Abstract
BACKGROUND
Sodium glucose cotransporter-2 inhibitors (SGLT2-I) are the most recently approved drugs for type 2 diabetes (T2D). Recent clinical trials of these compounds reported beneficial cardiovascular (CV) and renal outcomes. A major cause of vascular dysfunction and CV disease in diabetes is hyperglycemia associated with inflammation and oxidative stress. Pre-clinical studies demonstrated that SGLT2-I reduce glucotoxicity and promote anti-inflammatory effects by lowering oxidative stress.
AIM
To investigate the effects of SGLT2-I on markers of oxidative stress, inflammation, liver steatosis, and fibrosis in patients of T2D with non-alcoholic fatty liver disease (NAFLD).
METHODS
We referred fifty-two consecutive outpatients treated with metformin monotherapy and exhibiting poor glycemic control to our centre. We introduced the outpatients to an SGLT2-I (dapagliflozin, empagliflozin, or canagliflozin; n = 26) or a different hypoglycemic drug [other glucose-lowering drugs (OTHER), n = 26]. We evaluated circulating interleukins and serum hydroxynonenal (HNE)- or malondialdehyde (MDA)-protein adducts, fatty liver index (FLI), NAFLD fibrosis score, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, AST-to-platelet-ratio index (APRI), and fibrosis-4 on the day before (T0) and following treatment for six months (T1). We also performed transient elastography at T0 and T1.
RESULTS
Add-on therapy resulted in improved glycemic control and reduced fasting blood glucose in both groups. Of note, following treatment for six months, a reduction of FLI and APRI, as well as of the FibroScan result, was reported in patients treated with SGLT2-I, but not in the OTHER group; furthermore, in the SGLT2-I group, we reported lower circulating levels of interleukin (IL)-1β, IL-6, tumor necrosis factor, vascular endothelial growth factor, and monocyte chemoattractant protein-1, and higher levels of IL-4 and IL-10. We did not observe any modification in circulating interleukins in the OTHER group. Finally, serum HNE- and MDA-protein adducts decreased significantly in SGLT2-I rather than OTHER patients and correlated with liver steatosis and fibrosis scores.
CONCLUSION
The present data indicate that treatment with SGLT2-I in patients with T2D and NAFLD is associated with improvement of liver steatosis and fibrosis markers and circulating pro-inflammatory and redox status, more than optimizing glycemic control.
Keywords: Sodium glucose cotransporter-2 inhibitors, Non-alcoholic fatty liver disease, Oxidative stress, Type 2 diabetes, Liver fibrosis, Inflammation
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is the most common hepatic disorder, and it is often associated with type 2 diabetes mellitus (T2DM). Diabetic patients often suffer from advanced NAFLD and are keen on progressing toward severe fibrosis and end-stage liver disease. There is no approved treatment for NAFLD, but new drug classes introduced to treat T2DM can exert favorable effects beyond glucose control. This pilot study demonstrates that treatment with sodium glucose cotransporter-2 inhibitors in patients with T2DM and NAFLD is associated with improving liver steatosis and fibrosis markers and circulating pro-inflammatory and redox status.
INTRODUCTION
Patients affected by type 2 diabetes mellitus (T2DM) present with an increased risk of cardiovascular (CV) disease, which is associated with a high mortality rate and low quality of life[1,2]. T2DM is strongly associated with non-alcoholic fatty liver disease (NAFLD), whose prevalence in diabetic patients is over 60%[3,4]. NAFLD is the most common chronic liver disease, characterized by a broad spectrum of hepatic disorders, ranging from simple steatosis to steatohepatitis (non-alcoholic steatohepatitis), fibrosis, and cirrhosis[5]. The co-existence of NAFLD and T2DM pushes the progression of liver damage, increasing the risk of advanced fibrosis[6]. Besides, NAFLD is an independent risk factor of CV disease. Patients with T2DM and NAFLD present a higher CV risk than diabetic patients without NAFLD, suggesting that these conditions share common pathophysiological mechanisms, including low-grade systemic inflammation and oxidative stress[7].
Studies using new classes of antidiabetic drugs, such as sodium-glucose co-transporter-2 inhibitors (SGLT2-I) and glucagon-like peptide-1 receptor agonists (GLP1-RA), demonstrated definite CV advantage in patients with T2D[8]. Several clinical trials suggest that both classes also ameliorate liver steatosis and inflammation, potentially reversing fibrosis in NAFLD[9]. The currently approved SGLT2-I are dapagliflozin, canagliflozin, empagliflozin, and ertugliflozin, which increase urinary glucose excretion and improve glycemic control independent of insulin. Furthermore, these drugs reduce body weight, visceral adiposity, blood pressure, and arterial stiffness[10]. Real-world CVD-REAL and CVD-REAL 2 studies have demonstrated that the benefits of SGLT2-I on CV outcomes observed in clinical trials may be attributed to a class effect and may be extended to a broad range of patients[11,12]. Despite clinical evidence on the efficacy of SGLT2-I in both the reduction of CV events and the improvement of hepatic damage in NAFLD, human mechanistic trials remain elusive.
Pre-clinical studies demonstrated that dapagliflozin, empagliflozin, and canagliflozin attenuate inflammation in apolipoprotein E knockout mice[13-15], reduce oxidative stress and improve mitochondrial function through direct pleiotropic and epigenetic effects[16,17]. Furthermore, these compounds may also exert antifibrotic effects in diabetic and non-diabetic cardiopathy or nephropathy[18-20]. Such results strongly encourage clinical studies to clarify the impact of SGLT2-I on systemic inflammation, oxidative stress, and liver fibrosis in diabetic patients with NAFLD. Thus, the present investigation was aimed to evaluate the effects of SGLT2-I addition to metformin on circulating markers of inflammation and oxidative protein damage in patients affected by uncontrolled T2DM and NAFLD and compare these outcomes with other glucose-lowering drugs (OTHER).
MATERIALS AND METHODS
Study design
We collected and analyzed data from 204 patients affected by T2DM who underwent outpatient consultation between June 2017 and June 2018 at the University Internal Medicine clinic of the “Policlinico Riuniti” in Foggia (Italy). We designed the investigation as an observational pilot study considering the problematic setting, the limited scale, and the multiple outcome parameters. Patients aged > 18 years old who were: (1) Diagnosed with NAFLD; and (2) Presented with glycated hemoglobin equal to or greater than 7% after at least three months of treatment with metformin monotherapy at the maximal tolerated dosage were assessed for eligibility. NAFLD was suspected on previous ultrasound imaging and/or altered liver function tests[21]. We did not consider subjects diagnosed with viral or autoimmune hepatitis, atherosclerotic CV disease, chronic inflammatory disorders, or those diagnosed with active cancer for the study. Further exclusion criteria were alcohol consumption > 20 g/d (women) or > 30 g/d (men), anemia, severe hepatic failure, glomerular filtration rate < 60 mg/min/m2, use of drugs affecting redox balance, use of anti-inflammatory medications or corticosteroids during the observational period, use of medications associated with fatty liver (amiodarone, tamoxifen, sodium valproate, methotrexate), current smoker status, and prescription of a glucagon-like peptide-1 agonist (Supplementary Figure 1).
52 patients were finally referred to a combination therapy; of these, 26 patients were treated with an SGLT2-I, while 26 patients were treated with OTHER. The combination therapy was not randomized, and the second compound was chosen according to the standard of medical care in diabetes - 2017, considering the clinical characteristics of patients to maximize therapeutic advantages and minimize risks and side effects[22]. Compliance and adverse events were assessed by a verbal questionnaire. The frequency and distribution of the different compounds prescribed are reported in the Supplementary Table 1. For the study purposes, patients enrolled were assessed at baseline (T0) and after six months (T1). Our Institutional Review Board approved the study at the Policlinico Riuniti in Foggia (reference number 2325/2018) and performed it according to the Declaration of Helsinki. All patients gave written informed consent.
Laboratory measurements
Blood samples were obtained from a brachial vein between 8:00 and 9:00 AM, after an overnight fast, and immediately processed. Standard laboratory measurements included glycated hemoglobin A1c (HbA1c), fasting serum glucose, serum triglycerides, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (gamma-GT) activities, platelet count, and serum albumin. The concentrations of serum cytokines and growth factors, including several interleukins (IL), such as IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, tumor necrosis factor (TNF), interferon-γ (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF), were measured using the EV 3513 cytokine biochip array and competitive chemiluminescence immunoassays (Randox Laboratories Ltd, Crumlin, United Kingdom), according to the manufacturer’s instructions, using the Randox Evidence Investigator[23]. As previously reported, serum fluorescent adducts formed between peroxidation-derived aldehydes [hydroxynonenal (HNE) and malondialdehyde (MDA)] and proteins were measured by spectrofluorimetry[24].
Non-invasive markers of liver steatosis and fibrosis
Patients were assessed at baseline and after six months of treatment for the following parameters: Fatty liver index (FLI), calculated according to the formula ey / (1 + ey) × 100, where “y” = 0.953 × ln(triglycerides, mg/dL) + 0.139 × body mass index (BMI), kg/m2 + 0.718 × ln (gamma-GT, U/L) + 0.053 × waist circumference, cm - 15.745[25]; AST-to-platelet ratio index (APRI), calculated according to the formula AST, IU/L/AST upper limit of normal, IU/L/platelets, 109/L[26]; NAFLD fibrosis score, calculated according to the formula - 1.675 + 0.037 × age, years + 0.094 × BMI, kg/m2 + 1.13 × impaired fasting glucose or diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio - 0.013 × platelets, 109/L - 0.66 × albumin, g/dL[27]. The cut-off values chosen to categorize fibrosis grades F0-F2 or F3-F4 were < -1.455 and > 0.675, respectively; fibrosis-4 (FIB-4), calculated according to the formula (age, years × AST, IU/L)/(platelets, 109/L × rad ALT, IU/L)[28]. The cut-off values chosen to categorize fibrosis grades F0-F1 or F3-F4 were < -1.30 and > 2.67, respectively; AST/ALT ratio, whose cut-off value > 0.8 is associated with advanced disease[29].
Transient elastography
Transient elastography (TE) was performed by a Fibroscan (Echosense, Paris) on supine patients with the right arm elevated. The probe tip was put on the intercostal space at the level of the right liver lobe. The Fibroscan probe contains an ultrasound transducer and a mechanical device that provides a controlled vibrating external shot on the body surface to generate shear waves. TE measures liver stiffness (LS) in 1 cm cylindric volume (width: 25-65 mm, M probe; 35-75 mm, XL probe) below the skin surface. Criteria for a valid examination were as follows: (1) At least 10 valid measurements; (2) A success rate [(valid + invalid measurements)/total measurements] > 70%; and (3) An interquartile range < 30% of the median value. Measurements were expressed as KPa[30].
Statistical analysis
Data were expressed as count and percentages for categorical variables and as mean ± SDM for quantitative variables. Gaussian distribution of the samples was evaluated by Kolgomorov-Smirnov test. The significance of differences between the 2 treatment groups (SGLT2-I vs OTHER) at baseline was assessed by student’s t-test (continuous variables) or in contingency tables by Pearson’s Chi-squared test and Fisher’s exact test (categorical variables). The significance of differences between the 2 treatment groups between the beginning (T0) and the end of the observational period (T1) was assessed by the two-way analysis of variance to test the main effects of time and treatment as a between-subject factor; the interaction time × treatment was studied, and a Tukey test was applied as post hoc test for multiple comparisons. The correlation analysis between changes in non-invasive markers of hepatic steatosis and fibrosis with fasting serum glucose or HbA1c, serum interleukins, or serum HNE- and MDA-protein adducts was performed using Pearson correlation test followed by linear regression. All tests were 2-sided, and P < 0.05 were considered statistically significant. Statistical analysis was performed with the Statistical Package for Social Sciences version 23.0 (SPSS, Inc., Chicago, IL) and Graph-Pad Prism 6.0 for Windows (GraphPad Software, Inc., San Diego, CA).
RESULTS
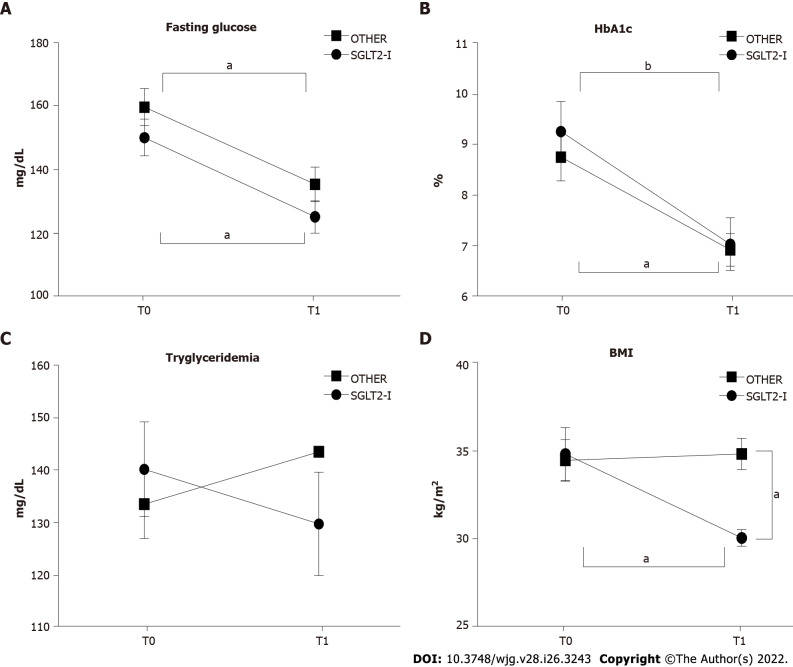
Baseline characteristics of the subjects included in the SGLT2-I or the OTHER group are represented in Table 1. The two groups were comparable in terms of clinical and biochemical features. No side effects in both groups were reported. After six months of treatment, a lowering effect on both fasting serum glucose (time factor: F(1, 100) = 16.04, P < 0.0001) and HbA1c (time factor: F(1, 100) = 14.83, P < 0.0001) was observed, with a significant reduction from T0 to T1 in both groups (Figures 1A and B). No significant variations were reported as regards serum triglycerides after 6 mo of treatment (Figure 1C). Interestingly, we observed an impact of time, treatment and interaction on body mass index (time factor: F(1, 100) = 4.146, P = 0.0444; treatment factor: F(1, 100) = 4.169, P = 0.0438; interaction factor: F(1, 100) = 5.650, P = 0.0194), and the post hoc analysis resulted in significant differences between the two treatment groups at T1, and between T0 and T1 in the SGLT2-I group (Figure 1D). These data suggest that 6 mo of add-on treatment to metformin improves glycemic control compared to baseline; nevertheless, this improvement is not related to a particular drug class. Furthermore, a beneficial impact on weight loss is exerted by SGLT2-I but not by other glucose-lowering agents.
Table 1.
Baseline characteristics of patients observed in the study (n = 52) and included in groups treated with the sodium-glucose co-transporter-2 inhibitors or other glucose lowering drugs
|
Variable
|
SGLT2-I (n = 26)
|
OTHER (n = 26)
|
P
value
|
| Age (yr) | 60.6 ± 6.78 | 63.4 ± 10.4 | 0.246 |
| Sex (male/female) | 15/11 | 15/11 | 1.000 |
| BMI (kg/m2) | 34.8 ± 7.7 | 34.5 ± 5.9 | 0.875 |
| No comorbidities, n (%) | 2 (7.7) | 2 (7.7) | 1.000 |
| Dyslipidemia, n (%) | 13 (50.0) | 15 (57.7) | 0.578 |
| Hypertension, n (%) | 10 (38.5) | 15 (57.7) | 0.165 |
| Chronic heart failure, n (%) | 8 (30.8) | 3 (11.5) | 0.089 |
| Chronic kidney disease, n (%) | 9 (34.6) | 4 (15.4) | 0.109 |
| AST (U/L) | 48.5 ± 26.6 | 54.7 ± 13.3 | 0.293 |
| ALT (U/L) | 49.6 ± 39.2 | 65.0 ± 18.7 | 0.077 |
| Gamma-GT (U/L) | 151.3 ± 87.2 | 179.2 ± 56.9 | 0.178 |
| Tryglycerides (mg/dL) | 140.2 ± 45.9 | 133.5 ± 33.2 | 0.549 |
| Fasting glucose (mg/dL) | 150.1 ± 45.9 | 159.7 ± 49.8 | 0.473 |
| HbA1c (%) | 9.24 ± 3.01 | 8.73 ± 2.31 | 0.496 |
| Creatininemia (mg/dL) | 1.01 ± 0.44 | 0.88 ± 0.32 | 0.229 |
| eGFR (mL/min/1.73 m2) | 61.4 ± 38.9 | 73.2 ± 24.6 | 0.197 |
| Albuminemia (g/dL) | 3.91 ± 0.44 | 4.08 ± 0.47 | 0.184 |
| Platelets (n, × 103/mm3) | 175.7 ± 113.1 | 179.7 ± 76.7 | 0.882 |
BMI: Body mass index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; Gamma-GT: Gamma-glutamyl transpeptidase; HbA1c: Hemoglobin A1c; eGFR: Epidermal growth factor receptor; SGLT2-I: Sodium-glucose co-transporter-2 inhibitor; OTHER: Other glucose lowering drug.
Figure 1.
Glycemic control and body weight in patients enrolled in the study and included in groups treated with the sodium-glucose co-transporter-2 inhibitors or other glucose-lowering drugs before (T0) and after 1 wk of treatment (T1). A: Serum fasting glucose; B: Serum hemoglobin A1c; C: Serum triglycerides; D: Body mass index in patients observed in the study, grouped according to the assigned treatment. Data in the graphs are represented as mean ± SEM. Two-way analysis of variance and Tukey assessed statistical differences as post hoc test. aP < 0.05, bP < 0.001. HbA1c: Hemoglobin A1c; SGLT2-I: Sodium-glucose co-transporter-2 inhibitor; OTHER: Other glucose lowering drug; BMI: Body mass index.
Effect of different add-on therapies on liver function tests and non-invasive markers of hepatic steatosis/fibrosis
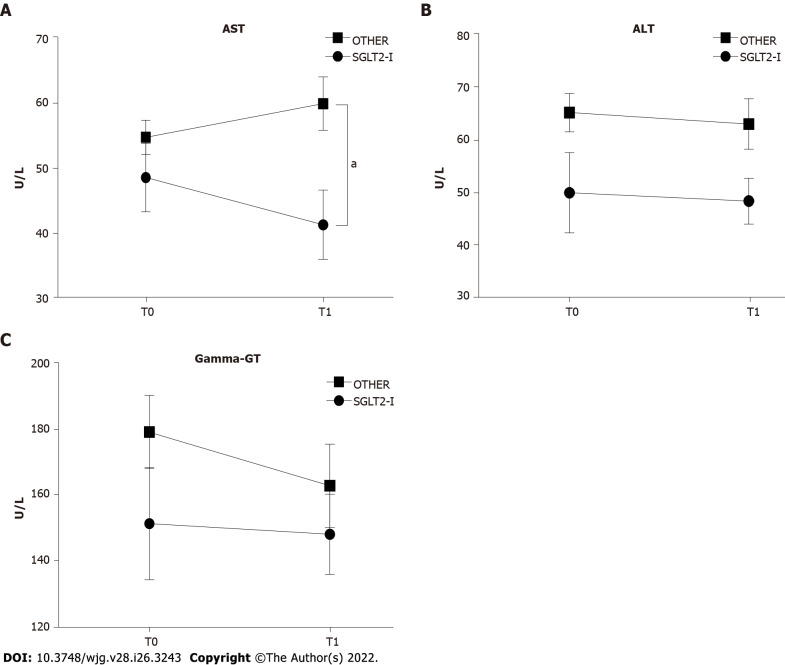
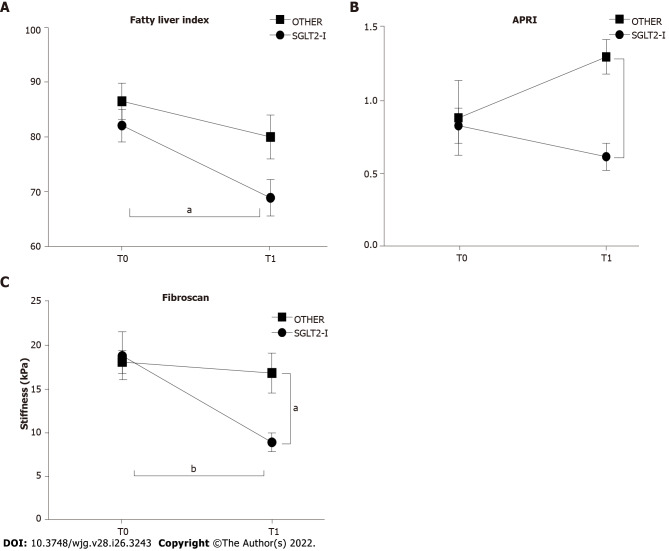
We then compared the impact of combined treatment with SGLT2-I vs OTHER on circulating liver enzymes. The effect of treatment was significant for serum AST level (F(1, 100) = 7.703, P = 0.0066), and the post hoc analysis showed lower values in SGLT2-I rather than the OTHER group at T1 (Figure 2A). No significant variations were reported for serum ALT and gamma-GT activities (Figures 2B and C). Non-invasive liver steatosis and fibrosis markers were further evaluated at baseline and after 6 mo of therapy in both groups. We observed the effect of both time and treatment on FLI (time factor: F(1, 100) = 8.279, P = 0.0049 and treatment factor: F(1, 100) = 5.113, P = 0.0259), but after 6 mo it decreased only in the SGLT2-I group - and not in OTHER patients - with respect to baseline (Figure 3A). A significant effect of treatment was also observed for the APRI (F(1, 100) = 5.309, P = 0.0233), with a lower value in SGLT2-I rather than OTHER group at T1 (Figure 3B). The proportion of patients affected by a fibrotic form of liver disease according to NAFLD fibrosis score, FIB-4, and AST/ALT ratio reduced significantly after 6 mo of therapy in the SGLT2-I group rather than in the OTHER group (Table 2). Finally, we observed a significant effect of time (F(1, 100) = 7.996, P = 0.0057) and interaction (F(1, 100) = 4.772, P = 0.0313) on hepatic elastometry, and LS in patients treated with SGLT2-I for six months was lower with respect to baseline and to the OTHER group (Figure 3C).
Figure 2.
Serum liver enzyme activities in patients enrolled in the study and included in groups treated with the sodium-glucose co-transporter-2 inhibitors or other glucose-lowering drugs before (T0) and after 1 wk of treatment (T1). A: Serum aspartate aminotransferase; B: Serum alanine aminotransferase; C: Serum gamma-glutamyl transpeptidase activities in patients observed in the study, grouped according to the assigned treatment. Data in the graphs are represented as mean ± SEM. Two-way analysis of variance and Tukey assessed statistical differences as posthoc test. aP < 0.05. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; Gamma-GT: Gamma-glutamyl transpeptidase; SGLT2-I: Sodium-glucose co-transporter-2 inhibitor; OTHER: Other glucose lowering drug.
Figure 3.
Non-invasive markers of liver steatosis and fibrosis in patients enrolled in the study and included in groups treated with the sodium-glucose co-transporter-2 inhibitors or other glucose glucose-lowering before (T0) and after 1 wk of treatment (T1). A: Fatty liver index; B: Aspartate aminotransferase-to-platelet ratio index; C: Hepatic elastometry in patients observed in the study grouped according to the assigned treatment. Data in the graphs are represented as mean ± SEM. Two-way analysis of variance and Tukey assessed statistical differences as posthoc test. aP < 0.05, bP < 0.01. SGLT2-I: Sodium-glucose co-transporter-2 inhibitor; OTHER: Other glucose lowering drug; APRI: Aspartate aminotransferase-to-platelet ratio index.
Table 2.
Non-invasive markers of hepatic fibrosis in patients observed in the study (n = 52) and included in groups treated with the sodium-glucose co-transporter-2 inhibitors or other glucose lowering drugs at baseline (T0) and after 6 mo of therapy (T1)
|
|
|
T0
|
T1
|
P
value
|
| NAFLD fibrosis score (F3/4), n (%) | SGLT2-I (n = 26) | 9 (34.6) | 4 (15.4) | 0.042 |
| OTHER (n = 26) | 7 (26.9) | 7 (26.9) | ||
| FIB-4 (F3/4), n (%) | SGLT2-I (n = 26) | 11 (42.3) | 6 (23.1) | 0.036 |
| OTHER (n = 26) | 7 (26.9) | 7 (26.9) | ||
| AST/ALT ≥ 0.8, n (%) | SGLT2-I (n = 26) | 15 (57.7) | 6 (23.1) | 0.001 |
| OTHER (n = 26) | 10 (38.5) | 11 (42.3) |
NAFLD: Non-alcoholic fatty liver disease; FIB-4: Fibrosis-4; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; SGLT2-I: Sodium-glucose co-transporter-2 inhibitor; OTHER: Other glucose lowering drug.
Effect of different add-on therapies on circulating markers of inflammation and oxidative stress
We further analyzed markers of systemic inflammation, such as circulating interleukins and growth factors. Table 3 summarizes the results related to serum levels of 12 different cytokines and growth factors evaluated at baseline (T0) and after 6 mo of therapy (T1) in both treatment groups. We could not observe any significant impact of time, treatment, or interaction on IL-1α, IL-2, IL-8, IFN-γ, and EGF. On the contrary, the time effect was observed for IL-1β, IL-4, IL-10, and TNF; the treatment effect was reported for IL-4, IL-6, IL-10, and TNF; the interaction effect was described for IL-1β, IL-6, IL-10, VEGF, and MCP-1. According to the post-hoc analysis, SGLT2-I patients at T1 showed: (1) Lower values of the pro-inflammatory cytokines IL-1β and TNF, and VEGF than T0; (2) Lower values of the pro-inflammatory cytokines IL-6 and TNF, VEGF, and MCP-1 than OTHER patients at T1; and (3) Higher values of the anti-inflammatory cytokines IL-4 and IL-10 as compared to SGLT2-I at T0 and OTHER at T1.
Table 3.
Circulating interleukin levels in patients enrolled in the study and included in groups treated with the sodium-glucose co-transporter-2 inhibitors or other glucose lowering drugs before (T0) and after 1 wk of treatment (T1)
|
Variable
|
SGLT2-I (n = 26)
|
OTHER (n = 26)
|
Fisher’s test (1, 100)
|
||||
|
T0
|
T1
|
T0
|
T1
|
Time
|
Treatment
|
Interaction
|
|
| IL-1α (pg/mL) | 1.35 ± 1.64 | 1.58 ± 1.30 | 1.44 ± 1.88 | 1.59 ± 1.56 | 0.373 | 0.026 | 0.016 |
| IL-1β (pg/mL) | 9.90 ± 2.39 | 7.31 ± 3.51e | 8.84 ± 2.54 | 9.15 ± 2.42 | 4.455a | 0.521 | 7.207b |
| IL-2 (U/mL) | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 | - | - | - |
| IL-4 (pg/mL) | 5.39 ± 4.20 | 8.54 ± 3.51d | 4.89 ± 3.54 | 5.94 ± 3.66 | 8.207b | 4.471a | 2.052 |
| IL-6 (pg/mL) | 9.41 ± 4.21 | 6.92 ± 1.98 | 11.4 ± 5.41 | 13.7 ± 9.01h | 0.007 | 15.4c | 4.516a |
| IL-8 (pg/mL) | 428 ± 216 | 409 ± 271 | 412 ± 296 | 449 ± 204 | 0.338 | 0.060 | 0.327 |
| IL-10 (pg/mL) | 1.33 ± 1.02 | 2.31 ± 1.12e | 1.29 ± 1.06 | 1.32 ± 1.04g | 5.894a | 6.130a | 5.214a |
| TNF (pg/mL) | 6.82 ± 3.02 | 4.61 ± 2.01d | 7.94 ± 3.14 | 7.81 ± 3.56g | 3.989a | 13.59c | 3.151 |
| VEGF (pg/mL) | 262 ± 162 | 142 ± 109d | 244 ± 201 | 268 ± 147f | 2.393 | 3.029 | 5.384a |
| IFN-γ (pg/mL) | 0.53 ± 0.23 | 0.57 ± 0.57 | 0.50 ± 0.39 | 0.61 ± 0.44 | 0.809 | 0.004 | 0.176 |
| MCP-1 (pg/mL) | 411 ± 168 | 291 ± 156 | 389 ± 154 | 414 ± 184f | 2.131 | 2.408 | 4.964a |
| EGF (pg/mL) | 25.7 ± 13.7 | 22.2 ± 12.1 | 24.9 ± 20.1 | 23.6 ± 18.6 | 0.553 | 0.009 | 0.116 |
P < 0.05.
P < 0.01.
P < 0.001.
P < 0.05 vs sodium-glucose co-transporter-2 inhibitor T0.
P < 0.01 vs sodium-glucose co-transporter-2 inhibitor T0.
P < 0.05 vs sodium-glucose co-transporter-2 inhibitor T1.
P < 0.01 vs sodium-glucose co-transporter-2 inhibitor T1.
P < 0.001 vs sodium-glucose co-transporter-2 inhibitor T1.
Data are expressed as mean ± SD. Statistical differences were assessed by two-way analysis of variance. VEGF: Vascular endothelial growth factor; MCP-1: Monocyte chemoattractant protein-1; EGF: Epidermal growth factor; SGLT2-I: Sodium-glucose co-transporter-2 inhibitor; OTHER: Other glucose lowering drug; IL: Interleukin; TNF: Tumor necrosis factor; IFN: Interferon.
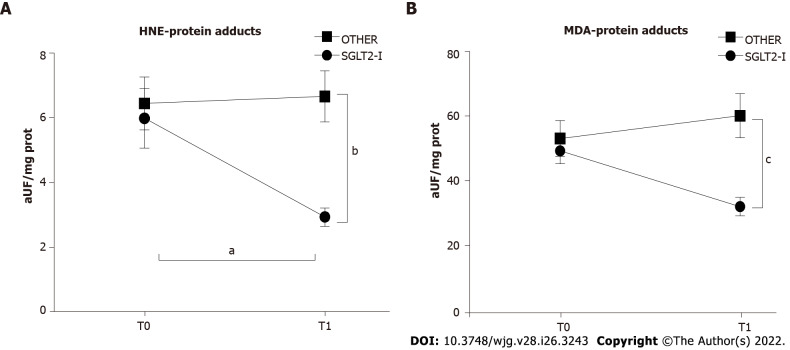
We evaluated systemic oxidative stress markers changes by measuring serum HNE- and MDA-protein adducts. A significant effect of treatment and interaction was observed for both HNE-protein adducts (treatment factor: F(1, 100) = 7.924, P = 0.0059; interaction factor: F(1, 100) = 4.820, P = 0.0305) and MDA-protein adducts (treatment factor: F(1, 100) = 10.17, P = 0.0019; interaction factor: F(1, 100) = 5.844, P = 0.0174). The post hoc analysis showed that, after 6 mo of therapy, circulating markers of oxidative stress were lower in the SGLT2-I group rather than OTHER patients; furthermore, HNE-protein adducts were significantly reduced from T0 to T1 in SGLT2-I patients (Figures 4A and B).
Figure 4.
Circulating markers of oxidative stress in patients enrolled in the study and included in groups treated with the sodium-glucose co-transporter-2 inhibitors or other glucose-lowering drugs before (T0) and after 1 wk of treatment (T1). Data in the graphs are represented as mean ± SEM. Two-way analysis of variance and Tukey assessed statistical differences as a post hoc test. aP < 0.05, bP < 0.01, cP < 0.001. HNE: Hydroxynonenal; MDA: Malondialdehyde; SGLT2-I: Sodium-glucose co-transporter-2 inhibitor; OTHER: Other glucose lowering drug.
Reduction of liver steatosis and fibrosis markers is associated with decreased circulating oxidative stress in patients treated with SGLT2-I
We then focused on patients treated with SGLT2-I. We performed a Pearson’s correlation analysis on non-invasive markers of hepatic steatosis and fibrosis, circulating parameters of glucose metabolism, inflammation, and oxidative stress after 6 mo of therapy. Of note, the FLI, the APRI, and LS showed: (1) A positive bivariate relationship with pro-inflammatory cytokines (IL-1β, IL6, TNF); and (2) A negative correlation with the anti-inflammatory cytokines (IL-4 and IL-10). Interestingly, all the non-invasive liver steatosis and fibrosis markers were strongly related to HNE- and MDA-protein adducts (Supplementary Table 2).
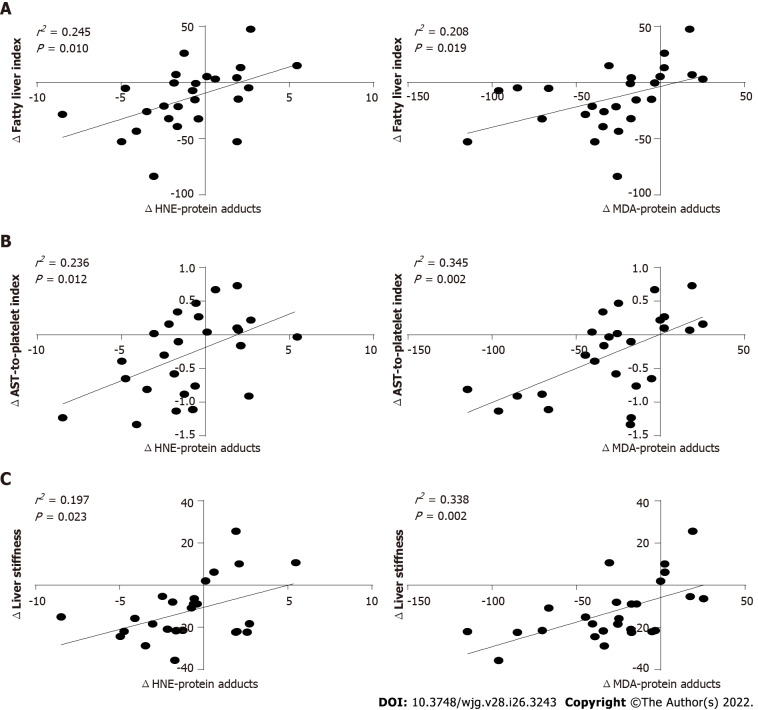
To verify whether the improvement of non-invasive markers of liver steatosis and fibrosis reported in patients treated with SGLT2-I after 6 mo of therapy was associated with the observed reduction of serum HNE- and MDA-protein adducts, a linear regression analysis on T1-T0 difference values was performed. Further, SGLT2-I treatment related alterations in both markers of circulating oxidative stress were directly related to variations in FLI, APRI, and LS (Figures 5A-C).
Figure 5.
Linear regression analysis between the variation of circulating oxidative stress parameters and non-invasive markers of hepatic steatosis or fibrosis in patients treated with the sodium-glucose co-transporter-2 inhibitors. Data in the graphs represent scatterplots of hydroxynonenal- or malondialdehyde-protein adducts A: Fatty liver index; B: Aspartate aminotransferase-to-platelet ratio index; C: Liver stiffness. HNE: Hydroxynonenal; AST: Aspartate aminotransferase; MDA: Malondialdehyd.
DISCUSSION
The present study demonstrates that the addition of an SGLT2-I - as compared to dipeptidyl peptidase-4 inhibitors (DPP4-I) or pioglitazone - to metformin monotherapy exerts a positive impact on systemic inflammation and circulating oxidative stress in patients with T2DM and NAFLD. It is associated with favorable changes in the non-invasive hepatic steatosis and fibrosis markers. The incidence of T2DM is exponentially increasing worldwide[31]. T2DM often presents associated with NAFLD since insulin resistance accounts for the alteration of lipid homeostasis, favoring hepatic fat accumulation by induction of lipogenesis and inhibition of very-low-density lipoprotein secretion[32,33]. Furthermore, hyperglycemia in diabetic patients worsens insulin resistance through mechanisms induced by glucose toxicity[34]. In addition, the efficacy of several antidiabetic drugs is lost during the time, leading to the progression of T2DM, which could worsen NAFLD[35]. There are currently no approved therapies for the treatment for NAFLD. Several compounds tested in phase 2 and phase 3 clinical trials target metabolic stress as a critical factor for the initiation and progression of hepatic injury. Data on the effects of antidiabetic drugs in NAFLD are limited although pioglitazone and GLP1-RA have demonstrated some protective effects[36]. SGLT2-I are molecules with direct action on the kidney, reduce the reabsorption of filtered glucose and significantly decrease blood glucose levels both in fasting and post-prandial conditions, with consequent decline of glucose toxicity and improvement of insulin resistance[37]. Moreover, these drugs may interfere with several mechanisms involved in the progression of T2DM, such as dysfunction or apoptosis of pancreatic β-cells[38].
Clinical studies testing the efficacy of SGLT2-I on NAFLD demonstrated that this class of drugs reduces both hepatic steatosis, as evaluated by several imaging techniques, and serum liver enzymes[39]. Our study confirms these observations since we report that the addition of SGLT2-I to metformin reduces both the FLI and serum AST after 6 mo; this effect is not observed when pioglitazone or DPP4-I are added to metformin. Furthermore, our data show that six months of therapy with an SGLT2-I are associated with reducing hepatic fibrosis - which occurs mainly in NAFLD patients with higher fibrosis grade - as suggested by decrease in NAFLD fibrosis score, FIB-4, as well as LS measured by TE. These results are comparable to the reported outcome in a similar study, suggesting that SGLT2-I are superior to other oral hypoglycemic agents in reducing hepatic steatosis and fibrosis[40].
Among other glucose-lowering agents, pioglitazone and GLP1-RA were demonstrated to reduce hepatic steatosis, inflammation, and fibrosis[41,42]. We designed this study by excluding patients treated with GLP1-RA, even though 38.5% of patients of the OTHER group were treated with pioglitazone added to metformin. Despite this subgroup of patients in the comparison group, SGLT2-I improved non-invasive liver steatosis and fibrosis markers. A previous randomized trial compared the SGLT2-I ipragliflozin against pioglitazone, showing that both treatments were equivalent in reducing liver fat infiltration and serum aminotransferase levels, even though ipragliflozin effected a reduction in body weight and abdominal fat area[43]. However, it is worth noting that more than one-third of patients included in our study showed a high grade of steatosis and fibrosis as assessed by non-invasive markers. In contrast, previous studies using pioglitazone enrolled patients with milder hepatic injury. More than potentially explaining differences between the present results and those of different studies, our results suggest that SGLT2-I treatment would be more beneficial in T2DM patients with advanced NAFLD.
Mechanisms explaining the benefits of SGLT2-I therapy in T2DM and NAFLD are primarily undefined, but the results of this study lead to several speculations. Even though SGLT2-I significantly controls blood glucose, an improvement in glucose metabolism was described in all patients treated with additional drugs. However, other studies could not demonstrate that amelioration of NAFLD after SGLT2-I treatment was dependent on improved circulating glucose concentration[44-46]. Weight loss induced by non-pharmacological interventions such as diet, exercise, or bariatric surgery, may ameliorate liver damage in NAFLD[47]. SGLT2-I decreases body weight and fat mass, reducing hepatic steatosis and serum liver enzymes[48]. Our data show that SGLT2-I effectively promoted weight loss after 6 mo of therapy in patients with T2DM and NAFLD. However, there was no relationship between BMI reduction and the improvement of non-invasive markers of hepatic steatosis and fibrosis.
Besides, previous studies have also shown improved liver function tests, and steatosis irrespective of weight loss in patients affected by T2DM and NAFLD treated with SGLT2-I[49,50]. The evidence so far indicated that SGLT2-I would induce different beneficial mechanisms than glucose control and weight loss in NAFLD. The present results further revealed that SGLT2-I - and not pioglitazone or DPP4-I - favorably modulate circulating cytokines, switching from pro-inflammatory to anti-inflammatory patterns, and reducing systemic markers of oxidative stress. These results are further buttressed by pre-clinical studies providing proving2-I inhibits pro-inflammatory cytokine secretion and reduces oxidative stress[16,51,52]. Our study clearly demonstrates a significant association between improving hepatic steatosis/fibrosis markers and reducing circulating oxidative stress in patients treated with SGLT2-I for 6 mo. On the other hand, we could not find any relationship between changes in circulating cytokines and reduction of liver injury markers. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) represent physiological products of cellular metabolism, which are normally counteracted by endogenous antioxidants. When ROS/RNS production overwhelms the antioxidant defense, oxidative stress occurs with consequent injury of macromolecules such as nucleic acids (DNA oxidation), lipids (lipoperoxidation), and proteins, which in turn leads to an impairment of normal cellular functions[53]. Oxidative stress promotes the generation of HNE and MDA, lipid peroxidation products which are able to generate adducts with cellular and circulating proteins, which may be used as systemic markers of injury. Oxidative stress is considered one of the main determinants of NAFLD pathogenesis and progression[54]. For the first time, our study provides evidence that the reduction of circulating oxidative stress induced by SGLT2-I is related to improved markers of hepatic damage in T2DM patients with NAFLD, suggesting a potential protective mechanism by this drug class. Studies to define how SGLT2-I modulate molecular pathways that impact redox balance need to be designed.
This study suffers from the following limitations: (1) It was not designed as a randomized placebo-controlled trial since the type of combined treatment was decided according to a patient-centered approach (of note, the newest Standards of Medical Care in Diabetes were not available at the time of enrolment); (2) Patients treated with GLP-1 agonists were not included; (3) Since this study was performed at a single-center, it may have presented some bias, resulting in slightly larger intervention effects than multicenter studies; (4) Our small pilot study was not designed to determine the impact of single compounds; and (5) Liver histology was not performed to evaluate steatosis, inflammation, and fibrosis.
CONCLUSION
In conclusion, treatment with SGLT2-I in T2DM patients affected by NAFLD is associated with a rapid improvement of non-invasive markers of hepatic steatosis and fibrosis, providing insights into the mechanisms by which such class of antidiabetic drugs may reduce liver damage in humans. More extensive randomized controlled trials are encouraged to confirm these preliminary observations, and fundamental studies are needed to define the molecular mechanisms underlying the effects of SGLT2-I in NAFLD.
ARTICLE HIGHLIGHTS
Research background
Clinical trials of sodium glucose cotransporter-2 inhibitors (SGLT2-I), recently approved drugs for type 2 diabetes (T2D), reported beneficial cardiovascular (CV) and renal outcomes.
Research motivation
Inflammation and oxidative stress are major causes of vascular dysfunction and CV disease in diabetes and pre-clinical studies demonstrated that SGLT2-I promote anti-inflammatory effects by lowering oxidative stress.
Research objectives
To investigate the effects of SGLT2-I on markers of oxidative stress, inflammation, liver steatosis, and fibrosis in patients of T2D with non-alcoholic fatty liver disease (NAFLD).
Research methods
Observational prospective study enrolling 52 consecutive outpatients treated with metformin monotherapy and exhibiting poor glycemic control, which were introduced to an SGLT2-I (n = 26) or a different hypoglycemic drug (n = 26). Circulating interleukins and serum hydroxynonenal (HNE)- or malondialdehyde (MDA)-protein adducts, fatty liver index (FLI), NAFLD fibrosis score, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio ratio, AST-to-platelet-ratio index (APRI), and fibrosis-4, as well as transient elastography (FibroScan) on the day before (T0) and following treatment for six months (T1) were evaluated.
Research results
With respect to other hypoglycemic drugs, treatment with SGLT2-I resulted in a reduction of FLI and APRI, as well as of the FibroScan result, as well as lower circulating levels of interleukins (IL)-1β, IL-6, tumor necrosis factor, vascular endothelial growth factor, and monocyte chemoattractant protein-1, higher levels of IL-4 and IL-10, decreased serum HNE- and MDA-protein adducts. Markers of circulating oxidative stress correlated with liver steatosis and fibrosis scores.
Research conclusions
This study indicates that, more than optimizing glucose control, treatment with SGLT2-I in patients with T2D and NAFLD is associated with improvement of liver steatosis and fibrosis markers and circulating pro-inflammatory and redox status.
Research perspectives
This study encourages extensive randomized controlled trials to confirm these preliminary observations, and basic investigations to define the molecular mechanisms underlying the effects of SGLT2-I in NAFLD.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board at the University of Foggia.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
STROBE statement: The Authors have read the STROBE statement - checklist of items, and the manuscript was prepared and revised according to the STROBE statement - checklist of items.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 10, 2022
First decision: March 8, 2022
Article in press: June 18, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boscá L , Spain; Zhao XK, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Francesco Bellanti, Department of Medical and Surgical Sciences, University of Foggia, Foggia 71122, Italy. francesco.bellanti@unifg.it.
Aurelio Lo Buglio, Department of Medical and Surgical Sciences, University of Foggia, Foggia 71122, Italy.
Michał Dobrakowski, Department of Biochemistry, Medical University of Silesia in Katowice, Zabrze 41-808, Poland.
Aleksandra Kasperczyk, Department of Biochemistry, Medical University of Silesia in Katowice, Zabrze 41-808, Poland.
Sławomir Kasperczyk, Department of Biochemistry, Medical University of Silesia in Katowice, Zabrze 41-808, Poland.
Palok Aich, School of Biological Sciences, National Institute of Science Education and Research, Khurdha 752050, India.
Shivaram P Singh, Department of Gastroenterology, SCB Medical College, Cuttack 753007, India.
Gaetano Serviddio, Department of Medical and Surgical Sciences, University of Foggia, Foggia 71122, Italy.
Gianluigi Vendemiale, Department of Medical and Surgical Sciences, University of Foggia, Foggia 71122, Italy.
Data sharing statement
No additional data are available.
References
- 1.Redekop WK, Koopmanschap MA, Stolk RP, Rutten GE, Wolffenbuttel BH, Niessen LW. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care. 2002;25:458–463. doi: 10.2337/diacare.25.3.458. [DOI] [PubMed] [Google Scholar]
- 2.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Chalasani NP, Anstee QM, Kowdley KV, George J, Goodman ZD, Lindor K. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68:361–371. doi: 10.1002/hep.29724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 5.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol. 2016;11:451–496. doi: 10.1146/annurev-pathol-012615-044224. [DOI] [PubMed] [Google Scholar]
- 6.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 7.Caussy C, Aubin A, Loomba R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr Diab Rep. 2021;21:15. doi: 10.1007/s11892-021-01383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajja AP, Dey AK, Guha A, Elnabawi Y, Joshi AA, Kalra A. SGLT-2 Inhibitors and GLP-1 Agonists: First-Line Therapy for Diabetes With Established Cardiovascular Disease. J Cardiovasc Pharmacol Ther. 2019;24:422–427. doi: 10.1177/1074248419838511. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty JA, Guirguis E, Thornby KA. A Systematic Review of Newer Antidiabetic Agents in the Treatment of Nonalcoholic Fatty Liver Disease. Ann Pharmacother. 2021;55:65–79. doi: 10.1177/1060028020935105. [DOI] [PubMed] [Google Scholar]
- 10.Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, Neal B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4:411–419. doi: 10.1016/S2213-8587(16)00052-8. [DOI] [PubMed] [Google Scholar]
- 11.Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Birkeland KI, Jørgensen ME, Thuresson M, Arya N, Bodegård J, Hammar N, Fenici P CVD-REAL Investigators and Study Group*. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors) Circulation. 2017;136:249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, Tangri N, Goh SY, Thuresson M, Chen H, Surmont F, Hammar N, Fenici P CVD-REAL Investigators and Study Group. Cardiovascular Events Associated With SGLT-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL 2 Study. J Am Coll Cardiol. 2018;71:2628–2639. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Leng W, Ouyang X, Lei X, Wu M, Chen L, Wu Q, Deng W, Liang Z. The SGLT-2 Inhibitor Dapagliflozin Has a Therapeutic Effect on Atherosclerosis in Diabetic ApoE-/- Mice. Mediators Inflamm. 2016;2016:6305735. doi: 10.1155/2016/6305735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB, Lim S. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE -/- mice fed a western diet. Diabetologia. 2017;60:364–376. doi: 10.1007/s00125-016-4158-2. [DOI] [PubMed] [Google Scholar]
- 15.Nasiri-Ansari Ν, Dimitriadis GK, Agrogiannis G, Perrea D, Kostakis ID, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E. Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice. Cardiovasc Diabetol. 2018;17:106. doi: 10.1186/s12933-018-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steven S, Oelze M, Hanf A, Kröller-Schön S, Kashani F, Roohani S, Welschof P, Kopp M, Gödtel-Armbrust U, Xia N, Li H, Schulz E, Lackner KJ, Wojnowski L, Bottari SP, Wenzel P, Mayoux E, Münzel T, Daiber A. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahnwong S, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc Diabetol. 2018;17:101. doi: 10.1186/s12933-018-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das NA, Carpenter AJ, Belenchia A, Aroor AR, Noda M, Siebenlist U, Chandrasekar B, DeMarco VG. Empagliflozin reduces high glucose-induced oxidative stress and miR-21-dependent TRAF3IP2 induction and RECK suppression, and inhibits human renal proximal tubular epithelial cell migration and epithelial-to-mesenchymal transition. Cell Signal. 2020;68:109506. doi: 10.1016/j.cellsig.2019.109506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quagliariello V, De Laurentiis M, Rea D, Barbieri A, Monti MG, Carbone A, Paccone A, Altucci L, Conte M, Canale ML, Botti G, Maurea N. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20:150. doi: 10.1186/s12933-021-01346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng S, Delic D, Chu C, Xiong Y, Luo T, Chen X, Gaballa MMS, Xue Y, Cao Y, Hasan AA, Stadermann K, Frankenreiter S, Yin L, Krämer BK, Klein T, Hocher B. Antifibrotic effects of low dose SGLT2 Inhibition with empagliflozin in comparison to Ang II receptor blockade with telmisartan in 5/6 nephrectomised rats on high salt diet. Biomed Pharmacother. 2022;146:112606. doi: 10.1016/j.biopha.2021.112606. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care. 2017;40:S4–S5. doi: 10.2337/dc17-S003. [DOI] [PubMed] [Google Scholar]
- 23.Molloy RM, Mc Connell RI, Lamont JV, FitzGerald SP. Automation of biochip array technology for quality results. Clin Chem Lab Med. 2005;43:1303–1313. doi: 10.1515/CCLM.2005.224. [DOI] [PubMed] [Google Scholar]
- 24.Bellanti F, Romano AD, Lo Buglio A, Castriotta V, Guglielmi G, Greco A, Serviddio G, Vendemiale G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas. 2018;109:6–12. doi: 10.1016/j.maturitas.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11:1201–1204. doi: 10.1016/j.cgh.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Tapper EB. The aspartate aminotransferase-to-platelet ratio and the evaluation of non-alcoholic fatty liver disease. Gastroenterol Rep (Oxf) 2014;2:326–327. doi: 10.1093/gastro/gou080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 28.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 30.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 32.Poulsen MK, Nellemann B, Stødkilde-Jørgensen H, Pedersen SB, Grønbæk H, Nielsen S. Impaired Insulin Suppression of VLDL-Triglyceride Kinetics in Nonalcoholic Fatty Liver Disease. J Clin Endocrinol Metab. 2016;101:1637–1646. doi: 10.1210/jc.2015-3476. [DOI] [PubMed] [Google Scholar]
- 33.Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, Okunade AL, Patterson BW, Nyangau E, Field T, Sirlin CB, Talukdar S, Hellerstein MK, Klein S. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130:1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo W, Ai L, Wang BF, Zhou Y. High glucose inhibits myogenesis and induces insulin resistance by down-regulating AKT signaling. Biomed Pharmacother. 2019;120:109498. doi: 10.1016/j.biopha.2019.109498. [DOI] [PubMed] [Google Scholar]
- 35.Evans JL, Goldfine ID. Aging and insulin resistance: just say iNOS. Diabetes. 2013;62:346–348. doi: 10.2337/db12-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder HS, Sakaan SA, March KL, Siddique O, Cholankeril R, Cummings CD, Gadiparthi C, Satapathy SK, Ahmed A, Cholankeril G. Non-alcoholic Fatty Liver Disease: A Review of Anti-diabetic Pharmacologic Therapies. J Clin Transl Hepatol. 2018;6:168–174. doi: 10.14218/JCTH.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao EC. SGLT-2 Inhibitors: A New Mechanism for Glycemic Control. Clin Diabetes. 2014;32:4–11. doi: 10.2337/diaclin.32.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, Eguchi J, Horiguchi CS, Nishii N, Yamada H, Takei K, Makino H. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. 2014;9:e100777. doi: 10.1371/journal.pone.0100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheen AJ. Effect of sodium-glucose cotransporter type 2 inhibitors on liver fat in patients with type 2 diabetes: hepatic beyond cardiovascular and renal protection? Ann Transl Med. 2018;6:S68. doi: 10.21037/atm.2018.10.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arai T, Atsukawa M, Tsubota A, Mikami S, Ono H, Kawano T, Yoshida Y, Tanabe T, Okubo T, Hayama K, Nakagawa-Iwashita A, Itokawa N, Kondo C, Kaneko K, Emoto N, Nagao M, Inagaki K, Fukuda I, Sugihara H, Iwakiri K. Effect of sodium-glucose cotransporter 2 inhibitor in patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus: a propensity score-matched analysis of real-world data. Ther Adv Endocrinol Metab. 2021;12:20420188211000243. doi: 10.1177/20420188211000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-analysis. JAMA Intern Med. 2017;177:633–640. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv X, Dong Y, Hu L, Lu F, Zhou C, Qin S. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) for the management of nonalcoholic fatty liver disease (NAFLD): A systematic review. Endocrinol Diabetes Metab. 2020;3:e00163. doi: 10.1002/edm2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, Akiyama Y, Morimoto Y, Noda M, Shimada A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care. 2017;40:1364–1372. doi: 10.2337/dc17-0518. [DOI] [PubMed] [Google Scholar]
- 44.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin vs glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 wk results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 45.Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 46.Lee PCH, Gu Y, Yeung MY, Fong CHY, Woo YC, Chow WS, Tan K, Lam KSL. Dapagliflozin and Empagliflozin Ameliorate Hepatic Dysfunction Among Chinese Subjects with Diabetes in Part Through Glycemic Improvement: A Single-Center, Retrospective, Observational Study. Diabetes Ther. 2018;9:285–295. doi: 10.1007/s13300-017-0355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hannah WN Jr, Harrison SA. Effect of Weight Loss, Diet, Exercise, and Bariatric Surgery on Nonalcoholic Fatty Liver Disease. Clin Liver Dis. 2016;20:339–350. doi: 10.1016/j.cld.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Koutoukidis DA, Koshiaris C, Henry JA, Noreik M, Morris E, Manoharan I, Tudor K, Bodenham E, Dunnigan A, Jebb SA, Aveyard P. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism. 2021;115:154455. doi: 10.1016/j.metabol.2020.154455. [DOI] [PubMed] [Google Scholar]
- 49.Komiya C, Tsuchiya K, Shiba K, Miyachi Y, Furuke S, Shimazu N, Yamaguchi S, Kanno K, Ogawa Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS One. 2016;11:e0151511. doi: 10.1371/journal.pone.0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia. 2018;61:2155–2163. doi: 10.1007/s00125-018-4702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mancini SJ, Boyd D, Katwan OJ, Strembitska A, Almabrouk TA, Kennedy S, Palmer TM, Salt IP. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep. 2018;8:5276. doi: 10.1038/s41598-018-23420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee DM, Battson ML, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17:62. doi: 10.1186/s12933-018-0708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.