Abstract
Baicalin is a natural bioactive compound derived from Scutellaria baicalensis, which is extensively used in traditional Chinese medicine. A literature survey demonstrated the broad spectrum of health benefits of baicalin such as antioxidant, anticancer, anti-inflammatory, antimicrobial, cardio-protective, hepatoprotective, renal protective, and neuroprotective properties. Baicalin is hydrolyzed to its metabolite baicalein by the action of gut microbiota, which is further reconverted to baicalin via phase 2 metabolism in the liver. Many studies have suggested that baicalin exhibits therapeutic potential against several types of hepatic disorders including hepatic fibrosis, xenobiotic-induced liver injury, fatty liver disease, viral hepatitis, cholestasis, ulcerative colitis, hepatocellular and colorectal cancer. During in vitro and in vivo examinations, it has been observed that baicalin showed a protective role against liver and gut-associated abnormalities by modifying several signaling pathways such as nuclear factor-kappa B, transforming growth factor beta 1/SMAD3, sirtuin 1, p38/mitogen-activated protein kinase/Janus kinase, and calcium/calmodulin-dependent protein kinase kinaseβ/adenosine monophosphate-activated protein kinase/acetyl-coenzyme A carboxylase pathways. Furthermore, baicalin also regulates the expression of fibrotic genes such as smooth muscle actin, connective tissue growth factor, β-catenin, and inflammatory cytokines such as interferon gamma, interleukin-6 (IL-6), tumor necrosis factor-alpha, and IL-1β, and attenuates the production of apoptotic proteins such as caspase-3, caspase-9 and B-cell lymphoma 2. However, due to its low solubility and poor bioavailability, widespread therapeutic applications of baicalin still remain a challenge. This review summarized the hepatic and gastrointestinal protective attributes of baicalin with an emphasis on the molecular mechanisms that regulate the interaction of baicalin with the gut microbiota.
Keywords: Baicalin, Biotransformation, Gut microbiota, Hepatobiliary and gastrointestinal disorders, Signaling pathways
Core Tip: Baicalin possesses therapeutic efficacy against hepatic and gastrointestinal diseases including hepatic fibrosis, xenobiotic-induced liver injury, fatty liver disease, viral hepatitis, cholestasis, ulcerative colitis, hepatocellular and colorectal cancer. The drug action is mediated through its interaction with the gut microbiota, modulation of several signaling pathways, and inflammatory factors. The limitations of low solubility, permeability, and bioavailability pose challenges in the therapeutic applications. The different modes of drug delivery used in the transport of baicalin for ready absorption have paved the way for its use as a pharmacological agent against hepato-intestinal disorders.
INTRODUCTION
The liver is the largest and central digestive organ in the body, which plays a vital role in several physiological processes including growth, nutrition, immunity, and metabolism of xenobiotics[1-3]. The hepatobiliary system mainly consists of the liver, and intra-hepatic and extra-hepatic bile ducts including the gall bladder. The liver in association with the intestine plays an essential role in digestion with the help of digestive enzymes, which facilitate the breakdown of larger biomolecules into simpler forms such as monosaccharides, amino acids, fatty acids, and glycerol. The intestinal microbiota also interacts with bile and other digestive juices, aiding the process of digestion. The complex network of molecular pathways and signal molecules that are involved in the functioning of the hepatobiliary system are also part of the immune cascade[4-6]. Therefore, any disruption in the gastrointestinal (GI) tract or gut microbiota results in the generation of an inflammatory response. Hepatic disorders such as fibrosis, viral hepatitis, non-alcoholic fatty liver, cirrhosis, cholestasis, and hepatocellular carcinoma (HCC) can be identified by alteration in the levels of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-1β, and nuclear factor-kappa B (NF-κB)[7,8]. Researchers in the past two decades have found numerous natural compounds, which have the ability to interact with the gut microbiome and aid in the treatment of diseases of the hepatobiliary system[9,10]. Natural products and their derivatives form a group of compounds known as secondary metabolites produced by the plants. Several such metabolites such as silymarin, ellagic acid, phyllanthin, rutin, and glycyrrhizin have been used to treat hepatic fibrosis, viral hepatitis, fatty liver disease, and cirrhosis[11,12].
Baicalin (5, 6-dihydroxy-7-O-glucuronide) is a flavonoid isolated predominantly from the roots of Scutellaria baicalensis (S. baicalensis), a Chinese medicinal herb that belongs to the family Lamiaceae and is widely known as Chinese skullcap[13]. The roots of S. baicalensis also contain several other significant bioactive molecules such as baicalein and wogonin[14]. Numerous in vitro and in vivo studies have indicated different pharmacological properties of baicalin, which include anti-oxidative, antiviral, anti-inflammatory, cardioprotective, hepatoprotective, neuroprotective, and pro-apoptotic properties. These biological activities can be attributed to the ability of baicalin to target multiple pathways and bind with several signaling molecules[15-18]. In addition, baicalin possesses anti-obese, antidyslipidemia, and pro-apoptotic effects, which help to improve hepatic function after injury, alleviate liver diseases due to alcohol abuse, and promote apoptosis of proliferating hepatocytes[19].
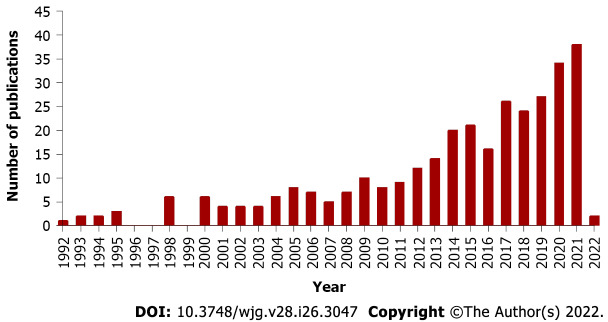
In the last two decades, there has been growing interest and research on the hepatoprotective and anticancer properties of baicalin indicated by the increasing number of publications on PubMed. In recent years (2017 to present), there has been a remarkable rise in the number of research and review articles on the biological potential of baicalin as shown in Figure 1. This review gives an account of the therapeutic effects of baicalin exerted on the hepatobiliary system and the mitigation of GI and liver-associated disorders. The use of baicalin alone or in combination with drugs in several in vitro and in vivo experiments in the last two decades, impact of baicalin on the gut microbiota, its interaction with molecules and receptors at different molecular pathways, and the range of doses at which baicalin has shown maximum activity have also been discussed.
Figure 1.
Increasing trend of publications on “hepatoprotective and anticancer properties of baicalin” indexed by PubMed.
SOURCES OF BAICALIN
Baicalin is the most abundant and important bioactive ingredient obtained from the roots of the medicinal plant S. baicalensis[20]. The roots of S. baicalensis possess baicalin in the range of 8% to 15%. Baicalin is also the main component of other species of Scutellaria such as S. rivularia, S. galericulata, and S. lateriflora[21,22]. Baicalin, chrysin, and its glucoside derivatives have also been obtained from various other parts of the popular Asian medicinal plant Oroxylum indicum, belonging to the family Bignoniaceae[23]. Baicalin, and its aglycone baicalein, are gaining increasing importance in the pharmaceutical, food, and cosmetics industries due to their remarkable biological properties. Baicalin and baicalein, in particular, have shown anti-inflammatory effects and the potential to ameliorate mitochondrial dysfunction[24], and combination strategies with baicalin or baicalein as chemotherapy adjuncts have been shown to be effective in various cancers and associated signaling pathways[25]. Due to growing interest in the properties of baicalin as a potential therapeutic agent, many studies in last few years have focused on developing appropriate techniques for the identification and quantification of baicalin in raw drug formulations including simple thin layer chromatography, and different modifications of the sophisticated technique of high-performance liquid chromatography[26,27].
CHEMISTRY AND BIOAVAILABILITY
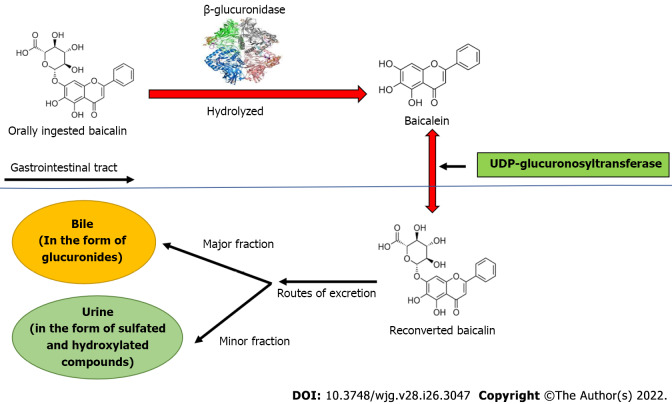
Baicalin is a flavone glycoside (molecular mass = 446.4 g/moL; melting point = 202-205 ºC), which is hydrolyzed to its aglycone baicalein in the stomach after ingestion. Baicalin is hydrolyzed to baicalein immediately after administration with the help of β-glucuronidase from gut bacteria. Baicalein is reconverted to baicalin in the systemic circulation by uridine 5'-diphospho(UDP)-glucuronosyltransferase-glucuronosyl transferase via phase 2 metabolism[21]. It is noteworthy that the circulating baicalin in the system is not the parent molecule but the conjugated metabolite of baicalein. Circulating baicalin returns to the GI system primarily by bile excretion in the form of glucuronides. The bile excretion of baicalin is mainly mediated by the multidrug resistance (MDR) protein 2 transporter. When baicalin and baicalein are given orally, the conjugated metabolites actually contribute to the in vivo effect because the glucuronide/sulfate of baicalin circulates predominantly in plasma[25]. Baicalin is moderately absorbed in the stomach and poorly absorbed in the small and large intestines (Figure 2).
Figure 2.
Biotransformation of baicalin after oral ingestion. Orally administered baicalin is hydrolyzed to baicalein by β-glucuronidase. Further, baicalein is reconverted to baicalin by uridine 5'-diphospho-glucuronosyltransferase in intestine. The major part of baicalin excretion takes place via the biliary route in the form of glucuronides, and a small fraction of baicalin is excreted via urine in the form of sulfated and hydroxylated compounds. UDP: Uridine 5'-diphospho.
PHARMACOKINETICS OF BAICALIN IN THE GI SYSTEM
The pharmacokinetic profile of baicalin in the GI system involves hydrolysis, enterohepatic recycling, carrier-mediated transport, and complex routes of metabolism with the interaction of gut microbiota. Baicalin administration is safe and endurable, and no evidence of liver or kidney toxicity has been recorded. The main obstacles to the clinical use of baicalin are its low water solubility (approximately 67.0 μg/mL) and bioavailability. Several nano-techniques such as solid nanocrystals, nanoemulsions, and lipid-based solid nanoparticles have been used to improve baicalin lysis, thus improving bioavailability[28-30]. Incomplete absorption in the GI system has emerged as the main barrier to bioavailability[31]. MDR protein 2 is the most important transporter of baicalin, which mediates bile outflow to hepatocytes[32]. In fact, biliary excretion of baicalin in rats with MDR protein 2 deficiency is significantly reduced with a significant increase in plasma baicalin levels[33]. Baicalin is also capable of crossing the blood-brain barrier and may be protective against a variety of neurodegenerative diseases[34,35].
β-glucuronidase and UDP-glucuronosyltransferase are important metabolic enzymes involved in the in vivo transformation of baicalin. In fact, five bile metabolites have been identified in rat liver after baicalin administration. The main metabolites include baicalein 6,7diβ-glucopyranuloside and 6oβ-glucopyranuronosyl baicalein-7 sulfate. These conjugated metabolites are hydrolyzed to baicalein in the GI tract by β-glucuronidase/sulfatase. Thirty-two baicalin metabolites have been reported in plasma, urine, and other rat tissues using more efficient approaches[36,37]. In terms of tissue distribution, liver and kidney contain most of the metabolites, indicating that these are the major sites of baicalin metabolism. Baicalin undergoes multiple chemical transformations in vivo including hydrolysis, methylation, hydroxylation, methoxylation, glucuronide conjugates, sulfate conjugates, and their combined reactions[37]. The pharmacokinetics of baicalin may help to understand its therapeutic implications in the liver. As a result of enterohepatic circulation, baicalin remains particularly concentrated in the liver and is thus beneficial in the treatment of hepatic anomalies. Baicalein also plays a crucial role in the treatment of hepatic diseases, indicating that the mechanism of baicalin's emphasis on liver-associated disorders may include the ameliorative effects of the metabolites as well. The major part of baicalin excretion takes place via the biliary route in the form of glucuronides, and a small fraction of baicalin is excreted via urine in the form of sulfated and hydroxylated compounds[38,39].
AMELIORATING EFFECTS OF BAICALIN AGAINST HEPATIC AND COLORECTAL DISEASES
Fatty liver syndrome
Fatty liver syndrome (FLS) occurs due to excess accumulation of non-esterified fatty acids in the hepatocytes. FLS accounts for approximately one-fourth of all liver-related anomalies in the world[40]. FLS causes several hepatic anomalies such as non-alcoholic fatty liver disease, non-alcoholic steatohepatitis (NASH), and advanced FLS may also lead to cirrhosis and ultimately hepatic failure[17,41]. Due to its antioxidant and hepatoprotective potential, baicalin is effective against FLS and associated diseases. Baicalin improves lipid metabolism and suppresses hepatic lipid production by inhibiting the calcium/calmodulin-dependent protein kinase kinase-β/adenosine monophosphate-activated protein kinase (AMPK)/acetyl-coenzyme A (CoA) carboxylase pathway[42,43]. Additionally, baicalin binds directly to carnitine palmitoyl-transferase-1α (CPT-1α) and promotes the influx of lipid into mitochondria, where it is oxidized[44]. Baicalin also downregulates lipid-producing genes such as fatty acid synthase (FASN), peroxisome proliferator-activated receptor-α (PPAR-α), and sterol regulatory element-binding protein-1c (SREBP-1c) to inhibit lipid accumulation in the liver[42,45]. Baicalin (200 mg/kg) has been found to reduce the expression of TNF-α, monocyte chemoattractant protein (MCP-1) and IL-1β, downregulate caspase-3 to alleviate NASH induced by a methionine- and choline-deficient (MCD) diet. In addition, treatment with baicalin can partially lessen the accumulation of lipids induced by the MCD diet in the liver by modulating the expression of SREBP-1c, FASN, PPAR-α, and CPT-1α[46]. Moreover, baicalin (50 mg/kg) also reduces the synthesis of inflammatory cytokines including TNF-α, IL-6, and IL-1β, and suppresses the Toll-like receptor 4 (TLR-4) signaling pathways in MCD diet fed mice to inhibit fat accumulation in liver. Thus, baicalin also acts as an anti-inflammatory compound in the attenuation of non-alcoholic FLS[47]. In a study of high-fat diet induced FLS, it was found that baicalin (25-100 mg/kg) exerted a substantial ameliorating effect on FLS by activating the expression of hepatic PPAR-γ receptors[48]. In addition, baicalin (5 g/kg) also alleviates FLS by reducing the levels of serum hepatic enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and inflammatory mediators such as TNF-α and MCP-1. Moreover, baicalin also inhibited the phosphorylation of Janus kinase (JNK), and suppressed the production of inflammatory enzyme cyclooxygenase-2 and pro-oxidative enzyme CYP-2E1 in the liver of a mouse model[49]. In another study of orotic acid-induced FLS, baicalin (12.5-50 mg/kg) downregulated SREBP-1c and upregulated AMPK to reduce the toxic effects of free fatty acids, subsequently inhibiting fat accumulation in the liver[45]. In an in vitro study of palmitic acid-induced FLS in AML-12 hepatocytes, baicalin (6.25-25 μM) alleviated FLS by reducing endoplasmic reticulum stress and suppression of the thioredoxin-interacting protein/Nod-like receptor protein 3 pathway[50].
Liver injury
The liver is the most important organ for the metabolism and elimination of toxins from the human body. Normal liver function can be deterred with excessive hepatic injuries, which occur due to a variety of factors including alcohol intake, chemical contaminants, hepatocellular ischemia, and drug damage[50,51]. Liver damage is a complex process that can manifest extensive hepatocellular apoptosis[52]. Baicalin (120 mg/kg) has been shown to alleviate alcohol-induced liver injury in a rat model via the reduced expression of inflammatory cytokines such as TNF-α, IL-6 and IL-1β and enhances the activity of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), which is further regulated by blocking the sonic-hedgehog signaling pathway[19]. Similarly, baicalin (30 mg/kg) has been shown to produce anti-inflammatory effects by downregulating the expression of IL-17 in a rat model with acetaminophen-induced liver injury[53]. Baicalin (80 and 200 mg/kg) activates heme oxygenase-1 via the nuclear respiratory factor (NRF)-2 antioxidant pathway and inhibits the activity of reactive oxygen species (ROS) generating enzymes in a liver injury model. Baicalin plays an important role in blocking the combination of NRF-2 and Kelch-like ECH-associated protein 1 (Keap-1) that causes phosphorylation of NRF-2, thus reversing liver damage[54,55]. Furthermore, baicalin (60 mg/kg) also downregulates extracellular signal-regulated kinase (ERK) in acetaminophen-induced liver injury[56]. In addition, baicalin (100 mg/kg) downregulates caspase-3 and caspase-9 and also increases the expression of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2). This helps to significantly reduce NG-nitro-L-arginine methyl ester-induced liver injury in rats[57].
The therapeutic effect of baicalin on reducing liver damage via the apoptotic pathway has also been demonstrated via in vitro experiments (Table 1). Baicalin at 100 μmol/L regulates apoptotic proteins such as caspase-3, caspase-9, and Bcl-2 associated X (Bax), and has a considerable therapeutic impact on the hypoxic model of L02 human hepatocytes[58]. Another in vitro study with L02 hepatocytes and acetaldehyde-treated HepG2 cells were used to identify the adverse effects of baicalin (20-100 mmol/L) on progression of the epithelial-mesenchymal transition (EMT) in the liver, which is an indicator of liver fibrosis and inflammation. This study demonstrated that baicalin considerably suppressed the progression of EMT by downregulating the transforming growth factor-β (TGF-β)/Smad signaling pathway, thus ameliorating liver damage[59]. Studies have shown that baicalin (60 μM) can repair liver injury in nanosecond pulse electric field (ns-PEF)-induced damage to L02 hepatocytes by stabilizing mitochondrial transmembrane potential and prevent excess ROS production[60]. Furthermore, baicalin (5 and 25 μM) can suppress the oxidation and nitrification of the hemin/nitrite/hydrogen peroxide system and protect HepG2 cells by inhibiting lipid peroxidation and GSH depletion[61]. Another study emphasized that baicalin administration (74 mg/kg) can restore the metabolism of amino acids to normal, improve the tricarboxylic acid cycle and ameliorate acute lipopolysaccharide (LPS)-induced sepsis in mice model[62].
Table 1.
In vitro hepatoprotective effects of baicalin on different cell lines
|
Disease/type of study
|
Cell line
|
Dose
|
Mechanism/target pathways
|
Ref.
|
| Palmitic acid-induced fatty liver | AML-12 hepatocytes | 6.25-25 μM | ER stress↓; TXNIP/NLRP-3 pathway↓ | [50] |
| Hypoxic liver injury | L02 human hepatocytes | 100 μmol/L | Caspase-3, caspase-9, and Bax↓ | [58] |
| Acetaldehyde induced EMT | HepG2 cells | 20-100 mmol/L | TGF-β/Smad pathway↓ | [59] |
| ns-PEF induced liver injury | L02 hepatocytes | 60 μM | MTP stabilization, ROS↓ | [60] |
| Hemin-nitrite-H2O2 induced liver injury | HepG2 cells | 5 μM and 25 μM | Lipid peroxidation↓; GSH depletion↓ | [61] |
| PDGF-BB induced fibrosis | HSCT6 hepatocytes | 150 μM | miR-3595↓; ACSL-4↑ | [69] |
| BDL-induced fibrosis | HSCs | 67.5-270 μM | Wnt pathway↓; PPAR-γ↓ | [70] |
| LPS-induced hepatitis | L20, THLE cell lines | 25-100 μM | TUG-1↑; p38-MAPK↓; JNK pathway↓ | [82] |
| Viral hepatitis | HuH7, HepG2 cells | 75 μg/mL | NF-κB pathway↓ | [83] |
| pHBV1.2HepG2 cells | 10 μM | HNF-4α/HNF-1α↓ | [84] | |
| HepG2.2.15 cells | 10 μg/kg | HBsAg, HBeAg↓ | [85] | |
| PBMCs | 50-200 mg/mL | Mitochondrial pathway↑; Caspase 3↑ | [87] | |
| HCC | HepG2-HCC | 100 μmol/L | ER-mediated TF-6↑; S-2P protein↑ | [94] |
| SMMC7721-HCC cells | 160 μM | CD47↓ | [95] | |
| SMMC7721, HepG2-HCC cells | 40 μM | STAT3, IFN-γ↓; Block PDL-1/PD-1 pathway | [96,97] |
ACSL-4: Long chain fatty acid CoA ligase 4; AML: Alpha mouse liver; BDL: Bile duct ligation; EMT: Epithelial-mesenchymal transition; ER: Endoplasmic reticulum; GSH: Reduced glutathione; HCC: Hepatocellular carcinoma; HSCs: Hepatic stellate cells; LPS: Lipopolysaccharide; MAPK: Mitogen-activated protein kinase; MTP: Mitochondrial transmembrane potential; NLRP-3: Nod-like receptor protein 3; nsPEF: Nanosecond-pulse electric field; PBMCs: Peripheral blood mononuclear cells; PDGF-BB: Platelet-derived growth factor BB; PPAR-γ: Peroxisome proliferator-activated receptor-γ; ROS: Reactive oxygen species; THLE: Transformed human liver epithelial; TUG-1: Taurine upregulated-1; TXNIP: Thioredoxin-interacting protein.
Liver fibrosis
Liver fibrosis occurs due to excess deposition of extracellular matrix proteins and collagen. It is characterized by hepatic tissue degeneration, inflammatory cell infiltration and hepatic cell necrosis[63,64]. It has also been confirmed that activation of hepatic stellate cells (HSCs) plays a central role in liver fibrosis[65]. In the absence of appropriate treatment, advanced liver fibrosis causes chronic hepatitis subsequently leading to liver cancer or cirrhosis[66,67]. In a recent study, it was shown that baicalin (200 mg/kg) decreased the expression of fibrotic genes such as α-smooth muscle actin (SMA) and connective tissue growth factor and inflammatory cytokines such as TNF-α, macrophage inflammatory protein-1α (MIP-1α), IL-1β, MIP-2, thereby effectively suppressing liver fibrosis induced by bile duct ligation (BDL) in mice. In addition, an in vitro study also showed the efficacy of baicalin in reducing the activation of HSCs and downregulating the expression of SMA, fibronectin, tissue inhibitor of metalloproteinase-1 (TIMP1) protein andcollagen-1[68]. Similarly, baicalin (150 μM) reduced microRNA (miR)-3595 expression and increased the activity of the enzyme long-chain fatty acid CoA ligase 4, significantly inhibiting the activity of HSCs leading to a reduction in fibrosis in HSCT6 hepatocyte cell lines caused by platelet-derived growth factor BB[69]. Moreover, baicalin (67.5-270 μM) helps in the reduction of BDL-induced activity of HSCs by inhibiting PPAR-γ via Wnt signaling, leading to alleviation of liver fibrosis[70]. Baicalin (100 mg/kg) attenuated carbon tetrachloride-induced hepatic fibrosis in mice by regulating the rise in TGF-β1, hydroxyproline, type III collagen, and hyaluronic acid laminin (Table 2). In addition, baicalin also attenuates liver fibrosis by suppressing the activity of antioxidant enzymes SOD and GSH-Px[71]. Similarly, baicalin (25-100 mg/kg) evidently reduces the level of PPAR-γ, suppresses the activity of HSCs, and downregulates the expression of TGF-β1, causing inhibition of hepatic fibrosis[72].
Table 2.
In vivo protective effects of baicalin on various hepatobiliary and colorectal disorders
|
Disease/type of study
|
Dose
|
Mechanism/target pathway
|
Ref.
|
| MCD induced NASH | 200 mg/kg | TNF-α, MCP-1, IL-1β↓; Caspase-3↓; SREBP-1c, FASN, PPAR-α, CPT-1α↓ | [46] |
| 50 mg/kg | TNF-α, IL-6, IL-1β↓; TLR-4 pathway↓ | [47] | |
| High fat diet induced non- alcoholic FLS | 25-100 mg/kg | PPAR-γ receptors↑ | [48] |
| 5 g/kg | AST, ALT↓; TNF-α, MCP-1↓; JNK-P↓; COX-2, CYP-2E1↓ | [49] | |
| Orotic acid induced FLS | 12.5-50 mg/kg | SREBP-1c↓; AMPK↑ | [45] |
| Alcohol-induced liver injury | 120 mg/kg | TNF-α, IL-6, IL-1β↓; SOD, GSH-Px↑; Block sonic-hedgehog pathway | [19] |
| 200 mg/kg | HO-1, NRF-2 pathway↑ | [54] | |
| Acetaminophen-induced liver injury | 30 mg/kg | IL-17↓ | [53] |
| 80 mg/kg | NRF-2, Keap-1↓ | [55] | |
| 60 mg/kg | ERK↓ | [56] | |
| NG-nitro-L-arginine methyl ester induced liver injury | 100 mg/kg | Caspases-3 and 9↓; Bcl-2↑ | [57] |
| LPS-induced sepsis | 74 mg/kg | Amino acid metabolism↑; TCA cycle↑ | [62] |
| BDL-induced liver fibrosis | 200 mg/kg | SMA, CTGF↓; TNF-α, MIP-1α, IL-1β, MIP-2↓ | [68] |
| CCl4-induced fibrosis | 100 mg/kg | TGF-β1, hydroxyproline, type III collagen, hyaluronic acid laminin↑; SOD, GSH-Px↓ | [71] |
| 25-100 mg/kg | PPAR-γ↓; TGF-β1↓ | [72] | |
| 17α- ethinyl estradiol-induced cholestasis | 50-200 mg/kg | TBA, AST, ALT, ALP↓; TNF-α, IL-6 and IL-1β↓ | [75,76] |
| Sirt1/HNF-1α/FXR pathway↓ | [77] | ||
| Hepatitis B in young duck model | 10 μg/kg | HBsAg, HBeAg↓; HNF-4α/HNF-1α↓ | [85] |
| Hepatitis in male BALB/c mouse model | 100-200 mg/kg | TNF-α, IL-6 and IFN-γ↓ | [86] |
| Hepatitis in male Sprague-Dawley rat model | 0.5-5.0 mg/kg | ALT, AST↓ | [86] |
| HCC | 50 mg/kg | RelB/p52 pathway↑ | [93] |
| CRC in mice | 100, 200 mg/kg | TGF-β/Smad pathway↓ | [100] |
| TNBS-induced UC | 30-90 mg/kg | IL-1β, TNF-α↓; Caspase 9, Bcl-2↓; IKK/IKB/NF-κB pathway↓ | [107] |
| 5-20 mg | IL-1β, TNF-α, IL-6↓; TLR4/NF-κB pathway↓ | [108] | |
| 30-120 mg/kg | Catalase, GSH-PX, SOD↑; Bcl-2↑; MDA↓; TGF-β, Bax↓ | [109] | |
| HTHE-induced UC | 100 mg/kg | NF-κB, MAPK pathways↓ | [110] |
| DSS-induced UC | 50-150 mg/kg | MPO, NO↓; IL-1β, TNF-α and IL-6↑ | [111] |
| 100 mg/kg | TLR-4/NF-κB-p65/IL-6 pathway↓; TNF-α, IL-6, IL-13↓ | [112] | |
| TNBS-induced UC | 10 mg/kg | MIF, MCP-1, MIP-3a↓ | [113] |
| 20-100mg/kg | Maintain Th17/Treg balance | [114] |
ALP: Alkaline phosphatase; ALT: Alanine transaminase; AST: Aspartate transaminase; AMPK; AMP-activated protein kinase; Bcl-2: B-cell lymphoma 2; CCL4: Carbon tetrachloride; COX-2: Cyclooxygenase-2; CPT-1α: Carnitine palmitoyl-transferase-1α; CRC: Colorectal cancer; CTGF: Connective tissue growth factor; CYP-2E1: Cytochrome P450 2E1; DSS: Dextran sulfate sodium; ERK: Extracellular signal-regulated kinase; FASN: Fatty acid synthase; FLS: Fatty liver syndrome; FXR: Farnesoid X receptor; GSH-Px: Glutathione peroxidase; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; HNF: hepatic nuclear factor; HO-1: Heme oxygenase 1; HTHE: High temperature and humid environment; IL-1: Interleukin-1; JNK: c-Jun N-terminal kinase; Keap-1: Kelch-like ECH-associated protein 1; MCD: Methionine- and choline-deficient; MCP-1: Monocyte chemoattractant protein-1; MIP-1α: Macrophage inflammatory protein-1 alpha; NASH: Non-alcoholic steatohepatitis; NF-κB: Nuclear factor-kappa B; NRF2: Nuclear factor-erythroid factor 2-related factor 2; PPAR-γ: Peroxisome proliferator-activated receptor-γ; Sirt: Sirtuin; SMA: Smooth muscle actin; SOD: Superoxide dismutase; SREBP: Sterol regulatory element-binding protein; TBA: Total bile acid; TCA: Tricarboxylic acid cycle; Th17: T helper 17 cells; TLR-4: Toll-like receptor 4; TNBS: 2,4,6-trinitrobenzenesulfonic acid; TNF-α: Tumor necrosis factor-alpha; Treg: Regulatory T cells; UC: Ulcerative colitis.
Cholestasis
Cholestasis is a condition that occurs due to the obstruction or complete blockage of bile secretion through the intrahepatic or extrahepatic bile ducts[73]. Consequently, there is excess accumulation of conjugated bilirubin, bile salts, and cholesterol in the liver, which leads to hepatic injury and damage to the human body[74]. Baicalin plays an important role at several stages to mitigate cholestasis and related hepatic damage. Baicalin specifically targets nuclear factor-erythroid factor 2-related factor 2 (NRF-2) in reversing cholestasis. Recent studies in a mouse model indicate that the interaction of baicalin (50 mg/kg) with NRF-2, inflammatory cytokines, and oxidative stress regulatory elements forms the central pathway of reducing cholestasis induced hepatic damage. Baicalin is capable of activating SMA, TIMP1, and collagen, resulting in amelioration of liver fibrosis due to BDL-induced cholestasis[68]. Another pharmacokinetic study in a rat model indicated that administration of baicalin (50-200 mg/kg) has therapeutic potential for cholestasis. It significantly increases bile excretion rates, which lead to a decline in serum levels of total bile acids as well as hepatic enzymes such as AST, ALT, and alkaline phosphatase in 17α-ethinyl estradiol-induced cholestasis[75,76]. In estrogen-induced cholestasis, it has been shown that baicalin targets NF-κB and inhibits the expression of inflammatory markers such as TNF-α, IL-6, and IL-1β, thereby increasing the activity of hepatic bile acid-metabolizing enzymes. Reports have also shown that baicalin alleviates 17α-ethinyl estradiol-induced cholestasis in mice by suppressing the expression of multidrug resistance protein 2 and bile salt export pump genes via the sirtuin 1/nuclear hepatic receptor 1α (HNF-1α)/farnesoid X receptor (FXR) pathway[77].
Hepatitis
Hepatitis caused by hepatitis A virus (HAV), hepatitis B virus (HBV), and other viral hepatitis are prevalent infectious diseases worldwide[78,79]. Liver-specific proteins and immune complex hypersensitivity have multiple roles in hepatitis[80]. Subsequent studies have proven that baicalin plays a key role in the attenuation of hepatitis by lowering the levels of hepatitis B surface antigen (HBsAg), viral antigen protein hepatitis B e-antigen (HBeAg), and hepatitis B virus (HBV)-DNA, and regulating oxidative stress, inflammation, and apoptosis in hepatic cells[80,81]. It has been found that baicalin (25-100 μM) significantly increases taurine upregulated 1 gene expression to suppress inflammation and apoptosis by downregulating the p38-mitogen activated protein kinase (MAPK) and JNK signaling pathways in L20 and transformed human liver epithelial cell lines to thwart LPS-induced hepatitis[82]. Another recent study showed that baicalin (75 μg/mL) significantly suppressed HBV replication and inflammation by downregulating the NF-κB signaling pathway in HepG2 and HuH7 cells[83]. Furthermore, it has also been confirmed that baicalin (10 μM) strongly suppresses the transcription of HBV by downregulating the liver-specific HNF-4α/HNF-1α axis in pHBV1.2 HepG2 cells[84]. In another experiment, baicalin (10 μg/kg) significantly reduced HBsAg and HBeAg levels in HepG2 cells, wild-type HBV cells, and young duck models infected with HBV by downregulating the HNF-4α/HNF-1α axis[85]. In addition, baicalin pre-treatment (100-200 mg/kg) attenuated elevated levels of plasma cytokines such as TNF-α, IL-6, and interferon-γ (IFN-γ) in male BALB/c mouse models, resulting in alleviation of hepatocyte necrosis and apoptosis[86]. Similarly in an in vivo study, pre-treatment with baicalin (0.5-5.0 mg/kg) considerably reduced serum levels of ALT and AST and lowered hepatic oxidative stress in a male Sprague-Dawley rat model[86]. Another study demonstrated the pro-oxidant properties of baicalin (50-200 mg/mL) by upregulating the mitochondrial signaling pathway in human peripheral blood mononuclear cells, thereby inducing the activation of caspase-3 and apoptosis[87]. Furthermore, baicalin has often been co-administered with other bioactive flavonoids in combination studies, to yield better results at prevention of hepatitis. For instance, combination of the alkaloid oxymatrine (1 g/L) with baicalin showed more efficacy against HBV than oxymatrine alone[88]. Below 31.50 μg/mL, the baicalin-phospholipid complex exhibits direct anti-duck HAV-1 activity by preventing the adsorption, replication, and release of duck HAV-1 and indirectly by promoting immunity in ducklings[89]. Baicalin (20 μg/mL) regulates the immunomodulatory effects and anti-HAV-1 reproduction by reducing the adsorption and release of HAV-1 in duck-suppressed embryonic hepatocytes[90].
HCC
HCC ranks sixth amongst the most common malignant tumors worldwide, and is the fourth highest cause of mortality due to malignancies[91]. The present modes of treatment for HCC include radiation therapy, local resection therapy, surgery, and liver transplantation. However, these treatments typically cause several side effects and have adverse consequences[91,92]. Several studies have reported that baicalin can effectively ameliorate HCC by indirectly inducing autophagy in liver tumor cells. Studies have revealed that baicalin (50 mg/kg) promotes the polarization of tumor-related macrophages into M1-like macrophages, subsequently increasing autophagy in cancerous cells to make them non-proliferating. Additionally, baicalin mediated anti-cancer effects may also be closely associated with activation of the RelB/p52 signaling pathway[93]. Similarly, baicalin (100 μmol/L) promotes apoptosis in HepG2-HCC cells by activating the ER-mediated TF-6 signaling cascade combined with S-2P protein[94]. Moreover, baicalin (160 μM) suppresses cluster of differentiation 47 and activates apoptosis and autophagy in SMMC7721-HCC cells[95]. Although nsPEFs have been developed as a new mode of treatment for cancer, they also result in the elimination of normal hepatocytes. Therefore, a study was designed combining nsPEF and baicalin for the treatment of HCC, which revealed that baicalin suppresses the proliferation of HCC cells, and protects normal liver cells by increasing mitochondrial membrane potential and reducing ROS production[59]. In recent times, cancer immunotherapy has emerged as a significant line of treatment for HCC. Baicalin (40 μM) is capable of reducing the activity of signal transducer and activator of transcription 3 protein and IFN-γ, thereby blocking the programmed death-ligand 1/programmed cell death protein 1 pathway. This increases the sensitivity of the immune system to hepatic cancerous cells and thus further activates T cells against hepatic cancer cells[96,97]. Based on several in vitro and in vivo studies, the protective effect of baicalin on many hepatic disorders is summarized in Tables 1 and 2.
Colorectal cancer
Colorectal cancer (CRC) is also a common malignant tumor worldwide, and is primarily due to genetic inheritance, colon polyps, and ulcerative colitis (UC)[98,99]. Baicalin, due to its pro-apoptotic properties, results in the killing of CRC cells. Researchers have used the human colon cancer cell line (HCT-166) and transplanted colon tumors into mice to conduct simultaneous in vivo and in vitro experiments to examine the antitumor mechanism of baicalin (100 and 200 mg/kg). Baicalin induces apoptosis in colon tumors by inhibiting the cells at the G1 stage and arresting EMT protein expression by blocking the TGF-β/Smad pathway[100]. Baicalin (40 mmol/L) promotes Dickkopf protein expression, suppresses the expression of proteins β-catenin and c-Myc, and inhibits miR-217 expression, thereby leading to the apoptosis of HCT-166 cancer cells by inhibition of the Wnt signaling pathway[101]. Another similar study demonstrated that apoptosis of HT-29 colon cancer cells was induced by baicalin (50-200 μM) via inhibition of c-Myc expression and regulation of the miR-10a, miR-23a, miR-31, miR-151a, and miR-205 mechanism[14]. Similarly, it has been reported that the antioxidant properties of baicalin (40 μM) increase progesterone expression in the intestine and leads to the apoptosis of HCT-116 colon cancer cells by activating the Ras/Raf/MEK/ERK pathways[102]. Reports have also shown that the antitumor activity of baicalin can be further enhanced by glycosidase pre-treatment[103]. Several studies have revealed that the development of CRC is closely associated with genetic mutations. In a study on colon cancer cells SW-480, baicalin (50-400 μg/mL) inhibited the expression of the transcription factor SP-1, leading to the apoptosis of cancer cells[104].
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a collective term that refers to the chronic inflammation of GI tract. The two major types of IBD are Crohn’s disease (CD) and UC. CD and UC are nonspecific chronic IBDs that cause inflammation and ulcers on the inner lining of the large intestine[105-107]. Baicalin plays an important role in the treatment of IBD, as it is capable of suppressing oxidative stress, immune regulation, and its anti-inflammatory properties. In addition, baicalin is capable of regulating NF-κB activation, which modulates both autophagic and inflammatory processes in intestinal epithelial cells, subsequently leading to enhancement in paracellular permeability. Baicalin alleviates dextran sulfate sodium (DSS)-induced UC by modulating the polarization of M1 macrophages to the M2 phenotype[107]. Dose-dependent administration of baicalin (30-90 mg/kg) has been found to largely downregulate the inflammatory cytokines IL-1β and TNF-α, and apoptotic genes Bcl-2 and caspase-9 in the colon tissue of rats affected by 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced UC. In addition, baicalin also inhibits the inhibitory κB (IKB) kinase (IKK)/IKB/NF-κB signaling pathway, leading to the alleviation of IBD[107,108]. Likewise, in another study, rats with TNBS-induced UC were given baicalin (5-20 mg) which resulted in the downregulation of inflammatory factors TNF-α, IL-6 and IL-1β in rat intestine and inhibition of the TLR4/NF-κB signaling pathway, leading to alleviation of UC[109]. Furthermore, baicalin (30-120 mg/kg) has also been effective in the treatment of TNBS-induced UC by promoting antioxidant enzymes such as catalase, SOD and GSH-PX, and reducing malondialdehyde (MDA). Baicalin also suppresses the regulation of apoptosis by upregulating Bcl-2 and downregulating TGF-β and Bax[110]. In a UC model generated by high temperature and humid environment, baicalin (100 mg/kg) significantly reduced the serum levels of IL-6, IL-1β, and IL-17, and inhibited SOD, GSH-PX, and MDA. That study attributed the anti-inflammatory effect of baicalin to suppression of the NF-κB and MAPK pathways[111]. In an in vivo study, baicalin (50-150 mg/kg) reduced myeloperoxidase activity, nitric oxide content, and elevated IL-1β, TNF-α and IL-6 levels in the colon of DSS-induced UC rats[112]. Another study revealed that baicalin (100 mg/kg) attenuated DSS-induced UC by blocking the TLR-4/NF-κB-p65/IL-6 signaling pathway and suppressing TNF-α, IL-6, and IL-13 mRNA expression[113]. Furthermore, baicalin (10 mg/kg) downregulated the expression of macrophage migration inhibitory factor, MCP-1 and MIP-3a in the colon tissue of TNBS-induced UC rat model[114]. Several reports have suggested the association of T helper 16 cell (Th17)/regulatory T cell (Treg) equilibrium with UC. Baicalin (20-100 mg/kg) regulates the Th17/Treg balance by inhibiting the rise in ROS and MDA, whereas simultaneously reducing GSH and SOD levels and regulating the expression of Th17-related factors IL-6 and IL-17 in TNBS-induced UC rats[115,116]. In a clinical study of UC patients, baicalin promoted the production of immune cells like CD4+ and CD29+ and induced immunomodulation to alleviate UC[117] (Figure 3).
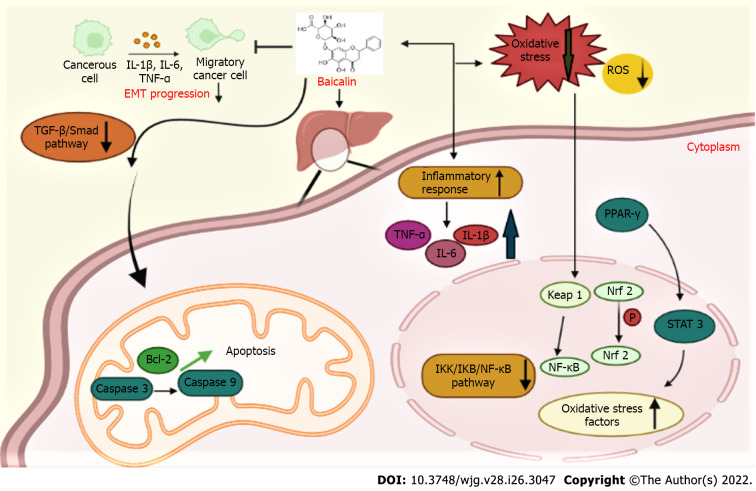
Figure 3.
Mechanism of baicalin action against hepatobiliary diseases. Baicalin downregulated peroxisome proliferator-activated receptor-α and activated the nuclear respiratory factor-2 antioxidant pathway to reduce oxidative stress in the hepatocytes. Baicalin suppressed epithelial-mesenchymal transition progression by downregulating the transforming growth factor-β/Smad pathway, inhibited the inhibitory κB (IKB) kinase/IKB/nuclear factor-kappa B pathway, reduced the elevated levels of inflammatory factors such as tumor necrosis factor-α, interleukin-6 (IL-6) and IL-1β, and attenuated the apoptotic proteins caspase-3, caspase-9, B-cell lymphoma 2, which led to the alleviation of liver diseases. ROS: Reactive oxygen species; STAT: Signal transducer and activator of transcription; PPAR-γ: Peroxisome proliferator-activated receptor-γ; IL: Interleukin; Nrf2: Nuclear respiratory factor-2; TGF-β: Transforming growth factor-β; NF-κB: Nuclear factor-kappa B; TNF-α: Tumor necrosis factor-alpha; Bcl-2: B-cell lymphoma 2; EMT: Epithelial-mesenchymal transition; IKK: Inhibitory κB kinase; IKB: Inhibitory κB.
INTERACTION OF BAICALIN WITH THE INTESTINAL MICROBIOTA
In the past decade, the intestinal microbiota has become an emerging aspect of research for the evaluation of several diseases. Besides playing an important role in the metabolism and breakdown of biomolecules into simpler molecules like fatty acids, amino acids, vitamins and bile salts, the gut microbiota is also capable of interacting with the host and affect the functioning of various organs including the liver and kidney to regulate homeostasis and disease development[5,118]. Baicalin, a flavonoid, exerts many therapeutic effects by modulating gut microbiota homeostasis. Intake of high fat diet causes imbalance of the gut microbiota, leading to several metabolic syndromes. Baicalin administered (200 mg/kg/d) to mice with high fat diet induced metabolic syndrome led to an increase in short-chain fatty acid (SCFA)-producing gut bacteria, thereby effectively reducing the metabolic syndrome in mice[119]. Baicalin also reduces damage to the intestinal barrier caused due to hypertension. A study reported that baicalin (100 mg/kg) significantly increased the number of SCFA-producing bacteria and altered the intestinal microflora, leading to a reduction in damage of the intestinal barrier in rats caused by hypertension[120]. Another study revealed that baicalin (25-100 mg/kg) helped increase SCFA-producing bacteria such as Eubacterium spp, Subdoligranulum spp, and Butyricimonas spp, thereby ameliorating TNBS-induced UC[114]. In some cases, the gut microbiome can also downregulate the therapeutic efficacy of baicalin[121]. The gut microbiota regulates hepatobiliary homeostasis via the gut-hepatic axis, and although it can regulate baicalin activity, baicalin can also modulate the gut microbiota[122]. Consequently, baicalin has the potential to exert a therapeutic role in liver and gut diseases by modulating FXR and TGR5-mediated crosstalk involving bile acids associated with the gut microbiome.
DRUG DELIVERY, CLINICAL TRIAL, AND FUTURE PROSPECTS
The clinical application of baicalin in pharmacology has been challenging, due to its low solubility and bioavailability. In the last decade, many researchers have designed novel delivery strategies for baicalin that include phospholipid complex, liposomes, solid baicalin nanocrystals, and micelle formation[123]. The dissolution and solubility of baicalin is considerably enhanced when administered in combination with other molecules in complex form. For instance, baicalin has exhibited improved oral bioavailability, distribution, targeting, and therapeutic efficacy when combined with polyethylene glycol and folic acid in the form of liposomes. β-Cyclodextrin complex has also been used as an effective formulation to facilitate the effective delivery of baicalin with wide range of therapeutic outcomes[123,124]. Moreover, baicalin is commonly used as adjuvant therapy for hepatitis. In a clinical study, single dose baicalin (500 mg/kg) in combination with cyclosporin A was found to be safe and well tolerated in adult human subjects without any severe adverse effects[125]. However, the co-administration of baicalin with other herbal formulations or drugs might impede baicalin’s in vivo actions and consequently its efficacy. Therefore, it is important to thoroughly study the clinically approved doses of baicalin which can be administered in combination with other compounds that help to improve the absorption and effectiveness of baicalin.
CONCLUSION
GI disorders have emerged as the leading cause of mortality in the recent years across the world. Baicalin, a major flavone obtained from S. baicalensis, exerts protective effect against hepatobiliary and colorectal disorders by modulating signaling pathways. Further, novel delivery strategies that are used in the transport of baicalin for ready absorption including phospholipid complex, liposomes, solid baicalin nanocrystals, and β-cyclodextrin complex have paved way for its widespread use as a pharmacological alternative in hepatic and GI disorders.
ACKNOWLEDGEMENTS
All of the authors acknowledge DST-FIST and UGC-SAP facilities of the Department of Biochemistry, University of Allahabad, Prayagraj, India.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 16, 2022
First decision: March 8, 2022
Article in press: May 21 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sitkin S, Russia; Wan XH, China A-Editor: Ribeiro IB, Brazil S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
Contributor Information
Risha Ganguly, Department of Biochemistry, University of Allahabad, Allahabad (Prayagraj) 211002, Uttar Pradesh, India.
Ashutosh Gupta, Department of Biochemistry, University of Allahabad, Allahabad (Prayagraj) 211002, Uttar Pradesh, India.
Abhay K Pandey, Department of Biochemistry, University of Allahabad, Allahabad (Prayagraj) 211002, Uttar Pradesh, India. akpandey23@rediffmail.com.
References
- 1.Gupta A, Pandey AK. Aceclofenac-induced hepatotoxicity: An ameliorative effect of Terminalia bellirica fruit and ellagic acid. World J Hepatol. 2020;12:949–964. doi: 10.4254/wjh.v12.i11.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma UK, Kumar R, Ganguly R, Gupta A, Pandey AK. Cinnamaldehyde, an active component of cinnamon provides protection against food color induced oxidative stress and hepatotoxicity in albino wistar rats. Vegetos. 2018;31:123–129. [Google Scholar]
- 3.Gupta A, Kumar R, Ganguly R, Singh AK, Rana HK, Pandey AK. Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicol Rep. 2021;8:44–52. doi: 10.1016/j.toxrep.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S. Intestinal microbiota regulate xenobiotic metabolism in the liver. PLoS One. 2009;4:e6958. doi: 10.1371/journal.pone.0006958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Kumar R, Dwivedi A, Pandey AK. In vitro antioxidant, antibacterial, and cytotoxic activity and in vivo effect of Syngonium podophyllum and Eichhornia crassipes leaf extracts on isoniazid induced oxidative stress and hepatic markers. Biomed Res Int. 2014;2014:459452. doi: 10.1155/2014/459452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Q, Zhang W, Wu Z, Tian X, Xiang J, Li L, Li Z, Peng X, Wei S, Ma X, Zhao Y. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol Res. 2021;165:105444. doi: 10.1016/j.phrs.2021.105444. [DOI] [PubMed] [Google Scholar]
- 7.Xiong F, Guan YS. Cautiously using natural medicine to treat liver problems. World J Gastroenterol. 2017;23:3388–3395. doi: 10.3748/wjg.v23.i19.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajaratnam M, Prystupa A, Lachowska-Kotowska P, Załuska W, Filip R. Herbal medicine for treatment and prevention of liver diseases. J Pre Clin Clin Res. 2014;8:55–60. [Google Scholar]
- 9.Kumar Singh A, Cabral C, Kumar R, Ganguly R, Kumar Rana H, Gupta A, Rosaria Lauro M, Carbone C, Reis F, Pandey AK. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients. 2019;11:2216. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma UK, Kumar R, Gupta A, Ganguly R, Singh AK, Ojha AK, Pandey AK. Ameliorating efficacy of eugenol against metanil yellow induced toxicity in albino Wistar rats. Food Chem Toxicol. 2019;126:34–40. doi: 10.1016/j.fct.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Sharma AK, Kumar S, Chashoo G, Saxena AK, Pandey AK. Cell cycle inhibitory activity of Piper longum against A549 cell line and its protective effect against metal-induced toxicity in rats. Indian J Biochem Biophys. 2014;51:358–364. [PubMed] [Google Scholar]
- 12.Sharma UK, Sharma AK, Gupta A, Kumar R, Pandey A, Pandey AK. Pharmacological activities of cinnamaldehyde and eugenol: antioxidant, cytotoxic and anti-leishmanial studies. Cell Mol Biol (Noisy-le-grand) 2017;63:73–78. doi: 10.14715/cmb/2017.63.6.15. [DOI] [PubMed] [Google Scholar]
- 13.Waisundara VY, Hsu A, Tan BK, Huang D. Baicalin reduces mitochondrial damage in streptozotocin-induced diabetic Wistar rats. Diabetes Metab Res Rev. 2009;25:671–677. doi: 10.1002/dmrr.1005. [DOI] [PubMed] [Google Scholar]
- 14.Tao Y, Zhan S, Wang Y, Zhou G, Liang H, Chen X, Shen H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci Rep. 2018;8:14477. doi: 10.1038/s41598-018-32734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng WX, Wang F, Cao XL, Pan HY, Liu XY, Hu XM, Sun YY. Baicalin protects PC-12 cells from oxidative stress induced by hydrogen peroxide via anti-apoptotic effects. Brain Inj. 2014;28:227–234. doi: 10.3109/02699052.2013.860469. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Xu Y, Wang J, Zhao W, Ruan H. Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation and oxidative stress in rat. Int J Clin Exp Pathol. 2015;8:10139–10147. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong X, Liu H. Baicalin attenuates diet induced nonalcoholic steatohepatitis by inhibiting inflammation and oxidative stress via suppressing JNK signaling pathways. Biomed Pharmacother. 2018;98:111–117. doi: 10.1016/j.biopha.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Lei K, Shen Y, He Y, Zhang L, Zhang J, Tong W, Xu Y, Jin L. Baicalin represses C/EBPβ via its antioxidative effect in Parkinson's Disease. Oxid Med Cell Longev. 2020;2020:8951907. doi: 10.1155/2020/8951907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Zhang Y, Bai R, Wang M, Du S. Baicalin attenuates alcoholic liver injury through modulation of hepatic oxidative stress, inflammation and sonic hedgehog pathway in rats. Cell Physiol Biochem. 2016;39:1129–1140. doi: 10.1159/000447820. [DOI] [PubMed] [Google Scholar]
- 20.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Noh K, Kang Y, Nepal MR, Jeong KS, Oh DG, Kang MJ, Lee S, Kang W, Jeong HG, Jeong TC. Role of Intestinal Microbiota in Baicalin-Induced Drug Interaction and its pharmacokinetics. Molecules. 2016;21:337. doi: 10.3390/molecules21030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Neuroprotective and cognitive enhancement potentials of baicalin: a review. Brain Sci. 2018;8 doi: 10.3390/brainsci8060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinda B, SilSarma I, Dinda M, Rudrapaul P. Oroxylum indicum (L.) Kurz, an important Asian traditional medicine: from traditional uses to scientific data for its commercial exploitation. J Ethnopharmacol. 2015;161:255–278. doi: 10.1016/j.jep.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 24.Mu X, He G, Cheng Y, Li X, Xu B, Du G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol Biochem Behav. 2009;92:642–648. doi: 10.1016/j.pbb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Gao Y, Wu J, Chen Y, Chen B, Hu J, Zhou J. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014;354:5–11. doi: 10.1016/j.canlet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin M, Tsai MJ, Wen KC. Supercritical fluid extraction of flavonoids from Scutellariae Radix. J Chromatogr A. 1999;830:387–395. [Google Scholar]
- 27.Yang R, Zeng HJ, Wang QW, Guo C, Li JJ, Qu LB. Simultaneous determination of eight active components in Chinese medicine 'JiangYaBiFeng' tablet by HPLC coupled with diode array detection. J Pharm Biomed Anal. 2011;55:552–556. doi: 10.1016/j.jpba.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Taiming L, Xuehua J. Investigation of the absorption mechanisms of baicalin and baicalein in rats. J Pharm Sci. 2006;95:1326–1333. doi: 10.1002/jps.20593. [DOI] [PubMed] [Google Scholar]
- 29.Hao J, Wang F, Wang X, Zhang D, Bi Y, Gao Y, Zhao X, Zhang Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur J Pharm Sci. 2012;47:497–505. doi: 10.1016/j.ejps.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhao L, Wei Y, Huang Y, He B, Zhou Y, Fu J. Nanoemulsion improves the oral bioavailability of baicalin in rats: in vitro and in vivo evaluation. Int J Nanomedicine. 2013;8:3769–3779. doi: 10.2147/IJN.S51578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore OA, Gao Y, Chen AY, Brittain R, Chen YC. The extraction, anticancer effect, bioavailability, and nanotechnology of baicalin. J Nutr Med Diet Care. 2016;2 doi: 10.23937/2572-3278.1510011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalapos-Kovács B, Magda B, Jani M, Fekete Z, Szabó PT, Antal I, Krajcsi P, Klebovich I. Multiple ABC transporters efflux baicalin. Phytother Res. 2015;29:1987–1990. doi: 10.1002/ptr.5477. [DOI] [PubMed] [Google Scholar]
- 33.Akao T, Sato K, Hanada M. Hepatic contribution to a marked increase in the plasma concentration of baicalin after oral administration of its aglycone, baicalein, in multidrug resistance-associated protein 2-deficient rat. Biol Pharm Bull. 2009;32:2079–2082. doi: 10.1248/bpb.32.2079. [DOI] [PubMed] [Google Scholar]
- 34.Tang Y, Zhu H, Zhang Y, Huang C. Determination of human plasma protein binding of baicalin by ultrafiltration and high-performance liquid chromatography. Biomed Chromatogr. 2006;20:1116–1119. doi: 10.1002/bmc.655. [DOI] [PubMed] [Google Scholar]
- 35.Liang W, Huang X, Chen W. The effects of baicalin and baicalein on cerebral ischemia: a review. Aging Dis. 2017;8:850–867. doi: 10.14336/AD.2017.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abe K, Inoue O, Yumioka E. Biliary excretion of metabolites of baicalin and baicalein in rats. Chem Pharm Bull (Tokyo) 1990;38:209–211. [PubMed] [Google Scholar]
- 37.Zhang J, Cai W, Zhou Y, Liu Y, Wu X, Li Y, Lu J, Qiao Y. Profiling and identification of the metabolites of baicalin and study on their tissue distribution in rats by ultra-high-performance liquid chromatography with linear ion trap-Orbitrap mass spectrometer. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;985:91–102. doi: 10.1016/j.jchromb.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Lai CC, Huang PH, Yang AH, Chiang SC, Tang CY, Tseng KW, Huang CH. Baicalein reduces liver injury induced by myocardial ischemia and reperfusion. Am J Chin Med. 2016;44:531–550. doi: 10.1142/S0192415X16500294. [DOI] [PubMed] [Google Scholar]
- 39.Dong Y, Xing Y, Sun J, Sun W, Xu Y, Quan C. Baicalein alleviates liver oxidative stress and apoptosis induced by high-level glucose through the activation of the PERK/Nrf2 signaling pathway. Molecules. 2020;25 doi: 10.3390/molecules25030599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akuta N, Kawamura Y, Suzuki F, Saitoh S, Arase Y, Fujiyama S, Sezaki H, Hosaka T, Kobayashi M, Suzuki Y, Ikeda K, Kumada H. Analysis of association between circulating miR-122 and histopathological features of nonalcoholic fatty liver disease in patients free of hepatocellular carcinoma. BMC Gastroenterol. 2016;16:141. doi: 10.1186/s12876-016-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xi Y, Wu M, Li H, Dong S, Luo E, Gu M, Shen X, Jiang Y, Liu Y, Liu H. Baicalin attenuates high fat diet-induced obesity and liver dysfunction: dose-response and potential role of CaMKKβ/AMPK/ACC pathway. Cell Physiol Biochem. 2015;35:2349–2359. doi: 10.1159/000374037. [DOI] [PubMed] [Google Scholar]
- 42.Guo HX, Liu DH, Ma Y, Liu JF, Wang Y, Du ZY, Wang X, Shen JK, Peng HL. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol Sin. 2009;30:1505–1512. doi: 10.1038/aps.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y, Yang F, Wang Y, Du Z, Liu D, Guo H, Shen J, Peng H. CaMKKβ is involved in AMP-activated protein kinase activation by baicalin in LKB1 deficient cell lines. PLoS One. 2012;7:e47900. doi: 10.1371/journal.pone.0047900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai J, Liang K, Zhao S, Jia W, Liu Y, Wu H, Lv J, Cao C, Chen T, Zhuang S, Hou X, Zhou S, Zhang X, Chen XW, Huang Y, Xiao RP, Wang YL, Luo T, Xiao J, Wang C. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc Natl Acad Sci U S A. 2018;115:E5896–E5905. doi: 10.1073/pnas.1801745115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q, Liu M, Yu H, Li J, Wang S, Zhang Y, Qiu F, Wang T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J Nat Med. 2018;72:655–666. doi: 10.1007/s11418-018-1199-5. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Zhang H, Deng X, Zhang N, Liu B, Xin S, Li G, Xu K. Baicalin attenuates non-alcoholic steatohepatitis by suppressing key regulators of lipid metabolism, inflammation and fibrosis in mice. Life Sci. 2018;192:46–54. doi: 10.1016/j.lfs.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Yuan Y, Gong X, Zhang L, Zhou Q, Wu S, Zhang X, Hu J, Kuang G, Yin X, Wan J. Baicalin and its nanoliposomes ameliorates nonalcoholic fatty liver disease via suppression of TLR4 signaling cascade in mice. Int Immunopharmacol. 2020;80:106208. doi: 10.1016/j.intimp.2020.106208. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W, Liu L, Wang Y, Mao T, Li J. Effects of a combination of puerarin, baicalin and berberine on the expression of proliferator-activated receptor-γ and insulin receptor in a rat model of nonalcoholic fatty liver disease. Exp Ther Med. 2016;11:183–190. doi: 10.3892/etm.2015.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, Zhang H, Deng X, Zhang Y, Xu K. Baicalin protects AML-12 cells from lipotoxicity via the suppression of ER stress and TXNIP/NLRP3 inflammasome activation. Chem Biol Interact. 2017;278:189–196. doi: 10.1016/j.cbi.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Zhang HY, Wang HL, Zhong GY, Zhu JX. Molecular mechanism and research progress on pharmacology of traditional Chinese medicine in liver injury. Pharm Biol. 2018;56:594–611. doi: 10.1080/13880209.2018.1517185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Real M, Barnhill MS, Higley C, Rosenberg J, Lewis JH. Drug-induced liver injury: highlights of the recent literature. Drug Saf. 2019;42:365–387. doi: 10.1007/s40264-018-0743-2. [DOI] [PubMed] [Google Scholar]
- 52.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 53.Liao CC, Day YJ, Lee HC, Liou JT, Chou AH, Liu FC. Baicalin attenuates IL-17-mediated acetaminophen-induced liver injury in a mouse model. PLoS One. 2016;11:e0166856. doi: 10.1371/journal.pone.0166856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He P, Wu Y, Shun J, Liang Y, Cheng M, Wang Y. Baicalin ameliorates liver injury induced by chronic plus binge ethanol feeding by modulating oxidative stress and inflammation via CYP2E1 and NRF2 in mice. Oxid Med Cell Longev. 2017;2017:4820414. doi: 10.1155/2017/4820414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi L, Hao Z, Zhang S, Wei M, Lu B, Wang Z, Ji L. Baicalein and baicalin alleviate acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: The involvement of ERK1/2 and PKC. Biochem Pharmacol. 2018;150:9–23. doi: 10.1016/j.bcp.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 56.Liao CC, Day YJ, Lee HC, Liou JT, Chou AH, Liu FC. ERK signaling pathway plays a key role in baicalin protection against acetaminophen-induced liver injury. Am J Chin Med. 2017;45:105–121. doi: 10.1142/S0192415X17500082. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Jia Y, Yang X, Liang B, Gao H, Yang T. A potential role of Baicalin to inhibit apoptosis and protect against acute liver and kidney injury in rat preeclampsia model. Biomed Pharmacother. 2018;108:1546–1552. doi: 10.1016/j.biopha.2018.09.107. [DOI] [PubMed] [Google Scholar]
- 58.Liu F, Zhang J, Qian J, Wu G, Ma Z. Baicalin attenuates liver hypoxia/reoxygenation injury by inducing autophagy. Exp Ther Med. 2018;16:657–664. doi: 10.3892/etm.2018.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu T, Liu T, Xing L, Ji G. Baicalin and puerarin reverse epithelial-mesenchymal transition via the TGF-β1/Smad3 pathway in vitro. Exp Ther Med. 2018;16:1968–1974. doi: 10.3892/etm.2018.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Yin S, Zhou Y, Zhou W, Chen T, Wu Q, Zhou L, Zheng S. Dual-function of Baicalin in nsPEFs-treated hepatocytes and hepatocellular carcinoma cells for different death pathway and mitochondrial response. Int J Med Sci. 2019;16:1271–1282. doi: 10.7150/ijms.34876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Li H, Gao Z, Gong Y, Xu H. Effects of flavonoids extracted from Scutellaria baicalensis Georgi on hemin-nitrite-H2O2 induced liver injury. Eur J Pharmacol. 2006;536:192–199. doi: 10.1016/j.ejphar.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 62.Liao S, Li P, Wang J, Zhang Q, Xu D, Yang M, Kong L. Protection of baicalin against lipopolysaccharide induced liver and kidney injuries based on 1H NMR metabolomic profiling. Toxicol Res (Camb) 2016;5:1148–1159. doi: 10.1039/c6tx00082g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512–518. doi: 10.1002/jhbp.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klingenberg M, Groß M, Goyal A, Polycarpou-Schwarz M, Miersch T, Ernst AS, Leupold J, Patil N, Warnken U, Allgayer H, Longerich T, Schirmacher P, Boutros M, Diederichs S. The long noncoding RNA cancer susceptibility 9 and RNA binding protein heterogeneous nuclear ribonucleoprotein L form a complex and coregulate genes linked to AKT signaling. Hepatology. 2018;68:1817–1832. doi: 10.1002/hep.30102. [DOI] [PubMed] [Google Scholar]
- 65.Dawood RM, El-Meguid MA, Salum GM, El Awady MK. Key players of hepatic fibrosis. J Interferon Cytokine Res. 2020;40:472–489. doi: 10.1089/jir.2020.0059. [DOI] [PubMed] [Google Scholar]
- 66.Lin L, Zhou F, Shen S, Zhang T. Fighting liver fibrosis with naturally occurring antioxidants. Planta Med. 2018;84:1318–1333. doi: 10.1055/a-0757-0008. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Z, Lin CY, Cheng K. siRNA- and miRNA-based therapeutics for liver fibrosis. Transl Res. 2019;214:17–29. doi: 10.1016/j.trsl.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen K, Feng X, Pan H, Zhang F, Xie H, Zheng S. Baicalin ameliorates experimental liver cholestasis in mice by modulation of oxidative stress, inflammation, and NRF2 transcription factor. Oxid Med Cell Longev. 2017;2017:6169128. doi: 10.1155/2017/6169128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu X, Zhi F, Lun W, Deng Q, Zhang W. Baicalin inhibits PDGF-BB-induced hepatic stellate cell proliferation, apoptosis, invasion, migration and activation via the miR-3595/ACSL4 axis. Int J Mol Med. 2018;41:1992–2002. doi: 10.3892/ijmm.2018.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang MD, Chiang YM, Higashiyama R, Asahina K, Mann DA, Mann J, Wang CC, Tsukamoto H. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor γ in hepatic stellate cells for their antifibrotic effect. Hepatology. 2012;55:1271–1281. doi: 10.1002/hep.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei XL, Fang RT, Yang YH, Bi XY, Ren GX, Luo AL, Zhao M, Zang WJ. Protective effects of extracts from Pomegranate peels and seeds on liver fibrosis induced by carbon tetrachloride in rats. BMC Complement Altern Med. 2015;15:389. doi: 10.1186/s12906-015-0916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiao H, Han H, Hong D, Ren Z, Chen Y, Zhou C. Protective effects of baicalin on carbon tetrachloride induced liver injury by activating PPARγ and inhibiting TGFβ1. Pharm Biol. 2011;49:38–45. doi: 10.3109/13880209.2010.493179. [DOI] [PubMed] [Google Scholar]
- 73.de Vries E, Bolier R, Goet J, Parés A, Verbeek J, de Vree M, Drenth J, van Erpecum K, van Nieuwkerk K, van der Heide F, Mostafavi N, Helder J, Ponsioen C, Oude Elferink R, van Buuren H, Beuers U Netherlands association for the study of the liver-cholestasis working group. Fibrates for Itch (FITCH) in fibrosing cholangiopathies: a double-blind, randomized, placebo-controlled trial. Gastroenterology. 2021;160:734–743.e6. doi: 10.1053/j.gastro.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Salas-Silva S, Simoni-Nieves A, Razori MV, López-Ramirez J, Barrera-Chimal J, Lazzarini R, Bello O, Souza V, Miranda-Labra RU, Gutiérrez-Ruiz MC, Gomez-Quiroz LE, Roma MG, Bucio-Ortiz L. HGF induces protective effects in α-naphthylisothiocyanate-induced intrahepatic cholestasis by counteracting oxidative stress. Biochem Pharmacol. 2020;174:113812. doi: 10.1016/j.bcp.2020.113812. [DOI] [PubMed] [Google Scholar]
- 75.Zhang CL, Xu YJ, Xiang D, Yang JY, Lei K, Liu D. Pharmacokinetic characteristics of baicalin in rats with 17α-ethynyl-estradiol-induced intrahepatic cholestasis. Curr Med Sci. 2018;38:167–173. doi: 10.1007/s11596-018-1861-x. [DOI] [PubMed] [Google Scholar]
- 76.Gupta A, Kumar R, Bhattacharyya P, Bishayee A, Pandey AK. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: A systematic and comprehensive review. Phytomedicine. 2020;77:153278. doi: 10.1016/j.phymed.2020.153278. [DOI] [PubMed] [Google Scholar]
- 77.Yang J, Xiang D, He W, Liu Y, Lan L, Li G, Jiang C, Ren X, Liu D, Zhang C. Baicalin protects against 17α-ethinylestradiol-induced cholestasis via the sirtuin1/hepatic nuclear receptor-1α/farnesoid X receptor pathway. Front Pharmacol. 2019;10:1685. doi: 10.3389/fphar.2019.01685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lanini S, Ustianowski A, Pisapia R, Zumla A, Ippolito G. Viral hepatitis: etiology, epidemiology, transmission, diagnostics, treatment, and prevention. Infect Dis Clin North Am. 2019;33:1045–1062. doi: 10.1016/j.idc.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki K, Suda G, Yamamoto Y, Furuya K, Baba M, Nakamura A, Miyoshi H, Kimura M, Maehara O, Yamada R, Kitagataya T, Yamamoto K, Shigesawa T, Ohara M, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Ohnishi S, Sakamoto N NORTE Study Group. Tenofovir-disoproxil-fumarate modulates lipid metabolism via hepatic CD36/PPAR-alpha activation in hepatitis B virus infection. J Gastroenterol. 2021;56:168–180. doi: 10.1007/s00535-020-01750-3. [DOI] [PubMed] [Google Scholar]
- 80.Lim HK, Jeffrey GP, Ramm GA, Soekmadji C. Pathogenesis of viral hepatitis-induced chronic liver disease: role of extracellular vesicles. Front Cell Infect Microbiol. 2020;10:587628. doi: 10.3389/fcimb.2020.587628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robertson BH. Viral hepatitis and primates: historical and molecular analysis of human and nonhuman primate hepatitis A, B, and the GB-related viruses. J Viral Hepat. 2001;8:233–242. doi: 10.1046/j.1365-2893.2001.00295.x. [DOI] [PubMed] [Google Scholar]
- 82.Huang Y, Sun M, Yang X, Ma A, Ma Y, Zhao A. Baicalin relieves inflammation stimulated by lipopolysaccharide via upregulating TUG1 in liver cells. J Physiol Biochem. 2019;75:463–473. doi: 10.1007/s13105-019-00698-0. [DOI] [PubMed] [Google Scholar]
- 83.Pollicino T, Musolino C, Irrera N, Bitto A, Lombardo D, Timmoneri M, Minutoli L, Raimondo G, Squadrito G, Squadrito F, Altavilla D. Flavocoxid exerts a potent antiviral effect against hepatitis B virus. Inflamm Res. 2018;67:89–103. doi: 10.1007/s00011-017-1099-2. [DOI] [PubMed] [Google Scholar]
- 84.Xia C, Tang W, Geng P, Zhu H, Zhou W, Huang H, Zhou P, Shi X. Baicalin down-regulating hepatitis B virus transcription depends on the liver-specific HNF4α-HNF1α axis. Toxicol Appl Pharmacol. 2020;403:115131. doi: 10.1016/j.taap.2020.115131. [DOI] [PubMed] [Google Scholar]
- 85.Huang H, Zhou W, Zhu H, Zhou P, Shi X. Baicalin benefits the anti-HBV therapy via inhibiting HBV viral RNAs. Toxicol Appl Pharmacol. 2017;323:36–43. doi: 10.1016/j.taap.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Hwang JM, Tseng TH, Tsai YY, Lee HJ, Chou FP, Wang CJ, Chu CY. Protective effects of baicalein on tert-butyl hydroperoxide-induced hepatic toxicity in rat hepatocytes. J Biomed Sci. 2005;12:389–397. doi: 10.1007/s11373-005-1572-8. [DOI] [PubMed] [Google Scholar]
- 87.Ueda S, Nakamura H, Masutani H, Sasada T, Takabayashi A, Yamaoka Y, Yodoi J. Baicalin induces apoptosis via mitochondrial pathway as prooxidant. Mol Immunol. 2002;38:781–791. doi: 10.1016/s0161-5890(01)00115-8. [DOI] [PubMed] [Google Scholar]
- 88.Cheng Y, Ping J, Xu HD, Fu HJ, Zhou ZH. Synergistic effect of a novel oxymatrine-baicalin combination against hepatitis B virus replication, alpha smooth muscle actin expression and type I collagen synthesis in vitro. World J Gastroenterol. 2006;12:5153–5159. doi: 10.3748/wjg.v12.i32.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wan JY, Gong X, Zhang L, Li HZ, Zhou YF, Zhou QX. Protective effect of baicalin against lipopolysaccharide/D-galactosamine-induced liver injury in mice by up-regulation of heme oxygenase-1. Eur J Pharmacol. 2008;587:302–308. doi: 10.1016/j.ejphar.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y, Zeng L, Yang J, Wang Y, Yao F, Wu Y, Wang D, Hu Y, Liu J. Anti-DHAV-1 reproduction and immuno-regulatory effects of a flavonoid prescription on duck virus hepatitis. Pharm Biol. 2017;55:1545–1552. doi: 10.1080/13880209.2017.1309554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh AK, Kumar R, Pandey AK. Hepatocellular carcinoma: causes, mechanism of progression and biomarkers. Curr Chem Genom Transl Med. 2018;12:9–26. doi: 10.2174/2213988501812010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su L, Zhou T, Zhang Z, Zhang X, Zhi X, Li C, Wang Q, Jia C, Shi W, Yue Y, Gao Y, Cheng B. Optimal staging system for predicting the prognosis of patients with hepatocellular carcinoma in China: a retrospective study. BMC Cancer. 2016;16:424. doi: 10.1186/s12885-016-2420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan HY, Wang N, Man K, Tsao SW, Che CM, Feng Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015;6:e1942. doi: 10.1038/cddis.2015.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu Z, Luo X, Wang C, Ye J, Liu S, Xie L, Wang F, Bao J. Baicalin promoted site-2 protease and not site-1 protease in endoplasmic reticulum stress-induced apoptosis of human hepatocellular carcinoma cells. FEBS Open Bio. 2016;6:1093–1101. doi: 10.1002/2211-5463.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Tang X, Liu H, Li L, Hou Q, Gao J. Autophagy induced by baicalin involves downregulation of CD147 in SMMC-7721 cells in vitro. Oncol Rep. 2012;27:1128–1134. doi: 10.3892/or.2011.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giraud J, Chalopin D, Blanc JF, Saleh M. Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Front Immunol. 2021;12:655697. doi: 10.3389/fimmu.2021.655697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gupta A, Singh AK, Kumar R, Ganguly R, Rana HK, Pandey PK, Sethi G, Bishayee A, Pandey AK. Corilagin in cancer: a critical evaluation of anticancer activities and molecular mechanisms. Molecules. 2019;24 doi: 10.3390/molecules24183399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kurilova I, Bendet A, Petre EN, Boas FE, Kaye E, Gonen M, Covey A, Brody LA, Brown KT, Kemeny NE, Yarmohammadi H, Ziv E, D'Angelica MI, Kingham TP, Cercek A, Solomon SB, Beets-Tan RGH, Sofocleous CT. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clin Colorectal Cancer. 2021;20:e82–e95. doi: 10.1016/j.clcc.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 99.Singh AK, Bishayee A, Pandey AK. Targeting histone deacetylases with natural and synthetic agents: an emerging anticancer strategy. Nutrients. 2018;10 doi: 10.3390/nu10060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang B, Bai H, Sa Y, Zhu P, Liu P. Inhibiting EMT, stemness and cell cycle involved in baicalin-induced growth inhibition and apoptosis in colorectal cancer cells. J Cancer. 2020;11:2303–2317. doi: 10.7150/jca.37242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia Y, Chen L, Guo S, Li Y. Baicalin induced colon cancer cells apoptosis through miR-217/DKK1-mediated inhibition of Wnt signaling pathway. Mol Biol Rep. 2019;46:1693–1700. doi: 10.1007/s11033-019-04618-9. [DOI] [PubMed] [Google Scholar]
- 102.Wang Z, Ma L, Su M, Zhou Y, Mao K, Li C, Peng G, Zhou C, Shen B, Dou J. Baicalin induces cellular senescence in human colon cancer cells via upregulation of DEPP and the activation of Ras/Raf/MEK/ERK signaling. Cell Death Dis. 2018;9:217. doi: 10.1038/s41419-017-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu C, Zhang Z, Zhang H, Zhen Z, Calway T, Wang Y, Yuan CS, Wang CZ. Pretreatment of baicalin and wogonoside with glycoside hydrolase: a promising approach to enhance anticancer potential. Oncol Rep. 2013;30:2411–2418. doi: 10.3892/or.2013.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma W, Liu X, Du W. Baicalin induces apoptosis in SW480 cells through downregulation of the SP1 transcription factor. Anticancer Drugs. 2019;30:153–158. doi: 10.1097/CAD.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neurath MF, Leppkes M. Resolution of ulcerative colitis. Semin Immunopathol. 2019;41:747–756. doi: 10.1007/s00281-019-00751-6. [DOI] [PubMed] [Google Scholar]
- 106.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rizzo V, Ferlazzo N, Currò M, Isola G, Matarese M, Bertuccio MP, Caccamo D, Matarese G, Ientile R. Baicalin-induced autophagy preserved LPS-stimulated intestinal cells from inflammation and alterations of paracellular permeability. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22052315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shen J, Cheng J, Zhu S, Zhao J, Ye Q, Xu Y, Dong H, Zheng X. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int Immunopharmacol. 2019;73:193–200. doi: 10.1016/j.intimp.2019.04.052. [DOI] [PubMed] [Google Scholar]
- 109.Cui L, Feng L, Zhang ZH, Jia XB. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int Immunopharmacol. 2014;23:294–303. doi: 10.1016/j.intimp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 110.Yao J, Cao X, Zhang R, Li YX, Xu ZL, Zhang DG, Wang LS, Wang JY. Protective effect of baicalin against experimental colitis via suppression of oxidant stress and apoptosis. Pharmacogn Mag. 2016;12:225–234. doi: 10.4103/0973-1296.186342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liang S, Deng X, Lei L, Zheng Y, Ai J, Chen L, Xiong H, Mei Z, Cheng YC, Ren Y. The comparative study of the therapeutic effects and mechanism of baicalin, baicalein, and their combination on ulcerative colitis rat. Front Pharmacol. 2019;10:1466. doi: 10.3389/fphar.2019.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang CL, Zhang S, He WX, Lu JL, Xu YJ, Yang JY, Liu D. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci. 2017;186:125–132. doi: 10.1016/j.lfs.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Feng J, Guo C, Zhu Y, Pang L, Yang Z, Zou Y, Zheng X. Baicalin down regulates the expression of TLR4 and NFkB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int J Clin Exp Med. 2014;7:4063–4072. [PMC free article] [PubMed] [Google Scholar]
- 114.Dai SX, Zou Y, Feng YL, Liu HB, Zheng XB. Baicalin down-regulates the expression of macrophage migration inhibitory factor (MIF) effectively for rats with ulcerative colitis. Phytother Res. 2012;26:498–504. doi: 10.1002/ptr.3581. [DOI] [PubMed] [Google Scholar]
- 115.Zhu L, Xu LZ, Zhao S, Shen ZF, Shen H, Zhan LB. Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl Microbiol Biotechnol. 2020;104:5449–5460. doi: 10.1007/s00253-020-10527-w. [DOI] [PubMed] [Google Scholar]
- 116.Zou Y, Dai SX, Chi HG, Li T, He ZW, Wang J, Ye CG, Huang GL, Zhao B, Li WY, Wan Z, Feng JS, Zheng XB. Baicalin attenuates TNBS-induced colitis in rats by modulating the Th17/Treg paradigm. Arch Pharm Res. 2015;38:1873–1887. doi: 10.1007/s12272-014-0486-2. [DOI] [PubMed] [Google Scholar]
- 117.Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. Effects of baicalin in CD4 + CD29 + T cell subsets of ulcerative colitis patients. World J Gastroenterol. 2014;20:15299–15309. doi: 10.3748/wjg.v20.i41.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feng W, Ao H, Peng C, Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 119.Ju M, Liu Y, Li M, Cheng M, Zhang Y, Deng G, Kang X, Liu H. Baicalin improves intestinal microecology and abnormal metabolism induced by high-fat diet. Eur J Pharmacol. 2019;857:172457. doi: 10.1016/j.ejphar.2019.172457. [DOI] [PubMed] [Google Scholar]
- 120.Wu D, Ding L, Tang X, Wang W, Chen Y, Zhang T. Baicalin protects against hypertension-associated intestinal barrier impairment in part through enhanced microbial production of short-chain fatty acids. Front Pharmacol. 2019;10:1271. doi: 10.3389/fphar.2019.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dey P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol Res. 2019;147:104367. doi: 10.1016/j.phrs.2019.104367. [DOI] [PubMed] [Google Scholar]
- 122.Chopyk DM, Grakoui A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology. 2020;159:849–863. doi: 10.1053/j.gastro.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ganguly R, Kumar R, Pandey AK. Baicalin provides protection against fluoxetine-induced hepatotoxicity by modulation of oxidative stress and inflammation. World J Hepatol. 2022;14:729–743. doi: 10.4254/wjh.v14.i4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J, Jiang Q, Deng P, Chen Q, Yu M, Shang J, Li W. The formation of a host-guest inclusion complex system between β-cyclodextrin and baicalin and its dissolution characteristics. J Pharm Pharmacol. 2017;69:663–674. doi: 10.1111/jphp.12708. [DOI] [PubMed] [Google Scholar]
- 125.Ibrahim A, Nasr M, El-Sherbiny IM. Baicalin as an emerging magical nutraceutical molecule: Emphasis on pharmacological properties and advances in pharmaceutical delivery. J Drug Deliv Sci Technol . 2022;70:1773–2247. [Google Scholar]