L-pre), and at 0.5 min (L-post-0.5) and 3.5 min (L-post-3.5) after completing the WT, an evaluator extracted a blood sample (5 μl) from the tip of the index finger of the left hand for BLa determination using a Pro 2 LT-1710 analyser (Arkray Factory Inc., KDK Corporation, Shiga, Japan).
Neuromuscular fatigue
NMF changes were determined through performance of a CMJ measured before and after the WT following a previously described method [23]. Using a force platform (Quattro Jump model 9290AD; Kistler Instruments, Winterthur, Switzerland), each participant performed two CMJs, separated by a 45 s rest period, at three time points: before (CMJ pre) the WT, just after finishing the WT (CMJ post), and after 3 min later (CMJ post-3). These variables recorded in this test were average values of the two CMJs for jump total time (TT), mean power (MP) and peak power (PP).
Statistical analysis
Data are presented as mean ± standard deviation (SD). A t-test for related samples was performed for normally distributed variables (tested with the Kolmogorov-Smirnov test) and the Wilcoxon test for those showing a non-normal distribution (as confirmed by the Kolmogorov-Smirnov test) in the WT. A Supplementation by Time analysis of variance for repeated measures (ANOVA-RM) was applied for BLa and NMF, whereas a Supplementation by Split ANOVA-RM was applied for split-specific average power. Greenhouse-Geiser corrections were applied for non-spherical distributions, evaluated using Mauchly’s test, and Bonferroni corrections were applied for post-hoc comparisons. Cohen’s d effect size was calculated for pairwise comparisons, with values > 0.8, 0.5–0.8, 0.2–0.5 and < 0.2 considered as large, moderate, small, and trivial, respectively [24]. Partial eta squared () was calculated for ANOVA-RM where < 0.25, 0.26–0.63 and > 0.63 were considered small, medium and large effect sizes, respectively [25]. Tests were performed using IBM SPSS Statistics for Mac, version 20.0 (IBM Corp., Armonk, NY, USA). Significance was set at p ≤ 0.05.
RESULTS
Wingate test performance
Increased Wpeak (p = 0.04; d = 0.56) and Wmean (p = 0.02; d = 0.64), along with reduced T_Wpeak (p = 0.001; d = 1.08), were observed in the WT for the CAFF condition, compared to PLAC. No differences were observed in Wmin (p = 0.76) (see Table 1).
TABLE 1.
Effects of CAFF supplementation on power output during WT. Values are represented as mean ± standard deviation [95% confidence interval].
| PLAC |
CAFF |
Δ% | t | p | d | |||
|---|---|---|---|---|---|---|---|---|
| M + SD [95% CI] | CV (%) | M + SD [95 % CI] | CV (%) | |||||
| Wpeak (W) | 885.88 ± 155.1 [803.23, 968.52] |
17.5 | 914.38 ± 153.16 [832.76, 995.99] |
16.8 | + 3.22 | -2.241 | 0.040* | 0.56 |
| T_Wpeak (s) | 8.69 ± 1.45 [7.92, 9.46] |
16.7 | 7.06 ± 1.29 [6.38, 7.75] |
18.3 | -18.76 | 4.333 | 0.001* | 1.08 |
| Wmean (W) | 677.05 ± 106.12 [620.51, 733.6] |
15.7 | 695.33 ± 110.74 [636.32, 754.34] |
15.9 | + 2.7 | -2.568 | 0.020* | 0.64 |
| Wmin (W) | 477 ± 77.53 [435.69, 518.31] |
16.3 | 471.56 ± 99 [418.81, 524.31] |
21.0 | -1.14 | 0.312 | 0.760 | 0.08 |
Note: Wpeak: peak power; T_Wpeak: time to reach Wpeak; Wmean: mean power; Wmin: minimum power; M: mean; SD: standard deviation; 95% CI: 95% confidence interval; CV (%): coefficient of variation; Δ%: percentage difference from PLAC; d: Cohen’s d effect size.
An effect of Supplementation (F1,15 = 12.19; p = 0.003; = 0.45) and Time (F1.7, 25.6 = 101.21; p < 0.001; = 0.87), with no Supplementation by Time interaction (F1.2, 17.7 = 2.7; p = 0.113; = 0.15), was found for mean power recorded in 6 splits of 5 seconds each during the WT. Overall, greater mean power output was observed for CAFF, compared to PLAC (725.73 ± 130.16 vs. 697.54 ± 131.6 W). Table 2 details the results of comparing average data among splits.
TABLE 2.
Mean power output registered in splits of 5 s during Wingate test. Values are represented as mean ± standard deviation [95% confidence interval].
| PLAC |
CAFF |
Statistical significance (p-value) |
|||||
|---|---|---|---|---|---|---|---|
| M ± DS [CI 95%] | CV (%) | M ± DS [CI 95%] | CV (%) | S | T | S × T | |
| split0–5 | 585.18 ± 124.20 [519, 651.36] |
21.22 | 625.35 ± 124.37 [559.08, 691.62] |
19.89 | |||
| split5–10 # | 724.95 ± 135.33 [652.83, 797.06] |
18.67 | 760.33 ± 131.95 [690.02, 830.64] |
17.35 | |||
| split10–15 #π | 750.20 ± 130.58 [680.62, 819.78] |
17.41 | 779.61 ± 127.23 [711.82, 847.41] |
16.32 | 0.003 | < 0.001 | 0.113 |
| split15–20 #γ | 737.82 ± 123.05 [672.25, 803.39] |
16.68 | 762.44 ± 122.17 [697.34, 827.54] |
16.02 | |||
| split20–25 #γ δ | 710.03 ± 113.69 [649.45, 770.62] |
16.01 | 731.32 ± 116.00 [669.5, 793.13] |
15.86 | |||
| split25–30 #π γ δ τ | 677.05 ± 106.12 [620.51, 733.6] |
15.67 | 695.33 ± 110.74 [636.32, 754.34] |
15.93 | |||
Note: PLAC: placebo; CAFF: caffeine; S: supplementation; T: time; M: mean; SD: standard deviation; 95% CI: 95% confidence interval; CV (%): coefficient of variation;
: significantly different (p < 0.001) from split0–5;
: significantly different (p < 0.001) from split5–10;
: significantly different (p < 0.001) from split10–15;
: significantly different (p < 0.001) from split15–20;
: significantly different (p < 0.001) from split20–25.
Blood lactate concentration
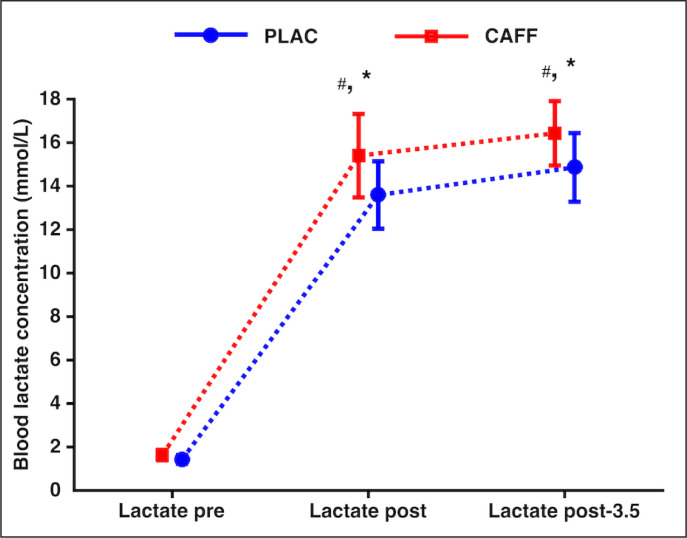
Our analysis revealed a Supplementation by Time interaction (F2,30 = 3.837; p = 0.033; = 0.204), and an effect of Supplementation (F1,15 = 8.621; p = 0.01; = 0.365) and Time (F2,30 = 291.912; p < 0.001; = 0.951). BLa was found to be increased (p < 0.001) at L-post-0.5 and L-post-3.5, under both the CAFF and PLAC conditions. In addition, BLa was greater in the CAFF condition at L-post-0.5 (Δ% = 13.29; p = 0.009) and L-post-3.5 (Δ% = 10.51; p = 0.044), vs. PLAC (see Figure 2).
FIG. 2.
Mean ± 95% confidence intervals for blood lactate concentration registered at Lactate pre, Lactate post-0.5, and Lactate post-3.5 are shown in this figure. *: significant differences from Lactate pre (p < 0.05); #: significant differences between CAFF and PLAC (p < 0.05).
Neuromuscular fatigue
A Time effect was observed for CMJ TT (F2,30 = 3.380; p = 0.047; = 1.84), with larger CMJ post values recorded compared to CMJ post-3 (0.80 ± 0.14 vs. 0.75 ± 0.11; p = 0.049). No Supplementation by Time interaction (F2,30 = 0.034; p = 0.967; = 0.002) or Supplementation effect (F1,15 = 0.116; p = 0.739; = 0.008) was observed.
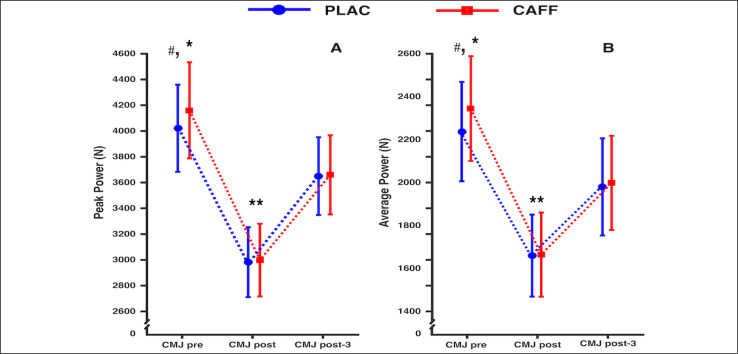
A Supplementation by Time interaction (F2,30 = 3.318; p = 0.05; = 0.181) and Time effect (F1.298,19.385 = 130.209; p < 0.001; = 0.897) were found for PP, with no Supplementation effect (F1,15 = 3.531; p = 0.08; = 0.191) (Figure 3A). For both conditions, CAFF and PLAC, greater values (p < 0.001) were found at the time point CMJ pre, compared to CMJ post and CMJ post-3, and smaller values (p < 0.001) were noted at CMJ post, compared to CMJ post-3. Moreover, a higher PP was recorded for CAFF at CMJ pre, compared to PLAC (4160.21 ± 700.23 W vs. 4021.79 ± 634.29 W; p = 0.003; Figure 3A).
FIG. 3.
Mean ± 95% confidence intervals for PP (A) and MP (B) registered at CMJ pre, CMJ post, and CMJ post-3 are shown in this figure. *: significant difference (p < 0.001) compared to post and post-3 for CAFF and PLAC; **: significant difference (p < 0.001) compared to post-3 for CAFF and PLAC; #: significant difference (p < 0.01) between CAFF and PLAC.
A Supplementation by Time interaction (F2,30 = 3.975; p = 0.029; = 0.209), and Time effect (F1.391, 20.862 = 104.279; p < 0.001; = 0.874) were observed for MP, with no Supplementation effect (F1,15 = 3.458; p = 0.083; = 0.187). Greater values were observed for CAFF and PLAC at CMJ pre, compared to CMJ post and CMJ post-3 (p < 0.001; Figure 3B), with larger MP at CMJ pre for CAFF, compared to PLAC (2344.55 ± 457.9 W vs. 2237.54 ± 433.98 W; p = 0.006; Figure 3B). Moreover, a lower MP was detected for CAFF and PLAC were found at CMJ post, compared to CMJ post-3 (p < 0.001).
DISCUSSION
This present study revealed an effect of acute CAFF supplementation on peak power (Wpeak), time to reach peak power (T_Wpeak), and mean power (Wmean) recorded during a WT, as well as on PP and MP measured in a pre-exercise CMJ test. After the WT (L-post- and L-post-3.5), BLa concentrations were also higher in participants subjected to CAFF supplementation compared to PLAC. However, no differences between CAFF and PLAC conditions were found for muscle fatigue measured through PP and MP at the points CMJ post- and CMJ post-3 conditions. Collectively, these results suggest that CAFF increases sport performance in a high-intensity anaerobic effort (WT) without affecting the fatigue levels, which would in turn favour athletes’ functional adaptions to training.
Several studies have confirmed the ergogenic effects of CAFF when executing a WT [10, 11]. Our results indicate that CAFF (6 mg·kg-1) increased Wpeak (+ 3.22%) and Wmean (+ 2.7%) and reduced T_Wpeak (-18.76%) in the WT. The results are in agreement with those of a meta-analysis [11] reporting increases of 3% and 4% in Wpeak and Wmean in WT performance after CAFF. When examining the kinetics of power output production during the WT, our results are in line with those of a study by San Juan et al. [26], where Wpeak was observed in the 2nd split (5–10 s) whereas the maximum mean power output was reached in the 3rd split (10–15 s), preceding the power descent until the end of the WT. These data suggest that Wpeak occurs when PCr stores are the main energy source [27]. During the second part of the WT, the critical reduction in PCr pools in the muscle promotes ADP accumulation, which is associated with an increase in the contribution of the glycolytic pathway to obtain ATP. This might be explained by a potential accumulation of metabolic end-products (i.e., H +), which is reflected in the diminished power output production observed in the last splits of this study. H + ions may compete with Ca2 + for the myosin-binding site, impairing the muscle contraction process, as well as inhibiting PCr resynthesis and phosphofructokinase activity, which are key parts of the glycolytic metabolism [8]. The sum of all these factors could partially explain muscle fatigue during the WT [10], and the consequent decline in performance. Considering that CAFF enhances neuromuscular recruitment [28] and increases Ca2 + bioavailability in the myoplasm [29], it could be possible that the increased Wpeak observed after CAFF supplementation is mediated by enhanced muscular function. In fact, improved performance in a time-to-exhaustion cycling test has recently been reported [30], revealing a lower electromyographic signal which suggests enhanced muscle contraction efficiency. The improvement in Wmean in response to CAFF might be related to greater mobilization of Ca2 + in the sarcoplasmic reticulum [11], which would offset the adverse effects of H + [8] and facilitate muscle contraction. However, mechanisms that could explain caffeine’s ergogenic effects seem to take place outside the muscle cell [31]. In this sense, the central factors associated with the inhibitory mechanisms of adenosine receptors [3] that lead to reduced perceived exertion [4] seem to be related to the increased Wmean. Since lactate production is related to higher demands of ATP production by the glycolytic and phosphagen systems [32], the higher BLa concentrations detected after CAFF compared to PLAC at L-post-0.5 and L-post-3.5 could reflect the improved performance observed for CAFF during the WT.
Regarding CMJ performance, enhanced PP and MP production after CAFF intake was observed in the CMJ pre. Our data are in agreement with those of previous studies that reported increased power production in male [33] and female [34] volleyball players, female soccer players [35], and in young [36], and recreationally trained athletes [37]. A recent study analysed the effect of CAFF on CMJ performance and the duration of jump phases, reporting increased jump height, MP, PP and maximal velocity before taking off, without changes in the duration of the different jump phases (i.e., eccentric, isometric and concentric phases) [38]. The exact mechanism responsible for the effect of caffeine ingestion on jump height performance is not known. Since PCr stores and RPE cannot affect performance at CMJ pre, we speculate that the positive effect of CAFF on intra- and inter-muscular coordination during muscle contractions may result from improved motor unit recruitment due to increased Ca2 + bioavailability in the myoplasm, which could act as a synergistic mechanism leading to enhanced jump ability [38]. However, using CMJ performance as an indicator of fatigue, no differences between the CAFF and PLAC conditions were observed for MP and PP at CMJ post- and CMJ post-3, which in turn were both lower compared to CMJ pre. These results are in line with a previous study where reduced CMJ performance was detected after a WT in elite athletes, with no differences emerging between CAFF and PLAC conditions [26]. It is plausible that the accumulation of ADP and H + could affect muscular contraction function during CMJ performed after the WT. At the metabolic level, the absence of PCr after the WT could partially explain the impaired performance observed in this study at CMJ post and CMJ post-3. Similarly, considering the PCr resynthesis kinetics, the small recovery in jump ability observed at CMJ post-3 is likely the consequence of a partial resynthesis of PCr reserves [39], whereas the decreased performance compared to CMJ pre would reflect NMF [23]. The results observed for jump ability in this study and those previously reported by San Juan et al. [26] represent important findings for sports training, as the participants of the present study who ingested caffeine were able to perform the WT with higher workloads, being their levels of fatigue (measured through the CMJ) similar to those recorded in the placebo condition, and thus leading to the suggestion that CAFF may mitigate the fatigue associated with high-intensity exercise. Although caffeine interventions can help support higher physiological workloads during high-intensity training sessions [40], it is important to highlight that in our study there was no mitigating effect of CAFF on decreasing fatigue. Therefore, the use of CAFF repeatedly in high-intensity sessions should be carefully considered to avoid the risk of negative adaptations to training [41] which in the medium term could lead to symptoms of non-functional overreaching [42].
Our results indicate that acute caffeine supplementation (6 mg·kg-1) enhances performance and increases workload without affecting fatigue levels. Thus, CAFF could be a nutritional strategy used together with a short period of overload training (i.e., functional overreaching). In this regard, athletes could be able to train with higher workloads and enhance the supercompensation effects after an adequate recovery period. Nevertheless, this study has some limitations. The effect of CAFF on Ca2 + bioavailability has been observed in vitro and so it is necessary to confirm this effect in humans during exercise. In this regard, different studies have reported that CAFF may affect several psychophysiological mood variables and psychological responses, including a modification of the relationship between workload and RPE during high-intensity anaerobic efforts [43]. Therefore, future studies should assess the potential influence of the psychological responses to CAFF supplementation on the post-exercise fatigue levels.
CONCLUSIONS
Acute supplementation with 6 mg·kg-1 of caffeine improved performance in a Wingate test (i.e., Wpeak, T_Wpeak and Wmean), and increased pre-exercise jump ability (CMJ) compared to placebo, with no effect on post-exercise muscular fatigue. Hence, CAFF could increase workload without affecting fatigue levels. Thus, using CAFF, athletes might be able to train with higher workloads and enhance the supercompensation effects after an adequate recovery period.
Conflict of Interest Declaration
We declare that there are no conflicts of interest relevant to the content of this article.
REFERENCES
- 1.Tallis J, Clarke N, Morris R, Richardson D, Ellis M, Eyre E, Duncan M, Noon M. The prevalence and practices of caffeine use as an ergogenic aid in English professional soccer. Biol Sport. 2021; 38(4):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber A, Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968; 52(5):750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Müller CE. Antinociceptive effects of novel a2b adenosine receptor antagonists. J Pharmacol Exp Ther. 2004; 308(1):358–366. [DOI] [PubMed] [Google Scholar]
- 4.Doherty M, Smith P. Effects of caffeine ingestion on rating of perceived exertion during and after exercise: A meta-analysis. Scand J Med Sci Sports. 2005; 15(2):69–78. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzo Calvo J, Fei X, Domínguez R, Pareja-Galeano H. Caffeine and Cognitive Functions in Sports: A Systematic Review and Met nalysis. Nutrients. 2021; 13(3):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Southward K, Rutherfurd-Markwick KJ, Ali A. The effect of acute caffeine ingestion on endurance performance: a systematic review and meta–analysis. Sports Med. 2018; 48(8):1913–1928. [DOI] [PubMed] [Google Scholar]
- 7.Junior AHL, de Salles Painelli V, Saunders B, Artioli GG. Nutritional strategies to modulate intracellular and extracellular buffering capacity during high-intensity exercise. Sports Med. 2015; 45(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ament W, Verkerke G. Exercise and fatigue. Sports Med. 2009; 39(5):389–422. [DOI] [PubMed] [Google Scholar]
- 9.Patton J, Murphy M, Frederick F. Maximal power outputs during the Wingate anaerobic test. Int J Sports Med. 1985; 6(2):82–85. [DOI] [PubMed] [Google Scholar]
- 10.Lopes-Silva JP, Reale R, Franchini E. Acute and chronic effect of sodium bicarbonate ingestion on Wingate test performance: A systematic review and meta-analysis. J Sports Sci. 2019; 37(7):762–771. [DOI] [PubMed] [Google Scholar]
- 11.Grgic J. Caffeine ingestion enhances Wingate performance: A meta-analysis. Eur J Sport Sci. 2018; 18(2):219–225. [DOI] [PubMed] [Google Scholar]
- 12.Enoka RM, Duchateau J. Translating fatigue to human performance. Med Sci Sports Exerc. 2016; 48(11):2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gathercole RJ, Sporer BC, Stellingwerff T, Sleivert GG. Comparison of the capacity of different jump and sprint field tests to detect neuromuscular fatigue. J Strength Cond Res. 2015; 29(9):2522–2531. [DOI] [PubMed] [Google Scholar]
- 14.Wu PP-Y, Sterkenburg N, Everett K, Chapman DW, White N, Mengersen K. Predicting fatigue using countermovement jump force-time signatures: Pca can distinguish neuromuscular versus metabolic fatigue. PloS one. 2019; 14:e0219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gathercole RJ, Stellingwerff T, Sporer BC. Effect of acute fatigue and training adaptation on countermovement jump performance in elite snowboard cross athletes. J Strength Cond Res. 2015; 29(1):37–46. [DOI] [PubMed] [Google Scholar]
- 16.Gathercole R, Sporer B, Stellingwerff T, Sleivert G. Alternative countermovementjump analysis to quantify acute neuromuscular fatigue. Int J Sports Physiol Perform. 2015; 10(1):84–92. [DOI] [PubMed] [Google Scholar]
- 17.Bosco C, Luhtanen P, Komi PV. A simple method for measurement of mechanical power in jumping. Eur J Appl Physiol Occup Physiol. 1983; 50(2):273–282. [DOI] [PubMed] [Google Scholar]
- 18.Claudino JG, Cronin J, Mezêncio B, McMaster DT, McGuigan M, Tricoli V, Amadio AC, Serrão JC. The countermovement jump to monitor neuromuscular status: A metaanalysis. J Sci Med Sport. 2017; 20(4):397–402. [DOI] [PubMed] [Google Scholar]
- 19.Mora-Rodríguez R, Pallarés JG, López-Gullón JM, López-Samanes Á, Fernández-Elías VE, Ortega JF. Improvements on neuromuscular performance with caffeine ingestion depend on the time-of-day. J Sci Med Sport. 2015; 18(3):338–342. [DOI] [PubMed] [Google Scholar]
- 20.McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. 2016; 71:294–312. [DOI] [PubMed] [Google Scholar]
- 21.Real Farmacopea Española . Ministerio de Sanidad y Consumo. Centro de Publicaciones: Madrid; 1997. [Google Scholar]
- 22.Magkos F, Kavouras SA. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit Rev Food Sci Nutr. 2005; 45(7–8):535–562. [DOI] [PubMed] [Google Scholar]
- 23.Maté-Muñoz JL, Lougedo JH, Barba M, García-Fernández P, Garnacho-Castaño MV, Domínguez R. Muscular fatigue in response to different modalities of crossfit sessions. PloS one. 2017; 12(7):e0181855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J: Statistical power analysis for the behavioral sciences. 2nd ed. Academic press; 1988. [Google Scholar]
- 25.Richardson JT. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. 2011; 6(2):135–147. [Google Scholar]
- 26.San Juan AF, López-Samanes Á, Jodra P, Valenzuela PL, Rueda J, Veiga-Herreros P, Pérez-López A, Domínguez R. Caffeine supplementation improves anaerobic performance and neuromuscular efficiency and fatigue in olympic-level boxers. Nutrients. 2019; 11(9):2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovell D, Kerr A, Wiegand A, Solomon C, Harvey L, McLellan C. The contribution of energy systems during the upper body wingate anaerobic test. Appl Physiol Nutr Metab. 2013; 38(2):216–219. [DOI] [PubMed] [Google Scholar]
- 28.Kalmar J, Cafarelli E. Central excitability does not limit postfatigue voluntary activation of quadriceps femoris. J Appl Physiol. 2006; 100(6):1757–1764. [DOI] [PubMed] [Google Scholar]
- 29.Rousseau E, Ladine J, Liu Q-Y, Meissner G. Activation of the Ca2 + release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988; 267(1):75–86. [DOI] [PubMed] [Google Scholar]
- 30.de Albuquerque Melo A, Basto ilva VJ, Moura FA, Bini RR, Lim ilva AE, de Araujo GG. Caffeine mouth rinse enhances performance, fatigue tolerance and reduces muscle activity during moderate-intensity cycling. Biol Sport. 2021; 38(4):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astorino TA, Roberson DW. Efficacy of acute caffeine ingestion for short-term highintensity exercise performance: A systematic review. J Strength Cond Res. 2010; 24(1):257–265. [DOI] [PubMed] [Google Scholar]
- 32.Jackman M, Wendling P, Friars D, Graham T. Metabolic, catecholamine, and endurance responses to caffeine during intense exercise. J Appl Physiol. 1996; 81(4):1658–1663. [DOI] [PubMed] [Google Scholar]
- 33.Zbiden-Foncea H, Rada I, Gomez J, Kokaly M, Stellingwerff T, Deldicque L, Peñailillo L. Effects of caffeine on countermovement-jump performance variables in elite male volleyball players. Int J Sports Physiol Perform. 2018; 13(2):145–150. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-López A, Salinero JJ, Abian-Vicen J, Valadés D, Lara B, Hernandez C, Areces F, González C, Del Coso J. Caffeinated energy drinks improve volleyball performance in elite female players. Med Sci Sports Exerc. 2015; 47(4):850–856. [DOI] [PubMed] [Google Scholar]
- 35.Lara B, Gonzalez-Millán C, Salinero JJ, Abian-Vicen J, Areces F, Barbero-Alvarez JC, Muñoz V, Portillo LJ, Gonzalez-Rave JM, Del Coso J. Caffeine-containing energy drink improves physical performance in female soccer players. Amino acids. 2014; 46(5):1385–1392. [DOI] [PubMed] [Google Scholar]
- 36.Abian-Vicen J, Puente C, Salinero JJ, González-Millán C, Areces F, Muñoz G, Muñoz-Guerra J, Del Coso J. A caffeinated energy drink improves jump performance in adolescent basketball players. Amino Acids. 2014; 46(5):1333–1341. [DOI] [PubMed] [Google Scholar]
- 37.Gant N, Ali A, Foskett A. The influence of caffeine and carbohydrate coingestion on simulated soccer performance. Int J Sport Nutr Exerc Metab. 2010; 20(3):191–197. [DOI] [PubMed] [Google Scholar]
- 38.Lago-Rodríguez A, Jodra P, Bailey S, DomÍnguez R. Caffeine improves performance but not duration of the countermovement jump phases. J Sports Med Phys Fitness. 2021; 61(2):199–204. [DOI] [PubMed] [Google Scholar]
- 39.McMahon S, Jenkins D. Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med. 2002; 32(12):761–784. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro BG, Morales AP, Sampai orge F, Barth T, de Oliveira MB, Coelho GM, Leite TC. Caffeine attenuates decreases in leg power without increased muscle damage. J Strength Cond Res. 2016; 30(8):2354–2360. [DOI] [PubMed] [Google Scholar]
- 41.Lastella M, Vincent GE, Duffield R, Roach GD, Halson SL, Heales LJ, Sargent C. Can sleep be used as an indicator of overreaching and overtraining in athletes? Front Physiology. 2018; 9:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellinger P. Functional overreaching in endurance athletes: A necessity or cause for concern? Sports Med. 2020; 50(6):1059–1073. [DOI] [PubMed] [Google Scholar]
- 43.Domínguez R, Veiga-Herreros P, Sánchez-Oliver AJ, Montoya JJ, Ramos-Álvarez JJ, Miguel-Tobal F, Lago-Rodríguez Á, Jodra P. Acute effects of caffeine intake on psychological responses and high-intensity exercise performance. Int J Environ Res Public Health. 2021; 18(2):584. [DOI] [PMC free article] [PubMed] [Google Scholar]