Abstract
MRI features are presented in a multicenter retrospective series of five patients with a unilateral masslike lesion seen in the genitourinary diaphragm at MRI performed for known or suspected prostate cancer. In all cases, the lesion appeared as an encapsulated 1.3 to 3.0 cm mass of heterogeneous low or intermediate T2 signal intensity in the genitourinary diaphragm, and targeted biopsy demonstrated benign Cowper’s gland tissue. This entity is a potential imaging pitfall that could result in a diagnosis of an exophytic nodule of benign prostatic hyperplasia or local spread of prostate cancer. We present these cases to facilitate correct identification of Cowper’s gland hyperplasia as an occasional finding at MRI of the prostate.
Keywords: MRI, Prostate, Cowper’s glands
1. Introduction
The normal Cowper’s or bulbourethral glands are bilateral accessory sex organs measuring about 0.5 cm in diameter located within the genitourinary diaphragm just inferior to the prostate (Figure 1) [1]. The gland’s function is to produce thick clear mucus prior to ejaculation and drain via paired ducts to the ventral bulbar urethra. Cowper’s gland pathology includes syringocele of the ducts, infection, inflammation, and neoplasm [1, 2]. Given their location, the Cowper’s glands are routinely included in MRI performed for known or suspected prostate cancer, but normal Cowper’s glands are not routinely identified [3]. Radiologists who interpret these studies should be familiar with the associated imaging pitfalls [4, 5] and with the radiological manifestations of periprostatic pathology, including diseases of the Cowper’s glands [6–12]. We have recently encountered five cases of a masslike appearance within the genitourinary diaphragm at MRI of the prostate where targeted biopsy of the lesion demonstrated benign Cowper’s gland. In combination, the radiological and pathological findings suggest a diagnosis of Cowper’s gland hyperplasia, which has only been recently described in a single case report [6]. We present these five cases to expand the description of this occasional finding at MRI and to facilitate correct identification that may prevent unnecessary workup of this entity.
Figure 1:-.
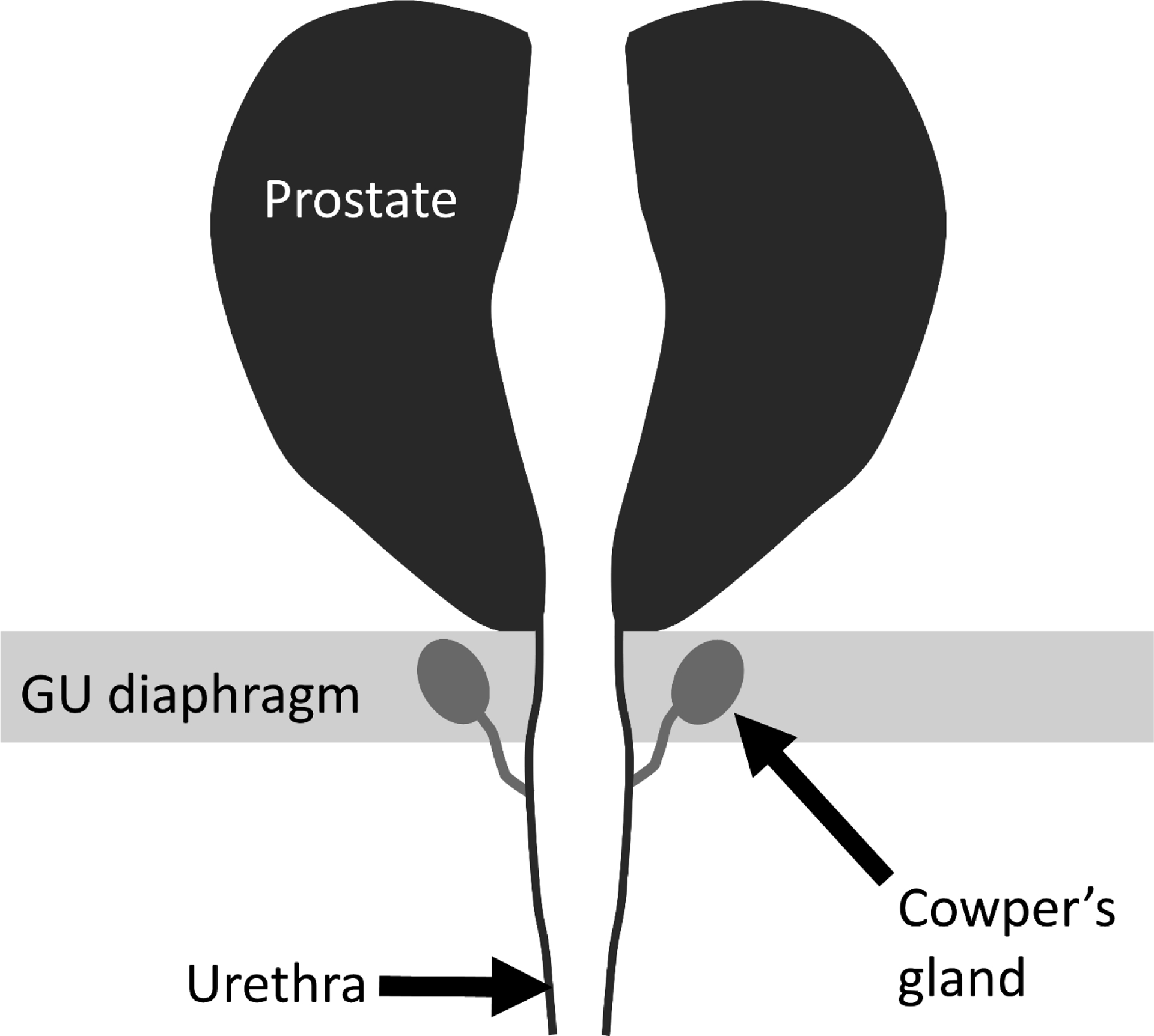
Schematic coronal diagram illustrating the normal anatomic relationship of the Cowper’s glands, genitourinary (GU) diaphragm, prostate, and urethra.
2. Case description
2.1. Methods and patients
We retrospectively identified five patients with a masslike appearance of the Cowper’s gland seen incidentally on MRI of the prostate where targeted biopsy demonstrated benign Cowper’s gland tissue. Two cases were identified at our institution and three additional cases were identified through communication with the Prostate Disease-Focused Panel of the Society of Abdominal Radiology (three members of the Panel each identified one case from each of their respective institutions).
2.2. Results
Pertinent clinical, radiological, and pathological findings are summarized in Table 1, with a representative case illustrated in Figure 1. In all five cases, the lesion appeared as an encapsulated 1.3 to 3.0 cm mass of heterogeneous low or intermediate T2 signal intensity in the genitourinary diaphragm, and targeted biopsy demonstrated benign Cowper’s gland tissue. The overall radiological and histological appearances were considered consistent with Cowper’s gland hyperplasia. No records of lower urinary tract symptoms that might be attributable to enlargement of the Cowper’s glands were identified.
Table 1:
Pertinent clinical, radiological, and pathological findings in the five patients with a pathologically confirmed diagnosis of Cowper’s gland hyperplasia.
| Case | Age (yrs) | History | MRI technique | Lesion location | Size (cm) | T1 signal | T2 | Diffusion | Perfusion | Tissue sampling |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | Recently diagnosed Gleason score 4+4 prostate cancer. PSA: 11.8 ng/ml. | 3T with endorectal coil | Left genitourinary diaphragm | 1.6 × 1.3 × 2.3 | Low | Low, encapsulated, mildly heterogeneous | Mildly restricted | Mildly enhancing | In-bore MRI targeted biopsy |
| 2 | 73 | Recently diagnosed Gleason score 3+4 prostate cancer. PSA: 5.4 ng/ml. | 3T with endorectal coil | Left genitourinary diaphragm | 1.9 × 1.4 × 2.0 | Low | Low, encapsulated, mildly heterogeneous | Markedly restricted | Not covered | In-bore MRI targeted biopsy |
| 3 | 69 | Recently diagnosed Gleason score 3+3 prostate cancer. PSA: 4.9 ng/ml | 3T without endorectal coil | Left genitourinary diaphragm | 1.9 × 1.4 × 1.9 | Low | Intermediate, encapsulated, bilobed, heterogeneous | Markedly restricted | Avidly enhancing | MRI-US fusion biopsy |
| 4 | 64 | Elevated PSA: 5.2 ng/ml with prior negative biopsy. | 3T with endorectal coil | Right genitourinary diaphragm | 3.0 × 2.8 × 2.4 | Low | Low, partially encapsulated, mildly heterogeneous | Mildly restricted | Avidly enhancing | In-bore MRI targeted biopsy |
| 5 | 70 | Elevated PSA: 5.3 ng/ml. Family history of prostate cancer | 3T with endorectal coil | Left genitourinary diaphragm | 2 × 1.6 × 1.8 | Low | Low, encapsulated, heterogeneous | Mildly restricted | Enhancing | MRI-US fusion biopsy |
PSA: Prostate specific antigen.
Size dimensions are given as transverse × anteroposterior × craniocaudal dimensions.
3. Discussion
This multicenter study describes a series of five patients in which Cowper’s gland hyperplasia was seen as an incidental finding in the genitourinary diaphragm at MRI of the prostate performed for known or suspected prostate cancer. The finding of an encapsulated 1.3 to 3.0 cm mass of heterogeneous low or intermediate T2 signal intensity with restricted diffusion in the genitourinary diaphragm seems to be a distinctive appearance for this entity, and correct recognition of this recently reported diagnosis should avoid erroneous differential considerations such as an exophytic nodule of benign prostatic hyperplasia or local spread of prostate cancer [4–6]. We believe the appearance of a well circumscribed encapsulated small mass of heterogeneous low or intermediate T2 signal intensity with restricted diffusion in the genitourinary diaphragm can be prospectively reported as probable Cowper’s gland hyperplasia and managed conservatively, with options including surveillance imaging to confirm stability.
Our review of the literature revealed a single recent case report of Cowper’s gland hyperplasia confirmed by MRI guided biopsy [6]. The reported lesion showed an intermediate heterogenous T2 signal, homogenous avid enhancement, and no diffusion restriction. The appearances were consistent with the MRI features of the five cases included in our series. True solid neoplasms of the Cowper’s gland are very rare, with fewer than 15 reported cases of primary carcinoma [7–13]. Most of the cases were reported before the wide availability of MRI [7–10] and so the MRI findings of primary carcinoma of the Cowper’s gland are not well established. Reported clinical and other imaging findings include a hard mass on digital rectal examination, intraluminal filling defect on urethrography, and a “prostate mass” at CT [12]. Knowledge of prostate tumor mimics and incidental pathology in or around the prostate is essential for the correct interpretation of MRI of the prostate [14, 15]. Our case series, along with the recently published case report, expands the differential diagnosis for Cowper’s gland masses and adds to the list of potential incidental benign findings at MRI of the prostate. Our study has the limitation of being a retrospective study with a small sample size. Larger and more comprehensive studies will be required to address these limitations.
4. Conclusion
In conclusion, Cowper’s gland hyperplasia can have a mass-like appearance in the genitourinary diaphragm that may simulate malignancy. We describe MR features of five cases which facilitates correct recognition of this benign entity at MRI of the prostate and avoids unnecessary work up.
Figure 2A:-.

Axial T2-weighted endorectal MR image in a 73 year old man with a recently diagnosed Gleason score 3+4 prostate cancer after detection of an elevated screening PSA of 5.4 (patient #2 in Table 1). An encapsulated low signal mass (arrow) is visible in the left genitourinary diaphragm.
Figure 2B:-.

Coronal T2-weighted endorectal MR image shows the mass is located in the genitourinary diaphragm inferior to the apex of the prostate.
Figure 2C:-.

Axial apparent diffusion coefficient map demonstrates restricted diffusion within the mass (arrow).
Figure 2D:-.

Photomicrograph of a hematoxylin and eosin-stained slide of an 18G core specimen obtained from the mass at an in-bore MRI targeted biopsy shows benign Cowper’s gland tissue.
Figure 3A:-.

Axial T2-weighted endorectal MR image in a 67 year old man with recently diagnosed Gleason score 4+4 prostate cancer after detection of a rising PSA of 11.8 while on active surveillance for previously diagnosed Gleason score 3+3 cancer (patient #1 in Table 1). An encapsulated low signal mass (arrow) is visible in the left genitourinary diaphragm.
Figure 3B:-.
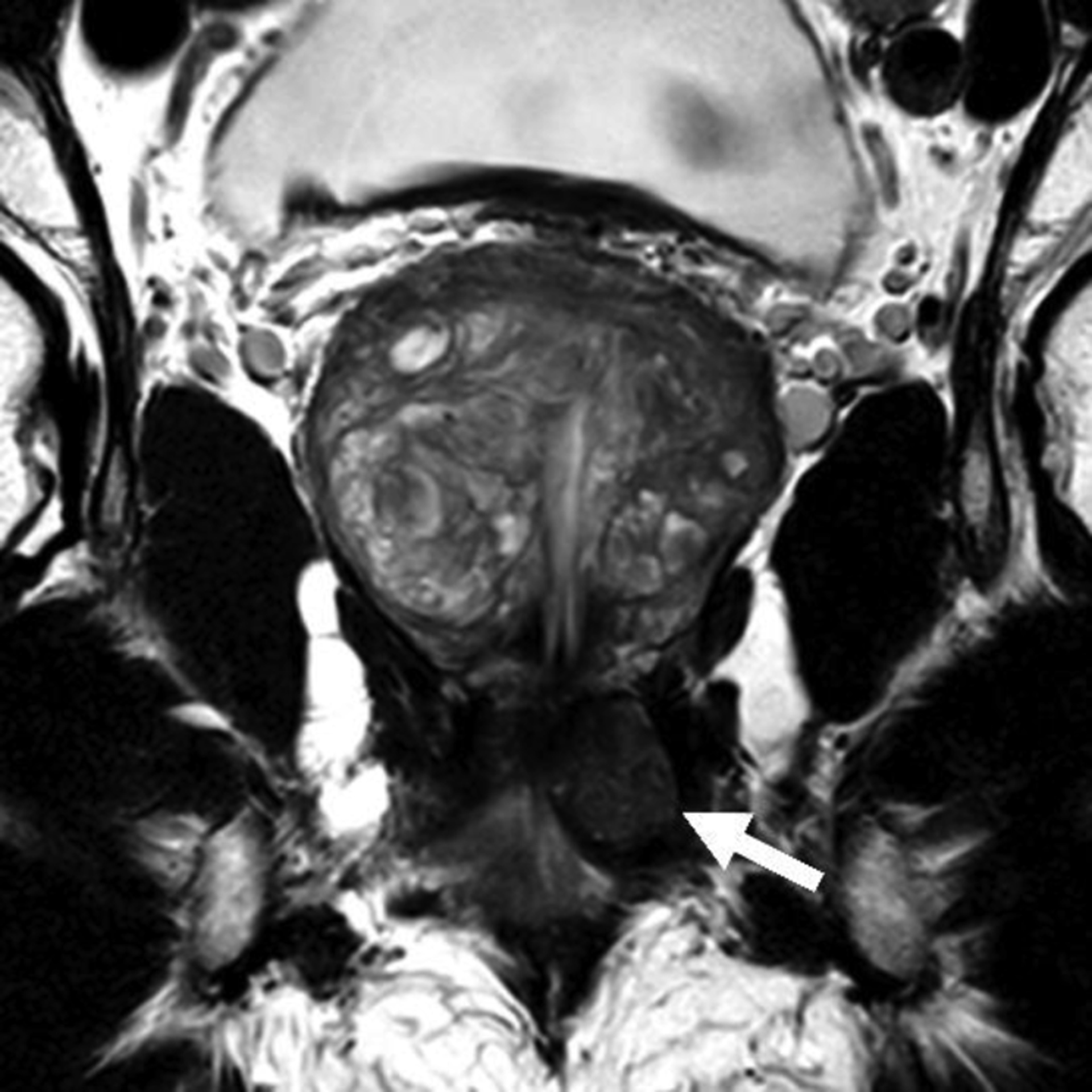
Coronal T2-weighted endorectal MR image shows the mass is located in the genitourinary diaphragm inferior to the apex of the prostate.
Figure 3C:-.

Axial apparent diffusion coefficient map demonstrates restricted diffusion within the mass (outlined).
Figure 3D:-.

Axial gadolinium-enhanced T1-weighted endorectal MR image demonstrates mild enhancement within the mass (outlined).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chughtai B, et al. , A neglected gland: a review of Cowper’s gland. Int J Androl, 2005. 28(2): p. 74–7. [DOI] [PubMed] [Google Scholar]
- 2.Sharbaugh AJ, et al. , Cowper’s Gland Syringocele. Urology, 2018. 119: p. e3–e4. [DOI] [PubMed] [Google Scholar]
- 3.Coakley FV, et al. , ACR Appropriateness Criteria(®) Prostate Cancer-Pretreatment Detection, Surveillance, and Staging. J Am Coll Radiol, 2017. 14(5s): p. S245–s257. [DOI] [PubMed] [Google Scholar]
- 4.Khanna A, et al. , Exophytic benign prostatic hyperplasia presenting with refractory retention: A rare entity. Indian J Urol, 2018. 34(3): p. 229–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong HL, Shi H, and Koh LT, Solitary metastasis to the penis from prostate adenocarcinoma - a case report. J Radiol Case Rep, 2019. 13(12): p. 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai S, et al. , Rare prostate cancer mimic on multiparametric MRI: Cowper’s gland hyperplasia. Urology Case Reports, 2021: p. 101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derrick FC Jr. and Lynch KM Jr., Cowper’s gland carcinoma. Report of a case. J S C Med Assoc, 1968. 64(3): p. 82–4. [PubMed] [Google Scholar]
- 8.Bourque JL, et al. , Primary carcinoma of Cowper’s gland. J Urol, 1970. 103(6): p. 758–61. [DOI] [PubMed] [Google Scholar]
- 9.Keen MR, et al. , Carcinoma of Cowper’s gland treated with chemotherapy. J Urol, 1970. 104(6): p. 854–9. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter AA and Bernardo JR Jr., Adenoid cystic carcinoma of Cowper’s gland: case report. J Urol, 1971. 106(5): p. 701–3. [DOI] [PubMed] [Google Scholar]
- 11.Small JD, et al. , Adenoid cystic carcinoma of Cowper’s gland. J Urol, 1992. 147(3): p. 699–701. [DOI] [PubMed] [Google Scholar]
- 12.Myers CE, et al. , Metastatic Cancer of Cowper’s Gland: A Rare Cancer Managed Successfully by Molecular Profiling. Case Rep Oncol, 2014. 7(1): p. 52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, et al. , Adenoid cystic carcinoma of the urethra/Cowper’s gland with concurrent high-grade prostatic adenocarcinoma: a detailed clinicopathologic case report and review of the literature. Hum Pathol, 2016. 58: p. 138–144. [DOI] [PubMed] [Google Scholar]
- 14.Rosenkrantz AB and Taneja SS, Radiologist, be aware: ten pitfalls that confound the interpretation of multiparametric prostate MRI. AJR Am J Roentgenol, 2014. 202(1): p. 109–20. [DOI] [PubMed] [Google Scholar]
- 15.Trivedi J, Sutherland T, and Page M, Incidental findings in and around the prostate on prostate MRI: a pictorial review. Insights into Imaging, 2021. 12(1): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]


