Abstract
The genetic protective factors for cognitive decline in aging remain unknown. Predicting an individual’s rate of cognitive decline—or with better cognitive resilience—using genetics will allow personalized intervention for cognitive enhancement and the optimal selection of target samples in clinical trials. Here, using genome-wide polygenic scores (GPS) of cognitive capacity as the genomic indicators for variations of human intelligence, we analyzed the 18-year records of cognitive and behavioral data of 8511 European-ancestry adults from the Wisconsin Longitudinal Study (WLS), specifically focusing on the cognitive assessments that were repeatedly administered to the participants with their average ages of 64.5 and 71.5. We identified a significant interaction effect between age and cognitive capacity GPS, which indicated that a higher cognitive capacity GPS significantly correlated with a slower cognitive decline in the domain of immediate memory recall (β = 1.86 × 10−1, p-value = 1.79 × 10−3). The additional phenome-wide analyses identified several associations between cognitive capacity GPSs and cognitive/behavioral phenotypes, such as similarities task (β = 1.36, 95% CI = (1.22, 1.51), p-value = 3.59 × 10−74), number series task (β = 0.94, 95% CI = (0.85, 1.04), p-value = 2.55 × 10−78), IQ scores (β = 1.42, 95% CI = (1.32, 1.51), p-value = 7.74 × 10−179), high school classrank (β = 1.86, 95% CI = (1.69, 2.02), p-value = 3.07 × 10−101), Openness from the BIG 5 personality factor (p-value = 2.19 × 10−14, β = 0.57, 95% CI = (0.42, 0.71)), and leisure activity of reading books (β = 0.50, 95% CI = (0.40, 0.60), p-value = 2.03 × 10−21), attending cultural events, such as concerts, plays, or museums (β = 0.60, 95% CI = (0.49, 0.72), p-value = 2.06 × 10−23), and watching TV (β = −0.48, 95% CI = (−0.59, −0.37), p-value = 4.16 × 10−18). As the first phenome-wide analysis of cognitive and behavioral phenotypes, this study presents the novel genetic protective effects of cognitive ability on the decline of memory recall in an aging population.
Keywords: cognitive genetics, aging genetics, phenome-wide association study, genome-wide polygenic score, sociogenomics
1. Introduction
The magnitudes of cognitive decline in aging, a major health concern in contemporary society, differ substantially across individuals [1,2]. Existing literature suggests that the heterogeneity of cognitive decline is partially owing to the fact that some adults are more resilient to neuropathological changes than others [3,4,5]. Cognitive resilience is conceptualized as an individual’s capacity to overcome negative effects or stress on cognitive functioning despite aging or neuropathologic changes [6,7]. Diverse factors have been hypothesized to explain cognitive resilience, including brain structural features, genetic factors, and personality attributes acquired over the lifespan that offset the negative effects (i.e., cognitive decline) of brain aging, insult, or pathologies [8,9]. Elucidating the underlying mechanisms that may differentiate and identify aging adults with high or low cognitive resilience is essential to inform the homogeneity of adults in clinical trials for risk stratification and providing preventive intervention.
Studying cognitive resilience in the elderly is of special interest because the finding may provide insights into maintaining good cognition and “aging well” without developing dementia due to Alzheimer’s disease or other causes. However, while studies have reported the genetic risk factors of accelerated cognitive decline among individuals with dementia [10,11,12], we know very little about the genetic protective factors against cognitive decline that are present in the normal aging population. Recent studies have identified several genes and proteins that are assumed to be associated with cognitive resilience [9,13,14], but no study has yet aggregated multiple genetic variants into a single score that may summarize individual-specific indices of cognitive capacity and their impacts on cognitive resilience and other related attributes. In addition, most studies have not assessed the inherited genetic profile of resilient people, nor have they associated these genetic factors with the rate of cognitive decline or other behavioral life course outcomes, which may help us understand the genetic backgrounds of resilient and non-resilient people, along with how variations in genetic profile affect life course alterations other than cognition. Genome-wide polygenic scores (GPS) leverage the fact that most human traits are the result of the aggregated influence of many genetic variants, both common and rare [15,16,17]. By aggregating the minuscule effects of millions of genetic variants into a single score, GPS allows researchers to stratify individuals by their genomic propensity for a particular trait and select individuals with extremely high or low GPS for further research. The recent large genome-wide association studies (GWAS) of educational attainment, an often-used proxy phenotype for human intelligence, identified 1271 independent autosomal loci reaching genome-wide significance [18]. These findings suggest that several biological pathways related to brain development or neuron-to-neuron communication contribute to human intelligence. While the GWAS revealed many genetic variants associated with cognitive capacity phenotypes (such as cognitive performance, math ability, and highest math class taken) [18,19,20,21,22,23,24,25], the genomic contribution to specific cognitive domains remains unknown, as does their relationship to cognitive changes with aging.
Since general cognitive ability is known to be highly heritable (50–70%) and polygenic [26,27], we utilized GPS to account for the genome-wide factors underlying cognitive capacity and its changes with aging [21,24,28,29]. We leveraged the comprehensive phenotype information of a 50+ year social longitudinal database for phenome-wide association studies (PheWAS). The Wisconsin Longitudinal Study (WLS), the longest-running social longitudinal study in the United States [30,31], encompasses a detailed and broad lifelog of cognition, personality, financial, health, and socioeconomic status. The surveys have been repeatedly administered the same cognitive ability tests with the time interval of ~10 years in their latest survey rounds, as well as collected the genotype data of the participants, which creates a deep genotype-phenotype catalog of an individual’s cognitive and behavioral traits over their adult lives (Files S1).
Herein, we hypothesize that the polygenic influence of the cognitive capacity can explain certain patterns of cognitive abilities and the rate of their decline during aging and other socio-behavioral phenotypes that might be affected by the genetics of cognitive abilities. We tested the associations between the longitudinal observations of individual cognitive/behavioral phenomes and the GPSs of four different cognitive phenotypes (educational attainment, cognitive performance, math ability, and highest math class taken), focusing on the secular changes in cognitive test scores. The approach was designed to systematically address the following research questions: first, whether a particular cognitive domain was more impacted by polygenic influence than other cognitive domains; secondly, whether individuals with different GPSs showed different patterns of cognitive decline during aging, which suggests whether attributes of cognitive resilience are genetically inherited; and, thirdly, the extent to which the phenotypic variances of the behavioral/personality attributes could be explained by the genetic liability of the cognitive capacities which may help us understand how the different genetic profiles of resilient people affect their life course alterations other than cognition. We aimed to investigate not just whether the lower cognitive test scores of certain groups of individuals were associated with the GPS itself, but also whether the rate of cognitive decline was influenced by the joint interaction effect between time and GPSs, which are themselves differentiated over time.
2. Materials and Methods
2.1. Data
The WLS is based on 10,317 individuals surveyed in 1957—representing a 1/3 random sample of Wisconsin high school graduates that year—with randomly-selected siblings empaneled later. The study has collected 27,000+ phenotypic variables of the participants, ranging from cognition, personality, financial, and socioeconomic to genotype data during 6 waves of data collection over 60 years. The cohort represents non-Hispanic White Americans who completed at least 12 years of high school education in the United States. The participants underwent in-person, telephone structured interviews or mail-in questionnaires for each survey round after providing informed consent. All the instruments and operations were approved by the Institutional Review Board of the University of Wisconsin-Madison.
2.2. Genotype Data and Quality Control Process
From 2007–2008, saliva samples were collected by mail or during a home interview, and 9019 individuals were successfully genotyped at the Johns Hopkins University Center for Inherited Disease Research (CIDR) using the Illumina HumanOmniExpress-24 v.1.1 array designed for human genome build 37/hg19. The subsequent quality control process filtered individuals with (i) genotype missingness rate > 0.05 in all chromosomes, (ii) mismatch between recorded sex and genetically determined sex, (iii) high genetic relatedness with other individuals (>0.025), (iv) outlier in heterozygosity/homozygosity test, and (v) non-European ancestry outliers. Non-European individuals were identified by visually inspecting the principal component analysis (PCA) plot of the covariance matrix of the WLS genotype data with 1000 Genomes reference populations [32]. Additionally, SNPs with (i) genotype call rate < 0.95, (ii) Hardy-Weinberg exact test p-value < 1.0 × 10−5, and (iii) minor allele frequency < 0.01 were excluded from the data, resulting in 607,469 autosomal SNPs in 8527 European-ancestry individuals considered for further analysis. The data was then imputed to the Haplotype Reference Consortium (HRC) v1.1 European reference panel [32] and resulted in 39,127,657 variants. The detailed imputation and QC report are available separately [33,34].
2.3. Construction of Cognitive Capacity GPS
A set of cognitive ability-related GPSs were constructed based on four large-scale GWAS MTAG summary statistics on educational attainment (EA, n = 1,131,881), cognitive performance (CP, n = 257,841), self-reported math ability (MA, n = 564,698), and highest-level math class taken (HM, n = 430,445) from Lee et al. [18] and available from the WLS website upon request [30]. We downloaded the set of GPSs that was calculated with PLINK 1.9 [35] using the SNP weights adjusted for linkage disequilibrium using LDpred software [36]. All the SNP weights were obtained from cognitive GWAS discovery samples that did not contain the WLS participants.
2.4. Outcome Measures
2.4.1. Cognitive Phenotypes
The participants’ cognition was assessed longitudinally using various tasks and structured questionnaires throughout the survey period of 60+ years. Our analysis used the participants’ cognition data from the four WLS survey rounds (taken in 1957, 1992–1994, 2003–2003, and 2011). The WLS data included the IQ scores of the participants from the Henmon-Nelson Test of Mental Ability with 90 items collected in their high school junior years in 1957, which measured general verbal, quantitative, and spatial knowledge [37,38,39], and their high school class rank percentile, which was based on the mean grade taken throughout the high school courses. The years of education (educational attainment) were calculated from the highest educational degree held by each participant at their middle age. We also included the cognition component of the Health Utilities Index 3 (HUI3 cognition level) which asked the subjects about their self-perceived cognitive status at the time of the interview.
Beginning in 1992–1994, 10 types of cognitive tasks were systematically proposed to the subjects at three time points over an 18–19 year period, including similarities (administered at survey timepoint 1/2/3), letter fluency (timepoint 2/3), category fluency (timepoint 2/3), immediate recall (timepoint 2/3), delayed recall (timepoint 2/3), digit ordering (timepoint 2/3), number series (timepoint 3), linguistic function (timepoint 3), including two health literacy assessments, the Newest Vital Sign (NVS) Health Literacy Assessment (timepoint 3) and the Short Test of Functional Health Literacy in Adults (STOFHLA) (timepoint 3). The phenotypes selected for the phenome-wide analysis are denoted with italics throughout the manuscript and their measurement criteria are available in the Supplementary Material. All the raw scores were z-scored for the analysis.
2.4.2. Behavioral Phenotypes
The participants’ personality traits were assessed with the Big 5 Factor Model of Personality inventory test [40] in the WLS 1992–1994 collection wave. The five personality traits are known as one of the most common and influential models in the field of personality research and remain relatively stable over a lifetime. The Big 5 Factor Model of Personality test describes an individual’s personality in five basic dimensions: extraversion, openness, neuroticism, conscientiousness, and agreeableness. A higher score on each scale indicates the person has higher tendencies and behaviors representing the personality traits.
The subjects were asked to report on the time they spent participating in different leisure activities in hours per week or year. We compared various types of leisure activities including reading, writing letters, watching movies/TV, light or vigorous physical activity (alone or together), doing crafts, hunting/fishing, playing a crossword puzzle/other word game, attending cultural events, etc. The description of each leisure activity is provided in the Supplementary Material. To correct for outliers with extreme hours of certain activities, we took the natural logarithm of the reported hours for each activity and used it for the analysis.
In addition, we included two occupational standing variables collected in the WLS 2003–2005 wave based on their current or past employment information. The occupational education score was a numeric value of the types of industry or class-of-worker categories based on the 1990 US Census data, which indicated a percentage of persons who had at least a year of college education, ranging from 0 to 999. The occupational income score was calculated from the 1990-basis occupational earning scores, representing the percentage of persons in the 1990 US Census data in an industry or class-of-work category who earned more than $14.30/h in 1989, ranging from 37 to 876.
Since the IQ data of the participants’ spouses were available, we also included this variable for the analysis, hypothesizing that the behavior of assortative mating is associated with the GPSs of the cognitive abilities. Previous literature suggests the psychiatric hypothesis of assortative mating in academic achievements and IQ [41,42,43,44,45].
2.5. Statistical Analysis
2.5.1. Cognitive/Behavioral PheWAS
Linear regression was used to investigate the associations between the four types of cognitive capacity GPSs (EA, CP, HM, and MA) and the normalized variables of the cognitive and behavioral phenotypes. Each cognitive capacity GPS was tested in separate models. We adjusted for biological sex, age, and the first 10 PCs of genetic ancestry and estimated each GPS’ significance (p-value), effect size (β), 95% confidence interval (CI), and proportion of variance explained (R2) for the target outcomes. Bonferroni-adjusted significance level of 2.60 × 10−4 was used to correct for multiple testing (48 tested phenotypes * 4 cognitive capacity GPS).
2.5.2. Cognitive Changes
We selected 7 repetitive measures administered to the participants among aforementioned cognitive assessments, with an average interval of 6.5 years. We investigated its interaction effects with the cognitive capacity GPSs as the participants aged, including similarities, letter fluency, category fluency, immediate recall, delayed recall, digit ordering, and HUI3 cognition level (timepoint 2/3). Linear mixed-effects regressions were nested by the individual ID and each survey round (random effect) and we included the following fixed covariates in the analysis: age at the survey time point, biological sex, the first 10 ancestrally-informative principal components (PC1-10) of the genotype data and years of education. Bonferroni’s correction was used to adjust for multiple testing, and the scores were normalized except for the ordinal variable, HUI3 cognition level. We hypothesized that the contribution of genetic factors to the cognitive phenotypes was associated with the different degrees of cognitive decline in a particular cognitive domain. The analyses were performed in the R 3.5.1 environment, and the linear mixed-effect model was run with lme4 package [46]. We calculated Schielzeth and Nakagawa’s R2 for generalized linear mixed effect models using r.squaredGLMM function from MuMIn R package [47,48,49].
3. Results
3.1. Participant Demographics
Our study included 8511 European-ancestry individuals with DNA genotype data, behavioral questionnaire data and cognitive assessment data available, including seven different cognitive ability tasks administered repetitively with an average interval of 6.5 years (SD = 1.25 year). The average age of the study participants was 48.6 at the time of the first round of the cognitive assessment (WLS survey round 4 (survey timepoint 1), 1992–1994, SD = 15.4 years), 64.2 at the second assessment (WLS survey round 5 (survey timepoint 2), 2003–2005, SD = 4.1 years), and 70.7 at the time of the last assessment (WLS survey round 6 (survey timepoint 3), 2011, SD = 4.2 years). The sample was 51.8% female, 47.8% completed high school or less than one year of college (number of years of education), and 78.2% were born in Wisconsin, USA.
3.2. PheWAS of Cognitive GPSs in the Cognitive/Behavioral Phenome
3.2.1. Cognitive Phenotypes
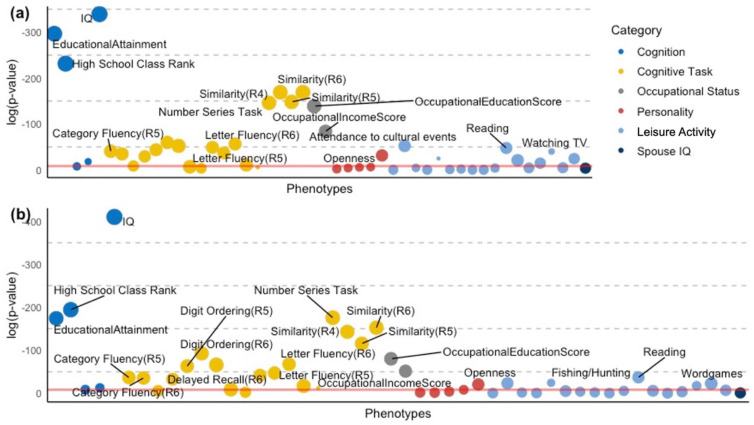
Across all of the PheWAS results, IQ score showed the strongest association with the four cognitive GPSs in terms of the p-value and the increased proportion of variance explained (strongest with CP GPS, p-value = 7.74 × 10−179, β = 1.42, 95% CI = (1.32, 1.51)) (Figure 1, Table 1, Supplementary Table S1). The variance of the IQ scores explained by the CP GPS was 10.4% (Adjusted R2), whereas the baseline covariate model without the GPS variable explained 0.8% of the IQ score variance.
Figure 1.
PheWAS plots of the Educational attainment (EA) and Cognitive Performance (CP) GPS in the Cognitive/behavioral phenome of the WLS participants. (a) PheWAS plot of Educational Attainment (EA) GPS. (b) PheWAS plot of Cognitive Performance (CP) GPS. The cognitive/behavioral phenotypes are presented on the x-axis. The phenotype variables were retrieved from the WLS survey data, primarily from the cognition and leisure activity modules in the 1957, 1992–1994, 2003–2005, and 2011 waves. The red line represents the phenome-wide significance level, og10 of the Bonferroni corrected p-value for multiple testing corrections (α = 0.05/(48 tested phenotypes * 4 GPS) = 2.60 × 10−4). The size of each point is proportional to the effect size of each cognitive capacity GPS-phenotype association.
Table 1.
Phenome-wide association studies (PheWAS) analysis for the four cognitive capacity GPSs with the cognitive/behavioral phenotypes. The table presents only the phenotypes significantly associated with all four cognitive capacity GPSs (Educational attainment (EA), Cognitive Performance (CP), Math Ability (MA), and Highest Math Class (HM)). The top cognitive capacity GPS-phenotype associations are presented from the full PheWAS results (available in Supplementary Table S1). Positive β (effect size) indicates that the genetic contribution to cognitive capacities is positively correlated with a higher score for each measurement module. The significance level of p < 2.60 × 10−4 was used according to the Bonferroni correction.
| Category | Strongest Cognitive Capacity GPS | β | 95% CI* (lower) | 95% CI* (upper) | p | Adjusted R2 | |
|---|---|---|---|---|---|---|---|
| IQ | Cognition | CP GPS | 1.42 | 1.32 | 1.51 | 7.74 × 10−179 | 10.4% |
| High School Class Rank | Cognition | EA GPS | 1.86 | 1.69 | 2.02 | 3.07 × 10−101 | 17.1% |
| EducationalAttainment | Cognition | EA GPS | 1.73 | 1.59 | 1.87 | 1.62 × 10−129 | 12.0% |
| HUI3 Cognition Level (R6)** | Cognition | HM GPS | −0.32 | −0.42 | −0.22 | 2.63 × 10−10 | 1.2% |
| Immediate Recall (R6) | Cognitive Task | EA GPS | 0.80 | 0.64 | 0.96 | 5.29 × 10−22 | 8.6% |
| Similarity (R4) | Cognitive Task | EA GPS | 1.35 | 1.20 | 1.49 | 3.86 × 10−74 | 5.1% |
| Similarity (R5) | Cognitive Task | EA GPS | 1.27 | 1.12 | 1.41 | 5.05 × 10−65 | 5.6% |
| Similarity (R6) | Cognitive Task | EA GPS | 1.36 | 1.22 | 1.51 | 3.59 × 10−74 | 6.1% |
| Number Series Task | Cognitive Task | HM GPS | 0.94 | 0.85 | 1.04 | 2.55 × 10−78 | 7.9% |
| Category Fluency (R5) | Cognitive Task | EA GPS | 0.95 | 0.74 | 1.16 | 2.03 × 10−18 | 4.9% |
| Category Fluency (R6) | Cognitive Task | CP GPS | 0.61 | 0.46 | 0.75 | 3.45 × 10−16 | 6.0% |
| Delayed Recall (R6) | Cognitive Task | CP GPS | 0.45 | 0.34 | 0.56 | 5.00 × 10−15 | 7.9% |
| Digit Ordering (R5) | Cognitive Task | CP GPS | 0.65 | 0.53 | 0.76 | 4.51 × 10−28 | 3.3% |
| Digit Ordering (R6) | Cognitive Task | CP GPS | 0.78 | 0.67 | 0.89 | 8.63 × 10−41 | 5.0% |
| Health Literacy Task (NVS) | Cognitive Task | CP GPS | 0.92 | 0.76 | 1.07 | 2.28 × 10−29 | 10.0% |
| Letter Fluency (R5) | Cognitive Task | EA GPS | 0.73 | 0.56 | 0.90 | 7.98 × 10−17 | 4.2% |
| Letter Fluency (R6) | Cognitive Task | CP GPS | 0.61 | 0.50 | 0.71 | 5.01 × 10−30 | 5.5% |
| OccupationalEducationScore | Occupational Status | EA GPS | 1.22 | 1.08 | 1.36 | 4.77 × 10−61 | 4.8% |
| OccupationalIncomeScore | Occupational Status | EA GPS | 0.90 | 0.76 | 1.03 | 3.50 × 10−37 | 13.4% |
| Watching TV | Leisure Activity | EA GPS | −0.48 | −0.59 | −0.37 | 4.16 × 10−18 | 1.9% |
| Reading | Leisure Activity | EA GPS | 0.50 | 0.40 | 0.60 | 2.03 × 10−21 | 4.8% |
| Fishing/Hunting | Leisure Activity | EA GPS | −0.59 | −0.77 | −0.42 | 1.72 × 10−11 | 15.3% |
| Attendance to cultural events | Leisure Activity | EA GPS | 0.60 | 0.49 | 0.72 | 2.06 × 10−23 | 4.8% |
| Openness | Personality | EA GPS | 0.57 | 0.42 | 0.71 | 2.19 × 10−14 | 4.0% |
* CI = Confidence interval; ** Only for HUI3 Cognition Level module, lower score indicates better cognition level.
The years of educational attainment measure (strongest with EA GPS, p-value = 1.62 × 10−129, β = 1.73, 95% CI = (1.59, 1.87)) and the high school class rank (strongest with EA GPS, p-value = 3.07 × 10−101, β = 1.86, 95% CI = (1.69, 2.02)) also significantly associated with all four cognitive GPSs, following the IQ score. The variance of high school class rank explained by the EA GPS was 17.1% (Adjusted R2), whereas the baseline covariate model without the GPS variable explained 9.2% of the high school class rank variance.
Among the cognitive tasks, the similarities task presented the strongest statistical significance and positive effect size with the cognitive GPSs in all three rounds (strongest with timepoint3 similarities and EA GPS, p-value = 3.59 × 10−74, β = 1.36, 95% CI = (1.22, 1.51)). The cognitive GPSs also showed robust associations with the number series (strongest with HM GPS, p-value = 2.55 × 10−78, β = 0.94, 95% CI = (0.85, 1.04)) and digit ordering tasks (strongest with CP GPS, p-value = 8.63 × 10−41, β = 0.78, 95% CI = (0.67, 0.89)) across the different cognitive GPSs. Several cognitive tasks were also consistently and significantly associated across the cognitive GPSs with positive effect sizes, including letter fluency (strongest association with timpoint3 letter fluency and CP GPS, p-value = 5.01 × 10−30, β = 0.61, 95% CI = (0.50, 0.71)), category fluency (strongest association with timpoint3 category fluency and EA GPS, p-value = 2.03 × 10−18, β = 0.95, 95% CI = (0.74, 1.16)), immediate recall (strongest association with timpoint3 immediate recall and EA GPS, p-value = 5.29 × 10−22, β = 0.80, 95% CI = (0.64, 0.96)), delayed recall (strongest association with timpoint3 delayed recall and CP GPS, p-value = 5.00 × 10−15, β = 0.45, 95% CI = (0.34, 0.56)), NVS Health Literacy assessments (strongest with CP GPS, p-value = 2.28 × 10−29, β = 0.92, 95% CI = (0.76, 1.07)), which indicated that genetic contribution to cognitive abilities was positively correlated with higher cognitive scores for several assessments (Table 1).
3.2.2. Behavioral Phenotypes
Among the Big 5 Personality traits, all the four GPS associations of openness (strongest with EA GPS, p-value = 2.19 × 10−14, β = 0.57, 95% CI = (0.42, 0.71)) met phenome-wide significance with positive effect sizes. In addition to openness, the HM and MA GPSs presented significant associations with neuroticism (strongest with MA GPS, p-value = 1.92 × 10−6, β = −0.32, 95% CI = (−0.45, −0.19)), showing negative effect sizes.
Leisure activities, such as reading books, magazines, newspapers or other reading material (strongest with EA GPS, p-value = 2.03 × 10−21, β = 0.50, 95% CI = (0.40, 0.60)) and attending cultural events (strongest with EA GPS, p-value = 2.06 × 10−23, β = 0.60, 95% CI = (0.49, 0.72)) presented phenome-wide significant associations across the cognitive GPSs with positive directions. Notably, watching TV (strongest with EA GPS, p-value = 4.16 × 10−18, β = −0.48, 95% CI = (−0.59, −0.37)) and fishing/hunting (strongest with EA GPS, p-value = 1.72 × 10−11, β = −0.59, 95% CI = (−0.77, −0.42)) showed significant negative associations with all the cognitive GPSs. Other phenome-wide significant activities included writing letters (strongest with EA GPS, p-value = 2.28 × 10−11, β = 0.32, 95% CI = (0.23, 0.41)), working on crosswords or word games (strongest with CP GPS, p-value = 1.27 × 10−10, β = 0.39, 95% CI = (0.27, 0.51)), and vigorous physical activities (alone) (strongest with EA GPS, p-value = 7.39 × 10−10, β = 0.61, 95% CI = (0.42, 0.80)) (Table 1, Figure 1).
Occupational education scores (strongest with EA GPS, p-value = 4.77 × 10−61, β = 1.22, 95% CI = (1.08, 0.36)) and occupational income scores (strongest with EA GPS, p-value = 3.50 × 10−37, β = 0.90, 95% CI = (0.76, 1.03)) presented positive relationships across all the cognitive GPSs. The association of the spouse’s IQ did not reach phenome-wide significance with any of the cognitive GPSs. The full PheWAS results of the phenome-wide significant associations are available in Supplementary Table S1.
3.3. Cognitive GPSs Correlate with Immediate Recall Changes
Our linear mixed effect model identified a significant age-x-GPS interaction effect on the immediate recall task. All four cognitive capacity GPSs showed significant interactions with the participants’ age (Age: GPS) for the immediate recall test scores (strongest with EA GPS, p-value = 1.79 × 10−3, β = 1.86 × 10−1) (Table 2). Their positive effect sizes suggested that an individual with a higher EA GPS tended to show fewer changes in the cognitive assessments as the individual aged.
Table 2.
Linear mixed-effects model analysis results for the temporal changes of the immediate recall assessment score of the WLS participants according to the cognitive capacity GPSs, including Educational attainment (EA), Cognitive Performance (CP), Math Ability (MA), and Highest Math Class (HM) with the age interaction effect. The effect size of each variable is presented with the 95% confidence interval in parentheses.
| Dependent Variable | ||||
|---|---|---|---|---|
| Immediate Recall | ||||
| Educational Attainment GPS | Cognitive Performance GPS | Math Ability GPS | Highest Math Class Taken GPS | |
| Age | −0.108 *** | −0.161 *** | −0.160 *** | −0.161 *** |
| (−0.149, −0.066) | (−0.185, −0.136) | (−0.185, −0.136) | (−0.185, −0.136) | |
| GPS | 0.204 *** | 0.032 *** | 0.021* | 0.028 ** |
| (0.077, 0.331) | (0.013, 0.051) | (0.002, 0.040) | (0.009, 0.047) | |
| Years of Educational Attainment | 0.069 *** | 0.070 *** | 0.072*** | 0.070 *** |
| (0.061, 0.077) | (0.062, 0.078) | (0.064, 0.079) | (0.062, 0.078) | |
| Sex | 0.148 *** | 0.149 *** | 0.150 *** | 0.149 *** |
| (0.111, 0.186) | (0.111, 0.186) | (0.112, 0.187) | (0.111, 0.187) | |
| Age:GPS interaction | 0.186 *** | 0.027 ** | 0.026 ** | 0.035 *** |
| (0.069, 0.303) | (0.009, 0.046) | (0.007, 0.044) | (0.017, 0.053) | |
| Log likelihood | −15,805.540 | −15,809.350 | −15,812.970 | −15,808.150 |
| Akaike Inf. Crit. | 31,649.08 | 31,656.71 | 31,663.94 | 31,654.31 |
| Bayesian Inf. Crit. | 31,788.53 | 31,796.16 | 31,803.39 | 31,793.75 |
Note: ** p < 0.01; *** p < 0.002, 0.002 = Bonferroni-adjusted Significance Level with 7 cognitive modules * 4 GPS tested.
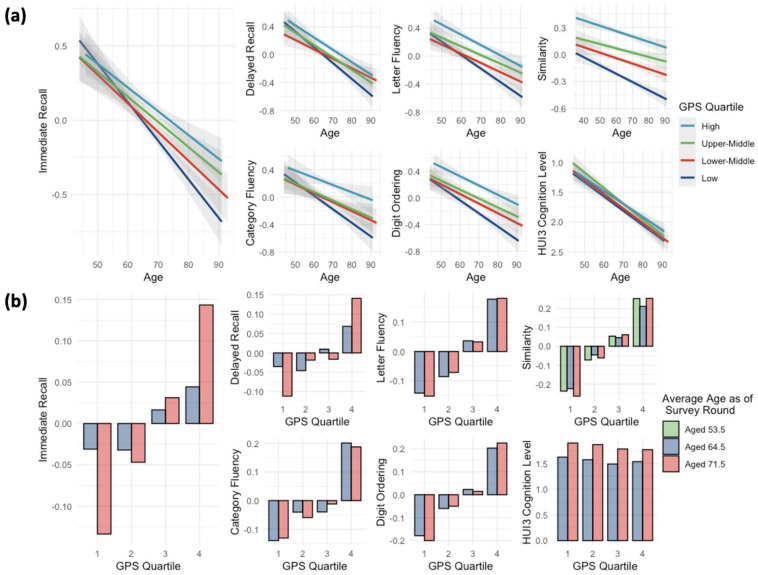
Compared to the individuals in the lowest GPS quartile, individuals in the highest GPS quartile showed a smaller decrease in their immediate recall score changes in later survey rounds. The slope of participants’ age in the lowest GPS quartile (β= −1.97 × 10−1, 95% CI = (−0.231, −0.163), p-value of slope = 8.61 × 10−31) distinctively showed a more intense decrease compared to the highest GPS quartile group (β = −1.24 × 10−1, 95% CI = (−0.158, −0.090), p-value of slope = 6.67 × 10−13) (Figure 2a). The pseudo-R2 of our linear mixed models, explaining the immediate recall by the cognitive capacity GPS, was up to 0.063 with fixed effects and was 0.170 with both fixed and random effects (both with EA GPS).
Figure 2.
Graphical results of the linear mixed-effect model analysis showing that individuals with a higher cognitive GPS presented a slower trajectory of memory decline than those with a lower GPS. Changes in seven cognitive assessments (immediate recall task, category fluency task, digit ordering task, delayed recall task, letter fluency task, similarities task, and health utility index (HUI) level 3 cognition level) and the interaction effects of the CP GPS are shown. The selected seven cognitive assessments were repeatedly administered to 8511 European ancestry individuals between the average age of mid−50s (survey timepoint 1) and mid−70s (survey timepoint 3). (a) Interaction plots showing the different slopes of age−dependent interaction effects by the cognitive capacity GPS on the cognitive assessments. The x−axis indicates the age of the WLS participants at the survey timepoint, while the y-axis indicates each cognitive assessment score (z−scored). The four lines indicate the different slopes of the individuals’ cognitive changes stratified by GPS. The gray area represents the 95% confidence interval of each slope. The similarities task was the only task that was repeatedly administered to the participants since timepoint 1 (Average participants’ age 48.6). (b). Bar plots showing the stratification performance of the cognitive capacity GPS in each cognitive assessment module. Quartile 1 on the x-axis includes the individuals with the lowest cognitive GPS (bottom 25%) and Quartile 4 includes the individuals with the highest. The number on the y-axis represented the average phenotypic scores by each GPS quartile.
To visually depict the degree of cognitive changes according to GPS, we divided the cohort into four quartiles based on the GPS of each individual and analyzed the average phenotypic changes of each group over time. The average immediate recall task scores of the individuals in the highest GPS quartile were 0.044 (z-score) at timepoint 2 (average age of participants 64.5), and this increased to 0.073 (z-score) at timepoint 3 (average age 71.5) (1.65-fold increase). In contrast, the average task scores of the individuals in the lowest GPS quartile were −0.031 (z-score) at timepoint 2 and decreased to −0.077 (z-score) at timepoint 3 (2.48-fold decrease) (Figure 2b).
4. Discussion
In this study, we assessed the genetic influence of general cognitive abilities on cognitive and behavioral phenome using an integrative approach of GPS-based PheWAS on longitudinal observations in the aging population. We hypothesized that the contribution of genetic factors to cognitive capacities is associated with specific cognitive or behavioral phenotypes, and even different degrees of cognitive decline in certain cognitive domains.
Our study identified that the effects of the age-x-GPS interactions were significantly positive across all four cognitive capacity GPSs (Table 2), and individuals with a higher cognitive GPS had a slower trajectory of memory decline than those with a lower GPS (Figure 2a). This result indicates that the portion of the cognitive ability under genetic influence may serve as a ‘buffer’ against memory decline in aging. These observations align well with existing studies on the protective effect of education and intelligence on the occurrence of dementia [50]. A close relationship between early-life education and intelligence with cognitive decline has been reported for dementia and Alzheimer’s disease (AD) [51]. Even though it is not yet clear how early-life education and intelligence moderate the risk for dementia, our findings suggest that individual variations of memory decline are closely associated with the polygenic influences of cognitive abilities.
Among the repeated assessments of the seven cognitive domains with an average interval of 6.5 years, a decline in immediate memory recall during aging significantly correlated with the cognitive GPS. Memory recall, assessed by the immediate and delayed recall tests of words, is hippocampus-dependent [52,53,54]. We did not observe a significant interaction effect in the domain of delayed recall. It is interesting that the discovered genetic protective effect exerted specifically on the hippocampus-related immediate memory recall. There are two implications worth noting. Firstly, given the specificity of the correlations among the various cognitive domains, the genetic protective factor of immediate memory decline may be mediated via the hippocampus. Indeed, the hippocampus is the primary mediator of interventions for cognitive wellness or dementia, such as aerobic fitness [55], diet [56], and medication [57,58,59]. This is closely related to the unique role of the hippocampus in neurogenesis and synaptic plasticity [60,61]. Future research should thus test whether the hippocampus and hippocampal network underlies the genetic projective effect on immediate memory decline, but not in delayed recall, and if so seek to elucidate the mechanisms involved. Secondly, given the role of hippocampal memory impairment in the pathophysiology of AD, our finding may lead to a potential link of the inherited genetic factor of cognitive resilience to the individual differences in hippocampal degeneration, as well as memory decline in AD [4,62]. Testing this link will allow better stratification of AD and monitor the disease’s course by the individual-specific genetic profiles of cognitive resilience.
Our PheWAS identified several phenome-wide associations between cognitive capacity GPSs and cognitive assessments. The similarities task from WAIS, number series task, and digit ordering task showed the strongest associations across the four cognitive capacity GPSs regarding effect size (β) and p-value (Figure 1, Figure S1). These findings suggest that the cognitive components required to successfully complete the similarities, number series, or digit ordering tasks might strongly overlap with the genetic components of cognitive capacities primarily exhibited by the domain of fluid intelligence. The series of cognitive components involved in the similarities and Number series tasks, such as logical memory, symbol search, and reasoning, might be closely linked to early-life cognition, all of which may serve as phenotypic indicators for fluid intelligence. Our findings are backed up by the previous knowledge that fluid intelligence is considered to be more dependent on biological influences and less dependent on past learning experiences than crystallized intelligence [63].
Our analysis identified several phenome-wide significant associations of cognitive capacity GPSs with several early-life cognitive phenotypes, including IQ scores, educational attainment, or high school class rank. The significant genetic association between cognitive capacity and IQ scores or educational attainment has been well established in several GWAS studies on human intelligence [18,22,23,24,25]. The IQ scores of the WLS respondents were derived from the Henmon-Nelson test of mental ability, which is regarded as a general measure of overall intelligence, capturing both fluid and crystallized intelligence.
PheWAS of the behavioral phenome identified several behavioral traits highly related to genetic factors of cognitive capacity. All of the tested cognitive capacity GPSs positively correlated with openness among the Big 5 Personality factors, and some GPSs negatively correlated with neuroticism (Supplementary Table S1). The finding presents an interesting cross-trait hypothesis in which variances in personality dimensions may be partially explained by the genomic components of cognitive capacity or vice versa. ‘Openness’ could be regarded as the attitude and tendency to explore, detect, understand, and appreciate complicated new information patterns through both the senses and in the abstract [64]. Previous studies support our findings, concluding that an overall open-minded attitude might positively influence the long-term variances of cognitive abilities with the willingness to explore [65]. Not only for the Big 5 Personality factors, but overall, we believe that our PheWAS findings could be developed further for examining several cross-trait hypotheses related to human cognition in future studies.
No significant associations between spouse IQ and cognitive abilities were identified, which indicates that the behavioral associations between assortative mating and cognitive abilities are unclear. In addition, a strong relationship between occupational income and several cognitive capacity GPSs was found, which supports existing studies demonstrating a strong association between general mental ability and job performance [66].
A few limitations of this study should be noted. The WLS included two time points for measuring changes in their cognitive assessments with an average interval of 6.5 years. Adding more cognitive measurements through time will strengthen our findings by more thoroughly monitoring cognitive changes over a lifetime. Also, the unexplored impact of other sociodemographic variables such as socioeconomic status, educational environment, lifestyles, or family structure, should be considered to better connect our theoretical findings with the phenome-wide expression of cognitive abilities. In addition, we used European-ancestry-specific summary statistics to construct the cognitive capacity GPSs and applied them to the participants of European ancestry. Researchers the should note that application of our findings to non-European populations could be different, thus the results should be interpreted with caution. Future investigation is needed to elucidate the generalizability of our findings across diverse ancestry groups. Lastly, shared variance among the cognitive and behavioral phenome may interrupt the discovered genotype-phenotype associations. However, our correlation analysis (Supplementary Figure S2), revealed only a few extreme correlations (r > 0.5) among the distinct phenotypes except when comparing measurements to different instances of the same test. The results should be interpreted with caution considering the shared variances of tested phenotypes. Our findings could serve as the first cognitive-phenome map that describes the functional boundaries and behavioral implications of human cognition from a genetic perspective, and the map could be further expanded with the advanced phenotyping of human cognition and behavior traits.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes13081320/s1, Figure S1: Distribution of the cognitive/behavioral phenotypes; Figure S2: Correlation matrix of the cognitive/behavioral phenotypes and the cognitive capacity GPSs. Table S1: Phenome-wide Significant Results of Cognitive Capacity GPSs on the Cognitive/Behavioral Phenome (p < 2.60 × 10−4, Bonferroni corrected); File S1: Supplementary Method: survey Instruments for creating the Cognitive/Behavioral Phenome [37,38,39,40,41,42,43,44,45,67,68,69,70,71,72].
Author Contributions
Y.Y.J., J.C., J.F. and M.G.H. designed the study; Y.Y.J., J.F. and M.G.H. acquired genotype and/or phenotype data; Y.Y.J. performed statistical analyses; Y.Y.J., J.C., J.F. and M.G.H. interpreted the results; Y.Y.J. and J.C. drafted the manuscript; Y.Y.J., J.C., J.F. and M.G.H. critically reviewed the manuscript; Y.Y.J. and M.G.H. obtained the funding. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Seoul National University (E2009/002-007 on 14 September 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data/analyses presented in the current publication have been deposited in and are available from the dbGaP database under dbGaP accession phs001157.v1.p1.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) (R01AG041868) and the Basic Science Research Program of the National Research Foundation of Korea (NRF) (2021R1I1A1A01054995).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deary I.J., Yang J., Davies G., Harris S.E., Tenesa A., Liewald D., Luciano M., Lopez L.M., Gow A.J., Corley J., et al. Genetic contributions to stability and change in intelligence from childhood to old age. Nature. 2012;482:212–215. doi: 10.1038/nature10781. [DOI] [PubMed] [Google Scholar]
- 2.Sharp E.S., Gatz M. Relationship between education and dementia: An updated systematic review. Alzheimer Dis. Assoc. Disord. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002;8:448–460. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- 4.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melikyan Z.A., Corrada M.M., Leiby A.M., Sajjadi S.A., Bukhari S., Montine T.J., Kawas C.H. Cognitive resilience to three dementia-related neuropathologies in an oldest-old man: A case report from The 90+ Study. Neurobiol. Aging. 2022;116:12–15. doi: 10.1016/j.neurobiolaging.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton A., Yaroush R., Staal M., Bourne L., Jr. Biobehavioral Resilience to Stress. Routledge; London, UK: 2008. Cognitive Performance and Resilience to Stress; pp. 259–299. [Google Scholar]
- 8.Yu L., Petyuk V.A., Gaiteri C., Mostafavi S., Young-Pearse T., Shah R.C., Buchman A.S., Schneider J.A., Piehowski P.D., Sontag R.L., et al. Targeted brain proteomics uncover multiple pathways to Alzheimer’s dementia. Ann. Neurol. 2018;84:78–88. doi: 10.1002/ana.25266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu L., Tasaki S., Schneider J.A., Arfanakis K., Duong D.M., Wingo A.P., Wingo T.S., Kearns N., Thatcher G.R.J., Seyfried N.T., et al. Cortical Proteins Associated With Cognitive Resilience in Community-Dwelling Older Persons. JAMA Psychiatry. 2020;77:1172–1180. doi: 10.1001/jamapsychiatry.2020.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews S.J., Das D., Cherbuin N., Anstey K.J., Easteal S. Association of genetic risk factors with cognitive decline: The PATH through life project. Neurobiol. Aging. 2016;41:150–158. doi: 10.1016/j.neurobiolaging.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Morley J.F., Xie S.X., Hurtig H.I., Stern M.B., Colcher A., Horn S., Dahodwala N., Duda J.E., Weintraub D., Chen-Plotkin A.S., et al. Genetic influences on cognitive decline in Parkinson’s disease. Mov. Disord. 2012;27:512–518. doi: 10.1002/mds.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raj T., Chibnik L.B., McCabe C., Wong A., Replogle J.M., Yu L., Gao S., Unverzagt F.W., Stranger B., Murrell J., et al. Genetic architecture of age-related cognitive decline in African Americans. Neurol. Genet. 2017;3:e125. doi: 10.1212/NXG.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zammit A.R., Yu L., Petyuk V., Schneider J.A., De Jager P.L., Klein H.U., Bennett D.A., Buchman A.S. Cortical Proteins and Individual Differences in Cognitive Resilience in Older Adults. Neurology. 2022;98:e1304–e1314. doi: 10.1212/WNL.0000000000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mostafavi S., Gaiteri C., Sullivan S.E., White C.C., Tasaki S., Xu J., Taga M., Klein H.-U., Patrick E., Komashko V. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nat. Neurosci. 2018;21:811–819. doi: 10.1038/s41593-018-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furlong L.I. Human diseases through the lens of network biology. Trends Genet. 2013;29:150–159. doi: 10.1016/j.tig.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Hindorff L.A., Sethupathy P., Junkins H.A., Ramos E.M., Mehta J.P., Collins F.S., Manolio T.A. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl. Acad. Sci. USA. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarti A., Turner T.N. Revealing rate-limiting steps in complex disease biology: The crucial importance of studying rare, extreme-phenotype families. Bioessays. 2016;38:578–586. doi: 10.1002/bies.201500203. [DOI] [PubMed] [Google Scholar]
- 18.Lee J.J., Wedow R., Okbay A., Kong E., Maghzian O., Zacher M., Nguyen-Viet T.A., Bowers P., Sidorenko J., Karlsson Linner R., et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 2018;50:1112–1121. doi: 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rietveld C.A., Medland S.E., Derringer J., Yang J., Esko T., Martin N.W., Westra H.J., Shakhbazov K., Abdellaoui A., Agrawal A., et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science. 2013;340:1467–1471. doi: 10.1126/science.1235488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okbay A., Beauchamp J.P., Fontana M.A., Lee J.J., Pers T.H., Rietveld C.A., Turley P., Chen G.B., Emilsson V., Meddens S.F., et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533:539–542. doi: 10.1038/nature17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plomin R., von Stumm S. The new genetics of intelligence. Nat. Rev. Genet. 2018;19:148–159. doi: 10.1038/nrg.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trampush J.W., Yang M.L.Z., Yu J., Knowles E., Davies G., Liewald D.C., Starr J.M., Djurovic S., Melle I., Sundet K., et al. GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: A report from the COGENT consortium. Mol. Psychiatry. 2017;22:1651–1652. doi: 10.1038/mp.2017.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sniekers S., Stringer S., Watanabe K., Jansen P.R., Coleman J.R.I., Krapohl E., Taskesen E., Hammerschlag A.R., Okbay A., Zabaneh D., et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat. Genet. 2017;49:1107–1112. doi: 10.1038/ng.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies G., Marioni R.E., Liewald D.C., Hill W.D., Hagenaars S.P., Harris S.E., Ritchie S.J., Luciano M., Fawns-Ritchie C., Lyall D., et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N = 112,151) Mol. Psychiatry. 2016;21:758–767. doi: 10.1038/mp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies G., Armstrong N., Bis J.C., Bressler J., Chouraki V., Giddaluru S., Hofer E., Ibrahim-Verbaas C.A., Kirin M., Lahti J., et al. Genetic contributions to variation in general cognitive function: A meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53,949) Mol. Psychiatry. 2015;20:183–192. doi: 10.1038/mp.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies G., Tenesa A., Payton A., Yang J., Harris S.E., Liewald D., Ke X., Le Hellard S., Christoforou A., Luciano M., et al. Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol. Psychiatry. 2011;16:996–1005. doi: 10.1038/mp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouchard T.J., Jr., McGue M. Genetic and environmental influences on human psychological differences. J. Neurobiol. 2003;54:4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- 28.Rietveld C.A., Esko T., Davies G., Pers T.H., Turley P., Benyamin B., Chabris C.F., Emilsson V., Johnson A.D., Lee J.J., et al. Common genetic variants associated with cognitive performance identified using the proxy-phenotype method. Proc. Natl. Acad. Sci. USA. 2014;111:13790–13794. doi: 10.1073/pnas.1404623111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trampush J.W., Lencz T., Knowles E., Davies G., Guha S., Pe’er I., Liewald D.C., Starr J.M., Djurovic S., Melle I., et al. Independent evidence for an association between general cognitive ability and a genetic locus for educational attainment. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015;168B:363–373. doi: 10.1002/ajmg.b.32319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herd P., Carr D., Roan C. Cohort profile: Wisconsin longitudinal study (WLS) Int. J. Epidemiol. 2014;43:34–41. doi: 10.1093/ije/dys194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carney A.K. Wisconsin longitudinal study. Int. J. Aging Hum. Dev. 2014;79:332–333. doi: 10.1177/0091415015574179. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy S., Das S., Kretzschmar W., Delaneau O., Wood A.R., Teumer A., Kang H.M., Fuchsberger C., Danecek P., Sharp K., et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016;48:1279–1283. doi: 10.1038/ng.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okbay A., Benjamin D., Visscher P. Documentation for the Data of Educational Attainment, Cognitive Performance and Math-Related Scores. [(accessed on 1 June 2021)]. Available online: https://www.ssc.wisc.edu/wlsresearch/documentation/GWAS/Lee_et_al_(2018)_PGS_WLS.pdf.
- 34.Center T.U.o.W.G.A. A Longitudinal Resource for Genetic Research in Behavioral and Health Sciences—Imputation Report Wisconsin Longitudinal Study Nov 2, 2016. 2016. [(accessed on 1 June 2021)]. Available online: https://www.ssc.wisc.edu/wlsresearch/documentation/GWAS/Herd_1000G_IMPUTE2report.pdf.
- 35.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilhjalmsson B.J., Yang J., Finucane H.K., Gusev A., Lindstrom S., Ripke S., Genovese G., Loh P.R., Bhatia G., Do R., et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am. J. Hum. Genet. 2015;97:576–592. doi: 10.1016/j.ajhg.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauser R.M., Palloni A. Adolescent IQ and Survival in the Wisconsin Longitudinal Study. J. Gerontol. Ser. B. 2011;66B((Suppl. S1)):i91–i101. doi: 10.1093/geronb/gbr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henmon V. Henmon-Nelson Tests of Mental Ability, High School Examination-Grades 7 to 12-Forms a, b, and c. Teacher’s Manual. Houghton-Mifflin Company; Boston, MA, USA: 1946. [Google Scholar]
- 39.Henmon V.A.C., Holt F.O. A Report on the Administration of Scholastic Aptitude Tests to 34,000 High. School Seniors in Wisconsin in 1929 and 1930: Prepared for the Committee on Cooperation, Wisconsin Secondary Schools and Colleges. Bureau of Guidance and Records of the University of Wisconsin; Madison, WI, USA: 1931. [Google Scholar]
- 40.John O.P., Donahue E.M., Kentle R.L. The Big Five Inventory—Versions 4a and 54. University of California, Berkeley, Institute of Personality; Berkeley, CA, USA: 1991. [Google Scholar]
- 41.Halpin B. Educational homogamy in Ireland and Britain: Trends and patterns. Br. J. Sociol. 2003;54:473–496. doi: 10.1080/0007131032000143546. [DOI] [PubMed] [Google Scholar]
- 42.Mascie-Taylor C.G., Vandenberg S.G. Assortative mating for IQ and personality due to propinquity and personal preference. Behav. Genet. 1988;18:339–345. doi: 10.1007/BF01260934. [DOI] [PubMed] [Google Scholar]
- 43.Watson D., Klohnen E.C., Casillas A., Simms E.N., Haig J., Berry D.S. Match makers and deal breakers: Analyses of assortative mating in newlywed couples. J. Pers. 2004;72:1029–1068. doi: 10.1111/j.0022-3506.2004.00289.x. [DOI] [PubMed] [Google Scholar]
- 44.Hur Y.M. Assortive mating for personaltiy traits, educational level, religious affiliation, height, weight, adn body mass index in parents of Korean twin sample. Twin Res. 2003;6:467–470. doi: 10.1375/136905203322686446. [DOI] [PubMed] [Google Scholar]
- 45.Pan Y., Wang K. Spousal concordance in academic achievements and IQ a principal component analysis. Open J. Psychiatry. 2011;1:15–19. doi: 10.4236/ojpsych.2011.12003. [DOI] [Google Scholar]
- 46.Bates D., Mächler M., Bolker B., Walker S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 47.Johnson P.C.D. Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods Ecol. Evol. 2014;5:944–946. doi: 10.1111/2041-210X.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakagawa S., Johnson P.C., Schielzeth H. The coefficient of determination R 2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface. 2017;14:20170213. doi: 10.1098/rsif.2017.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa S., Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013;4:133–142. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- 50.Plassman B.L., Welsh K.A., Helms M., Brandt J., Page W.F., Breitner J.C. Intelligence and education as predictors of cognitive state in late life: A 50-year follow-up. Neurology. 1995;45:1446–1450. doi: 10.1212/WNL.45.8.1446. [DOI] [PubMed] [Google Scholar]
- 51.Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 1993;43:13–20. doi: 10.1212/WNL.43.1_Part_1.13. [DOI] [PubMed] [Google Scholar]
- 52.Griffith H.R., Pyzalski R.W., O’Leary D., Magnotta V., Bell B., Dow C., Hermann B., Seidenberg M. A controlled quantitative MRI volumetric investigation of hippocampal contributions to immediate and delayed memory performance. J. Clin. Exp. Neuropsychol. 2003;25:1117–1127. doi: 10.1076/jcen.25.8.1117.16731. [DOI] [PubMed] [Google Scholar]
- 53.Golomb J., Kluger A., de Leon M.J., Ferris S.H., Convit A., Mittelman M.S., Cohen J., Rusinek H., De Santi S., George A.E. Hippocampal formation size in normal human aging: A correlate of delayed secondary memory performance. Learn. Mem. 1994;1:45–54. doi: 10.1101/lm.1.1.45. [DOI] [PubMed] [Google Scholar]
- 54.Hackert V.H., den Heijer T., Oudkerk M., Koudstaal P.J., Hofman A., Breteler M.M. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage. 2002;17:1365–1372. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- 55.Erickson K.I., Prakash R.S., Voss M.W., Chaddock L., Hu L., Morris K.S., White S.M., Wojcicki T.R., McAuley E., Kramer A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Latimer C.S., Brewer L.D., Searcy J.L., Chen K.C., Popovic J., Kraner S.D., Thibault O., Blalock E.M., Landfield P.W., Porter N.M. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc. Natl. Acad. Sci. USA. 2014;111:E4359–E4366. doi: 10.1073/pnas.1404477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goveas J.S., Xie C., Ward B.D., Wu Z., Li W., Franczak M., Jones J.L., Antuono P.G., Li S.J. Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer’s disease patients treated with donepezil assessed by resting-state fMRI. J. Magn. Reson. Imaging. 2011;34:764–773. doi: 10.1002/jmri.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin K., Peel A.L., Mao X.O., Xie L., Cottrell B.A., Henshall D.C., Greenberg D.A. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnan K.R., Charles H.C., Doraiswamy P.M., Mintzer J., Weisler R., Yu X., Perdomo C., Ieni J.R., Rogers S. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am. J. Psychiatry. 2003;160:2003–2011. doi: 10.1176/appi.ajp.160.11.2003. [DOI] [PubMed] [Google Scholar]
- 60.Toda T., Gage F.H. Review: Adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 2018;373:693–709. doi: 10.1007/s00441-017-2735-4. [DOI] [PubMed] [Google Scholar]
- 61.Snyder J.S., Kee N., Wojtowicz J.M. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 62.Scarmeas N., Albert S.M., Manly J.J., Stern Y. Education and rates of cognitive decline in incident Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2006;77:308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akshoomoff N., Beaumont J.L., Bauer P.J., Dikmen S.S., Gershon R.C., Mungas D., Slotkin J., Tulsky D., Weintraub S., Zelazo P.D., et al. VIII. NIH Toolbox Cognition Battery (CB): Composite scores of crystallized, fluid, and overall cognition. Monogr. Soc. Res. Child Dev. 2013;78:119–132. doi: 10.1111/mono.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeYoung C.G. APA Handbook of Personality and Social Psychology, Volume 4: Personality Processes and Individual Differences. APA Handbooks in Psychology®. American Psychological Association; Washington, DC, USA: 2015. Openness/intellect: A dimension of personality reflecting cognitive exploration; pp. 369–399. [Google Scholar]
- 65.DeYoung C.G., Peterson J.B., Higgins D.M. Sources of openness/intellect: Cognitive and neuropsychological correlates of the fifth factor of personality. J. Pers. 2005;73:825–858. doi: 10.1111/j.1467-6494.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt F.L., Hunter J. General mental ability in the world of work: Occupational attainment and job performance. J. Pers. Soc. Psychol. 2004;86:162–173. doi: 10.1037/0022-3514.86.1.162. [DOI] [PubMed] [Google Scholar]
- 67.Wechsler D. The Wechsler Adult Intelligence Acale—Revised. The Psychological Corporation; New York, NY, USA: 1981. [Google Scholar]
- 68.Wechsler D. Administration and Scoring Manual. The Psychological Corporation; New York, NY, USA: 1997. WAIS-III, Wechsler Adult Intelligence Scal. [Google Scholar]
- 69.Hubbard R.C. Newest Vital Sign (NVS) [(accessed on 1 June 2021)]. Available online: https://pfe-pfizercom-prod.s3.amazonaws.com/health/nvs_flipbook_english_final.pdf.
- 70.Maenner M.J., Greenberg J.S., Mailick M.R. Association Between Low IQ Scores and Early Mortality in Men and Women: Evidence from a Population-Based Cohort Study. Am. J. Intellect. Dev. Disabil. 2015;120:244–257. doi: 10.1352/1944-7558-120.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henmon V.A.C., Nelson M.J. Manual for Administration. Vol. 171. Houghton-Mifflin Company; Boston, MA, USA: 1954. The Henmon-Nelson tests of mental ability; pp. 297–318. [Google Scholar]
- 72.Willingham W.W., Pollack J.M., Lewis C. Grades and test scores: Accounting for observed differences. J. Educ. Meas. 2002;39:1–37. doi: 10.1111/j.1745-3984.2002.tb01133.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data/analyses presented in the current publication have been deposited in and are available from the dbGaP database under dbGaP accession phs001157.v1.p1.