Abstract
BACKGROUND
Irritable bowel syndrome (IBS) is a highly prevalent gastrointestinal disorder in children and adults, which increased over the past twenty years. The Mediterranean diet is a well-known diet full of antioxidants and anti-inflammatory ingredients.
AIM
To evaluate the safety, tolerability, and effects of adherence to the Mediterranean diet on disease patterns in children and adolescents with IBS.
METHODS
This prospective, cross-sectional case-controlled study included 100 consecutive IBS patients diagnosed according to Rome IV criteria, aged 12-18 years. Patients were subdivided into two groups (50 patients each); Group I received a Mediterranean diet, and Group II on their regular diet for six months. Besides IBS scores (IBS-SSS, IBS-QoL, and total score), different clinical and laboratory parameters were evaluated at the start and end of the study.
RESULTS
The Mediterranean diet was safe and well-tolerated in IBS patients. IBS children and adolescents with good adherence to the Mediterranean diet (KIDMED Score ≥ 8 points); group I showed significant improvement in IBS scores. IBS-SSS in the Mediterranean diet group was 237.2 ± 65 at the beginning of the study and decreased to 163.2 ± 33.8 at the end of the study (P < 0.001). It did not show a significant improvement in the group with a regular diet (248.3 ± 71.1 at the beginning of the study compared to 228.5 ± 54.3 at the study end with P < 0.05). The mean IBS-SSS in the Mediterranean diet group significantly improved compared with the group with a regular diet. Mean IBS-QoL in group I improved from 57.3 ± 12.9 at the start of the study to 72.4 ± 11.2 at the study end (P < 0.001) and significantly improved when compared to its level in group II at the study end (59.2 ± 12.7 with P < 0.001), while group II showed no significant improvement in IBS-QoL at the study end when compared to the beginning of the study (59.2 ± 11.7 with P >0.05). The mean total IBS score in group I became 28.8 ± 11.2 at the end of our study compared to 24.1 ± 10.4 at the start (P < 0.05) and significantly improved when compared to its level in group II at the end of the study (22.1 ± 12.5 with P < 0.05), while in group II, non-significant improvement in the total score at the end of our study compared to its mean level at the start of the study (22.8 ± 13.5 with P > 0.05).
CONCLUSION
The Mediterranean diet was safe and associated with significant improvement in IBS scores in children and adolescent patients with IBS.
Keywords: Mediterranean diet, Irritable bowel syndrome, Children and adolescents, Safety, Tolerability
Core Tip: Diet is an essential factor in the pathogenesis and management of irritable bowel syndrome (IBS) patients. Studies involving different modalities of diets in IBS are lacking with contradictory results. The Mediterranean diet is a well-known balanced diet with anti-inflammatory properties. We prospectively studied 100 pediatric and adolescent patients with IBS, divided into two equal groups: group I received a Mediterranean diet, and group II had a regular diet for six months. Different clinical and laboratory parameters besides IBS scores were evaluated at the start and end of the study. The current study showed that the Mediterranean diet is a safe and effective low-cost new strategy in pediatric and adolescent patients with IBS.
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the highly prevalent gastrointestinal disorders in children and adults, which increased over the past twenty years. It has a significant effect on the lives of affected children and their families and poses a substantial burden on healthcare systems[1]. It is classified as one of the functional gastrointestinal disorders; characterized by varying degrees of abdominal pain or discomfort, abdominal distension, altered bowel habits, and flatulence, and can be divided into four subtypes; IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), IBS with mixed bowel habits (IBS-M) and IBS unsubtyped (IBS-U)[2].
IBS pathogenesis is a poorly recognized disorder. Many theories were proposed to explain its pathogenesis[3]. It could be related to low-grade inflammation of the bowel mucosa. Dysbiosis with dysregulation of brain-gut axis function and bacterial overgrowth are commonly seen in IBS and are accepted theories that can explain the occurrence of IBS. Immune activation and visceral hypersensitivity are possible pathogenetic mechanisms associated with disturbed gastrointestinal motility[4,5]. A possible mechanism is dysregulated neurotransmitters such as cholecystokinin, vasoactive intestinal peptides, and serotonin with the abnormal gut-brain axis[6]. Moreover, food allergy or vitamin deficiency may play a role[7,8].
A few potential therapeutic modalities are available to treat children with IBS, and fewer of them have shown some benefits. Additionally, most of the described pathophysiological mechanisms and treatment choices are adult studies. These have surfaced as challenges when dealing with pediatric IBS, and they need to be overcome for the effective management of children with IBS[9].
The Mediterranean diet is characterized by many vegetables, fruits, bread, and other forms of cereal, rice, beans, and nuts. It also includes virgin olive oil as the principal source of fat, moderate amounts of dairy products (basically cheese and yogurt), moderate amounts of fish, and red meat in low quantities. The value of this dietary model is related to being a balanced and diverse diet that can provide most of the recommended macronutrients in proper proportions. It is characterized by a low content of saturated fatty acids, high monounsaturated fatty acids, high amounts of fiber, complex carbohydrates, and essential antioxidants[10]. They play a crucial role in preventing cardiovascular and cerebrovascular diseases, diabetes, obesity, neurodegenerative illnesses, and cancer[11,12].
Data suggests that the Mediterranean diet might be beneficial in alleviating the functional gastrointestinal symptoms through increased fiber and antioxidant consumption and a low intake of saturated fats and oligosaccharides[13,14]. However, information about the compliance and efficacy of the Mediterranean diet in children and adolescents with IBS is lacking. We aimed to study the effects of the Mediterranean diet on the symptoms of IBS in children and adolescents.
MATERIALS AND METHODS
We designed the study to evaluate the Mediterranean diet's tolerability, safety, and potential efficacy in children and adolescent patients with IBS. After explaining the study design, goals, and rights, all patients/caregivers provided written consent or permission. We conducted the study according to the Helsinki Declaration of 1975. This prospective randomized, case-controlled study was carried out in the Pediatric and Gastroenterology departments, Tanta University Hospital, Egypt, between September 2020 and July 2021. We included one hundred consecutive children and adolescents with IBS diagnosed according to Rome IV criteria[15], aged 12-18 years old. We divided the patients into two groups (50 patients each); the group I received a Mediterranean diet with good adherence (KIDMED Score ≥ 8 points), and Group II received a regular diet. Allocation to the groups was done using simple randomization. The study was not blind as we need to do patient and family education about the Mediterranean diet.
Inclusion criteria
Patients aged 12–18 years were diagnosed with childhood irritable bowel syndrome according to ROME IV criteria[15].
Exclusion criteria
Exclusion criteria include recent changes in IBS therapy, gastrointestinal infection, history of gut surgery or radiation, celiac disease, overweight or underweight according to the centile curve[16], chronic diseases such as renal failure or diabetes mellitus, and patients not adherent to the dietary protocol.
Study intervention: During the study period (6 mo), the patients in group I had the Mediterranean diet as a sole intervention besides their regular treatment. Patients (and their caregivers) received one-to-one education and counseling by a dietitian trained in the Mediterranean diet during each visit. Before each visit, patients and their families completed a three-day food intake record to help assure compliance with the diet. We closely followed up with the patients with the study team, including the dietitian, research pediatrician, and research gastroenterologist, for questions and problem intervention during the study period.
All participants had complete history taking, including dietetic history, thorough clinical examination, and anthropometric measurements such as height, weight, and body mass index (BMI). All participants with IBS filled out the IBS symptoms severity score (IBSSSS) questionnaire[17]. IBSSSS consists of 5 items (severity and frequency of abdominal pain, bloating, satisfaction with bowel habits, and quality of life) collected by direct interview using the visual analog scale (VAS). We scored each item on a scale from 0–to 100. A score below 75 means that the patient is in remission. The mild, moderate, and severe boundary scores are 75–175, 175–300, and above 300. A decrease in the score of 50 or more was considered a significant improvement. The patients also had an IBS quality of life (IBSQoL) questionnaire[18]. Effectiveness, reliability, and sensitivity of IBSSSS to treatment are verified by IBSQoL, which has 34 items, using a 5-choice scale (0–4). We transformed the summed total score to a 100-scale ranging from 0 (lowest) to 100 (highest). A total score of IBS measured by a VAS of 100 scales is used to evaluate the real IBS symptoms' impact on the quality of life, which was done at the same frequency as IBSSSS and IBSQoL scores.
The patients also had routine laboratory investigations such as complete blood count (CBC), erythrocyte sedimentation rate (ESR), serum calcium, random blood sugar, renal and hepatic functions, serum proteins, urine, and stool analysis. Fecal calprotectin was measured, and fecal blood in the stool was done in all included patients to exclude patients with inflammatory bowel disease. Follow-up visits were done at one, three, and six months. All IBS scores, laboratory parameters, and growth parameters (body weight, height, and BMI) were repeated at the end of our study.
KIDMED test: The Mediterranean diet quality index for children and teenagers (KIDMED test) is an instrument developed and validated by Serra-Majem et al[19]. It is used to evaluate the adherence of children and youths to the Mediterranean diet. The index ranges from 0 to 12. It is based on a 16-questions test that can be self-administered or conducted by interview (pediatrician, dietitian, etc.). Questions indicating a negative association concerning the Mediterranean diet are assigned a value of -1, and those with a positive aspect are given a value of +1. The total values from the processed test are categorized into three degrees: (1) ≥ 8, optimal adherence to the Mediterranean diet; (2) 4–7, adherence improvement is needed to adjust intake to Mediterranean patterns; and (3) ≤ 3, poor adherence to the Mediterranean diet[20].
The primary outcome of the current study was to assess the effects of adherence to the Mediterranean diet for six months on the IBS symptoms and severity score. The secondary outcome was to evaluate the safety and tolerability of the Mediterranean diet in children and adolescents with IBS.
Ethical considerations
This clinical study was conducted following the principles of the Declaration of Helsinki. At the beginning of the study, all subjects (and caregivers) were fully informed about the study objectives and their rights. They signed a written informed consent to participate in the study. The local Ethical Committee approved the study. The study is registered with the registration number PACTR202008711997928. All authors had access to the study data and have reviewed and approved this final manuscript.
Statistical analysis
A sample size of 45 IBS patients in each group was required to achieve a power of more than 80 to detect a difference of 60 in the mean of the primary outcome point (IBSSSS) based on a previous study[21]. We recruited more than the estimated sample size, expecting a possible lack of adherence to the Mediterranean diet or withdrawal from the study, undermining our results. We collected and analyzed the data using SPSS version 17 (SPSS Inc., Chicago, IL, United States). We expressed the continuous data as mean ± SD. We used the paired t-test to compare the same group before and after treatment. An independent t-test was used for comparison between group 1 and group 2. We expressed the categorical variables as numbers and percentages and analyzed them using the Chi-square test. We used the Pearson correlation to evaluate the correlation between the Mediterranean diet with IBS scores. The statistical significance was defined as P < 0.05.
RESULTS
This study included 100 children and adolescent patients with IBS aged 12-18 years; divided into two groups included 50 patients. Group-I had 27 males and 23 females with a mean age of 15.5 ± 1.8 years, and group II had 26 males and 24 females with a mean age of 15.2 ± 1.5 years. Before the study, the average duration of IBS symptoms was 34.4 ± 9.1 mo in group I and 35.3 ± 9.8 mo in group II. We illustrated the demographic, growth parameters, clinical subtypes, IBS severity, treatment drugs, and IBS scores in both groups in Table 1. We found no significant differences between the two groups in all measured parameters at the start of our study.
Table 1.
Demographic data and clinical characteristics in irritable bowel syndrome patients' groups before the start of the Mediterranean diet
|
Variable
|
Group I (Mediterranean diet) (n = 50)
|
Group II (n = 50)
|
P
value
|
| Age (yr) | 15.50 ± 1.80 | 15.2 ± 1.5 | 0.881 |
| Sex (M: F) | 27:23 | 26:24 | 0.902 |
| Height (z-score) | 0.04 ± 1 | 0.04 ± 1.00 | 0.661 |
| Weight (z-score) | 0.14 ± 0.99 | 0.12 ± 0.89 | 0.831 |
| BMI (z-score) | 0.18 ± 0.88 | 0.17 ± 1.02 | 0.771 |
| IBS subtypes | 0.472 | ||
| IBS-C | 22 (44 %) | 21 (42 %) | |
| IBS-D | 20 (40 %) | 21 (42 %) | |
| IBS-M | 4 (8 %) | 5 (10 %) | |
| IBS-U | 2 (4 %) | 3 (6 %) | |
| IBS severity | 0.552 | ||
| Mild | 12 (24%) | 14 (28 %) | |
| Moderate | 33 (66%) | 31 (62 %) | |
| Severe | 5 (10%) | 5 (10 %) | |
| Duration of IBS symptoms (mo) | 34.40 ± 9.10 | 35.30 ± 9.80 | 0.581 |
| Treatment drugs3 | 0.661 | ||
| Gastroprokinetic | 50 | 50 | |
| Antidepressants | 11 | 12 | |
| Antacids | 18 | 20 | |
| Antibioticsprobiotics | 924 | 723 | |
| IBS-SSS | 237.20 ± 65 | 248.30 ± 71.10 | 0.681 |
| IBS-QoL | 57.30 ± 12.90 | 59.10 ± 11.70 | 0.711 |
| Total score | 24.10 ± 10.40 | 22.80 ± 13.50 | 0.821 |
Independent t-test.
Chi-square test.
Treatment drugs for one month before starting the study and during the whole study period.
BMI: Body mass index; IBS: Irritable bowel syndrome; IBS-C: IBS constipation; IBS-D: IBS diarrhea; IBS-M: IBS mixed; IBS-U: IBS unsubtyped; IBS-SSS: IBS symptoms severity score questionnaire; IBS-QoL: IBS quality of life questionnaire.
Basic laboratory data in all patients done at start of our study (Table 2) with non-significant differences between both groups regarding serum albumin(4.1 ± 0.9 g/dL in Group-I and 4.3 ± 0.88 in Group-II with P = 0.93) ,serum triglycerides(120.7 ± 45.6 mg/dL in Group I and 112.9 ± 49.4 in Group-II with P = 0.44), serum cholesterol (154.0 ± 36.6 mg/dL in Group I and 163.6 ± 44.1 in Group II with P = 0.35),random blood glucose level (86.20 ± 20.20 mg/dL in Group I and 85.7 ± 9.70 in Group II with P = 0.91), hemoglobin level (13.10 ± 1.60 g/dL in Group-I and 13.6 ± 1.80 in Group II with P = 0.47). Fecal calprotectin was normal in both groups (12 ± 9.10 μg/g in group I and 11 ± 8.80 in Group II with p 0.52), and it was done to exclude patients with inflammatory bowel disease.
Table 2.
Laboratory data of all patients at the start of the study
|
Variable
|
Group I (Mediterranean diet) (n = 50)
|
Group II (n = 50)
|
P
value
|
| Albumin (g/dL) | 4.10 ± 0.90 | 4.30 ± 0.88 | 0.93 |
| Triglycerides (mg/dL) | 120.70 ± 45.60 | 112.90 ± 49.40 | 0.44 |
| Cholesterol (mg/dL) | 154.00 ± 36.60 | 163.60 ± 44.10 | 0.35 |
| Glucose (mg/dL) | 86.20 ± 20.20 | 85.70 ± 9.70 | 0.91 |
| Hemoglobin (g/dL) | 13.10 ± 1.60 | 13.60 ± 1.80 | 0.47 |
| Fecal calprotectin (μg/g) n < 50 | 12 ± 9.10 | 11 ± 8.80 | 0.52 |
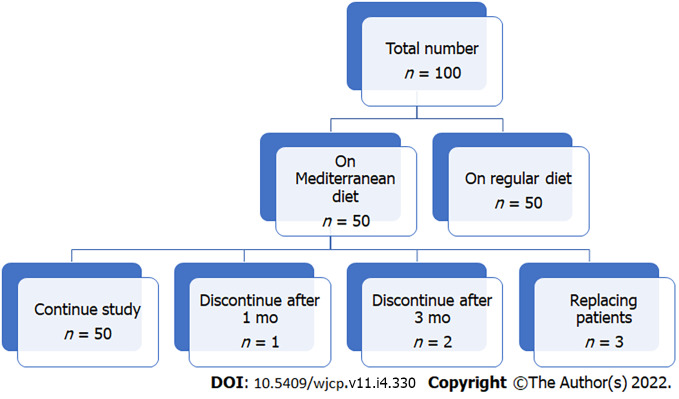
The Mediterranean diet was well tolerated in IBS patients. Only three patients could not tolerate it and were withdrawn from the study (one after one month and two patients after three months, replaced by other patients; Figure 1). No adverse events regarding the Mediterranean diet were reported as reflected by non-significant changes in growth parameters (height, weight, and BMI), laboratory parameters (serum albumin, triglycerides, cholesterol, glucose, and hemoglobin levels) at the end of our study when compared to the same parameters at the start of the research and when compared to group-II at the end of our study (Table 3). At the end of our research, there was a significant improvement in all IBS scores in IBS patients who received a Mediterranean diet (group I) compared to such scores at the start of the study and when compared to group II at the end of the study (Table 3). The mean IBS-SSS in group-I became 163.20 ± 33.80 at the study end compared to 237.20 ± 65 at the start (P < 0.001), with significant improvement when compared to group-II at the study end (228.50 ± 54.30) with P < 0.001, while in group-II, there was no substantial improvement in IBS-SSS at the study end compared to its mean level at the start of the study (228.50 ± 54.30 vs. 248.30 ± 71.10 with P = 0.29). Mean IBS-QoL in group-I became 72.40 ± 11.20 at the end of our study compared to 57.30 ± 12.90 at the start (P < 0.001) and significantly improved when compared to its level in group II at the end of the study (59.20 ± 12.70 with P < 0.001), while in group II, non-significant improvement in IBS-QoL at the end of our study compared to its mean level at the start of the study (59.20 ± 12.70 vs 59.10 ± 11.70 with P = 0.77). The mean total score in group I became 28.80 ± 11.20 at the end of our study compared to 24.10 ± 10.40 at the start (P = 0.02) and significantly improved when compared to its level in group II at the end of the study (22.10 ± 12.50) with P = 0.03, while in group II, non-significant improvement in the total score at the end of our study compared to its mean level at the start of the study (22.10 ± 12.50 vs. 22.80 ± 13.50 with P = 0.94). These changes reflect the Mediterranean diet's positive impact on the symptoms and lifestyle of IBS children and adolescents.
Figure 1.
The flow chart of the study.
Table 3.
Growth parameters, laboratory data, and irritable bowel syndrome scores in all patients at the start versus at the end of the study
|
Variables
|
Group I (Mediterranean diet) (n = 50)
|
Group II (n = 50)
|
P
value
1
|
|||||
|
Start
|
End
|
P
value
|
Start
|
End
|
P
value
|
|||
| Growthparameters | Height (z-score) | 0.04 ± 1 | 0.04 ± 0.92 | 0.88 | 0.04 ± 1.00 | 0.04 ± 0.99 | 0.63 | 0.18 |
| Weight (z-score) | 0.14 ± 0.99 | 0.13 ± 1.0 | 0.54 | 0.12 ± 0.89 | 0.12 ± 0.55 | 0.61 | 0.36 | |
| BMI (z-score) | 0.18 ± 0.88 | 0.17 ± 0.69 | 0.6 | 0.17 ± 1.02 | 0.16 ± 1.08 | 0.80 | 0.55 | |
| Laboratory data | Albumin (g/dL) | 4.10 ± 0.90 | 4.3 ± 0.94 | 0.77 | 4.30 ± 0.88 | 4.50 ± 0.91 | 0.49 | 0.54 |
| Triglycerides (mg/dL) | 120.7 0 ± 45.60 | 118.50 ± 47.10 | 0.90 | 112.90 ± 49.40 | 115.20 ± 50.40 | 0.51 | 0.65 | |
| Cholesterol (mg/dL) | 154 ± 36.60 | 155.80 ± 32.20 | 0.56 | 163.60 ± 44.1 | 168.10 ± 42.90 | 0.63 | 0.28 | |
| Glucose (mg/dL) | 86.2 ± 20.20 | 81.90 ± 24.50 | 0.27 | 85.7 ± 9.7 | 87.30 ± 11.20 | 0.28 | 0.73 | |
| Hemoglobin (g/dL) | 13.1 ± 1.60 | 14.00 ± 1.10 | 0.66 | 13.60 ± 1.80 | 13.20 ± 1.50 | 0.71 | 0.33 | |
| Fecal calprotectin (μg/g) n < 50 | 12 ± 9.10 | 11.30 ± 9.90 | 0.81 | 11 ± 8.80 | 10.80 ± 9.20 | 0.88 | 0.62 | |
| IBS scores | IBS-SSS | 237.20 ± 65 | 163.20 ± 33.80 | 0.0011 | 248.3 ± 71.1 | 228.50 ± 54.30 | 0.29 | < 0.0011 |
| IBS-QoL | 57.30 ± 12.9 | 72.40 ± 11.2 | <0.0011 | 59.1 ± 11.7 | 59.20 ± 12.70 | 0.77 | < 0.0011 | |
| Total score | 24.10 ± 10.4 | 28.80 ± 11.20 | 0.021 | 22.8 ± 13.50 | 22.10 ± 12.50 | 0.94 | 0.031 | |
P value is for group I vs group II at the end of the study.
BMI: Body mass index; IBS: Irritable bowel syndrome; IBS-SSS: IBS symptoms severity score questionnaire; IBS-QoL: IBS quality of life questionnaire.
DISCUSSION
What should we eat? This question is one of the most frequently asked questions by patients with IBS and their caregivers. Many patients also seek dietary guidelines because the diet is considered safer than medical therapies. Treating IBS symptoms by modifying the patient's diet has been one of the most desirable therapeutic strategies for a long time. Unfortunately, the scarcity of high-quality evidence supporting a specific dietary intervention resulted in the unnecessary exclusion of diets despite lacking evidence of efficacy and safety, especially in pediatric age groups[22]. The current study found that the Mediterranean diet was safe and well-tolerated in IBS patients. Compared to the control group, good adherence to the Mediterranean diet resulted in significant improvement in IBS scores and IBS-QoL and total IBS scores. Many previous studies on children and adolescents showed a negative correlation between compliance with the Mediterranean diet and the development of various diverse pathological conditions, such as obesity, asthma, and recurrent cold[23,24].
Considering the potential association of adherence to the Mediterranean diet with the development of functional gastrointestinal disorders (FGIDs), much data from the adult population supports the beneficial effect of the Mediterranean diet on preventing the development of or lessening the gastrointestinal (GI) symptoms in patients with GI disorders, both functional (IBS, functional dyspepsia, gastroesophageal reflux) or organic (inflammatory bowel disease)[25]. Elmaliklis et al[26] showed that adherence to the Mediterranean diet (including functional foods containing probiotics, prebiotics, antioxidants, fiber, vitamins, minerals) was significantly lower in adult patients with various gastrointestinal disorders such as IBS, Crohn's disease, ulcerative colitis, and gastroesophageal reflux than in controls.
Another Southern Italian study by Zito et al[27] investigated the association between adherence to the Mediterranean diet and the onset of symptoms in adults with functional dyspepsia or IBS. They demonstrated a negative correlation between compliance with the Mediterranean diet and the development of gastrointestinal symptoms. They concluded that good adherence to the Mediterranean diet could prevent the development of gastrointestinal symptoms in adults. Moreover, they showed that patients with functional dyspepsia and IBS between 17 and 24 years had significantly poorer Mediterranean diet adherence than the age-matched controls. Interestingly, Strisciuglio et al[28] studied the adherence to the Mediterranean diet in children and adolescents suffering from inflammatory bowel disease with an age-matched population with FGIDs (gastroesophageal reflux and functional constipation). It was found that children/adolescents with inflammatory bowel syndrome had poorer adherence to the Mediterranean diet than those with FGIDs. However, there is no data on the association of Mediterranean diet adherence with the prevalence of FGIDs in children and adolescents.
In the current study, the Mediterranean diet was well tolerated in IBS patients; only three patients could not tolerate it and were withdrawn from the study (one after one month, two patients after three months, and replaced by other patients). No adverse events regarding the Mediterranean diet were reported as reflected by non-significant changes in growth parameters (height, weight, and BMI), laboratory parameters (serum albumin, triglycerides, cholesterol, glucose, and hemoglobin levels) at the end of our study when compared to the same parameters at the start of the research and when compared to group II at the end of our study.
Our study found positive effects of the Mediterranean diet in children and adolescents with IBS, with significant improvements in all IBS scores compared to the patients on the regular diet. These effects may be due to the specific components of the Mediterranean diet, which is characterized by a high intake of plant-based foods (vegetables, legumes, fruits, nuts, whole grain cereals), olive oil as the primary fat source, moderate amounts of dairy products (yogurt and cheese), and low or moderate cuts of fish and meat, with well-known antioxidant and anti-inflammatory properties[10]. Regular consumption of such products induces an accumulation of nitrate/nitrite/NO, polyunsaturated fatty acids (PUFA), and polyphenolic compounds, such as resveratrol, in the human body[12]. The most important dietary sources of NO3− for the human body include green vegetables such as spinach, lettuce, collard greens and radishes, beets, and meat. At the organ level, NO2−-dependent vasorelaxation plays a role in hypoxic blood flow regulation and improves tissue microcirculation[29,30].
The Mediterranean diet traditionally includes an abundance of vegetables and fish; both contain a substantial amount of diverse PUFA (ω-3, 6, 9). Briefly, PUFAs are divided into three classes based on the position of the first double bond from the methyl carbon, labeled "ω": ω-3 (DHA-docosahexaenoic, EPA-eicosapentaenoic, and ALA-α-linolenic), ω-6 (LA-linoleic, GLA-γ-linolenic, and AA-arachidonic); and ω-9 (OA-oleic). Extensive studies have revealed that the protective effects of EPA and DHA could be mediated by forming reactive lipid molecules called Resolvins[31]. Resolvins (E1 and D1) have a well-known anti-inflammatory property by preventing polymorphonuclear neutrophil (PMN) activation and translocation into the tissue[32,33]. Resolvin E1 regulates cytokine/chemokine production[34] and inhibits TNFα-induced nuclear translocation of NF-kB[35]. Freeman et al[36] characterized several electrophilic oxo-derivatives of DHA and EPA, synthesized in activated macrophages via the cyclooxygenase-2 dependent pathway. Like Resolvins, these also possess strong anti-inflammatory properties.
Regular consumption of grape wine is an integral element of the Mediterranean diet. The anti-inflammatory benefits of grape wine could be attributed to its phenolic components. Polyphenolic compounds such as quercetin, resveratrol, or catechins are potent antioxidants; thus, one of the mechanisms of protection they provide might be the inhibition of oxidative stress[37]. Moreover, the effect of the Mediterranean diet on gut microbiota may be an additional factor. Previous studies demonstrated that good adherence to the Mediterranean diet was associated with lower Escherichia coli (E. coli ) counts and a higher Bifidobacteria to E. coli ratio[38].
The strength of the current study is that it is the first report on the association between adherence to the Mediterranean diet and IBS symptoms in children and adolescents. The main limitation is the cross-sectional design, which allows the assessment of good associations but not conclusions on causality. The study was also from a single center, so the data cannot be generalized.
CONCLUSION
Results of the current study indicate that good adherence to the Mediterranean diet is safe and associated with significant improvement in IBS-score in children and adolescents. The mechanisms underlying this association and the causality between the Mediterranean diet and IBS need further clarification. If other studies with extensive metabolomic analysis and microbiome assessments confirm the current study's findings, this will complete the picture of the diet–health interaction and relationship. Until then, we should encourage children and adolescents to follow the Mediterranean diet to have a place among other measures in minimizing the symptoms.
ARTICLE HIGHLIGHTS
Research background
Irritable bowel syndrome (IBS) has a significant effect on the lives of affected children and their families and poses a substantial burden on healthcare systems. A few potential therapeutic modalities are available to treat children with IBS, and fewer of them have shown some benefits.
Research motivation
A few potential therapeutic modalities are available to treat children with IBS, and fewer of them have shown some benefits. The authors need to conduct more studies to help patients with IBS alleviate their symptoms.
Research objectives
The authors aimed to study the effects of the Mediterranean diet on the symptoms of IBS in children and adolescents.
Research methods
The authors studied one hundred consecutive IBS patients diagnosed according to Rome IV criteria, aged 12-18 years old. The authors divided the patients into two groups (50 patients each), the group I received a Mediterranean diet with good adherence (KIDMED Score ≥ 8 points), and Group II received a regular diet.
Research results
IBS children and adolescents with good adherence to the Mediterranean diet (KIDMED Score ≥ 8 points); group I showed significant improvement in IBS scores. IBS-SSS in the Mediterranean diet group was 237.2 ± 65 at the beginning of the study and decreased to 163.2 ± 33.8 at the end of the study (P < 0.001). It did not show a significant improvement in the group with a regular diet (248.3 ± 71.1 at the beginning of the study compared to 228.5 ± 54.3 at the study end with P < 0.05). The mean IBS-SSS in the Mediterranean diet group significantly improved compared with the group with a regular diet. Mean IBS-QoL in group I improved from 57.3 ± 12.9 at the start of the study to 72.4 ± 11.2 at the study end (P < 0.001) and significantly improved when compared to its level in group II at the study end (59.2 ± 12.7) with P < 0.001, while group II showed no significant improvement in IBS-QoL at the study end when compared to the beginning of the study (59.2 ± 11.7 with P > 0.05). The mean total IBS score in group I became 28.8 ± 11.2 at the end of our study compared to 24.1 ± 10.4 at the start (P < 0.05) and significantly improved when compared to its level in group II at the end of the study (22.1 ± 12.5) with P < 0.05, while in group II, non-significant improvement in the total score at the end of our study compared to its mean level at the start of the study (22.8 ± 13.5) with P > 0.05.
Research conclusions
Mediterranean diet was safe and associated with significant improvement in IBS scores in children and adolescent patients with IBS.
Research perspectives
The authors need to extend our study for a longer duration. We also need to investigate the effects of the Mediterranean diet on the various GIT functions, including bowel movement, stool consistency, and the impact on the gut microbiota.
ACKNOWLEDGEMENTS
We thank the anonymous referees and editors for their valuable suggestions.
Footnotes
Institutional review board statement: We performed to study according to the latest version of Helsinki's Declaration. The Research and Ethics Committee at the Ministry of Health, Kingdom of Bahrain, approved the study.
Informed consent statement: An informed written consent was signed by all subjects (and their caregivers).
Conflict-of-interest statement: None of the authors had potential undisclosed conflicts of interest.
STROBE statement: The authors have read the STROBE statement, and the manuscript was prepared and revised according to the STROBE statement.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 13, 2021
First decision: March 24, 2022
Article in press: April 28, 2022
Specialty type: Pediatrics
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soares RLS, Brazil; Ulasoglu C, Turkey S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
Contributor Information
Mohammed Al-Biltagi, Department of Pediatrics, University Medical center, King Abdulla Medical City, Arabian Gulf University, Manama 26671, Bahrain. mbelrem@hotmail.com; Department of Pediatrics, Faculty of Medicine, Tanta University, Tanta 31527, Al Gharbia, Egypt; Department of Pediatrics, University Medical Center, Dr. Sulaiman Al-Habib Medical Group, Manama 26671, Bahrain.
Doaa El Amrousy, Department of Pediatrics, Tanta University, Faculty of Medicine, Tanta 31527, Alghrabia, Egypt.
Heba El Ashry, Department of Tropical Medicine, Faculty of Medicine, Tanta University, Tanta 31527, Alghrabia, Egypt.
Sara Maher, Department of Immunology, Theodor Bilharz Research Institute, Cairo 12411, Egypt.
Mahmoud A Mohammed, Department of Industrial Pharmacy, Faculty of Pharmacy, Assiut University, Assiut 71515, Egypt.
Samir Hasan, Department of Pediatrics, Tanta University, Faculty of Medicine, Tanta 31527, Alghrabia, Egypt.
Data sharing statement
Data are available upon reasonable request.
References
- 1.Ahlawat R, Weinstein T, Pettei MJ. Vitamin D in pediatric gastrointestinal disease. Curr Opin Pediatr. 2017;29:122–127. doi: 10.1097/MOP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 2.Sprake EF, Grant VA, Corfe BM. Vitamin D3 as a novel treatment for irritable bowel syndrome: single case leads to critical analysis of patient-centred data. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oświęcimska J, Szymlak A, Roczniak W, Girczys-Połedniok K, Kwiecień J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62:17–30. doi: 10.1016/j.advms.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Parkes GC, Brostoff J, Whelan K, Sanderson JD. Gastrointestinal microbiota in irritable bowel syndrome: their role in its pathogenesis and treatment. Am J Gastroenterol. 2008;103:1557–1567. doi: 10.1111/j.1572-0241.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 5.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 7.O'Malley T, Heuberger R. Vitamin D status and supplementation in pediatric gastrointestinal disease. J Spec Pediatr Nurs. 2011;16:140–150. doi: 10.1111/j.1744-6155.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- 8.Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:1256–1270. doi: 10.1111/apt.13167. [DOI] [PubMed] [Google Scholar]
- 9.Devanarayana NM, Rajindrajith S. Irritable bowel syndrome in children: Current knowledge, challenges and opportunities. World J Gastroenterol. 2018;24:2211–2235. doi: 10.3748/wjg.v24.i21.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donini LM, Serra-Majem L, Bulló M, Gil Á, Salas-Salvadó J. The Mediterranean diet: culture, health and science. Br J Nutr. 2015;113 Suppl 2:S1–S3. doi: 10.1017/S0007114515001087. [DOI] [PubMed] [Google Scholar]
- 11.Bulló M, Lamuela-Raventós R, Salas-Salvadó J. Mediterranean diet and oxidation: nuts and olive oil as important sources of fat and antioxidants. Curr Top Med Chem. 2011;11:1797–1810. doi: 10.2174/156802611796235062. [DOI] [PubMed] [Google Scholar]
- 12.Nadtochiy SM, Redman EK. Mediterranean diet and cardioprotection: the role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition. 2011;27:733–744. doi: 10.1016/j.nut.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev. 1997;55:383–389. doi: 10.1111/j.1753-4887.1997.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 14.Iaccarino Idelson P, Scalfi L, Valerio G. Adherence to the Mediterranean Diet in children and adolescents: A systematic review. Nutr Metab Cardiovasc Dis. 2017;27:283–299. doi: 10.1016/j.numecd.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional Disorders: Children and Adolescents. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 18.Bao C, Zhang J, Liu J, Liu H, Wu L, Shi Y, Li J, Hu Z, Dong Y, Wang S, Zeng X, Wu H. Moxibustion treatment for diarrhea-predominant irritable bowel syndrome: study protocol for a randomized controlled trial. BMC Complement Altern Med. 2016;16:408. doi: 10.1186/s12906-016-1386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serra-Majem L, García-Closas R, Ribas L, Pérez-Rodrigo C, Aranceta J. Food patterns of Spanish schoolchildren and adolescents: The enKid Study. Public Health Nutr. 2001;4:1433–1438. doi: 10.1079/phn2001234. [DOI] [PubMed] [Google Scholar]
- 20.Serra-Majem L, Ribas L, García A, Pérez-Rodrigo C, Aranceta J. Nutrient adequacy and Mediterranean Diet in Spanish school children and adolescents. Eur J Clin Nutr. 2003;57 Suppl 1:S35–S39. doi: 10.1038/sj.ejcn.1601812. [DOI] [PubMed] [Google Scholar]
- 21.Jalili M, Hekmatdoost A, Vahedi H, Poustchi H, Khademi B, Saadi M, Zemestani M, Janani L. Co-Administration of Soy Isoflavones and Vitamin D in Management of Irritable Bowel Disease. PLoS One. 2016;11:e0158545. doi: 10.1371/journal.pone.0158545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esteban-Cornejo I, Izquierdo-Gomez R, Gómez-Martínez S, Padilla-Moledo C, Castro-Piñero J, Marcos A, Veiga OL. Adherence to the Mediterranean diet and academic performance in youth: the UP&DOWN study. Eur J Nutr. 2016;55:1133–1140. doi: 10.1007/s00394-015-0927-9. [DOI] [PubMed] [Google Scholar]
- 23.Calatayud-Sáez FM, Calatayud Moscoso Del Prado B, Gallego Fernández-Pacheco JG, González-Martín C, Alguacil Merino LF. Mediterranean diet and childhood asthma. Allergol Immunopathol (Madr) 2016;44:99–105. doi: 10.1016/j.aller.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Calatayud FM, Calatayud B, Gallego JG, González-Martín C, Alguacil LF. Effects of Mediterranean diet in patients with recurring colds and frequent complications. Allergol Immunopathol (Madr) 2017;45:417–424. doi: 10.1016/j.aller.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Reddavide R, Rotolo O, Caruso MG, Stasi E, Notarnicola M, Miraglia C, Nouvenne A, Meschi T, De' Angelis GL, Di Mario F, Leandro G. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed. 2018;89:60–75. doi: 10.23750/abm.v89i9-S.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elmaliklis IN, Liveri A, Ntelis B, Paraskeva K, Goulis I, Koutelidakis AE. Increased Functional Foods' Consumption and Mediterranean Diet Adherence May Have a Protective Effect in the Appearance of Gastrointestinal Diseases: A Case⁻Control Study. Medicines (Basel) 2019;6 doi: 10.3390/medicines6020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zito FP, Polese B, Vozzella L, Gala A, Genovese D, Verlezza V, Medugno F, Santini A, Barrea L, Cargiolli M, Andreozzi P, Sarnelli G, Cuomo R. Good adherence to mediterranean diet can prevent gastrointestinal symptoms: A survey from Southern Italy. World J Gastrointest Pharmacol Ther. 2016;7:564–571. doi: 10.4292/wjgpt.v7.i4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strisciuglio C, Giugliano F, Martinelli M, Cenni S, Greco L, Staiano A, Miele E. Impact of Environmental and Familial Factors in a Cohort of Pediatric Patients With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2017;64:569–574. doi: 10.1097/MPG.0000000000001297. [DOI] [PubMed] [Google Scholar]
- 29.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ologhobo AD, Adegede HI, Maduagiwu EN. Occurrence of nitrate, nitrite and volatile nitrosamines in certain feedstuffs and animal products. Nutr Health. 1996;11:109–114. doi: 10.1177/026010609601100203. [DOI] [PubMed] [Google Scholar]
- 31.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with anti-inflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal anti-inflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 36.Koenitzer JR, Freeman BA. Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann N Y Acad Sci. 2010;1203:45–52. doi: 10.1111/j.1749-6632.2010.05559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O'Toole PW, Ercolini D. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.