Abstract
The study of transporters is highly challenging, as they cannot be isolated or studied in suspension, requiring a cellular or vesicular system, and, when mediated by more than one carrier, difficult to interpret. Nucleoside analogues are important drug candidates, and all protozoan pathogens express multiple equilibrative nucleoside transporter (ENT) genes. We have therefore developed a system for the routine expression of nucleoside transporters, using CRISPR/cas9 to delete both copies of all three nucleoside transporters from Leishmania mexicana (ΔNT1.1/1.2/2 (SUPKO)). SUPKO grew at the same rate as the parental strain and displayed no apparent deficiencies, owing to the cells’ ability to synthesize pyrimidines, and the expression of the LmexNT3 purine nucleobase transporter. Nucleoside transport was barely measurable in SUPKO, but reintroduction of L. mexicana NT1.1, NT1.2, and NT2 restored uptake. Thus, SUPKO provides an ideal null background for the expression and characterization of single ENT transporter genes in isolation. Similarly, an LmexNT3-KO strain provides a null background for transport of purine nucleobases and was used for the functional characterization of T. cruzi NB2, which was determined to be adenine-specific. A 5-fluorouracil-resistant strain (Lmex5FURes) displayed null transport for uracil and 5FU, and was used to express the Aspergillus nidulans uracil transporter FurD.
Keywords: equilibrative nucleoside transporter, nucleobase transport, Trypanosoma cruzi, Trichomonas vaginalis, FurD uracil transporter, Aspergillus nidulans, expression system
1. Introduction
Nucleoside and/or nucleobase transporters are essential to the growth and proliferation of all protozoan parasites. All parasitic protozoa are deficient in de novo purine biosynthesis and several anaerobic protozoa are also deficient in pyrimidine biosynthesis [1]. Purine and pyrimidine transporters are also important conduits for chemotherapeutic agents, particularly in trypanosomes, and thus also play a role in drug resistance [2,3,4]. A well-studied example is the role of the TbAT1/P2 aminopurine transporter of T. brucei in the uptake of melaminophenyl arsenicals and diamidines [5,6,7]. In recent years, purine antimetabolites have been shown to be among the most effective classes of antiprotozoal compounds, with promising activities against Trypanosoma brucei [8,9,10,11], T. congolense [12], T. cruzi [13], Trichomonas vaginalis [14], and Leishmania spp. [15], among others, placing further emphasis on the pharmacological importance of nucleoside carriers. In T. brucei, both P1- and P2-type nucleoside transporters are implicated in the uptake of purine antimetabolites [8,11]. Similarly, in Leishmania species, cytotoxic purine antibiotics tubercidin and formycin B are documented to be transported by NT1 and NT2, respectively [16,17], and in Toxoplasma gondii, the antimetabolite adenine arabinoside (AraA) is taken up by the AT1 carrier [18].
However, in many pathogenic protozoa and other pathogens, knowledge of purine and pyrimidine nucleoside and nucleobase transporters is almost nonexistent, for a variety of reasons related to the fact that transport function can only be studied in a cellular system, in contrast to most enzymes, which can be purified and studied in appropriate cell-free systems. For instance, some species are not easily available in the required amounts and purity from host cell material, and some transporters are only expressed in some life-cycle stages. Another common complication is the expression of multiple similar transporters in the same cell type, leading to major challenges in assigning observed transport to any specific gene product and in determining true kinetic parameters for any one transporter. The cloning and heterologous expression of single genes is an obvious solution, but consensus around a suitable expression system has not materialized. Early studies of protozoan nucleoside transporter genes universally opted for expression in oocytes of the toad Xenopus laevis [16,18,19,20,21], following the practice with human nucleoside transporters [22]. While this has undoubtedly been of great impact to the field, this system has significant disadvantages. For instance, it requires a breeding colony of X. laevis from which the oocytes are harvested by surgery; the oocytes need to have the right maturity and the expression requires microinjection of cDNA, making experiments with large numbers of time points or concentrations difficult, and for each experiment this must be repeated. All this is expensive. Moreover, the oocytes themselves express multiple nucleobase and nucleobase transporters, complicating the analysis. An alternative has been expression in the yeast Saccharomyces cerevisiae [23,24], which had the clear advantages of easy culture, improved reproducibility, and lower cost. Different S. cerevisiae strains, lacking specific transporters, can be used for the heterologous expression of specific transporters. However, well-characterized null-backgrounds for the expression of nucleobase and nucleoside transporters in an easy-to-culture protozoan cell type would be much preferable. We have previously expressed protozoan transporters in T. brucei lacking either the TbAT1 or the NT8.1/NT8.2/NT8.3 nucleobase transporter locus [25,26,27,28,29].
Here, we present a full set of Leishmania mexicana cell lines collectively deficient in the transport of all the purine and pyrimidine nucleobases and nucleosides. Thus, a single cloning of an unknown transporter gene into the expression vector allows screening for uptake of any of the natural purine and pyrimidine substrates as well as pharmacological analogues in a null background. We propose this as the standard system for the expression of protozoan nucleoside/base transporters. The promastigote cells are easy to culture in any amount, and the expression is highly stable as long as the selection antibiotic is included. We demonstrate the utility of the system by successfully expressing a range of known and unknown transporters in the cells, including the Aspergillus nidulans uracil transporter FurD [30], which demonstrates that the expression system has utility beyond protozoan transporters only.
2. Results
2.1. Construction and Verification of ΔNT1.1/Δ1.2 with a Single Transfection
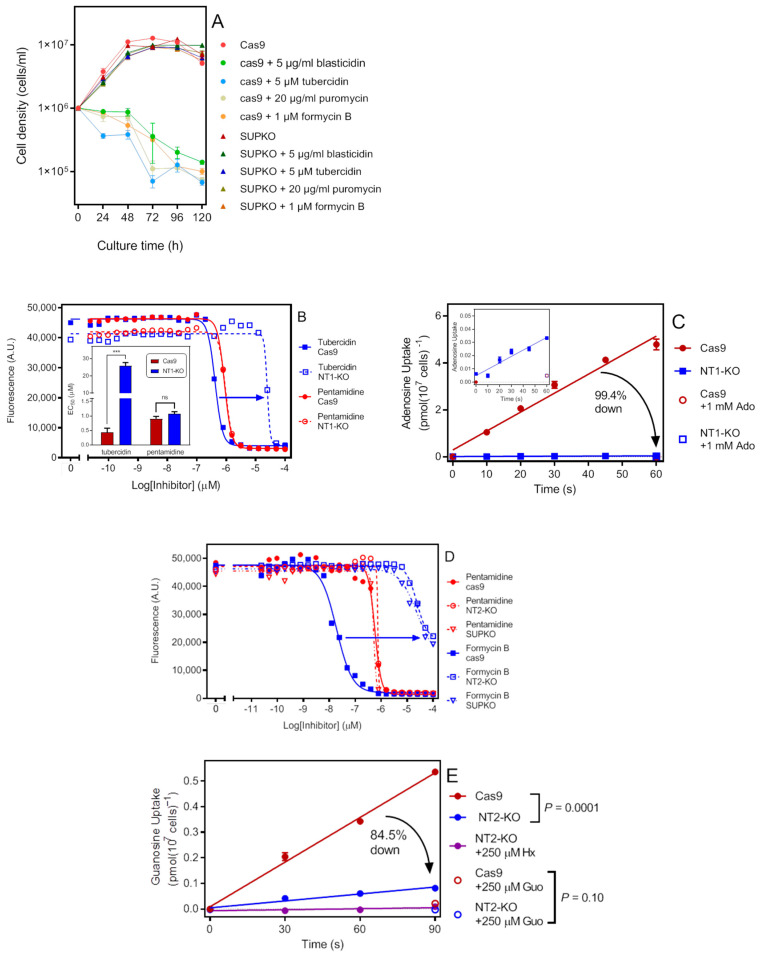
Leishmania mexicana NT1.1 (LmxM.15.1230) and NT1.2 (LmxM.15.1240) are situated in tandem on chromosome 15, so a CRISPR/cas9 strategy was devised to delete this locus and replace it with a blasticidin selection marker. In order to select for the deletion of both alleles in one transfection, 5 μM of tubercidin and 5 μg/mL blasticidin were both added to the selection medium, and sensitivity of the cas9 cells to blasticidin and tubercidin at this concentration was confirmed in a growth curve (Figure 1A). Tubercidin is an adenosine analogue and a known substrate of NT1, and has been used previously to select for NT1 mutants [17] and identify the gene [19].
Figure 1.
Functional confirmation of NT1 and NT2 knockout in L. mexicana. (A) Growth curve of cas9 control and SUPKO cells in the presence of selection agents (average of three determinations and SEM). All selection agents led to a progressive decline of cas9 cells but did not impede the growth of SUPKO. (B) Resazurin assay with the Cas9 and NT1-KO cell lines. The trace is of a single experiment in duplicate and representative of three identical experiments, the average and SEM of which are depicted in the inset bar chart. *** p < 0.001, unpaired t-test. (C) Transport of 50 nM [3H]-adenosine by Cas9 and NT1-KO cells. The rate of uptake was 99.4% lower in NT1-KO (0.00048 ± 0.00007 pmol(107 cells)−1s−1, r2 = 0.93) than in Cas9 cells (0.081 ± 0.006 pmol(107 cells)−1s−1, r2 = 0.98), but both slopes were significantly different from zero (p = 0.0021 and p = 0.0002, respectively, F-test); the two lines were significantly different p < 0.0001. Inset provides a zoom-in of the NT1-KO line. Symbols are the average of triplicate determinations and error bars are SEM; lines were calculated by linear regression. (D) Resazurin assay with the Cas9 and NT2-KO cell lines. The trace is of a single experiment in duplicate and representative of three identical experiments, the average and SEM of which are depicted in the inset bar chart. *** p < 0.001, unpaired t-test. (E) Transport of 100 nM [3H]-guanosine by cas9 and NT2-KO. The rate of uptake was 84.5% lower in the NT2-KO cells (0.00090 ± 0.00014 (r2 = 0.99) vs. 0.0058 ± 0.0003 pmol(107 cells)−1s−1 (r2 = 0.95)). The residual uptake in NT2-KO cells was significantly non-zero (p = 0.023) and completely inhibited by 250 µM hypoxanthine or 250 µM guanosine (neither significantly different from zero, p > 0.1).
L. mexicana Cas9/T7 RNAP cells [31], which constitutively express the humanized Streptococcus pyogenes Cas9 nuclease gene and T7 RNA polymerase, were transfected with the sgRNAs (Supplemental Table S1) and the PCR-amplified blasticidin resistance cassette from the pTBlast plasmid for integration into the NT1.1/NT1.2 locus (see Section 4 for details).
The resistance of the NT1-KO to tubercidin was confirmed using a resazurin assay, which showed a 60-fold shift in tubercidin sensitivity (p < 0.001 by unpaired t-test) whereas the EC50 for pentamidine, used as internal control, was unchanged (Figure 1B). Moreover, the rate of uptake of 50 nM [3H]-adenosine in the NT1-KO was 99.4% lower than in the parental cas9 cells (Figure 1C).
2.2. Construction and Verification of ΔNT2 with a Single Transfection
The NT2 locus (LmxM.36.1940) was knocked out also in one round of transfection, employing 20 µg/mL puromycin and 1 µM formycin B selection for the selection pressure; sensitivity of the cas9 cells to these concentrations was confirmed with a growth curve (Figure 1A). Details of the gene deletion are given in Section 4. The deletion was confirmed by PCR and qRT-PCR targeting the NT2 open reading frame.
NT2 is an inosine/guanosine transporter and formycin B is a known substrate that has been used before in the cloning and knockout of Leishmania donovani NT2 [16,32]. The NT2-KO strain was strongly resistant to formycin B compared to the cas9 line (EC50 63.5 ± 1.9 vs. 0.018 ± 0.002 µM, n = 3; p < 0.0001) (Figure 1D). Uptake of [3H]-guanosine was diminished by 84.5% in NT2-KO (p = 0.0001), but still significantly different from zero over 90 s (p = 0.023). This residual uptake was completely inhibited by 250 µM of either guanosine or hypoxanthine (Figure 1E). This seems to indicate that a hypoxanthine-sensitive transporter, presumably NT3, is able to transport trace amounts of guanosine in the absence of competing nucleobases—a situation similar to the situation in T. b. brucei bloodstream forms, where the high-affinity H2 hypoxanthine-guanine displays a moderate affinity for guanosine, but not for inosine [33].
2.3. Construction and Verification of Δ(NT1.1/NT1.2/NT2) (SUPKO)
The construction of SUPKO followed the exact protocols outlined above for the construction of ΔNT1 and ΔNT2, but sequentially, meaning that the ΔNT1 cell line was transfected with the guide RNAs, selection cassette, and primers for the deletion of NT2, and the ΔNT2 cells transfected with the constructs for the deletion of the NT1.1–NT1.2 locus. Selection was with blasticidin + tubercidin and puromycin + formycin B, respectively, as above. Both approaches yielded viable transfectants, and deletion of all three nucleoside transporter genes was confirmed by PCR and qRT-PCR.
2.4. Characterization of Purine and Pyrimidine Transport in SUPKO
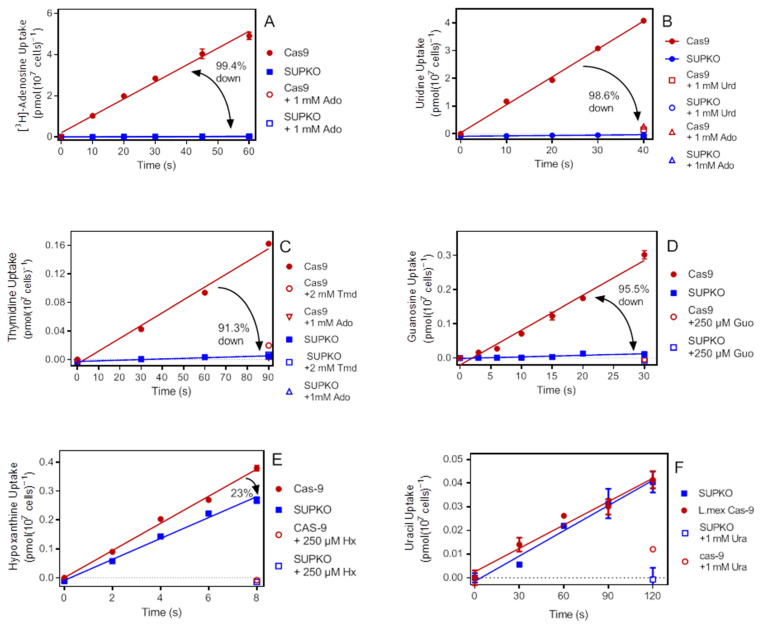
The rate of uptake of radiolabeled nucleosides was compared side by side in the SUPKO and cas9 control cell lines. Transport of 0.05 µM [3H]-adenosine in cas9 cells over 60 s was linear, with a rate of 0.082 ± 0.004 pmol(107 cells)−1s−1; this rate was 99% lower in SUPKO (Figure 2A). However, the rate in SUPKO was still significantly non-zero (p < 0.0001), the very small amount just measurable and possibly taken up by the LmexNT3 transporter.
Figure 2.
Nucleoside transport in SUPKO compared to Cas9 cells. (A) Uptake of 0.1 µM [3H]-adenosine was >99% reduced in SUPKO compared to Cas9 (0.00046 ± 0.00002 vs. 0.082 ± 0.004 pmol(107 cells)−1s−1 (both r2 > 0.99; p < 0.0001). The uptake in SUPKO was still significantly non-zero over 60 s (p < 0.0001). (B) 100 nM [3H]-uridine was taken up robustly by cas9 cells, but significantly less in SUPKO (p < 0.0001; 0.10 ± 0.003 vs. 0.0014 ± 0.00009 pmol(107 cells)−1s−1 (r2 = 0.997 and 0.989, respectively). Uptake in SUPKO was significantly non-zero (p = 0.0005). (C) Uptake of 50 nM [3H]-thymidine was lower in SUPKO than in cas9 cells (p = 0.0003; 8.7 × 10−5 ± 1.2 × 10−5 vs. 0.0018 ± 0.0001 pmol(107 cells)−1s−1 (r2 = 0.962 and 0.988, respectively). The residual thymidine uptake in SUPKO was significantly non-zero (p = 0.019). (D) Uptake of 0.1 µM [3H]-guanosine was different in Cas9 and SUPKO cells (p < 0.0001; 0.010 ± 0.0006 vs. 0.00046 ± 0.00011 pmol(107 cells)−1s−1; r2 = 0.98 and 0.78, respectively). Residual guanosine uptake in SUPKO was significantly non-zero (p = 0.0085). (E) Uptake of 100 µM [3H]-hypoxanthine was lower in SUPKO than in cas9 cells. The rates were not significantly different over 3 experiments by unpaired t-test, but in the experiment shown, the difference between the two linear regression lines was significant on F-test (GraphPad Prism 9), p = 0.0068). (F) Uptake of 100 nM [3H]-uracil was identical in SUPKO and cas9 cells (0.00032 ± 0.00003 vs. 0.00031 ± 0.00002 pmol(107 cells)−1s−1, n = 3; p = 0.81). One representative experiment in triplicate is shown.
Apart from adenosine, L. mexicana NT1 transports pyrimidine nucleosides [29]. In three independent experiments, transport of 0.1 µM [3H]-uridine was lower in SUPKO than cas9 cells, by 88.3%, 98.6%, and 97.5%, respectively (p < 0.0001), but in each case a significant amount of uridine uptake (p < 0.05) could just be discerned (Figure 2B). The very small residual uptake was likely mediated by the U1 uracil transporter, as the uridine Ki for the L. major U1 transporter is 10.9 ± 3.2 µM compared to 0.32 ± 0.07 for uracil [34]. Similarly, uptake of 0.1 µM [3H]-thymidine was much reduced in SUPKO cells (95.7 ± 1.8%, n = 5, p < 0.0001), fully inhibited by adenosine, and the residual in SUPKO barely measurable (Figure 2C).
Liu et al. [32] showed that knockout of LdNT2 resulted in a virtual null transport phenotype for inosine and xanthosine, but not adenosine. Here, we show that uptake of [3H]-guanosine was also very strongly inhibited relative to the cas9 cells (95.5%; p < 0.0001) (Figure 2D). As an additional control, we measured the uptake of 100 nM [3H]-hypoxanthine, which is known not to be a substrate of L. mexicana NT1 [29] and is undetectable over background binding in an L. major NT3 knockout strain [35]. We surmised that hypoxanthine uptake, mediated by NT3, might be upregulated after the deletion of the nucleoside transporters, but in fact hypoxanthine uptake in SUPKO tended to be somewhat lower than in Cas9 cells (Figure 2E), with an average rate of 0.045 ± 0.004 vs. 0.064 ± 0.007 pmol(107 cells)−1s−1, n = 3), although this did not reach statistical significance (p = 0.12, unpaired t-test). Although the difference in hypoxanthine uptake was not significant, we followed up with uptake of a pyrimidine base, [3H]-uracil, which is taken up by a separate transporter in L. major [34] and in T. brucei [36] that is presumed not to be encoded by an ENT-family transporter [37]. In three separate experiments, we found that the uptake of 100 nM [3H]-uracil in SUPKO or NT1-KO cells was identical to that in cas9 (p = 0.81) (Figure 2F).
We conclude that SUPKO is a low-uptake background for purine and pyrimidine nucleosides, but not for purine and pyrimidine bases and should be a sensitive system for the expression of nucleoside transporters.
2.5. Re-Expression of Leishmania mexicana NT1.1, NT1.2, and NT2 in SUPKO
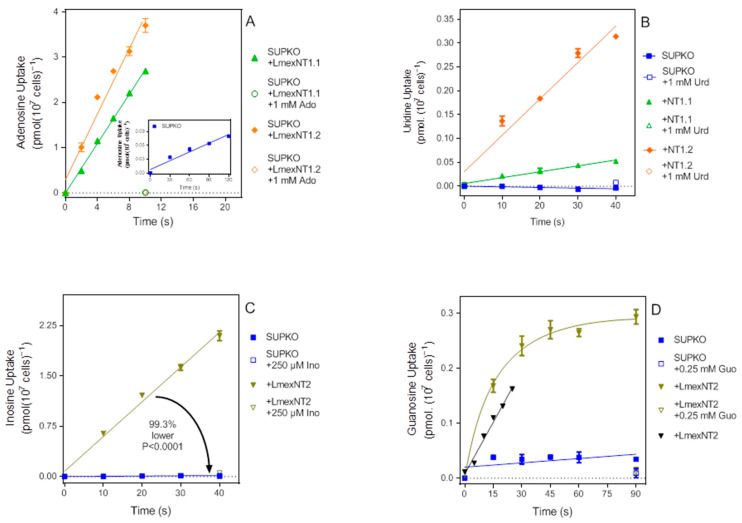
In order to test SUPKO as an expression system, we first expressed each of the L. mexicana nucleoside transporters separately as “add-back” controls that should restore the respective transport activities. Figure 3A shows linear uptake of SUPKO expressing LmexNT1.1 or LmexNT1.2, with rates of 0.27 ± 0.006 and 0.36 ± 0.03 pmol(107 cells)−1s−1, respectively (compare 0.00063 for SUPKO (Figure 3A, inset). Uridine uptake was also restored in the transfectants, but taken up 6.3-fold faster by cells expressing NT1.2 than NT1.1 (p = 0.0004) (Figure 3B). Similarly, expressing NT2 in SUPKO restored the uptake of inosine (Figure 3C) and guanosine (Figure 3D).
Figure 3.
Re-expression of L. mexicana nucleoside transporters in SUPKO. (A) 50 nM [3H]-Adenosine uptake in SUPKO cells expressing LmexNT1.1 or LmexNT1.2. Rates were 0.27 ± 0.006 and 0.36 ± 0.03 pmol(107 cells)−1s−1, respectively. The two slopes were significantly different (p = 0.031; F-test). Inset: Uptake in SUPKO was measured over 120 s instead of 10 s because of the lower rate, which was still significantly different from zero (p = 0.004) (B) Uptake of 50 nM [3H]-uridine in SUPKO and the same cells expressing either LmexNT1.1 or LmexNT1.2. The SUPKO rate was not significantly different from zero. Rates for LmexNT1.1 and LmexNT1.2 were 0.0012 ± 0.0001 and 0.0076 ± 0.0009 pmol(107 cells)−1s−1, respectively, and significantly different from each other (p = 0.0004). (C) Uptake of 100 nM [3H]-inosine by SUPKO and cells expressing LmexNT2. Rates were 0.00039 ± 0.00005 and 0.052 ± 0.002 pmol(107 cells)−1s−1, respectively, and significantly different (p < 0.0001). (D) Uptake of 100 nM [3H]-guanosine by SUPKO or cells expressing LmexNT2. In the initial experiment, over 90 s, the uptake in the cells expressing LmexNT2 was not linear and the experiment was repeated with the same number of points over just 25 s (black down-triangles). Rates were 0.00039 ± 0.00005 and 0.052 ± 0.002 pmol(107 cells)−1s−1, respectively, and significantly different (p < 0.0001). The rate in SUPKO was not significantly different from zero in this experiment (p = 0.24).
2.6. Expression of TvagENT3 in SUPKO Equals Expression in T. brucei
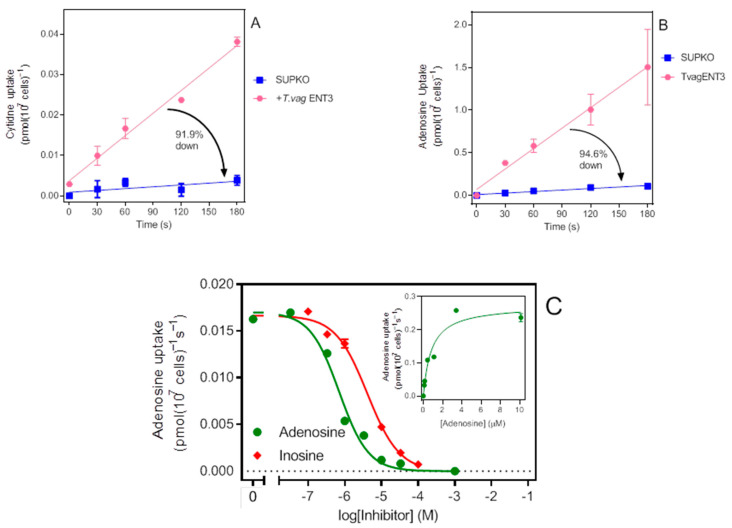
As further validation of the expression system, we next tested a known non-kinetoplastid nucleoside transporter, the Trichomonas vaginalis transporter TvagENT3, which we recently characterized by heterologous expression in Trypanosoma brucei [26]. In that study, TvagENT3 was characterized in the T. brucei cell line TbAT1-KO, which lacks the P2/AT1 transporter, but not the array of P1-type nucleoside transporters [38], using [3H]-cytidine as the probe. Figure 4A shows that TvagENT3 did strongly increase uptake of 500 nM [3H]-cytidine uptake in SUPKO. However, the characterization in T. brucei showed that this transporter had the highest affinity for adenosine, followed by inosine, which we were unable to assess in that system because of the expression of multiple P1-type purine nucleoside transporters [39,40,41]. In contrast, the expression in SUPKO showed a very strong induction of [3H]-adenosine uptake over background upon expression of TvagENT3 (Figure 4B). The adenosine Km value was determined as 0.90 ± 0.13 µM (n = 3), identical to the Ki value found in T. brucei (0.87 ± 0.15 µM), and the inosine Ki as 2.7 ± 0.5 µM (value obtained in T. brucei 3.3 ± 0.07 µM; [26]) (Figure 4C).
Figure 4.
Functional expression of T. vaginalis ENT3 in SUPKO. (A) 500 nM of [3H]-cytidine was significantly higher after expression of TvagENT3 in SUPKO (1.8 × 10−4 ± 1.3 × 10−5 vs. 1.5 × 10−5 ± 9 × 10−6 pmol(107 cells)−1s−1, respectively; p < 0.0001). The cytidine uptake in SUPKO was not significantly different from zero (p = 0.18). (B) Uptake of 50 nM [3H]-adenosine was 94.6% lower in SUPKO than in the same cells expressing TvagENT3 (0.00060 ± 0.00007 vs. 0.0080 ± 0.0004 pmol(107 cells)−1s−1; p < 0.0001). Both slopes were significantly different from zero (p = 0.004 and 0.0003, respectively). (C) Uptake of 50 nM [3H]-adenosine in SUPKO over 60 s in the presence of variable concentrations of unlabelled adenosine and inosine. Inset: Conversion of adenosine inhibition data to a Michaelis–Menten saturation plot.
2.7. Nucleobase and Nucleoside Transport by NT3-KO Cells
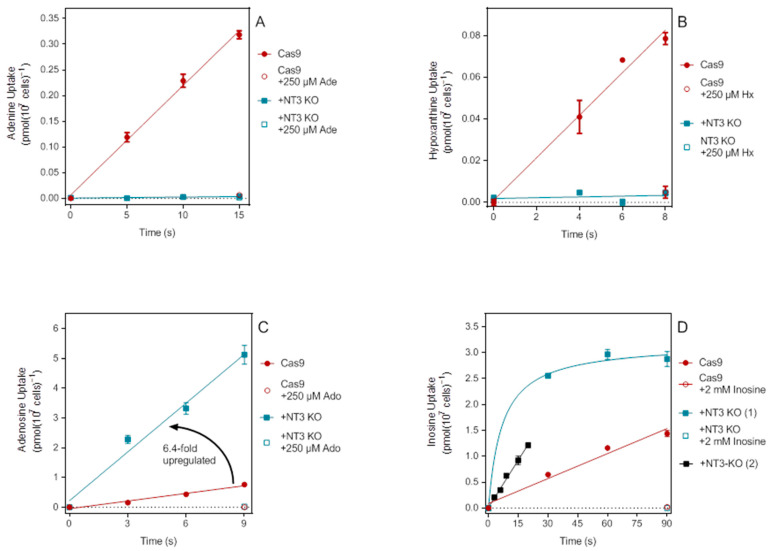
An L. mexicana knockout strain of NT3 was recently generated using CRISPR technology by Dr Richard Burchmore and his group [42] and was made available to us for characterization with respect to nucleobase and nucleoside transport. A similar NT3-KO cell line was first made in L. major by Ortiz et al. [35], after the identification of the gene by the same group [43]. Here, we are primarily concerned with its uses as an expression system for purine nucleobase transporters and as such verified the levels of uptake of a number of purines. Uptake of 100 nM [3H]-adenine in NT3-KO was >100-fold lower than in cas9 cells over 15 s and not significantly different from zero (p = 0.15) over 15 s (Figure 5A). Uptake of 100 nM [3H]-hypoxanthine was particularly fast in cas9 cells and was measured over 8 s (Figure 5B); no significant hypoxanthine uptake was detected in the NT3-KO cells (p = 0.72). It thus appears that the NT4 nucleobase transporter, which in L. major contributes to adenine uptake at neutral pH but is more important in the intra-macrophage amastigote stage [35], does not play a significant role in nucleobase salvage in L. mexicana promastigotes. This probably explains the pronounced growth defect of the L. mexicana NT3-KO (see below) also observed for the L. major NT3-KO, which was restored to wild-type growth rates upon episomal expression of LmajNT3 [35]. The loss of purine nucleobase transport in the LmexNT3-KO was partially compensated by the upregulation of NT1 and NT2 activity. Figure 5C shows the strong upregulation of 100 nM [3H]-adenosine uptake in the NT3-KO. In two separate experiments, the rate increase was 4.0- and 6.4-fold relative to cas9 cells. In separate experiments the uptake rates of [3H]-thymidine and [3H]-cytidine were also upregulated, by 3.45 and by 3.0- and 3.7-fold, respectively; all these are substrates of the NT1 transporters [29]. Similarly, uptake of the NT2 substrates inosine and guanosine was also upregulated. Figure 5D shows the upregulation of inosine uptake by 4-fold in NT3-KO cells, and guanosine uptake was upregulated by 1.7- and 2.1-fold (both p < 0.0001) in two separate experiments. In summary, the uptake of purine nucleobases in LmexNT3-KO cells was not significant over the course of the assay time and, conversely, uptake of nucleosides through NT1 and NT2 was consistently found to be higher than in the cas9 control cells.
Figure 5.
Characterization of purine transport in LmexNT3-KO. (A) Transport of 100 nM [3H]-adenine by L. mexicana cas9 and NT3-KO promastigotes. In NT3-KO cells the rate of adenine transport was <1% of that in cas9 cells (0.000207 ± 0.000094 vs. 0.0213 ± 0.0009 pmol(107 cells)−1s−1; p < 0.0001) and not significantly different from zero (p = 0.16), whereas in cas9 cells the slope was significantly different from zero (p = 0.0019). (B) No significant uptake of 100 nM [3H]-hypoxanthine could be detected in NT3-KO cells (p = 0.72), but this was highly significant in the cas9 parental cells (p = 0.075). The respective rates were 0.00019 ± 0.00045 and 0.0102 ± 0.0009 pmol(107 cells)−1s−1 (p = 0.0006). (C) Upregulation of [3H]-adenosine uptake (100 nM) in NT3-KO cells compared to cas9 cells. Adenosine uptake in the two cell lines was significantly different (p = 0.0010): 0.55 ± 0.05 vs. 0.086 ± 0.009 pmol(107 cells)−1s−1. Uptake in both strains was significantly different from zero (p = 0.0094 and 0.012, respectively). (D) Uptake of 100 nM [3H]-inosine in cas9 and NT3-KO cells. In a first experiment, transport was measured at 30, 60, and 90 s, which linear for cas9 cells (r2 = 0.97; not significantly non-linear by runs test p = 0.67; 0.016 ± 0.002 pmol(107 cells)−1s−1) while the uptake in NT3-KO cells was clearly non-linear over this interval. A reexamination of 100 nM inosine uptake in NT3-KO cells over 20 s showed linearity (r2 = 0.99, not significantly nonlinear, p > 0.999, and almost 4-fold higher than in the cas9 cells (0.061 ± 0.002 pmol(107 cells)−1s−1).
2.8. Expression of TcrNB2 in NT3-KO
Ortiz et al. [35] have already demonstrated that the episomal expression of NT3 restores wild-type levels of nucleobase transport in L. major promastigotes. Instead of repeating this experiment, we chose to introduce the Trypanosoma cruzi NB2 transporter into the L. mexicana NT3-KO cells instead. We have previously reported the characterization of three of the four T. cruzi ENTs by expression in T. brucei procyclics, but the substrate for TcrNB2 could not be identified in that expression system [27]. However, phylogenetic analysis showed that TcrNB2 aligned closest to Leishmania NT4, which is an adenine transporter at neutral pH [35,44]. Unfortunately, the T. brucei NBT-KO strain in which the transporter was expressed retained a high background rate of adenine uptake, although its main locus of three ENT-family nucleobase transporters was deleted [45] and uptake of other purine nucleobases was very low in these cells [27]. The availability of the LmexNT3-KO cell line now allowed us to test our hypothesis. TcrNB2 was cloned into pNUS, and introduced into the NT3-KO and the NT1-KO strains. Clones with high expression were selected by qRT-PCR.
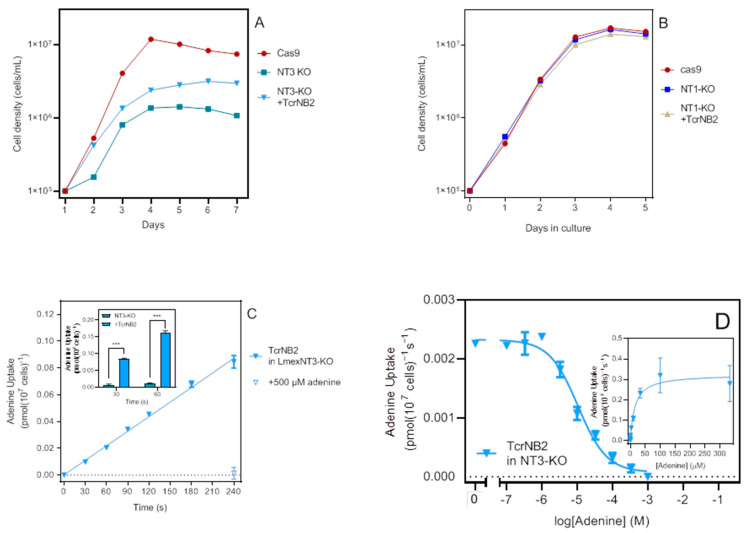
The expression of TcrNB2 increased the growth rate of the LmexNT3-KO cells, although not to the level of the cas9 parental cell line (Figure 6A), but did not change the growth rate of NT1-KO cells (Figure 6B). Whereas TcrNB2 did not increase the uptake of 50 nM [3H]-adenosine, 100 nM [3H]-hypoxanthine or 100 nM [3H]-uracil when expressed in NT1-KO cells (data not shown), [3H]-adenine uptake in NT3-KO cells was readily detectable, linear, and significantly different from zero (p < 0.0001) (Figure 6C) as well as highly significantly different from NT3-KO cells (p < 0.0001; Figure 6C, inset). An average Km value of 14.2 ± 2.6 µM and a Vmax of 0.26 ± 0.04 pmol(107 cells)−1s−1 was obtained (n = 3) (Figure 6D). Consistent with our observations in the T. brucei expression system [27], TcrNB2 appears to be an adenine-only transporter, in that it displayed very low affinity for other purine nucleobases (hypoxanthine) and for adenosine, with 1 mM of these compounds inhibiting adenine uptake by only 31.1 ± 4.3% and 15.5 ± 11.7%, respectively.
Figure 6.
Characterization of TcrNB2 in LmexNT3-KO cells. (A) Growth curve of cas9, NT3-KO and NT3-KO expressing TcrNB2. Symbols are the average of triplicate determinations. (B) Growth curve of cas9, NT1-KO and NT1-KO expressing TcrNB2. (C) Uptake of 100 nM [3H]-adenine in NT3-KO expressing TcrNB2 was linear (r2 = 0.996; not significantly non-linear, p = 0.20) and displayed a rate of 0.00036 ± 0.00001 pmol(107 cells)−1s−1. Inset: separate experiment (n = 3) of [3H]-adenine uptake by NT3-KO and NT3-KO+TcrNB2 cells. *** p < 0.0001 by unpaired t-test. (D) Uptake of 100 nM [3H]-adenine in the presence of various concentrations of unlabelled adenine. Inset: conversion to a Michaelis–Menten saturation plot. The experiment shown is one of three independent repeats, yielding a Km of 14.99 µM; incubation time was 120 s.
2.9. Expression of the Aspergillus nidulans FurD Transporter in the L. mexicana Cell Line 5FU-Res
We have previously described the adaptation of L. mexicana promastigotes to 5F-uracil and the characterization of the resulting Lmex-5FURes strain, which was almost completely deficient in the uptake of [3H]-uracil and [3H]-5F-uracil [29]. It was shown that this is due to the loss of the U1 uracil-specific transporter [34], as uridine uptake was unaffected in Lmex-5FURes cells and the cells should therefore be an ideal background for the expression and characterization of (putative) uracil transporters. In order to test this possibility, we subcloned the Aspergillus nidulans uracil transporter FurD [30] into the pNUS-HcN plasmid for episomal expression in Lmex-5FURes. Three independent clones were generated and the expression levels were screened using qRT-PCR, the highest-expressing clone being selected.
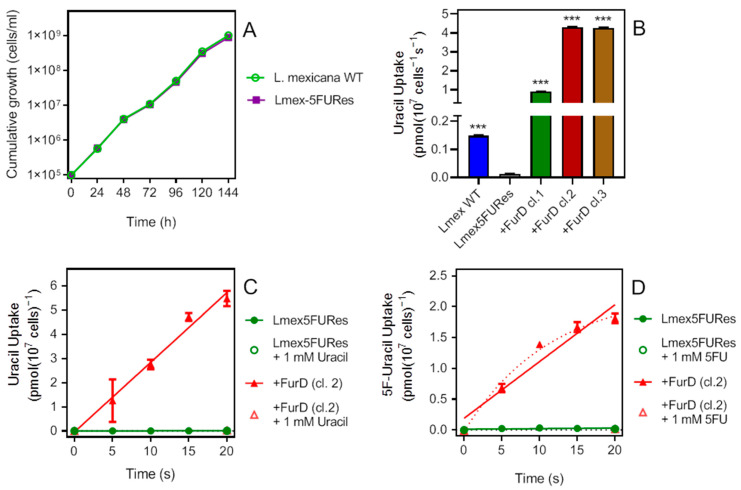
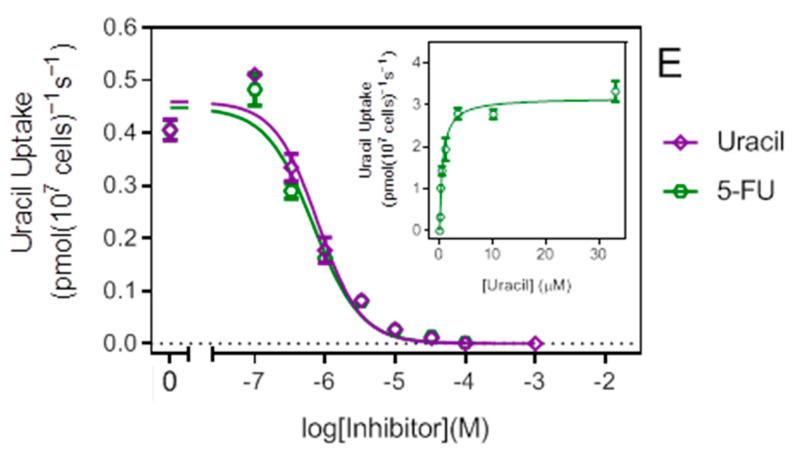
AnFurD differs from the other heterologously expressed transporters used in this study, in that it is a member of the NCS1 gene family [46] rather than the ENT transporter family, which, to date, has not been found in any protozoan species [47,48]. Figure 7A shows that promastigotes of the L. mexicana WT and the derived 5FURes strain displayed the same growth rate in standard HOMEM medium. The expression of this Aspergillus transporter greatly increased uracil uptake in all three clonal strains tested (p < 0.0001), very much above even the level in WT L. mexicana (Figure 7B). Over 20 s, uracil was very robust in the cells expressing FurD but virtually undetectable in the Lmex5FURes at 0.02% of the rate (Figure 7C). Similarly, uptake of 5FU was rapid in the +FurD cells but not significantly different from zero in the control (Figure 7D). The nonlinearity of the FurD-expressing cells for 5FU uptake can be ascribed to a low rate of metabolic incorporation of 5FU compared to uracil, allowing a buildup of the free substrate in the cell. The Km for uracil was determined at 0.89 ± 0.20 µM and the Ki for 5FU as 0.70 ± 0.25 µM, showing that the transporter does not discriminate by binding affinity between the two substrates (p = 0.65) (Figure 7E). This compares with a reported Km of 0.45 µM for uracil and Ki of 0.46 for 5FU in A. nidulans [30], showing that L. mexicana is an excellent expression system for Aspergillus transporters.
Figure 7.
Characterization of Aspergillus nidulans FurD in L. mexicana 5FURes cells. (A) Growth curve of cultures of 5FURes and the parental WT strain. Cells were seeded at a density of 1 × 105 cells/mL, and grown in standard HOMEM supplemented with 10% FCS and 1% penicillin/streptomycin. Cell densities were determined every 24 h. This result represents data from three similar independent repeats. (B) Uptake of 100 nM [3H]-uracil by WT L. mexicana promastigotes and by promastigotes of Lmex5FURes and three clones of that strain expressing A. nidulans FurD. Incubation time was 120 s; *** p < 0.0001 relative to Lmex5FUres, unpaired t-test. (C) Uptake of 100 nM [3H]-uracil Lmex5FUres and the same cells expressing FurD, clone 2 (cl.2). Rates were 0.00057 ± 0.0001 and 0.29 ± 0.02 pmol(107 cells)−1s−1 (p < 0.0001). (D) Like frame C but using 100 nM [3H]-5FU as probe. 5-FU uptake was non-linear (dotted line); uptake in Lmex5FURes was not significantly different from zero (p = 0.27). (E) Uptake of 100 nM [3H]-uracil by Lmex5FURes+FurD cl.2 over 10 s. Inset is the conversion of the uracil inhibit plot to a Michaelis–Menten saturation plot.
3. Discussion
In this paper, we report the construction and/or characterization of a series of L. mexicana cell lines that display null or extremely low background uptake of either adenosine and pyrimidine nucleosides (NT1-KO), oxopurine nucleosides (NT2-KO), all purine nucleosides (SUPKO), purine nucleobases (NT3-KO), or uracil and 5FU (Lmex5FURes). The construction of the NT1-KO and NT2-KO and SUPKO are reported herein and utilized the CRISPR/cas9 cell lines, tools and vectors developed by Beneke et al. [31]. These were also used to make the NT3-KO cell line, as reported [42], whereas 5FURes was developed through adaptation to gradually increased levels of 5FU in vitro [29].
Kinetoplastids are useful systems for the heterologous expression of transporters. For instance, we have previously expressed the T. congolense transporter TcoAT1 in the T. brucei cell line lacking the TbAT1/P2 transporter [25], showing it did not have the proposed role in diminazene resistance [49], a conclusion recently confirmed by meticulous work on diamidine uptake in T. congolense [50]. We have also recently published the first characterization of Trichomonas vaginalis nucleoside transporters by expression in T. brucei [26] and reported a unique substrate binding mode for Toxoplasma gondii oxopurine transporter Tg244440 [28] expressed in a T. brucei cell line from which the main nucleobase transporter locus has been knocked out [27]. The characterization of individually expressed transporters is important because it unambiguously links the transport activity to a specific gene product, and disentangles the (potentially) multiple gene products contributing to the uptake of a certain substrate. T. brucei, for instance, encodes a dozen ENT transporters [1] as well as additional carriers for uracil [36,51], adenine and hypoxanthine that are apparently not encoded by any of the ENT members [37,45]. Only when it is certain that a single transport function is being measured can a meaningful characterization of its kinetic parameters, substrate specificity and binding mode be determined.
Beyond the direct insights into parasite biochemistry and physiology that studies of single transporters provide, they have potential pharmacological significance. The study of nucleoside transporters in protozoa was greatly incentivized with the discovery that the TbAT1/P2 transporter of T. brucei was responsible for the internalization of essential arsenical [5,52] and diamidine drugs [53,54,55]. It has also long been known that protozoan nucleoside transporters mediate the uptake of cytotoxic nucleoside analogues including formycin B and tubercidin used in this study but also, e.g., adenosine arabinoside in T. gondii [18] and cordycepin in T. brucei [10,56]. Very recently, a class of 7-substituted,7-deaza analogues of adenosine and inosine have shown great promise against multiple protozoan pathogens [9,12,13,14,57,58], which is clearly mediated by uptake through nucleoside transporters [8,9,10].
The Leishmania mexicana cell lines we describe here seem highly suitable for the characterization of single transporters. Stable expression of single L. mexicana transporters NT1.1, NT1.2. and NT2 will allow the unambiguous characterization of binding motifs and substrate selectivity of each transporter—something that is much harder in the wild-type cells, or even by cloning each gene separately in the more complex background of TbAT1-KO T. brucei [29]. Moreover, we expressed T. cruzi NB2 in the LmexNT3-KO as phylogenetic analysis had indicated it might be an adenine transporter. We had previously expressed it in the T. brucei NB-KO strain but this retains a high background for adenine [27,45]. That study found no evidence that TcrNB2 was able to transport nucleosides or oxopurine nucleobases. Here, we show that TcrNB2 is indeed an adenine transporter, with almost no affinity for adenosine or hypoxanthine. This work also shows that expression of a purine nucleobase transporter in the NT3-KO rescues the significant growth phenotype, and the strain can thus be used to screen for putative nucleobase transporter genes. The confirmation of TcrNB2 as an adenine-specific transporter concludes the characterization of the four T. cruzi ENT genes that we identified in its genome: TcrNB1 is a hypoxanthine/guanine carrier, TcrNT1 is an inosine/guanosine transporter and TcrNT2 is a thymidine transporter [27].
Expression of non-kinetoplastid transporters is also possible: T. vaginalis ENT3 was successfully expressed in SUPKO and the kinetic parameters matched those of its initial characterization [26]. Further characterization of the nine TvagENTs is now in progress in these cell lines. Finally, we expressed the main uracil transporter from the filamentous fungus Aspergillus nidulans in the 5FURes line. This induced very strong transport activity for both uracil and the cytotoxic analogue 5FU, with Km and Ki values very close to those first reported in A. nidulans [30]. This leads to the idea that uncharacterized fungal transporters, including proteins of important pathogens (e.g., A. fumigatus, Candida species, antibiotic-resistant Mucorales, etc.) can be kinetically studied in specific protozoan cell lines lacking similar endogenous transport activities, as those described herein. From a more fundamental point of view, the nearly identical kinetic characteristics of functionally expressed fungal transporters in protozoa strongly suggest that subcellular tracking mechanisms and specific transporter–membrane lipid interactions [59,60] are functionally conserved between fungi and protozoa, opening a new avenue of cell biology, and biochemical and structural studies of fungal and maybe metazoan transporters in protozoa.
In summary, we report here the characterization of a set of nucleoside and nucleobase transport-deficient L. mexicana strains that can be utilized for the functional expression of protozoan and fungal transporters. These tools will be particularly useful for organisms that are not easily cultured or otherwise obtained in large amounts for transporter studies. As such, we have cloned multiple Trypanosoma vivax ENT genes for expression in our L. mexicana strains. Moreover, some protozoan transporters will only be expressed in life-cycle stages that are not readily available, e.g., T. gondii bradyzoites, multiple Plasmodium liver and insect stages, T. brucei metacyclics or the obligate intracellular amastigotes of trypanosomatids incl. T. cruzi, so heterologous expression is certainly the best way for such studies. The characterized transport activities can then be linked back to the life-cycle stages by expression profiles.
4. Materials and Methods
4.1. Strains and Cultures
Our wild-type L. mexicana strain is MNY/BZ/62/M379, and the 5FURes strain was developed from it by in vitro adaptation to gradually increasing concentrations of 5-fluorouracil (5FU) in vitro [29]. The L. mexicana-Cas9 T7 strain (derived from L. mexicana WT promastigotes by expression of the Streptococcus pyogenes Cas9 nuclease gene) is named cas9 in this study and maintained on 32 μg/mL hygromycin [31]. The NT1-KO (ΔNT1.1,ΔNT1.2), NT-2-KO (ΔNT2) and SUPKO strains were made from this using the CRISPR/cas9 protocols described [31], details below. The NT3-KO strain (ΔNT3) was similarly produced from the cas9 strain [42].
All Leishmania strains were grown as promastigotes, in standard HOMEM (GIBCO, Life Technologies, Paisley, UK) supplemented with 10% heat-inactivated fetal bovine serum (FBS; PAA Laboratories, Linz, Austria) and 1% of a penicillin–streptomycin solution (Life Technologies) at 25 °C, as described [61]. In order to prepare stabilates of L. mexicana promastigotes, cell cultures were added to an equivalent volume of HOMEM containing 30% sterilized glycerol in cryogenic tubes. Before the cryogenic tubes were transferred to liquid nitrogen store, they were frozen at a temperature of −80 °C for 72 h. For the recovery of stabilates, the cryogenic tubes were brought out from the liquid nitrogen store, thawed at 25 °C and then cultivated in HOMEM. The cells were renewed after 20 passages by bringing out fresh cells from the liquid nitrogen store.
4.2. Radiochemicals
The following radiolabeled compounds were used during the project: [methyl-3H]-thymidine (20 Ci/mmol) and [3H(G)]-hypoxanthine (16.1 Ci/mmol) were obtained from PerkinElmer (Waltham, MA, USA). [2,8-3H]-adenine (32.2 Ci/mmol) was from NEN Life Science Products (Boston, MA, USA). [8-3H]-guanosine (12.9 Ci/mmol) and [6-3H]-5-Fluorouracil (20 Ci/mmol) were from Moravek Biochemicals (Brea, CA, USA). [2,8-3H]-adenosine (40 Ci/mmol), [5,6-3H]-uridine (60 Ci/mmol), [8-3H]-inosine (20 Ci/mmol), [5,6-3H]-uracil (40 Ci/mmol), and [5-3H]-cytidine (20 Ci/mmol) were from American Radiolabeled Chemicals (ARC, St-Louis, MO, USA).
4.3. Growth Curves
The growth rates of the L. mexicana promastigotes were determined in the standard HOMEM medium supplemented with 10% FBS, in 12-well plates, in triplicate. After every 24 h, cells were counted in a sample of the culture, using either a Neubauer hemocytometer chamber (Hawksley, Birmingham, UK) or by a coulter particle counter and size analyzer (Beckman, Brea, CA, USA) to count the cells in triplicate. An average of the triplicate readings was taken and plotted using GraphPad Prism 8 software to obtain the growth curves.
4.4. Molecular Cloning Techniques
The sequences of the nucleotide and amino acid for a gene of interest were obtained from the TritrypDB (tritrypdb.org/tritrypdb; accessed 18 February 2020) website [62]. The sequence alignments and the primers that were used in this project were designed by using the CLC Genomics Workbench version 7.0 software package (CLC bio, Qiagen, Singapore). All the primers used in this project were synthesized by Eurofins MWG Operon (Ebersberg, Germany) and Sigma-Aldrich (Dorset, UK). PCR amplification for sequencing or cloning was performed using Phusion High-Fidelity DNA Polymerase (New England BioLabs, Hitchin, UK), whereas routine screening of gene presence would use GoTaq DNA Polymerase (Promega, Southampton, UK). PCR reaction products were separated on a 1% or 2% of agarose gel with 50 μL/L of SYBR Safe DNA gel stain (Invitrogen) and visualized under UV light.
Expression of genes in L. mexicana used the episomal expression vector pNUS-HcN which contains a G418 resistance gene [63]. These included T. cruzi NB2 (tritrypdb TcCLB.506445.110; TcrNB2), T. vaginalis ENT3 (TVAG_271560; TvagENT3), L. mexicana NT1.1 (tritrypdb LmxM.15.1230), L. mexicana NT1.2 (LmxM.15.1240), L. mexicana NT2 (LmxM.36.1940) and A. nidulans FurD (ENA accession number ABR22526). The appropriate restriction enzymes were used to digest the pNUS-HcN plasmid and the gene of interest (GOI), ligation was performed with T4 DNA Ligase (Promega) or used the NEBBuilder HiFi DNA Assembly Cloning Kit (New England BioLabs), and followed by transfection into E. coli XL1-blue cells (Agilent, Stockport, UK) by heat shock, following the manufacturer’s protocol. Colonies were screened by PCR screening using the forward primer of the GOI and the reverse primer of pNUS-HcN plasmid (HDK340). Plasmid from PCR-positive clones were sent to Source Bioscience (Livingston, United Kingdom) for Sanger sequencing. 25 µg of the verified plasmids (pHDK245 ‘FurD’ and pHDK271 ‘TcrNB2′) were precipitated by ethanol and resuspended in 15 µL sterile water.
L. mexicana promastigotes (5 × 107 cells) were washed with 100 µL transfection buffer and mixed with 10 µg DNA of the expression construct. The cells were electroporated with the Amaxa Nucleofector (Amaxa AG, Cologne, Germany), using Program U-033. Transfected cells were transferred to 20 mL HOMEM medium containing 10% FBS and allowed to recover overnight at 25 °C after which 50 μg/mL of G418 was added to the culture as a selection agent. To select single cells by limiting dilution the cells were plated out in a 96-well plate to produce individual clones. PCR was used to screen the positive clones, the forward primer of the GOI and the reverse primer of pNUS-HcN plasmid (HDK340) were used to confirm the presence of the target gene in the new strain. The Macherey-Nagel NucleoSpin Tissue kit (Fisher Scientific, Loughborough, UK) was used to extract genomic DNA, according to the instructions given by the manufacturer.
4.5. Construction of Knockouts Using CRISPR/cas9
Knockout clones of ΔNT1.1,ΔNT1.2 (NT1-KO) and ΔNT2 (NT2-KO) were prepared using the CRISPR/cas9 system developed by Beneke et al. [31]. The LmexNT1.1/NT1.2 locus was replaced with a blasticidin selection cassette (pTBlast) in the presence of 5 μM tubercidin, which selected for the replacement of both copies of the NT1 locus, as the retention of one allele will allow sufficient tubercidin uptake to be lethal. Similarly, the LmexNT2 allele was replaced with a puromycin (pTPuro)-resistant cassette in the presence of 1 μM formycin B, a cytotoxic inosine analogue taken up by the NT2 transporter. Primers and single guide RNAs (sgRNAs) were designed using the online platform LeishGEdit and are listed in Supplemental Table S1.
For the PCR reaction for the amplification of 5′ sgRNA and 3′ sgRNA templates (50 µL total volume), PCR steps were 30 s at 98 °C followed by 35 cycles of 10 s at 98 °C, 30 s at 60 °C, and 15 s at 72 °C. This reaction (3 µL) was run on a 2% agarose gel to check for the presence of the expected product. The remainder was heat-sterilized at 94 °C for 5 min and transfected without further purification. The pTBlast and pTPuro plasmids were amplified using primer pairs HDK1742/1743 and HDK1755/1756, respectively, as follows: 110 °C, followed 5 min at 94°C followed by 40 cycles of 10 s at 94 °C, 30 s at 65 °C, 20 s at 72 °C. This reaction (3 µL) was run on a 1% agarose gel to check for the presence of the expected product. The remainder was heat-sterilized at 94 °C for 5 min and transfected without further purification.
For the transfection, 1 × 107 cells of cell line L. mexicana Cas9 T7 promastigotes (maintained in the presence of 32 μg/mL of hygromycin) were washed with 150 µL of transfection buffer and mixed with 100 µL of the heat-sterilized mixture of the sgRNA templates and the resistance cassettes in 2 mm electroporation cuvettes. Electroporation was carried out using an Amaxa Nucleofector, program X-001 (Amaxa AG, Cologne, Germany). Immediately, the cells were transferred to 20 mL HOMEM medium with 10% FBS and incubated at 25 °C. The cells were allowed to recover overnight, after which 5 μg/mL of blasticidin and 5 μM of tubercidin (NT-KO) or 1 μM formycin B plus 20 μg/mL of puromycin (NT2-KO). The culture was diluted (1:10, 1:25, 1:100) in a 24-well plate to grow individual clones. After 7–14 days, positive clones were grown. The positive clones were then picked from the 24-well plate and transferred to 10 mL of fresh preheated HOMEM media/10% FBS plus the appropriate selection agents. Gene deletion was confirmed using diagnostic PCR primers HDK1748/1749 and HDK1750/1751 for NT1-KO and NT2-KO, respectively (Supplemental Table S2). After confirmation, the process of NT1 allele deletion was repeated with a NT2-KO clone and, conversely, NT2-deletion with a NT1-KO clone was performed exactly as described above. Confirmation of the second-round knockout (generation of SUPKO) used the same primer pairs as used in the generation of the single gene-deletion strains.
4.6. qRT-PCR of Heterologously Expressed Genes
RNA was extracted using the NucleoSpin RNA kit (Macherey-Nagel, Düren, Germany) in accordance with the manufacturer’s instructions and quantified using a NanoDrop ND-1000 spectrophotometer; samples were stored at −80 °C until further use. qRT-PCR primers were designed using Primer Express 3.0 software (Supplemental Table S3). A total volume of 20 μL was obtained for each sample by diluting 4 μg of RNA in RNase-free water and cDNA was synthesized using the Precision nanoScript 2 Reverse Transcription kit (PrimerDesign Ltd., Eastleigh, UK) and stored at −20 °C. Primer efficiency was established using the method of Pfaffl [64]. The cDNA was amplified using the PrecisionPLUS OneStep RT-qPCR Master Mix kit (PrimerDesign Ltd., Eastleigh, UK) in a 7500 Real-Time PCR System coupled to a desktop computer (Thermo Fisher Scientific, Oxford, UK). Samples without reverse transcriptase (RT) or cDNA were used in the experiment as negative controls and the gene expression was normalized to GPI8 expression, which is a standard reference gene in L. mexicana [65]. Relative quantification was calculated using the delta delta ct method. Applied Biosystems 7500 Fast Real-Time PCR System Software (Thermo Fisher Scientific, Oxford, UK) was used for analysis of data. Each experiment was carried out with three independent determinations.
4.7. Alamar Blue Assay for Cell Survival and Drug Sensitivity
The assay is based on the reduction of the dye resazurin (Alamar blue) by live but not dead cells [66] and was performed with various strains of Leishmania mexicana promastigotes, exactly as described [67]. Briefly, doubling dilutions starting at 100 µM were set up over 2 rows of a 96-well plate and 2 × 105 cells were added to each well, followed by incubation at 25 °C. After 72 h, 20 µL of 125 µg/mL resazurin solution was added and the plates incubated for a further 24 h. Fluorescence was determined in a FLUOstar OPTIMA plate reader (BMG Labtech, Ortenberg, Germany), reading the fluorescence intensity in arbitrary units (A.U.) at 544 nm wavelength for excitation and 620 nm wavelength for emission. The EC50 values and fluorescence data were determined and plotted to a sigmoid curve with variable slope, using the GraphPad Prism 8 software package. All EC50 values were determined on at least three independent occasions and are presented as average ± SEM.
4.8. Transport Assays
Transport assays were performed exactly as described previously [2]. Briefly, 107 cells in 100 μL of assay buffer (AB) were mixed with an equal volume of radiolabel at 2× concentration for a preset time. The incubation was terminated by the addition of 700 μL of “Stop solution”, being ice-cold 1 mM solution of unlabelled permeant, followed by immediate centrifugation through an oil layer in a microfuge at 13,000 rpm. This causes the cell pellet to reside under the oil layer and isolated from residual radiolabel in the assay buffer. The tubes were flash-frozen in liquid nitrogen and the bottom tip, containing the cell pellet, cut off and collected in a scintillation tube. Cells were solubilized by gentle shaking after addition of a 2% SDS solution, before the addition of scintillation fluid (Scintilogic U, Lablogic, Sheffield, UK); the tubes were left to stand in the dark overnight before counting in a 300SL Hidex Scintillation Counter (Lablogic). Linear and nonlinear regression analyses were carried out using GraphPad Prism versions 8.0 and higher. Statistical analyses and parameters of linear regression included the coefficient of determination r2, test for whether the slope is significantly different from zero (F-test), test for whether the line is significantly non-linear (runs test) and test whether two slopes are significantly different (F-test). Whether two data points of at least three replicates were significantly different from each other was determined by unpaired t-test. In this paper, individual experiments, always performed in triplicate or quadruplicate, are shown in the figures. However, all parameters, including Km, Vmax, Ki, given are the average of at least three separate, independent determinations; errors given are standard errors, unless otherwise specified.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23158139/s1.
Author Contributions
Conceptualization, H.P.D.K.; methodology, M.J.N., R.J.S.B. and H.P.D.K.; formal analysis, H.P.D.K.; investigation, M.M.A., T.A.A., H.A.A.E., M.J.N., I.A.A., G.D.C. and B.S.; resources, R.J.S.B. and G.D.; writing—original draft preparation, H.P.D.K.; writing—review and editing, R.J.S.B. and G.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by a Wellcome Trust Institutional Strategic Support Fund (ISSF) Award (ref 317019); by studentships from the Libyan Government to M.M.A.; by a fellowship from the Government of Saudi Arabia to M.J.N.; by studentship from the Saudi Arabian Government to T.A.A. and I.A.A.; and by Science Without Borders with a scholarship to G.D.C. (206385/2014-5, CNPq, Brazil).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Koning H.P., Bridges D.J., Burchmore R. Purine and pyrimidine transport in protozoa: From biology to therapy. FEMS Microbiol. Rev. 2005;29:987–1020. doi: 10.1016/j.femsre.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Wallace L.J.M., Candlish D., De Koning H.P. Different substrate recognition motifs of human and trypanosome nucleobase transporters: Selective uptake of purine antimetabolites. J. Biol. Chem. 2002;277:26149–26156. doi: 10.1074/jbc.M202835200. [DOI] [PubMed] [Google Scholar]
- 3.Carter N.S., Barrett M.P., De Koning H.P. A drug resistance determinant from Trypanosoma brucei. Trends Microbiol. 1999;7:469–471. doi: 10.1016/S0966-842X(99)01643-1. [DOI] [PubMed] [Google Scholar]
- 4.Munday J.C., Settimo L., De Koning H.P. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Front. Pharmacol. 2015;6:32. doi: 10.3389/fphar.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter N.S., Fairlamb A.H. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature. 1993;361:173–176. doi: 10.1038/361173a0. [DOI] [PubMed] [Google Scholar]
- 6.Collar C.J., Al-Salabi M.I., Stewart M.L., Barrett M.P., Wilson W.D., De Koning H.P. Predictive computational models of substrate binding by a nucleoside transporter. J. Biol. Chem. 2009;284:34028–34035. doi: 10.1074/jbc.M109.049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munday J.C., Tagoe D.N.A., Eze A.A., Krezdorn J.A., Rojas López K.E., Alkhaldi A.A.M., McDonald F., Still J., Alzahrani K.J., Settimo L., et al. Functional analysis of drug resistance-associated mutations in the Trypanosoma brucei adenosine transporter 1 (TbAT1) and the proposal of a structural model for the protein. Mol. Microbiol. 2015;96:887–900. doi: 10.1111/mmi.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulpia F., Campagnaro G.D., Scortichini M., Van Hecke K., Maes L., De Koning H.P., Caljon G., Van Calenbergh S. Revisiting tubercidin against kinetoplastid parasites: Aromatic substitutions at position 7 improve activity and reduce toxicity. Eur. J. Med. Chem. 2019;164:689–705. doi: 10.1016/j.ejmech.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 9.Hulpia F., Mabille D., Campagnaro G.D., Schumann G., Maes L., Roditi I., Hofer A., De Koning H.P., Galjon G., Van Calenbergh S. Combining tubercidin and cordycepin scaffolds results in highly active candidates to treat late-stage sleeping sickness. Nat. Commun. 2019;10:5564. doi: 10.1038/s41467-019-13522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vodnala S.K., Lundbäck T., Yeheskieli E., Sjöberg B., Gustavsson A.L., Svensson R., Olivera G.C., Eze A.A., De Koning H.P., Hammarström L.G.J., et al. Structure-activity relationships of synthetic cordycepin analogues as experimental therapeutics for Africa trypanosomiasis. J. Med. Chem. 2013;56:9861–9873. doi: 10.1021/jm401530a. [DOI] [PubMed] [Google Scholar]
- 11.Ranjbarian F., Vodnala M., Alzahrani K.J.H., Ebiloma G.U., De Koning H.P., Hofer A. 9-(2-Deoxy-2-fluoro-ß-D-arabinofuranosyl) is a potent antitrypanosomal adenosine analogue that circumvents transport-related drug resistance. Antimicrob. Agents Chemother. 2017;61:e0271-16. doi: 10.1128/AAC.02719-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabille D., Ilbeigi K., Hendrickx S., Ungogo M.A., Hulpia F., Lin C., Maes L., De Koning H.P., Van Calenbergh S., Caljon G. Nucleoside analogues for the treatment of animal African trypanosomiasis. Int. J. Parasitol. Drugs Drug Resist. 2022;19:21–30. doi: 10.1016/j.ijpddr.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin C., Ferreira de Almeida Fiuza L., Cardoso Santos C., Ferreira Nunes D., Cruz Moreira O., Bouton J., Karalic I., Maes L., Caljon G., Hulpia F., et al. 6-Methyl-7-Aryl-7-Deazapurine Nucleosides as Anti-Trypanosoma cruzi Agents: Structure-Activity Relationship and in vivo Efficacy. ChemMedChem. 2021;16:2231–2253. doi: 10.1002/cmdc.202100144. [DOI] [PubMed] [Google Scholar]
- 14.Natto M.J., Hulpia F., Kalkman E.R., Baillie S., Alhejeli A., Miyamoto Y., Eckmann L., Van Calenbergh S., De Koning H.P. Deazapurine nucleoside analogues for the treatment of Trichomonas vaginalis. ACS Infect. Dis. 2021;7:1752–1764. doi: 10.1021/acsinfecdis.1c00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C., Jaén Batista D.D.G., Mazzeti A.L., Donola Girão R., de Oliveira G.M., Karalic I., Hulpia F., Soeiro M.N.C., Maes L., Caljon G., et al. N6-modification of 7-Deazapurine nucleoside analogues as anti-Trypanosoma cruzi and anti-Leishmania agents: Structure-activity relationship exploration and in vivo evaluation. Eur. J. Med. Chem. 2022;231:114165. doi: 10.1016/j.ejmech.2022.114165. [DOI] [PubMed] [Google Scholar]
- 16.Carter N.S., Drew M.E., Sanchez M., Vasudevan G., Landfear S.M., Ullman B. Cloning of a novel inosine–guanosine transporter gene from Leishmania donovani by functional rescue of a transport-deficient mutant. J. Biol. Chem. 2000;275:20935–20941. doi: 10.1074/jbc.M002418200. [DOI] [PubMed] [Google Scholar]
- 17.Iovannisci D.M., Kaur K., Young L., Ullman B. Genetic analysis of nucleoside transport in Leishmania donovani. Mol. Cell. Biol. 1984;4:1013–1019. doi: 10.1128/mcb.4.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang C.W., Carter N., Sullivan W.J., Jr., Donald R.G., Roos D.S., Naguib F.N., el Kouni M.H., Ullman B., Wilson C.M. The adenosine transporter of Toxoplasma gondii. Identification by insertional mutagenesis, cloning, and recombinant expression. J. Biol. Chem. 1999;274:35255–35261. doi: 10.1074/jbc.274.49.35255. [DOI] [PubMed] [Google Scholar]
- 19.Vasudevan G., Carter N.S., Drew M.E., Beverley S.M., Sanchez M.A., Seyfang A., Ullman B., Landfear S.M. Cloning of Leishmania nucleoside transporter genes by rescue of a transport-deficient mutant. Proc. Natl. Acad. Sci. USA. 1998;95:9873–9878. doi: 10.1073/pnas.95.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downie M.J., Saliba K.J., Bröer S., Howitt S.M., Kirk K. Purine nucleobase transport in the intraerythrocytic malaria parasite. Int. J. Parasitol. 2008;38:203–209. doi: 10.1016/j.ijpara.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Parker M.D., Hyde R.J., Yao S.Y., McRobert L., Cass C.E., Young J.D., McConkey G.A., Baldwin S.A. Identification of a nucleoside/nucleobase transporter from Plasmodium falciparum, a novel target for anti-malarial chemotherapy. Biochem. J. 2000;349:67–75. doi: 10.1042/bj3490067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffith D.A., Jarvis S.M. Nucleoside and nucleobase transport systems of mammalian cells. Biochim. Biophys. Acta. 1996;1286:153–181. doi: 10.1016/S0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 23.Mäser P., Sütterlin C., Kralli A., Kaminsky R. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science. 1999;285:242–244. doi: 10.1126/science.285.5425.242. [DOI] [PubMed] [Google Scholar]
- 24.Burchmore R., Wallace L.J.M., Candlish D., Al-Salabi M.I., Beal P., Barrett M.P., Baldwin S.A., De Koning H.P. Cloning, heterologous expression, and in situ characterization of the first high affinity nucleobase transporter from a protozoan. J. Biol. Chem. 2003;278:23502–23507. doi: 10.1074/jbc.M301252200. [DOI] [PubMed] [Google Scholar]
- 25.Munday J.C., Rojas López K.E., Eze A.A., Delespaux V., Van Den Abbeele J., Rowan T., Barrett M.P., Morrison L.J., De Koning H.P. Functional expression of TcoAT1 reveals it to be a P1-type nucleoside transporter with no capacity for diminazene uptake. Int. J. Parasitol. Drugs Drug Resist. 2013;3:69–76. doi: 10.1016/j.ijpddr.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natto M.J., Miyamoto Y., Munday J.C., AlSiari T.A., Al-Salabi M.I., Quashie N.B., Eze A.A., Eckmann L., De Koning H.P. Comprehensive characterization of purine and pyrimidine transport activities in Trichomonas vaginalis and functional cloning of a trichomonad nucleoside transporter. Mol. Microbiol. 2021;116:1489–1511. doi: 10.1111/mmi.14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campagnaro G.D., de Freitas Nascimento J., Girard R.B.M., Silber A.M., De Koning H.P. Cloning and characterisation of the Equilibrative Nucleoside Transporter family of Trypanosoma cruzi: Ultra-high affinity and selectivity to survive in the intracellular niche. Biochim. Biophys. Acta Gen. Subj. 2018;1862:2750–2763. doi: 10.1016/j.bbagen.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Campagnaro G.D., Elati H.A.A., Balaska S., Martin Abril M.E., Natto M.J., Hulpia F., Lee K., Sheiner L., Van Calenbergh S., De Koning H.P. A Toxoplasma gondii oxopurine transporter binds nucleobases and nucleosides using different binding modes. Int. J. Mol. Sci. 2022;23:710. doi: 10.3390/ijms23020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alzahrani K.J.H., Ali J.A.M., Eze A.A., Looi W.L., Tagoe D.N.A., Creek D.J., Barrett M.P., De Koning H.P. Functional and genetic evidence that nucleoside transport is highly conserved in Leishmania species: Implications for pyrimidine-based chemotherapy. Int. J. Parasitol. Drugs Drug Resist. 2017;7:206–226. doi: 10.1016/j.ijpddr.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amillis S., Hamari Z., Roumelioti K., Scazzocchio C., Diallinas G. Regulation of expression and kinetic modeling of substrate interactions of a uracil transporter in Aspergillus nidulans. Mol. Membr. Biol. 2007;24:206–214. doi: 10.1080/09687860601070806. [DOI] [PubMed] [Google Scholar]
- 31.Beneke T., Madden R., Makin L., Valli J., Sunter J., Gluenz E. A CRISPR Cas9 high-throughput genome editing toolkit for kinetoplastids. R. Soc. Open Sci. 2017;4:170095. doi: 10.1098/rsos.170095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W., Boitz J.M., Galazka J., Arendt C.S., Carter N.S., Ullman B. Functional characterization of nucleoside transporter gene replacements in Leishmania donovani. Mol. Biochem. Parasitol. 2006;150:300–307. doi: 10.1016/j.molbiopara.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Koning H.P., Jarvis S.M. Purine nucleobase transport in bloodstream forms of Trypanosoma brucei brucei is mediated by two novel transporters. Mol. Biochem. Parasitol. 1997;89:245–258. doi: 10.1016/S0166-6851(97)00129-1. [DOI] [PubMed] [Google Scholar]
- 34.Papageorgiou I.G., Yakob L., Al-Salabi M.I., Diallinas G., Soteriadou K., De Koning H.P. Identification of the first pyrimidine nucleobase transporter in Leishmania: Similarities with the Trypanosoma brucei U1 transporter and antileishmanial activity of uracil analogues. Parasitology. 2005;130:275–283. doi: 10.1017/S0031182004006626. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz D., Sanchez M.A., Pierce S., Hermann T., Kimblin N., Bouwer H.G.A., Landfear S.M. Molecular genetic analysis of purine nucleobase transport in Leishmania major. Mol. Microbiol. 2007;64:1228–1243. doi: 10.1111/j.1365-2958.2007.05730.x. [DOI] [PubMed] [Google Scholar]
- 36.De Koning H.P., Jarvis S.M. A highly selective, high affinity transporter for uracil in Trypanosoma brucei brucei; evidence for proton-dependent transport. Biochem. Cell Biol. 1998;76:853–858. doi: 10.1139/o98-086. [DOI] [PubMed] [Google Scholar]
- 37.De Koning H.P. Pyrimidine transporters of protozoa—A class apart? Trends Parasitol. 2007;23:190. doi: 10.1016/j.pt.2007.03.004. [DOI] [Google Scholar]
- 38.Matovu E., Stewart M., Geiser F., Brun R., Mäser P., Wallace L.J.M., Burchmore R.J., Enyaru C.K., Barrett M.P., Kaminsky R., et al. Mechanisms of Arsenical and Diamidine Uptake and Resistance in Trypanosoma brucei. Eukaryot. Cell. 2003;2:1003–1008. doi: 10.1128/EC.2.5.1003-1008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Koning H.P., Watson C.J., Jarvis S.M. Characterisation of a nucleoside/proton symporter in procyclic Trypanosoma brucei brucei. J. Biol. Chem. 1998;273:9486–9494. doi: 10.1074/jbc.273.16.9486. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez M.A., Tryon R., Green J., Boor I., Landfear S.M. Six related nucleoside/nucleobase transporters from Trypanosoma brucei exhibit distinct biochemical functions. J. Biol. Chem. 2002;277:21499–21504. doi: 10.1074/jbc.M202319200. [DOI] [PubMed] [Google Scholar]
- 41.Al-Salabi M.I., Wallace L.J.M., Lüscher A., Mäser P., Candlish D., Rodenko B., Gould M.K., Jabeen I., Ajith S.N., De Koning H.P. Molecular interactions underlying the unusually high adenosine affinity of a novel Trypanosoma brucei nucleoside transporter. Mol. Pharmacol. 2007;71:921–929. doi: 10.1124/mol.106.031559. [DOI] [PubMed] [Google Scholar]
- 42.Kabli A.M.M. Ph.D. Thesis. University of Glasgow; Glasgow, UK: 2020. Polyomic Analyses for Rational Antileishmanial Vaccine Development: A Role for Membrane Transporters? [Google Scholar]
- 43.Sanchez M.A., Tryon R., Pierce S., Vasudevan G., Landfear S.M. Functional expression and characterization of a purine nucleobase transporter gene from Leishmania major. Mol. Membr. Biol. 2004;21:11–18. doi: 10.1080/0968768031000140845. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz D., Sanchez M.A., Koch H.P., Larsson H.P., Landfear S.M. An acid-activated nucleobase transporter from Leishmania major. J. Biol. Chem. 2009;284:16164–16169. doi: 10.1074/jbc.M109.006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campagnaro G.D., Alzahrani K.J.H., Munday J.C., De Koning H.P. Trypanosoma brucei bloodstream forms express highly specific and separate transporters for adenine and hypoxanthine; evidence for a new protozoan purine transporter family? Mol. Biochem. Parasitol. 2018;220:46–56. doi: 10.1016/j.molbiopara.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Krypotou E., Evangelidis T., Bobonis J., Pittis A.A., Gabaldón T., Scazzocchio C., Mikros E., Diallinas G. Origin, diversification and substrate specificity in the family of NCS1/FUR transporters. Mol. Microbiol. 2015;96:927–950. doi: 10.1111/mmi.12982. [DOI] [PubMed] [Google Scholar]
- 47.Patching S.G. Recent developments in nucleobase cation symporter-1 (NCS1) family transport proteins from bacteria, archaea, fungi and plants. J. Biosci. 2018;43:797–815. doi: 10.1007/s12038-018-9780-3. [DOI] [PubMed] [Google Scholar]
- 48.Campagnaro G.D., De Koning H.P. Purine and pyrimidine transporters of pathogenic protozoa—Conduits for therapeutic agents. Med. Res. Rev. 2020;40:1679–1714. doi: 10.1002/med.21667. [DOI] [PubMed] [Google Scholar]
- 49.Delespaux V., De Koning H.P. Transporters in anti-parasitic drug development and resistance. In: Jäger T., Koch O., Flohe L., editors. Trypanosomatid Diseases: Molecular Routes to Drug Discovery. Wiley-Blackwell; Weinheim, Germany: 2013. pp. 335–349. [Google Scholar]
- 50.Ungogo M.A., Campagnaro G.D., Alghamdi A.H., Natto M.J., De Koning H.P. Differences in transporters rather than drug targets are the principal determinants of the different innate sensitivities of Trypanosoma congolense and Trypanozoon subgenus trypanosomes to diamidines and melaminophenyl arsenicals. Int. J. Mol. Sci. 2022;23:2844. doi: 10.3390/ijms23052844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gudin S., Quashie N.B., Candlish D., Al-Salabi M.I., Jarvis S.M., Ranford-Cartwright L.C., De Koning H.P. Trypanosoma brucei: A survey of pyrimidine transport activities. Exp. Parasitol. 2006;114:103–108. doi: 10.1016/j.exppara.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 52.De Koning H.P., MacLeod A., Barrett M.P., Cover B., Jarvis S.M. Further evidence for a link between melarsoprol resistance and P2 transporter function in African trypanosomes. Mol. Biochem. Parasitol. 2000;106:181–185. doi: 10.1016/S0166-6851(99)00206-6. [DOI] [PubMed] [Google Scholar]
- 53.De Koning H.P., Anderson L.F., Stewart M., Burchmore R.J.S., Wallace L.J.M., Barrett M.P. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter; additional insights in diamidine resistance in African trypanosomes. Antimicrob. Agents Chemother. 2004;48:1515–1519. doi: 10.1128/AAC.48.5.1515-1519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart M.L., Burchmore R.J.S., Clucas C., Hertz-Fowler C., Brook K., Tait A., McLeod A., Turner C.M.R., De Koning H.P., Wong P.E., et al. Multiple genetic mechanisms lead to the loss of functional TbAT1 expression in drug resistant trypanosomes. Eukaryot. Cell. 2010;9:336–343. doi: 10.1128/EC.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward C.P., Wong P.E., Burchmore R.J., De Koning H.P., Barrett M.P. Trypanocidal furamidine analogues: Influence of pyridine nitrogens on trypanocidal activity, transport kinetics and resistance patterns. Antimicrob. Agents Chemother. 2011;55:2352–2361. doi: 10.1128/AAC.01551-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geiser F., Lüscher A., De Koning H.P., Seebeck T., Mäser P. Molecular pharmacology of adenosine transport in Trypanosoma brucei: P1/P2 revisited. Mol. Pharmacol. 2005;68:589–595. doi: 10.1124/mol.104.010298. [DOI] [PubMed] [Google Scholar]
- 57.Hulpia F., Van Hecke K., França da Silva C., da Gama Jaen Batista D., Maes L., Caljon G., Correia Soeiro M.N., Van Calenbergh S. Discovery of novel 7-aryl 7-deazapurine 3’-deoxy-ribofuranosyl nucleosides with potent activity against Trypanosoma cruzi. J. Med. Chem. 2018;61:9287–9300. doi: 10.1021/acs.jmedchem.8b00999. [DOI] [PubMed] [Google Scholar]
- 58.Hulpia F., Bouton J., Campagnaro G.D., Alfayez I.A., Mabille D., Maes L., De Koning H.P., Caljon G., Van Calenbergh S. C6-O-Alkylated 7-deazainosine nucleoside analogues: Discovery of potent and selective anti-sleeping sickness agents. Eur. J. Med. Chem. 2020;18:112018. doi: 10.1016/j.ejmech.2019.112018. [DOI] [PubMed] [Google Scholar]
- 59.Diallinas G., Martzoukou O. Transporter membrane traffic and function: Lessons from a mould. FEBS J. 2019;286:4861–4875. doi: 10.1111/febs.15078. [DOI] [PubMed] [Google Scholar]
- 60.Dimou S., Diallinas G. Life and death of fungal transporters under the challenge of polarity. Int. J. Mol. Sci. 2020;21:5376. doi: 10.3390/ijms21155376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Salabi M.I., Wallace L.J.M., De Koning H.P. A Leishmania major nucleobase transporter responsible for allopurinol uptake is a functional homologue of the Trypanosoma brucei H2 transporter. Mol. Pharmacol. 2003;63:814–820. doi: 10.1124/mol.63.4.814. [DOI] [PubMed] [Google Scholar]
- 62.Aslett M., Aurrecoechea C., Berriman M., Brestelli J., Brunk B.P., Carrington M., Depledge D.P., Fischer S., Gajria B., Gao X., et al. TriTrypDB: A functional genomic resource for the Trypanosomatidae. Nucleic Acids Res. 2010;38:D457. doi: 10.1093/nar/gkp851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tetaud E., Lecuix I., Sheldrake T., Baltz T., Fairlamb A.H. A new expression vector for Crithidia fasciculata and Leishmania. Mol. Biochem. Parasitol. 2002;120:195–204. doi: 10.1016/S0166-6851(02)00002-6. [DOI] [PubMed] [Google Scholar]
- 64.Pfaffl M.W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucl. Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang X., Szallies A., Rawer M., Echner H., Duszenko M. GPI anchor transamidase of Trypanosoma brucei: In vitro assay of the recombinant protein and VSG anchor exchange. J. Cell. Sci. 2002;115:2529–2539. doi: 10.1242/jcs.115.12.2529. [DOI] [PubMed] [Google Scholar]
- 66.Gould M.K., Vu X.L., Seebeck T., De Koning H.P. Propidium iodide-based methods for monitoring drug action in the kinetoplastidae: Comparison with the Alamar Blue assay. Anal. Biochem. 2008;382:87–93. doi: 10.1016/j.ab.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 67.Anyam J.V., Daikwo P.E., Ungogo M.A., Nweze N.E., Igoli N.P., Gray A.I., De Koning H.P., Igoli J.O. Two New Diterpenes from the roots of Acacia nilotica. Front. Chem. 2021;9:624741. doi: 10.3389/fchem.2021.624741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.