Abstract
Most peripheral serotonin (5-HT) is synthesized in enterochromaffin cells, and most circulating 5-HT is stored in platelets. As a monoamine, 5-HT has several functions in various non-neuronal and neuronal systems. In the central nervous system, it functions as a neurotransmitter to modulate feeding behavior and mood. Numerous clinical trials have focused on increasing 5-HT activation in the central nervous system, including those involving anti-obesity drugs currently in the market, although severe side effects on peripheral system can lead to the withdrawal of certain drugs. Recent studies have revealed that both the peripheral and central serotonergic systems play a vital role in diabetes and its complications. This review summarizes the roles of the serotonergic system in blood glucose regulation, diabetic macroangiopathy, diabetic peripheral neuropathy, and diabetic encephalopathy, indicating its potential clinical significance as a therapeutic target for the treatment of diabetes and its complications.
Keywords: serotonin, 5-HT, diabetes, neuropathy, 5-HT receptor, hyperglycemia
Introduction
5-hydroxytryptamine (5-HT), also known as serotonin, has numerous functions in non-neuronal and neuronal systems. 5-HT was discovered in 1918 and was considered to be a vasoconstrictor stored in platelets. In 1937, 5-HT was discovered in enterochromaffin cells of the gastrointestinal tract and was called enteramine, since it was considered to induce smooth muscle contraction in the intestine (Erspamer and Asero, 1952). 5-HT was reported to be a neurotransmitter in 1952 (Brodie and Shore, 1957), and has been linked to appetite, behavior, mood, and sleep cycle regulation (Zucker, 1944). It is now known to function as a mitogen and hormone as well.
Most 5-HT in the periphery is synthesized by enterochromaffin cells (Sumara et al., 2012). As the rate-limiting enzyme for 5-HT production, tryptophan hydroxylase causes the hydroxylation of tryptophan to synthesize 5-HT. The synthesis of 5-HT occurs in two steps. In the first step, tryptophan hydroxylase (the rate-limiting enzyme for 5-HT production) metabolizes tryptophan to 5-hydroxytryptophan. In the second step, 5-hydroxytryptophan is decarboxylated to 5-HT by a nonspecific enzyme (aromatic L-amino acid decarboxylase). Thus, 5-HT synthesis is modulated by the availability of tryptophan and the activity of tryptophan hydroxylase. One of the two tryptophan hydroxylase isoforms, tryptophan hydroxylase 1, is mainly expressed in the peripheral tissues and also in the pineal gland, while the other, tryptophan hydroxylase 2, is chiefly expressed in a subset of neurons of the enteric and central nervous system, including those in the raphe nuclei of the brain stem (Malek et al., 2005; Li et al., 2011; Yabut et al., 2019). Although tryptophan hydroxylase 1 is extensively expressed in peripheral tissues, more than 90% of the total 5-HT in the body is synthesized in the intestine (Berger et al., 2009). Most peripheral 5-HT is stored in platelets and controls the hemodynamics upon platelet activation. Several recent studies have shown that 5-HT can be produced in other peripheral tissues, including in the pancreas, heart, and adipose tissue, and plays a role in the manner of cell-autonomous (Kim et al., 2010; Ponicke et al., 2012; Oh et al., 2015). 5-HT also acts autonomously on various cell types in the gastrointestinal, hematopoietic, cardiovascular, and immune systems, and in the liver, bone, and placenta (Amireault et al., 2013). Within the enteric and central nervous systems, 5-HT is produced and stored in presynaptic neurons. As 5-HT cannot physically cross the blood-brain barrier, the peripheral serotonergic system is functionally separate from the central serotonergic system.
5-HT modulates many physiological and pathological processes via several membrane-bound 5-HT receptors; moreover, the biological functions of 5-HT are restricted by its uptake into cells via 5-HT transporters (Wade et al., 1996). More than 14 5-HT receptors belonging to seven families mediate the different functions of 5-HT (Mohammad-Zadeh et al., 2008). The 5-HT3 receptor is a ligand-gated cation channel, while all other 5-HT receptors are G-protein-coupled receptors (Peroutka and Howell, 1994; Reeves and Lummis, 2002). Recently, a novel detection technology was used in zebra fish model to show that 5-HT concentration significantly decreases in early diabetes, and that 5-HT can be used as an effective biomarker for the early diagnosis of diabetes (Khoshnevisan et al., 2021).
Several recent reviews have discussed the role of 5-HT and its receptors in neurological diseases and metabolism (Politis and Niccolini, 2015; Tu et al., 2015; Fakhoury, 2016; Oh et al., 2016; Lalut et al., 2017; Yohn et al., 2017; Yabut et al., 2019; Jones et al., 2020). Especially one review has summarized that 5-HT is produced in some peripheral tissues, including pancreas, adipose tissue, and liver; 5-HT and its receptors are involved in several metabolic pathways in peripheral tissues in the manner of cell-autonomous, such as insulin secretion and cell proliferation during development of β-cells, lipolysis in adipocytes, gluconeogenesis and glucose uptake in hepatocytes (Oh et al., 2016). So this review only summarizes the recent results regarding the roles of the peripheral and central serotonergic systems in diabetes and its complications, especially diabetic macroangiopathy, diabetic peripheral neuropathy, and diabetic encephalopathy.
Roles of the peripheral serotonergic system in blood glucose level regulation
Obese humans synthesize and release more 5-HT from the proximal small intestine, which is strongly associated with glycemic control and higher body mass. The 5-HT produced in the intestine is believed to be a vital driver of pathogenesis in human obesity and dysglycemia (Young et al., 2018). Pharmacological or genetic reductions in gut 5-HT protect against diet-induced obesity, hepatic steatosis and glucose intolerance (Sumara et al., 2012; Crane et al., 2015; Oh et al., 2015; Choi et al., 2018), illustrating a causative role of elevated gut-derived 5-HT in driving metabolic dysfunctions. Both the density of enterochromaffin cells and the expression of tryptophan hydroxylase 1 are increased in human obesity (Young et al., 2018); however, mechanisms driving such changes remain unknown. Interactions between intestine-produced 5-HT and gut microbiota have recently been hypothesized to regulate host glucose metabolism. Pharmacological suppression or knockout of tryptophan hydroxylase to abolish intestinal 5-HT synthesis in mice, with or without antibiotic-related microbiota elimination, shows remarkable amelioration of glucose clearance (Martin et al., 2019). The amelioration in host glucose clearance caused by antibiotic-induced alterations in microbiota composition are dependent on the production of intestine-derived 5-HT (Martin et al., 2019).
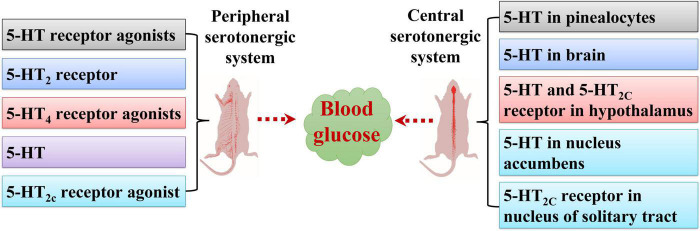
With regard to insulin resistance, inhibition of intestine-secreted 5-HT improves glucose tolerance in high-fat diet-induced mice (Sumara et al., 2012). In hepatocytes, intestine-secreted 5-HT signaling via the 5-HT2B receptor increases gluconeogenesis. Furthermore, intestine-derived 5-HT inhibits glucose uptake into hepatocytes through glucose transporter 2; hence, intestine-specific tryptophan hydroxylase 1 knockout mice and liver-specific 5-HT2B receptor knockout mice show improved glucose tolerance compared to wild-type mice (Sumara et al., 2012). Glycemic regulation is also ameliorated in tryptophan hydroxylase 1 knockout mice, although the glucose uptake rate is the same as in the liver, muscle, and heart, suggesting that brown adipose tissue makes a major contribution to the increased basal metabolic rate (Crane et al., 2015). The effects of the peripheral and central serotonergic systems on blood glucose levels are shown in Table 1 and Figure 1.
TABLE 1.
Summary of the role of serotonergic system in diabetes and its complication.
| Serotonergic system | Target | Effects | References |
| Intestine-secreted 5-HT | 5-HT2B receptor in hepatocytes | Increases gluconeogenesis, increases serum glucose level | Sumara et al., 2012 |
| Inhibits glucose uptake into hepatocytes via glucose transporter 2 to increase serum glucose level | Sumara et al., 2012 | ||
| 8-hydroxy-2-(di-n-propylamino)tetralin 5-HT1A receptor agonist |
Central nervous system | Increased corticosterone leads to increased plasma glucose levels | Gehlert and Shaw, 2014 |
| 5-carboxamidotryptamine Peripherally acting nonselective 5-HT receptor agonist |
Peripherally 5-HT7 receptor (>0.05 mg/kg) | Hyperglycemia in rats | Yamada et al., 1998 |
| Mirtazapine 5-HT2 receptor antagonists |
Peripheral | Worsens insulin sensitivity | Gilles et al., 2005 |
| Fluoxetine Selective serotonin reuptake inhibitor |
Peripheral | Ameliorates insulin sensitivity | Breum et al., 1995 |
| Sertraline and paroxetine Selective serotonin reuptake inhibitor |
Peripheral | Improves glycemic control | Goodnick et al., 1997; Ueno et al., 2010 |
| Mosapride or prucalopride 5-HT4 receptor agonist |
Pancreatic tissue and islet cells of rats in vitro | Increases insulin release; elevates the serum insulin level accompanied by a decrease in blood glucose | Chen et al., 2016 |
| 5-HT4 receptor | Nucleus accumbens | Reduces the physiological drive to eat | Jean et al., 2007 |
| Lorcaserin 5-HT2C receptor agonist |
Peripheral | Loses weight and ameliorates glycemic parameters in prediabetes patients, and prevents progression to type 2 diabetes and accelerates reversion to normal blood glucose levels | Nesto et al., 2016 |
| Lorcaserin 5-HT2C receptor agonist |
Brainstem nucleus of solitary tract | Decreasing food intake when activated | D’Agostino et al., 2018 |
| Glucagon-like peptide 1 receptor | Dorsal raphe Hypothalamus |
Body weight reduction, anorexia, and fat mass loss; 5-HT turnover and expression of 5-HT2A and 5-HT2C receptors | Anderberg et al., 2017 |
| M-chlorophenylpiperazine 5-HT2C receptor agonist |
Peripheral | Ameliorates insulin sensitivity and glucose homeostasis | Berglund et al., 2013 |
| Antagonists or genetic deletion of 5-HT2C receptors | Pro-opiomelanocortin neurons | Compromises glucose homeostasis | Berglund et al., 2013 |
| Transcutaneous auricular vagus nerve stimulation (2/15 Hz, 2 mA) | Inhibits the progression of nociceptive hypersensitivity in Zucker diabetic fatty rats, this advantageous impact on nociceptive behavior is associated with an increase in 5-HT plasma levels and upregulated expression of the 5-HT1A receptor in the hypothalamus | Li et al., 2018 | |
| Duloxetine Inhibitor of serotonin and norepinephrine reuptake |
Systemic or intrathecal injection | Alleviates tactile allodynia in diabetic rats | Mixcoatl-Zecuatl and Jolivalt, 2011 |
FIGURE 1.
Effects of peripheral and central serotonergic systems on blood glucose levels. 5-HT, 5-HT2 receptor, 5-HT receptor agonists, 5-HT4 receptor agonists, and 5-HT2c receptor agonists regulate blood glucose levels via the peripheral serotonergic system. 5-HT in the brain, pinealocytes, nucleus accumbens, and hypothalamus and 5-HT2C receptors in the hypothalamus and nucleus of the solitary tract are involved in modulating blood glucose levels via the central serotonergic system.
5-HT receptor agonists induce hyperglycemia in rats
5-carboxamidotryptamine, a peripherally acting nonselective 5-HT receptor agonist, induces significant hyperglycemia in rats at doses higher than 0.05 mg/kg. The above mentioned dose of 5-carboxamidotryptamine causing hyperglycemia can induce other pharmacological effects (drinking or hypophagia) in rats (Yamada et al., 1998). 5-carboxamidotryptamine-induced hyperglycemia is mediated by the facilitation of adrenaline secretion. Moreover, 5-carboxamidotryptamine causes hyperglycemia via 5-HT7 receptors, but not 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2, 5-HT3, 5-HT4, and 5-HT5 receptors. This indicates that the 5-HT7 receptor may participate in adrenaline secretion like other 5-HT receptor subtypes (Yamada et al., 1998). 8-hydroxy-2-(di-n-propylamino)tetralin, a 5-HT1A receptor agonist, causes hyperphagia and increases plasma glucose levels. It increases plasma corticosterone levels through a central nervous system mechanism, and increased corticosterone leads to higher plasma glucose levels (Gehlert and Shaw, 2014).
Role of the 5-HT2 receptor in glucose metabolism
The 5-HT2 receptor participates directly in glucose metabolism in humans (Gilles et al., 2005). This is closely related to the effects of pharmacological agents that function as 5-HT2 receptor antagonists (such as mirtazapine), which can worsen insulin sensitivity and, in vulnerable patients or those with accompanying weight gain, may elevate the risk of impaired glucose tolerance and eventually diabetes. These observations confirm the hypothesis that serotonergic antidepressants directly deteriorate insulin sensitivity (Gilles et al., 2005). The 5-HT2A receptor is expressed on both human skeletal muscle cells (Hajduch et al., 1999b) and in adult rat skeletal muscle (Hajduch et al., 1999a). It can stimulate rapid glucose uptake in rat skeletal muscle in vitro, particularly in L6 myotubes (Hajduch et al., 1999b). Therefore, several drugs may damage insulin sensitivity at the level of 5-HT2 receptor-mediated modulation of glucose transporters in muscle. To date, the antagonist property at 5-HT2A receptors of clozapine and olanzapine have been associated with antipsychotic-induced diabetes (Henderson, 2002). This may be due to the obvious drug-related weight gain (Nasrallah, 2003), highlighting another disadvantageous effect of impaired insulin sensitivity on metabolism. However, some data suggest that all 5-HT2 receptor antagonists, including both these drugs, may pose a risk associated with insulin sensitivity (Gilles et al., 2005).
Fluoxetine, a selective serotonin reuptake inhibitor, induces the inhibition of 5-HT2c receptors, and its short-term treatment is linked to weight loss; however, it also appears to ameliorate insulin sensitivity beyond the efficacy produced via weight loss by a likely stimulating effect on glycogen synthase activity in skeletal muscle (Breum et al., 1995). Sertraline and paroxetine, selective serotonin reuptake inhibitors, though lacking direct 5-HT2c inhibiting properties, may also improve glycemic control (Goodnick et al., 1997; Paile-Hyvarinen et al., 2003).
5-HT2C receptor agonists prevent type 2 diabetes
Lorcaserin (Belviq, lorcaserin hydrochloride), a 5-HT2C receptor agonist, was the first drug approved by the U.S. Food and Drug Administration-to treat obesity in 2010 (2012). Several studies have analyzed the impact of lorcaserin on the progression from prediabetes to type 2 diabetes and on the restoration of prediabetes blood glucose levels to normal levels (Higgins et al., 2020). It can result in weight loss and ameliorate glycemic parameters in prediabetes patients, and can prevent progression to type 2 diabetes and accelerate reversion to normal blood glucose levels (Nesto et al., 2016). It has also been shown to ameliorate glycemic control in relation to weight loss in obese patients with type 2 diabetes (Burke et al., 2017). Brain pro-opiomelanocortin peptides are essential and adequate neurochemical mediators of lorcaserin’s glucoregulatory influence. Lorcaserin dose-dependently ameliorates glycemic control in type 2 diabetes mice without decreasing food intake or body weight. It needs functional melanocortin-4 receptors on cholinergic preganglionic neurons to trigger its effects on glucose homeostasis (Burke et al., 2017). In contrast, melanocortin-4 receptors on cholinergic preganglionic neurons do not affect lorcaserin’s influence on feeding, suggesting a diversity in the neurocircuitry underpinning lorcaserin’s curative glycemic and anorectic effects. A hyperinsulinemic-euglycemic clamp study revealed that lorcaserin decreases hepatic glucose synthesis, raises glucose disposal, and ameliorates insulin sensitivity (Burke et al., 2017). In its recently CAMELLIA-TIMI 61 trail (Scirica et al., 2019), there is a small raise in cancer in lorcaserin group, so the U.S. Food and Drug Administration asks the manufacturer to withdraw lorcaserin from the U.S. market in February 2021 (Mathai, 2021).
5-HT4 receptor agonists reduce blood glucose level
Gastrointestinal diseases, including constipation and gastroparesis, are common in diabetic patients. As an agonist of the 5-HT4 receptor, mosapride ameliorates these symptoms and controls the glycemic response (Ueno et al., 2010). The 5-HT4 receptor is expressed in β-cells in mice, rats, pigs, and humans. Stimulation of the 5-HT4 receptor with mosapride or prucalopride (a selective, high-affinity 5-HT4 receptor agonist) induces insulin release in both pancreatic tissue and islet cells of rats in vitro. In rats, it also elevates the serum insulin level accompanied by a decrease in blood glucose (Chen et al., 2016).
Role of the central serotonergic system in eating and blood glucose regulation
Central serotonergic activity may modulate glucose metabolism via neuroendocrine effectors (Trento et al., 2010). There are obvious alterations in 5-HT levels in the prefrontal cortex, and in γ-aminobutyric acid and glutamate levels in the hippocampus in Spontaneously Diabetic Torii fatty rats compared with those in control rats (Sakimura et al., 2018). Hypothalamic 5-hydroxyindoleacetic acid concentrations in Goto-Kakizaki rats, a spontaneous diabetic model, were not different from those in control rats, whereas hypothalamic 5-HT concentrations in the streptozotocin-induced diabetic rats were significantly decreased (Gotoh et al., 2006). In addition, the expression of 5-HT1A receptors in the hypothalamus can be modulated by serum glucose levels (Jhanwar-Uniyal et al., 1994).
5-HT in feeding and anorexigen action
Maintaining the energy balance requires modulation of the amount and timing of food intake, and eating disorders are emerging as a critical health issue in several developed countries. As confirmed in the central nervous system, 5-HT is a fundamental neurotransmitter regulating several physiological processes that affect food intake. The most important features of the serotonergic effects on energy balance pathways are linked to the regulation of the arcuate nucleus pro-opiomelanocortin and agouti-related peptide/neuropeptide Y neuronal populations. Previous studies have established that 5-HT hyperpolarizes and suppresses agouti-related peptide/neuropeptide Y neurons and decreases the suppressive drive onto pro-opiomelanocortin cells by stimulation of 5-HT1B receptors. The 5-HT also activates pro-opiomelanocortin/cocaine- and amphetamine-regulated transcript neurons through activation of 5-HT2C receptors (Heisler et al., 2006), which results in a reciprocal increase in α-melanocyte stimulating hormone secretion and decline in agouti-related peptide secretion at melanocortin 4 receptors in the target areas. Subsequent elevation of 5-HT neurotransmission also modulates the hypothalamic-pituitary-adrenal axis (HPA) upstream of the corticotropin-secreting hormone (Heisler et al., 2007). CP-809101 (a selective 5-HT2C agonist) suppresses responding motivated by both food and nicotine in rats. CP-809101 inhibits the discriminative stimulus characteristics of nicotine with the same as Ro 60-0175 and lorcaserin (two structurally distinct 5-HT2C receptor agonists). Behaviors including hypolocomotion, ptosis, and chewing become evident after treated with both higher doses of lorcaserin and CP-809101. These results indicate that the use of 5-HT2C agonists as a therapeutic drug to treat nicotine dependence (Higgins et al., 2013).
The nucleus accumbens is a brain structure implicated in reward. Direct activation of 5-HT4 receptors in the nucleus accumbens suppresses the physiological initiation to feed and upregulates mRNA expression of cocaine- and amphetamine-regulated transcript in food-deprived and fed mice (Jean et al., 2007). Knockdown of 5-HT4 receptor with siRNA or injecting 5-HT4 receptor antagonist into the nucleus accumbens causes hyperphagia uniquely in fed but not in food-deprived mice. In both food-deprived and fed mice, this hyperphagia is not linked to alteration in mRNA expression of cocaine- and amphetamine-regulated transcript in the nucleus accumbens. 5-HT4 receptor regulates mRNA expression of cocaine- and amphetamine-regulated transcript into the nucleus accumbens through a cyclic adenosine monophosphate/protein kinase A signaling pathway (Jean et al., 2007). The nucleus accumbens-5-HT4 receptor/cocaine- and amphetamine-regulated transcript pathway tightly connects between hyperactivity and anorexia, indicating the presence of a fundamental functional unit susceptible to inhibit overeating linked to resting abiding by the rules of homeostasis (Jean et al., 2012).
Decreased 5-HT levels in pinealocytes of diabetic pigs
Streptozotocin-induced diabetes significantly impacts sympathetic neurotransmission and the metabolism of melatonin synthesis-associated indoles in the pineal gland of pigs. The most prominent effect of diabetes on pig pinealocytes is the reduction in 5-HT levels. However, the synthesis of 5-HT is not influenced, since there is no change in 5-hydroxytryptophan content; therefore, two mechanisms that could result in the reduction in 5-HT content should be taken into account—increased utilization of 5-HT for N-acetylserotonin synthesis and insufficient storage of 5-HT (Lewczuk et al., 2018). The N-acetylserotonin level in the pineal gland of diabetic pigs is remarkably higher than that in control pigs; however, this is not reflected in an elevated melatonin content. Moreover, there were no discrepancies in the basal and adrenergic-induced secretion of melatonin and N-acetylserotonin between the pineal glands of control and diabetic pigs in vitro (Lewczuk et al., 2018).
Elevating brain 5-HT levels to treat diabetes
Intranasal 5-HT administration reduces the body weight of diabetic rats and improves glucose tolerance, insulin-induced glucose utilization, and lipid metabolism (Derkach et al., 2015). Moreover, it restores the hormonal modulation of adenylyl cyclase activity in the hypothalamus and normalizes adenylyl cyclase activation by β-adrenergic agonists in the myocardium. The same administration induces metabolic and hormonal changes in control rats, some of which resemble those in type 2 diabetes, but to a lesser degree. The elevation of brain 5-HT content may be considered an effective method to treat type 2 diabetes and its complications (Derkach et al., 2015). Several other studies show that the intranasal delivery of peptides or proteins is a potential method to overcome the obstacles of the blood–brain barrier (Lochhead and Thorne, 2012). But the pathways and mechanisms of molecules delivery to central nervous system from the nasal passages still need completely understood.
Role of hypothalamic 5-HT in hypoglycemic regulation
Insulin-induced hypoglycemia activates widespread 5-HT release in several forebrain areas, such as the ventromedial hypothalamus, perifornical hypothalamus, paraventricular hypothalamus, cerebral cortex, and paraventricular thalamic nucleus (Otlivanchik et al., 2015). In conscious rats, bilateral perifornical hypothalamic glucoprivation with 5-thioglucose significantly triggers adrenal medullary epinephrine secretion and feeding, while clamping perifornical hypothalamic glucose levels in the postprandial brain blunts the epinephrine response to hypoglycemia by 30%. The perifornical hypothalamus modulates adrenomedullary and feeding responses in the case of a metabolic emergency in freely behaving conscious rats. These responses are partly mediated by perifornical hypothalamic orexin neurons and 5-HT signaling (Otlivanchik et al., 2015). The perifornical hypothalamus has glucose-excited and glucose-inhibited neurons—glucose-excited neurons are principally excited, while glucose-inhibited neurons can be inhibited or excited by 5-HT at hypoglycemic glucose levels in vitro. 5-HT also stimulates lactate generation in hypothalamic astrocytes in vitro. Depleting perifornical hypothalamus 5-HT blunts the epinephrine (but not feeding) response to the focal perifornical hypothalamus and systemic glucoprivation, while raising the perifornical hypothalamus 5-HT level amplifies the epinephrine response to hypoglycemia by 32% (Otlivanchik et al., 2015). Perifornical hypothalamus 5-HT promotes the adrenomedullary response, and that perifornical hypothalamus orexin neurons change the electrophysiological properties of adrenal premotor neurons facilitating adrenaline release in response to glucopenia response to local and systemic glucose deficit (Korim et al., 2014). Selective serotonin reuptake inhibitors increase the counterregulatory response to acute hypoglycemia and avoid the blunting of the counterregulatory response after recurrent hypoglycemia in rats (Sanders et al., 2008) and humans (Briscoe et al., 2008a,b), indicating that 5-HT plays a vital role in mediating this response. These results suggest a critical role for 5-HT in strengthening the counterregulatory response to hypoglycemia. But it is still unknown how serotonergic system is activated in response to insulin-induced hypoglycemia. And whether serotonergic neurons are activated by either decreased glucose level or increased insulin level is still unknown.
Role of nucleus accumbens 5-HT in blood glucose homeostasis
The nucleus accumbens is part of the reward circuitry that regulates feeding behavior. It contains glucose-sensing neurons (Papp et al., 2007) and provides input to the lateral hypothalamus (Groenewegen and Russchen, 1984), another region involved in glucose control (Yi et al., 2009; Morgan et al., 2015). Electrical stimulation of the shell nucleus accumbens using deep brain stimulation raises plasma levels of glucagon and glucose and stimulates neurons in the lateral hypothalamus (Diepenbroek et al., 2013), indicating a role for the shell nucleus accumbens in the modulation of glucose homeostasis. Locally infusing fluoxetine, a 5-HT reuptake inhibitor, in the shell nucleus accumbens of rats elevates blood glucose levels without overall altering glucoregulatory hormones, such as glucagon, insulin, and corticosterone. Its mechanism is not via the activity of the autonomic nervous system, since the glucagon level is not impacted. The effect on glucose levels might be due to the combined effects of higher endogenous glucose production and direct effect of 5-HT availability in the shell area of the nucleus accumbens on peripheral glucose uptake. Since peripheral glucose uptake is regulated by hypothalamus (Sudo et al., 1991) and the hypothalamus receives dense projections from the shell area of the nucleus accumbens (Sano and Yokoi, 2007). These results establish a role for the shell nucleus accumbens in systemic glucose metabolism and indicate that 5-HT may play a vital role in mediating these effects (Diepenbroek et al., 2017).
5-HT2C receptors in the nucleus of the solitary tract and hypothalamus modulate food intake and glucose homeostasis
Selective stimulation of 5-HT2C receptors in the nucleus of the solitary tract in the brainstem reduces feeding and is adequate to mediate acute food intake decrease induced by the use of lorcaserin, a 5-HT2C receptor agonist, as an obesity medication (D’Agostino et al., 2018). A subpopulation of hypothalamic neurons in the brainstem nucleus of solitary tract resemble pro-opiomelanocortin neurons in the hypothalamic arcuate nucleus and co-express 5-HT2C receptors and are stimulated by 5-HT2C receptor agonists. Deletion of 5-HT2C receptors in the hypothalamic arcuate nucleus inhibits the acute appetite-inhibitive function of lorcaserin; conversely, deletion in the hypothalamic arcuate nucleus prevents the total anorectic effect. These results indicate that 5-HT2C receptors in the brainstem nucleus of the solitary tract represent a subpopulation of 5-HT2C receptors that are capable of decreasing food intake when activated, and reveal that 5-HT2C receptor agonists as obesity medications need pro-opiomelanocortin in the nucleus of the solitary tract and the arcuate nucleus to reduce food intake (D’Agostino et al., 2018).
Mice lacking 5-HT2C receptors, especially in pro-opiomelanocortin neurons, show normal body weight but exhibit glucoregulatory defects, such as hyperglycemia, hyperglucagonemia, hyperinsulinemia, and insulin resistance (Berglund et al., 2013). In addition, these mice have no anorectic responses to serotonergic agents that inhibit appetite and gradually become hyperphagic and obese after being fed a high-fat/high-sugar diet. The demand for 5-HT2C receptors in pro-opiomelanocortin neurons to maintain normal energy and glucose homeostasis is further indicated by the effects of 5-HT2C knockout in pro-opiomelanocortin neurons in adult mice using a tamoxifen-inducible pro-opiomelanocortin-Cre system (Berglund et al., 2013). Treatment with m-chlorophenylpiperazine, a non-selective 5-HT2C receptor agonist, ameliorates insulin sensitivity and glucose homeostasis, and antagonists [ritanserin (a 5-HT2 and 5-HT1c antagonist) and MDL 72222 (a 5-HT antagonist)] or genetic deletion of 5-HT2C receptors compromises glucose homeostasis (Berglund et al., 2013). These results show that 5-HT2C receptor-expressing pro-opiomelanocortin neurons are required to regulate energy and glucose homeostasis and imply that pro-opiomelanocortin neurons are the target for the effect of 5-HT2C receptor agonists on weight reduction and glycaemia regulation (Berglund et al., 2013). Postsynaptic receptors, such as 5-HT1B, 5-HT2C, and possibly 5-HT2B receptors, are assumed to be directly stimulated by dexfenfluramine-induced 5-HT transporter-dependent and 5-HT2B receptor-dependent 5-HT secretion (Banas et al., 2011).
5-HT2C receptors expressed by pro-opiomelanocortin neurons of hypothalamic arcuate nucleus modulate food intake, energy homeostasis, and glucose metabolism. A subpopulation (about 25%) of the hypothalamic arcuate nucleus neurons, which areas are differ from leptin-activated, are depolarized by the 5-HT2C receptors agonist (m-chlorophenylpiperazine) through activation of the putative transient receptor potential C channels (Sohn et al., 2011). With this consistent mechanism, to record the brainstem nucleus of the solitary tract neurons in a voltage clamp and treated with tetrodotoxin to antagonize the inhibitory effect of 5-HT, 5-HT, and lorcaserin excite the brainstem nucleus of the solitary tract neurons through the activation of a post-synaptic, mixed cationic current (D’Agostino et al., 2018).
5-HT as a substrate of glucagon-like peptide 1 to impact energy homeostasis
5-HT depletion impairs the effect of exendin-4, a glucagon-like peptide 1 analog, to reduce body weight in rats, indicating that 5-HT is a vital mediator of the energy balance-related effect of glucagon-like peptide 1 receptor stimulation. 5-HT turnover and expression of 5-HT2A and 5-HT2C receptors in the hypothalamus are also altered by glucagon-like peptide 1 receptor activation (Anderberg et al., 2017). The 5-HT2A receptor is significantly associated with body weight reduction, anorexia, and fat mass loss caused by central glucagon-like peptide 1 receptor activation. Importantly, 5-HT2A receptors in the brain are also needed for peripherally administered liraglutide, a glucagon-like peptide-1 receptor agonist, to decrease feeding and body weight (Anderberg et al., 2017). The dorsal raphe harbors the cell bodies of 5-HT-synthesizing neurons that provide 5-HT to the hypothalamic nuclei. Glucagon-like peptide 1 receptor activation in the dorsal raphe is sufficient to lower hypophagia and elevate the electrical activity of serotonergic neurons in the dorsal raphe. This identifies 5-HT as a novel pivotal neural substrate for glucagon-like peptide 1 in influencing energy homeostasis and amplifies the current map of brain regions affected by glucagon-like peptide 1 receptor activation (Anderberg et al., 2017).
Role of the serotonergic system in diabetic macroangiopathy
5-HT is a significant vasoactive monoamine in the cardiovascular system. Reduction of 5-HT levels in platelets and elevation in plasma concentration may reflect increased secretion of 5-HT by hyperactive platelets. This elevation in plasma 5-HT levels may lead to the pathogenesis of vasospasm and atherosclerosis (Barradas et al., 1988), an important feature of cardiovascular diseases in diabetes (Forbes and Cooper, 2013).
Plasma 5-HT levels are elevated in diabetic patients, and this elevation is associated, at least in part, with platelet hyperfunction (Hasegawa et al., 2002). Although 5-HT-induced platelet aggregation is enhanced in diabetic patients, with an increase in serum advanced glycation end-product levels, there is no association between platelet aggregation and either fasting blood glucose or hemoglobin A1c levels. 5-HT-stimulated platelet aggregation is dose-dependently increased by advanced glycation end-products. Adenosine diphosphate-activated platelet aggregation is also enhanced by advanced glycation end-products; moreover this increase is abolished by the action of sarpogrelate, a selective 5-HT2A receptor antagonist (Hasegawa et al., 2002). These results suggest that advanced glycation end-products enhance platelet aggregation via the 5-HT receptor and may influence the development of thrombotic complications in diabetic patients (Hasegawa et al., 2002).
5-HT2A receptors in diabetes-related vascular complications
5-HT, stimulated by damaged vascular endothelial cells, participates in the pathological process of vascular complications such as carotid artery contraction (Matsumoto et al., 2014) and arteriogenesis (Bir et al., 2008) in diabetes via the 5-HT2A receptor. Sarpogrelate, a 5-HT2A receptor antagonist, attenuates diabetic cardiovascular complications; it decreases the blood glucose concentration (Matsumoto et al., 2014), inhibits the production of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 (Su et al., 2013), and reduces 5-HT-triggered contraction in aortas via PI3K pathways (Matsumoto et al., 2014). Combined sarpogrelate and sustained-release basic fibroblast growth factor therapy is effective in inducing neovascularization to restore arteriogenesis and tissue blood perfusion in diabetic mice (Bir et al., 2008). High glucose levels increase intercellular adhesion molecule-1 expression and decrease superoxide dismutase activity; these effects are partially abolished by sarpogrelate hydrochloride administration (Lin et al., 2007).
Enhanced contraction of arteries in type 2 diabetic mice is chiefly mediated by smooth muscle signaling pathway alterations that modulate calcium sensitivity of contractile proteins, rather than an alteration in endothelial-derived vasoactive factors or special 5-HT receptor subtypes (Nelson et al., 2012). 5-HT causes contraction via the activation of both 5-HT2A and 5-HT2B receptors in both diabetic and control mice. 5-HT1B receptor expression is detectable, but does not participate in the contraction in response to 5-HT in either diabetic or control mice, because there is no response to the 5-HT1B receptor agonist CP93129 (Nelson et al., 2012). Aberrant endothelial factors modulated by nitric oxide synthase and cyclooxygenase are also not involved in the increased contractions in response to 5-HT since endothelium removal; similarly, nitro-L-arginine and indomethacin do not have any obvious impact on contractions in response to 5-HT in the aorta in either diabetic or control mice. Contractions increase in response to all 5-HT2A and 5-HT2B receptor agonists, and suppression of both 5-HT2A and 5-HT2B receptors inhibits contraction response to 5-HT in arteries from diabetic mice. This occurs due to altered expression of either receptor subtype in diabetic arteries (Nelson et al., 2012). Contractions in response to 5-HT and both 5-HT2A and 5-HT2B receptor agonists are diminished by inhibiting Rho kinase. Receptor-independent contractions of diabetic arteries to KCl are also enhanced and partly mediated by Rho kinase (Nelson et al., 2012). In most arteries, serotonin-induced contractions are mediated by 5HT2A receptors on vascular smooth muscle, which activates the Rho/Rho kinase pathway to regulate phosphorylation of myosin light chain phosphatase and myosin light chain (Dreja et al., 2002; Nuno et al., 2007). These results suggest that an alteration in rho kinase modulation of calcium sensitivity, but not 5-HT receptor expression, may mediate the hypercontractility of arteries from diabetics.
5-HT1B receptors in peripheral arterioles
Cardiopulmonary bypass is linked to a decline in the contractile response of peripheral arterioles to 5-HT, which is exacerbated in diabetes. This 5-HT-stimulated contractile response of peripheral arterioles is mediated by the 5-HT1B receptor in both non-diabetic and diabetic patients (Sabe et al., 2018).
5-HT2A receptor antagonists in thrombosis
Polyethylene tube-induced thrombus formation was significantly elevated in streptozotocin-induced diabetic rats compared to that in normal rats (Yamada et al., 2012). Both 5-HT and high glucose upregulate the expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells, and this upregulation is further enhanced by the combination of 5-HT and high glucose. Sarpogrelate, a 5-HT2A receptor antagonist, but not aspirin, inhibits the increased expression of vascular cell adhesion molecule-1 triggered by 5-HT and high glucose (Yamada et al., 2012). These results imply that 5-HT drives the increased thrombogenesis in diabetes and that 5-HT2A receptor antagonists may be potential novel candidates to treat diabetic complications.
5-HT receptors in retinal superoxide generation
Reactive oxygen species play a crucial role in the pathogenesis of diabetic retinopathy. Incubating cells or retinal explants in 30 mmol/L glucose significantly activated superoxide generation compared to incubation in 5 mmol/L glucose (Du et al., 2015). This response is diminished or blocked by pharmacological inhibition of the α1-adrenergic receptor (a Gq-coupled receptor) or Gs-coupled 5-HT receptors (5-HT4, 5-HT6, and 5-HT7) or by activation of the Gi-coupled α2-adrenergic receptor. Links between different G protein-coupled receptor pathways regarding superoxide generation may originate from hyperglycemia-induced elevations in cytosolic Ca2+ levels (Du et al., 2015).
5-HT in diabetic vasculopathy
Levels of blood 5-HT in diabetic retinopathy patients are significantly lower than those in non-diabetic retinopathy patients (Pietraszek et al., 1992), while plasma 5-HT concentration is significantly elevated in diabetes. This is linked to vascular alterations in the retina. Platelets in diabetic patients take up less 5-HT than those in non-diabetic patients, spontaneous secretion of 5-HT from platelets is concomitantly enhanced, and platelets show an increased response to 5-HT. However, 5-HT-stimulated aggregation is not related to the presence of retinopathy (Pietraszek et al., 1992). These data indicate that 5-HT may be involved in the pathogenesis of diabetic vasculopathy.
Role of selective serotonin reuptake inhibitors in diabetes-related cardiovascular complications
Escitalopram, a selective serotonin reuptake inhibitor, improves high-fat/high-fructose diet- and streptozotocin-induced metabolic and cardiac impairment characteristics, such as inflammation, oxidative stress, apoptosis, fibrosis, hypertrophy, and impaired conduction (Ahmed et al., 2020). These benefits may be secondary to its primary beneficial effects on blood glucose control and, consequently, the downregulated expression of receptor for advanced glycation end-product. Escitalopram can be deemed a profitable antidepressant drug in diabetic patients because it ameliorates blood glucose control in diabetes and can also prevent diabetes-related cardiovascular complications (Ahmed et al., 2020).
Roles of the serotonergic syste in diabetic peripheral neuropathy
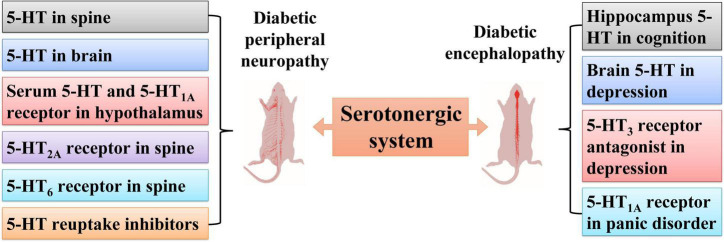
Hyperglycemia is no longer regarded as a unique etiological factor for diabetic peripheral neuropathy (Nelson et al., 2012; Sabe et al., 2018), as painful neuropathy is also a common comorbidity in patients with diabetes. The roles of the serotonergic system in diabetic peripheral neuropathy and diabetic encephalopathy are described in Figure 2.
FIGURE 2.
Roles of the serotonergic system in diabetic peripheral neuropathy and diabetic encephalopathy. Serotonergic systems involving 5-HT in serum, spine, brain, the 5-HT2A receptor and 5-HT6 receptor in the spine, and the 5-HT1A receptor in the hypothalamus participate in diabetic peripheral neuropathy. The serotonergic system is also involved in diabetic encephalopathy, including hippocampal 5-HT in cognition dysfunction, brain 5-HT in depression, 5-HT3 receptor antagonists in depression, and the 5-HT1A receptor in panic disorder.
Spinal 5-HT
Painful diabetic neuropathy activates neuronal hyperactivity in the periaqueductal gray and spinal cord. As a critical region in descending nociceptive regulation, the periaqueductal gray makes use of relay stations in the noradrenergic and serotonergic brainstem regions. Streptozotocin-induced diabetic rats present with chemical allodynia and mechanical hyperalgesia, coupled with higher spinal levels of noradrenaline and 5-HT and more neurons expressing tryptophan hydroxylase in the rostral ventrolateral medulla and tyrosine hydroxylase in the A5 noradrenergic cell group (Morgado et al., 2011). Administration of insulin growth factor 1 prevents the behavioral signs of painful diabetic neuropathy and restores the neuronal hyperactivity in the spinal cord and ventrolateral periaqueductal gray and the neurochemical alterations in the brainstem and spinal cord. Based on the facilitatory role of noradrenergic and serotonergic descending regulation during chronic pain, the elevated 5-HT and noradrenaline innervation of the dorsal horn in streptozotocin-induced diabetic rats may explain the pain reinforcement during painful diabetic neuropathy. The protective effects of insulin growth factor 1 in painful diabetic neuropathy are likely due to the inhibition of increased peripheral input to the somatosensory system, but direct central effects cannot be excluded (Morgado et al., 2011).
Serum 5-HT and 5-HT1A receptors in the hypothalamus
Zucker diabetic fatty (fa/fa) rats progress to type 2 diabetes spontaneously with aging and exhibit nociceptive hypersensitivity at 13-week of age. Daily 30-min transcutaneous auricular vagus nerve stimulation (2/15 Hz, 2 mA) for 27 consecutive days significantly inhibited the progression of nociceptive hypersensitivity in Zucker diabetic fatty rats, as measured based on mechanical allodynia and thermal hyperalgesia in the hindpaw. This advantageous impact on nociceptive behavior is associated with an increase in 5-HT plasma levels and upregulated expression of the 5-HT1A receptor in the hypothalamus (Li et al., 2018).
5-HT2A receptors in the spine
Systemic or intrathecal injection of duloxetine, a dual inhibitor of serotonin and norepinephrine reuptake, alleviates tactile allodynia in diabetic rats (Mixcoatl-Zecuatl and Jolivalt, 2011). The effect of systemic duloxetine administration is inhibited by intrathecal injection of ketanserin (a non-selective 5-HT2A receptor antagonist) or pruvanserin (a selective 5-HT2A receptor antagonist), suggesting the involvement of spinal 5-HT2A receptors in the mechanism of action of duloxetine. Compared with spinal injection, local peripheral and systemic administration of ketanserin or pruvanserin relieve tactile allodynia in diabetic rats. This effect is restored immediately after local or systemic administration of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride, a 5-HT receptor agonist (Mixcoatl-Zecuatl and Jolivalt, 2011). These data suggest a role of spinal 5-HT2A receptors in the effect of duloxetine in improving painful diabetic neuropathy. These results also show that the role of 5-HT2A receptors relies on the neuraxis level where activation occurs, with peripheral activation resulting in tactile allodynia in diabetic rats; conversely, spinal activation of this receptor relieves tactile allodynia.
Lower urinary tract impairment is one the most common diabetic complications. The 5-HT2A receptor in spinal micturition control is involved in urethane anesthetized diabetes rats. Intravenous injection of the 5-HT2A/2C receptor agonist (2,5-methoxy-4-iodoamphetamine) triggers high frequency oscillations and ameliorates micturition (Tu et al., 2015). Expressions of 5-HT2A receptor and 5-HT2C receptor are raised in lumbosacral cord motoneurons, and the number of serotonergic paraneurons are declined in the urethra in diabetes rats (Cao et al., 2019). The function of lower number of urethral paraneurons in diabetes still needs to be clarified but may be associated with the decreased urethral sensation induced by diabetes.
5-HT6 receptors in the spine
The 5-HT6 receptor accurately regulates critical neuro-developmental processes, such as migration and differentiation of neuron (Chaumont-Dubel et al., 2020). The activation of 5-HT6 receptors leads to the activation of adenylyl cyclase and may have an excitatory efficiency on neuronal activity (Ruat et al., 1993). Although there are very few studies on the role of 5-HT6 receptors in nociception, these receptors seem to mediate pronociceptive efficacies in the spinal cord and the periphery. Pharmacological suppression of 5-HT6 receptors diminishes formalin-stimulated nociceptive behavior (Finn et al., 2007), and spinal and local peripheral 5-HT6 receptors have a pronociceptive role in formalin-triggered pain (Castaneda-Corral et al., 2009).
Spinal nerve damage results in a loss of descending serotonergic neurons (Leong et al., 2011); in contrast, it does not alter spinal 5-HT6 receptor expression (Morgado et al., 2011). Differentiation from nerve injury-caused neuropathy, and 5-HT6 receptor expression is obviously downregulated in diabetes-induced neuropathy, which can be due to the effect of intrathecal injection of 5-HT6 receptor antagonist SB-258585 to ameliorate thermal hyperalgesia in diabetic rats (Sari et al., 2019). Systemic, but not spinal, inhibition of 5-HT6 receptors relieved thermal hyperalgesia in diabetic mice. These results indicate that 5-HT6 receptor antagonists may be therapeutic targets for pharmacotherapy of diabetic neuropathy (Sari et al., 2019).
Selective 5-HT reuptake inhibitors
The antiallodynic efficacies of intrathecal injections of the antidepressants milnacipran, paroxetine, and fluvoxamine were measured in two rat models of neuropathic pain—streptozotocin-induced diabetic neuropathy and chronic sciatic nerve constriction injury (Ikeda et al., 2009). Intrathecal administration of milnacipran, a serotonin and noradrenaline reuptake inhibitor, has antiallodynic efficacy in both chronic constriction injury and streptozotocin-induced diabetic rat models in a dose-dependent manner. Intrathecal injection of both fluvoxamine and paroxetine, which are selective 5-HT reuptake inhibitors, has antiallodynic effects in streptozotocin-induced diabetic rats in a dose-dependent manner, but elicited little antiallodynic effects in rats with chronic constriction injury (Ikeda et al., 2009). These results suggest that the two antidepressants (paroxetine and fluvoxamine) may be effective for the treatment of diabetic neuropathic pain.
Role of the serotonergic system in diabetic encephalopathy
Hippocampal 5-HT in cognitive impairment
Rats with streptozotocin-induced type 1 diabetes exhibit cognitive dysfunction during acquisition sessions and long-term retention in the active avoidance test. Diabetic rats also exhibit cognitive dysfunctions in terms of spatial learning, reference, and working memory in the Morris water maze. Streptozotocin remarkably reduced norepinephrine levels in the cortex and dopamine levels in the hippocampus, but increased the levels of 5-HT and dopamine in the cortex 35 days after injection. The 5-HT level in the hippocampus was also significantly elevated (Lin et al., 2018). As a microbial deamination metabolite of tryptophan, 3-indolepropionic acid is an effective neuroprotective antioxidant. Administration of both 3-indolepropionic acid and 5-HT significantly improve cognitive dysfunction in diabetic mice (Liu et al., 2020). Moreover, higher plasma levels of 3-indolepropionic acid are associated with a lower risk of type 2 diabetes (Tuomainen et al., 2018).
Antidepressant-like action of brain 5-HT
Neuropathological depletion of brain monoaminergic activity, due to chronic diabetes, specifically with regard to the 5-HT system, may result in mood- and behavior-related complications that further reduce the quality of life. Hypothalamic-pituitary-adrenal activity, insulin signaling, and glycogen synthase kinase 3 modulations are hampered and interlinked to the serotonergic system following diabetic progression (Prabhakar et al., 2015).
Disorder of the serotonergic system is a sign of diabetes-related depression. Reduced central 5-HT levels can be mediated by diabetes-induced inflammation and oxidative stress in the central nervous system. Oxidative stress results in dysfunction of the glucocorticoid receptor, negative feedback impairment, and altered functioning of the HPA axis. Hyperactivity of the HPA axis increases glucocorticoid secretion, which in turn inhibits 5-HT synthesis (Prabhakar et al., 2015). On the contrary, neuroinflammation in diabetes modulates the secretion of cytokines, which decreases 5-HT synthesis, increases 5-HT reuptake, and finally reduces its availability (Felger and Lotrich, 2013). In diabetic rats, the levels of 5-HT and brain-derived neurotrophic factor in the cortex and hippocampus are reduced, and serum corticosterone levels are elevated, which suggests hyperactivity of the HPA axis.
A single facial injection of botulinum neurotoxin A induces a quick and extended amelioration of depression-like behaviors in naïve and space-restriction-stressed mice, with reduced duration of immobility in behavioral despair tests. Botulinum neurotoxin A significantly increases the 5-HT levels in numerous brain areas, such as the hippocampus and hypothalamus, in space-restriction-stressed mice (Li et al., 2019). It was also shown to increase the expression of N-methyl-D-aspartate receptor subunits NR1 and NR2B in the hippocampus, which are significantly downregulated in space-restriction-stressed mice. Furthermore, botulinum neurotoxin A significantly increases the expression of brain-derived neurotrophic factor in the hypothalamus, hippocampus, amygdala, and prefrontal cortex, which are downregulated in space-restriction-stressed mice. Botulinum neurotoxin A also momentarily upregulates the expression of phosphorylated extracellular signal-regulated kinase and phosphorylated cAMP-response element binding protein, which are downregulated in the hippocampus of space-restriction-stressed mice (Li et al., 2019). These data demonstrate that botulinum neurotoxin A administration has an antidepressant-like effect on mice, which is linked to elevated 5-HT levels and the activation of brain-derived neurotrophic factor/extracellular signal-regulated kinase/cAMP-response element binding protein pathways in the hippocampus, backing further research on botulinum neurotoxin A treatment in depression.
5-HT3 receptor antagonists in diabetes-induced depression
5-HT is one of the most frequently imbalanced neurotransmitters in the etiology of major depressive disorder, and this system is the chief target of most medications for the treatment of depression (Yohn et al., 2017). Antidepressants treatment in adults increases the risk of developing new-onset type 2 diabetes. Proof from human and animal studies indicates a relationship between increased risk of diabetes and the use of antidepressants that act on 5-HT signaling, such as 5-HT-norepinephrine reuptake inhibitors, 5-HT antagonist and reuptake inhibitors, and noradrenergic and specific serotonergic antidepressants (De Long et al., 2015). Spontaneously Diabetic Torii fatty rats exhibit depression-like behaviors and altered baseline HPA activity. In addition, neurotransmitter levels are dysregulated in the brain areas involved in the pathophysiology of depression (Sakimura et al., 2018).
Diabetic mice also show remarkable behavioral deficiencies, such as anxiety-like behavior in the open field test, depression-like behavior in the forced swim test, and sociability deficits in the social interaction test, accompanied by an obvious decline in 5-HT content in the associated brain areas (Gupta et al., 2016). Similar to fluoxetine, a selective serotonin reuptake inhibitor, N-(3-chloro-2-methylphenyl)quinoxalin-2-carboxamide (1 mg/kg), prevents these behavioral deficits and normalizes brain 5-HT levels. N-(3-chloro-2-methylphenyl)quinoxalin-2-carboxamide (0.5 mg/kg) ameliorated only diabetes-induced depressive-like behavior and 5-HT decrease, but not anxiety-like effects. 1-(m-chlorophenyl)-biguanide, a 5-HT3 receptor agonist, blunted the N-(3-chloro-2-methylphenyl)quinoxalin-2-carboxamide-activated behavioral response and elevated brain 5-HT levels (Gupta et al., 2016). These results suggest that N-(3-chloro-2-methylphenyl)quinoxalin-2-carboxamide prevents diabetes-induced depressive phenotypes in mice, which may be attributed to the antagonism of 5-HT3 receptors and elevation in 5-HT levels in different brain areas.
5-HT1A receptors in panic disorder in type 1 diabetes
Diabetic animals exhibit dysregulation of the serotonergic system in some brain regions linked to anxiety-like responses. In one study, 4 weeks after diabetic identification, the threshold of electric stimulation of the dorsal periaqueductal gray to reduce escape behavior in diabetic rats was found to be lower than that in normoglycemic rats (Gambeta et al., 2017); open-field test results indicated that there were no negative effects on locomotor activity in diabetic rats compared with normoglycemic rats. Intra-dorsal administration of the 5-HT1A receptor agonist (±)-8-hydroxy-2-(di-n-propylamino)tetralin in the periaqueductal gray increases the increment threshold in both diabetic and normoglycemic rats, indicating a panicolytic-like effect. Diabetic rats present with a more significant panicolytic-like response than normoglycemic rats, since a higher increment threshold was measured after 8-hydroxy-2-(di-n-propylamino)tetralin treatment, which could be a result of the upregulated expression of 5-HT1A receptors in the dorsal periaqueductal gray in diabetic rats (Gambeta et al., 2017). These data suggest that a lack of serotonergic regulation of the dorsal periaqueductal gray is involved in initiating the panic attacks and that 5-HT1A receptors might be critical for the panicolytic-like response.
Conclusion
There are two serotonergic systems in the human body—the central system and the peripheral system. With regard to the central serotonergic system, elevating 5-HT signaling has been used to therapeutically reduce body weight by inhibiting appetite. 5-HT is also thought to function in vasospasm and increase platelet aggregability, which can cause atherosclerosis. In peripheral tissues, inhibiting 5-HT signaling may represent a novel anti-obesity treatment by reducing energy expenditure and ameliorating insulin resistance (Crane et al., 2015; Oh et al., 2015). Systemic tryptophan hydroxylase 1 inhibitors and a peripheral tryptophan hydroxylase 1 inhibitor (LP-533401) have been patented for use in treating diabetes and obesity (Kolodziejczak et al., 2015; Abg Abd Wahab et al., 2019). However, there are still no clinical trials on the use of these drugs for the treatment of diabetes.
Upregulated expression of 5-HT2C receptors in both β-cells and the hypothalamus can be a protective strategy to avoid redundant energy intake. 5-HT2C receptor-expressing pro-opiomelanocortin neurons are required to regulate energy and glucose homeostasis (Berglund et al., 2013). Central 5-HT2C receptors control glucose homeostasis and could be a rational target for the treatment of type 2 diabetes. 5-HT2A receptors are broadly expressed in the central nervous system (Zhang and Stackman<suffix>Jr.</suffix>, 2015) and are needed for peripherally administered liraglutide to decrease feeding and body weight (Anderberg et al., 2017). Brain serotonergic deficiencies are linked to depressive-like behavior in diabetes and selective serotonin reuptake inhibitors prevent these behavioral deficits and normalizes brain 5-HT levels (Gupta et al., 2016).
There is limited options for the investigation of the role of central serotonergic system in diabetes in humans in vivo. The data in the present review are focused mostly on the results obtained from different animal models of diabetes. More experimental data on humans are expected to support this view in the future.
Author contributions
JZ, YC, and XL conceptualized the topics. JZ, YC, XL, and HZ wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81770806), the Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0249), and Special Project for Enhancing Science and Technology Innovation Ability of Army Medical University (No. 2019XYY16).
References
- Abg Abd Wahab D. Y., Gau C. H., Zakaria R., Muthu Karuppan M. K., Bs A. R., et al. (2019). Review on cross talk between neurotransmitters and neuroinflammation in striatum and cerebellum in the mediation of motor behaviour. Biomed. Res. Int. 2019:1767203. 10.1155/2019/1767203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed L. A., Shiha N. A., Attia A. S. (2020). Escitalopram ameliorates cardiomyopathy in type 2 diabetic rats via modulation of receptor for advanced glycation end products and its downstream signaling cascades. Front. Pharmacol. 11:579206. 10.3389/fphar.2020.579206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amireault P., Sibon D., Cote F. (2013). Life without peripheral serotonin: insights from tryptophan hydroxylase 1 knockout mice reveal the existence of paracrine/autocrine serotonergic networks. ACS Chem. Neurosci. 4 64–71. 10.1021/cn300154j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderberg R. H., Richard J. E., Eerola K., Lopez-Ferreras L., Banke E., Hansson C., et al. (2017). Glucagon-Like peptide 1 and its analogs act in the dorsal raphe and modulate central serotonin to reduce appetite and body weight. Diabetes Metab. Res. Rev. 66 1062–1073. 10.2337/db16-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas S. M., Doly S., Boutourlinsky K., Diaz S. L., Belmer A., Callebert J., et al. (2011). Deconstructing antiobesity compound action: requirement of serotonin 5-HT2B receptors for dexfenfluramine anorectic effects. Neuropsychopharmacology 36 423–433. 10.1038/npp.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barradas M. A., Gill D. S., Fonseca V. A., Mikhailidis D. P., Dandona P. (1988). Intraplatelet serotonin in patients with diabetes mellitus and peripheral vascular disease. Eur. J. Clin. Invest. 18 399–404. 10.1111/j.1365-2362.1988.tb01030.x [DOI] [PubMed] [Google Scholar]
- Berger M., Gray J. A., Roth B. L. (2009). The expanded biology of serotonin. Annu. Rev. Med. 60 355–366. 10.1146/annurev.med.60.042307.110802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund E. D., Liu C., Sohn J. W., Liu T., Kim M. H., Lee C. E., et al. (2013). Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Invest. 123 5061–5070. 10.1172/JCI70338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bir S. C., Fujita M., Marui A., Hirose K., Arai Y., Sakaguchi H., et al. (2008). New therapeutic approach for impaired arteriogenesis in diabetic mouse hindlimb ischemia. Circ. J. 72 633–640. 10.1253/circj.72.633 [DOI] [PubMed] [Google Scholar]
- Breum L., Bjerre U., Bak J. F., Jacobsen S., Astrup A. (1995). Long-term effects of fluoxetine on glycemic control in obese patients with non-insulin-dependent diabetes mellitus or glucose intolerance: influence on muscle glycogen synthase and insulin receptor kinase activity. Metabolism 44 1570–1576. 10.1016/0026-0495(95)90077-2 [DOI] [PubMed] [Google Scholar]
- Briscoe V. J., Ertl A. C., Tate D. B., Davis S. N. (2008a). Effects of the selective serotonin reuptake inhibitor fluoxetine on counterregulatory responses to hypoglycemia in individuals with type 1 diabetes. Diabetes Metab. Res. Rev. 57 3315–3322. 10.2337/db08-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe V. J., Ertl A. C., Tate D. B., Dawling S., Davis S. N. (2008b). Effects of a selective serotonin reuptake inhibitor, fluoxetine, on counterregulatory responses to hypoglycemia in healthy individuals. Diabetes Metab. Res. Rev. 57 2453–2460. 10.2337/db08-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie B. B., Shore P. A. (1957). A concept for a role of serotonin and norepinephrine as chemical mediators in the brain. Ann. N. Y. Acad. Sci. 66 631–642. 10.1111/j.1749-6632.1957.tb40753.x [DOI] [PubMed] [Google Scholar]
- Burke L. K., Ogunnowo-Bada E., Georgescu T., Cristiano C., De Morentin P. B. M., Valencia Torres L., et al. (2017). Lorcaserin improves glycemic control via a melanocortin neurocircuit. Mol. Metab. 6 1092–1102. 10.1016/j.molmet.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao N., Huang J., Ni J., Si J., Gu B., Wang Z., et al. (2019). Streptozotocin-induced diabetes causes upregulation of serotonin (5-HT)(2A/C) receptors in lumbosacral cord motoneurons and down regulation of serotonergic paraneurons in the urethra. Brain Res. 1715 21–26. 10.1016/j.brainres.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Castaneda-Corral G., Rocha-Gonzalez H. I., Araiza-Saldana C. I., Ambriz-Tututi M., Vidal-Cantu G. C., Granados-Soto V. (2009). Role of peripheral and spinal 5-HT6 receptors according to the rat formalin test. Neuroscience 162 444–452. 10.1016/j.neuroscience.2009.04.072 [DOI] [PubMed] [Google Scholar]
- Chaumont-Dubel S., Dupuy V., Bockaert J., Becamel C., Marin P. (2020). The 5-HT6 receptor interactome: new insight in receptor signaling and its impact on brain physiology and pathologies. Neuropharmacology 172:107839. 10.1016/j.neuropharm.2019.107839 [DOI] [PubMed] [Google Scholar]
- Chen H., Hong F., Chen Y., Li J., Yao Y. S., Zhang Y., et al. (2016). Activation of islet 5-HT4 receptor regulates glycemic control through promoting insulin secretion. Eur. J. Pharmacol. 789 354–361. 10.1016/j.ejphar.2016.07.024 [DOI] [PubMed] [Google Scholar]
- Choi W., Namkung J., Hwang I., Kim H., Lim A., Park H. J., et al. (2018). Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat. Commun. 9:4824. 10.1038/s41467-018-07287-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J. D., Palanivel R., Mottillo E. P., Bujak A. L., Wang H., Ford R. J., et al. (2015). Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 21 166–172. 10.1038/nm.3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino G., Lyons D., Cristiano C., Lettieri M., Olarte-Sanchez C., Burke L. K., et al. (2018). Nucleus of the solitary tract serotonin 5-HT2C receptors modulate food intake. Cell Metab. 28 619–630.e5. 10.1016/j.cmet.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Long N. E., Stepita R. A., Taylor V. H., Holloway A. C. (2015). Major depressive disorder and diabetes: does serotonin bridge the gap? Curr. Diab. Rev. 11 71–78. 10.2174/1573399811666150223123053 [DOI] [PubMed] [Google Scholar]
- Derkach K. V., Bondareva V. M., Chistyakova O. V., Berstein L. M., Shpakov A. O. (2015). The effect of long-term intranasal serotonin treatment on metabolic parameters and hormonal signaling in rats with high-fat diet/low-dose streptozotocin-induced type 2 diabetes. Int. J. Endocrinol. 2015:245459. 10.1155/2015/245459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepenbroek C., Rijnsburger M., Eggels L., Van Megen K. M., Ackermans M. T., Fliers E., et al. (2017). Infusion of fluoxetine, a serotonin reuptake inhibitor, in the shell region of the nucleus accumbens increases blood glucose concentrations in rats. Neurosci. Lett. 637 85–90. 10.1016/j.neulet.2016.11.045 [DOI] [PubMed] [Google Scholar]
- Diepenbroek C., Van Der Plasse G., Eggels L., Rijnsburger M., Feenstra M. G., Kalsbeek A., et al. (2013). Alterations in blood glucose and plasma glucagon concentrations during deep brain stimulation in the shell region of the nucleus accumbens in rats. Front. Neurosci. 7:226. 10.3389/fnins.2013.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreja K., Voldstedlund M., Vinten J., Tranum-Jensen J., Hellstrand P., Swärd K. (2002). Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler. Thromb. Vasc. Biol. 22 1267–1272. 10.1161/01.atv.0000023438.32585.a1 [DOI] [PubMed] [Google Scholar]
- Du Y., Cramer M., Lee C. A., Tang J., Muthusamy A., Antonetti D. A., et al. (2015). Adrenergic and serotonin receptors affect retinal superoxide generation in diabetic mice: relationship to capillary degeneration and permeability. FASEB J. 29 2194–2204. 10.1096/fj.14-269431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V., Asero B. (1952). Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169 800–801. 10.1038/169800b0 [DOI] [PubMed] [Google Scholar]
- Fakhoury M. (2016). Revisiting the serotonin hypothesis: implications for major depressive disorders. Mol. Neurobiol. 53 2778–2786. 10.1007/s12035-015-9152-z [DOI] [PubMed] [Google Scholar]
- Felger J. C., Lotrich F. E. (2013). Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246 199–229. 10.1016/j.neuroscience.2013.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn D. P., Fone K. C., Beckett S. R., Baxter J. A., Ansell L., Marsden C. A., et al. (2007). The effects of pharmacological blockade of the 5-HT(6) receptor on formalin-evoked nociceptive behaviour, locomotor activity and hypothalamo-pituitary-adrenal axis activity in rats. Eur. J. Pharmacol. 569 59–63. 10.1016/j.ejphar.2007.05.020 [DOI] [PubMed] [Google Scholar]
- Forbes J. M., Cooper M. E. (2013). Mechanisms of diabetic complications. Physiol. Rev. 93 137–188. 10.1152/physrev.00045.2011 [DOI] [PubMed] [Google Scholar]
- Gambeta E., Sestile C. C., Fogaca M. V., Guimaraes F. S., Audi E. A., Da Cunha J. M., et al. (2017). A serotonergic deficit in the dorsal periaqueductal gray matter may underpin enhanced panic-like behavior in diabetic rats. Behav. Pharmacol. 28 558–564. 10.1097/FBP.0000000000000332 [DOI] [PubMed] [Google Scholar]
- Gehlert D. R., Shaw J. (2014). 5-Hydroxytryptamine 1A (5HT1A) receptors mediate increases in plasma glucose independent of corticosterone. Eur. J. Pharmacol. 745 91–97. 10.1016/j.ejphar.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Gilles M., Wilke A., Kopf D., Nonell A., Lehnert H., Deuschle M. (2005). Antagonism of the serotonin (5-HT)-2 receptor and insulin sensitivity: implications for atypical antipsychotics. Psychosom. Med. 67 748–751. 10.1097/01.psy.0000174994.91245.34 [DOI] [PubMed] [Google Scholar]
- Goodnick P. J., Kumar A., Henry J. H., Buki V. M., Goldberg R. B. (1997). Sertraline in coexisting major depression and diabetes mellitus. Psychopharmacol. Bull. 33 261–264. [PubMed] [Google Scholar]
- Gotoh M., Li C., Yatoh M., Okabayashi N., Habu S., Hirooka Y. (2006). Hypothalamic monoamine metabolism is different between the diabetic GK (Goto-Kakizaki) rats and streptozotocin-induced diabetic rats. Brain Res. 107 497–501. 10.1016/j.brainres.2005.12.022 [DOI] [PubMed] [Google Scholar]
- Groenewegen H. J., Russchen F. T. (1984). Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J. Comp. Neurol. 223 347–367. 10.1002/cne.902230303 [DOI] [PubMed] [Google Scholar]
- Gupta D., Thangaraj D., Radhakrishnan M. (2016). A novel 5HT3 antagonist 4i (N-(3-chloro-2-methylphenyl)quinoxalin-2-carboxamide) prevents diabetes-induced depressive phenotypes in mice: modulation of serotonergic system. Behav. Brain Res. 297 41–50. 10.1016/j.bbr.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Hajduch E., Dombrowski L., Darakhshan F., Rencurel F., Marette A., Hundal H. S. (1999a). Biochemical localisation of the 5-HT2A (serotonin) receptor in rat skeletal muscle. Biochem. Biophys. Res. Commun. 257 369–372. 10.1006/bbrc.1999.0471 [DOI] [PubMed] [Google Scholar]
- Hajduch E., Rencurel F., Balendran A., Batty I. H., Downes C. P., Hundal H. S. (1999b). Serotonin (5-Hydroxytryptamine), a novel regulator of glucose transport in rat skeletal muscle. J. Biol. Chem. 274 13563–13568. 10.1074/jbc.274.19.13563 [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Suehiro A., Higasa S., Namba M., Kakishita E. (2002). Enhancing effect of advanced glycation end products on serotonin-induced platelet aggregation in patients with diabetes mellitus. Thromb. Res. 107 319–323. 10.1016/s0049-3848(02)00348-1 [DOI] [PubMed] [Google Scholar]
- Heisler L. K., Jobst E. E., Sutton G. M., Zhou L., Borok E., Thornton-Jones Z., et al. (2006). Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 51 239–249. 10.1016/j.neuron.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Heisler L. K., Pronchuk N., Nonogaki K., Zhou L., Raber J., Tung L., et al. (2007). Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J. Neurosci. 27 6956–6964. 10.1523/JNEUROSCI.2584-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D. C. (2002). Atypical antipsychotic-induced diabetes mellitus: how strong is the evidence? CNS Drugs 16 77–89. 10.2165/00023210-200216020-00001 [DOI] [PubMed] [Google Scholar]
- Higgins G. A., Fletcher P. J., Shanahan W. R. (2020). Lorcaserin: a review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol. Ther. 205:107417. 10.1016/j.pharmthera.2019.107417 [DOI] [PubMed] [Google Scholar]
- Higgins G. A., Silenieks L. B., Lau W., De Lannoy I. A., Lee D. K., Izhakova J., et al. (2013). Evaluation of chemically diverse 5-HT2c receptor agonists on behaviours motivated by food and nicotine and on side effect profiles. Psychopharmacology (Berl.) 226 475–490. 10.1007/s00213-012-2919-2 [DOI] [PubMed] [Google Scholar]
- Ikeda T., Ishida Y., Naono R., Takeda R., Abe H., Nakamura T., et al. (2009). Effects of intrathecal administration of newer antidepressants on mechanical allodynia in rat models of neuropathic pain. Neurosci. Res. 63 42–46. 10.1016/j.neures.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Jean A., Conductier G., Manrique C., Bouras C., Berta P., Hen R., et al. (2007). Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc. Natl. Acad. Sci. U S A. 104 16335–16340. 10.1073/pnas.0701471104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A., Laurent L., Bockaert J., Charnay Y., Dusticier N., Nieoullon A., et al. (2012). The nucleus accumbens 5-HTR(4)-CART pathway ties anorexia to hyperactivity. Trans. Psychiatry 2:e203. 10.1038/tp.2012.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M., Moorjani B., Kahn A. H. (1994). Indications of pre- and post-synaptic 5-HT1A receptor interactions in feeding behavior and neuroendocrine regulation. Brain Res. 646 247–257. 10.1016/0006-8993(94)90086-8 [DOI] [PubMed] [Google Scholar]
- Jones L. A., Sun E. W., Martin A. M., Keating D. J. (2020). The ever-changing roles of serotonin. Int. J. Biochem. Cell Biol. 125:105776. 10.1016/j.biocel.2020.105776 [DOI] [PubMed] [Google Scholar]
- Khoshnevisan K., Baharifar H., Torabi F., Sadeghi Afjeh M., Maleki H., Honarvarfard E., et al. (2021). Serotonin level as a potent diabetes biomarker based on electrochemical sensing: a new approach in a zebra fish model. Anal. Bioanal. Chem. 413 1615–1627. 10.1007/s00216-020-03122-5 [DOI] [PubMed] [Google Scholar]
- Kim H., Toyofuku Y., Lynn F. C., Chak E., Uchida T., Mizukami H., et al. (2010). Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 16 804–808. 10.1038/nm.2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczak M., Bechade C., Gervasi N., Irinopoulou T., Banas S. M., Cordier C., et al. (2015). Serotonin modulates developmental microglia via 5-HT2B receptors: potential implication during synaptic refinement of retinogeniculate projections. ACS Chem. Neurosci. 6 1219–1230. 10.1021/cn5003489 [DOI] [PubMed] [Google Scholar]
- Korim W. S., Bou Farah L., Mcmullan S., Verberne A. J. (2014). Orexinergic activation of medullary premotor neurons modulates the adrenal sympathoexcitation to hypothalamic glucoprivation. Diab. Metab. Res. Rev 63 1895–1906. 10.2337/db13-1073 [DOI] [PubMed] [Google Scholar]
- Lalut J., Karila D., Dallemagne P., Rochais C. (2017). Modulating 5-HT4 and 5-HT6 receptors in Alzheimer’s disease treatment. Future Med. Chem. 9 781–795. 10.4155/fmc-2017-0031 [DOI] [PubMed] [Google Scholar]
- Leong M. L., Gu M., Speltz-Paiz R., Stahura E. I., Mottey N., Steer C. J., et al. (2011). Neuronal loss in the rostral ventromedial medulla in a rat model of neuropathic pain. J. Neurosci. 31 17028–17039. 10.1523/JNEUROSCI.1268-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk B., Prusik M., Ziolkowska N., Dabrowski M., Martniuk K., Hanuszewska M., et al. (2018). Effects of streptozotocin-induced diabetes on the pineal gland in the domestic pig. Int. J. Mol. Sci. 19:3077. 10.3390/ijms19103077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Sun C., Rong P., Zhai X., Zhang J., Baker M., et al. (2018). Auricular vagus nerve stimulation enhances central serotonergic function and inhibits diabetic neuropathy development in Zucker fatty rats. Mol. Pain 14:1744806918787368. 10.1177/1744806918787368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu J., Liu X., Su C. J., Zhang Q. L., Wang Z. H., et al. (2019). Antidepressant-Like action of single facial injection of botulinum neurotoxin a is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci. Bull. 35 661–672. 10.1007/s12264-019-00367-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Chalazonitis A., Huang Y. Y., Mann J. J., Margolis K. G., Yang Q. M., et al. (2011). Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 31 8998–9009. 10.1523/JNEUROSCI.6684-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. W., Tsai F. S., Yang W. T., Lai S. C., Shih C. C., Lee S. C., et al. (2018). Differential change in cortical and hippocampal monoamines, and behavioral patterns in streptozotocin-induced type 1 diabetic rats. Iranian J. Basic Med. Sci. 21 1026–1034. 10.22038/IJBMS.2018.29810.7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. J., Shyue S. K., Shih M. C., Chu T. H., Chen Y. H., Ku H. H., et al. (2007). Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis 190 124–134. 10.1016/j.atherosclerosis.2006.02.044 [DOI] [PubMed] [Google Scholar]
- Liu Z., Dai X., Zhang H., Shi R., Hui Y., Jin X., et al. (2020). Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 11:855. 10.1038/s41467-020-14676-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead J. J., Thorne R. G. (2012). Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 64 614–628. 10.1016/j.addr.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Malek Z. S., Dardente H., Pevet P., Raison S. (2005). Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur. J. Neurosci. 22 895–901. 10.1111/j.1460-9568.2005.04264.x [DOI] [PubMed] [Google Scholar]
- Martin A. M., Yabut J. M., Choo J. M., Page A. J., Sun E. W., Jessup C. F., et al. (2019). The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc. Natl. Acad. Sci. U S A. 116 19802–19804. 10.1073/pnas.1909311116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai M. L. (2021). Has the bloom gone out of lorcaserin following the CAMELLIA-TIMI61 trial? Expert Opin. Pharmacother. 22 261–264. 10.1080/14656566.2020.1858795 [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Watanabe S., Taguchi K., Kobayashi T. (2014). Mechanisms underlying increased serotonin-induced contraction in carotid arteries from chronic type 2 diabetic Goto-Kakizaki rats. Pharmacol. Res. 87 123–132. 10.1016/j.phrs.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Mixcoatl-Zecuatl T., Jolivalt C. G. (2011). A spinal mechanism of action for duloxetine in a rat model of painful diabetic neuropathy. Br. J. Pharmacol. 164 159–169. 10.1111/j.1476-5381.2011.01334.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad-Zadeh L. F., Moses L., Gwaltney-Brant S. M. (2008). Serotonin: a review. J. Vet. Pharmacol. Ther. 31 187–199. 10.1111/j.1365-2885.2008.00944.x [DOI] [PubMed] [Google Scholar]
- Morgado C., Silva L., Pereira-Terra P., Tavares I. (2011). Changes in serotoninergic and noradrenergic descending pain pathways during painful diabetic neuropathy: the preventive action of IGF1. Neurobiol. Dis. 43 275–284. 10.1016/j.nbd.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Mcdaniel L. N., Yin T., Khan M., Jiang J., Acevedo M. R., et al. (2015). Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes Metab. Res. Rev. 64 1976–1987. 10.2337/db14-1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah H. (2003). A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology 28 (Suppl. 1), 83–96. 10.1016/s0306-4530(02)00114-2 [DOI] [PubMed] [Google Scholar]
- Nelson P. M., Harrod J. S., Lamping K. G. (2012). 5HT(2A) and 5HT(2B) receptors contribute to serotonin-induced vascular dysfunction in diabetes. Exp. Diab. Res. 2012:398406. 10.1155/2012/398406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesto R., Fain R., Li Y., Shanahan W. (2016). Evaluation of lorcaserin on progression of prediabetes to type 2 diabetes and reversion to euglycemia. Postgrad. Med. 128 364–370. 10.1080/00325481.2016.1178590 [DOI] [PubMed] [Google Scholar]
- Nuno D. W., Korovkina V. P., England S. K., Lamping K. G. (2007). RhoA activation contributes to sex differences in vascular contractions. Arterioscler. Thromb. Vasc. Biol. 27 1934–1940. 10.1161/atvbaha.107.144675 [DOI] [PubMed] [Google Scholar]
- Oh C. M., Namkung J., Go Y., Shong K. E., Kim K., Kim H., et al. (2015). Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat. Commun. 6:6794. 10.1038/ncomms7794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C. M., Park S., Kim H. (2016). Serotonin as a new therapeutic target for diabetes mellitus and obesity. Diab. Metab. J. 40 89–98. 10.4093/dmj.2016.40.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otlivanchik O., Le Foll C., Levin B. E. (2015). Perifornical hypothalamic orexin and serotonin modulate the counterregulatory response to hypoglycemic and glucoprivic stimuli. Diab. Metab. Res. Rev 64 226–235. 10.2337/db14-0671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paile-Hyvarinen M., Wahlbeck K., Eriksson J. G. (2003). Quality of life and metabolic status in mildly depressed women with type 2 diabetes treated with paroxetine: a single-blind randomised placebo controlled trial. BMC Fam. Pract. 4:7. 10.1186/1471-2296-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp S., Lukats B., Takacs G., Szalay C., Karadi Z. (2007). Glucose-monitoring neurons in the nucleus accumbens. Neuroreport 18 1561–1565. 10.1097/WNR.0b013e3281667eca [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Howell T. A. (1994). The molecular evolution of G protein-coupled receptors: focus on 5-hydroxytryptamine receptors. Neuropharmacology 33 319–324. 10.1016/0028-3908(94)90060-4 [DOI] [PubMed] [Google Scholar]
- Pietraszek M. H., Takada Y., Takada A., Fujita M., Watanabe I., Taminato A., et al. (1992). Blood serotonergic mechanisms in type 2 (non-insulin-dependent) diabetes mellitus. Thromb. Res. 66 765–774. 10.1016/0049-3848(92)90052-c [DOI] [PubMed] [Google Scholar]
- Politis M., Niccolini F. (2015). Serotonin in Parkinson’s disease. Behav. Brain Res. 277 136–145. 10.1016/j.bbr.2014.07.037 [DOI] [PubMed] [Google Scholar]
- Ponicke K., Gergs U., Buchwalow I. B., Hauptmann S., Neumann J. (2012). On the presence of serotonin in mammalian cardiomyocytes. Mol. Cell. Biochem. 365 301–312. 10.1007/s11010-012-1270-6 [DOI] [PubMed] [Google Scholar]
- Prabhakar V., Gupta D., Kanade P., Radhakrishnan M. (2015). Diabetes-associated depression: the serotonergic system as a novel multifunctional target. Indian J. Pharmacol. 47 4–10. 10.4103/0253-7613.150305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D. C., Lummis S. C. (2002). The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel (review). Mol. Membr. Biol. 19 11–26. 10.1080/09687680110110048 [DOI] [PubMed] [Google Scholar]
- Ruat M., Traiffort E., Arrang J. M., Tardivel-Lacombe J., Diaz J., Leurs R., et al. (1993). A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem. Biophys. Res. Commun. 193 268–276. 10.1006/bbrc.1993.1619 [DOI] [PubMed] [Google Scholar]
- Sabe S. A., Feng J., Liu Y., Scrimgeour L. A., Ehsan A., Sellke F. W. (2018). Decreased contractile response of peripheral arterioles to serotonin after CPB in patients with diabetes. Surgery 164 288–293. 10.1016/j.surg.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K., Maekawa T., Sasagawa K., Ishii Y., Kume S. I., Ohta T. (2018). Depression-related behavioural and neuroendocrine changes in the Spontaneously Diabetic Torii (SDT) fatty rat, an animal model of type 2 diabetes mellitus. Clin. Exp. Pharmacol. Physiol. Online ahead of print. 10.1111/1440-1681.12965 [DOI] [PubMed] [Google Scholar]
- Sanders N. M., Wilkinson C. W., Taborsky G. J., Jr., Al-Noori S., Daumen W., et al. (2008). The selective serotonin reuptake inhibitor sertraline enhances counterregulatory responses to hypoglycemia. Am. J. Physiol. Endocrinol. Metab. 294 E853–E860. 10.1152/ajpendo.00772.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Yokoi M. (2007). Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. J. Neurosci. 27 6948–6955. 10.1523/jneurosci.0514-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari C. C., Gunduz O., Ulugol A. (2019). Spinal serotonin and 5HT6 receptor levels during development of neuropathy and influence of blockade of these receptors on thermal hyperalgesia in diabetic mice. Drug Res (Stuttg) 69 428–433. 10.1055/a-0817-5464 [DOI] [PubMed] [Google Scholar]
- Scirica B. M., Bohula E. A., Dwyer J. P., Qamar A., Inzucchi S. E., Mcguire D. K., et al. (2019). Lorcaserin and renal outcomes in obese and overweight patients in the CAMELLIA-TIMI 61 trial. Circulation 139 366–375. 10.1161/CIRCULATIONAHA.118.038341 [DOI] [PubMed] [Google Scholar]
- Sohn J. W., Xu Y., Jones J. E., Wickman K., Williams K. W., Elmquist J. K. (2011). Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71 488–497. 10.1016/j.neuron.2011.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Mao N., Li M., Dong X., Lin F. Z., Xu Y., et al. (2013). Sarpogrelate inhibits the expression of ICAM-1 and monocyte-endothelial adhesion induced by high glucose in human endothelial cells. Mol. Cell. Biochem. 373 195–199. 10.1007/s11010-012-1490-9 [DOI] [PubMed] [Google Scholar]
- Sudo M., Minokoshi Y., Shimazu T. (1991). Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Am. J. Physiol. 261 E298–E303. 10.1152/ajpendo.1991.261.3.E298 [DOI] [PubMed] [Google Scholar]
- Sumara G., Sumara O., Kim J. K., Karsenty G. (2012). Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 16 588–600. 10.1016/j.cmet.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trento M., Kucich C., Tibaldi P., Gennari S., Tedesco S., Balbo M., et al. (2010). A study of central serotoninergic activity in healthy subjects and patients with Type 2 diabetes treated by traditional one-to-one care or Group Care. J. Endocrinol. Invest. 33 624–628. 10.1007/BF03346660 [DOI] [PubMed] [Google Scholar]
- Tu H., Cao N., Gu B., Si J., Chen Z., Andersson K. E. (2015). Serotonin (5-HT)2A/2C receptor agonist (2,5-dimethoxy-4-idophenyl)-2-aminopropane hydrochloride (DOI) improves voiding efficiency in the diabetic rat. BJU Int. 116 147–155. 10.1111/bju.12684 [DOI] [PubMed] [Google Scholar]
- Tuomainen M., Lindstrom J., Lehtonen M., Auriola S., Pihlajamaki J., Peltonen M., et al. (2018). Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutrition Diab. 8:35. 10.1038/s41387-018-0046-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno N., Inui A., Satoh Y. (2010). The effect of mosapride citrate on constipation in patients with diabetes. Diab. Res. Clin. Pract. 87 27–32. 10.1016/j.diabres.2009.09.024 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (2012). FDA approves Belviq to treat some overweight or obese adults. Home Healthc. Nurse 30 443–444. [PubMed] [Google Scholar]
- Wade P. R., Chen J., Jaffe B., Kassem I. S., Blakely R. D., Gershon M. D. (1996). Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J. Neurosci. 16 2352–2364. 10.1523/JNEUROSCI.16-07-02352.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabut J. M., Crane J. D., Green A. E., Keating D. J., Khan W. I., Steinberg G. R. (2019). Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule. Endocr. Rev. 40 1092–1107. 10.1210/er.2018-00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J., Sugimoto Y., Noma T., Yoshikawa T. (1998). Effects of the non-selective 5-HT receptor agonist, 5-carboxamidotryptamine, on plasma glucose levels in rats. Eur. J. Pharmacol. 359 81–86. 10.1016/s0014-2999(98)00617-7 [DOI] [PubMed] [Google Scholar]
- Yamada K., Niki H., Nagai H., Nishikawa M., Nakagawa H. (2012). Serotonin potentiates high-glucose-induced endothelial injury: the role of serotonin and 5-HT(2A) receptors in promoting thrombosis in diabetes. J. Pharmacol. Sci. 119 243–250. 10.1254/jphs.12009fp [DOI] [PubMed] [Google Scholar]
- Yi C. X., Serlie M. J., Ackermans M. T., Foppen E., Buijs R. M., Sauerwein H. P., et al. (2009). A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diab. Metab. Res. Rev. 58 1998–2005. 10.2337/db09-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn C. N., Gergues M. M., Samuels B. A. (2017). The role of 5-HT receptors in depression. Mol. Brain 10:28. 10.1186/s13041-017-0306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. L., Lumsden A. L., Martin A. M., Schober G., Pezos N., Thazhath S. S., et al. (2018). Augmented capacity for peripheral serotonin release in human obesity. Int. J. Obesity 42 1880–1889. 10.1038/s41366-018-0047-8 [DOI] [PubMed] [Google Scholar]
- Zhang G., Stackman R. W., Jr. (2015). The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol. 6:225. 10.3389/fphar.2015.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. B. (1944). A study of the substances in blood serum and platelets which stimulate smooth muscle. Am. J. Physiology-Legacy Content 142 12–26. 10.1152/ajplegacy.1944.142.1.12 [DOI] [Google Scholar]